Abstract
Background
Obstructive sleep apnoea‐hypopnoea (OSAH) is a syndrome characterised by recurrent episodes of partial or complete upper airway obstruction during sleep that are usually terminated by an arousal. Nasal continuous positive airway pressure (CPAP) is the primary treatment for OSAH , but many patients are unable or unwilling to comply with this treatment. Oral appliances (OA) are an alternative treatment for OSAH.
Objectives
The objective was to review the effects of OA in the treatment of OSAH in adults.
Search methods
We searched the Cochrane Airways Group Specialised Register. Searches were current as of June 2008. Reference lists of articles were also searched.
Selection criteria
Randomised trials comparing OA with control or other treatments in adults with OSAH .
Data collection and analysis
Two authors independently extracted data and assessed trial quality. Study authors were contacted for missing information.
Main results
Seventeen studies (831 participants) met the inclusion criteria. All the studies had some shortcomings, such as small sample size, under‐reporting of methods and data, and lack of blinding. OA versus control appliances (six studies): OA reduced daytime sleepiness in two crossover trials (ESS score ‐1.81; 95%CI ‐2.72 to ‐0.90), and improved apnoea‐hypopnoea index (AHI) (‐10.78 events/hr; 95% CI‐15.53 to ‐6.03 parallel group data ‐ five studies). OA versus CPAP (ten studies): There was no statistically significant difference in symptoms for either parallel or crossover studies, although OAs were less effective than CPAP in reducing apnoea‐hypopnoea index in parallel and crossover studies. CPAP was more effective at improving minimum arterial oxygen saturation during sleep compared with OA. In two small crossover studies, participants preferred OA therapy to CPAP. OA versus corrective upper airway surgery (one study): Symptoms of daytime sleepiness were initially lower with surgery, but this difference disappeared at 12 months. AHI did not differ significantly initially, but did so after 12 months in favour of OA.
Authors' conclusions
There is increasing evidence suggesting that OA improves subjective sleepiness and sleep disordered breathing compared with a control. CPAP appears to be more effective in improving sleep disordered breathing than OA. The difference in symptomatic response between these two treatments is not significant, although it is not possible to exclude an effect in favour of either therapy. Until there is more definitive evidence on the effectiveness of OA in relation to CPAP, with regard to symptoms and long‐term complications, it would appear to be appropriate to recommend OA therapy to patients with mild symptomatic OSAH, and those patients who are unwilling or unable to tolerate CPAP therapy. Future research should recruit patients with more severe symptoms of sleepiness, to establish whether the response to therapy differs between subgroups in terms of quality of life, symptoms and persistence with usage. Long‐term data on cardiovascular health are required.
Plain language summary
Oral appliances for treating sleepiness, quality of life and markers of sleep disruption in people with obstructive sleep apnoea/hypopnoea (OSAH)
OSAH is characterized by recurrent episodes of partial or complete upper airway obstruction during sleep, leading to a variety of symptoms including excessive daytime sleepiness. The current first choice therapy is CPAP that keeps the upper airway patent during sleep. However, this treatment can be difficult for some patients to tolerate and comply with on a long‐term basis. OA are now widely used as an alternative to CPAP therapy. They are designed to keep the upper airway open by either advancing the lower jaw forward or by keeping the mouth open during sleep. This review found that OA should not be considered as first choice therapy for OSAH, where symptoms and sleep disruption are severe. There has not been a sufficient amount of research that examines the effects of OA compared with CPAP in terms of symptoms and quality of life. Although CPAP was clearly more effective at reducing the disruption to sleep, some people with OSAH may prefer using them if they are found to be tolerable and more convenient than CPAP. When an active OA was compared with an inactive OA , there were improvements in daytime sleepiness and apnoea/hypopnoea severity. OA may be more effective than corrective upper airway surgery. Further research should consider whether people with more distinctly severe symptoms respond in a similar way to those patients represented in the studies we have included in the review.
Background
Obstruction of the upper airway during sleep may result in snoring, and reduction (hypopnoea) or cessation (apnoea) of airflow. In adults apnoea is defined as cessation of airflow for greater than 10 seconds. Hypopnoea is defined as a 50% or greater decrease in airflow, often accompanied by hypoxaemia or arousal. The obstructive sleep apnoea‐hypopnoea (OSAH) syndrome is defined as a patient suffering five or more apnoeas/hypopnoeas per hour of sleep with daytime symptoms, and is a relatively common condition occurring in 2 to 4% of males and 1 to 2 % of females in middle age (Young 1993).
The pathophysiology of OSAH involves factors that relate to the anatomical dimensions of the upper airway, upper airway resistance and upper airway muscle activity during sleep (Hudgel 1992).
The patient with OSAH often presents because of symptoms noticed by their bed partner, who will often report that the patient snores loudly followed by an apnoea associated with respiratory effort and terminated by an awakening and resumption of loud snoring. The patient then resumes sleep and the cycle may repeat itself many times during the night. Excessive daytime sleepiness and an impairment of cognitive function are often present due to sleep fragmentation and patients may experience other symptoms including mood disturbance, decreased libido and social withdrawal (ASDA 1995). Several epidemiological studies have reported associations between OSAH and health related outcomes such as cardiac arrhythmias, systemic and pulmonary hypertension, ischaemic heart disease and cerebrovascular disease (Shahar 2001). There is some evidence that OSAH may be linked to sleepiness and road traffic accidents which has medico legal implications, with some countries requiring drivers suffering with OSAH to report this to the appropriate licensing authority (RCP 1993; Wright 1997).
The diagnosis of OSAH is usually made by polysomnography, which also provides an indication of severity (ASDA 1995). Polysomnography involves recording during sleep of chest and abdominal movements, oxyhaemoglobin saturation, airflow, ECG tracing, sleep state (EEG, EOG and EMG), activity whilst asleep, and arousals. The number of episodes of apnoea and hypopnoea per hour of sleep is calculated from the polysomnogram and is referred to as the apnoea‐hypopnoea index (AHI). Severity of OSAH has two components: severity of daytime sleepiness and AHI (AASM 1999). Epworth Sleepiness Score is currently the most widely used assessment of subjective sleepiness and apnoea hypopnoea index is the most widely used assessment of sleep disordered breathing from overnight monitoring. Both measurements have their limitations but should be considered independent outcomes in evaluating the effectiveness of OSAH treatment.
Treatment options for OSAH include behavioural modification such as weight loss, alcohol avoidance and alteration of sleeping position (Shneerson 2001; Smith 2006); CPAP (Giles 2006); and a range of upper airway surgical procedures (Sundaram 2005). OA that modify the upper airway size are increasingly prescribed to patients with OSAH. Upper airway muscle activity decreases during sleep, leading to increased collapsibility of the pharyngeal tissues, mandibular opening and posterior displacement of the tongue. These changes result in narrowing of the oropharyngeal and hypopharyngeal airway (Hudgel 1992). A variety of OA are available whose primary actions are to advance the mandible or tongue and thus increase the upper airway size. Another, less accepted theory of their mode of action, is that OA cause stretch‐induced activation of the pharyngeal motor system, reducing soft tissue laxity and airway collapse (Ono 1996).
Side‐effects have been reported with the use of OA including discomfort in the temporo‐mandibular joint, teeth or facial musculature, bite change, excessive salivation or dryness of the mouth (Clark 2000). The effectiveness of OA in the treatment of patients with OSAH, and their relative efficacy in comparison to the other available modalities of treatment is unclear. Previous reviews of OAs in the treatment of OSAH have only reported evidence from case series reports (Schmidt‐Nowara 1995), or in comparison to CPAP (Giles 2006), and their place in guidelines suggests that they are not considered first‐line therapy (SIGN 2003).
Objectives
To determine the clinical effectiveness of OA in the treatment of OSAH in adults.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials.
Types of participants
Participants with a diagnosis of OSAH, as defined as five or more apnoeas or hypopnoeas per hour of sleep are eligible for inclusion. There are no gender restrictions. Participants will be restricted to those over the age of 16 years.
Types of interventions
Treatment group: any intraoral prostheses for OSAH Control group: other surgical or non‐surgical intervention, or no intervention.
Types of outcome measures
Primary outcomes
Primary outcome measures were daytime sleepiness as measured by a validated sleep apnoea symptom score and the number of apnoeas and hypopnoeas per hour of sleep (AASM 1999).
Secondary outcomes
Quality of life (using a validated scale);
Cognitive function (using a validated scale);
Side effects associated with use of OA;
Oxygen desaturation indices;
One year mortality;
Patient preference.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Airways Group Specialised Register, together with an additional search on MEDLINE for citations containing "sleep" and ("apnoea" or "apnea" or hypopnoea" or "hypopnea") or "sleep disordered breathing" or " sleep related respiratory disorder(s)" in any of the fields. We then identified any possible randomised controlled trials using the strategy developed by the Cochrane Collaboration.
The text words used in the initial searches were: oral OR intraoral OR dental OR tongue OR mandib* OR mandib* advancement AND splint OR appliance OR prosth* OR device OR continuous positive airway* pressure OR CPAP OR (adenotonsil*) OR (tonsil*) OR (adenoid*) OR (surg* and palate) OR (surg* and uvula) OR (surg* and pharynx) OR (uvulopharyngoplasty) OR (UPPP) OR (UVPP) OR (UPP) OR (palatoplasty) OR (pharyngoplasty) OR (palatopharyngoplasty) OR (PPP) OR (uvulopalatoplasty) OR (LAUP) OR (tracheostomy) OR (mini‐tracheostomy) OR (surg* and maxillo‐facial) OR (surg* and maxillofacial) OR (genioglossal advancement) OR (maxillo‐mandibular advancement) OR (maxillo‐mandibular osteotomy) OR (maxillary advancement) OR (maxillary osteotomy) OR (mandibular osteotomy) OR (intrapalatine resection) OR (tongue volume reduction) OR (inferior sagittal osteotomy) OR (hyoid bone suspension) OR (hyoid suspension) OR (hyoid myotomy) OR (surg* and upper‐airways) OR (surg* and nasal) OR (septoplasty) OR (polypectomy).
To update the review, we carried out further searches of the Specialised Register in June 2008 and the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 2, 2008). The search was amended and the text words used were:
(oral* OR intraoral* OR intra‐oral* OR dental* OR tongue* or mouth* or jaw* OR mandib* OR "mandib* advancement*" or splint* or prosth* or appliance or device)
Searching other resources
To locate additional RCTs we:
checked the reference lists of relevant review articles, and references of all identified RCTs;
contacted the NHS Centre for Reviews and Dissemination, the National Health Technology Assessment Programme the NHS National Research register and the Aggressive Research Intelligence Facility;.
contacted local and national sleep laboratories and experts in the fields of sleep and respiratory medicine, and ENT surgery;.
contacted other Cochrane Review Groups to identify citations found by handsearching of journals (such as surgical journals).
Data collection and analysis
Selection of studies
The authors (JL, TL) independently reviewed the titles, abstracts, and citations to assess potential relevance for full review. From the full text, the reviewers independently assessed studies for inclusion based on the criteria for population, intervention, study design and outcomes. There was no disagreement between the reviewers on the inclusion and exclusion of studies. Any disagreement over study inclusion would have been resolved by a third reviewer (JF).
Data extraction and management
The reviewers independently extracted data from included trials and entered results into the Cochrane Collaboration software program (Review Manager). No information regarding outcomes needed to be estimated from graphs. Should future studies be published with such presentation of data, two reviewers will estimate it independently. Data extraction included the following items:
Population: age, gender, number of patients studied, patient demographics, withdrawals.
Intervention: type (type of OA).
Control: medical (type, dose, route of delivery, duration), mechanical (type, delivery), other surgical (type).
Outcomes: as above
Design: method of randomisation and allocation concealment
Assessment of risk of bias in included studies
We assessed the bias protection of each study by assessing randomisation procedures and blinding. Given the nature of the questions we have addressed in this review, blinding is not likely to be possible for oral appliance and CPAP or surgical comparisons. This assessment is in line with guidance described in chapter 8 of the Cochrane Handbook (Handbook 2008).
Each study was assessed for validity on a 0‐5 scale, method of Jadad 1996: Was the study described as randomised? (1 = yes, 0 = no) Was the study described as double‐blind? (1 = yes, 0 = no) Was there a description of withdrawals and drop outs? (1 = yes, 0 = no) Was the method of randomisation well described and appropriate? (1 = yes, 0 = no) Was the method of double‐blinding well described and appropriate? (1 = yes, 0 = no) Deduct 1 point if methods of randomisation or blinding were inappropriate.
Data synthesis
We combined all included trials using Review Manager. For continuous variables, we calculated the results of individual studies as a fixed‐effect weighted mean difference (WMD) or standardised mean difference (SMD), with 95% confidence intervals (95% CI). For dichotomous variables, a fixed‐effect odds ratio (OR) with 95% CI, were calculated for individual studies. For pooled effects, heterogeneity was tested using the Breslow‐Day test; P < 0.1 was considered to be statistically significant. If heterogeneity was observed, we used a random‐effects model to calculate the results.
Subgroup analysis and investigation of heterogeneity
In the presence of heterogeneity, we performed the following sensitivity analysis using:
Females versus males;
Random‐effect versus fixed‐effect modelling.
No data stratified by severity were presented.
Results
Description of studies
Results of the search
For details of search history, see Table 1. An annual search update in June 2008 identified 26 new references. Four references were linked with previously included studies (Gotsopoulos 2002; Hoekema 2006). Three references were considered as new studies for this updated review but were excluded (Friedman 2008; Saletu 2007; van der Sweep 2006: see Characteristics of excluded studies).
