Abstract
House dust mites (HDMs) are a potent allergen source that are commonly found in human living environments. While HDMs are known to induce allergic diseases in humans, such as asthma, its other biological activities related to human health are less understood. Our laboratory recently purified the HDM protein PDI (protein disulfide isomerase). In this study, we assess the role of PDI in contributing to immune regulation. Using mass spectrometry, we analyzed the complexes of DEC205 and HDM extracts, and the role of PDI in the induction of tolerogenic dendritic cells (DCs) was assessed in human cell culture experiments and verified in a murine model. We found that more than 20 HDM-derived proteins, including PDI, bound to DCs by forming complexes with DEC205. Additionally, DEC205-mediated the endocytosis of PDI. HDM-derived PDI (HDM-PDI) promoted Foxp3 expression in DCs. HDM-PDI-primed DCs also showed tolerogenic properties that induced regulatory T cell development, indicating that the primed DCs were tolerogenic DCs. Our results suggested that the PDI/DEC205/TIEG1/Foxp3 signal pathway activation was involved in the HDM-PDI-induced Foxp3 expression in DCs. Finally, we found that HDM-PDI competitively counteracted the Th2 cytokines to restore DC’s tolerogenicity, and administration of HDM-PDI could suppress experimental asthma. In conclusion, our data suggest that HDM-PDI contributes to immune regulation by inducing tolerogenic DC development. Administration of HDM-PDI can alleviate experimental asthma. These findings demonstrate that HDM-PDI has translational potential to be used in the treatment of immune disorders such as asthma.
Keywords: asthma, allergy, house dust mite, protein disulfide isomerase, dendritic cell, immune tolerance, inflammation, Foxp3
Abbreviations: BALF, bronchoalveolar lavage fluid; Breg, regulatory B cell; FCS, flow cytometry; HDM, house dust mite; IL, interleukin; MS, mass spectrometry; PDI, protein disulfide isomerase; TGF, transforming growth factor; TolDC, tolerogenic dendritic cell; Treg, regulatory T cell
The immune tolerance indicates a condition that the immune system in the body does not respond to immune stimulation that should be. It is extremely important to maintain the immune tolerance system function properly in the body as it plays a crucial role in the maintenance of the homeostasis in the body. Many immune cells, such as tolerogenic dendritic cells (TolDC), regulatory T cells (Treg), and regulatory B cells (Breg), and cytokines, such as transforming growth factor (TGF)-β and interleukin (IL)-10, have been recognized in association with immune tolerance (1). The dysfunctional immune tolerance system is involved in many diseases, for example, the Treg deficiency elicits autoimmunity and heritable diseases of immune dysregulation (1). This mirrors an important fact that it has not fully understood the induction and maintenance of immune tolerance in the body.
House dust mite (HDM) extensively distributes to human living environment. It is well known that HDM is the major allergen of allergic asthma (2). HDM can induce aberrant Th2 response, the latter further induces specific IgE production to sensitize mast cells; re-exposure to specific antigens induces mast cells to release allergic mediators to trigger asthma attacks (2). However, besides being allergens to induce allergic diseases, other features of HDM related to human health are less understood. Our previous studies show that one of HDM components, phthalein proline cis/trans isomerase peptidyl-prolylisomerases (Pplase), has immune suppressive effects on airway allergic response (3), suggesting not all the HDM components are allergens, some of them may be of benefit to human beings. Thus, in the present study, we assessed the effects of HDM-derived factors on regulating DC activities. We found that the HDM-derived PDI (protein disulfide isomerase) could induce TolDCs. PDI is an enzyme that catalyzes disulfide formation and isomerization. With disulfide bonds, PDI contributes to individual protein stable or proteins joined covalently with each other in a mechanism of chemically cross-linked specific cysteines (4). PDI is associated with cancer pathology (5), vascular inflammation (6), or immune disorder pathogenesis (7). The present study expanded the above findings about PDI by showing that HDM-derived PDI (PDI, in short) has immune regulatory effects.
