Abstract
Background
Nowadays, the heterogeneity of chronic rhinosinusitis (CRS) has attracted extensive attention. The histological patterns and clinical characteristics may vary greatly in different areas and among different groups of people. Prior studies found a shift from the neutrophilic inflammatory pattern to the eosinophilic inflammatory pattern in Asian cities. This study set out with the aim of investigating the changes that have occurred in the past 18 years of southern China and exploring the causes.
Methods
Tissues, clinical, and demographic characteristics were obtained from 473 patients (91 in 2000–2001, 170 in 2010–2011, 212 in 2017–2018) who satisfied the criteria of diffuse (bilateral) chronic rhinosinusitis. The clinical characteristics, including the previous history of allergic rhinitis and asthma, and the major symptoms of rhinosinusitis, were collected. Formalin-fixed nasal tissue was obtained from each patient for calculating inflammatory cells. We also performed immunohistochemistry to evaluate the expression levels of eosinophilic cationic protein (ECP), IgE, myeloperoxidase (MPO), and other Type 1, Type 2, and Type 3 related inflammatory cytokines.
Results
The comorbidity of asthma and atopic disease was higher in 2017–2018 compared to 2000–2001. The histological characteristics revealed a significant increase in tissue eosinophils and decrease in neutrophils in 2017–2018 as compared with 2000–2001. Meanwhile, the proportion of eosinophilic CRS (eCRS) increased significantly from 2000 to 2001 to 2017–2018 (P = 0.03). The tissue eosinophil increase was higher in overweight patients (Body Mass Index, BMI≥24) as compared with non-overweight. There was an increasing trend of ECP, IL-13 and IL-17. Besides, IFN-γ and TNF-α decreased.
Conclusions
There was an eosinophilic shift of diffuse rhinosinusitis inflammatory pattern in southern China over the last 18 years. The proportion of eCRS and difficult-to-treat rhinosinusitis has steadily increased, which is associated with the increase of Type 2, Type 3 cytokines and the decrease of Type 1 cytokines. This study also provided firstly evidence of a strong relationship between overweight and eosinophil shift in the southern Chinese population.
Keywords: Chronic rhinosinusitis, Nasal polyps, Inflammatory pattern, Eosinophils, Southern China, Body mass index
Introduction
Chronic rhinosinusitis (CRS) is a common disease in otorhinolaryngology. The prevalence of CRS in China was 8% according to an epidemiological investigation performed in 2005.1 According to the European Position Paper on Rhinosinusitis and Nasal Polyps 2020 (EPOS2020),2 CRS can be divided into localized (unilateral) CRS and diffuse (bilateral) CRS based on the anatomic distribution.
Increasing evidence indicates that bilateral CRS is a heterogeneous disease with different kinds of inflammatory patterns.3 Based on the infiltration of eosinophils, diffuse CRS can be divided into eosinophilic CRS (eCRS) and non-eosinophilic CRS (non-eCRS). Approximately 11 years ago, eosinophilic inflammation was characterized in 65–90% of nasal polyps (NP) cases in Caucasians, but only 50% in China.4,5
The inflammatory pattern may have changed during the recent 2 decades in Asia with the development of industrial processes.6,7 In northern and central China, the infiltration of eosinophils increased in patients with diffuse CRS, according to recent studies.8,9 However, the increase in eosinophils varied across China. Meanwhile, with the increasing of eosinophil infiltration, the expression level of Th2 bias inflammatory markers, such as IL-5, ECP and local IgE, were also increased in eCRS in central China. However, changes in other inflammatory markers were not clear. Because most Asian patients exhibited a Th1/Th17 bias inflammatory pattern in the past,10 a careful definition of the multiple inflammatory cytokines in diffuse CRS is essential to elucidate the reason for the change of inflammatory pattern in Chinese patients.
This study aimed to explore the shift in clinical and histological characteristics in the recent 2 decades in southern China, to elucidate the probable reason for the change of inflammatory pattern in Chinese patients.
Materials and methods
Subject
Ethics Committee for Clinical Research and animal trials approved the study. We retrospectively investigated patients who received endoscopic sinus surgery in our hospital. The basic information and history of patients were obtained from medical records and inspection results. If the patients declared previous sinus surgery history, the surgery performed in this research hospital would be defined as revision surgery. The diagnosis of CRS was made according to the European EAACI Position Paper on Rhinosinusitis and Nasal Polyps.5 An atopic status was evaluated via skin prick test or serum allergen test. The diagnosis of allergic rhinitis was based on typical clinical manifestation and special IgE examination or positive skin prick test. The diagnosis of asthma and aspirin sensitivity was based on history and physician diagnosis. According to a previous study in China, the optimal cut-off point of body mass index (BMI) for adults to present overweight was 24kg/m2. All patients over the age of 18 and who satisfied the diagnosis criteria of bilateral diffuse CRS were included in the study. At the same time, subjects who had localized CRS, cystic fibrosis, fungal sinusitis, vasculitis, or primary ciliary dyskinesia were excluded. Our subjects were not treated with oral steroids for at least 4 weeks before surgery. Patients from 2000 to 2001, which were the earliest with complete clinical information, patients from 2010 to 2011, and patients from 2017 to 2018 were selected and analyzed to include samples separated by the longest possible period.
Histological analysis
Data were not available for all subjects because some of the tissue sections could not be obtained. All specimens were sampled during the sinus surgery and stored in the pathology department and were collected in 2019. To reduce the deviation of staining, after collecting the paraffin embedded samples from the pathology department, all samples were processed in the same laboratory techniques, including paraffin section, and stained with hematoxylin-eosin (HE). NP tissue sections (5 μm) were numbered continuously by a researcher and the clinical information, time period of each patient, and corresponding slide number were stored in a separate list. Then, the HE staining slides were mixed together and observed disorderly by 2 independent investigators who were blind to the clinical data and the information list. The numbers of eosinophils, neutrophils, plasma cells, lymphocytes, and total inflammatory cells in the lamina propria (LP) per high power field (HPF) ( × 400) were quantified. Ten fields were randomly selected, and the average number per field was analyzed by 2 independent researchers. Finally, after calculating the inflammatory cells of each slide, the previous researcher would match the slide number with the patient information according to the list. There were different opinions about the precise definition of eCRS. According to previous studies of eCRS in China, we defined eCRS by measuring the infiltration level of tissue eosinophils the cut-off eosinophil percentage≥10%, as proposed by Cao et al,4 and the cut-off eosinophil percentage ≥27%, which was proposed by Zhang et al.11
Immunohistochemistry analysis
For immunohistochemistry, we deparaffinized and rehydrated the sample and placed the tissue sections in ethylenediaminetetraacetic acid (pH 9.0) antigen repair solution and placed in a pressure cooker with a certain amount of water. The electromagnetic oven was heated until the stomata started to air out, and the heating was stopped to release the pressure. Then 3% H2O2 was added to block endogenous peroxidase, and the sections were incubated in a dark place at room temperature. Slides were incubated with eosinophil cationic protein (ECP; 1:100, Abcam, Cambridge, United Kingdom), myeloperoxidase (MPO; 1:200, Abcam), interferon-γ (IFN-γ, 1:200, Abcam), tumor necrosis factor (TNF-α, 1:100, Abcam), IgE (1:100, Abcam), IL-4 (1:150, Abcam), IL-5 (1:300, Boosen Biotechnology, Beijing, China), IL-8 (1:1000, Abcam),IL-9 (1:500, Boosen Biotechnology), IL-13 (1:400, Abcam), IL-17 (1:500, Abcam), IL-22 (1:700, Abcam), IL-25 (1:200, Abcam), and IL-33 (1:400, Abcam), overnight at 4°C. Tissue sections were incubated with secondary antibodies (appropriately responding to primary antibody in species), labeled with Horseradish Peroxidase (HRP, Servicebio, Wuhan, China) and incubated at room temperature. Sections were dried slightly and freshly prepared 3′, 3′-diaminobenzidine (DAB) chromogenic reagent was added to marked tissue. Nuclei were counterstained with a hematoxylin staining solution. The slices were dehydrated and mounted with resin mounting medium. DAB-positive cell exhibited a brown-yellow nucleus. The number of positive cells per HPF was divided by the number of total inflammatory cells to analyze the percentage of positive cells per HPF.
