Abstract
Background
Each year, more than four million abdominal surgeries are performed in the US and over 250,000 in England. Acute respiratory failure, a common complication that can affect 30% to 50% of people after upper abdominal surgery, can lead to significant morbidity and mortality. Noninvasive ventilation has been associated with lower rates of tracheal intubation in adults with acute respiratory failure, thus reducing the incidence of complications and mortality. This review compared the effectiveness and safety of noninvasive positive pressure ventilation (NPPV) versus standard oxygen therapy in the treatment of acute respiratory failure after upper abdominal surgery.
Objectives
To assess the effectiveness and safety of noninvasive positive pressure ventilation (NPPV), that is, continuous positive airway pressure (CPAP) or bilevel NPPV, in reducing mortality and the rate of tracheal intubation in adults with acute respiratory failure after upper abdominal surgery, compared to standard therapy (oxygen therapy), and to assess changes in arterial blood gas levels, hospital and intensive care unit (ICU) length of stay, gastric insufflation, and anastomotic leakage.
Search methods
The date of the last search was 12 May 2015. We searched the following databases: the Cochrane Handbook for Systematic Reviews of Interventions (CENTRAL) (2015, Issue 5), MEDLINE (Ovid SP, 1966 to May 2015), EMBASE (Ovid SP, 1974 to May 2015); the physiotherapy evidence database (PEDro) (1999 to May 2015); the Cumulative Index to Nursing and Allied Health Literature (CINAHL, EBSCOhost, 1982 to May 2015), and LILACS (BIREME, 1986 to May 2015). We reviewed reference lists of included studies and contacted experts. We also searched grey literature sources. We checked databases of ongoing trials such as www.controlled‐trials.com/ and www.trialscentral.org/. We did not apply language restrictions.
Selection criteria
We selected randomized or quasi‐randomized controlled trials involving adults with acute respiratory failure after upper abdominal surgery who were treated with CPAP or bilevel NPPV with, or without, drug therapy as standard medical care, compared to adults treated with oxygen therapy with, or without, standard medical care.
Data collection and analysis
Two authors independently selected and abstracted data from eligible studies using a standardized form. We evaluated study quality by assessing allocation concealment; random sequence generation; incomplete outcome data; blinding of participants, personnel, and outcome assessors; selective reporting; and adherence to the intention‐to‐treat (ITT) principle.
Main results
We included two trials involving 269 participants. The participants were mostly men (67%); the mean age was 65 years. The trials were conducted in China and Italy (one was a multicentre trial). Both trials included adults with acute respiratory failure after upper abdominal surgery. We judged both trials at high risk of bias. Compared to oxygen therapy, CPAP or bilevel NPPV may reduce the rate of tracheal intubation (risk ratio (RR) 0.25; 95% confidence interval (CI) 0.08 to 0.83; low quality evidence) with a number needed to treat for an additional beneficial outcome of 11. There was very low quality evidence that the intervention may also reduce ICU length of stay (mean difference (MD) ‐1.84 days; 95% CI ‐3.53 to ‐0.15). We found no differences for mortality (low quality evidence) and hospital length of stay. There was insufficient evidence to be certain that CPAP or NPPV had an effect on anastomotic leakage, pneumonia‐related complications, and sepsis or infections. Findings from one trial of 60 participants suggested that bilevel NPPV, compared to oxygen therapy, may improve blood gas levels and blood pH one hour after the intervention (partial pressure of arterial oxygen (PaO2): MD 22.5 mm Hg; 95% CI 17.19 to 27.81; pH: MD 0.06; 95% CI 0.01 to 0.11; partial pressure of arterial carbon dioxide (PCO2) levels (MD ‐9.8 mm Hg; 95% CI ‐14.07 to ‐5.53). The trials included in this systematic review did not present data on the following outcomes that we intended to assess: gastric insufflation, fistulae, pneumothorax, bleeding, skin breakdown, eye irritation, sinus congestion, oronasal drying, and patient‐ventilator asynchrony.
Authors' conclusions
The findings of this review indicate that CPAP or bilevel NPPV is an effective and safe intervention for the treatment of adults with acute respiratory failure after upper abdominal surgery. However, based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology, the quality of the evidence was low or very low. More good quality studies are needed to confirm these findings.
Plain language summary
Noninvasive positive pressure ventilation for acute respiratory failure following upper abdominal surgery
Review question
The goal of this review was to evaluate the effectiveness and safety of noninvasive positive pressure ventilation (NPPV) (continuous positive airway pressure (CPAP) or bilevel NPPV) compared with oxygen therapy alone in adults with acute respiratory failure after upper abdominal surgery.
Background
Acute respiratory failure , where fluid builds up in the lungs stopping oxygen from crossing into the blood, is a relatively common complication after abdominal surgery and can increase the risk of dying (mortality).
NPPV is a type of treatment that helps to improve breathing without having to insert a tube inside a person's windpipe (tracheal intubation). This intervention is effective in several illnesses.
Study characteristics
We searched scientific databases for clinical trials looking at the treatment of adults with acute respiratory failure following abdominal surgery. The trials compared NPPV with usual care(oxygen therapy through a face mask). We included two trials involving 269 participants.The participants were mostly men (67%) and on average 65 years of age. One trial was conducted in several intensive care units (ICU). Both trials included adults with acute respiratory failure after upper abdominal surgery. The evidence is current to May 2015.
Key results
This review examined mortality, rate of tracheal intubation, length of stay in the ICU, length of hospital stay, complications after NPPV, and changes in the levels of gases within the blood (arterial blood gases).
Compared with oxygen therapy, NPPV decreased the rate of tracheal intubation. Out of every 1000 adults who developed acute respiratory failure after upper abdominal surgery, 181 adults treated with oxygen therapy would need to be intubated compared with 54 adults treated with NPPV.
When compared to oxygen therapy, NPPV tended to reduce mortality. However, since the number of participants included in the two trials was small, more studies are needed.
The use of NPPV also reduced the length of stay in the ICU by almost two days when compared to oxygen therapy. However, the mean length of stay in the hospital was similar in the two groups.
When compared to oxygen therapy, NPPV improved blood gas levels one hour after the intervention.
There was insufficient evidence to be certain that CPAP or NPPV had an effect on anastomotic (e.g. where two pieces of intestine are joined together) leakage, pneumonia related complications and sepsis (blood poisoning) or infections. However, adults treated with NPPV had lower rates these complications than adults treated with oxygen.
Quality of the evidence
There was low quality evidence for hospital mortality, low quality of evidence for rate of tracheal intubation, and very low quality of evidence for ICU length of stay.
Authors' conclusions
The findings of this review showed that NPPV is an effective and safe treatment for adults with acute respiratory failure after upper abdominal surgery. However, due to the low quality of the evidence, more good quality studies are needed to confirm these findings.
Summary of findings
Summary of findings for the main comparison. Noninvasive positive pressure ventilation (NPPV) compared to oxygen therapy for acute respiratory failure after upper abdominal surgery.
| Noninvasive positive pressure ventilation (NPPV) compared to oxygen therapy for acute respiratory failure after upper abdominal surgery | ||||||
|
Patient or population: adults (aged > 17 years) with acute respiratory failure after upper abdominal surgery Settings: hospital intensive care units in China and Italy Intervention: NPPV Comparison: oxygen therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oxygen therapy | NPPV | |||||
| Hospital mortality | Study population | RR 0.14 (0.01 to 2.71) | 209 (1 study) | ⊕⊝⊝ low1,2 | ‐ | |
| 29 per 1000 | 4 per 1000 (0 to 78) | |||||
| Moderate | ||||||
| ‐ | ‐ | |||||
| Rate of tracheal Intubation | Study population |
RR 0.25 (0.08 to 0.83) |
269 (2 studies) | ⊕⊕⊝⊝ low3,4 | Risks were calculated from pooled RR | |
| 134 per 1000 | 34 per 1000 (11 to 111) | |||||
| Moderate | ||||||
| 181 per 1000 | 45 per 1000 (14 to 150) | |||||
| ICU length of stay | The mean ICU length of stay in the control group was 4.8 days | The mean ICU length of stay in the intervention group was 2.7 days | MD ‐1.84 (‐3.53 to ‐0.15) | 269 (2 studies) | ⊕⊝⊝⊝ very low3,4,5 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CPAP: continuous positive airway pressure; ICU: intensive care unit; MD: mean difference; NPPV: noninvasive positive pressure ventilation; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Quality of evidence downgraded by one level due to serious risk of bias (study personnel were not blinded, and the study did not provide data on the allocation process). 2 Quality of evidence downgraded by one level due to serious imprecision (the sample size (209 participants) was lower than the calculated optimal (609 participants)).