Results
HDM-derived PDI binds DCs by forming complexes with DEC205
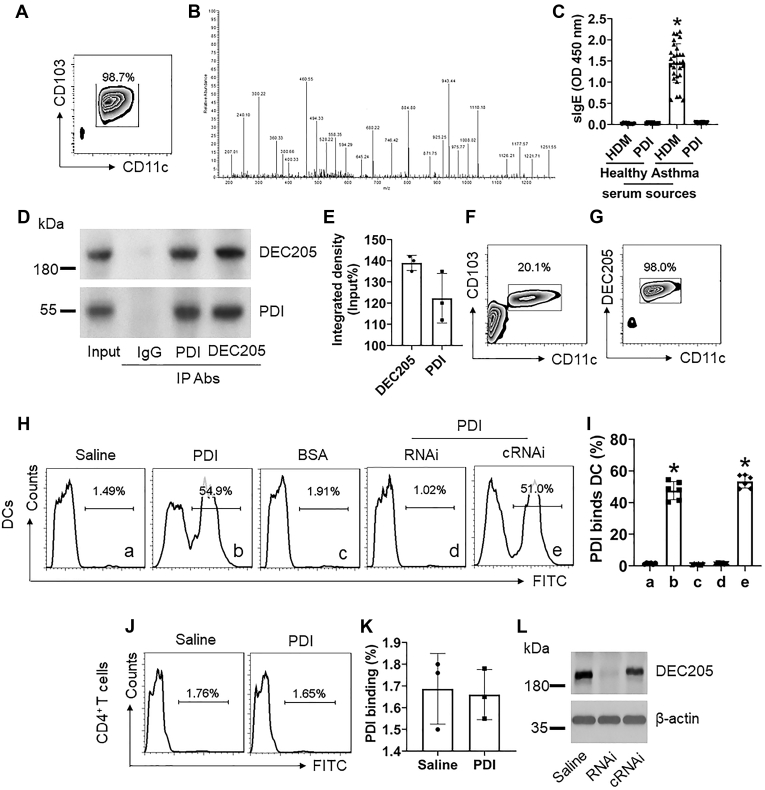
It is a consensus that HDM is the major source of allergen to trigger allergic asthma (2). DCs play a central role in the initiation of the immune response. Thus, it is of significance to further elucidate the interaction between HDM and DCs. To this end, we isolated DCs from healthy human blood samples (Fig. 1A). DCs were cultured in the presence of HDM extracts for 3 h. The DCs were harvested; proteins were extracted from the cells and analyzed by immunoprecipitation (IP) with anti-HDM pAb as a bait, followed by using anti-mouse Ab as the IP Ab. The IP complexes were purified and analyzed by mass spectrometry (MS). The MS results (Fig. 1B) showed that 27 HDM-derived proteins formed the complexes with DEC205 protein (Table S1 in supplementary materials), in which 25 allergens have been characterized by previous studies (references are presented in the table); one is PPlase that we characterized previously (3); another is the PDI (Table S1). As Haffman et al reported that ERp57 (one of PDI phenotypes) levels were significantly increased in airway epithelial cells of asthma patients and mice with experimental airway allergy and proposed that ERp57 plays a role in the pathogenesis of airway allergy (7), we assessed the interaction between HDM-derived PDI and human DCs. Based on the gene sequence (Table S3) we reported in our previous studies (8), we asked a biotech company (Sangon Biotech Inc, Shanghai, China) to produce the PDI protein through the molecular cloning approach with the E. coli system (the recombinant protein quality was verified by the company; the sequence verification is shown in Table S3) to be used in the present study. The PDI was used to coat ELISA plates to assess the PDI-specific IgE levels in serum samples obtained from 30 asthma patients who sensitized to HDM as shown by skin test (Table S2). The results showed that none of the 30 serum samples had detectable PDI-specific IgE (Fig. 1C). The results suggest that HDM-derived PDI is not an allergen. We further found that PDI formed a complex with DEC205 in DCs by co-IP assay, in which both DEC205 and PDI could be precipitated by either anti-DEC205 Ab or anti-PDI Ab (Fig. 1, D and E). To corroborate the results, we isolated DEC205+ DCs (Fig. 1, F and G) from healthy human blood samples to expose to FITC-labeled PDI in the culture for 3 h. We observed that PDI bound more than 50% DEC205+ DCs (Fig. 1, H and I), but not CD4+ T cells (Fig. 1, J and K); the binding was abolished by knocking down DEC205 in DCs (Fig. 1, H, I and L). The results suggest that PDI may modulate DC’s properties.
Figure 1.
HDM-derived PDI binds DCs by forming a complex with DEC205. A, DCs were isolated from the healthy human blood samples and exposed to HDM extracts (1 μg/ml) in the culture. B, DC protein extracts were precipitated with anti-HDM pAb as the first IP Ab and followed by using anti-mouse Ab to precipitate the HDM/DC protein complexes, the latter was analyzed by mass spectrometry (MS). Panel B shows a representative MS data sheet. C, blood samples were collected from healthy subjects (n = 10) and asthma patients (n = 30, sensitized to HDM, approved by HDM skin test). The serum was isolated from blood samples and analyzed by ELISA, the plates were coated with HDM or PDI, respectively. The bars show the specific IgE levels in the serum. D, immunoblots show the PDI/DEC205 complexes (analyzed by conventional co-IP; IgG: Isotype IgG). E, bars show integrated density of the immunoblots in panel D. F–G, DEC205+ DCs were prepared from human blood samples. FCS plots show the cell purity. H, DEC205+ DCs were exposed to PDI (1 μg/ml; labeled with FITC) in the culture for 3 h. Gated histograms show cell frequency bound by FITC-PDI. I, bars show mean ± SEM of FITC-PDI-bound DCs. J, gated histograms show the rate of PDI-bound CD4+ T cells. K, bars show summarized mean ± SEM of PDI-bound CD4+ T cell counts. L, immunoblots show DEC205 RNAi results. RNAi: DEC205 RNAi. cRNAi: DCs were treated with control RNAi. The data represent three or six independent experiments (each sample was analyzed in triplicate with average of the three readouts as one datum). ∗, p < 0.01, compared with the healthy-HDM group (C) or group a (I).
