Abstract
Objective
Primary outcome was to evaluate complete improvement at six months after acute treatment in NMOSD relapses.
Methods
Retrospective observational cohort study of patients with diagnosis of NMOSD admitted for acute attacks. We performed an explanatory analysis using the univariate, bivariate and multivariate logistic regression approach. We compared survival curves using the Kaplan Meier analysis and estimated the median time for the main outcome.
Results
In the univariate analysis, basal EDSS score, AQP4-IgG positivity, PLEX as a first-line treatment (IVMP + PLEX), less systemic complications related to acute treatment and total attack history were independently associated with complete improvement at six months. After adjusting for confounding variables and using multivariate analysis by Cox Regression, positive AQ4-IgG (HR 0.04, 95% CI: 0.02–0.66) and IVMP + PLEX (HR 5.1, 95% CI: 3.9–66.4), were kept as independent factors associated to time to complete improvement. Time from admission to PLEX initiation and complete improvement at six months had a median of seven days (95% CI: 5.2–8.8). In secondary effects, there were no statistical differences between the groups.
Conclusions
PLEX + IVMP is the treatment of choice for NMOSD relapses and should be initiated as early as possible.
Keywords: Devic's syndrome, Autoimmune diseases, Transverse myelitis, Optic neuritis, All demyelinating disease (CNS)
Devic's syndrome; Autoimmune diseases; Transverse myelitis; Optic neuritis; All Demyelinating disease (CNS).
1. Introduction
Neuromyelitis optica spectrum disorders (NMOSD) are a group of central nervous-system (CNS) inflammatory diseases, characterized as autoimmune channelopathies with antibodies immunoglobulin G against the aquaporin-4 receptor (AQP4-IgG) in the astrocytes [1], and, more recently, IgG against myelin oligodendrocyte glycoprotein (MOG-IgG) in the oligodendrocytes [2]. Typical clinical manifestations include severe optic neuritis (ON), longitudinally extensive transverse myelitis (LETM), and occasionally, area postrema, brainstem, diencephalic and cortex syndromes [3]. In several Latin American cohorts, NMOSD is more prevalent in young women, with AQP4-IgG positivity and an African ancestry in 54% of the patients [4]. The first attack was described as ON followed by LETM, with a mean expanded disability status scale (EDSS) score of 4.1 [4,5].
Attacks are usually treated with high-dose intravenous metilprednisolone (IVMP) and in case of failure, plasma exchange (PLEX) is used as a rescue therapy [6, 7]. Nevertheless, this approach is deficient since most patients accumulate disability after attacks, which may be reduced with optimal relapse and chronic treatment [6, 8, 9, 10, 11, 12]. Recently, the possibility of starting with IVMP associated to PLEX as a first-line treatment has been raised in order to improve outcomes after severe outbreaks [6, 9, 12].
Prognostic factors associated with good outcome after PLEX are male sex, early initiation of PLEX, short disease duration and minimal disability at baseline [13, 14, 15, 16]; AQP4-IgG positivity has been controversial in terms of prognosis [12, 13, 17, 18]. Here, we report the outcomes of IVMP + PLEX (first or second line) vs IVMP in patients with attacks related to NMOSD and factors associated to good prognosis.
2. Methods
2.1. Standard protocol approvals, registrations, and patient consents
This is a retrospective observational cohort study of patients with a diagnosis of NMOSD, who were admitted for treatment of an acute relapse at the Instituto Neurológico de Colombia between July 2015 and July 2020. We defined the primary outcome as complete improvement at six months (Basal EDSS score). Secondary outcomes were factors associated to a good prognosis after PLEX treatment and its complications. The Institutional Ethics Committee approved this study and provides Class III evidence that, in patients with severe NMOSD attacks, IVMP plus PLEX as a first-line treatment is associated to complete improvement at six months.
2.2. Patients and imaging
2.2.1. Inclusion criteria
We included patients 18 years of age or older if they had been diagnosed with NMOSD as defined by the 2015 Wigerchuck diagnostic criteria [3] and presented an acute (≤30 days) exacerbation defined as new or worsening symptoms attributable to a new T2 or contrast-enhancing T1 lesion through magnetic resonance imaging (MRI). Each relapse what confirmed by MRI which was done in a Siemens Symphony 1.5 T. The standard protocol included Brain MRI: Axial T1 MT, SF orbits T1 SE FS, Axial, coronal T1 gadolinium, Axial and coronal T2 FS, DWI and ADC, axial and sagittal FLAIR, 3D sagittal FLAIR, Time of flight, gradient echo. Cervical and Thoracic MRI: Axial and sagittal T1 gadolinium, axial and sagittal T2, STIR.