3 Quality of evidence downgraded by one level due to serious risk of bias (one of the studies did not describe how randomization was done and neither provided data on the allocation process).
4 Quality of evidence downgraded by one level due to serious imprecision (although the total (cumulative) sample size is greater than the calculated optimal information size, only a very small number of participants and events were included; total sample size was fewer than 400).
5 Quality of evidence downgraded by one level due to serious inconsistency (the studies used different types of intervention (one used CPAP and other used bilevel NPPV) and for different durations of time).
Background
Description of the condition
More than four million abdominal surgeries are performed in the US every year and in England about 250,000 abdominal operations are performed per year (Brown 2005; Pasquina 2006). Adults undergoing upper abdominal surgery are at an increased risk of postoperative pulmonary complications (Pasquina 2006). Acute respiratory failure is a relatively common complication after abdominal surgery and is associated with significant morbidity and mortality (Conti 2007; Jaber 2005; Lawrence 1996). According to Michelet 2010, the development of respiratory complication may be explained by two pathological mechanisms. The first is linked to surgical complications, notably with the occurrence of anastomotic leakage leading to mediastinitis, septic shock, and acute respiratory distress. The second is of medical origin, with multifactorial impairment of respiratory function. Medical causes of respiratory complications can involve muscle dysfunction, alteration of pulmonary mechanics, and development of pulmonary atelectasis, leading to postoperative hypoxaemia and inducing the further development of complications such as pneumonia or acute respiratory failure.
Jaber et al, describe acute respiratory failure as severe respiratory distress with dyspnoea, a respiratory rate greater than 25 breaths/minute, contraction of accessory inspiratory muscles, paradoxical abdominal motion, peripheral oxygen saturation less than 92% while breathing at least 10 L/minute of oxygen, or partial pressure of arterial oxygen (PaO2) less than 60 mm Hg on room air or less than 80 mm Hg while breathing supplemental oxygen (Jaber 2005). Acute respiratory failure complicates the recovery of 30% to 50% of adults after upper abdominal surgery. Abdominal surgery can disturb respiratory function by causing alterations in diaphragmatic function and contractility that lead to a reduction in pulmonary volumes and airflow; impairment of pulmonary secretion clearance; and pain. Affected people are predisposed to lower‐lobe atelectasis, pneumonia, and other respiratory complications (Conti 2007; Hedenstierna 1990; Jaber 2005; Jansen 1990; Siafakas 1999; Squadrone 2005).
Most often, the management of acute respiratory failure after abdominal surgery in adults admitted to the intensive care unit (ICU) requires endotracheal intubation and mechanical ventilation (8% to 10% of cases) (Arozullah 2000; Conti 2007; Jaber 2005; Lång 2001; O'Donohue 1985; Thompson 2003). Conventional mechanical ventilation with endotracheal intubation has been associated with early and late complications of increased morbidity (such as tracheal injury, major functional losses due to restriction in bed, pneumonia associated with the use of invasive mechanical ventilation, among others) and mortality, prolonged ICU stay, prolonged hospital stay, and additional healthcare costs (Arozullah 2000; Chatila 2002; Conti 2007; Heyland 1999; Pasquina 2006; Pingleton 1988). Noninvasive positive pressure ventilation (NPPV) has been associated with lower rate of tracheal intubation in adults with acute respiratory failure, reducing the incidence of complications and mortality associated with invasive ventilation (Jaber 2005; Vital 2013).
Description of the intervention
NPPV includes forms of ventilatory support applied without the use of a tracheal tube. It includes continuous positive airway pressure (CPAP) with or without inspiratory pressure support (bilevel NPPV or BiPAP®, Respironics Inc, Murrysville, PA). CPAP is a breathing mode by which the person spontaneously breathes through a pressurized circuit against a threshold resistor that maintains positive airway pressure during both inspiration and expiration (Squadrone 2005). Bilevel NPPV combines inspiratory positive airway pressure with positive end‐expiratory pressure (PEEP). Consequently, bilevel NPPV differs from CPAP in providing inspiratory assistance to rest the muscles of respiration (Mehta 1997).
NPPV has been associated with lower rates of tracheal intubation in adults with acute respiratory failure, reducing the incidence of complications and mortality (Jaber 2005; Vital 2013). It improves gas exchange, minimizes atelectasis formation, and increases functional residual capacity and tidal volume (VT) (Kramer 1995; Nava 1993; Rusca 2003; Stock 1985; Vitacca 2001). NPPV also improves vital signs and sense of dyspnoea (Kramer 1995), and reduces respiratory rate (Vitacca 2001), need for sedation (Rathgeber 1997), length of stay in the ICU, and incidence of pneumonia (Nourdine 1999; Squadrone 2005).
Oesophageal and gastric surgery are usually considered contraindications to NPPV use (Jaber 2005). This is because one of the potential complications of NPPV is abdominal distention due to air forced into the stomach under positive pressure. Therefore, disruption of the surgical anastomosis can occur because of this abdominal distention (Penuelas 2007). NPPV can be used without adverse effects in adults after oesophageal surgery when the insufflation pressure level is less than 12 cm H2O (Brochard 2000; Jaber 2005; Joris 1997).
How the intervention might work
NPPV, unlike conventional invasive ventilation, is achieved with an oronasal or nasal airway, or a total face mask or helmet, connected to a ventilator and does not require an artificial airway (Burns 2010). By improving alveolar ventilation, NPPV may allow sufficient oxygenation without raising the partial pressure of arterial carbon dioxide (PaCO2). NPPV can be delivered noninvasively and assists each spontaneous breath. It is able to reduce the pressure‐time index of the respiratory muscles. This reduction in muscle effort is accompanied by an increase in VT and a reduction in breathing frequency; it ameliorates dyspnoea, improves gas exchange, and allows the person to partially rest his or her muscles of respiration (Jaber 2005; Kramer 1995; Nava 1993; Vitacca 2001).
Several studies have shown that NPPV may be associated with a reduction in complications, length of hospital stay, and mortality after upper abdominal surgery (Anand 2010; Nourdine 1999; Squadrone 2005).
Oxygen therapy is the administration of supplemental oxygen in order to raise or maintain oxygen saturation above 90%, correcting damage from hypoxaemia. However, when used in high concentrations for prolonged periods, oxygen can cause toxicity and atelectasis.
NPPV is less invasive than endotracheal intubation, but its use has risks and requires careful monitoring of vital parameters and possible adverse events such as mask or anastomotic leakage or abdominal distention and should always be applied by qualified professionals. Due to the risk of failure of NPPV, healthcare professionals should always carefully monitor the patient's vital signs and recognize its failure, as early as possible, in order to avoid delays in endotracheal intubation, increasing the risk of mortality in these patients (Holanda 2001; Stoltzfus 2006).
Why it is important to do this review
Acute respiratory failure is a relatively common complication after abdominal surgery (Conti 2007; Jaber 2005; Lawrence 1996). Noninvasive ventilation (NIV) has been associated with lower rates of tracheal intubation in adults with acute respiratory failure, reducing the incidence of complications and mortality (Jaber 2005).
This review compared NPPV with standard oxygen therapy in the treatment of acute respiratory failure after upper abdominal surgery.
This review is important to determine whether NPPV is effective and safe in adults with acute respiratory failure after upper abdominal surgery.
Objectives
To assess the effectiveness and safety of noninvasive positive pressure ventilation (NPPV), that is continuous positive airway pressure (CPAP) or bilevel NPPV, in reducing mortality and the rate of tracheal intubation in adults with acute respiratory failure after upper abdominal surgery, compared to standard therapy (oxygen therapy), and to assess changes in arterial blood gas levels, hospital and ICU length of stay, gastric insufflation, and anastomotic leakage.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) or quasi‐randomized controlled trials (QRCT). QRCT are those studies in which the method of allocating participants to different forms of care that is not truly random, for example, allocation by date of birth, day of the week, using odd‐even numbers, or according to the participant's social security number or medical record number.
Types of participants
We included adults, aged over 17 years, with acute respiratory failure after upper abdominal surgery of gastrointestinal origin.
We excluded studies involving other surgical approaches (e.g. abdominal aortic aneurysm surgery, bariatric surgery).
Types of interventions
Intervention
We included NPPV (CPAP or bilevel NPPV) associated with or without drug therapy as standard medical care.
Control
We included oxygen therapy with or without standard medical care. We defined standard medical care as drug therapy (e.g. bronchodilators, analgesics).
Types of outcome measures
Primary outcomes
Hospital mortality (measured starting at the date of randomization up to the time of discharge from hospital).
Rate of tracheal intubation, (measured in number of events occurred since the date of randomization up to the time of discharge from hospital).
Secondary outcomes
ICU length of stay, measured in days.