PDI does not induce airway allergy
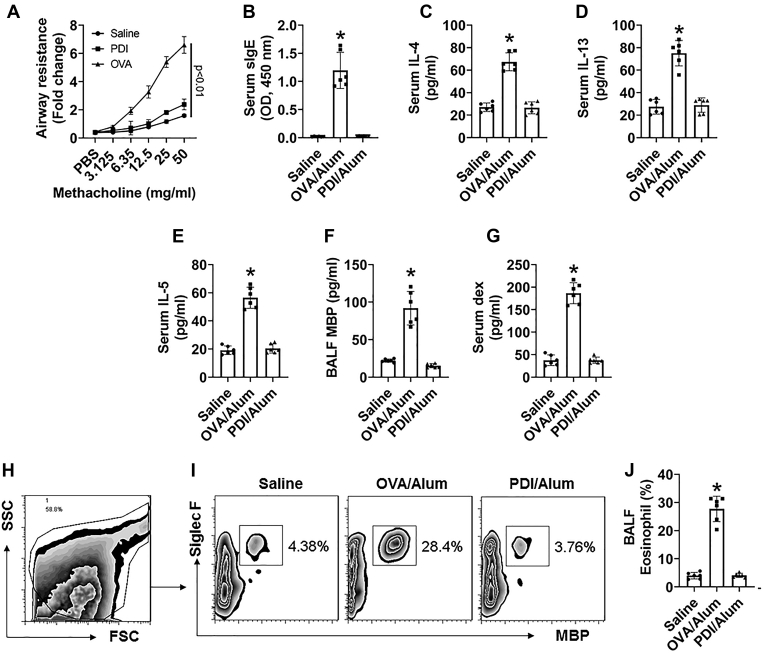
We then tested if HDM-derived PDI could induce airway allergy. BALB/c mice were immunized with PDI/Alum following established procedures (9). As compared with mice immunized with the ovalbumin (OVA)/Alum procedures, which show asthma-like symptoms, including airway hyperresponsiveness, high levels of serum specific IgE (sIgE), IL-4, IL-5, and IL-13, high major basic protein (MBP) in BALF (bronchoalveolar lavage fluids), high frequency of eosinophil in BALF, and airway epithelial barrier dysfunction, which did not occur in mice immunized with PDI/Alum (Fig. 2). The data confirm that HDM-derived PDI is not an allergen.
Figure 2.
PDI does not induce airway allergy. BALB/c mice were immunized with the PDI/Alum protocol or the OVA/Alum protocol. A, curves show airway resistance of mice. B–E, bars show serum levels of OVA- or PDI-specific IgE (B), IL-4 (C), IL-5 (D), or IL-13 (E). F, bars show MBP levels in BALF. G, bars show serum levels of FITC-dextran (dex). H–I, FCS results of eosinophil counts in BALF. J, bars show summarized eosinophil counts in BALF. The data of bars are presented as mean ± SEM. ∗, p < 0.01 (ANOVA with Dunnett’s test), compared with the saline group. Each group consists of six mice. The data represent three independent experiments (each sample was analyzed in triplicate with average of the three readouts as one datum).
PDI induces TolDCs
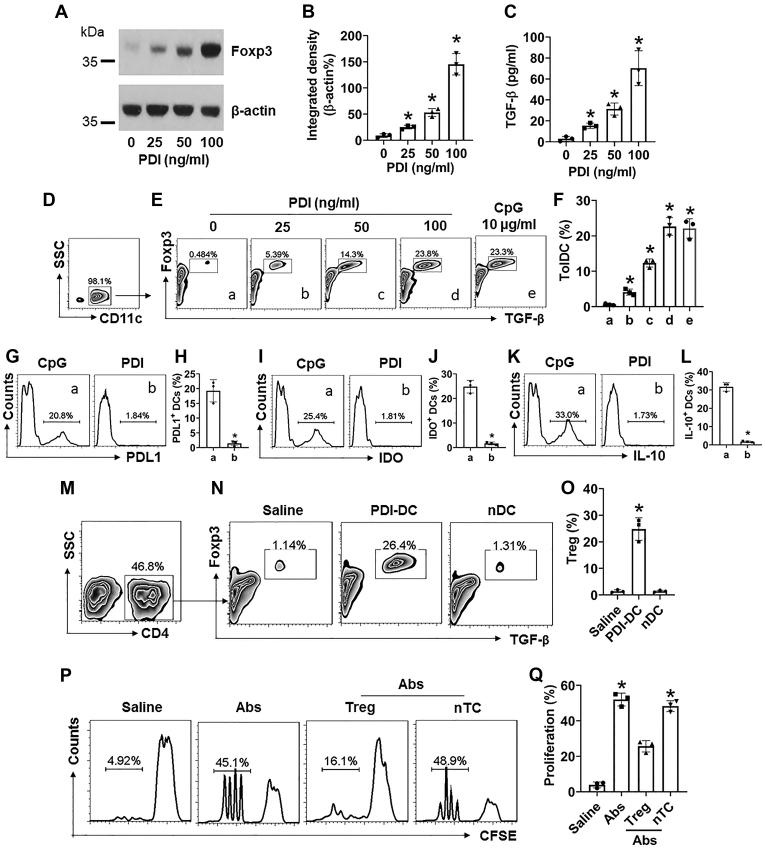
We then tested if PDI modulated DC’s properties. DEC205+ DCs were isolated from healthy human blood samples and exposed to PDI in the culture for 3 days. We found that the Foxp3 and TGF-β expression in DCs was markedly induced by PDI as well as by CpG (a positive control (10)) (Fig. 3, A–F). We also observed that exposure to CpG induced the expression of other immune tolerogenic factors, including PDL1, IDO, and IL-10, in DCs, but not in PDI-stimulated DCs (Fig. 3, G–L). Since the TGF-β expression indicates the tolerogenic feature of DCs (11), the results suggest that exposure to PDI induces TolDCs. To verify this, CD4+ CD25- T cells were isolated from the naïve mouse spleen and cocultured with PDI-primed DCs for 3 days. As analyzed by flow cytometry (FCS), coculture with PDI-primed DCs markedly increased the expression of Foxp3 and TGF-β in CD4+ T cells (Fig. 3, M–O). The results indicate that PDI-primed DCs can induce regulatory T cell (Treg) differentiation. These Tregs showed immune-suppressive effects on CD4+ T cell proliferation (Fig. 3, P–Q). The data demonstrate that PDI can induce TolDCs, the latter induces Tregs.