2.3. Data collection
We collected demographic data, disease characteristics, long-term immunotherapy treatment, clinical attack features, MRI findings, acute treatment and its complications. We extracted an EDSS score from available records or prospectively calculated prior to admission (baseline), at presentation, nadir of the admission, discharge and follow up (6–9 months). Eleven patients did not attend their six-month follow up. In order to understand the cause, they were reached telephonically. Four patients had changed neurologist, three were pending to schedule their appointment due to socioeconomic issues, two of them had died from complications related to NMOSD and we were unable to reach two patients. Seven patients stated that they were back to their baseline EDSS score.
Relapses were classified according to the type of acute treatment provided as IVMP or IVMP + PLEX. In the IVMP group, all relapses were treated with IVMP at a dose of 1000 mg daily for three to five days. In the IVMP + PLEX group, patients received five to seven sessions of PLEX simultaneously with IVMP (first line) or PLEX following IVMP treatment (second line) according to the neurologist's decision. Classically, PLEX was offered to relapses resistant to IVMP or reserved for severe attacks (high EDSS score); however, in the past few years after good results from PLEX studies have been published, neurologists have started using it as a first-line treatment and in most NMOSD attacks. PLEX was done by centrifugation with a central line. One to 1.5 volumes of plasma were exchanged against a 5% human albumin solution at each session every other day. When given concurrently, IVMP was given approximately 6 h after PLEX. We defined complete improvement as recovery of the EDSS score back to baseline.
2.4. Detection of AQP4-IgG
AQP4-IgG was processed by enzyme-linked immunosorbent assay (ELISA) in serum by an approved laboratory in Colombia. Positive results were above 3.0 U/ml.
2.5. Statistical analysis
Data are reported in frequencies, mean or median and their respective dispersion measurements according to the nature of the variables.
We used Chi Square Tests for comparison between clinical characteristics and binary outcome values. We performed an explanatory analysis using the univariate, bivariate and multivariate logistic regression approach. We compared survival curves using the Kaplan Meier analysis and estimated the median time for the main outcome.
We considered p values of <0.25 (Hosmer-Lemeshow) at univariate analysis as candidates for multivariate modelling, and we checked collinearity and interaction among variables. We used the multivariate proportional Cox regression method to model nominal outcomes of the variables selected during the previous step. Data are reported with the hazard ratio (HR) and 95% Confidence Interval (CI) and evaluated Goodness of Fit for the different models using Hosmer-Lemeshow criteria. We performed all statistical analyses using SPSS V25, and considered p values of ≤0.05 significant. Data was analyzed the epidemiologist and biostatistician from CES University.
2.6. Data availability
All authors have complete access to all data and take full responsibility for the veracity of the data and precision of the data analysis.
3. Results
3.1. Patient characteristics
A total of 119 attacks in 78 patients were included. Baseline characteristics are described (Table 1). AQP4-IgG were positive in 68 (87.2%) of the patients. The median duration of disease in years was seven (IQR 4–12). The basal EDSS score was higher in the groups that included PLEX: 1.87 in IVMP, 2.7 in PLEX as first line and 4.2 in PLEX as a second line. Time from admission to treatment initiation was also longer in the PLEX groups, compared to the IVMP group (5.4 vs 2). The majority of patients 60 (50.4%) presented myelitis, followed by optic neuritis 34 (28.6%). The most common chronic treatment was Rituximab in 49 (41%) patients (Table 1).
Table 1.