Hospital length of stay, measured in days.
Complications after NPPV included gastric insufflation, fistulae, anastomotic leakage, pneumothorax, bleeding, pneumonia, hospital infection, important leaks, mask discomfort, skin breakdown, eye irritation, sinus congestion, oronasal drying, and patient‐ventilator asynchrony.
Changes in arterial blood gas levels.
Search methods for identification of studies
Electronic searches
We searched the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 5, see Appendix 1 for search strategy), MEDLINE (OvidSP, 1966 to May 2015, see Appendix 2), EMBASE (OvidSP, 1974 to May 2015, see Appendix 3), the physiotherapy evidence database (PEDro) (1999 to May 2015), Cumulative Index to Nursing and Allied Health Literature (CINAHL, EBSCOhost, 1982 to May 2015, see Appendix 4), and LILACS (BIREME, 1986 to May 2015, see Appendix 5).
We imposed no language restrictions.
We combined our search strategy for MEDLINE with the Cochrane highly sensitive filter for identifying RCTs as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We adapted the search strategy for MEDLINE and the RCT filter according to the standards of each database.
We used terms of intervention and clinical condition without filters for RCT for CENTRAL, PEDro, and CINAHL.
Searching other resources
We searched the bibliography of all included studies. We checked the databases of ongoing trials such as www.controlled‐trials.com/ and www.trialscentral.org/. We did not search the grey literature as proposed in the protocol of this review (Faria 2011; see Differences between protocol and review).
In future updates of this review, we will also search the following clinical trial databases: ClinicalTrials.gov (www.clinicaltrials.gov/) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/).
Data collection and analysis
Selection of studies
We followed the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Two authors (DF, FV) analysed all relevant articles identified by the search strategy. In the case of disagreement, we consulted a third author (ES) to resolve the issue. If consensus could not be reached, we contacted the author of the trial for additional information.
Data extraction and management
Two authors (DF, FV) independently extracted all data. We contacted the authors of primary trials when there were any doubts regarding missing data or methodological details of a trial. We used a standard form to extract the following data:
characteristics of the study (design, allocation concealment, blinding, method of randomization, and withdrawals or drop‐outs);
participants (age, sex);
intervention (type of intervention, duration of therapy);
outcomes (types of outcome measures, timing, complications after NPPV).
Appendix 6 shows an example of the form.
One author (DF) entered the data into Review Manager 5 (RevMan 2014), and a second author (FV) identified and resolved any discrepancies in the data entry.
Assessment of risk of bias in included studies
To evaluate the methodological quality of selected studies, we independently assessed the methods section of the reports considering the following items that were associated with risk of bias:
random sequence generation;
allocation concealment;
incomplete outcome data;
blinding of participants and personnel;
blinding of outcome assessment;
selective reporting;
other sources of bias.
We classified each trial into the following categories: 'low risk', 'high risk', or 'unclear risk' by following the criteria set out in the Cochrane Handbook for Systematic Reviews of Interventions in order to estimate selection bias, reporting bias, attrition bias, and detection bias (Higgins 2011).
We analysed the risk of bias for outcome following the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology (GRADEpro 2011).
We generated 'Risk of bias' tables.
Measures of treatment effect
We analysed data from all trials using Review Manager 5 (RevMan 2014), or performing descriptive analyses.
We summarized continuous variables using the mean difference (MD) with 95% confidence interval (95% CI). We summarized dichotomous variables using risk ratios (RR) with 95% CIs. We calculated the number needed to treat for an additional beneficial outcome (NNTB) and risk difference (RD) for statistically significant results in order to clarify the magnitude of the intervention's clinical benefit.
Unit of analysis issues
We did not find cluster‐randomized trials or cross‐over trials.
Dealing with missing data
We used ITT analysis only for dichotomous data and according to the information available from the primary studies (Higgins 2011).
We followed the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions in dealing with a lack of data (Higgins 2011).
Whenever possible, we contacted the original investigators to request missing data.
We made explicit the assumptions of any methods used to cope with missing data.
We commented on the potential impact of missing data on the findings of the review, in the Discussion section of the review.
Assessment of heterogeneity
Clinical heterogeneity was variability in the participants, interventions, and outcomes studied, and methodological heterogeneity was variability in study design and risk of bias. We considered meta‐analysis when the studies were sufficiently homogeneous in terms of participants, interventions, and outcomes to provide a meaningful summary.
We assessed the impact of heterogeneity using the I2 statistic (Higgins 2002). This test illustrates the percentage of variability in effect estimates resulting from heterogeneity, as opposed to sampling error. We used the following limits for the interpretation of the I2 statistic:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
In the presence of the significant heterogeneity (greater than 50%) and a sufficient number of studies reporting the outcome of interest, we wanted to perform exploratory analyses to investigate potential sources of heterogeneity (e.g. participants, treatments, and study quality). We hypothesized that age, gender, and co‐morbidities may represent potential sources of heterogeneity among participants. Treatment heterogeneity may be related to the type of intervention, the levels of pressure applied with NPPV, or treatment duration. We considered P values < 0.05 to be statistically significant.
Assessment of reporting biases
We planned to assess publication bias using funnel plots and Egger's regression model (regressing the magnitude of the point estimate versus precision) if the number of included studies was more than 10 to avoid biases and small study effects (Egger 1997). However, we only included two studies in this review.
Data synthesis
In the presence of two or more randomized trials with comparable populations, we implemented a meta‐analysis using Review Manager 5 (RevMan 2014). We pooled data via a random‐effects model using an inverse variance weighting for both dichotomous (RR) and continuous (MD) data. We calculated summary point estimates with their 95% CIs for both RR and MD. Statistical significance for the summary statistics was declared if the z statistic had a P value < 0.05 and the summary statistic 95% CI did not cross the line of identity (RR = 1, MD = 0). For rare events, we estimated pooled effects using the Peto odds ratio (Higgins 2011). We used the GRADE approach to interpret findings. We constructed Table 1 using the GRADE principles. We used GRADEpro (GRADEpro 2011) to import data from Review Manager 5 to generate Table 1. This table provided outcome‐specific information concerning the overall quality of evidence from studies included the magnitude of effect of interventions examined, and the sum of available data on the outcomes that we considered.
For assessments of the overall quality of evidence or each outcome that included pooled data from RCTs, we downgraded the evidence from 'high quality' by one level for serious (or by two for very serious) study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias.
We included the following outcomes in Table 1:
hospital mortality;
rate of tracheal intubation;
ICU length of stay.
We rated quality separately for each outcome. The GRADE specifies four levels of quality:
high quality: further research is very unlikely to change our confidence in the estimate of effect;
moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate;
low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate;
very low quality: we are very uncertain about the estimate.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analysis but due to the small number of included trials (two) and the lack of some data from these, we could not perform subgroup analyses. If we add further trials in future updates of this review, we will use subgroup analysis to explore possible sources of heterogeneity. We will base this on the following:
age (In the event of a significant difference in the ages of the participants. For example, very old versus very young participants);
gender;
baseline disease (chronic obstructive pulmonary disease (COPD), obesity);
duration of intervention;
types of intervention (CPAP or bilevel NPPV);
type of surgery.
We assessed adverse effects with descriptive techniques in the Discussion section.
Sensitivity analysis
We planned to perform a sensitivity analysis excluding studies with high risk of bias. In future updates, if we include a larger number of trials, we will perform a sensitivity analysis excluding those trials with high risk of bias.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The electronic searches identified 1751 citations (264 in CENTRAL, 297 in MEDLINE, 789 in EMBASE, 10 in PEDro, 303 in CINAHL, and 88 in LILACS). We identified four additional studies through searches in bibliographic references. After screening the titles and abstracts of these citations, we selected 18 studies for full‐text reading. We excluded 16 studies and included two studies (Figure 1).
1.
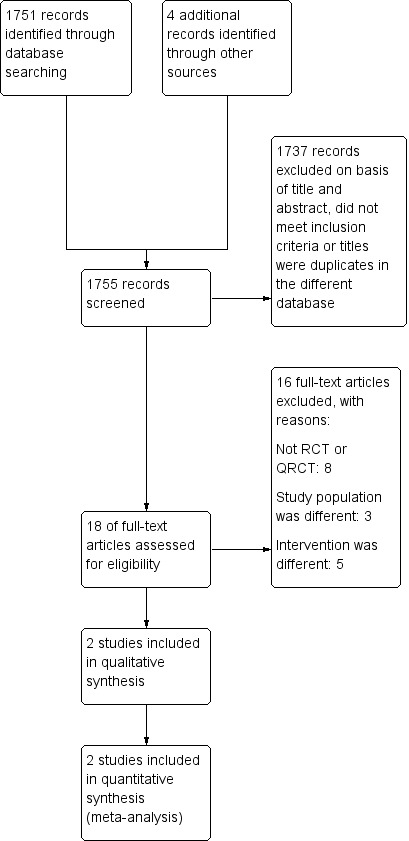
Study flow diagram.