Figure 3.
PDI induces TolDCs. A–C, DEC205+ DCs were isolated from the human blood samples and cultured in the presence of PDI at gradient concentrations for 48 h. Immunoblots show Foxp3 protein in DCs (A); bars show integrated density of immunoblots of A (B); bars show TGF-β levels in DC culture supernatant (C). D, gated plots show the purity of DCs. E, gated plots show TolDC frequency. F, bars show summarized data of TolDC counts. G–L, DCs were cultured in the presence of CpG (10 μg/ml) or DPI (100 ng/ml) for 48 h. Gated histograms show the frequency of PDL1+ DCs (G), or IDO+ DCs (I), or IL-10+ DCs (K). Bars show summarized positive cell counts of PDL1 (H), or IDO (J), or IL-10 (L). M–O, DCs were primed by exposing to PDI (100 ng/ml) in the culture for 48 h and then cocultured with naïve CD4+ CD25¯ T cells (DC:T cell = 1:1) in the presence of IL-2 (20 ng/ml) for 72 h. nDC: Naïve DC (controls). M, CD4+ T cells were gated first. N, gated FCS plots show Foxp3+ TGF-β+ Treg frequency. O, bars show mean ± SEM of 3 experiments of panel N. P–Q, Tregs were prepared as described in panel M-O and cocultured with naïve CD4+ CD25¯ T cells (labeled with CFSE) with the conditions denoted above each subpanel of P. Gated histograms show CD4+ T cell proliferation (P). Q, bars show summarized CD4+ T cell proliferation. Ab: Anti-CD3/CD28 Ab (5 μg/ml of each Ab). nTC: Naïve CD4+ T cells (controls). The data of bars are presented as mean ± SEM. ∗, p < 0.01 (ANOVA with Dunnett’s test), compared with the dose 0 (B, C) or group a (F), or the saline group (O, Q). The data represent three independent experiments (each sample was analyzed in triplicate with average of the three readouts as one datum).
Interaction of PDI/TIEG1 regulates TolDC development
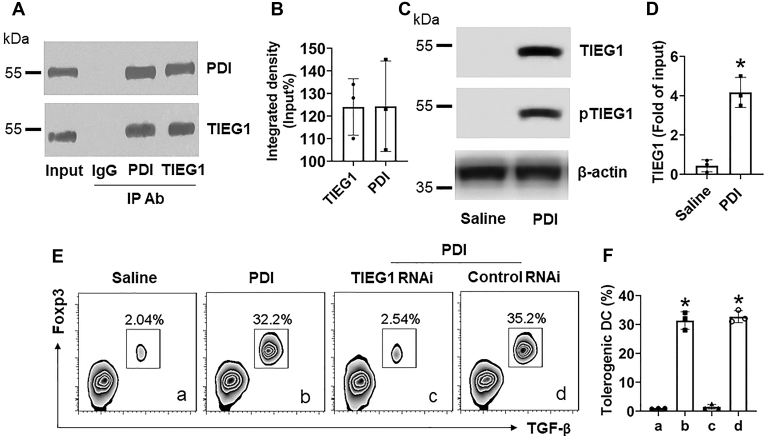
TIEG1 is associated with the Foxp3 expression in T cells (12). We wondered if TIEG1 was also involved in the PDI-induced Foxp3 expression in DCs. To this end, after exposure to PDI, DC protein extracts were analyzed by co-IP with anti-TIEG1 Ab or anti-PDI Ab as a bait. The results showed that a complex of PDI/TIEG1 was detected in the extracts by using either anti-PDI Ab or anti-TIEG1 Ab as the IP Ab (Fig. 4, A and B). Further analysis showed that the phosphorylated TIEG1 levels were upregulated in DCs by PDI exposure (Fig. 4C). The increase in TIEG1 was detected in the Foxp3 promoter (Fig. 4D). Depletion of TIEG1 also abolished the PDI-induced TolDC development. The results indicate that the DEC205/PDI/TIEG1 signal pathway is required in the PDI-induced TolDC development.
Figure 4.
TIEG1 is involved in PDI-induced tolerogenic DC development. A–D, DEC205+ DCs were isolated from the human blood samples and exposed to PDI (100 ng/ml) in the culture for 48 h. A, immune blots show that a complex of PDI/TIEG1 was detected in DC protein extracts. B, integrated density of the immunoblots in panel A. C, immunoblots show that phosphorylated TIEG1 levels are increased in DCs in response to PDI. D, bars show mean ± SEM of TIEG1 levels in the Foxp3 promoter. E, gated FCS plots show tolerogenic DC counts after exposing to PDI in the culture for 3 days. F, bars show mean ± SEM of tolerogenic DC counts. ∗, p < 0.01, compared with group saline (D) or group a (F). Statistical method: ANOVA with the Dunnett’s test. The data represent three independent experiments (each sample was analyzed in triplicate with average of the three readouts as one datum).