Statistical analysis
Statistical analysis of the data was performed using GraphPad Prism 8.0 for Windows. Continuous variables with a normal distribution were expressed as the mean and standard deviation (SD) and analyzed using Student t-test and the variables with a non-normal distribution were expressed as the medians and interquartile range and analyzed using Kruskal-Wallis test and Dunn's test for comparisons. The other clinical variables were expressed as frequencies and percentages and analyzed using chi-square test or Fisher exact test. Logistic regression analysis with forward stepwise model were performed to analyze the association between overweight and eCRS. The model was adjusted for patients' group, gender, age, allergic rhinitis status, asthma status, revision surgery status and smoking habit, odds ratio (OR) and 95% confidence interval (CI) were calculated for each independent risk factor. All tests were 2-tailed and statistical significance was accepted at P value less than 0.05.
Results
Clinical characteristics
A total of 473 adult diffuse CRS patients with complete clinical information undergoing endoscopic sinus surgery were enrolled in this study: 91 diffuse CRS patients from 2000 to 2001 were designated as Group A, 170 diffuse CRS patients from 2010 to 2011 were designated as Group B, and 212 diffuse CRS patients from 2017 to 2018 were designated as Group C. The basic information and clinical characteristics of patients were displayed in Table 1. The results demonstrated that the percentage of smoking patients continuously declined. The disease duration of patients also decreased significantly (P < 0.001). The percentage of revision surgery reduced. For allergic diseases, more patients were comorbid with atopic rhinitis over time (P = 0.02). The percentage of patients who were comorbid with asthma also increased (P = 0.02). As for subjective symptoms, headache/facial pain and epistaxis decreased significantly over time. We also collected information about inflammatory cells from blood samples. The absolute number and percentage of blood eosinophils increased significantly (Table 1). The absolute number and percentage of neutrophils were comparable among the 3 groups.
Table 1.
Changes in the Clinical characteristics of patients with diffuse CRS
| GroupA 2000–2001 |
GroupB 2010–2011 |
GroupC 2017–2018 |
P-value | |
|---|---|---|---|---|
| Subjects, n | 91 | 170 | 212 | / |
| Sex, male, n (%) | 60 (65.93) | 114 (67.06) | 155 (73.11) | 0.31 |
| Age (years), mean (SD) | 43.39 (15.45) | 45.12 (14.27) | 42.87 (13.54) | 0.28 |
| BMI(kg/m2), mean (SD) | 22.17 (3.71) | 23.09 (3.88) | 23.50 (3.21) | 0.01∗ |
| Overweight, n (%) | 23 (25.56) | 57 (33.53) | 99 (46.70) | 0.002∗∗ |
| Smoking, n | 47 (51.65) | 37 (21.76) | 25 (11.79) | <0.001∗∗∗ |
| Disease duration (years), median (IQR) | 10 (5,20) | 7.5 (2,13.25) | 4 (1,10) | <0.001∗∗∗ |
| Revision surgery patients, n (%) | 38 (41.76) | 44 (25.88) | 38 (17.92) | <0.001∗∗∗ |
| Patients with nasal polyps, n (%) | 14(84.62%) | 146(85.88%) | 183 (86.32%) |
0.926 |
| Patients with atopy, n (%) | 7 (7.69) | 24 (14.12) | 42 (19.81) | 0.02∗ |
| Patients with asthma, n (%) | 3 (3.30) | 16 (9.41) | 29 (13.68) | 0.02∗ |
| Symptom, n (%) | ||||
| Nasal obstruction | 91 (100) | 167 (98.24) | 210 (99.06) | 0.61 |
| Rhinorrhea | 90 (98.90) | 160 (94.12) | 197 (92.92)) | 0.11 |
| Purulent nasal drainage | 8 (8.79) | 14 (8.24) | 17 (8.02) | 0.98 |
| Headache/Facial pain | 41 (45.05) | 55 (32.35) | 46 (21.70) | <0.001∗∗∗ |
| Fever | 0 (0) | 0 (0) | 2 (0.94) | 0.68 |
| Sneezing | 24 (26.37) | 40 (23.53) | 49 (23.11) | 0.81 |
| Ear ringing | 3 (3.30) | 4 (2.35) | 1 (0.47) | 0.13 |
| Ear fulness | 4 (4.40) | 3 (1.76) | 2 (0.94) | 0.12 |
| Epistaxis | 8 (8.80) | 18 (10.59) | 7 (3.30) | 0.02∗ |
| Loss of smell | 55 (60.44) | 113 (66.47) | 137 (64.62) | 0.62 |
| Complete blood count parameters, median (IQR) | ||||
| Eosinophil percentage (%) | 0.026 (0.014–0.055) | 0.030 (0.011–0.056) | 0.035 (0.017–0.063) | 0.05 |
| Eosinophil absolute number | 0.18 (0.083–0.40) | 0.20 (0.075–0.36) | 0.27 (0.11–0.41) | 0.02∗ |
| Neutrophil percentage (%) | 0.56 (0.49–0.64) | 0.52 (0.46–0.62) | 0.55 (0.50–0.61) | 0.09 |
| Neutrophil absolute number | 3.78 (2.69–5.07) | 3.58 (2.78–4.81) | 3.93 (3.05–4.95) | 0.34 |
∗P < 0.05.
∗∗P < 0.01.
∗∗∗P < 0.001, SD, Standard deviation, IQR, Interquartile range.
Histological characteristics
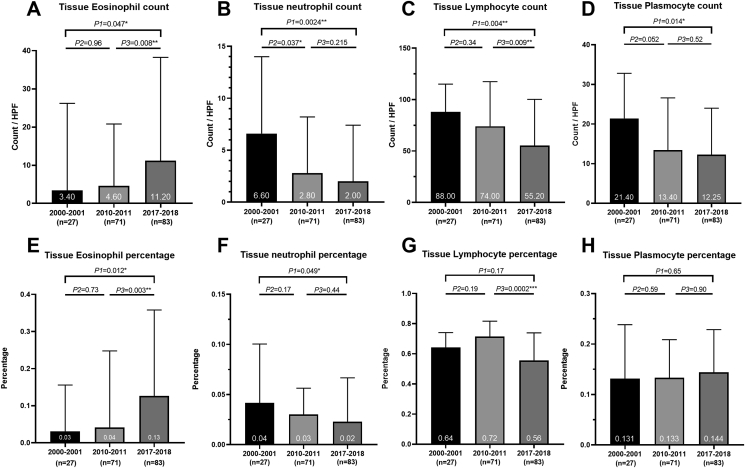
The number and percentage of tissue eosinophils increased remarkably (Fig. 1). On the contrary, the infiltration level of tissue neutrophils, plasmocyte, and lymphocytes declined significantly. Meanwhile, the total number of inflammatory cells in tissue also decreased significantly (Table S1).