Included studies
Study design
We included two RCTs comparing CPAP or bilevel NPPV with oxygen therapy with or without standard medical care. One trial involved 60 participants (Gao 2002), while the other trial involved 209 participants (Squadrone 2005). We reported the two trials in full (see Characteristics of included studies table). We found no QRCTs.
Population
The two included trials reported the use of CPAP or bilevel NPPV in participants residing in Italy and China.
Gao 2002 recruited participants in China.
Squadrone 2005 was a multicentric trial conducted in 15 ICUs of the Piedmont Intensive Care Units Network in Italy. Both trials included only adults with acute respiratory failure after upper abdominal surgery.
The mean (± standard deviation (SD)) age of the participants in both trials was 65 ± 10 years and the majority of the participants were men (67%).
Intervention
Gao 2002 compared bilevel NPPV to standard treatment (oxygen mask with a flow rate of 5 L/minute). In Gao 2002, the NPPV group (30 participants) received noninvasive mechanical ventilation using an Eviat4 ventilator, set on the pressure support ventilation (PSV) breathing mode, flow trigger of 3 L/minute, and fraction of inspired oxygen (FiO2) of 40%. The level of PSV was adjusted from 10 cm H2O until a VT greater than 7 mL/kg or a respiratory rate less than 25 breaths/minute was reached. PEEP was set at 3 to 5 cm H2O. The mean (± SD) level of PSV was 13.8 ± 2.7 cm H2O and the mean (± SD) PEEP was 4.3 ± 0.9 cm H2O for a mean (± SD) of 3 ± 2 days on NIV.
Squadrone 2005 compared NPPV (CPAP of 7.5 cm H2O) to standard treatment (oxygen through a Venturi mask at an FiO2 of 0.5). The authors reported that CPAP was generated using a flow generator with an adjustable inspiratory oxygen fraction set to deliver a flow of up to 140 L/minute (Whisperflow, Caradyne, Ireland) and a spring‐loaded expiratory pressure valve (Vital Signs Inc, Totoma, NJ) and applied using a latex‐free polyvinyl chloride transparent helmet (CaStar, Starmed, Italy). PEEP was 7.5 cm H2O. At the end of a six‐hour period, participants underwent a one‐hour screening test breathing oxygen through a Venturi mask with an FiO2 of 0.3. Participants returned to the assigned treatment if the PaO2/FiO2 ratio was 300 or lower. The mean (± SD) time of treatment required to obtain the oxygenation goal was 19 ± 22 hours in the CPAP group and 28 ± 27 hours in the control group (P value = 0.006).
Gao 2002 used a face mask for NIV and Squadrone 2005 used a helmet.
Outcomes
Gao 2002 reported tracheal intubation as their primary outcome. Their secondary outcomes were ICU length of stay, end changes in arterial blood gas results (arterial blood gases and pH measured one hour after the intervention), pH, PaCO2 (mm Hg), PaO2 (mm Hg), respiratory rate (breaths/minute), heart rate (beats/minute) and mean arterial pressure (mm Hg).
Squadrone 2005 reported tracheal intubation within the first seven days after surgery as their primary outcome. Their secondary outcomes were hospital mortality; ICU and hospital length of stay; and incidence of pneumonia, infection, and sepsis within the first month after surgery. Pneumonia, infection, and sepsis were diagnosed according to standard definitions.
We performed two meta‐analyses with the outcomes that were provided in the two RCTs: rate of tracheal intubation and ICU length of stay.
The dichotomous outcomes were described adhering to the ITT principle. Both studies reported their results using mean and SDs for continuous outcomes.
Excluded studies
We excluded 16 studies: eight were not RCTs or QRCTs (Benditt 2009; Conti 2007; Foster 2005; Frangos 2005; Jaber 2005; Lee 2011; Kindgen‐Milles 2000; Tarraza 2001), three had populations that did not fulfil our selection criteria (Cypel 2014; Delclaux 2000; Kiil 2003), and five had different interventions (Campbell 1986; Denehy 2001;Michelet 2006; Souza 2012; Zhang 2015).
See Characteristics of excluded studies table.
Ongoing studies
We found no ongoing studies.
Studies awaiting classification
We found no studies awaiting classification.
Risk of bias in included studies
Both of the two included trials had a low or unclear risk of bias.
Gao 2002 had low risk of bias for incomplete outcome data, selective reporting, and other bias. We judged it to have an unclear risk of bias for blinding of participants and personnel and blinding of outcome assessors.
Squadrone 2005 had low risk of bias for selection bias, incomplete outcome data, selective reporting, and other bias. We considered it to have an unclear risk of bias for blinding of outcome assessors and a high risk of bias for blinding of participants and personnel.
Figure 2 shows the 'Risk of bias' as percentages across the included studies. See Figure 3 for a 'Risk of bias' summary of our judgements about each risk of bias item for each included trial.
2.
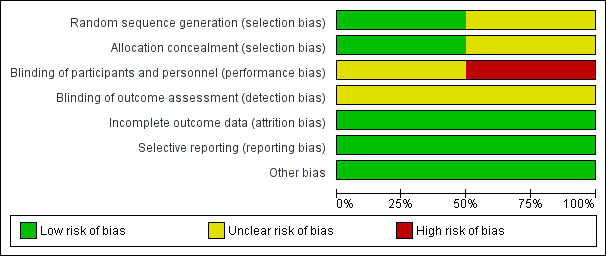
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
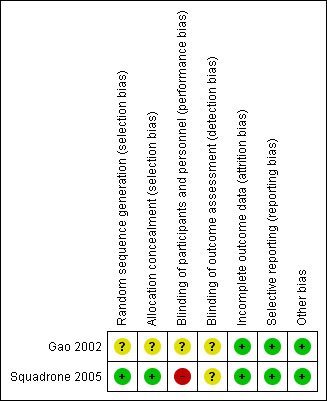
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Gao 2002 did not describe how allocation concealment was performed and so had unclear risk of bias for this domain. We wrote to the contact author asking about this procedure, but did not receive an answer.
Squadrone 2005 described that randomization was performed through a programme of random distribution of blocks generated by a computer and so had low risk of bias for this domain.
Blinding
Gao 2002 did not mention any attempt to blind the participants or professionals involved in the interventions.
The authors of Squadrone 2005 stated that allocation to treatment with oxygen or CPAP was not blinded. However, to minimize potential bias in the assessment of some of the study end points, the authors used measures such as objective criteria for tracheal intubation and standardization of all co‐interventions that could have influenced outcome variables such as anaesthesia, postoperative pain control, and respiratory physiotherapy.
Neither of the two included RCTs reported blinding of outcome assessors. We contacted the authors of both trials to ask about blinding of study participants, personnel, and outcome assessors but did not obtain an answer from them.
It is difficult to blind the allocation to treatment with oxygen or NPPV because the masks used are different. The person applying the intervention is also unlikely to be blinded. However, we assessed what the authors reported in their studies.
We categorized Gao 2002 as having an unclear risk of bias in blinding of participants and personnel (performance bias), because this was not reported in the text of the publication.
We judged Squadrone 2005 as having a high risk of bias because the authors reported that the professionals responsible for the intervention and the participants were not blinded.
Both trials had an unclear risk in blinding of outcome assessment (detection bias) because the authors did not describe whether outcome assessors were blinded to the intervention received by the participants.
Incomplete outcome data
Although Gao 2002 did not describe an ITT analysis, we classified this trial as having a low risk of attrition bias because all randomized participants were analysed and there were no losses.
Squadrone 2005 reported that 2.9% of their participants were lost to follow‐up but since they conducted an ITT analysis, we also classified this trial as having a low risk of bias for this domain.
Selective reporting
The two included trials reported the results of outcomes planned. We classified them both as having a low risk of bias for selective reporting.
Other potential sources of bias
We classified the two included trials as having a low risk of bias for other potential sources of bias. Both were RCTs. The participants were randomized. The two included trials were not cluster‐randomized or cross‐over trials and the authors did not deviate from the study protocols.
Effects of interventions
See: Table 1
Primary outcomes
Hospital mortality (measured from the date of randomization to the time of discharge from hospital)
Gao 2002 did not report hospital mortality. In the trial by Squadrone 2005, all participants treated with CPAP were discharged alive while three participants died in the group treated with oxygen alone (RR 0.14; 95% CI 0.01 to 2.71; P value = 0.19). This difference did not reach statistical significance.