PDI competitively binds DEC205 to prevent IL-4 from compromising TolDC’s tolerogenic properties
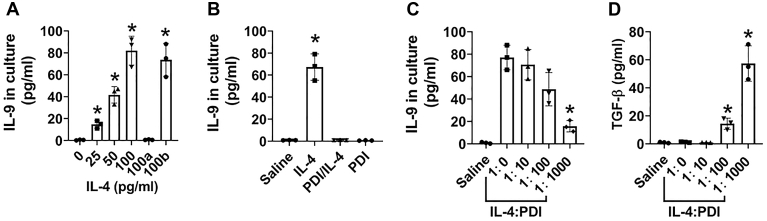
IL-4 plays a central role in Th2-dominant inflammation such as asthma. In a synergistic manner, IL-4 and TGF-β induce the IL-9 production; the latter also plays an important role in the pathogenesis of allergic diseases (13). We wondered if IL-4 could cooperate with the TGF-β in TolDCs to initiate the IL-9 production within the cells that compromise the TolDC properties. To this end, we exposed TolDCs to IL-4 in the culture. We found that IL-4 prevented the TGF-β production by TolDCs, instead, IL-4 induced TolDCs to produce IL-9, which was abolished by the depletion of DEC205 (Fig. 5A). The results suggest that IL-4 contacts TolDCs via DEC205 to induce the IL-9 production that prompted us to add PDI to the TolDC culture first, then IL-4. As expected, the IL-4 addition did not induce TolDCs to produce IL-9 (Fig. 5B). The results indicate that the PDI-occupying DEC205 can block IL-4 to bind DEC205 in DCs, suggesting a competitive relation between IL-4 and PDI in binding DEC205 on DC. PDI alone did not induce the IL-9 expression in DCs (Fig. 5B). Adding IL-4 to the TolDC culture first, then adding PDI in a ratio of 1:10 or 1:100 (IL-4:PDI; wt:wt), to the culture, no apparent inhibitory effects were found on the IL-9 production by TolDCs. However, increase in the dosage of PDI to 1:1000 showed inhibitory effects on the IL-9 production (Fig. 5C) as well as restored the TGF-β production (Fig. 5D). The results suggest that PDI may be used to restore the TolDCs’ function in allergic diseases by recovering DCs’ immune tolerogenic functions.
Figure 5.
PDI counteracts the IL-4-induced IL-9 from TolDCs. A, bars show mean ± SEM of IL-9 levels in TolDC culture supernatant of three experiments in the presence of IL-4 at indicated concentrations (denoted on the X axis; the ratio of IL-4:PDI is wt:wt) for 48 h. RNAi: TolDCs were treated with DEC205 RNAi. cRNAi: TolDCs were treated with control RNAi. B, TolDCs were treated with IL-4 (100 pg/ml) alone, or pretreated with PDI (100 ng/ml) for 30 min, then added IL-4 (100 pg/ml) to the culture for 48 h. Bars show mean ± SEM of IL-9 levels in TolDC culture supernatant of three experiments. C and D, TolDCs were treated with IL-4 (100 pg/ml) and gradient concentrations of PDI (denoted on the X axis) in the culture for 48 h. Bars show mean ± SEM of IL-9 (C) or TGF-β (D) in culture supernatant. ∗, p < 0.01 (ANOVA with Dunnett’s test), compared with the dose 0 (A), or the saline group (B), or the dose 1:0 (C, D). The data represent three independent experiments (each sample was analyzed in triplicate with average of the three readouts as one datum).
Administration of PDI inhibits experimental asthma by generating ToDCs in the lung
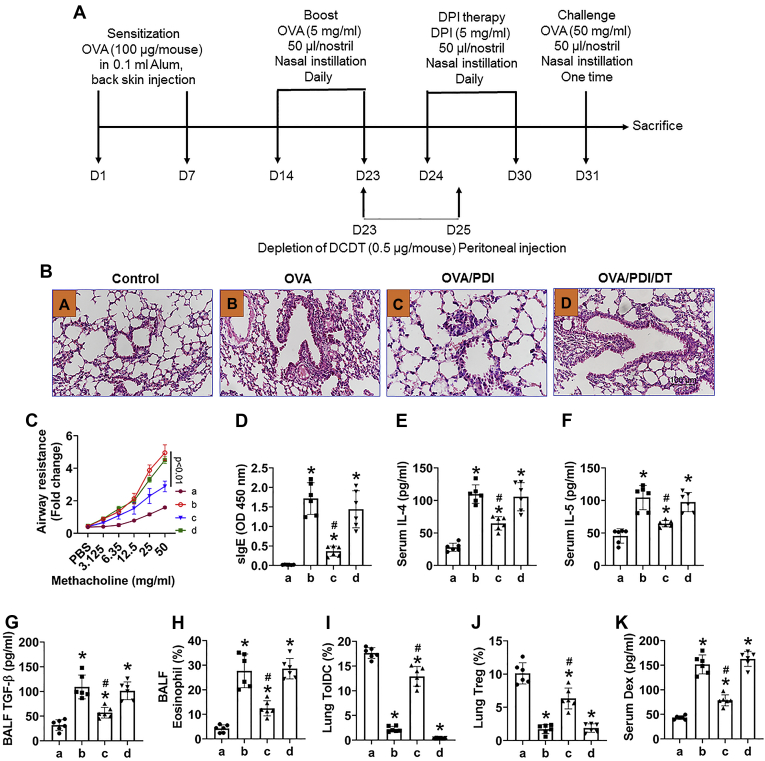
Finally, we developed a mouse model of asthma (Fig. 6A). The mice showed asthma-like symptoms, including hyperresponsiveness in the airways. High levels of specific IgE, Th2 cytokines, major basic proteins, high frequency of eosinophils in BALF (Figs. 6, S1 and S2), and increase in the epithelial barrier permeability. Administration of PDI (Fig. 6A) efficiently attenuated the asthma symptoms, restored TolDCs and Tregs in the lungs, which was abolished by depletion of DCs (Figs. 6, S1–S3). The results demonstrate that PDI can alleviate experimental asthma.