1. Search history.
| Date | Detail |
| All years to June 2005 | References identified: 705 Unique studies retrieved: 55 (three unpublished) Studies failing to meet the entry criteria of the review: 37 Studies included: 16 Awaiting translation: 2 |
| June 2005 to June 2007 | References identified: 38 Unique studies retrieved: 4 Studies not complete: 1 Studies failing to meet the entry criteria of the review: 2 Studies meeting review entry criteria: 1 |
Included studies
Seventeen individual trials (32 references) met the review inclusion criteria of the review. There was no disagreement about study inclusion between reviewers. One study is ongoing (Fairbairn 2004), and one study is available as an interim analysis and does not contribute data to this review (Cibele 2006). For a full description of each eligible trial see Characteristics of included studies.
Design
All included studies were randomised and controlled. Eleven trials were of crossover design and seven were parallel group trials (Blanco 2005; Fleetham 1998; Hans 1997; Hoekema 2006; Hoekema 2007a; Lam 2007; Tegelberg 1999). Observer blinding was reported in Engleman 2002.
Participants
The participants in the studies suffered from mixed severity of OSAH, from mild to severe, as defined by AHI. The majority of participants were men of middle‐age. In all studies there was polysomnographic confirmation of sleep apnoea, except for Johnston 2002 where home oximetry was used as the basis of determining oxygen desaturation indices.
Interventions
Ten trials compared an OA with CPAP (Barnes 2004; Engleman 2002; Ferguson 1997; Fleetham 1998; Hoekema 2006; Lam 2007; Olson 2002; Randerath 2002; Tan 2002), six trials compared an OA with a control OA (Blanco 2005; Durán 2002; Gotsopoulos 2002; Hans 1997; Johnston 2002; Mehta 2001) and one trial compared an OA with surgery (Tegelberg 1999). Active OA therapy consisted of mandibular advancement devices. Control OA therapy consisted of devices which were placed in the mouth that did not protrude the mandible. Only data from the OA versus CPAP comparison from (Barnes 2004) were entered in the review, as we felt that the data from the OA versus placebo pill comparison was not an adequate mean of establishing efficacy. Lam 2007 allocated participants to receive conservative management on top of either CPAP or OA, and also as a separate treatment arm.
Duration
The studies lasted for between two weeks to one year. One study conducted a four year follow‐up (Tegelberg 1999).
Risk of bias in included studies
Availability of information regarding the allocation of participants to treatment groups was generally poor. A summary of judgements for allocation generation, concealment and blinding is given in Figure 1. The information we have used as the basis for these judgements is provided in Characteristics of included studies. Overall there was a limited amount of information available which meant that many of our judgements were left as 'unclear'. Where information was available regarding randomisation procedures, they were adequate in protecting studies from selection bias.
1.
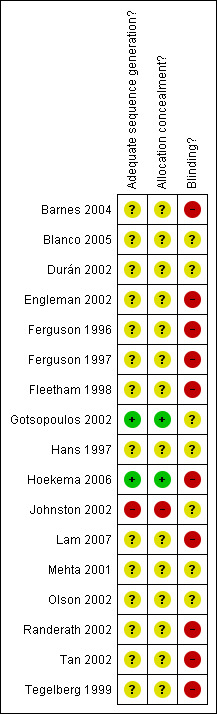
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Blinding procedures were not possible for CPAP or surgical comparisons. Barnes 2004 was a three arm crossover trial reporting the effects of OA, CPAP and a dummy pill. We have retained data only for the OA and CPAP comparisons since data from the OA and dummy pill comparison may be particularly prone to detection bias. Other studies describe a control appliance (Blanco 2005; Durán 2002; Hans 1997 ), or an attempt by study personnel to present an inactive appliance as an alternative therapy in a single‐blind manner (Gotsopoulos 2002; Johnston 2002; Mehta 2001).
Jadad scores are provided for each study in Characteristics of included studies.
Effects of interventions
We have used the different comparisons to display the results. Some unpublished data have been made available to the reviewers. Subgroup analysis by severity of OSAH was not possible due to the heterogeneous populations recruited in the studies. Random‐effects modelling has been compared with fixed‐effect modelling if heterogeneity was observed, in order to determine the impact of variability between studies on the pooled results. Data from four crossover studies (Gotsopoulos 2002; Johnston 2002; Mehta 2001; Randerath 2002) have been analysed as parallel and crossover group data as the trialists made first arm data available upon request. Crossover scores were extracted from the published article.
Active oral appliance versus control oral appliance
Six trials reported data for this comparison (Blanco 2005; Durán 2002; Hans 1997; Gotsopoulos 2002; Johnston 2002; Mehta 2001). Mandibular advancement devices were compared with devices that did not protrude the mandible.
Epworth Sleepiness Score
Parallel/First arm crossover studies (Blanco 2005; Gotsopoulos 2002; Hans 1997; Johnston 2002): Pooled analysis generated a heterogeneous but significant result (‐2.09; 95% CI ‐3.8 to ‐0.37, I2 = 70.2%, Figure 2). Random‐effects modelling gave a non‐significant result (‐2.95; 95% CI ‐6.69 to 0.79). The addition of data from Blanco 2005 may have introduced a degree of variation. This may be explained by the somewhat more censored nature of the data from this study. No intention‐to‐treat analysis was undertaken in this trial, and there was an attrition rate of 20%. The overall attrition rate could explain the more significant difference on symptoms in the active treatment group, whereby those who remained in the study perceived the most benefit.
2.
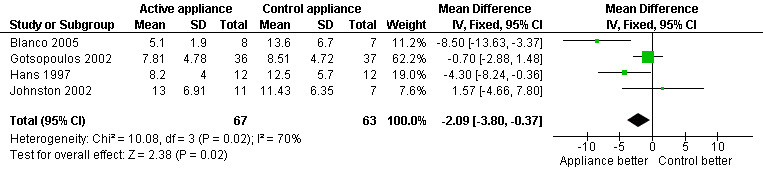
Forest plot of comparison: 1 Active oral appliance versus control appliance, outcome: 1.1 Epworth sleepiness score ‐ first arm data/parallel studies.
Crossover studies (Gotsopoulos 2002; Johnston 2002): Pooled analysis gave a significant difference in favour of active OAs of ‐1.81; 95%CI ‐2.72 to ‐0.90, Figure 3.
3.
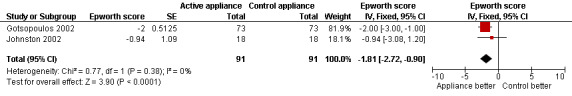
Forest plot of comparison: 1 Active oral appliance versus control appliance, outcome: 1.2 Epworth sleepiness score ‐ crossover studies.
Apnoea Hypopnea Index
Parallel/First arm crossover studies (Blanco 2005; Gotsopoulos 2002; Hans 1997; Johnston 2002; Mehta 2001): There was a significant effect in favour of active treatment (‐10.78 events/hrI; 95%CI ‐15.53 to ‐6.03, Analysis 1.3).
1.3. Analysis.
Comparison 1 Active oral appliance versus control appliance, Outcome 3 Apnoea Hypopnea Index ‐ first arm/parallel studies.
Crossover studies (combined scores) (Durán 2002; Gotsopoulos 2002; Johnston 2002;Mehta 2001): Data from Durán 2002; Gotsopoulos 2002; Johnston 2002 and Mehta 2001 and gave a significant treatment effect in favour of active OA (‐15.15 events/hr; 95% CI ‐19.40 to ‐10.89, Analysis 1.4).
1.4. Analysis.
Comparison 1 Active oral appliance versus control appliance, Outcome 4 Apnoea Hypopnea Index ‐ crossover studies.
Minimum arterial oxygen saturation
Parallel/First arm crossover studies (Gotsopoulos 2002; Johnston 2002; Mehta 2001): There was no significant effect in favour of active appliances (1.79%; 95% CI ‐0.29 to 3.87, Analysis 1.7).
1.7. Analysis.
Comparison 1 Active oral appliance versus control appliance, Outcome 7 Minimum saturation ‐ first arm data/parallel studies.
Crossover studies (combined scores) (Gotsopoulos 2002; Mehta 2001): There was a significant difference of 3.39%; 95% CI 2.25 to 4.54, Analysis 1.8).
1.8. Analysis.
Comparison 1 Active oral appliance versus control appliance, Outcome 8 Minimum saturation ‐ crossover studies.
Arousal Index
Parallel/First arm crossover studies (Blanco 2005; Gotsopoulos 2002; Mehta 2001): Overall there was a statistically significant effect in favour of active OA compared with control: WMD‐10.66 arousals/hr; 95% CI ‐16.03 to ‐5.29, Analysis 1.5.
1.5. Analysis.
Comparison 1 Active oral appliance versus control appliance, Outcome 5 Arousals ‐ first arm data/parallel studies.
Crossover studies (combined scores) (Gotsopoulos 2002; Mehta 2001): There was a significant difference of ‐10.72 arousals/hr; 95% CI ‐15.05 to ‐6.39, Analysis 1.6.
1.6. Analysis.
Comparison 1 Active oral appliance versus control appliance, Outcome 6 Arousals ‐ crossover studies.
Blood pressure outcomes
Crossover studies (Gotsopoulos 2002) Active oral appliance therapy led to lower blood pressure compared with control for certain measurements of diurnal/nocturnal blood pressure. Confirmatory work is required.
Withdrawals
Parallel/First arm crossover studies (Blanco 2005; Hans 1997; Johnston 2002) There was no significant difference between active and control OA (OR 0.83; 95% CI 0.24 to 2.86, Analysis 1.9).
1.9. Analysis.
Comparison 1 Active oral appliance versus control appliance, Outcome 9 Withdrawals ‐ first arm/parallel studies.
Crossover studies (combined data) (Gotsopoulos 2002) Gotsopoulos 2002 reported 12 withdrawals over the course of the study. Reasons cited were refusal to participate after receiving CPAP (one), work interference (three), permanent relocation interstate (two), extended overseas trip (one), self‐perceived improvement in OSAH symptoms with OA during phase 1 (one), self‐perceived improvement during acclimatisation phase (one).
Side effects/tolerability
Parallel/First arm crossover studies No data available.
Crossover studies (combined data) (Gotsopoulos 2002; Johnston 2002; Mehta 2001) Gotsopoulos 2002 reported that participants given the active OA suffered side effects more frequently than those given the control device. Side effects reported were jaw discomfort (p < 0.0001; control versus active OA); tooth tenderness (P < 0.0001; control versus active OA) and excessive salivation (P < 0.05; control versus active OA). Mehta 2001 reported that side effects were mild‐moderate, and included: excessive salivation (50%), gum irritation (20%), mouth dryness (46%), jaw discomfort (12.5%) and bruxism (12.5%). Control and active OA data were not separated. The active OA was well tolerated by 21/24 participants who completed the protocol. Control OA data were not reported. Johnston 2002 reported that 68% participants wore the OA every, or almost every, night. Eighty‐four per cent of participants complained that the device fell out of their mouth on more than two nights per week. Forty‐two per cent of participants complained of jaw discomfort on waking. Johnston 2002 did not report control OA data on tolerability.
Oral appliance versus CPAP
Ten trials were identified which compared an OA to CPAP (Barnes 2004; Engleman 2002; Ferguson 1996; Ferguson 1997; Fleetham 1998; Hoekema 2006; Lam 2007; Olson 2002; Randerath 2002; Tan 2002). Three had a parallel group design (Fleetham 1998; Hoekema 2006; Lam 2007) and the remaining studies were crossover trials.
Epworth Sleepiness Score
Parallel/First arm crossover studies (Fleetham 1998; Hoekema 2006; Lam 2007): No statistically significant difference: 0.64; 95% CI ‐0.57 to 1.86, Figure 4).
4.
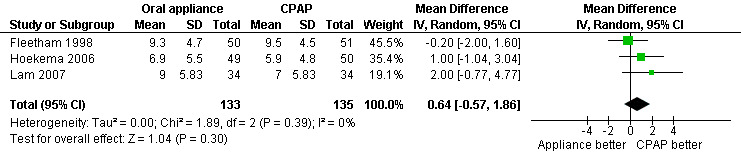
Forest plot of comparison: 2 Oral appliance versus continuous positive airways pressure, outcome: 2.1 Epworth sleepiness scale ‐ first arm data/parallel studies.
Crossover studies (combined scores) (Barnes 2004; Engleman 2002; Ferguson 1997; Tan 2002): There were conflicting scores for Epworth scores between the studies. There was a statistically significant effect in favour of CPAP over OAs in Engleman 2002 (of four units, P < 0.001). None of the three other trials reported a significant difference between the two groups on Epworth scores (Barnes 2004; Ferguson 1997;Tan 2002). There was a high degree of heterogeneity when pooled (I2 72.4%). With fixed‐effect modelling, the pooled estimate was 0.54; 95% CI ‐0.29 to 1.38, Figure 5. With random‐effects modelling the result was 0.98 (95% CI ‐0.8 to 2.76). Although neither result was significant, the variation between the studies may be attributed to different OA design or treatment adherence.
5.
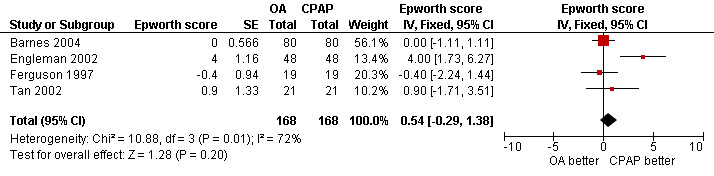
Forest plot of comparison: 2 Oral appliance versus continuous positive airways pressure, outcome: 2.2 Epworth sleepiness score ‐ crossover studies.
Randerath 2002 reported no difference on an in‐house symptom score.