Patient characteristics.
| Characteristics | Total | IVMP | IVMP + PLEX (first line) | IVMP then PLEX (second line) |
|---|---|---|---|---|
| Demographics N (%) | ||||
| Number of total relapses included | 119 | 81 (68) | 19 (16) | 19 (16) |
| Number of patients included | 78 | 53 | 14 | 11 |
| Number of relapses per patient Me (IQR) | 3 (1–12) | 3 (1–10) | 3 (1–8) | 3 (1–12) |
| Age in years mean (SD) | 49 (14.6) | 48 (14) | 51 (14) | 56 (13) |
| Female | 70 | 48 (68.5) | 13 (18.5) | 9 (13) |
| Latino | 69 (88.4) | 48 (61.5) | 12 (15.3) | 8 (10.2) |
| Duration of disease in years, Me (IQR) | 7 (4–12) | 7 (5–12.5) | 5 (2–12.5) | 8 (3–10) |
| Diagnosis N (%) | ||||
| AQP4-IgG+ | 74 (94) | 49 (60.5) | 14 (73.7) | 11 (57.9) |
| Basal EDSS score Me (IQR) | 1 (0–8.5) | 1 (0–8.5) | 2 (0–7.5) | 4 (0–8) |
| Time from admission to acute treatment initiation (days) | 2.0 | 5.4 | 5.4 | |
| Attacks N (%) | ||||
| Manifestations | ||||
| ON | 34 (28.6) | 26 (76.5) | 3 (8.8) | 5 (14.7) |
| Myelitis | 60 (50.4) | 36 (60) | 11 (18) | 13 (22) |
| ON + Myelitis | 16 (13.4) | 11 (68.8) | 4 (25) | 1 (6.3) |
| Others | 9 (7.6) | 8 (89) | 1 (11) | 0 (0) |
| MRI | ||||
| Optic nerve | ||||
| Bilateral | 12 (10) | 7 (58) | 3 (25) | 2 (17) |
| Unilateral | 39 (33) | 31 (79) | 4 (10.5) | 4 (10.5) |
| Optic chiasm involvement | 16 (13.4) | 8 (50) | 7 (44) | 1 (6) |
| Gd (+) | 44 (37) | 32 (73) | 7 (16) | 5 (11) |
| Spinal cord | ||||
| Cervical | 14 (12) | 9 (64.3) | 3 (21.4) | 2 (14.3) |
| Thoracic | 21 (17.5) | 16 (76) | 3 (14) | 2 (10) |
| Cervical + thoracic | 36 (30.3) | 22 (61) | 7 (19.5) | 7 (19.5) |
| Cervico-medullary | 11 (9.2) | 6 (55) | 3 (27) | 2 (18) |
| Gd (+) | 75 (63) | 46 (61) | 15 (20) | 14 (19) |
| Chronic treatment N (%) | ||||
| Rituximab | 49 (41.2) | 33 (67) | 8 (16.5) | 8 (16.5) |
| Azathioprine | 22 (18.5) | 16 (73) | 4 (18) | 2 (9) |
| Prednisone | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Cyclophosphamide | 1 (0.8) | 0 (0) | 1 (100) | 0 (0) |
| Mycophenolate | 1 (0.8) | 1 (100) | 0 (0) | 0 (0) |
| Tocilizumab | 1 (0.8) | 1 (100) | 0 (0) | 0 (0) |
Demographic characteristics, clinical and radiological manifestations and chronic treatment according to the type of acute treatment. AQP4-IgG: aquaporin 4 IgG; Gd: gadolinium; IVMP: intravenous methylprednisolone; Me (IQR): median (interquartile range); MRI: magnetic resonance imaging; ND: no data; ON: optic neuritis; PLEX: plasma exchange; (SD): mean (standard deviation).
3.2. Predictive factors of PLEX outcome
We achieved the primary outcome (Basal EDSS score) in 51 (42%) patients, 37 (45%) in the IVMP group, five (26%) in the IVMP + PLEX (first-line) group and eight (42%) in the IVMP then PLEX (second-line) group.
In the univariate analysis, the basal EDSS score, AQP4-IgG positivity, PLEX as a first-line treatment (IVMP + PLEX), less systemic complications related to acute treatment and total attack history were independently associated with complete improvement at six months (Table 2).
Table 2.
Bivariate analysis by Proportional Cox Regression.
| Bivariate analysis (Chi Square) |
Bivariate analysis (regression) |
|
|---|---|---|
| Dependent: complete improvement at six months | Time to PLEX on days | |
| Variable | p-value | p-value |
| Basal EDSS | 0.006 | 0.17 |
| AQP4 | 0.024 | 0.27 |
| IVMP + PLEX first line | 0.007 | 0.025 |
| Chronic medication | 0.27 | 0.23 |
| Topography | 0.53 | 0.26 |
| Type of attack | 0.24 | 0.61 |
| Treatment with PLEX | 0.74 | 0.031 |
| Attack history | 0.001 | 0.99 |
Independent factors associated to complete improvement at six months. Bold denotes significant values. AQP4-IgG: aquaporin 4 IgG; IVMP: intravenous methylprednisolone; PLEX: plasma exchange.