Fig. 1.
Shift in the inflammation cells of patients with diffuse CRS over 18 years. Fig. 1 Legend. (A) The change of tissue eosinophil count. The number of tissue eosinophils was significantly increased. (B) The change of tissue neutrophil count. The number of tissue neutrophil declined. (C) The change of tissue lymphocyte count. The number of lymphocytes significantly decreased. (D) The change of tissue plasmocyte count. There was more plasmocyte. (E) The change of tissue eosinophil percentage. The percentage of eosinophils also kept an upward tendency as the number of eosinophils. (F) The change of tissue neutrophil percentage. The percentage of neutrophil decreased significantly. (G) The change of tissue lymphocyte percentage. The percentage of lymphocytes decreased sharply. (H) The change of tissue plasmocyte percentage. The percentage of plasmocyte was comparable among the 3 groups.
When divided diffuse CRS into eCRS and non-eCRS based on the percentage of eosinophil infiltration in tissue. We made the cut-off eosinophil percentage≥10% as the diagnostic criterion for eCRS, as proposed by Cao et al.4 The percentage of eCRS in diffuse CRS increased significantly (Table 2). Meanwhile, the proportion of non-eCRS patients decreased sharply.
Table 2.
Changes in the tissue proportion of ECRS over 18 years
| Tissue EOS≥10% |
|||||||
|---|---|---|---|---|---|---|---|
| Group A 2000–2001 | Group B 2010–2011 | Group C 2017–2018 | P (ABC) | P (A VS B) | P (B VS C) | P (A VS C) | |
| ECRS,n, (%) | 7 (25.93%) | 27 (38.03%) | 44 (53.01%) | 0.03∗ | 0.3 | 0.06 | 0.01∗ |
| NECRS,n (%) |
20 (74.07%) |
44 (61.97%) |
39 (46.99%) |
||||
| Tissue EOS≥27% |
|||||||
| ECRS,n, (%) | 5 (18.52%) | 16 (22.54%) | 32 (38.55%) | 0.04∗ | 0.67 | 0.03∗ | 0.06 |
| NECRS,n (%) | 22 (81.48%) | 55 (77.46%) | 51 (61.45%) | ||||
eCRS, eosinophilic chronic rhinosinusitis, non-eCRS, non-eosinophilic chronic rhinosinusitis.
Zhang et al11 made the cut-off eosinophil percentage ≥27% as the diagnostic criterion for eCRS which was also the prognostic indicator of difficult-to-treat rhinosinusitis. The percentage of difficult-to-treat rhinosinusitis also increased remarkably (Table 2).
The number of eosinophils infiltrating the sinonasal mucosa may indicate the prognosis of CRS.12 Therefore, we further analyzed the distribution of tissue eosinophil infiltration in the 3 groups (see Table 3). The results demonstrated that the group of patients with 30–50% tissue eosinophils increased significantly over time, especially from Group A to Group C (P = 0.03). A similar trend appeared in patients with 10–50% tissue eosinophils infiltration with significant difference between each group.
Table 3.
Changes in the degree of tissue eosinophil infiltration
| Eos% | <10% | 10%–20% | 20%–30% | 30%–50% | >50% | 10%–30% | 10%–50% |
|---|---|---|---|---|---|---|---|
| Group A2000–2001 | 0.74 | 0.04 | 0.04 | 0.04 | 0.15 | 0.08 | 0.11 |
| Group B2010–2011 | 0.62 | 0.09 | 0.11 | 0.14 | 0.03 | 0.20 | 0.34 |
| Group C2017–2018 | 0.47 | 0.08 | 0.10 | 0.22 | 0.13 | 0.18 | 0.40 |
| P-Value | |||||||
| Group (ABC) | 0.03∗ | 0.70 | 0.52 | 0.07 | <0.05∗ | 0.33 | 0.02∗ |
| GroupA vs B | 0.3 | 0.42 | 0.25 | 0.15 | <0.05∗ | 0.14 | 0.03∗ |
| GroupB vs C | 0.06 | 0.99 | 0.74 | 0.22 | 0.02∗ | 0.80 | 0.45 |
| GroupA vs C | 0.01∗ | 0.41 | 0.33 | 0.03∗ | 0.84 | 0.18 | <0.01∗∗ |
∗P < 0.05.
∗∗P < 0.01.
Inflammatory factors in tissue
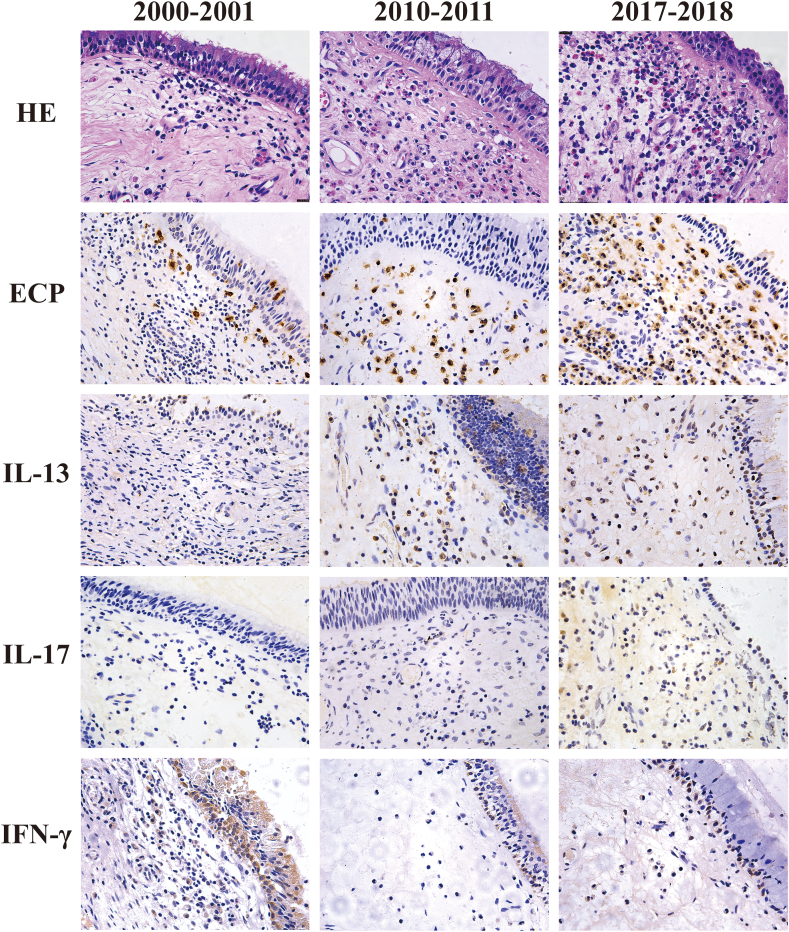
To investigate the shift of inflammatory patterns in diffuse CRS, we performed immunohistochemistry for some inflammatory molecules (Fig. 2 and Table 4). Consistent with the results of HE staining, the percentages of ECP + eosinophils increased significantly. For Type 1 related cytokines, the percentage of IFN-γ+ cells decreased significantly. For Type 2 related cytokines, the percentage of IL-13+ cells increased. For the Type 3 related cytokines, the number of IL-17+ cells increased significantly. Other cytokines were comparable between the 3 groups.
Fig. 2.