Apnoea Hypopnea Index
Parallel/First arm crossover studies (Fleetham 1998; Hoekema 2006; Lam 2007; Randerath 2002): CPAP was more effective in suppressing apnoea and hypopnea than OA (8.13 events/hr; 95% CI 5.57 to 10.69, Analysis 2.3). Although the level of statistical heterogeneity for this outcome was high (I2 60%), random effects modelling did not alter the statistical significance or the direction of the effect.
2.3. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 3 Apnoea Hypopnea Index ‐ first arm data/parallel studies.
Crossover studies (combined scores) (Barnes 2004; Engleman 2002; Ferguson 1996; Ferguson 1997; Olson 2002; Randerath 2002; Tan 2002). There were significant differences in AHI between OA and CPAP treated participants in favour of CPAP. When pooled these data generated a difference of 7.97 events/hr; 95% CI 6.38 to 9.56, Analysis 2.4.
2.4. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 4 Apnoea Hypopnea Index ‐ crossover studies.
Quality of life scores
Parallel/First arm crossover studies (Fleetham 1998; Lam 2007; Olson 2002): Overall, there was no statistically significant difference between treatment groups on the SAQLI score (‐0.03; 95% CI ‐0.35 to 0.28, Analysis 2.5).
2.5. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 5 Quality of life score (SAQLI) ‐ first arm data/parallel studies.
Crossover studies (combined scores) (Barnes 2004; Engleman 2002; Tan 2002): Tan 2002 used a scale where low scores were indicative of good health. Scores in the OA group were 10.20 +/‐9.90 in the OA group and 6.50 +/‐5.90 in the CPAP group (P < 0.001 after both treatments). Engleman 2002 reported component scores from the SF‐36, which showed a significant effect in favour of CPAP versus OA on health transition and mental component scores (health transition: OA: 2.9 +/‐ 0.8; CPAP: 2.4 +/‐0.8, P = 0.001; mental: OA: 48 +/‐11; CPAP: 52 +/‐10, P = 0.008). No significant difference was detected on the physical component score. The HADS anxiety and depression scores did not differ significantly. Barnes 2004 and Engleman 2002 also reported data on the Functional Outcomes of Sleep Questionnaire. There was no statistically significant difference between CPAP and OAs (‐0.18; 95% CI ‐0.42 to 0.07), but there was a high degree of heterogeneity (I2 88.8%). Random‐effects modelling gave a non‐significant result with wider confidence intervals (‐1.44 to 0.51). In the absence of additional data sets for this measurement, the numerous possible explanations of the conflicting results such as subtle differences in the design of the OA, study design and severity could influence the response to therapy. It is noteworthy that Engleman 2002 which reported superiority of CPAP over OA on other outcomes such as AHI, ESS also reported superiority over OA on FOSQ outcome data.
Tan 2002 also measured bed‐partner health and daytime sleepiness, but did not detect a significant difference between treatment with CPAP and OA. Both treatments improved these scores compared with baseline. Data were not entered as it was not clear whether all participants in the study had bed partners or not.
Cognitive performance
Crossover studies (combined scores) (Barnes 2004; Engleman 2002) Engleman 2002 did not detect a significant difference on cognitive performance scores. No other trial reported data on cognitive performance. Barnes 2004 reported no significant difference between CPAP and OA on word association, psychomotor vigilance, or maintenance of wakefulness tasks.
Minimum arterial oxygen saturation
Parallel/First arm crossover studies (Fleetham 1998; Lam 2007; Randerath 2002): There was a significant effect in favour of CPAP compared with OA (4.59%; 95% CI 2.55 to 6.64, Analysis 2.13).
2.13. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 13 Minimum saturation (%) ‐ first arm data/parallel studies.
Crossover studies (combined scores) (Barnes 2004; Ferguson 1996; Ferguson 1997; Randerath 2002): Although there was significant heterogeneity observed (I2 61.9%), both fixed and random‐effects modelling detected significant differences between the two treatment groups in favour of CPAP (Fixed: 5.16%; 95% CI 3.25 to 7.06); Random: 5.64%; 95% CI 2.48 to 8.8).
Arousal Index
Parallel/First arm crossover studies (Fleetham 1998; Lam 2007; Randerath 2002): When pooled these data were statistically significant in favour of CPAP with both fixed‐effects modelling (WMD 5.21 arousals/hr; 95% CI 2.48 to 7.94, Analysis 2.15). Crossover studies (combined scores) (Barnes 2004; Ferguson 1996; Ferguson 1997; Olson 2002; Randerath 2002; Tan 2002): There was a significant difference in favour of CPAP (2.24 arousals/hr; 95% CI 0.04 to 4.05, Analysis 2.16).
2.15. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 15 Arousals ‐ first arm data/parallel studies.
2.16. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 16 Arousals ‐ crossover studies.
Blood pressure outcomes
Parallel/First arm crossover studies (Lam 2007) No statistically significant differences were observed on systolic and diastolic blood pressure in Lam 2007.
Crossover studies (Barnes 2004) One study reported data for this outcome. There were no significant differences between CPAP and OA in mean 24 hour systolic and diastolic pressure. OA treatment led to lower mean nocturnal diastolic pressure.
Withdrawals
Parallel/First arm crossover studies (Barnes 2004; Engleman 2002; Hoekema 2006; Lam 2007; Fleetham 1998; Randerath 2002): There was a greater likelihood of withdrawal in those treated with OA compared with CPAP (OR 2.05; 95% CI 1.15 to 3.67, Analysis 2.21).
2.21. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 21 Withdrawals ‐ first arm data/parallel studies.
Crossover studies (Ferguson 1996; Ferguson 1997; Tan 2002): Ferguson 1996 reported one drop out occurred during wash‐in period and one dropped out in the first treatment period due to relocation (OA) and in Ferguson 1997 one drop‐out occurred in the OA period declining follow‐up. Three refused to crossover from appliance to CPAP, two of whom were treatment successes. Tan 2002 reported three dropouts from the study (two from the CPAP group and one from the OA group).
Side effects/tolerability
Parallel/First arm crossover studies (Fleetham 1998): Fleetham 1998 did not report data on tolerability of either treatment.
Crossover studies (combined scores) (Engleman 2002; Ferguson 1996; Ferguson 1997; Randerath 2002): Both treatment options lead to different, but noticeable and potentially important adverse effects. Jaw and oral pain occurred more frequently with OA than with CPAP (OR 18; 95% CI 8.62 to 37.57, two studies, N = 67). There were no differences in participants who were free from side effects (OR 0.57; 95% CI 0.24 to 1.36, two studies, N = 45). Individual studies reported higher rates of excessive salivation and appliance removal during sleep with OA, but higher rates of leak, dry upper airway, stuffy nose, and inconvenience with CPAP.
Patient preference
Barnes 2004; Engleman 2002; Ferguson 1996; Ferguson 1997; Olson 2002; Tan 2002): Due to the fact that preference outcomes from crossover studies are not paired, we have not pooled preference data. Out of 15 participants who were deemed treatment successes (reduction in AHI to < 10 and relief of symptoms) on both an OA and CPAP, 13 preferred treatment with an OA and two preferred treatment with CPAP (Olson 2002). The trial by Olson 2002 only considered the preferences from participants whose quality of life (SAQLI) score improved by one or more during either appliance or CPAP treatment. Engleman 2002 reported preference from all participants regardless of treatment success or failure. There was no difference between OA and CPAP preference (19 participants preferred OA and 25 preferred CPAP (four were unaccounted for), P = 0.194). Barnes 2004 reported that more participants preferred CPAP to OA (44% versus 30%), and that their bed partners also preferred this treatment option to OA (40% versus 36%). This was a large study and the expression of preference was made irrespective of treatment success of either option. A placebo pill was also introduced in this study, and such an option may have appealed to those who liked the convenience of this option, especially in milder patients who may not perceive as great a benefit as those with more severe OSAH.
Oral appliance versus surgery
One study (Tegelberg 1999) reported data for this comparison.
Subjective sleepiness score
An unvalidated questionnaire was developed in order to determine daytime sleepiness. No numerical values were reported, but a statistically significant difference was detected at six months, the group treated with surgery experienced less daytime sleepiness compared with the dental appliance group (P < 0.05). At twelve months this difference was not apparent.
Apnoea Hypopnea Index
Mean AHI was not statistically different between OA and surgery at six months (6.6 versus 8.6 respectively). At 12 months, there was a statistically significant difference between the groups, with a mean AHI in the OA group of 6.0 (95% CI 3.0 to 8.9) and an increase in the surgery group to 10.4 (7.6 to 13.2), P < 0.05. At four year follow‐up there was a significant difference reported in the success rate in AHI for the OA (72% versus 35% ‐ success defined as greater than 50% reduction in AHI). The difference between the two groups on mean scores was significant (7.2 versus 14.2 in the OA and uvulopalatopharyngoplasty groups respectively, P < 0.001).
Oxygen desaturation
Oxygen desaturation indices were reported. At six months, there was no significant difference (6.4 versus 8.0 in the appliance and surgery groups respectively). At 12 months the difference was non‐significant (6.1 versus 9.3 in the appliance and surgery groups). At four year follow‐up, oxygen desaturation index (ODI) was 6.7 and 13.1 in the OA and uvulopalatopharyngoplasty groups respectively, P < 0.01.
Quality of life
Quality of life was reported via three component sections of an overall questionnaire ‐ the Minor Symptoms Evaluation‐Profile (MSE‐P), which has been validated in the assessment of central nervous system related symptoms during cardiovascular pharmacotherapy among hypertensive patients, but not in the assessment of patients with OSA. The three sections reported were: vitality, contentment and sleep. There were improvements in both groups compared with baseline. Vitality improved by 5.4 (95% CI 0.1 to 10.7), P < 0.05 in the OA group compared with 9.7 (95% CI 5.0 to 14.4), P < 0.001 in the surgery group. Sleep improved by 19.5 (95% CI 13.5 to 25.5), P<0.001 in the OA group compared with 22.6 (95% CI 16.9 to 28.3), P < 0.001 in the surgery group. At 12 months, there was a significant difference detected in favour of surgery on the contentment component (33.7 versus 27.4 in appliance and surgery groups respectively, P < 0.05). No differences were detected between the groups on the other two components.
Withdrawals
Withdrawals were reported for the OA group as follows: 12 participants withdrew from the OA group ‐ four prior to treatment, two due to discomfort, one due to an allergic reaction with the OA , three due to perceived lack of efficacy one due to epilepsy and one due to maxillary cancer. Three participants withdrew from the group treated with surgery, two reversed their decision about participation and one was diagnosed with gastric cancer.
Discussion
OAs are widely prescribed for the treatment of OSAH both as primary therapy and as an alternative to patients who are unable to tolerate CPAP. There are currently a large number of different OAs available for the treatment of OSAH. Until the mid to late 1990s the majority of studies of the effectiveness of OAs in OSAH were short term, uncontrolled, small and retrospective. More recently the quality of OA clinical research has become more rigorous and this review has identified 17 randomised controlled trials involving 831 participants with varied severity of OSAH. Review of these trials suggests that OAs are effective in improving subjective sleepiness and indices of sleep disordered breathing in selected patients with OSAH, are less effective than CPAP in improving indices of sleep disordered breathing but that certain patients prefer them. We remain uncertain as to the equivalence of OA and CPAP in terms of symptoms; the confidence intervals include a potentially meaningful difference in symptoms, but they also include unity. One study measured bed‐partner health and sleepiness, another measured loudness of snoring rated by bed partners, and a third study incorporated preference data from bed partners. However, the data are not definitive because of the small patient numbers and inadequate assessment of efficacy with patient‐oriented outcomes in the assembled studies.
The issues concerning the effectiveness of OA treatment in OSAH are very similar to those outlined for CPAP therapy (Giles 2006). Ethical concerns have been expressed about the use of placebo controls in OSAH randomised controlled trials (Hans 1997). However, sham nasal CPAP has been successfully used in OSAH randomised controlled trials and has shown a measurable placebo effect (Jenkinson 1999). The best achievable placebo in an OA treatment study would be an OA that had no effect on the vertical mouth opening or mandibular position. However, the inconvenience of this without any likely benefit would tend to bias in favour of an OA which had an effect on the vertical mouth opening and/or mandibular position, unless the benefits could be measured after subtracting the side effects of the treatment.
Compared with control, OAs have been shown to reduce symptoms of sleepiness associated with OSAH, and also to reduce AHI, arousals and minimum saturation. There is some evidence that OAs lead to lower blood pressure from one study. This effect requires replication in future trials. Further assessment of OAs with control should consider quality of life measures.
There is increasing evidence that CPAP is effective in improving daytime sleepiness, quality of life and blood pressure (Giles 2006; Patel 2003). This review suggests that OAs are less effective than CPAP in improving indices of sleep disordered breathing, but the evidence on subjective outcomes is less certain. Symptoms and indices of quality of life (arguably more powerful indications of how patients perceive therapeutic benefit than indices of sleep disordered breathing) have shown equivocal or conflicting results. The number of studies, and the somewhat mixed severity of baseline symptoms reported in their respective populations restrict meaningful exploration via subgroup analysis. Further work in more distinct study populations will help to establish whether particular characteristics (such as disease severity and burden) predispose patients to accept one treatment modality over another, to accept them equally well, or to accept neither. If the superiority of CPAP in reducing AHI, arousals and minimum saturation reflects a more intensive, invasive intervention and is independent of improvement in symptoms, then people who start treatment with fewer symptoms do not stand to perceive the same degree of benefit as do those with more pronounced sleepiness. However, if sleep disordered breathing is left inadequately controlled, the long‐term risk of cardiovascular morbidity may be significant (Mooe 2001). Studies contributing data on AHI (11) outnumber those contributing data on ESS (seven) or quality of life (five). The use of subjective outcomes is a priority for additional research in this area.