Based on the GRADE methodology, we downgraded hospital mortality from high to low quality because the sample size (209 participants) was smaller than the calculated optimal information size (OIS) (609 participants). The published evidence was limited to a small number of trials and high risk of bias. (The trial did not describe how randomization was done and did not provide data on the allocation process and whether the personnel were blinded.)
Rate of tracheal intubation (measured in number of events occurred from the date of randomization to the time of discharge from hospital)
There was a statistically significant reduction in the rate of tracheal intubation for participants treated with NPPV (CPAP or bilevel NPPV) compared with oxygen therapy alone. Eighteen participants in the control group, compared to four participants in the NPPV group, needed tracheal intubation (RR 0.25; 95% CI 0.08 to 0.83; P value = 0.02 (I2 statistic = 17%; Analysis 1.1). This translates into a RD of ‐0.09 or 9% (95% CI ‐0.15 to ‐0.04) and an NNTB of 11.
1.1. Analysis.
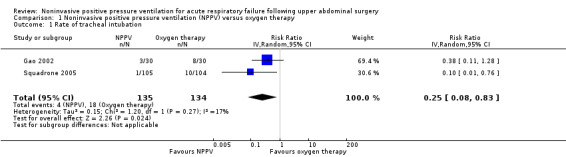
Comparison 1 Noninvasive positive pressure ventilation (NPPV) versus oxygen therapy, Outcome 1 Rate of tracheal intubation.
We downgraded rate of tracheal intubation from high to low quality, based on the GRADE methodology, because of the risk of bias (one of the trials did not describe how randomization was done (Gao 2002); and both trials did not provide data on the allocation process). The total (cumulative) sample size was greater (269 participants) than the calculated OIS (166 participants); however, only a very small number of participants and events were included, and the published evidence was limited to a small number of trials (two), although both showed benefits of the intervention.
Secondary outcomes
Intensive care unit length of stay (measured in days)
The meta‐analysis of the two trials (269 participants) showed a statistically significant reduction in ICU length of stay between NPPV (CPAP or bilevel NPPV) versus oxygen therapy alone (MD ‐1.84; 95% CI ‐3.53 to ‐0.15; P value = 0.03; Analysis 1.2). However, there was moderate heterogeneity in this meta‐analysis (I2 statistic = 59%). One possible cause for this moderate heterogeneity may have been the duration of the intervention or even the type of intervention. Gao 2002 used bilevel NPPV and Squadrone 2005 used CPAP. Due to the small number of included trials, we could not perform a subgroup analysis. If we find new trials in future updates, we will look for evidence of substantial heterogeneity and investigate and report the possible reasons for this.
1.2. Analysis.
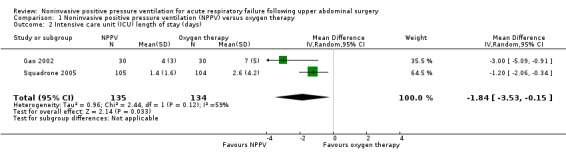
Comparison 1 Noninvasive positive pressure ventilation (NPPV) versus oxygen therapy, Outcome 2 Intensive care unit (ICU) length of stay (days).
According to the GRADE methodology, this outcome was downgraded from high to very low quality due to the risk of bias (one of the trials did not describe how randomization was done (Gao 2002), and both trials did not provide data on the allocation process and the personnel not were blinded), and the small number of trials (two), although all showed benefits of the intervention. Although total (cumulative) sample size was greater than the calculated OIS, the total population size was small (269 participants). The meta‐analysis showed moderate heterogeneity.
Hospital length of stay (measured in days)
Gao 2002 did not report hospital length of stay.
In Squadrone 2005, there was no statistically significant difference in the mean duration of hospital stay between the groups (15 ± 13 days with CPAP versus 17 ± 15 days with oxygen; P value = 0.10).
Complications after noninvasive positive pressure ventilation
We considered complications to be gastric insufflation, fistulae, anastomotic leakage, pneumothorax, bleeding, pneumonia, hospital infection, important leaks, mask discomfort, skin breakdown, eye irritation, sinus congestion, oronasal drying, and patient‐ventilator asynchrony.
Gao 2002 did not describe the rate of complications (60 participants).
Squadrone 2005 reported the following complications.
Pneumonia: two participants treated with oxygen plus CPAP had pneumonia versus 10 participants treated with oxygen alone (RR 0.19; 95% CI 0.04 to 0.88; P value = 0.02).
Infection: three participants treated with oxygen plus CPAP had infection versus 11 participants treated with oxygen alone (RR 0.27; 95% CI 0.07 to 0.94; P value = 0.03). These were all cases of surgical wound infection.
Sepsis: the number of cases of sepsis was lower in the group treated with oxygen plus CPAP (two participants) than in the group treated with oxygen alone (nine participants) and all occurred after the first week of surgery (RR 0.22; 95% CI 0.04 to 0.99; P value = 0.03). The causes of sepsis were anastomotic leakage (67%) and pneumonia (33%).
Anastomotic leakage: one participant treated with oxygen plus CPAP had anastomotic leakage versus six participants treated with oxygen alone (RR 0.17; 95% CI 0.02 to 1.35; P value = 0.09).
Intolerance to treatment:4/105 participants in the oxygen plus CPAP group and 2/104 participants in the oxygen only group developed intolerance to treatment (RR 1.98; 95% CI 0.37 to 10.58; P value = 0.42).
The differences were statistically significant for the outcomes pneumonia, infection, and sepsis, in favour of the CPAP plus oxygen group. There were no statistically significant differences between the groups for the outcomes anastomotic leakage and intolerance to treatment.
The two trials included in this systematic review did not present data on the following outcomes that we intended to assess: gastric insufflation, fistulae, pneumothorax, bleeding, skin breakdown, eye irritation, sinus congestion, oronasal drying, and patient‐ventilator asynchrony.
Changes in arterial blood gas levels
Gao 2002 analysed changes in blood gas levels one hour after the intervention (60 participants). The authors reported that, compared to the oxygen group, the group who had received bilevel NPPV had a significant increase in PaO2 levels (MD 22.5 mm Hg; 95% CI 17.19 to 27.81; P value < 0.00001) and in pH levels (MD 0.06; 95% CI 0.01 to 0.11; P value < 0.02), as well as a significant decrease in PCO2 levels (MD ‐9.8 mm Hg; 95% CI ‐14.07 to ‐5.53; P value < 0.00001).
Squadrone 2005 did not describe changes in arterial blood gas levels.
Discussion
Although we found only two RCTs with a relatively small number of participants, our findings showed that NPPV appears to be a safe and effective intervention that reduces the rate of tracheal intubation.
Acute respiratory failure can occur early in the postoperative course and 8% to 10% of these people may need endotracheal intubation and mechanical ventilation (Conti 2007; Ferreyra 2008; Pasquina 2006; Squadrone 2005). In people with acute respiratory failure, NPPV is an alternative to invasive mechanical ventilation that can reduce the rate of tracheal intubation as well as complications and mortality (Jaber 2005; Vital 2013).
Summary of main results
The results of our meta‐analysis showed that CPAP or bilevel NPPV, compared to oxygen therapy, is effective in reducing the need for intubation: 11 participants need to be treated with NPPV for an additional beneficial outcome (avoidance one intubation).
We performed the calculation of sample size for the outcome of rate of tracheal intubation. To calculate OIS we used a 5% alpha error and 80% power, with a tracheal intubation ratio of 13.43% in the control group and 2.96% in the intervention group. The study should have a sample of 166 participants (83 in each arm) to evaluate the rate of tracheal intubation (Lee 2011). Our sample was 269 participants, which is why we were able to show a significant difference in this outcome. We also found that, compared to oxygen therapy, NPPV significantly reduced ICU length of stay by almost two days. We calculated the sample size to achieve reduction of at least one day in the ICU and we found that the study should have a sample of 130 participants (65 in each arm). We used a 5% alpha error and 80% power and a SD of 2.3 (Lee 2011). Our sample was 269 participants.
Gao 2002 did not report hospital mortality. The second trial concluded that there was no significant difference hospital mortality between the control and the intervention groups (Squadrone 2005). The lack of statistical significance for mortality may be due to the small number of participants (209) and events (only three) in this trial. Based on these results, with a mortality rate of 2.88% in the control group and 0% in the intervention group, with a 5% alpha error and 80% power, approximately 610 participants (303 in each arm) would be necessary to detect a significant difference in mortality (Lee 2014).