The HE staining and immunohistochemistry staining sections of nasal polyp tissue. Fig. 2 Legend. The representative slides of HE, ECP, IL-13, IL-17 and IFN-γ. All pictures were taken at a magnification of 400 × .
Table 4.
Immunohistochemistry analysis
| Markers (Median %) (IQR) | Group A 2000–2001 (n = 10) | Group B 2010–2011 (n = 10) | Group C 2017–2018 (n = 10) | P (Group ABC) | P (Group A VS B) | P (Group B VS C) | P (Group A VS C) |
|---|---|---|---|---|---|---|---|
| ECP | 0.090 (0.075, 0.13) | 0.32 (0.17, 0.59) | 0.39 (0.18, 0.56) | <0.01∗ | <0.001∗∗ | 0.91 | <0.01∗ |
| MPO | 0.12 (0.065, 0.18) | 0.18 (0.061, 0.31) | 0.12 (0.049, 0.21) | 0.77 | 0.48 | 0.60 | 0.85 |
| IFN-γ | 0.12 (0.077, 0.15) | 0.010 (0.001, 0.054) | 0.072 (0.017, 0.12) | <0.05∗ | <0.001∗∗ | 0.11 | 0.12 |
| TNF-α | 0.10 (0.029, 0.22) | 0.056 (0.031, 0.11) | 0.053 (0.028, 0.085) | 0.53 | 0.42 | 0.80 | 0.27 |
| IgE | 0.076 (0.032, 0.11) | 0.095 (0.049, 0.22) | 0.11 (0.062, 0.17) | 0.09 | 0.68 | 0.80 | 0.49 |
| IL-4 | 0.12 (0.094, 0.21) | 0.15 (0.060, 0.24) | 0.21 (0.11, 0.25) | 0.40 | 0.60 | 0.39 | 0.18 |
| IL-5 | 0.058 (0.035, 0.12) | 0.087 (0.049, 0.23) | 0.091 (0.065, 0.12) | 0.40 | 0.31 | 0.78 | 0.20 |
| IL-8 | 0.052 (0.024, 0.16) | 0.066 (0.004, 0.27) | 0.11 (0.067, 0.20) | 0.30 | 0.88 | 0.20 | 0.17 |
| IL-9 | 0.15 (0.072, 0.24) | 0.10 (0.017, 0.42) | 0.20 (0.096, 0.25) | 0.82 | 0.92 | 0.64 | 0.55 |
| IL-13 | 0.05 (0.025, 0.078) | 0.23 (0.16, 0.28) | 0.12 (0.045, 0.17) | <0.01∗ | <0.001∗∗ | 0.054 | 0.24 |
| IL-17 | 0.019 (0, 0.10) | 0.018 (0.001, 0.074) | 0.088 (0.047, 0.14) | 0.05 | 0.99 | <0.05∗ | <0.05∗ |
| IL-22 | 0.081 (0.028, 0.14) | 0.037 (0.011, 0.052) | 0.068 (0.036, 0.098) | 0.11 | 0.07 | 0.06 | 0.93 |
| IL-25 | 0.13 (0.074, 0.16) | 0.07 (0.053, 0.17) | 0.05 (0.025, 0.12) | 0.30 | 0.60 | 0.33 | 0.13 |
| IL-33 | 0.20 (0.12, 0.28) | 0.23 (0.13, 0.48) | 0.31 (0.21, 0.41) | 0.27 | 0.42 | 0.42 | 0.10 |
∗P < 0.05.
∗∗P < 0.01.
∗∗∗P < 0.001, IQR, Interquartile range.
The impact of disease combination on clinical and histological characteristics
To explore the impact of disease combination on clinical and histological characteristics, we classified patients into CRS combined with allergic rhinitis (atopic CRS), CRS combined with asthma (asthmatic CRS), and patients who only suffered from CRS (NAR-AS). Because few patients in Group A combined with asthma or allergic rhinitis, for asthmatic CRS and atopic CRS, we only analyzed patients in Group B and Group C to avoid deviation (see Table 5).
Table 5.
The impact of combining diseases on clinical and histological characteristics
| Group B 2010–2011 |
Group C 2017–2018 |
|||||
|---|---|---|---|---|---|---|
| atopic CRS | asthmatic CRS | NAR-AS | atopic CRS | asthmatic CRS | NAR-AS | |
| Subjects, n | 24 | 16 | 135 | 42 | 29 | 152 |
| Symptom, n (%) | ||||||
| Nasal obstruction | 24 (100) | 16 (100) | 132 (97.78) | 42 (100) | 29 (100) | 150 (98.68) |
| Rhinorrhea | 23 (95.83) | 15 (93.75) | 126 (93.33) | 40 (95.24) | 27 (93.10) | 141 (92.76) |
| Purulent nasal drainage | 4 (16.67) | 2 (12.50) | 9 (6.67) | 8 (19.05) | 6 (20.69) | 7 (4.61) |
| Head/Facial pain | 7 (29.17) | 5 (31.25) | 45 (33.33) | 11 (26.19) | 8 (27.59) | 29 (19.08) |
| Sneezing | 16 (6.67) | 2 (12.50) | 22 (16.30) | 18 (42.86) | 11 (37.93) | 25 (16.45) |
| Loss of smell | 22 (91.67) | 15 (93.75) | 80 (59.26) | 22 (52.38) | 21 (72.41) | 102 (67.11) |
| Blood inflammatory cells, median (IQR) | ||||||
| Eosinophil percentage (%) | 0.041 (0.018,0.073) | 0.061 (0.014,0.11) | 0.026 (0.011,0.049) | 0.063 (0.039,0.088) | 0.056 (0.036,0.10) | 0.028 (0.014,0.052) |
| Eosinophil absolute number | 0.24 (0.10,0.47) | 0.46 (0.11,0.75) | 0.20 (0.073,0.32) | 0.39 (0.30,0.59) | 0.38 (0.30,0.64) | 0.20 (0.10,0.37) |
| Neutrophil percentage (%) | 0.49 (0.42,0.60) | 0.46 (0.41,0.67) | 0.53 (0.47,0.62) | 0.53 (0.46,0.58) | 0.51 (0.45,0.58) | 0.55 (0.51,0.62) |
| Neutrophil absolute number | 2.86 (2.14,4.52) | 3.69 (2.61,5.81) | 3.63 (2.91,4.82) | 3.51 (2.75,4.75) | 3.88 (3.02,4.67) | 3.99 (3.13,4.96) |
| Tissue inflammatory cells, median (IQR) | ||||||
| Eosinophil/HPF | 9.20 (1.80,41.0) | 4.40 (1.90,34.90) | 3.4 (1.05,15.65) | 23.10 (5.65,46.95) | 61.0 (12.0,73.60) | 7.0 (2.33,30.80) |
| Eosinophil % | 0.055 (0.01,0.31) | 0.055 (0.01,0.22) | 0.039 (0.0081,0.22) | 0.11 (0.045,0.38) | 0.33 (0.14,0.59) | 0.087 (0.016,0.31) |
| Neutrophil/HPF | 4.80 (1.40,13.80) | 16.40 (6.40,17.0) | 2.6 (0.80,6.15) | 4.40 (0.95,11.75) | 2.60 (2.0,6.60) | 3.0 (0.60,9.40) |
| Neutrophil % | 0.034 (0.023,0.22) | 0.075 (0.024,0.16) | 0.03 (0.009,0.52) | 0.024 (0.009,0.078) | 0.025 (0.011,0.048) | 0.04 (0.007,0.09) |
| Lymphocyte/HPF | 74.40 (34.80,120.2) | 88.60 (46.60,207.1) | 67.10 (49.05,103.6) | 82.50 (68.95,126.8) | 52 (35.50,84.80) | 64.00 (30.70,108.2) |
| Lymphocyte % | 0.61 (0.51,0.84) | 0.61 (0.42,0.74) | 0.72 (0.58,0.82) | 0.67 (0.53,0.76) | 0.41 (0.32,0.56) | 0.59 (0.46,0.77) |
| Plasmocyte/HPF | 10.40 (6.0,13.40) | 35.60 (23.70,60.50) | 13.70 (7.90,26.55) | 20.60 (11.80,29.75) | 14.40 (5.20,18.00) | 11.2 0 (4.90,24.33) |
| Plasmocyte % | 0.11 (0.037,0.14) | 0.21 (0.17,0.27) | 0.14 (0.1,0.22) | 0.15 (0.075,0.19) | 0.14 (0.042,0.14) | 0.1 (0.05,0.23) |
Asthmatic CRS, Chronic rhinosinusitis with asthma, atopic CRS, Chronic rhinosinusitis with allergic rhinitis, NAR-AS, Chronic rhinosinusitis patients without allergic rhinitis or asthma, IQR, Interquartile range, HPF, High power field, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
In Group B, more asthmatic and atopic CRS (P < 0.01) patients complained about olfactory dysfunction. The percentage and absolute count of blood eosinophil were significantly higher in the asthmatic CRS compared to the NAR-AS, but the results were comparable between the atopic CRS and NAR-AS. For the histological characteristics, the numbers of neutrophils and plasmocytes were significantly higher in asthmatic CRS than NAR-AS patients. The number and percentage (P < 0.01) of plasmocyte in asthmatic CRS were also higher than atopic CRS patients. The level of eosinophils infiltration and total inflammatory cells seemed similar in all patients in Group B.