The relative effects outlined above and the different side‐effect profiles of these treatments also shed some light on to some of the findings on study withdrawal and preference. It should be noted that there was a higher withdrawal rate in OA treatment groups compared with CPAP. Where this occurs in crossover studies, this alters the characteristic of the study population at the end of treatment, such that trialists collect data from participants who have adhered to both treatment regimens. This is compounded when preference data are sought, because this can only be recorded in participants who have been exposed to all treatments assessed, leaving preference in crossover trials theoretically prone to order effects.
Across the studies there was no consistent preference for one intervention over another, with individual study results indicating discordant directions and degrees of preference for the two treatments. Exploration of these differing effects between trials is difficult, but there have been within‐study subgroup analyses. Engleman 2002 suggested that preference for OA was stronger in those with milder daytime sleepiness, and less impairment in quality of life when compared with those expressing preference for CPAP. Those who preferred CPAP also used it for longer than those who preferred OA. Although baseline AHI did not correlate with preference in Engleman 2002, Barnes 2004 reported that in a mild subgroup of participants with AHI <15, more people expressed a preference for OAs than for CPAP. When studies considered preference in relation to a treatment response rather than baseline characteristic, there appeared to be a strong preference for OAs over CPAP (Ferguson 1996; Ferguson 1997; Olson 2002). The largest study to date has attempted to record overall preference irrespective of whether treatments were deemed successful or not (Barnes 2004). The number of participants preferring CPAP differed depending on whether the trialists asked which option was more convenient or which one was more effective, with many indicating that the placebo was easiest to use. There are clearly complex reasons why some people prefer OAs and others CPAP, and this may be related to baseline psychological factors and disease severity, as well as subsequent response to treatment and tolerance of side‐effects. Preference has been measured in numerous OSAH trials where different types of CPAP machines have been compared (Haniffa 2004). However, despite a numerically superior preference for auto‐CPAP over fixed pressure machines in these studies, this does not seem to lead to superior amounts of usage (Haniffa 2004). Whilst preference is a powerful, subjective way of determining which option was regarded as superior in participants in these studies, this reflects only short‐term exposure. Barnes 2004 also measured self‐reported compliance with OA, and this indicated that where the OA were thought to have been effective, they were reportedly used more. Continued acceptance with treatment may be predicted by psychological determinants, and evidence from qualitative sources would be helpful in elucidating the interaction between perceived treatment success, willingness to persist with treatment, and psychological and emotional factors (Wild 2004).
Self‐reported treatment compliance with OAs was high as reported in six studies (Barnes 2004; Ferguson 1997; Gotsopoulos 2002; Mehta 2001; Randerath 2002; Tegelberg 1999), though objective usage data are harder to capture for OAs than for CPAP.
Evidence from one trial comparing OA with a surgical procedure suggests that an OA was more effective than uvulopalatopharyngoplasty in improving indices of sleep disordered breathing. However, the significance of this finding is questionable as there is no definitive evidence of the effectiveness of this type of corrective upper airway surgery (Sundaram 2005). Further work is required to determine the most effective type of OA but until there is definitive evidence of the effectiveness of OA treatment the value of comparing different types of appliance is also debatable. Several studies have compared devices of different design (Bloch 2000; Luks 1996; Pitsis 2002; Rose 2002). Bloch 2000 reported no significant difference in efficacy between appliances, whilst Rose 2002 and Pitsis 2002 suggested that appliance design is important to treatment success and patient preference.
There are some data from a crossover study on blood pressure which indicates a reduction in certain measures when participants are treated with OA. In one measure, OA blood pressure was lower than CPAP. Repeated recording in future studies would help to quantify the effect compared with control. There is a need to monitor other conditions thought to be associated with OSAH. Successful long‐term management of this condition is likely to be defined not merely by subjective response and associated adherence, but also by cardiovascular morbidity. Although predictors of treatment success have been proposed they have never been systematically validated (Mehta 2001). There are limited data on long‐term complications. The results of this review justify well designed, large scale, randomised controlled trials to assess the effectiveness and cost effectiveness of OA treatment.
Authors' conclusions
Implications for practice.
Although the data are limited by the relatively small number of patients studied and methodological weaknesses, such as lack of blinding, there is increasing evidence that OA improves subjective sleepiness and indices of sleep disordered breathing over an inactive control. CPAP and OA both led to improvements in AHI compared with baseline, but the magnitude of improvement favoured CPAP. Symptomatic response varied between the studies, and reinforces the need for additional trials using symptom and quality of life outcomes in the future. Participants were more likely to withdraw from OA therapy than from CPAP, although patients who respond to both treatments appear to prefer the use of an OA over CPAP. There may be various factors which determine which patients are more likely to persist with CPAP and OA, and studies in more distinct patient subgroups, or exploration of patient subgroups within studies, would help to this end. On the basis of evidence in this review it would appear appropriate to offer OA therapy to patients with mild symptomatic OSAH, and those who are unwilling or unable to persist with CPAP therapy. This recommendation is drawn from evidence of limited duration. Long‐term effects of these two treatments and their impact on cardiovascular health are not currently evaluable.
Implications for research.
Although the evidence base to support the use of CPAP in OSAH is strong, its relative effects compared with OA require further elucidation.
Additional well designed, large scale randomised controlled trials comparing active and control OA are required in patients with OSAH to determine which groups of patients are most likely to benefit from OA treatment, how these patients can be identified, how much benefit can be achieved and with what cost, side effects and complications.
These trials should have patient and investigator blinding and perform an intention to treat analysis. Adequate procedures to protect studies against selection bias should be detailed in study reports.
Crossover studies should also include tests of carryover and period effects. These trials should also use standardised, validated instruments to measure subjective outcomes.
There is a need to clarify the relative effects of OAs and CPAP in terms of symptoms and quality of life. The effect estimate for ESS did not exclude a difference in favour of either intervention, and this outcome in particular would benefit from additional usage of ESS in future trials. These findings would provide valuable insights in to how patients offered either of these therapies respond subjectively, and whether they seek alternative treatments to manage their OSAH.
There are very few studies of sufficient duration or size to estimate whether CPAP or OA are effective in improving cardiovascular health such as reducing stroke and heart attacks. Further studies should consider measuring these outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 13 August 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 13 August 2008 | New search has been performed | Literature search run in June 2008. Additional data for an included study obtained from study investigators. |
| 1 June 2007 | New search has been performed | Literature search re‐run. One new trial was identified but did not contribute data to the review (Hoekema 2006). One study published in full and previously available as a conference abstract contributed data to the comparison OA versus CPAP. This did not alter the conclusions of the review. |
| 12 September 2005 | New citation required and conclusions have changed | Three new trials met the inclusion criteria of the review (Barnes 2004; Blanco 2005; Lam 2003). The inclusion of these data had a small impact on the findings of the review. More evidence has come to light that CPAP is more effective at suppressing apnoea, although the impact on symptoms between the two treatments remains not significantly different. Some data now call in to question whether our previous observation that OAs were preferred to CPAP. It appears that this is not always so, and that there are several important psychological factors which could influence preference over a short‐term trial, including perceived benefit. |
Acknowledgements
The authors would like to thank the staff of the Cochrane Airways Group editorial base for assistance with electronic searching, and retrieval of papers. We are grateful to member of the Airways Group Translation Network for invaluable input in the translation of clinical trials from Russian, Italian, German and French (Derek Scoins, Gianni Ferrara and Toby Lasserson). In addition we wish to thank authors who corresponded with us and provided unpublished data for their studies, namely: Dr Aarnoud Hoekema, Dr Chris Johnston, Prof Les Olson, Prof Stephen Spiro, Prof Winfried Randerath, Prof Peter Cistulli, Dr Helen Gotsopoulos and Prof Marie Walker‐Engstrom.
Data and analyses
Comparison 1. Active oral appliance versus control appliance.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Epworth sleepiness score ‐ first arm data/parallel studies | 4 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐2.09 [‐3.80, ‐0.37] |
| 2 Epworth sleepiness score ‐ crossover studies | 2 | 182 | Epworth score (Fixed, 95% CI) | ‐1.81 [‐2.72, ‐0.90] |
| 3 Apnoea Hypopnea Index ‐ first arm/parallel studies | 5 | 156 | Mean Difference (IV, Fixed, 95% CI) | ‐10.78 [‐15.53, ‐6.03] |
| 4 Apnoea Hypopnea Index ‐ crossover studies | 4 | 310 | AHI/hr (Fixed, 95% CI) | ‐15.15 [‐19.40, ‐10.89] |
| 5 Arousals ‐ first arm data/parallel studies | 3 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐10.66 [‐16.03, ‐5.29] |
| 6 Arousals ‐ crossover studies | 2 | 194 | Arousals/hr (Fixed, 95% CI) | ‐10.72 [‐15.05, ‐6.39] |
| 7 Minimum saturation ‐ first arm data/parallel studies | 3 | 117 | Mean Difference (IV, Fixed, 95% CI) | 1.79 [‐0.29, 3.87] |
| 8 Minimum saturation ‐ crossover studies | 2 | 194 | % (Fixed, 95% CI) | 3.39 [2.25, 4.54] |
| 9 Withdrawals ‐ first arm/parallel studies | 3 | 65 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.24, 2.86] |
| 10 Quality of life (FOSQ) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Blood pressure outcomes | 1 | mmHg (Fixed, 95% CI) | Totals not selected | |
| 11.1 Systolic BP (24hr) | 1 | mmHg (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Systolic BP (diurnal) | 1 | mmHg (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Systolic BP (nocturnal) | 1 | mmHg (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.4 Diastolic BP (24hr) | 1 | mmHg (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.5 Diastolic DP (diurnal) | 1 | mmHg (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.6 Diastolic BP (nocturnal) | 1 | mmHg (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.7 Mean BP (24hr) | 1 | mmHg (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.8 Mean BP (diurnal) | 1 | mmHg (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.9 Mean BP (nocturnal) | 1 | mmHg (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
Comparison 1 Active oral appliance versus control appliance, Outcome 1 Epworth sleepiness score ‐ first arm data/parallel studies.
1.2. Analysis.
Comparison 1 Active oral appliance versus control appliance, Outcome 2 Epworth sleepiness score ‐ crossover studies.
1.10. Analysis.
Comparison 1 Active oral appliance versus control appliance, Outcome 10 Quality of life (FOSQ).
1.11. Analysis.
Comparison 1 Active oral appliance versus control appliance, Outcome 11 Blood pressure outcomes.
Comparison 2. Oral appliance versus continuous positive airways pressure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Epworth sleepiness scale ‐ first arm data/parallel studies | 3 | 268 | Mean Difference (IV, Random, 95% CI) | 0.64 [‐0.57, 1.86] |
| 2 Epworth sleepiness score ‐ crossover studies | 4 | 336 | Epworth score (Fixed, 95% CI) | 0.54 [‐0.29, 1.38] |
| 3 Apnoea Hypopnea Index ‐ first arm data/parallel studies | 4 | 283 | Mean Difference (IV, Random, 95% CI) | 9.08 [4.78, 13.38] |
| 4 Apnoea Hypopnea Index ‐ crossover studies | 7 | 464 | AHI/hr (Fixed, 95% CI) | 7.97 [6.38, 9.56] |
| 5 Quality of life score (SAQLI) ‐ first arm data/parallel studies | 3 | 192 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.35, 0.28] |
| 6 Quality of life ‐ crossover studies | 1 | General Health score (Fixed, 95% CI) | Totals not selected | |
| 7 Functional outcomes of sleep questionnaire ‐ crossover studies | 2 | 256 | FOSQ Units (Fixed, 95% CI) | ‐0.18 [‐0.42, 0.07] |
| 7.1 Total Score | 2 | 256 | FOSQ Units (Fixed, 95% CI) | ‐0.18 [‐0.42, 0.07] |
| 8 Short‐form 36 (quality of life) ‐ crossover studies | 2 | SF36 units (Fixed, 95% CI) | Totals not selected | |
| 8.1 Physical component score | 1 | SF36 units (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Mental component score | 1 | SF36 units (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Total score | 1 | SF36 units (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Hospital Anxiety Depression Scale ‐ parallel group trials | 0 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Anxiety | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Depression | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Hospital Anxiety Depression Scale ‐ crossover studies | 1 | HADS Units (Fixed, 95% CI) | Totals not selected | |
| 10.1 Anxiety | 1 | HADS Units (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Depression | 1 | HADS Units (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Cognitive performance ‐ SteerClear | 1 | Cows hit (Fixed, 95% CI) | Totals not selected | |
| 12 Maintenance of Wakefulness test (MWT) ‐ crossover studies | 2 | 256 | Mins (Fixed, 95% CI) | 0.69 [‐1.56, 2.94] |
| 13 Minimum saturation (%) ‐ first arm data/parallel studies | 3 | 189 | Mean Difference (IV, Fixed, 95% CI) | 4.59 [2.55, 6.64] |
| 14 Minimum saturation ‐ crossover studies | 4 | 279 | % (Fixed, 95% CI) | 5.16 [3.25, 7.06] |
| 15 Arousals ‐ first arm data/parallel studies | 3 | 189 | Mean Difference (IV, Fixed, 95% CI) | 5.21 [2.48, 7.94] |
| 16 Arousals ‐ crossover studies | 6 | 367 | Arousals/hr (Fixed, 95% CI) | 2.24 [0.43, 4.05] |
| 17 Blood pressure outcomes ‐ crossover studies | 1 | mmHg (Fixed, 95% CI) | Totals not selected | |
| 17.1 Mean 24hr arterial pressure | 0 | mmHg (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 Mean 24hr systolic BP | 1 | mmHg (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.3 Mean 24hr diastolic BP | 1 | mmHg (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.4 Mean diurnal arterial pressure | 0 | mmHg (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.5 Mean nocturnal arterial pressure | 0 | mmHg (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.6 Mean nocturnal diastolic | 1 | mmHg (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 Blood pressure outcomes (parallel studies) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 18.1 Mean 24hr arterial pressure | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Mean 24hr systolic BP | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.3 Mean 24hr diastolic BP | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.4 Mean morning systolic BP | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.5 Mean evening systolic BP | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.6 Mean morning diastolic BP | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.7 Mean evening diastolic BP | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 Patient Preference | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20 Patient preference ‐ treatment success in both arms | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 21 Withdrawals ‐ first arm data/parallel studies | 6 | 411 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.05 [1.15, 3.67] |
| 22 Side‐effects by type ‐ crossover studies | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 22.1 Pressure on face | 1 | 38 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.20 [0.05, 0.85] |
| 22.2 Pressure in mouth | 1 | 38 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.81 [0.47, 129.75] |
| 22.3 TMJ pain | 2 | 134 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 18.00 [8.62, 37.57] |
| 22.4 Removal during sleep | 1 | 96 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.50 [1.43, 8.57] |
| 22.5 Sleep disruption | 1 | 96 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.28, 1.61] |
| 22.6 Excessive salivation | 1 | 96 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.88 [2.27, 34.79] |
| 22.7 Tooth damage | 1 | 96 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.71 [0.78, 75.97] |
| 22.8 Leak | 1 | 96 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.11 [0.03, 0.37] |
| 22.9 Dry upper airway | 1 | 96 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.02, 0.74] |
| 22.10 Stuffy nose | 1 | 96 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.03, 0.49] |
| 22.11 Inconvenience | 1 | 96 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.02, 0.63] |
| 23 Side‐effects by severity ‐ crossover studies | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 None | 2 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.24, 1.36] |
| 23.2 Mild | 2 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.19 [1.65, 16.30] |
| 23.3 Moderate | 2 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.35, 2.95] |
| 23.4 Severe | 2 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.04, 1.16] |
| 24 Preference ‐ treatment success in either treatment arm | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 1 Epworth sleepiness scale ‐ first arm data/parallel studies.