Gao 2002 did not report drop‐outs or withdrawals in their trial. Squadrone 2005 reported that two of the 104 participants in the control group and four of the 105 participants in the CPAP group developed an intolerance to the treatment. None of the participants who developed an intolerance to the treatment required intubation (Squadrone 2005). Squadrone 2005 stated that the use of a face or nasal mask appeared to be a particularly relevant factor that may limit the use of NPPV. Difficulties in fitting the mask, leaks, and patient discomfort can limit the continuous and long‐term use of noninvasive CPAP and may account for a large proportion of the failures. Squadrone 2005 used a helmet for CPAP. Conti 2007 reported that although improvement in oxygenation and functional residual capacity were similar, participants using helmets had lower rates of intolerance to ventilatory treatment as well as lower incidence of skin necrosis, gastric distension, and eye irritation than participants using a face mask. Therefore, the use of the helmet to deliver CPAP could explain the low rate of intolerance seen in Squadrone's trial (Squadrone 2005). More studies are needed to evaluate whether the use of more widely available devices for CPAP administration, such as full face or nasal masks, would produce results similar to those reported by Squadrone et al.
Gao 2002 used facial masks for bilevel NPPV and reported that appropriate masks were adopted to pass a nasogastric tube to prevent gastric distention. Squadrone 2005 did not report cases of anastomosis leakage due to gastric distention. One RCT investigating pulmonary complications reported that, depending on the flow rate, the use of CPAP may be associated with a risk of gastric distension and, therefore, should be recommended only in selected people (Fagevik 2002). However, Fagevik 2002 had no problems with gastric distention in their trial because the oesophageal conduit was decompressed by use of a nasogastric tube during the entire period of CPAP use. Oesophageal and gastric surgery are usually considered contraindications to NPPV use. This is because one of the potential complications of NPPV is abdominal distention due to air forced into the stomach under positive pressure. Disruption of the surgical anastomosis can occur because of this abdominal distention (Penuelas 2007). NPPV can be used without adverse effects in participants after oesophageal surgery when the insufflation pressure level is less than 12 cm H2O (Brochard 2000; Jaber 2005; Joris 1997). Joris 1997 used NPPV with an inspiratory pressure of 12 cm H2O and a PEEP of 4 cm H2O and reported no severe complications such as aspiration or gastric distension. Joris 1997 reported that gastric distension was successfully avoided by the placement of nasogastric tubes prior to the institution of NPPV and by regular decompression of the stomach. Moreover, they avoided higher ventilatory pressures because these are associated with increased risk of gastric distension, leaks around the face mask, and desynchronization between the participant's spontaneous breathing and the ventilator, which reduce the tolerance of NPPV. Jaber 2005 reported no complications related to the use of NPPV in their 60 participants. Neither of the two trials included in this review reported the occurrence of fistulae following the use of NPPV (Gao 2002; Squadrone 2005).
In one case‐control study, Michelet 2009 evaluated the safety and efficacy of NPPV in avoiding endotracheal intubation in participants with acute respiratory failure after oesophagectomy. The authors of that study reported that the participants in the NPPV group had a better and sustained improvement in oxygenation and a reduction in ICU length of stay. The use of NPPV was not associated with an increase in anastomotic leakage.
Although some investigators have reported adverse effects due to the use of NPPV, including changes in arterial blood gas, fistulae, anastomotic leakage, pneumothorax, bleeding, pneumonia, hospital infection, important leaks, mask discomfort, skin breakdown, eye irritation, sinus congestion, oronasal drying, and patient‐ventilator asynchrony (Pasquina 2006; Vital 2013), the two trials included in this review did not evaluate these findings. Gao 2002 did not report any complications and Squadrone 2005 reported that, compared with oxygen therapy alone, the use of NPPV reduced the incidence of pneumonia, surgical wound infection, and sepsis. However, the lack of reports of adverse effects does not mean that these cannot occur. If additional trials are included in the next update of this review, we will evaluate possible adverse effects due to the use of NPPV.
NPPV has been shown to be safe and effective in improving other clinical conditions such as COPD, acute cardiogenic pulmonary oedema, and respiratory distress. Due to the substantial worldwide morbidity and mortality of acute cardiogenic pulmonary oedema and acute COPD exacerbation, it is important to treat these people with effective therapies as early as possible (Mal 2014). One systematic review and meta‐analysis showed that CPAP or bilevel NPPV was effective in reducing hospital mortality, intubation rate, and ICU length of stay in participants with acute cardiogenic pulmonary oedema (Vital 2013). In addition, NPPV resulted in faster improvement and was better tolerated than standard medical care.
Another meta‐analysis also concluded that NIV (BiPAP and CPAP) could reduce in‐hospital mortality of adults with acute cardiogenic pulmonary oedema and could be used as part of the first‐line management strategies for these people (Sun 2014). A similar benefit was reported for participants with acute exacerbation of COPD. In‐hospital treatment of people with NPPV reduced mortality and the need for mechanical ventilation, compared with standard therapy (Mal 2014). However, as pointed out by another recent systematic review and meta‐analysis (Struik 2014), there is still insufficient evidence to support the routine use of NPPV in people with stable COPD.
The findings of the systematic review by Mal et al. suggest that, compared to standard therapy, out‐of‐hospital NPPV reduces the risk of hospital mortality and need for invasive ventilation in adults with severe respiratory distress (Mal 2014). The use of out‐of‐hospital NPPV in people presenting with dyspnoea does not appear to increase complication rates. The authors of that systematic review concluded that NPPV, compared to delayed NPPV, appears to be a safe and feasible therapy that can lead to faster improvement and may decrease the need for intubation in the emergency unit.
A consensus conference recommended that treatment of severe hypoxaemia with NIV should be performed in an ICU or within a system of care capable of providing high levels of monitoring, with immediate access to staff skilled in invasive airway management (Evans 2001). However, NPPV appears to be effective and safe in improving acute respiratory insufficiency and avoiding tracheal intubation and should be started as early as possible to prevent further complications. NPPV can be used in hospital wards as long as the patient is being monitored and serviced by qualified professionals.
Overall completeness and applicability of evidence
This review pooled the results of two RCTs (one comparing bilevel NPPV versus oxygen therapy and another comparing CPAP versus oxygen therapy) in participants with acute respiratory failure after upper abdominal surgery. Data from only two outcomes could be pooled in a meta‐analysis (rate of tracheal intubation and ICU length of stay) and it was not possible to perform subgroup analyses. Our meta‐analysis showed a significantly shorter mean ICU length of stay and a reduced rate of tracheal intubation in the CPAP or bilevel NPPV group than in the oxygen therapy group. The two trials included in this systematic review partially answer our research question with some limitations, mostly related to the small number of RCTs that assessed mortality. Our results show that, in adults with acute respiratory failure after upper abdominal surgery, NPPV is effective in reducing the rate of tracheal intubation and ICU length of stay and is safe because the intervention did not increase anastomotic leaks.
Quality of the evidence
This systematic review included two RCTs with 269 participants. We defined three relevant outcomes in order to assess the quality of evidence using the GRADE methodology (GRADEpro 2011). For the primary outcomes of hospital mortality and rate tracheal intubation, we rated the quality of the evidence low, which means that our confidence in the effect estimate is limited and that the true effect may be substantially different from the estimate of the effect. For the secondary outcome of ICU length of stay, we considered the quality of the evidence very low, which means that we have very little confidence in the effect estimate and that the true effect is likely to be substantially different from the estimate of effect (Table 1).
The main limiting factor, which was the reason for the downgrading of the levels of quality for the outcomes, was the inconsistency of results across the small number of included trials. Another limiting factor, which meant that we downgraded the quality of the evidence by one level, was the risk of bias of the trials included in this review. Some data were not well defined in the trials. One trial did not describe random sequence generation; allocation concealment; or blinding of participants, personnel, or outcome assessors and was therefore classified as having an unclear risk of bias (Gao 2002). The other trial reported that the personnel involved in the intervention were not blinded and, therefore, we classified it at high risk of bias (Squadrone 2005). Both trials had a low risk of bias for the other domains. We believe that the risk of bias of the included trials in this review is high or uncertain. For the outcome of ICU length of stay, we downgraded the quality of the evidence by more than one level because we found moderate heterogeneity in this outcome; therefore, the quality of the evidence was very low.
Therefore, according to the GRADE methodology (GRADEpro 2011), our overall rating for the quality of evidence in this systematic review is low.
Potential biases in the review process
The major weakness of our review is the relatively small number of trials (two) and participants (269) comparing NPPV with oxygen therapy and standard medical care.
In our protocol, we planned to assess the impact of heterogeneity, but due to the small number of included trials (two) and the lack of some data, it was not possible to perform this analysis (Faria 2011). In the next update of this review, if new trials are included, we will conduct analysis of heterogeneity to reduce the risk of bias.