In Group C, more asthmatic CRS patients had revision surgery compared to NAR-AS patients (P = 0.01). More patients complained about purulent nasal drainage and sneezing in the atopic CRS (purulent nasal drainage: P < 0.01; sneezing: P < 0.001) and asthmatic CRS (purulent nasal drainage: P = 0.008; sneezing: P < 0.01) while comparing to the NAR-AS group. There were no significant differences in other symptoms between the patients in Group C. Meanwhile, as Group B, comparing to NAR-AS patients, the percentage and number of blood eosinophils were also significantly elevated in the asthmatic CRS (percentage and number: P < 0.001) and atopic CRS (percentage and number: P < 0.001). In terms of histological characteristics, the percentage and number of eosinophils were also increased in the asthmatic CRS (percentage and number: P < 0.001) and atopic CRS (percentage: P = 0.11; number: P = 0.14) group comparing to the NAR-AS group. The percentage and number of lymphocytes were higher in the NAR-AS group comparing to the asthmatic CRS (percentage and number: P < 0.001). However, there was no significant difference between atopic CRS and asthmatic CRS groups in Group C.
We then investigated the characteristics of atopic CRS patients in the different groups. The total number of blood eosinophils increased (P = 0.02), but the percentage of blood eosinophils remained similar in each group. There were no significant differences in the histological characteristics or symptoms between these 2 groups.
Furthermore, we investigated the characteristics of asthmatic CRS patients in different groups. There were no significant differences in clinical characteristics between Group B and Group C. For the histological characteristics, the percentage of eosinophils increased (P = 0.01), while the number of neutrophils decreased slightly (P = 0.08). On the other hand, the infiltration level of plasmocytes (P < 0.05) and lymphocyte (P = 0.03) decreased. The total number of inflammatory cells also decreased (P < 0.05).
For NAR-AS patients (see Table S2), the number of revision surgeries decreased significantly. In terms of symptoms, the proportion of patients who complained about headache/facial pain sharply decreased over time. There was no obvious shift of the blood cell among the 3 groups. However, as for histological characteristics, the percentage of eosinophils in tissue increased, the number of neutrophils, lymphocytes, and plasmocytes decreased. The total number of inflammatory cells was decreased.
The impact of body mass index on histological characteristics
The BMI of diffuse CRS patients increased significantly from Group A to Group C (P = 0.01, Table 1). Based on the BMI standard in China (low weight means BMI<18.5, normal weight means BMI not less than 18.5 and less than 24.0, overweight means BMI not less than 24.0, pre-obese means BMI not less than 24.0 and less than 28.0, and obese means BMI not less than 28.0), we found that the proportion of overweight CRS patients increased over time (P = 0.002). We further divided patients into 7 subgroups based on different BMI cutoff points (See Figure S1). The tissue eosinophil percentage was sharply increased in patients with BMI of more than 24.0 kg/m2. To further analyze the association between overweight and eCRS (with tissue eosinophil percentage not less than 27%), we performed a multivariable logistic regression with forward stepwise model and adjusted for patients’ group, gender, age, Allergic rhinitis status, Asthma status, revision surgery status, and smoking habit. The multivariate analysis revealed that Asthma status and overweight were independent risk factors for eCRS. Compared to non-overweight patients (BMI<24kg/m2), overweight patients had a significantly higher ratio of eCRS (adjusted OR: 2.45, 95% CI: 1.22–4.94, P = 0.012). Meanwhile, in accordance with previous research, asthma patients had a higher risk of eCRS (adjusted OR: 4.67, 95% CI 1.73, 12.59, P < 0.001).
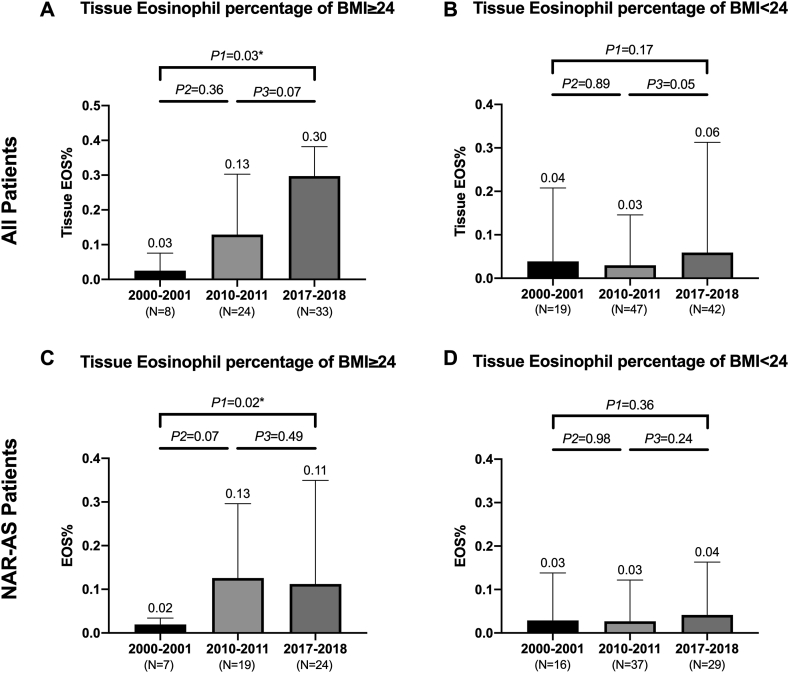
Subgroup analyses were established to explore whether BMI status (overweight/non-overweight) might influence the infiltration of tissue eosinophils (Fig. 3). The results showed that the increase of tissue eosinophils was only noted in overweight patients but not in non-overweight patients. Since BMI might related to allergic disease and asthma,13 we focused on patients without asthma or allergic disease (NAR-AS patients). The same trend was observed for NAR-AS patients. Meanwhile, in Group C, higher BMI was observed in difficult-to-treat rhinosinusitis (tissue eosinophil percentage ≥27%) while comparing to non-difficult-to-treat rhinosinusitis (24.75 vs 22.69 kg/m2, P = 0.01).