Figure 6.
Administration of PDI alleviates experimental asthma by generating TolDCs in the lung. An asthma mouse model was developed with ovalbumin (OVA) as an antigen and mice were treated with PDI by nasal instillation (A). B, representative lung histology images (original magnification: × 200). C, the curves show mouse airway resistance records in response to methacholine challenge. C–F, bars show the serum levels of OVA-specific IgE (sIgE, D), IL-4 (E), and IL-5 (F). G, bars show the TGF-β levels in BALF. H, bars show eosinophil frequency in cellular components of BALF. I and J, bars show frequencies of TolDC (I) and Treg (J) in lung mononuclear cells. K, bars show serum FITC-dextran (Dex) levels. FCS plots of H–J, are presented in Figs. S1–S3. Group labels in panel C–K are the same as those in panel B. The data of bars are presented as mean ± SEM. Each group consists of six mice. ∗, p < 0.01 (ANOVA with Dunnett’s test), compared with group a. #, p < 0.01 (the Student’s t-test), compared with group b. The data represent three independent experiments (each sample was analyzed in triplicate with average of the three readouts as one datum).
Discussion
This study found a previously undescribed phenomenon that HDM-derived PDI contributed to immune regulation by activating the PDI/DEC205/TIE2 signal pathway to induce the Foxp3 expression in DCs. Since Foxp3 is the transcription factor of TGF-beta, the latter is a critical effective factor of immune regulation, the data suggest that the interaction between PDI and DCs can confer DCs the immune tolerogenic properties. Indeed, PDI-primed DCs could induce Treg generation. Administration of PDI efficiently alleviated the experimental asthma.
Our previous work showed that more than 20,000 active genes were identified in HDM (8). Among these gene products, over 20 allergens have been identified (14). Thus, the functions of majority of HDM body components have not been fully characterized yet. In fact, not all the HDM-derived proteins are allergens; some components may have different functions. For example, the HDM-derived PPlase protein has the immune-suppressive function (3). The present study has expanded the previous findings by showing that the HDM-derived PDI has immune regulatory functions by inducing TolDCs. Thus, it is worthy to further characterize the properties of those unanalyzed HDM-derived proteins.
We found that DEC205 molecule mediated the interaction between PDI and DCs. DEC205 is an endocytic receptor on DCs and thymus epithelial cells (15). Some exogenous molecules can bind DEC205 to regulate DC’s activities. For example, DEC205 can mediate the effects of cartilage proteoglycan on modulating DC’s activities to elicit the immune regulatory activities that alleviate experimental arthritis (16). By MS approach, we found over 20 HDM-derived proteins bound to DEC205 including PDI, among which the known allergens have been described (14) except PDI. By employing the serum obtained from asthma patients with HDM sensitization as well as tested in a murine model, we found that PDI was not an allergen as no PDI-specific IgE was detectable in the HDM-specific IgE positive serum and PDI could not induce experimental airway allergy.
We in turn found that PDI had immune regulatory activities. By exposing naïve DCs to PDI in the culture, both Foxp3 and TGF-β were detected in those DCs. Since TGF-β is an important immune regulatory cytokine (17), the data indicate that PDI can induce TolDCs. This was verified by further experimental data that the PDI-induced TolDCs could generate Tregs. Although many studies found that IL-10-producing DCs were TolDCs, TGF-β-producing DCs were also found as a fraction of TolDC that was detailed by a review paper (11). The TGF-β-producing TolDCs naturally exist in the body; such as a portion of mesentery lymph node–derived CD103+ DCs express TGF-β; their immune regulatory functions can be promoted by retinoic acid, a dietary metabolite (18). The TGF-β-producing TolDCs also can be induced, such as exposure to TGF-β can induce CD14+ monocytes with granulocyte–monocyte colony stimulating factor, IL-4, and TGF-β to TGF-β-producing TolDCs (19). Our data provide a novel mechanism for TGF-β-producing TolDCs by exposing DEC205+ DCs to PDI in the culture.
Our data show that PDI promotes the Foxp3 expression in DCs. The key point in the induction of TGF-β expression is that the Foxp3 expression must be activated first as Foxp3 is the transcription factor of TGF-β (20). The present data show that PDI has such a function; by inducing TIEG1, the transcription factor of Foxp3, phosphorylation, PDI activates the Foxp3 expression in DCs. PDI has multiple functions. Fox examples, it activates platelets to promote thrombosis and induces vascular inflammation (6). PDI is also associated with the survival, proliferation, and metastasis of several cancers (21). These features need to be considered in further investigations in PDI studies.
The data show that IL-4 and PDI competently bind DEC205 to modulate TolDC’s activities. Exposure to IL-4 induces TolDCs to produce IL-9 instead of TGF-β. IL-9 is one of the Th2 cytokines that plays a role in the pathogenesis of Th2 pattern inflammation such as allergic asthma (13). IL-4 in synergy with TGF-β initiates the IL-9 expression (13). TolDCs produce TGF-β. Thus, it is logical that exposure to IL-4 induces TolDCs to produce IL-9. Fortunately, such a status can be modulated by exposing the TolDCs to higher PDI concentrations. This suggests that administration of PDI may restore DC’s tolerogenic function in certain environment such as allergic asthma. Indeed, this inference was verified by an experimental asthma model. Administrating PDI efficiently alleviated airway allergic inflammation in a mouse model via restoring TolDC and Treg numbers in the lungs.