We hypothesized that age, gender, and co‐morbidities may represent potential sources of heterogeneity among participants. Treatment heterogeneity may be related to the type of intervention, the levels of pressure applied with NPPV, or treatment duration. We also planned to perform analysis of subgroups to identify possible problems caused by a difference between the groups; however, this was not possible due to the small number of trials and participants.
We sent an email to the contact authors of the two included trials to request information on any missing data and minimize biases, but they did not reply.
The strengths of this review include the methods used to reduce bias as we conducted a wide search of the literature, used a multimodal search strategy without language restrictions, and did not restrict the dates of research. We undertook duplicate independent screening and data extraction and pre‐specified criteria for appraising methodological quality, all of which are typical of Cochrane reviews. We have presented and discussed all the outcomes originally described in the protocol of this review that were available for analysis, whether statistically significant or not (Faria 2011).
Agreements and disagreements with other studies or reviews
Despite the suggestive title "Continuous Positive Airway Pressure for treatment of respiratory Complications After Abdominal Surgery: a Systematic Review and Meta‐Analysis", the systematic review by Ferreyra 2008 assessed CPAP for the prevention of, and not for the treatment of, pulmonary complications after major abdominal surgery. Our systematic review evaluated the use of CPAP or bilevel NPPV for the treatment of acute respiratory failure after upper abdominal surgery. Although Ferreyra et al. included the Squadrone 2005 study, they also included eight RCTs that evaluated the prevention of pulmonary complications with CPAP. Despite these differences, these authors also concluded that CPAP reduced the risk of intubation when used prophylactically or therapeutically in acute respiratory failure and that more studies should be conducted to assess the efficacy of NPPV on mortality. They also concluded that the use of CPAP in the early postoperative period of abdominal surgery decreased pulmonary complications such as atelectasis and pneumonia, supporting its use in clinical practice.
Authors' conclusions
Implications for practice.
Data from two randomized controlled trials (RCTs) showed that in adults with acute respiratory insufficiency after upper abdominal surgery, continuous positive airway pressure (CPAP) and bilevel noninvasive positive pressure ventilation (NPPV) is effective in the reduction of intubation rates and intensive care unit (ICU) length of stay and to improve blood gases and blood pH one hour after the intervention. The intervention also reduced the risks of pneumonia, sepsis, and surgical wound infection and is apparently safe because it does not lead to anastomotic leakages. However, because of the small number of studies and their low quality, more good quality studies are needed to increase the reliability of the evidence.
Implications for research.
New RCTs should be conducted in adults who develop respiratory insufficiency after upper abdominal surgery to compare the effects of CPAP or bilevel NPPV versus oxygen therapy on hospital mortality and hospital length of stay and to detect possible adverse events such as gastric insufflation, fistulae, pneumothorax, bleeding, skin breakdown, eye irritation, sinus congestion, oronasal drying, and patient‐ventilator asynchrony. We calculated that studies involving approximately 610 participants are needed to detect a significant difference in hospital mortality.
More studies are also needed to assess whether treatment with CPAP is superior to treatment with bilevel NPPV in the treatment of respiratory insufficiency after upper abdominal surgery.
Notes
We would like to thank Mathew Zacharias (content editor), Nathan Pace (statistical editor), and Dean Hess and Felix Ram (peer reviewers) for their help and editorial advice during the preparation of the protocol (Faria 2011) for this systematic review.
Acknowledgements
We thank the Cochrane Anaesthesia, Critical and Emergency Care Group for their help during the preparation of this systematic review. In particular, we would like to thank Rodrigo Cavallazzi (content editor); Nathan Pace (statistical editor); and Andrew MacDuff, Sachin Sud, and Regina El Dib (peer reviewers) for their help and editorial advice during the preparation of this systematic review.
We would also like to thank Peng Lihua for help in finding the full paper for Gao 2002 and Yuguang Huang and Ruquan Han for their help in undertaking the translation of this article for us.
Appendices
Appendix 1. Search strategy for CENTRAL (The Cochrane Library)
#1 MeSH descriptor: [Positive‐Pressure Respiration] explode all trees #2 MeSH descriptor: [Continuous Positive Airway Pressure] explode all trees #3 (pressure near (airway or positive or respirat* or ventilat*)) #4 #1 or #2 or #3 #5 MeSH descriptor: [Biliary Tract Surgical Procedures] explode all trees #6 MeSH descriptor: [Digestive System Surgical Procedures] explode all trees #7 (abdom* near (surg* or oprat* or procedur* or preoperativ* or intraoperativ* or perioperativ* or peroperativ* or elective)) #8 #5 or #6 or #7 #9 #4 and #8
Appendix 2. Search strategy for MEDLINE (OvidSP)
1. exp Positive‐Pressure Respiration/ or exp Continuous Positive Airway Pressure/ or (pressure adj3 (airway or positive or respirat* or ventilat*)).af. 2. ((exp Abdomen/ or exp Stomach/ or exp biliary tract/ or exp bile ducts/ or exp gallbladder/ or exp gastrointestinal tract/ or exp liver/ or exp pancreas/) and (exp General Surgery/ or exp Surgical Procedures, Operative/ or exp Surgical Procedures, Elective/)) or exp Biliary Tract Surgical Procedures/ or exp Digestive System Surgical Procedures/ or (abdom* adj6 surg*).ti,ab. or (abdom* adj3 (surg* or oprat* or procedur* or preoperativ* or intraoperativ* or perioperativ* or peroperativ*)).af. 3. 1 and 2 4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 3. Search strategy for EMBASE (OvidSP)
1. exp positive end expiratory pressure/ or (pressure adj3 (airway or positive or respirat* or ventilat*)).af. 2. ((exp abdomen/ or exp stomach/ or exp hepatobiliary system/ or exp bile duct/ or exp gallbladder/ or exp gastrointestinal tract/ or exp liver/ or exp pancreas/) and (exp general surgery/ or exp surgery/ or exp elective surgery/)) or exp biliary tract surgery/ or exp abdominal surgery/ or (abdom* adj6 surg*).ti,ab. or (abdom* adj3 (surg* or oprat* or procedur* or preoperativ* or intraoperativ* or perioperativ* or peroperativ*)).af. 3. 1 and 2 4. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or factorial* or placebo* or volunteer* or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*))).ti,ab.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 4. CINAHL (EBSCOhost) search strategy
S1 ((MH "Positive Pressure Ventilation") OR (MH "Continuous Positive Airway Pressure") OR (MH "Intermittent Positive Pressure Ventilation")) OR ((pressure and (airway or positive or respirat* or ventilat*))) S2 ((MH "Biliary Tract Surgical Procedures+") OR (MH "Surgery, Digestive System+")) OR ( (abdom* and (surg* or operat* or procedur* or preoperativ* or intraoperativ* or perioperativ* or peroperativ* or elective))) S3 S1 and S2
Appendix 5. LILACS (BIREME iAH) search strategy
("pressure" and ("airway" or "positive" or "respirat$" or "ventilat$")) and ("abdom$" and ("surg$" or "operat$" or "procedur$" or "preoperativ$" or "intraoperativ$" or "perioperativ$" or "peroperativ$" or "elective"))
Appendix 6. Data extraction form
Noninvasive positive pressure ventilation for upper abdominal surgery
| Author / year of publication: | |
| ACTION: |
| METHODS | Randomization procedure: | ||||
| Allocation: | |||||
| Blinding: | |||||
| Duration: | |||||
| Design: | |||||
| Multicentre: | |||||
| Analysis (ITT): | |||||
| Informed consent: | |||||
| PARTICIPANTS | N: | ||||
| Setting: | |||||
| Age: | O: | C: | B: | ||
| Gender: | O: | C: | B: | ||
| Diagnostic criteria: | |||||
| History: | |||||
| Excluded: | |||||
| NOTES | |||||
|
INTERVENTIONS OUTCOMES |
Intervention: | ( ) CPAP ( ) bilevel NPPV ( ) Other | |||
| PEEP level: | C: | B: | |||
| PS level: | B: | ||||
| N: | C: | B: | |||
| Time: | C: | B: | |||
| Co‐intervention: | |||||
| Control group: | ( ) O2 ( ) Co‐intervention ( ) Other | ||||
| N: | |||||
| Mask: | ( ) oro‐nasal ( ) nasal ( ) total face ( ) other | ||||
| Mortality: | O: | C: | B: | ||
| Tracheal intubation rate: | O: | C: | B: | ||
| ICU length of stay: | O: | C: | B: | ||
| Hospital length of stay: | O: | C: | B: | ||
| Treatment failure: | O: | C: | B: | ||
| Adverse effects: | O: | C: | B: | ||
| Patient compliance : | O: | C: | B: | ||
| Drop‐outs / withdrawals: | O: | C: | B: | ||
| Notes | |||||
Key
O: Oxygen; C: CPAP; NPPV B: bilevel NPPV
Data and analyses
Comparison 1. Noninvasive positive pressure ventilation (NPPV) versus oxygen therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of tracheal intubation | 2 | 269 | Risk Ratio (IV, Random, 95% CI) | 0.25 [0.08, 0.83] |
| 2 Intensive care unit (ICU) length of stay (days) | 2 | 269 | Mean Difference (IV, Random, 95% CI) | ‐1.84 [‐3.53, ‐0.15] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gao 2002.