Fig. 3.
The BMI and Tissue eosinophil percentage of all patients and NAR-AS patients. Fig. 3 Legend. (A) The change of all patients tissue eosinophil percentage of BMI≥24. The percentage of eosinophil kept an upward tendency. (B) The change of all patients' tissue eosinophil percentage BMI<24. The percentage of eosinophil was comparable among the 3 groups. (C) The change of NAR-AS patients tissue eosinophil percentage BMI≥24. The percentage of eosinophil increased significantly (D) The change of NAR-AS tissue eosinophil percentage BMI<24. There was no significant difference of eosinophil percentage among the 3 groups.
Discussion
This study aimed to explore the shift in clinical and histological characteristics in the recent 2 decades in southern China. In our study, the infiltration of tissue eosinophils increased, and the infiltration of tissue neutrophils, lymphocytes, and plasmocytes decreased significantly over time. Recent studies also demonstrated the similar eosinophil shift in other Asian cities.6, 7, 8, 9 When we adopted an eosinophil percentage of >10% as the diagnostic criterion for eCRS, the percentage of eCRS increased from 25.93% to 53.01% in our study. However, while comparing to previous studies, the percentage increased from 59.1% to 73.7% in northern China7 and from 15.7% to 44% in central China.9 Since socioeconomic status may influence the frequency of immunological diseases,14,15 we further searched the gross domestic product (GDP) in the National Bureau of statistics of China,16 and found common trends in the regional average GDP and the proportion of eCRS. Therefore, economic status could be one of the reasons that caused heterogeneity of eCRS.
Accumulating evidences have demonstrated a steady rise in the prevalence of allergic and autoimmune diseases in developed countries.17,18 Previous studies reported an increasing prevalence of allergic rhinitis and asthma in China.17,19 The results of our study also showed that the prevalence of comorbid diseases such as allergic rhinitis and asthma increased significantly in the last 2 decades. Allergic rhinitis and asthma were known to correlate to eosinophil infiltration, blood eosinophilia, and the expression of Type 2 cytokines.20,21 Notably, we then analyzed patients without comorbid diseases (NAR-AS patients), although the blood inflammatory cell remained unchanged, the infiltration of tissue eosinophils increased significantly and the neutrophils decreased sharply in this NAR-AS population. Therefore, the increase of allergic diseases and asthma may only explain part of the eosinophilic shift. According to a cluster analysis performed by Lou et al,12 patients with tissue eosinophils between 30% and 54.5% and lymphocytes less than 64% may indicate a mixed inflammatory cell pattern, and patients with lymphocyte proportion ≥64% or plasmocyte ≥20% may indicate a milder degree of lower airway inflammation. Our study demonstrated that Group A had more lymphocytes and fewer eosinophils, while Group C had fewer lymphocytes, plasmocytes, and more eosinophils. Then we analyzed the changes in the degree of eosinophil infiltration. Patients with tissue eosinophils less than 10% decreased sharply; more interestingly, patients with 10–50% and, 30–50% tissue eosinophils increased significantly over time. However, the percentage of patients with more than 50% eosinophil infiltration was similar in the Group A and Group C. Therefore, more patients shifted from a lower airway inflammation pattern to mix inflammatory cell pattern rather than a severe eosinophilic inflammatory cell pattern. This shift may explain the increase in eosinophil infiltration in the NAR-AS population.
Previous studies characterized IFN-γ as a Type 1 marker, ECP and IL-13 as Type 2 markers, IL-17 as Type 3 marker in chronic rhinosinusitis patients.22,23 According to previous studies, the major inflammatory type of diffuse CRS was neutrophilic inflammation with Th1/Th17 expression in Chinese patients.4 The results of this study indicated an increase in Type 2 inflammatory cytokine, and a decrease in Type 1 inflammatory cytokine. A significant increase in Type 3 inflammatory cytokine was also observed. Steven et al demonstrated that the presence of Type 2 inflammation negatively associated with headache and migraine in diffuse CRS and the presence of purulent nasal drainage positively associated with the Type 3 inflammatory pattern.22 The changes in the clinical characteristics in our study were consistent with the changes in the inflammatory pattern. The proportion of patients with headache and facial pain decreased significantly as time passed, and patients comorbid with asthma or allergic rhinitis also complained more about purulent nasal drainage while comparing to NAR-AS patients. Meanwhile, the reduction of Type 1 inflammatory cytokines might explain the decrease in neutrophil infiltration in diffuse CRS patients. Therefore, our results showed that the inflammatory patterns of diffuse CRS patients changed from solely Type 1 inflammation to a Type 2/Type 3 mix inflammatory pattern in past 2 decades. Wang et al24 also showed that the inflammatory pattern of diffuse CRS primarily demonstrated Th2/Th1/Th17 mixed patterns, which is partially in consistent with our study. A recent study demonstrated that environmental factors such as pollution might increase the Th17 reaction, and Th17 cytokines could enhance the expression of Th2 cytokines.25,26 Therefore, the increasement of Th17 and Th2 reaction in Chinese diffuse CRS patients might be the consequence of the interaction between environmental factors and human upper airway mucosa.
Our research also firstly revealed that the BMI and the percent of overweight CRS patients had a tendency to increase in last 2 decades which was consistent with that of previous studies in other chronic diseases, such as cardiovascular disease and diabetes.27 The prevalence of overweight has been increasing in China for several years.28 The sustainable economic growth throughout China has brought about great changes in lifestyle and nutrition status, such as the increased intake of animal-source food and remarkable change in eating patterns.29 All of these resulted in a rapid increase in the distribution of the BMI of the Chinese population. Besides, several studies have hypothesized a relationship between overweight and respiratory disease such as asthma and allergic diseases which were strongly related to eCRS.13 Our study found that the infiltration level of tissue eosinophils increased significantly in overweight patients and the same trend was observed for NAR-AS patients. Therefore, overweight might contribute to the significant increase in eCRS and difficult-to-treat rhinosinusitis patients. Overweight might contribute to immune disorders and increase the expression levels of inflammatory mediators, such as leptin, osteopontin.30,31 The osteopontin and leptin could mediate the migration and activation of eosinophil in allergic rhinitis, and the serum osteopontin level was correlated with the blood eosinophilia.32,33 Furthermore, the leptin/osteopontin axis also promoted Th17 response,32 and the results of our study also showed the expression of IL-17 increased overtime. Remarkably, overweight was strongly correlated with the distribution and the abundance of gut and respiratory microbiome, which might associate with allergic diseases and other chronic diseases.34,35 Recent studies focus on Staphylococcus aureus and the impact of staphylococcal enterotoxins was demonstrated as a major factor contributing to the change in inflammatory patterns.6 However, only one-third of polyps from southern China contained IgE antibodies to Staphylococcus aureus enterotoxins.23,36 Therefore, further studies are needed to distinguish the correlation between overweight and eCRS and to explore the potential mechanism.