The associated variables after the bivariate logistic regression approach were all treatments that included PLEX and IVMP + PLEX as a first-line treatment. We also included chronic immunosuppressive medication, multifocal attack type and topography as candidate variables for multivariate analysis (Table 2).
After adjusting for confounding variables and using multivariate analysis by Cox Regression, we kept positive AQP4-IgG (HR 0.04, 95% CI: 0.02–0.66) and PLEX as a first-line treatment (IVMP + PLEX) (HR 5.1, 95% CI: 3.9–66.4) as independent factors associated to time to complete improvement (Table 3).
Table 3.
Cox proportional hazards models and median time to PLEX initiation.
| Variable | Proportional Cox Regression |
IC 95% |
||
|---|---|---|---|---|
| p-value | HR | Lower limit | Upper limit | |
| AQP4 | 0.025 | 0.04 | 0.02 | 0.66 |
| IVMP + PLEX second line | 0.006 | 4.7 | 1.6 | 14.02 |
| IVMP + PLEX first line |
0.003 |
5.1 |
3.9 |
66.4 |
| Median time to PLEX in days | ||||
| Median |
Lower limit |
Upper limit |
||
| 7.000 | 5.2 | 8.8 | ||
Candidates by the enter method associated to complete improvement at six months. Median time to PLEX initiation from admission: seven days. IVMP: intravenous methylprednisolone; PLEX: plasma exchange.
We developed three Cox proportional hazard models with an enter method. The first candidate was the type of treatment [IVMP, IVMP + PLEX (first line), IVMP then PLEX (second line)] plus the variable AQP4-IgG. Next were candidates with chronic immunosuppressive medication, topography and systemic complications. Of all of them, only the IVMP + PLEX (first line) remained with statistically significant association (HR 5.1, 95% CI: 3.9–66.4), indicating that it is a determining factor to achieve complete improvement at six months (Table 3).
3.3. Clinical outcome according to PLEX delay
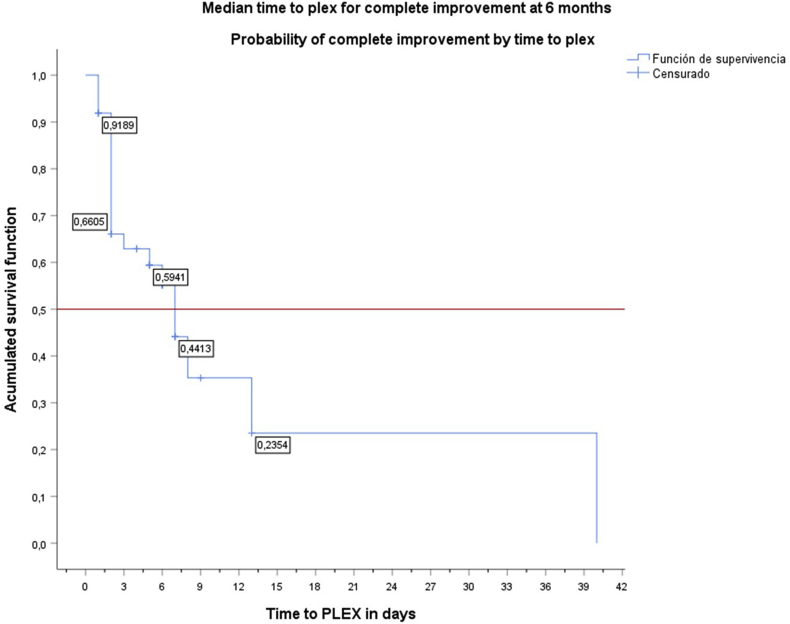
The analysis of time from admission to PLEX initiation and complete improvement at six months had a median of seven days (95% CI 5.2–8.8), meaning that the probability of achieving the primary outcome (complete improvement at six months) started decreasing to less than 60% at day seven (Figure 1).
Figure 1.