One of the previous studies indicates that human airway epithelial cells also express PDI (7). The levels of PDI are increased in airway epithelial cells of asthma patients. The paper suggests that PDI contributes to fibrosis in the airway tissues. Depletion of the PDI expression could not induce airway allergy in mice (7), whereas our data show that PDI can alleviate experimental asthma. Such a difference between this report and the present study may be because that study and our project focus on different cell types. Hoffman et al observed PDI in airway epithelial cells; the present study investigated into the role of PDI in regulating DC’s tolerogenic properties. On the other hand, the similarity of PDI amino acid sequence between HDM and human is 27% (AAC50401.1) and 44% (CAA89996.1), respectively; the molecular structures are different between HDM PDI and human PDI. Furthermore, we treated mice with PDI by nasal instillation, which resulted in improvement of the epithelial barrier function. The fact demonstrates that exposure to PDI does not impair the airway epithelial barrier function; instead, it makes it better. It should be mentioned that there are several subtypes of PDI in mammals; some of them can promote the development of allergic disorders such as asthma (7). The present study shows a PDI from HDM. By analyzing the amino acid sequence of PDI between HDM and human, the similarity is 44%. This may be the basis of functional difference between the HDM-derived PDI and human PDI.
In summary, the present data show that HDM-derived PDI can contribute to immune tolerance by inducing TolDCs and restoring TolDCs’ functions in an allergic environment. Administration of PDI can efficiently alleviate experimental airway allergy. The data suggest that PDI may have translation potential for the treatment of airway allergy.
Experimental procedure
Ethical statement
The use of human tissues and animals in the present study was approved by the Human and Animal Ethical Committee at Shenzhen University. The human studies were abided by the Declaration of Helsinki principles.
Isolation of DC from human blood samples
Peripheral blood samples (20 ml per person) were obtained from human subjects by ulnar vein puncture. Mononuclear cells (PBMC) were isolated from blood samples by the gradient Percoll density centrifugation. PBMCs were stained with fluorochrome-labeled Abs of CD45, CD11c, and CD103. CD11c+ CD103+ DCs were purified by FCS. Purity of isolated DCs was greater than 96% as assessed by FCS. The DCs were used in further experiments.
A murine asthma model development
Following established procedures (22), BALB/c mice were immunized with ovalbumin (OVA; 0.1 mg/mouse in 0.1 ml Alum) through subcutaneous injection on the back skin on day 0 and day 7, respectively. The mice were boosted by nasal instillation with OVA solution (50 μl per nostril; 5 mg/ml) daily from day 14 to day 23. Mice were sacrificed on day 23. The asthma symptoms were assessed with the procedures as we reported previously.
PDI therapy
Asthma mice were treated with PDI solution (5 mg/ml) through nasal instillation (50 μl/nostril) daily from day 24 to day 30. Mice were challenged with OVA (50 mg/ml) via nasal instillation (50 μl/nostril) followed by asthma symptom assessment.
Depletion of DC in mice
Mice were peritoneal injection with diphtheria toxin (DT; 0.5 μg/mouse) in 0.1 ml saline. The injection was repeated once 2 days later.
Statistics
Each experimental group consisted of six mice. Each experiment was repeated at least three times. Each sample was analyzed in triplicate (the average of three readouts was used as one datum). The data are presented as mean ± SEM. The difference between two groups was determined by the Student’s t-test. ANOVA followed by Dunnett’s test or Bonferroni test was performed for multiple comparisons. p < 0.05 was set as a significant criterion.
Data availability
All the data are included in the main text and the supplemental materials.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
This study was supported by grants of the National Nature and Science Foundation of China (32090052, 31570932, U1801286, 82071807, 82004046), Guangdong Provincial Key Laboratory of Regional Immunity and Diseases (2019B030301009), The Funding of Medical and Health Technology Project of Guangzhou (20171A010042), Shenzhen Nanshan District Oversea Research Personnel Initiative Group Fund (LHTD20180007), and Shenzhen science, technology and innovation committee (KQTD20170331145453160, GJHZ20180418190535757, KQJSCX20180328095619081) and Shenzhen Key Medical Discipline Construction Fund (No.SZXK039).
Author contributions
X. L., Y. W., D. C., S. J., L. T. Y., Q. H., L. G., K. C., D. L., R. Y., C. O., T. Y. H., and Z. Q. L. performed the experiments, analyzed the data, and reviewed the article. P. C. Y., Z. G. L., B. S., and G. X. organized the study and supervised the experiments. P. C. Y. and Z. G. L. designed the project. P. C. Y. prepared the article.
Edited by Ursula Jakob
Contributor Information
Baoqing Sun, Email: sunbaoqing@vip.163.com.
Zhi-Gang Liu, Email: lzg@szu.edu.cn.
Ping-Chang Yang, Email: pc2356@163.com.