We also found that the proportion of smoking patients decreased over time. Cigarette smoking is closely related to respiratory diseases, although the mechanism of how smoking influences nasal mucosa is not yet clear. Based on previous studies, cigarette smoke might induce the sputum neutrophils in asthma patients37 and also cause an accumulation of neutrophils in human lung tissue.38 However, recent studies also found that the expression of IL-17A was increased in the nasal tissue of smokers with asthma and CRS.39 Meanwhile, other studies showed that smoke can augment Th2 dependent response and increase eosinophil accumulation in nasal tissue.40 The decrease of smoking patients might indicate an increase of health awareness in the population. However, in our study, we did not find any significant differences between smoking and non-smoking patients (data not shown); since smoking alters multiple signal pathway and immune reaction, further research should focus on the how cigarette smoking contributes to the immunological disruption in CRS patients.
Finally, one interesting result was obtained in this study. Generally, the elevation of eosinophil infiltration indicates a higher recurrence rate and a greater patient need for revision surgery.3,11,41,42 However, the proportion of patients requiring revision surgery in our study decreased significantly over time. While tracing the development of sinus surgery in China, some patients in Group A had sinus surgery under local anesthesia, and these patients had only removal of the nasal polyps in the nasal cavity. Functional endoscopic sinus surgery (FESS) has been used in the treatment of chronic rhinosinusitis since the 1990s and became popular in 2008,43 but the technological level was inconsistent. With the efforts of many otorhinolaryngologists in the past 2 decades, the procedure of endoscopic sinus surgery became standard and consistent. Therefore, the optimization of surgery decreased the rate of revision surgery over time.
There are several limitations in our research. First, we lacked the visual analogy (VAS), 22-item Sino-Nasal Outcome Test (SNOT-22), endoscopy, and computed tomography (CT) scores. Second, this study was a single center retrospective study; even though the center is one of the largest otorhinolaryngology centers in Southern China and the patients came from various areas in Southern China, the power of this study was limited. Third, there was a deficiency in the number of immunohistochemistry analysis. Fourth, according to the results of this study, we found the lymphocytes declined significantly from 2000 to 2017, but since we did not perform immunochemistry staining for the specific markers to differentiate T cells, B cells, and other lymphoid cells, we could not differentiate the subgroup of lymphocyte in detail. Further study should focus on the changes of lymphocytes.
Many factors may contribute to the changes in inflammatory pattern in southern China; further research about fat metabolism with diffuse CRS will be developed.
Conclusion
There was an inflammatory pattern shift associated with diffuse chronic rhinosinusitis in southern China in the last 18 years. Our study demonstrated that asthmatic CRS and atopic CRS patients increased over time. The infiltration level of tissue eosinophils and the proportion of eCRS increased dramatically, which was associated with the increase of Type 2, Type 3 cytokines and the decrease of Type 1 cytokines. Furthermore, our research also revealed that overweight might contribute to the significant increase in eCRS and difficult-to-treat rhinosinusitis patients. This study proved that the eosinophil shift appeared in the southern China and firstly implicated the correlation between overweight and eosinophilic chronic rhinosinusitis in Chinese population.
Abbreviations
BMI: body mass index, CRS: chronic rhinosinusitis; diffuse CRS: diffuse chronic rhinosinusitis, eCRS: eosinophilic chronic rhinosinusitis, non-eCRS: Non-eosinophilic chronic rhinosinusitis, asthmatic CRS: Chronic rhinosinusitis with asthma, atopic CRS: Chronic rhinosinusitis with allergic rhinitis, NAR-AS: Chronic rhinosinusitis patients without allergic rhinitis or asthma
Ethics approval
All data obtained from the First Affiliated Hospital of Sun Yat-sen University. Ethics approved by Ethics Committee for Clinical Research and animal trials of the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China approved the study (Number: [2019]163).This study was a retrospective study and did not sign an informed consent.
Availability of data and material
All data generated or analyzed during this study are included in this published article and its Supplemental file. More related data of the current study are available from the corresponding author on reasonable request.
Consent for publication
All authors have seen and approved the last version and agreed to publication of the work.
Conflict of interest statement
All authors report no conflicts of interest.
Funding
This study was supported by the National Outstanding Youth Science Fund Project of National Natural Science Foundation of China (81300814). The funding organization had no role in the design of the study, in the collection, analysis, and interpretation of data or in writing the manuscript.
Author contributions
All authors were involved in the study. Prof. Yinyan Lai and Prof. Jianbo Shi designed the study. Xin Luo, Zhaofeng Xu and Jie Deng collected the patients’ information. The collection of nasal polyp section and evaluation of histological characteristics was completed by Xin Luo, Lei Xu, Lijie Jiang and Zhaoqi Huang. Kejun Zuo and Wenxiang Gao controlled the quality of the study. Xin Luo, Zhaofeng Xu and Yinyan Lai finished the analysis of all data.
Acknowledgments
The authors express appreciation for the participation of all people who contributed to the design, analysis, and interpretation of the manuscript.
Footnotes
Full list of author information is available at the end of the article https://doi/org/10.1016/j.waojou.2021.100531
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2021.100531.
Contributor Information
Jianbo Shi, Email: tsjbent@163.com.
Yinyan Lai, Email: Laiyy3@mail.sysu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Shi J.B., Fu Q.L., Zhang H. Epidemiology of chronic rhinosinusitis: results from a cross-sectional survey in seven Chinese cities. Allergy. 2015;70:533–539. doi: 10.1111/all.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fokkens W.J., Lund V.J., Hopkins C. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58:1–464. doi: 10.4193/Rhin20.600. [DOI] [PubMed] [Google Scholar]
- 3.Lou H., Zhang N., Bachert C., Zhang L. Highlights of eosinophilic chronic rhinosinusitis with nasal polyps in definition, prognosis, and advancement. International Forum of Allergy & Rhinology. 2018;8:1218–1225. doi: 10.1002/alr.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao P., Li H., Wang B. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009;124:478–484. doi: 10.1016/j.jaci.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Fokkens W.J., Lund V.J., Mullol J. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012;23(3):1–298. [PubMed] [Google Scholar]
- 6.Katotomichelakis M., Tantilipikorn P., Holtappels G. Inflammatory patterns in upper airway disease in the same geographical area may change over time. Am J Rhinol Allergy. 2013;27:354–360. doi: 10.2500/ajra.2013.27.3922. [DOI] [PubMed] [Google Scholar]
- 7.Kim S.J., Lee K.H., Kim S.W., Cho J.S., Park Y.K., Shin S.Y. Changes in histological features of nasal polyps in a Korean population over a 17-year period. Otolaryngology-Head Neck Surg (Tokyo) 2013;149:431–437. doi: 10.1177/0194599813495363. [DOI] [PubMed] [Google Scholar]
- 8.Wang W., Gao Y., Zhu Z. Changes in the clinical and histological characteristics of Chinese chronic rhinosinusitis with nasal polyps over 11 years. International Forum of Allergy & Rhinology. 2019;9:149–157. doi: 10.1002/alr.22234. [DOI] [PubMed] [Google Scholar]
- 9.Jiang W.X., Cao P.P., Li Z.Y. A retrospective study of changes of histopathology of nasal polyps in adult Chinese in central China. Rhinology Journal. 2019;57(4):261–267. doi: 10.4193/Rhin18.070. [DOI] [PubMed] [Google Scholar]
- 10.Zhang N., Van Zele T., Perez-Novo C. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122:961–968. doi: 10.1016/j.jaci.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Lou H., Meng Y., Piao Y., Wang C., Zhang L., Bachert C. Predictive significance of tissue eosinophilia for nasal polyp recurrence in the Chinese population. Am J Rhinol Allergy. 2015;29:350–356. doi: 10.2500/ajra.2015.29.4231. [DOI] [PubMed] [Google Scholar]
- 12.Lou H., Meng Y., Piao Y. Cellular phenotyping of chronic rhinosinusitis with nasal polyps. Rhinology. 2016;54:150–159. doi: 10.4193/Rhino15.271. [DOI] [PubMed] [Google Scholar]
- 13.Ciprandi G., Pistorio A., Tosca M., Ferraro M.R., Cirillo I. Body mass index, respiratory function and bronchial hyperreactivity in allergic rhinitis and asthma. Respir Med. 2009;103:289–295. doi: 10.1016/j.rmed.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Blanchard J.F., Bernstein C.N., Wajda A., Rawsthorne P. Small-area variations and sociodemographic correlates for the incidence of Crohn's disease and ulcerative colitis. Am J Epidemiol. 2001;154:328–335. doi: 10.1093/aje/154.4.328. [DOI] [PubMed] [Google Scholar]
- 15.von Mutius E., Martinez F.D., Fritzsch C., Nicolai T., Roell G., Thiemann H.H. Prevalence of asthma and atopy in two areas of West and East Germany. Am J Respir Crit Care Med. 1994;149:358–364. doi: 10.1164/ajrccm.149.2.8306030. [DOI] [PubMed] [Google Scholar]
- 16.National Bureau of statistics of China, http://data.stats.gov.cn/.