Survival function of time to PLEX initiation since admission in days Median time to PLEX initiation from admission: seven days. The probability of complete improvement started decreasing to less than 60% at day seven. PLEX: plasma exchange. Y: Accumulate survival; X: time to PLEX initiation since admission in days..
3.4. Time between attacks and PLEX
Although not statistically significant (p = 0.75), it seems clinically relevant that PLEX was also associated to increased time between upcoming attacks (5.8 years) vs 2.92 years in the IVMP group.
3.5. Secondary effects associated to acute treatment in NMOSD
Minor complications associated to acute treatment were present in 47 (39.5%) patients; there were no statistical differences between the groups. The most prevalent complications associated to PLEX were low fibrinogen and anemia in 15 and five patients, respectively. In the IVMP group, infection in 11 patients was the most frequent. There were no deaths and there was only one patient with a local complication (hemopneumothorax), which was associated to the PLEX catheter implantation and rapidly treated (Table 4).
Table 4.
Secondary effects by the type of acute treatment.
| Total | IVMP | PLEX | |
|---|---|---|---|
| Complications N (%) | 47 (39.5) | 17 (36) | 30 (64) |
| Systemic | 44 (37) | 17 (39) | 27 (61) |
| Anemia | 6 (5) | 1 (17) | 5 (83) |
| Bradycardia | 2 (1.7) | 1 (50) | 1 (50) |
| Electrolyte imbalance | 3 (2.5) | 0 (0) | 3 (100) |
| Hyperglycemia | 5 (4.2) | 1 (20) | 4 (80) |
| Hypotension | 5 (4.2) | 0 (0) | 5 (100) |
| Infection | 21 (17.6) | 11 (52) | 10 (48) |
| Low fibrinogen | 16 (13.4) | 0 (0) | 16 (100) |
| Local | |||
| Hemopneumothorax | 1 (0.8) | 0 (0) | 1 (100) |
Local and systemic secondary effects according to the type of acute treatment. IVMP: intravenous methylprednisolone; PLEX: plasma exchange.
4. Discussion
In this retrospective Latin American NMOSD cohort study, PLEX as a first-line treatment was associated to complete improvement at six months in comparison to IVMP alone. Furthermore, it demonstrated that delaying PLEX initiation in severe attacks beyond seven days may decrease the chances of complete improvement. These results are consistent with previous analyses, which have shown that severe NMOSD relapses should be considered an emergency and should be treated aggressively from admission [6, 9, 19, 20, 21]. The benefits of adding PLEX to NMOSD attack treatments could be explained by the fact that most of the astrocyte and neural destruction is caused by the deposition of AQP4-IgG and subsequent complement activation. PLEX removes circulating antibodies, complement and cytokines from the blood, which may shorten the action of antibodies and lessen further inflammation and necrosis.
PLEX was also associated to increased time between upcoming attacks. This benefit has been described by several authors using monthly or yearly PLEX sessions to avoid relapses in NMOSD patients, seeing that the removal of the humoral autoimmunity – in addition to modulation of cellular inflammation by IVMP – may increase the interval between relapses [22, 23, 24, 25, 26].
The independent factors associated to a good outcome after PLEX found in this cohort were: PLEX + IVMP as a first-line treatment, AQP4-IgG positivity, a low basal EDSS score, and a small number of previous attacks. This agrees with the literature since in recent reports, early initiation of PLEX has been a key factor to disability improvement in severe NMOSD attacks, given that most patients respond better to attack treatments at the beginning of the disease and accumulate disability as relapses develop [9, 13, 14, 16]. Moreover, a low basal EDSS score and a small number of total attacks have also been described in previous reports as predictors of a good prognosis [13, 15, 20].
AQP4-IgG serostatus has been controversial in terms of prognosis, considering that some articles have found worse outcomes when it is positive [17, 20, 27]. This might be explained by the fact that AQP4-IgG positivity was related to attack recurrence more than to PLEX response [11, 27, 28]. Moreover, it has been associated to intrathecal IgG synthesis, lower complement levels and an earlier age of onset [29, 30]. On the other hand, recent studies have found good or no correlation between AQP4-IgG and PLEX treatment, which evidences the clearance of AQP4-IgG and might suggest the presence of different antibodies in the seronegative patients that are also cleared by PLEX [12, 14, 19, 25, 31, 32, 33]. Also, around 25–40% of NMOSD seronegative patients are informed as MOG-IgG positive, which are less severe and respond better to relapse treatments [10, 34].