Supporting information
References
- 1.Alroqi F.J., Chatila T.A. T regulatory cell Biology in health and disease. Curr. Allergy Asthma Rep. 2016;16:27. doi: 10.1007/s11882-016-0606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson J.M., Platts-Mills T.A.E. Home environmental Interventions for House dust mite. J. Allergy Clin. Immunol. Pract. 2018;6:1–7. doi: 10.1016/j.jaip.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H., Mo L., Xiao X., An S., Liu X., Ba J., Wu W., Ran P., Yang P., Liu Z. Pplase of Dermatophagoides farinae promotes ovalbumin-induced airway allergy by modulating the functions of dendritic cells in a mouse model. Sci. Rep. 2017;7:43322. doi: 10.1038/srep43322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilkinson B., Gilbert H.F. Protein disulfide isomerase. Biochim. Biophys. Acta. 2004;1699:35–44. doi: 10.1016/j.bbapap.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Stopa J.D., Zwicker J.I. The intersection of protein disulfide isomerase and cancer associated thrombosis. Thromb. Res. 2018;164(Suppl 1):S130–S135. doi: 10.1016/j.thromres.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho J. Protein disulfide isomerase in thrombosis and vascular inflammation. J. Thromb. Haemost. 2013;11:2084–2091. doi: 10.1111/jth.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman S.M., Chapman D.G., Lahue K.G., Cahoon J.M., Rattu G.K., Daphtary N., Aliyeva M., Fortner K.A., Erzurum S.C., Comhair S.A., Woodruff P.G., Bhakta N., Dixon A.E., Irvin C.G., Janssen-Heininger Y.M. Protein disulfide isomerase-endoplasmic reticulum resident protein 57 regulates allergen-induced airways inflammation, fibrosis, and hyperresponsiveness. J. Allergy Clin. Immunol. 2016;137:822–832.e827. doi: 10.1016/j.jaci.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan T.F., Ji K.M., Yim A.K., Liu X.Y., Zhou J.W., Li R.Q., Yang K.Y., Li J., Li M., Law P.T., Wu Y.L., Cai Z.L., Qin H., Bao Y., Leung R.K. The draft genome, transcriptome, and microbiome of Dermatophagoides farinae reveal a broad spectrum of dust mite allergens. J. Allergy Clin. Immunol. 2015;135:539–548. doi: 10.1016/j.jaci.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H.P., Sun Y.X., Lin Z., Yang G., Liu J.Q., Mo L.H., Geng X.R., Song Y.N., Zeng H.T., Zhao M., Li G.S., Liu Z.G., Yang P.C. CARsomes inhibit airway allergic inflammation in mice by inducing antigen-specific Th2 cell apoptosis. Allergy. 2020;75:1205–1216. doi: 10.1111/all.14157. [DOI] [PubMed] [Google Scholar]
- 10.Volpi C., Fallarino F., Pallotta M.T., Bianchi R., Vacca C., Belladonna M.L., Orabona C., De Luca A., Boon L., Romani L., Grohmann U., Puccetti P. High doses of CpG oligodeoxynucleotides stimulate a tolerogenic TLR9-TRIF pathway. Nat. Commun. 2013;4:1852. doi: 10.1038/ncomms2874. [DOI] [PubMed] [Google Scholar]
- 11.Esebanmen G.E., Langridge W.H.R. The role of TGF-beta signaling in dendritic cell tolerance. Immunol. Res. 2017;65:987–994. doi: 10.1007/s12026-017-8944-9. [DOI] [PubMed] [Google Scholar]
- 12.Peng D.J., Zeng M., Muromoto R., Matsuda T., Shimoda K., Subramaniam M., Spelsberg T.C., Wei W.Z., Venuprasad K. Noncanonical K27-linked polyubiquitination of TIEG1 regulates Foxp3 expression and tumor growth. J. Immunol. 2011;186:5638–5647. doi: 10.4049/jimmunol.1003801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch S., Sopel N., Finotto S. Th9 and other IL-9-producing cells in allergic asthma. Semin. Immunopathol. 2017;39:55–68. doi: 10.1007/s00281-016-0601-1. [DOI] [PubMed] [Google Scholar]
- 14.Cui Y., Wang Q., Jia H. Consideration of methods for identifying mite allergens. Clin. Transl Allergy. 2018;8:14. doi: 10.1186/s13601-018-0200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang W., Swiggard W.J., Heufler C., Peng M., Mirza A., Steinman R.M., Nussenzweig M.C. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 16.Spiering R., Margry B., Keijzer C., Petzold C., Hoek A., Wagenaar-Hilbers J., van der Zee R., van Eden W., Kretschmer K., Broere F. DEC205+ dendritic cell-Targeted tolerogenic Vaccination promotes immune tolerance in experimental Autoimmune arthritis. J. Immunol. 2015;194:4804–4813. doi: 10.4049/jimmunol.1400986. [DOI] [PubMed] [Google Scholar]
- 17.Sanjabi S., Oh S.A., Li M.O. Regulation of the immune response by TGF-β: From Conception to autoimmunity and Infection. Cold Spring Harb Perspect. Biol. 2017;9:a022236. doi: 10.1101/cshperspect.a022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coombes J.L., Siddiqui K.R., Arancibia-Cárcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abediankenari S., Ghasemi M. Generation of immune inhibitory dendritic cells and CD4+T regulatory cells inducing by TGF-beta. Iran J. Allergy Asthma Immunol. 2009;8:25–30. [PubMed] [Google Scholar]
- 20.Kanamori M., Nakatsukasa H., Okada M., Lu Q., Yoshimura A. Induced regulatory T cells: Their development, Stability, and Applications. Trends Immunol. 2016;37:803–811. doi: 10.1016/j.it.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Lee E., Lee D.H. Emerging roles of protein disulfide isomerase in cancer. BMB Rep. 2017;50:401–410. doi: 10.5483/BMBRep.2017.50.8.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin J., Huang N., Li J., Liu X., Xiong Q., Hu C., Chen D., Guan L., Chang K., Li D., Tsui S.K., Zhong N., Liu Z., Yang P.C. Cross-reactive antibodies against dust mite-derived enolase induce neutrophilic airway inflammation. Eur. Respir. J. 2021;57:1902375. doi: 10.1183/13993003.02375-2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data are included in the main text and the supplemental materials.