- 17.Zhang Y., Zhang L. Increasing prevalence of allergic rhinitis in China. Allergy, Asthma & Immunology Research. 2019;11:156. doi: 10.4168/aair.2019.11.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bach J.F. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 19.Lin J., Wang W., Chen P. Prevalence and risk factors of asthma in mainland China: the CARE study. Respir Med. 2018;137:48–54. doi: 10.1016/j.rmed.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Woodruff P.G., Modrek B., Choy D.F. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McHugh T., Levin M., Snidvongs K., Banglawala S.M., Sommer D.D. Comorbidities associated with eosinophilic chronic rhinosinusitis: a systematic review and meta-analysis. Clin Otolaryngol. 2020;45:574–583. doi: 10.1111/coa.13536. [DOI] [PubMed] [Google Scholar]
- 22.Stevens W.W., Peters A.T., Tan B.K. Associations between inflammatory endotypes and clinical presentations in chronic rhinosinusitis. J Allergy Clin Immunol: In Pract. 2019;7(8):2812–2820. doi: 10.1016/j.jaip.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomassen P., Vandeplas G., Van Zele T. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137:1449–1456. doi: 10.1016/j.jaci.2015.12.1324. [DOI] [PubMed] [Google Scholar]
- 24.Wang X., Zhang N., Bo M. Diversity of T H cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138:1344–1353. doi: 10.1016/j.jaci.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 25.van Voorhis M., Knopp S., Julliard W. Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor. PloS One. 2013;8 doi: 10.1371/journal.pone.0082545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M., Zhang N., Zheng M. Cross-talk between TH2 and TH17 pathways in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2019;144:1254–1264. doi: 10.1016/j.jaci.2019.06.023. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds K., Gu D., Whelton P.K. Prevalence and risk factors of overweight and obesity in China. Obesity. 2007;15:10–18. doi: 10.1038/oby.2007.527. [DOI] [PubMed] [Google Scholar]
- 28.Gordon-Larsen P., Wang H., Popkin B.M. Overweight dynamics in Chinese children and adults. Obes Rev. 2014;15:37–48. doi: 10.1111/obr.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popkin B.M. Will China's nutrition transition overwhelm its health care system and slow economic growth? Health Aff. 2008;27:1064–1076. doi: 10.1377/hlthaff.27.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medoff B.D., Okamoto Y., Leyton P. Adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol. 2009;41:397–406. doi: 10.1165/rcmb.2008-0415OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung S.Y., Park D.C., Kim S.H., Yeo S.G. Role of obesity in otorhinolaryngologic diseases. Curr Allergy Asthma Rep. 2019;19:34. doi: 10.1007/s11882-019-0865-3. [DOI] [PubMed] [Google Scholar]
- 32.Liu W., Zeng Q., Zhou L., Li Y., Chen Y., Luo R. Leptin/osteopontin axis contributes to enhanced T helper 17 type responses in allergic rhinitis. Pediatr Allergy Immunol. 2018;29:622–629. doi: 10.1111/pai.12926. [DOI] [PubMed] [Google Scholar]
- 33.Liu W., Zeng Q., Chen Y., Luo R.Z. Role of leptin/osteopontin Axis in the function of eosinophils in allergic rhinitis with obesity. Mediat Inflamm. 2018;2018:9138904. doi: 10.1155/2018/9138904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tun H.M., Konya T., Takaro T.K. Exposure to household furry pets influences the gut microbiota of infant at 3-4 months following various birth scenarios. Microbiome. 2017;5:40. doi: 10.1186/s40168-017-0254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alderete T.L., Jones R.B., Chen Z. Exposure to traffic-related air pollution and the composition of the gut microbiota in overweight and obese adolescents. Environ Res. 2018;161:472–478. doi: 10.1016/j.envres.2017.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang N., Holtappels G., Claeys C., Huang G., van Cauwenberge P., Bachert C. Pattern of inflammation and impact of Staphylococcus aureus enterotoxins in nasal polyps from southern China. Am J Rhinol. 2006;20:445–450. doi: 10.2500/ajr.2006.20.2887. [DOI] [PubMed] [Google Scholar]
- 37.Chalmers G.W., MacLeod K.J., Thomson L., Little S.A., McSharry C., Thomson N.C. Smoking and airway inflammation in patients with mild asthma. Chest. 2001;120:1917–1922. doi: 10.1378/chest.120.6.1917. [DOI] [PubMed] [Google Scholar]
- 38.Moerloose K.B., Pauwels R.A., Joos G.F. Short-term cigarette smoke exposure enhances allergic airway inflammation in mice. Am J Respir Crit Care Med. 2005;172:168–172. doi: 10.1164/rccm.200409-1174OC. [DOI] [PubMed] [Google Scholar]
- 39.Huang Y., Lou H., Wang C., Zhang L. Impact of cigarette smoke and IL-17A activation on asthmatic patients with chronic rhinosinusitis. Rhinology. 2019;57:57–66. doi: 10.4193/Rhin18.131. [DOI] [PubMed] [Google Scholar]
- 40.Strzelak A., Ratajczak A., Adamiec A., Feleszko W. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int J Environ Res Publ Health. 2018;15 doi: 10.3390/ijerph15051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gurrola J.N., Borish L. Chronic rhinosinusitis: endotypes, biomarkers, and treatment response. J Allergy Clin Immunol. 2017;140:1499–1508. doi: 10.1016/j.jaci.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Nakayama T., Asaka D., Yoshikawa M. Identification of chronic rhinosinusitis phenotypes using cluster analysis. Am J Rhinol Allergy. 2012;26:172–176. doi: 10.2500/ajra.2012.26.3749. [DOI] [PubMed] [Google Scholar]
- 43.Han D., Zhang L. Functional endoscopic sinus surgery in China. ORL J Otorhinolaryngol Relat Spec. 2008;70:80–83. doi: 10.1159/000114529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Supplemental file. More related data of the current study are available from the corresponding author on reasonable request.