2.2. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 2 Epworth sleepiness score ‐ crossover studies.
2.6. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 6 Quality of life ‐ crossover studies.
2.7. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 7 Functional outcomes of sleep questionnaire ‐ crossover studies.
2.8. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 8 Short‐form 36 (quality of life) ‐ crossover studies.
2.10. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 10 Hospital Anxiety Depression Scale ‐ crossover studies.
2.11. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 11 Cognitive performance ‐ SteerClear.
2.12. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 12 Maintenance of Wakefulness test (MWT) ‐ crossover studies.
2.14. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 14 Minimum saturation ‐ crossover studies.
2.17. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 17 Blood pressure outcomes ‐ crossover studies.
2.18. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 18 Blood pressure outcomes (parallel studies).
2.19. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 19 Patient Preference.
2.20. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 20 Patient preference ‐ treatment success in both arms.
2.22. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 22 Side‐effects by type ‐ crossover studies.
2.23. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 23 Side‐effects by severity ‐ crossover studies.
2.24. Analysis.
Comparison 2 Oral appliance versus continuous positive airways pressure, Outcome 24 Preference ‐ treatment success in either treatment arm.
Comparison 3. Oral appliance versus surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Apnoea Hypopnea Index | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 4 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Oxygen desaturation index | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 4 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Withdrawals/loss to follow up | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.
Comparison 3 Oral appliance versus surgery, Outcome 1 Apnoea Hypopnea Index.
3.2. Analysis.
Comparison 3 Oral appliance versus surgery, Outcome 2 Oxygen desaturation index.
3.3. Analysis.
Comparison 3 Oral appliance versus surgery, Outcome 3 Withdrawals/loss to follow up.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barnes 2004.
| Methods | Three‐way crossover trial. Patients randomised to three arms CPAP, MAS and Placebo. | |
| Participants | 80 middle aged patients with mild to moderate OSA. Male 64 females 16, mean age 47; baseline ESS mean 10.7; AHI: 21.3. Inclusion criteria: AHI 5‐30; |
|
| Interventions | Nasal CPAP versus OA versus Placebo tablet. Study duration: 12 weeks |
|
| Outcomes | ESS, FOSQ, ODI 4%, AHI, MWT, SF‐36 | |
| Notes | Two week wash out period between each treatment. Intention‐to‐treat analysis Jadad Score=2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | OA & CPAP compared with dummy pill (single‐blind) |
Blanco 2005.
| Methods | Randomised, parallel group trial. Method of allocation: not clear. | |
| Participants | 24 participants randomised. Data presented on 15 participants who completed the study. Mean age: 55 years; BMI: 26.8; AHI: 24‐33; ESS: 14.7‐16.3. Inclusion criteria: AHI >/= 10; two OSA symptoms. Exclusion criteria: Poor dentition; >75years; BMI: >40 |
|
| Interventions | OA versus control OA. Control OA same as active without advancement | |
| Outcomes | AHI; symptoms; quality of life; tolerability | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Presentation of control intervention different; information on whether it was described as being an alternative treatment to intervention not available |
Durán 2002.
| Methods | Randomised, crossover trial. Method of allocation: not clear. | |
| Participants | 44 participants recruited. 38 participants completed the study (4 women). Mean age: 46.5 (SEM 9.2); BMI: 27.7 (SEM 3.2); AHI: 15.3 (SEM 10) Inclusion criteria: Mild OSA and snoring (AHI >5). |
|
| Interventions | OA versus OA in centric occlusion. Study duration: unclear. Study preceded by a 12‐18 week acclimatisation period. |
|
| Outcomes | AHI; symptoms; tolerability | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Presentation of control intervention different; information on whether it was described as being an alternative treatment to intervention not available |
Engleman 2002.
| Methods | Randomised crossover study comparing oral appliance and CPAP. Randomisation was stratified by severity of OSA defined by AHI of less than or equal to 15. Randomisation was conducted by blocks of 4. | |
| Participants | 12 women and 36 men completed the trial (51 were recruited). Baseline age 46 +/‐9, baseline AHI 31+/‐26, baseline Epworth 14 +/‐4. Inclusion criteria: Age 18‐70, AHI 5 or more, 2 or more symptoms of OSA including sleepiness (Epworth score of 8 or more) and driving impairment. Exclusion criteria: Patients with fewer than 4 teeth remaining in either arch, coexisting narcolepsy, periodic limb movement (more than 10 per hour), major medical illness, shift work, or residency more than 50 miles from Edinburgh. | |
| Interventions | Participants randomised to either CPAP or one of two OA devices (occlusal and non‐occlusal coverage). Duration: 4 months (2 months on each treatment). |
|
| Outcomes | AHI, subjective efficacy, symptom score, Epworth score, FOSQ, SF‐36 health transition, physical and mental component scores. | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | OA and CPAP compared |
Ferguson 1996.
| Methods | Randomised, prospective, crossover study. Unblinded comparison of oral appliance and nasal CPAP. Study duration 8 months | |
| Participants | 24 male and 3 female participants were recruited. Age range 25‐72. Inclusion criteria AHI 15‐50, patients residing in the metropolitan Vancouver area. Exclusion criteria <10 teeth in both maxillary and mandibular arches. | |
| Interventions | After a two week wash‐in period participants were randomised to either oral appliance or CPAP for 4 months. A 2 week wash‐out period was followed by a second 4 month crossover treatment period. | |
| Outcomes | AHI, Apnoea index, Total sleep time, desaturations <90%, minimum SaO2, sleep efficiency, arousals, symptom score (in‐house), patient satisfaction, | |
| Notes | Jadad score 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | OA and CPAP compared |
Ferguson 1997.
| Methods | Randomised, prospective, crossover study. Unblinded comparison of oral appliance and nasal CPAP. Study duration 8 months | |
| Participants | 19 male and 5 female participants were recruited. Age mean (SD) = 44(10.6). BMI: 32 +/‐8.2; AHI 26.8 +/‐11.9. Inclusion criteria AHI 15‐50. Exclusion criteria <10 teeth in either maxillary or mandibular arches. |
|
| Interventions | After a two week run‐in period participants were randomised to either oral appliance or CPAP for 4 months. A 2 week wash‐out period was followed by a further 4 month crossover treatment period. | |
| Outcomes | AHI, Apnea index, total sleep time, desaturations <90%, minimum SaO2, sleep latency, NREM, REM, arousals, Epworth sleepiness score. | |
| Notes | Jadad score 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | OA and CPAP compared |
Fleetham 1998.
| Methods | Randomised, prospective, unblinded parallel group study comparing oral appliance with nasal CPAP. Study duration 3 months | |
| Participants | 101 patients were recruited. Inclusion criteria AHI>10. 51 participants were randomised to receive CPAP and 50 were randomised to receive OA therapy. 96 men were recruited. Baseline demographics: AHI: CPAP: 37.6 +/‐22.8; OA: 38.7 +/‐22.2. Min SaO2: CPAP: 75.8 +/‐12.7; OA: 73.6 +/‐11.8. Age: CPAP: 49.0 +/‐9.4; OA: 46.2 +/‐11.3. ESS: CPAP: 12.8+/‐4.1; OA: 11.1 +/‐4.9. BMI: CPAP: 32.0 +/‐5.5; OA: 31.4 +/‐5.7. SAQLI: CPAP: 4.2 +/‐1.1; OA: 4.2 +/‐1.0. |
|
| Interventions | Participants were randomised to either oral appliance or nCPAP for a period of three months. | |
| Outcomes | AHI, Epworth sleepiness score, minimum SaO2, Quality of life index. | |
| Notes | Jadad score 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | OA and CPAP compared |
Gotsopoulos 2002.
| Methods | Randomised, crossover study. Double‐blind comparison of oral appliance with inactive control device. 4 week duration. Randomisation was conducted by random number generator in blocks of 4. | |
| Participants | 73 participants analysed out 85 recruited. 59 were males. Mean age: 48 +/‐11. Baseline AHI: 27 +/‐2; Min Sao2: 86 +/‐1%. ESS: 11 Inclusion criteria: RDI >10, with symptoms of OSAS. Age >20, mandibular protrusion >3 mm. Exclusion criteria: Central SA, psychiatric disease, narcotics + sedatives, dental disease, exaggerated gag reflex. |
|
| Interventions | Active or inactive oral appliance (active device was mandibular advancement splint). 1 week wash‐in period followed by 4 weeks of treatment. 1 week wash‐out followed by second treatment regimen. | |
| Outcomes | AHI, Epworth sleepiness score, Min Sa O2, Sleep architecture, snoring, multiple sleep latency test, self‐reported compliance, tolerability, treatment satisfaction. | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated randomisation schedule |
| Allocation concealment? | Low risk | Investigators unaware as to order of treatment group assignment |
| Blinding? All outcomes | Unclear risk | Two treatments not identical in presentation, but control treatment described as alternative treatment to participants (single‐blind) |
Hans 1997.
| Methods | Randomised parallel group study of oral appliance and minimally active oral appliance. Study duration two weeks. | |
| Participants | 24 adult volunteers were recruited. Age 51.9(12.3)Inclusion criteria RDI<30. Exclusion criteria systemic diseases other than OSAS, pregnant women, prisoners, minors, mentally disabled, edentulous, previous surgical treatments for snoring or apnoea, significant non‐OSAS sleep disorders, RDI >30. | |
| Interventions | Participants were randomised to either active oral appliance or minimally active oral appliance. | |
| Outcomes | RDI, Epworth sleepiness score, | |
| Notes | Jadad score 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Presentation of control intervention different; information on whether it was described as being an alternative treatment to intervention not available |
Hoekema 2006.
| Methods | Randomised parallel group trial of CPAP and OA. Study duration: 8 weeks. Method of randomisation: not clear. Blinding: not performed. | |
| Participants | 103 participants. Local outpatient unit Inclusion criteria: >20 years; polysomnographically confirmed sleep apnoea; Exclusion criteria: Prior treatment for OSA; clearly reversible morphological airway abnormalities; endocrine dysfunction; severe cardia disease; periodic limb movement; extensive periodontal disease/tooth decay; active temporomandibular joint disease (including severe bruxism); restrictions in mouth opening or advancement of mandible; partial/complete edentulism (< 8 teeth in upper or lower jaw). |
|
| Interventions | Oral appliance versus CPAP | |
| Outcomes | Treatment success; ESS; AHI; FOSQ; SF‐36; HADS | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'The clinical epidemiologist (BS) for the study made computer‐generated randomization sequences, balancing for disease severity. The randomization sequences were used for selecting random permuted blocks with lengths of 2, 4, and 6 |
| Allocation concealment? | Low risk | 'The randomization sequences were concealed and administered by Department of Oral and Maxillofacial Surgery staff. After each person’s serial number and diagnosis of disease severity were provided, the treatment was disclosed. Each serial number could be provided only once.' |
| Blinding? All outcomes | High risk | OA and CPAP compared |
Johnston 2002.
| Methods | Randomised crossover trial of oral appliance versus 'placebo' appliance. Study duration: 4‐6 weeks followed by change to other appliance. Method of randomisation: toss of coin. Participants informed that two different types of oral appliance would be tested ‐ one with proven efficacy and one without. Statistical test: paired t test | |
| Participants | 21 participants recruited from a dedicated sleep clinic. Mean age: 55.10 +/‐6.87. 16 M. OSA diagnosis confirmed by home oximetry study (10 + desaturations per hour). AHI: 31.93 +/‐21.18; ESS: 13.90 +/‐6.90; ODI: 30.69 +/‐18.82; BMI: 31.63 +/‐5.94. Inclusion criteria: >/= 10 desaturations per hour Exclusion criteria: Concurrent pulmonary disease; inadequate number of sound teeth. |
|
| Interventions | Mandibular Advancement Appliance (MAA) versus placebo device | |
| Outcomes | AHI, ODI, ESS, tolerability, compliance | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Coin toss |
| Allocation concealment? | High risk | No concealment of allocation |
| Blinding? All outcomes | Unclear risk | Two treatments not identical in presentation, but control treatment described as alternative treatment to participants (single‐blind) |
Lam 2007.