In our study, PLEX was not associated to complication occurrence compared to IVMP. There was only one major adverse effect associated to catheter implantation, namely hemopneumothorax. The rest of the side effects were mostly anemia, electrolyte imbalance and low fibrinogen, which were easily corrected before the next PLEX session (Table 4). Similar findings have been previously described [35, 36]. PLEX treatment should not be delayed because of safety.
Limitations of this study are that it is retrospective in nature and only included patients from one center. Also, AQP4-IgG was mostly done by ELISA, which is less sensitive than cell-based assay [37]. The small sample size of a single center limits the statistical power to compare differences across each group; however, NMOSD is a rare disease and the number of patients and AQP4-IgG seropositive percentage are similar to previous reports, which suggest we selected the patient group properly. We expect greater results with larger cohorts. Another limitation is that patients were not tested for MOG antibodies. Finally, randomized multicenter double-blinded studies are required to confirm our results.
5. Conclusion
PLEX + IVMP is the treatment of choice for NMOSD relapses and should be initiated as early as possible to increase the probabilities of complete improvement at six months. Further studies are needed to confirm the discrepancies in the literature.
Declarations
Author contribution statement
Carolina Restrepo-Aristizábal, Lilliana María Giraldo and María Isabel Zuluaga: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Yessica María Giraldo: Analyzed and interpreted the data.
Felipe Álvarez-Gómez and Angélica María Pino-Pérez: Contributed reagents, materials, analysis tools or data.
César Augusto Franco, José Vladimir Tobón and José Luis Ascencio: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Lennon V.A., Kryzer T.J., Pittock S.J., Verkman A.S., Hinson S.R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 2005;202(4):473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kitley J., Woodhall M., Waters P., Leite M.I., Devenney E., Craig J. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. 2012;79(12):1273–1277. doi: 10.1212/WNL.0b013e31826aac4e. [DOI] [PubMed] [Google Scholar]
- 3.Wingerchuk, Barwell Dean M., Brenda, Bennett J. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2016;86(5):491–492. doi: 10.1212/WNL.0000000000002366. [DOI] [PubMed] [Google Scholar]
- 4.Fragoso Y.D., Sousa N.A.C., Alves-Leon S.V., Dias R.M., Pimentel M.L.V., Gomes S. Clinical characteristics of 153 Brazilian patients with neuromyelitis optica spectrum disorder (NMOSD) Mult Scler Relat Disord. 2019;27(November 2018):392–396. doi: 10.1016/j.msard.2018.11.031. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 5.Díaz Becerra C.A., Zarco Montero L.A., Lasalvia P. Clinical, paraclinical and imaging characterization of a population of Colombian patients with neuromyelitis optica spectrum disorder at the hospital universitario san ignacio, bogotá, Colombia. Acta Neurol. Colomb. 2019;35(4):179–185. [Google Scholar]
- 6.Abboud H., Petrak A., Mealy M., Sasidharan S., Siddique L. 2015. Treatment of Acute Relapses in Neuromyelitis Optica : Steroids Alone versus Steroids Plus Plasma Exchange; pp. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srisupa - Olan T., Siritho S., Kittisares K., Jitprapaikulsan J., Sathukitchai C., Prayoonwiwat N. Beneficial effect of plasma exchange in acute attack of neuromyelitis optica spectrum disorders. Mult Scler Relat Disord. 2018;20(January):115–121. doi: 10.1016/j.msard.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Bichuetti D.B. Archives of NeurologyArchives of neurology. Neuromyelitis Optica Treatment. 2010;67(9):1131–1136. doi: 10.1001/archneurol.2010.203. [DOI] [PubMed] [Google Scholar]
- 9.Bonnan M., Valentino R., Debeugny S., Merle H., Fergé J.L., Mehdaoui H. Short delay to initiate plasma exchange is the strongest predictor of outcome in severe attacks of NMO spectrum disorders. J. Neurol. Neurosurg. Psychiatry. 2018;89(4):346–351. doi: 10.1136/jnnp-2017-316286. [DOI] [PubMed] [Google Scholar]