| Methods | Randomised parallel group trial of CPAP versus oral appliance therapy. Study duration: 10 weeks. Method of randomisation: not specified. | |
| Participants | 68 participants with OSA (mean baseline AHI 22). Inclusion criteria: AHI >5–40 and ES >9 for those with AHI 5–20. Exclusion criteria: Excessive sleepiness, unstable medical diseases, coexistence of sleep disorders, upper airway surgery, pregnancy |
|
| Interventions | OA versus CPAP (conservative management given as a control group and not considered by this review) | |
| Outcomes | AHI, Quality of life, sleepiness | |
| Notes | Jadad score 2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | OA versus CPAP compared |
Mehta 2001.
| Methods | Randomised crossover trial of oral appliance and control oral plate. Study duration 3 weeks. Patients were blinded as to likely superior efficacy of the double plate appliance over the single plate. | |
| Participants | 28 adult participants were recruited. Age 48 (9), BMI 29.4(3.1), baseline AHI 27(17), min SaO2 85(8). Inclusion criteria at least 2 symptoms of OSA and AHI>10. Exclusion criteria periodontal disease, esentulism, exaggerated gag reflex, regular use of sedatives. | |
| Interventions | Participants were randomised to three periods (ABB/BAA) of oral appliance or control plate after an acclimatization period. | |
| Outcomes | AHI, minimum SaO2, snoring frequency,mean snoring intensity, maximum snoring intensity, total sleep time, REM, NREM, total sleep time spent supine, arousal index, sleep efficiency. | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Two treatments not identical in presentation, but control treatment described as alternative treatment to participants (single‐blind) |
Olson 2002.
| Methods | Randomised crossover study. Comparison of oral appliance and nasal CPAP. Study duration 14 weeks | |
| Participants | 24 patients were included. Baseline AHI 8.1‐36.9. Inclusion criteria AHI>15, or apnoea index >5, or AHI>5 and arousal index >15. Exclusion criteria poor dentition, temporomandibular joint pain, or previous treatment with oral appliances or nCPAP | |
| Interventions | Participants were randomised to six week treatment periods of oral appliance or CPAP separated by a two week wash‐out period. | |
| Outcomes | Total sleep time, sleep efficiency, %REM sleep, AHI, Arousal index, Sleep apnoea quality of life index | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | OA versus CPAP compared |
Randerath 2002.
| Methods | Randomised, crossover single centre trial. Comparison of CPAP with oral appliance. Study duration 12 weeks. | |
| Participants | 20 Participants with mild‐moderate sleep apnoea were included in the study. 16 men; mean age: 56.5 +/‐10.2; BMI: 31.2 +/‐6.4; AHI: 17.5 +/‐7.7. Inclusion criteria: AHI: 5‐30, clinical symptoms of OSAS. Exclusion criteria: AHI >30, temporomandibular joint disorders, bruxism, participants with gaps in their teeth preventing fitting of device. | |
| Interventions | Participants underwent 1 night polysomnography with both treatment modes, followed by 6 weeks treatment with either OA or CPAP in random order. Participants then crossed over to the other treatment. | |
| Outcomes | AHI; Snoring (epochs/h); SaO2 (%); TST (min); Wake after sleep onset; Sleep stage 1, 2, 3, 4; REM sleep; Arousals per/h; Respiration‐induced arousals, per/h of TST. | |
| Notes | Jadad score 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | OA versus CPAP compared |
Tan 2002.
| Methods | Randomised crossover study. Comparison of oral appliance and CPAP. Study duration 4 months | |
| Participants | 24 patients were recruited. Mean age: 50.9 +/‐ 10.1.Epworth sleepiness score: 13.4 +/‐ 4.6; AHI: 22.6 +/‐ 9.6; O2 desaturation: 7.1 +/‐ 2.7. Arousals/hr: 19.3 +/‐ 9.6. Three participants withdrew. | |
| Interventions | Two months of CPAP and oral appliance in random order. Two week wash‐out. | |
| Outcomes | AHI, O2 desaturation, Epworth sleepiness score, general symptoms, daytime somnolence score, partner's assessment, duration of apnoeas, arousals/hr, sleep efficiency, REM sleep. | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | OA versus CPAP compared |
Tegelberg 1999.
| Methods | Randomised, parallel group study. Single‐blind comparison of surgery versus oral appliance. Study duration: 1 year. | |
| Participants | 95 male participants were recruited. Age: 20‐65, baseline AHI: 15.7‐23.3. Inclusion criteria: AHI between 5 and 25, age between 20 and 65. Exclusion criteria: Mental illness, drug misuse, significant nasal obstruction, insufficient teeth, pronounced dental malocclusion, severe cardiovascular disease, neurological disease, respiratory disease. At 4 year follow‐up, OA group: N = 32, UPPP group: N = 40. | |
| Interventions | Participants were randomised to either oral appliance or surgical intervention (uvulopalatopharyngoplasty). Participants randomised to receive UPP were followed up at regular intervals. | |
| Outcomes | AHI, AI, oxygen desaturation index, snoring index, clinical dysfunction score, QOL scores | |
| Notes | Jadad score: 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | OA versus surgery |
OSAS = Obstructive Sleep Apnoea Syndrome; AI = Apnoea Index; AHI = Apnoea Hypopnea Index; CPAP = Continuous Positive Airways Pressure; nCPAP = nasal CPAP; OA = Oral Appliance; BMI = Body Mass Index; SAQLI = Sleep Apnoea Quality of Life Index; ESS = Epworth Sleepiness Score; RDI = Respiratory Disturbance Index; ODI = Oxygen Desaturation Index; UPPP = Uvulopalatopharyngoplasty; TST = Total Sleep Time; SF‐36 = Short‐form 36; HADS: Hospital anxiety and depression score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Athen 1999 | Case series |
| Bloch 2000 | Comparison of two types of mandibular devices |
| Bushell 1991 | Randomised cross over which excluded OSA. |
| Cistulli 1998 | Before and after study |
| Clark 1996 | Non‐randomised allocation to treatment groups |
| David 2000 | Before and after study. |
| Dort 2003 | Tongue‐retaining device. |
| Eckhart 1998 | Review article of splint characteristics |
| Eroshina 2001 | Non‐randomised before and after study. |
| Eveloff 1994 | Before and after study |
| Friedman 2008 | Study of surgically inserted palatal implants |
| Fritsch 2000 | Review article |
| Ichioka 1995 | Case series |
| Kato 2000 | Before and after study |
| Lamont 1998 | Non‐randomised case series |
| Lawton 2005 | Comparison of two types of mandibular devices |
| Liu 2000 | Case series. |
| Lowe 2000 | Before and after study |
| Luks 1996 | Comparison of two types of mandibular devices |
| Marklund 1998 | Non‐randomised before and after study ‐ identified from additional electronic search |
| Menn 1996 | Non‐randomised before and after study ‐ identified from bibliography of Gotsopoulos 2002 |
| Moore 2000 | New product report, no data regarding efficacy |
| Neill 2002 | Split night protocol |
| O'Sullivan 1993 | Uncontrolled randomised study |
| O'Sullivan 1995 | Uncontrolled randomised study |
| Ono 1996 | Case control trial reporting genioglossus EMGs |
| Pillar 2004 | Different OAs compared |
| Pirila‐Parkkinen '99 | Case controlled trial in children |
| Pitsis 2002 | Comparison of two types of mandibular devices |
| Rose 2002 | Comparison of two types of mandibular devices |
| Rose 2002b | Long‐term assessment of oral appliance in non‐randomised cohort study |
| Saletu 2007 | Laboratory based short term crossover study |
| Schonhofer 1997 | Review article |
| Schonhofer 1997b | Before and after study |
| Sjoholm 1994 | Unable to determine suitability based on full text article. No reply from study investigators. |
| Skinner 2004 | Study assessing the effects of a collar. Whilst this was a form of mandibular advancement, it was not within the scope of the review which was specifically concerned with oral appliances. |
| Turk 2005 | Not randomised. |
| van der Sweep 2006 | Assessment of chin straps in CPAP users. |
| van der Veken 2005 | Comparison of two different oral devices. |
| Villa 2002 | Randomised trial in children |
| Walker‐Engstrom 2003 | Randomised study ‐ this was excluded as there was no placebo/inactive control. This study compared different degrees of mandibular advancement (50% versus 75%) |
| Winkels 1997 | Non‐randomised case series |
| Yoshida 1998 | Case series |
Characteristics of ongoing studies [ordered by study ID]
Cibele 2006.
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Fairbairn 2004.
| Trial name or title | |
| Methods | |
| Participants | Patients with Obstructive sleep apnoea syndrome (OSAS) |
| Interventions | CPAP versus MAS |
| Outcomes | Steering simulation |
| Starting date | |
| Contact information | |
| Notes |
Contributions of authors
JL developed the protocol with editorial input from JW. TL and JL went through search results, extracted and entered data. TL and JL developed the analysis with additional input from JF. TL , JL and JF contacted study authors and obtained unpublished data. The analysis was developed by JL and TL. JF and JL wrote and developed the discussion and conclusions. JW offered editorial support for the analysis and interpretation of the studies.
Sources of support
Internal sources
No sources of support supplied
External sources
NHS Research and Development, UK.
Declarations of interest
Prof John Fleetham was a trial investigator on several of the trials and has been the lead investigator in one study.
Edited (no change to conclusions)
References
References to studies included in this review
Barnes 2004 {published data only}
- Barnes M, McEvoy D, Banks S, Tarquinio N, Murray C, Vowles N, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine 2004;170(6):656‐64. [DOI] [PubMed] [Google Scholar]
Blanco 2005 {published data only}
- Blanco J, Zamarron C, Abeleira Pazos MT, Lamela C, Suarez Quintanilla D. Prospective evaluation of an oral appliance in the treatment of obstructive sleep apnea syndrome. Sleep Breath 2005;9(1):20‐5. [DOI] [PubMed] [Google Scholar]
Durán 2002 {unpublished data only}
- Duran JJ, Esnaola S, Rubio R, Torre G, Anitua E, Zubia S, et al. A randomised, double blind, crossover, placebo‐controlled trial of mandibular advancement device for the treatment of snoring and mild obstructive sleep apnoe‐hypopnoea syndrome. European Respiratory Journal 2002;20(Suppl 38):102s. [Google Scholar]
Engleman 2002 {published and unpublished data}
- Engleman HM, McDonald JP, Graham D, Lello GE, Kingshott RN, Coleman EL, et al. Randomized crossover trial of two treatment for sleep apnea/hypopnea syndrome. American Journal of Respiratory & Critical Care Medicine 2002;166:855‐9. [DOI] [PubMed] [Google Scholar]
Ferguson 1996 {published data only}
- Ferguson KA, Ono T, Lowe AA, Keenan SP, Fleetham J. A randomized crossover study of an oral appliance versus nasal‐continuous positive airway pressure. Chest 1996;109(5):1269‐75. [DOI] [PubMed] [Google Scholar]
Ferguson 1997 {published data only}
- Ferguson K, Ono T, Lowe A, Sulaiman AM, Love L, Fleetham J. A short term controlled trial of an adjustable oral appliance for the treatment of mild to moderate obstructive sleep apnoea. Thorax 1997;52:362‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleetham 1998 {published and unpublished data}
- Fleetham JA, Lowe A, Vazquez JC, Ferguson K, Flemons W, Remmers J. A long term randomised parallel multicentre study of an oral appliance vs nCPAP in the treatment of obstructive sleep apnea. American Thoracic Society International Conference; April 24‐29; Chicago, Illinois. 1998:P613.
Gotsopoulos 2002 {published data only}
- Gotsopoulos H, Chen C, Qian J, Cistulli P. Oral appliance therapy improves symptoms in obstructive sleep apnea. American Journal of Respiratory Critical Care Medicine 2002;166:743‐8. [DOI] [PubMed] [Google Scholar]
- Gotsopoulos H, Kelly JJ, Cistulli P. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: a randomized controlled trial. Sleep 2004;27(5):934‐41. [DOI] [PubMed] [Google Scholar]
- Gotsopoulos H, Mowbray J, Lawson J, Chen C, Qian J, Durston M, et al. Effect of mandibular advancement splint (MAS) therapy on blood pressure in obstructive sleep apnoea syndrome (OSAS). Thoracic Society of Australia and New Zealand Annual Scientific Meeting. 2001:A84.
- Naismith SL, Winter VR, Hickie IB, Cistulli PA. Effect of oral appliance therapy on neurobehavioral functioning in obstructive sleep apnoea: a randomized controlled trial. Journal of Clinical Sleep Medicine 2005;1(4):374‐80.. [PubMed] [Google Scholar]
Hans 1997 {published data only}
- Hans MG, Nelson S, Luks VG, Lorkovich P, Baek SJ. Comparison of two dental devices for treatment of obstructive sleep apnea syndrome (OSAS). American Journal of Orthodontics and Dentofacial Orthopedics 1997;111:562‐70. [DOI] [PubMed] [Google Scholar]
Hoekema 2006 {published data only}
- Hoekema A. Personal communication 2006.
- Hoekema A. Efficacy and comorbidity of oral appliances in the treatment of obstructive sleep apnea‐hypopnea: a systematic review and preliminary results of a randomized trial. Sleep and breathing 2006;10(2):102‐3. [DOI] [PubMed] [Google Scholar]
- Hoekema A, Doff MH, Bont LG, Hoeven JH, Wijkstra PJ, Pasma HR, et al. Predictors of obstructive sleep apnea‐hypopnea treatment outcome. Journal of Dental Research 2007;86(12):1181‐6. [DOI] [PubMed] [Google Scholar]
- Hoekema A, Stegenga B, Bakker M, Brouwer WH, Bont LGM, Wijkstra PJ, et al. Simulated driving in obstructive sleep apnoea‐hypopnoea; effects of oral appliances and continuous positive airway pressure. Sleep and Breathing 2007;11(3):129‐38. [DOI] [PubMed] [Google Scholar]
- Hoekema A, Stegenga B, Wijkstra PJ, Hoeven JH, Meinesz AF, Bont LGM. Effectiveness of obstructive sleep apnea therapy: a randomized parallel clinical trial of oral‐appliance versus continuous positive airway pressure therapy. Sleep Medicine 2006;7:14s. [Google Scholar]
- Hoekema A, Stel A‐L, Stegenga B, Hoeven JH, Wijkstra PJ, Driel MF, et al. Sexual function and obstructive sleep apnea‐hypopnea: A randomized clinical trial evaluating the effects of oral‐appliance and continuous positive airway pressure therapy. Journal of Sexual Medicine 2007;4(4 II):1153‐62. [DOI] [PubMed] [Google Scholar]
- Hoekema A, Voors AA, Wijkstra PJ, Stegenga B, Hoeven JH, Tol CG. Effects of oral appliances and CPAP on the left ventricle and natriuretic peptides. International Journal of Cardiology 2008;128:232‐9. [DOI] [PubMed] [Google Scholar]
Johnston 2002 {published data only}
- Johnston CD, Gleadhill IC, Cinnamond MJ, Gabbey J, Burden DJ. Mandibular advancement appliances and obstructive sleep apnoea: a randomized clinical trial. European Journal of Orthodontics 2002;24:251‐62. [DOI] [PubMed] [Google Scholar]
Lam 2007 {published data only}
- Lam B, Sam K, Cheung MT, Mok W, Lam J, Tsang KWT, et al. A randomized controlled study for the treatment of mild/moderate onstructive sleep apnea (OSA) [Abstract]. Sleep Medicine 2003;4(Suppl 1):S26. [Google Scholar]
- Lam B, Sam K, Mok WYW, Cheung MT, Fong DYT, Lam JCM, et al. Randomised study of three non‐surgical treatments in mild to moderate obstructive sleep apnoea. Thorax 2007;62(4):354‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehta 2001 {published data only}
- Mehta A, Qian J, Petocz P, Ali Darandeliler M, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnoea. American Journal of Respiratory Critcal Care Medicine 2001;163:1457‐61. [DOI] [PubMed] [Google Scholar]
Olson 2002 {unpublished data only}
- Olson LG, Ambrogetti A, Trevillian Z. A randomised crossover comparison of nasal CPAP and a mandibular advancement splint in mild obstructive sleep apnea. Personal communication 2002.
Randerath 2002 {published data only}
- Randerath W, Heise M, Galetke W, Hinz R, Rühle KH. Prospective randomised comparison of a three dimensional adjustabale protrusion device with CPAP in mild obstrictive sleep apnoea [Prospektiver randomiserter Vergleich einer dreidimnsional‐variablen Protrusionsschiene mit CPAP beim leichtgradigen obstruktiven Schlafapnoe‐Synfrom]. Pneumologie 2001;55:73s [poster: P101]. [Google Scholar]
- Randerath WJ, Heise M, Hinz R, Ruehle KH. An individually adjustable oral appliance vs continuous positive airway pressure in mild‐to‐moderate obstructive sleep apnea syndrome. Chest 2002;122(2):569‐75. [DOI] [PubMed] [Google Scholar]
Tan 2002 {published data only}
- Tan YK, L'Estrange PR, Grant HR, Smith C, Simonds AK, Spiro SG. A randomised crossover study of continuous positive airway pressure (CPAP) and a mandibular advancement splint (MAS) in a randomised crossover study of patients with mild or moderate obstructive sleep apnoea. Thorax 1998;53:S15. [Google Scholar]
- Tan YK, L'Estrange PR, Luo YM, Smith C, Grant HR, Simonds AK, et al. Mandibular advancement splints and continuous positive airway pressure in patients with obstructive sleep apnoea. European Journal of Orthodontics 2002;24:239‐49. [DOI] [PubMed] [Google Scholar]
Tegelberg 1999 {published data only}
- Ringqvist M, Walker‐Engstrom ML, Tegelberg A, Ringqvist I. Dental and skeletal changes after 4 years of obstructive sleep apnea treatment with a mandibular advancement device: a prospective, randomized study. American Journal of Orthodontics & Dentofacial Orthopedics 2003;124(1):53‐60. [DOI] [PubMed] [Google Scholar]
- Tegelberg A, Wilhelmsson B, Walker‐Engstrom ML, Ringqvist M, Andersson L, Krekamanov L, et al. Effects and adverse events of a dental appliance for treatment of obstructive sleep apnoea. Swedish Dental Journal 1999;23(4):117‐26. [PubMed] [Google Scholar]
- Tegelberg AS, Vestling O, Wilhelmsson BJ, Walker‐Engström ML. Treatment effects in obstructive sleep apnea. Comparison of two standardized degrees of mandibular advancement with a dental appliance [Abstract]. Journal of Dental Research 2003;82(Special Issue B):B‐368 (Abs 2869). [Google Scholar]
- Walker‐Engstrom ML. Treatment effects with a mandibular advancement appliance and uvulopalatopharyngoplasty in obstructive sleep apnea: Randomised controlled trials [PhD Thesis]. Sweden: Uppsala Univeritet, 1999. [Google Scholar]
- Walker‐Engstrom ML, Tegelberg A, Wilhelmsson B, Ringqvist I. 4‐year follow‐up of treatment with dental appliance or uvuloplaltopharyngoplasty in patients with obstructive sleep apnoea. Chest 2002;121(3):739‐46. [DOI] [PubMed] [Google Scholar]
- Walker‐Engstrom ML, Wilhelmsson B, Tegelberg A, Dimenas E, Ringqvist I. Quality of Life measurements of treatment with dental appliance or UPPPin patients with mild to moderate obstructive slep apnoea. A prospective randomized 1 year follow‐up study. Journal of Sleep Research 2000;9(3):303‐8. [DOI] [PubMed] [Google Scholar]
- Wilhelmsson B, Tegelberg A, Walker‐Engstrom ML, Ringqvist M, Andersson L, Krekmanov L, et al. A prospective randomized study of a dental appliance compared with uvulppalatopharngoplasty in the treatment of obstructive sleep apnoea. Acta‐Oto‐laryngologica 1999;119:503‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Athen 1999 {published data only}
- Athen B, Athen B. Intraoral treatment of borderline levels of sleep‐related respiratory disorders by modified protrusion fixation of the lower jaw [Intraorale therapie grenzwertiger schlafbezogener atemstorungen mit einer modifizierten protrusions‐fixation des unterkiefers]. Pneumologie 1999;53(2):88‐91. [PubMed] [Google Scholar]
Bloch 2000 {published data only}
- Bloch K, Iseli A, Zhang JN, Xie X, Kaplan V, Stockeli PW, et al. A randomized, controlled corssover trial of two oral appliances for sleep apnoea treatment. American Journal of Respiratory Critical Care Medicine 2000;162:246‐51. [DOI] [PubMed] [Google Scholar]
Bushell 1991 {published data only}
- Bushell M, Baldock P, Antic R, Thornton A, McEvoy RD. Obligatory nasal breathing:effects on snoring and sleep apnoea. Medical Journal of Australia 1991;155:83‐5. [DOI] [PubMed] [Google Scholar]
Cistulli 1998 {published data only}
- Cistulli PA, Palmisano RG, Poole MD. Treatment of obstructive sleep apnea syndrome by rapid maxillary expansion. Sleep 1998;21(8):831‐5. [DOI] [PubMed] [Google Scholar]
Clark 1996 {published data only}
- Clark GT, Blumfeld I, Yoffe N, Peled E, Lavie P. A crossover study comparing the efficacy of continuous positive airway pressure with anterior mandibular positioning devices on patients with obstructive sleep apnea. Chest 1996;109:1477‐83. [DOI] [PubMed] [Google Scholar]
David 2000 {published data only}
- David M, Bou Saba S, Liistro G, Rodenstein D, Rombaux P. Oral appliances in the treatment of sleep apnoea: a cephalometric and polysomnographic study [Les appareils orthodontiques au service de l'apnee du sommeil: une etude cephalomterique et polysomnographique]. Bulletin du Groupement International pour la Recherche Scientifique en Stomatologie & Odontologie 2000;42:73‐81. [PubMed] [Google Scholar]
Dort 2003 {published data only}
- Dort LC, Hussein J. Efficacy of a non‐customized tongue retaining device: a randomized, controlled cross‐over trial [Abstract]. Sleep 2003;26:A255. [Google Scholar]
Eckhart 1998 {published data only}
- Eckhart JE. Comparisons of oral devices for snoring. Journal of the California Dental Association 1998;26(8):611‐23. [PubMed] [Google Scholar]
Eroshina 2001 {published data only}
- Eroshina VA, Gasilin VS, Buzunov RV, Kalinkin AL. Efficiency of intraoral applicator UPLH‐01 in snore and obstructive sleep apnea [Otsenka effektivnosti primeneniia vnutrirotovogo applikatora UPLKH‐01 pri khrape i sindrome obstruktivnogo apnoe sna]. Klinicheskaia Meditsina 2001;79(4):44‐7. [PubMed] [Google Scholar]
Eveloff 1994 {published data only}
- Eveloff SE, Rosenberg CL, Carlisle CC, Millman RP. Efficacy of a Herbst mandibular advancement device in obstructive sleep apnea. American Journal of Respiratory & Critical Care Medicine 1994;149:905‐9. [DOI] [PubMed] [Google Scholar]
Friedman 2008 {published data only}
- Friedman M, Schalch P, Lin HC, Kakodkar KA, Joseph NJ, Mazloom N. Palatal implants for the treatment of snoring and obstructive sleep apnoea/hypopnea syndrome. Otolaryngology ‐ Head & Neck Surgery 2008;138(2):209‐16. [DOI] [PubMed] [Google Scholar]
Fritsch 2000 {published data only}
- Fritz K, Bloch KE. Noninvasive alternatives to CPAP in therapy of obstructive sleep apnea syndrome [Nichtoperative alternativen zum CPAP in der Therapie des obstruktiven schlafapnoe syndroms]. Therapeutische Umschau 2000;57(7):449‐53. [DOI] [PubMed] [Google Scholar]
Ichioka 1995 {published data only}
- Ichioka M, Hasegawa M, Nakagawa K, Kataoka N, Inase N, Tojo N, et al. Effects of a prosthetic dental device used to treat obstructive sleep apnea syndrome, and compliance of patients using the device. Japanese Journal of Thoracic Diseases 1995;33(11):1191‐7. [PubMed] [Google Scholar]
Kato 2000 {published data only}
- Kato J, Isono S, Tanaka A, Watanabe T, Araki D, Tanzawa H, et al. Dose‐dependent effects of mandibular advancement on pharyngeal mechanics and nocturnal oxygenation in patients with sleep‐disordered breathing. Chest 2000;117:1065‐72. [DOI] [PubMed] [Google Scholar]
Lamont 1998 {published data only}
- Lamont J, Baldwin DR, Hay KD, Veale AG. Effect of two types of mandibular advancement splints on snoring and obstructive sleep apnoea. European journal of orthodontics 1998;20(3):293‐7. [DOI] [PubMed] [Google Scholar]
Lawton 2005 {published data only}
- Lawton HM, Battagel JM, Kotecha B. A comparison of the Twin Block and Herbst mandibular advancement splints in the treatment of patients with obstructive sleep apnoea: a prospective study. European Journal of Orthodontics 2005;27(1):82‐90. [DOI] [PubMed] [Google Scholar]
Liu 2000 {published data only}
- Liu Y, Zeng X, Fu M, Huang X, Lowe A. Effects of mandibular repositioner on obstructive sleep apnoea. American Journal of Orthodontics and Dentofacial Orthopaedics 2000;118(3):248‐56. [DOI] [PubMed] [Google Scholar]
Lowe 2000 {published data only}
- Lowe AA, Sjoholm TT, Ryan CF, Fleetham JA, Ferguson KA, Remmers JE. Treatment, airway and compliance effects of a titratable oral appliance. Sleep 2000;23(Suppl 4):S172‐8. [PubMed] [Google Scholar]
Luks 1996 {unpublished data only}
- Luks V, Lorkovich P, Hans M, Nelson S. Comparing three dental devices for treatment of OSAS [Abstract]. Journal of Dental Research 1996;75(Special Issue 1):19. [Google Scholar]
Marklund 1998 {published data only}
- Marklund M, Franklin KA, Sahlin C, Lundgren R. The effect of a mandibular advancement device on apneas and sleep in patients with obstructive sleep apnea. Chest 1998;113(3):707‐13. [DOI] [PubMed] [Google Scholar]
Menn 1996 {published data only}
- Menn SJ, Loube DI, Morgan TD, Mitler MM, Berger JS, Erman MK. The mandibular repositioning device: role in the treatment of obstructive sleep apnea. Sleep 1996;19(10):794‐800. [DOI] [PubMed] [Google Scholar]
Moore 2000 {published data only}
- Moore RW, Hart WT. OPAP‐ a new approach to the management of obstructive sleep apnea. The functional orthodontist 2000;17(1):29‐30. [PubMed] [Google Scholar]
Neill 2002 {published data only}
- Neill A, Whyman R, Bannan S, Jeffrey O, Campbell A. Mandibular advancement splint improves indices of obstructive sleep apnoea and snoring but side‐effects are common. New Zealand Medical Journal 2002;115(1156):289‐92. [PubMed] [Google Scholar]
O'Sullivan 1993 {published data only}
- O'Sullivan RA, Hillman DR, Mateljan R, Pantin C, Finucane KE. Mandibular advancement splint: the effects on snoring and obstructive sleep apnea. Sleep 1993;16:S143. [DOI] [PubMed] [Google Scholar]
O'Sullivan 1995 {published data only}
- O'Sulliva RA, Hillman DR, Mateljan R, Pantin C, Finucane KE. Mandibular advancement splint: an appliance to treat snoring and obstructive sleep apnea. American Journal of Respiratory & Critical Care Medicine 1995;151:194‐8. [DOI] [PubMed] [Google Scholar]
Ono 1996 {published data only}
- Ono T, Lowe AA, Ferguson KA, Pae EK, Fleetham J. The effect of the tongue retaining device on awake genioglossus muscle activity in patients with obstructive sleep apnea. American Journal of Orthodontics and Dentofacial Orthopedics 1996;110:28‐35. [DOI] [PubMed] [Google Scholar]
Pillar 2004 {published data only}
- Pillar G. Substantial improvement in obstructive sleep apnea using two oral appliances [Abstract]. AAO‐HNSF Annual Meeting & OTO EXPO, New York, Spetember 19‐22. 2004.