- 10.Lin C.W., Lin I.H., Chen T.C., Jou J.R., Woung L.C. Clinical course and treatment response of neuromyelitis optica spectrum disease: an 8-year experience. Asia-Pacific J Ophthalmol. 2019;8(3):206–210. doi: 10.22608/APO.2018247. [DOI] [PubMed] [Google Scholar]
- 11.Stellmann J.P., Krumbholz M., Friede T., Gahlen A., Borisow N., Fischer K. Immunotherapies in neuromyelitis optica spectrum disorder: efficacy and predictors of response. J. Neurol. Neurosurg. Psychiatry. 2017;88(8):639–647. doi: 10.1136/jnnp-2017-315603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleiter I., Gahlen A., Borisow N., Fischer K., Wernecke K.D., Hellwig K. Apheresis therapies for NMOSD attacks A retrospective study of 207 therapeutic interventions. Neurol Neuroimmunol NeuroInflammation. 2018;5(6) doi: 10.1212/NXI.0000000000000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aungsumart S., Apiwattanakul M. Clinical outcomes and predictive factors related to good outcomes in plasma exchange in severe attack of NMOSD and long extensive transverse myelitis: case series and review of the literature. Mult Scler Relat Disord. 2017;13(December 2016):93–97. doi: 10.1016/j.msard.2017.02.015. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 14.Kleiter I., Gahlen A., Borisow N., Fischer K., Wernecke K.D., Wegner B. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann. Neurol. 2016;79(2):206–216. doi: 10.1002/ana.24554. [DOI] [PubMed] [Google Scholar]
- 15.Lim Y.M., Pyun S.Y., Kang B.H., Kim J., Kim K.K. Factors associated with the effectiveness of plasma exchange for the treatment of NMO-IgG-positive neuromyelitis optica spectrum disorders. Mult. Scler. J. 2013;19(9):1216–1218. doi: 10.1177/1352458512471875. [DOI] [PubMed] [Google Scholar]
- 16.Mealy M.A., Mossburg S.E., Kim S.H., Messina S., Borisow N., Lopez-Gonzalez R. Long-term disability in neuromyelitis optica spectrum disorder with a history of myelitis is associated with age at onset, delay in diagnosis/preventive treatment, MRI lesion length and presence of symptomatic brain lesions. Mult Scler Relat Disord. 2019;28(July 2018):64–68. doi: 10.1016/j.msard.2018.12.011. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wingerchuk D.M. Evidence for humoral autoimmunity in neuromyelitis optica. Neurol. Res. 2006;28(3):348–353. doi: 10.1179/016164106X98260. [DOI] [PubMed] [Google Scholar]
- 18.Kessler R.A., Mealy M.A., Jimenez-Arango J.A., Quan C., Paul F., López R. Anti-aquaporin-4 titer is not predictive of disease course in neuromyelitis optica spectrum disorder: a multicenter cohort study. Mult Scler Relat Disord. 2017;17:198–201. doi: 10.1016/j.msard.2017.08.005. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 19.Keegan M., Pineda A.A., McClelland R.L., Darby C.H., Rodriguez M., Weinshenker B.G. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology. 2002;58(1):143–148. doi: 10.1212/wnl.58.1.143. [DOI] [PubMed] [Google Scholar]
- 20.Jiao Y., Cui L., Zhang W., Zhang Y., Wang W., Zhang L. Plasma exchange for neuromyelitis optica spectrum disorders in Chinese patients and factors predictive of short-term outcome. Clin. Therapeut. 2018;40(4):603–612. doi: 10.1016/j.clinthera.2018.03.007. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 21.Stiebel-Kalish H., Hellmann M.A., Mimouni M., Paul F., Bialer O., Bach M. Does time equal vision in the acute treatment of a cohort of AQP4 and MOG optic neuritis? Neurol Neuroimmunol NeuroInflammation. 2019;6(4):1–7. doi: 10.1212/NXI.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyamoto K., Kusunoki S. Intermittent plasmapheresis prevents recurrence in neuromyelitis optica. Ther. Apher. Dial. 2009;13(6):505–508. doi: 10.1111/j.1744-9987.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- 23.Oji S., Nomura K. Immunoadsorption in neurological disorders. Transfus. Apher. Sci. 2017;56(5):671–676. doi: 10.1016/j.transci.2017.08.013. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 24.Schwartz J. Guidelines on the use of therapeutic apheresis in clinical practice—evidence-based approach from the writing committee of the American society for apheresis: the seventh special issue. J. Clin. Apher. 2016;31(2016):149–162. doi: 10.1002/jca.21470. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 25.Lipphardt M., Wallbach M., Koziolek M.J. Plasma exchange or immunoadsorption in demyelinating diseases: a meta-analysis. J. Clin. Med. 2020;9(5):1597. doi: 10.3390/jcm9051597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khatri B.O. Maintenance plasma exchange therapy for steroid-refractory neuromyelitis optica. J. Clin. Apher. 2012;27(4):183–192. doi: 10.1002/jca.21215. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 27.Weinshenker B.G., Wingerchuk D.M., Vukusic S., Linbo L., Pittock S.J., Lucchinetti C.F. Neuromyelitis optica IgG predicts relapse after longitudinally extensive transverse myelitis. Ann. Neurol. 2006;59(3):566–569. doi: 10.1002/ana.20770. [DOI] [PubMed] [Google Scholar]
- 28.Contentti E.C., De Virgiliis M., Hryb J.P., Gomez A., Morales S., Celso J. Aquaporin-4 serostatus and visual outcomes in clinically isolated acute optic neuritis. J. Neuro Ophthalmol. 2019;39(2):165–169. doi: 10.1097/WNO.0000000000000668. [DOI] [PubMed] [Google Scholar]
- 29.Qin C., Chen B., Tao R., Chen M., Ma X., Shang K. The clinical value of complement proteins in differentiating AQP4-IgG-positive from MOG-IgG-positive neuromyelitis optica spectrum disorders. Mult Scler Relat Disord. 2019;35(June):1–4. doi: 10.1016/j.msard.2019.06.035. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y., Li R., Wu A.M., Shu Y.Q., Lu Z.Q., Hu X.Q. The complement and immunoglobulin levels in NMO patients. Neurol. Sci. 2014;35(2):215–220. doi: 10.1007/s10072-013-1481-y. [DOI] [PubMed] [Google Scholar]
- 31.Bonnan M., Valentino R., Bonnan M., Mehdaoui H., Smadja D., Cabre P. Plasma exchange in severe spinal attacks associated with neuromyelitis optica spectrum disorder. Mult. Scler. 2009;15(4):487–492. doi: 10.1177/1352458508100837. [DOI] [PubMed] [Google Scholar]
- 32.Sepúlveda M., Armangué T., Sola-Valls N., Arrambide G., Meca-Lallana J.E., Oreja-Guevara C. Neuromyelitis optica spectrum disorders: comparison according to the phenotype and serostatus. Neurol Neuroimmunol NeuroInflammation. 2016;3(3) doi: 10.1212/NXI.0000000000000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishimura H., Enokida H., Sakamoto T., Takahashi T., Hayami H., Nakagawa M. Immunoadsorption plasmapheresis treatment for the recurrent exacerbation of neuromyelitis optica spectrum disorder with a fluctuating anti-aquaporin-4 antibody level. J. Artif. Organs. 2018;21(3):378–382. doi: 10.1007/s10047-018-1044-3. [DOI] [PubMed] [Google Scholar]
- 34.Reindl M., Jarius S., Rostasy K., Berger T. Myelin oligodendrocyte glycoprotein antibodies: how clinically useful are they? Curr. Opin. Neurol. 2017;30(3):295–301. doi: 10.1097/WCO.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 35.Morgan S. Therapeutic plasma exchange in neuromyelitis optica: a case series. J. Clin. Apher. 2014;29(2014):171–177. doi: 10.1002/jca.21304. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 36.Veerachit-O-larn T., Siritho S., Prayoonwiwat N. Retrospective study of the adverse events of the treatment for an acute attack of neuromyelitis optica spectrum disorder. Ther. Apher. Dial. 2020;24(4):453–460. doi: 10.1111/1744-9987.13456. [DOI] [PubMed] [Google Scholar]
- 37.Waters P., Reindl M., Saiz A., Schanda K., Tuller F., Kral V. Multicentre comparison of a diagnostic assay: aquaporin-4 antibodies in neuromyelitis optica. J. Neurol. Neurosurg. Psychiatry. 2016;87(9):1005–1015. doi: 10.1136/jnnp-2015-312601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All authors have complete access to all data and take full responsibility for the veracity of the data and precision of the data analysis.
Data included in article/supplementary material/referenced in article.