- Spiegel E, Pillar G. Substantial improvement in obstructive sleep apnea using two different oral appliances assessed by the Watch‐PAT ambulatory device. Sleep 2004;27(Suppl):A178. [Google Scholar]
Pirila‐Parkkinen '99 {published data only}
- Pirila‐Parkkinen K, Pirttiniemi P, Nieminen P, Lopponen H, Tolonen U, Uotila R, et al. Cervical headgear therapy as a factor in obstructive sleep apnea syndrome. Pediatric Dentistry 1999;21(1):39‐45. [PubMed] [Google Scholar]
Pitsis 2002 {published data only}
- Pitsis AJ, Darendeliler MA, Gotsopoulos H, Petocz P, Cistulli PA. Effect of vertical dimension on efficacy of oral appliance therapy in obstructive sleep apnea. American Journal of Respiratory & Critical Care Medicine 2002;166(6):860‐4. [DOI] [PubMed] [Google Scholar]
Rose 2002 {published data only}
- Rose E, Staats R, Virchow C, Jonas IE. A comparative study of two mandibular advancement appliances for the treatment of obstructive sleep apnoea. European Journal of Orthodontics 2002;24:191‐8. [DOI] [PubMed] [Google Scholar]
Rose 2002b {published data only}
- Rose EC, Barthlen GM, Staats R, Jonas IE. Therapeutic efficacy of an oral appliance in the treatment of obstructive sleep apnea: a 2‐year follow‐up. American Journal of Orthodontics & Dentofacial Orthopedics 2002;121(3):273‐9. [DOI] [PubMed] [Google Scholar]
Saletu 2007 {published data only}
- Saletu A, Anderer P, Parapatics S, Matthai C, Matejka M, Saletu B. Effects of a mandibular repositioning appliance on sleep structure, morning behavior and clinical symptomatology in patients with snoring and sleep‐disordered breathing. Neuropsychobiology 2007;55(3‐4):184‐93. [DOI] [PubMed] [Google Scholar]
Schonhofer 1997 {published data only}
- Schonhofer B, Wenzel M, Barchfeld T, Siemon K, Rager H, Kohler D. Value of intra‐ and extraoral devices in the treatment of obstructive sleep apnoea and snoring [Wertigkeit verscheidener intra‐ und extraoraler Therapieverfahren fur die behandlung der obstruktiven schalafapnoe und des schnarchens]. Medizinische Klinik 1997;92(3):167‐74. [DOI] [PubMed] [Google Scholar]
Schonhofer 1997b {published data only}
- Schonhofer B, Rager H, Wenzel G, Kohler D. Does SnorEx(TM) act as ApnoeaEx? A study of a new intraoral prothesis as a form of treatment of the obstructive sleep apnoea syndrome [Ist SnorEx auch ApnoeEx? Eine studie zu einer neuen intraoralen prothese als therapieform des obstruktiven schlafapnoesyndroms]. Pneumologie 1997;51(Suppl 3):804‐8. [PubMed] [Google Scholar]
Sjoholm 1994 {published data only}
- Sjoholm TT, Polo OJ, Rauhala ER, Vuoriluoto J, Helenius HYM. Mandibular advancement with dental appliances in obstructive sleep apnoea. Journal of oral rehabilitation 1994;21:595‐603. [DOI] [PubMed] [Google Scholar]
Skinner 2004 {published data only}
- Skinner M, Kingshott R, Jones D, Taylor D. Efficacy of a cervico‐mandibular support collar in the management of obstructive sleep apnoea. The New Zealand Medical Journal 2003;116(1186):A5‐A6. [Google Scholar]
- Skinner MA, Kingshott RN, Jones DR, Taylor DR. Lack of efficacy for a cervicomandibular support collar in the management of obstructive sleep apnea. Chest 2004;125(1):118‐26. [DOI] [PubMed] [Google Scholar]
Turk 2005 {published data only}
- Turk AJ, Iseli A, Russi EW, Bloch KE. Long term treatment of obstructive sleep apnea with mandibular advancement devices. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005.
van der Sweep 2006 {published data only}
- Zweep V, Copland J, Romios H, Jayamaha J, Ho M. Randomized crossover study to assess the use of chin support straps for mouth leaks in. [obstructive sleep apnoea] OSA patients. Internal Medicine Journal 2006;36:A32. [Google Scholar]
van der Veken 2005 {published data only}
- vanderVeken OM, Marklund M, Braem MJ, Devolder A, Boudewyns AN, Verbraecken JA. Comparison of a custom made and a prefabricated mandibular advancement device for treatment of sleep disordered breathing, a prospective randomized cross over trial. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:C29, Poster 522.
Villa 2002 {published data only}
- Villa MP, Bernkopf E, Pagani J, Broia V, Montesano M, Ronchetti R. Randomised controlled study of an oral jaw‐positioning appliance for the treatment of obstructive sleep apnea in children with malocclusion. American Journal of Respiratory & Critical Care Medicine 2002;165:123‐7. [DOI] [PubMed] [Google Scholar]
Walker‐Engstrom 2003 {published data only}
- Tegelberg A, Walker‐Engstrom ML, Vestling O, Wilhelmsson B. Two different degrees of mandibular advancement with a dental appliance in treatment of patients with mild to moderate obstructive sleep apnea. Acta Odontologica Scandinavica 2003;61(6):356‐62. [DOI] [PubMed] [Google Scholar]
- Vestling O, Walker Engstrom ML, Wilhelmsson B, Tegelberg A. Treatment of obstructive sleep apnea (OSA) with two different degrees of mandibular advancement of dental appliance ‐ a prospective and randomised study [Abstract]. Swedish Dental Journal 2001;25(4):180. [Google Scholar]
- Walker‐Engstrom ML, Ringqvist I, Vestling O, Wilhelmsson B, Tegelberg A. Prospective randomized study comparing two different degrees of mandibular advancement with a dental appliance in treatment of severe obstructive sleep apnea. Sleep & Breathing 2003;7(3):119‐30. [DOI] [PubMed] [Google Scholar]
Winkels 1997 {published data only}
- Winkels W, Klutmann M, Bias R, Wessolowski T, Simon H. Effectivity of an anto‐snoring prothesis in obstructive sleep apnoea syndrome [Wirsamkeit einer antischarchprothese beim obstruktiven schalafapnoesyndrom]. Pneumologie 1997;51:802‐3. [PubMed] [Google Scholar]
Yoshida 1998 {published data only}
- Yoshida K. Effect of a prosthetic appliance for treatment of sleep apnea syndrome on masticatory and tongue muscle activity. Journal of Prosthetic Dentistry 1998;79:537‐44. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Ninomiya 2004 {published data only}
- Ninomiya K. The follow up study of oral appliance therapy for obstructive sleep apnoea syndrome: the patients keep the device for more than a year. Therapeutic Research 2004;25(10):2081‐3. [Google Scholar]
Sakakibara 2005 {published data only}
- Sakakibara H. Treatment of sleep apnoea syndrome with oral appliances. Respiration & Circulation 2005;53(3):301‐8. [Google Scholar]
References to ongoing studies
Cibele 2006 {published data only}
- Cibele D‐F, Garbuio SA, D'Almeida V, Santos RF, Bittencourt LRS, Tufik S. Efficacy of an oral appliance (OA) compared to nCPAP over oxidative stress parameters in obstructive sleep apnoea (OSA) patients ‐ preliminary results. Sleep Medicine 2006;7:93s [P412]. [Google Scholar]
Fairbairn 2004 {published data only}
- Fairbairn S. Steering simulation in severe obstructive sleep apnoea, and the effect of therapeutic intervention in the form of mandibular advancement splint, and continuous positive airways pressure (CPAP). National Research Register 2004.
Additional references
AASM 1999
- American Academy of Sleep Medicine Task Force Sleep Related Breathing Disorders in Adults. Recommendations for syndrome definition and measurement techniques in clinical research. Sleep 1999;5:667‐9. [PubMed] [Google Scholar]
ASDA 1995
- American Sleep Disorders Association. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances. Sleep 1995;18(6):511‐3. [DOI] [PubMed] [Google Scholar]
Clark 2000
- Clrk GT, Sohn JW, Hong CN. Treating obstructive sleep apnea and snoring : assessment of an anterior mandibular positioning device. Journal of American Dental Association 2000;131(6):765‐71. [DOI] [PubMed] [Google Scholar]
Giles 2006
- Giles TL, Lasserson TJ, Smith BJ, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea (Cochrane review). Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001106.pub3] [DOI] [PubMed] [Google Scholar]
Handbook 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2008. [Google Scholar]
Haniffa 2004
- Haniffa M, Lasserson TJ, Smith I. Interventions to improve compliance with continuous positive airway pressure for obstructive sleep apnoea (Cochrane Review). Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD003531.pub2] [DOI] [PubMed] [Google Scholar]
Hudgel 1992
- Hudgel DW. The role of upper airway anatomy and physiology in obstructive sleep apnea. Clinics in Chest Medicine 1992;13(3):383‐98. [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomised controlled trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Jenkinson 1999
- Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and sub‐therapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet 1999;353(9170):2100‐5. [DOI] [PubMed] [Google Scholar]
Mooe 2001
- Mooe T, Franklin KA, Holmstrom K, Rabben T, Wiklund U. Sleep‐disordered breathing and coronary artery disease: long‐term prognosis. American Journal of Respiratory & Critcal Care Medicine 2001;164(10):1910‐3. [DOI] [PubMed] [Google Scholar]
Patel 2003
- Patel SR, White D, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnoea: results of a meta‐analysis. Archives of Internal Medicine 2003;163(5):565‐71. [DOI] [PubMed] [Google Scholar]
RCP 1993
- Royal College of Physicians. Sleep apnoea and related conditions: Summary of a report of a working party of the Royal College of Physicians. Journal of the Royal College of Physicians of London 1993;27(4):363‐4. [PMC free article] [PubMed] [Google Scholar]
Schmidt‐Nowara 1995
- Schmidt‐Nowara W, Lowe A, Wiegand L, Cartwright R, Perez‐Guerra F, Menn S. Oral appliances for the treatment of snoring and obstructive sleep apnea : a review. Sleep 1995;18(6):501‐10. [DOI] [PubMed] [Google Scholar]
Shahar 2001
- Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, et al. Sleep‐disordered breathing and cardiovascular disease: cross‐sectional results of the Sleep Heart Health Study. American Journal of Respiratory & Critical Care Medicine 2001;163(1):19‐25. [DOI] [PubMed] [Google Scholar]
Shneerson 2001
- Shneerson J, Wright J. Lifestyle modification for obstructive sleep apnoea (Cochrane Review). Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD002875] [DOI] [PMC free article] [PubMed] [Google Scholar]
SIGN 2003
- Scottish Intercollegiate Guideline Network. Management of obstructive sleep apnoea/hypopnoea syndrome in adults (guideline no. 73). www.sign.ac.uk.
Smith 2006
- Smith I, Lasserson TJ, Wright J. Drug treatments for obstructive sleep apnoea (Cochrane Review). Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003002.pub2] [DOI] [PubMed] [Google Scholar]
Sundaram 2005
- Sundaram S, Lim J, Lasserson TJ. Surgery for obstructive sleep apnoea (Cochrane Review). Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD001004.pub2] [DOI] [PubMed] [Google Scholar]
Wild 2004
- Wild MR, Engleman HM, Douglas NJ, Espie CA. Can psychological factors help us to determine adherence to CPAP? A prospective study. European Respiratory Journal 2004;24(3):461‐5. [DOI] [PubMed] [Google Scholar]
Wright 1997
- Wright J, Johns R, Watt I, Melville A, Sheldon T. Health effects of obstructive sleep apnoea and the effectiveness of continuous positive airways pressure: a systematic review of the research evidence. BMJ 1997;314(7084):851‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 1993
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep‐disordered breathing among middle‐aged adults. New England Journal of Medicine 1993;328:1230‐5. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lim 2004
- Lim J, Lasserson TJ, Fleetham J, Wright J. Oral appliances for obstructive sleep apnoea. Cochrane Database of Systematic Reviews 2004, Issue 4. [Art. No.: CD004435] [DOI] [PubMed] [Google Scholar]