| Methods |
Randomization procedure: concealed randomization Allocation: not reported Blinding: not reported Design: randomized, controlled Duration: not reported Multicentre: no Analysis (ITT): not reported Informed consent: not reported |
|
| Participants |
Number of participants: 60 Setting: ICU Age (mean ± SD): O: 64 ± 8.1 years; NPPV: 65 ± 10.4 years Gender (M/W): O: 21/9; NPPV: 23/7 Diagnostic criteria: elderly participants with respiratory rate over 30 breaths/minute after upper abdominal surgery in an ICU Exclusion criteria: indication of endotracheal intubation PaO2 ≤ 60 mm Hg or PaCO2 ≥ 50 mm Hg under oxygen mask or NPPV with oxygen flow rate of 5 L/min Number excluded: not reported |
|
| Interventions |
Control: routine oxygen mask with oxygen flow rate of 5 L/min Intervention: NPPV. Assisted mechanical ventilation using an oxygen mask. The ventilator was Evita 4, PSV pattern was used, with the flow trigger 3 L/min and FiO2 40%. The PSV levels were adjusted from 10 cm H2O until VT > 7 mL/kg or respiratory rate < 25 breaths/min. PEEP was set at 3‐5 cm H2O Number of participants per group: O: 30; NPPV: 30 Mask: appropriate facial mask was adopted that allowed the stomach tube to pass through |
|
| Outcomes |
Primary outcome: endotracheal intubation Secondary outcome: ICU and hospital length of stay; incidence of pneumonia, infection, and sepsis within the first month after surgery; and hospital mortality. Pneumonia, infection, and sepsis were identified using standard definitions Mortality: not reported Tracheal intubation rate: O: 8 NPPV: 3 ICU length of stay: O: 7 ± 5 days; NPPV: 4 ± 3 days Hospital length of stay: not reported Treatment failure: not reported Adverse effects: not reported Participant compliance: not reported Drop‐outs/withdrawals: not reported |
|
| Notes |
Declarations of interest: none reported Funding sources for the study: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method used was not reported. We wrote to the contact author asking about this procedure but did not receive an answer |
| Allocation concealment (selection bias) | Unclear risk | The method used was not reported. We wrote to the contact author asking about this procedure but did not receive an answer |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. We contacted the authors of this study to ask about blinding of study participants and personnel but did not obtain an answer |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. We contacted the authors of this study to ask about blinding of study outcome assessors but did not obtain an answer |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review were reported in the pre‐specified way |
| Other bias | Low risk | This study was not a cluster randomized or a cross‐over trial. This study was a randomized controlled trial. The participants were randomized. The distribution of the participants was balanced between the control and intervention groups. The authors did not deviate from the study protocol |
Squadrone 2005.
| Methods |
Randomization procedure: concealed randomization was conducted centrally through a dedicated web site using a computer‐generated block randomization schedule Allocation: not reported Blinding: allocation to treatment with oxygen or oxygen plus CPAP was not blinded. To minimize potential bias in the assessment of some of the study end points, we used measures such as objective criteria for endotracheal intubation and standardization of all co‐interventions that could have influenced outcome variables such as anaesthesia, postoperative pain control, and respiratory physiotherapy Design: randomized, controlled, unblinded study with concealed allocation Duration: June 2002 to November 2003 Multicentre: participants recruited from the 15 ICUs of the Piedmont Intensive Care Units Network in Italy Analysis (ITT): all analyses conducted on an ITT basis Informed consent: ethics committees approved the protocol and written informed consent was obtained from the participants |
|
| Participants |
Number of participants: 209 Setting: 15 ICU Age (mean ± SD): O: 65; CPAP + O: 66 Gender (M/W): O: 64/40; CPAP: 71/34 Diagnostic criteria: participants scheduled for elective abdominal surgery and general anaesthesia were eligible to participate in the study if they met the following criteria: abdominal surgery requiring laparotomy and time of viscera exposure > 90 minutes. At the end of the surgical procedure, participants were extubated and underwent a 1‐hour screening test breathing oxygen through a Venturi mask at an inspiratory fraction of 0.3. Participants were included in the study if they developed a PaO2/FiO2 ratio of ≤ 300 Exclusion criteria: participants were excluded if before surgery they were > 80 or < 18 years; had a New York Heart Association functional class of II, III, or IV; had valvular heart disease, history of dilated cardiomyopathy, implanted cardiac pace maker, unstable angina, or myocardial infarction and cardiac surgery within the previous 3 months; had a history of chronic obstructive pulmonary disease, asthma, or sleep disorders; had preoperative infection, sepsis, or both; had a body mass index > 40; had a presence of tracheostomy, facial, neck, or chest wall abnormalities; required an emergency procedure (operation that must be performed as soon as possible and no longer than 12 hours after admission); or had undergone abdominal aortic aneurysm surgery, chemotherapy, or immunosuppressive therapy within the previous 3 months. Participants were also excluded if before randomization they had arterial pH < 7.3 with a PaCO2 > 50 mm Hg; arterial oxygen saturation < 80% with the maximal fraction of inspiratory oxygen; clinical signs of acute myocardial infarction; systolic arterial pressure < 90 mm Hg under optimal fluid therapy; presence of criteria for acute respiratory distress syndrome; haemoglobin < 7 g/dL, serum albumin < 3 g/dL; creatinine > 3.5 mg/dL (309 μmol/L); or a Glasgow Coma Scale < 12 Number excluded: O: 2; CPAP: 4 |
|
| Interventions |
Control: treated for 6 hours with oxygen through a Venturi mask at an FiO2 of 0.5 Intervention: treated with oxygen at an FiO2 of 0.5 plus a CPAP of 7.5 cm H2O Number of participants per group: O: 104;CPAP: 105 Mask: helmet |
|
| Outcomes |
Primary outcome: endotracheal intubation within the first 7 days after surgery Secondary outcome: ICU and hospital length of stay; incidence of pneumonia, infection, and sepsis within the first month after surgery; hospital mortality. Pneumonia, infection, and sepsis were identified using standard definitions Mortality: O: 3CPAP: 0 Tracheal intubation rate: O: 10; CPAP: 1 ICU length of stay: O: 2.6; CPAP: 1.4 Hospital length of stay: O: 17; CPAP: 15 Treatment failure: O: 2 CPAP: 4 Adverse effects: not reported Participant compliance: O: 30 CPAP: 7 Drop‐outs/withdrawals: O: 2; CPAP: 4 |
|
| Notes |
Declarations of interest: none reported Funding sources for the study: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomly allocated |
| Allocation concealment (selection bias) | Low risk | Concealed randomization was conducted centrally through a dedicated web site using a computer‐generated block randomization schedule |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible. Allocation to treatment with oxygen or oxygen plus CPAP was not blinded. To minimize potential bias in the assessment of some of the study end points, the study author used measures such as objective criteria for endotracheal intubation and standardization of all co‐interventions that could have influenced outcome variables such as anaesthesia, postoperative pain control, and respiratory physiotherapy. We contacted the authors of this study to ask about blinding of study participants and personnel but did not obtain an answer from them |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported. The authors only stated that the people responsible for the interventions were not involved in the study. We contacted the authors of this study to ask about blinding of study outcome assessors but did not obtain an answer |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review were reported in the pre‐specified way |
| Other bias | Low risk | This study was not a cluster randomized trial or a cross‐over trial. This study was a randomized controlled trial. The participants were randomized. The distribution of the participants was balanced between the control and intervention groups. The authors did not deviate from the study protocol |
CPAP: continuous positive airway pressure; FiO2: fraction of inspired oxygen; ICU: intensive care unit; ITT: Intention‐to‐treat; M: men; min: minute; NPPV: noninvasive positive pressure ventilation; O: oxygen; PaCO2: partial pressure of carbon dioxide; PaO2: partial pressure of oxygen; PEEP: positive end‐expiratory pressure; pH: hydrogen potential; PSV: pressure support ventilation; SD: standard deviation; VT: tidal volume; W: women.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Benditt 2009 | Not a randomized controlled trial. It is a review of the literature |
| Campbell 1986 | The study intervention (PEEP) was different from the intervention included in this review CPAP or bilevel NPPV compared to oxygen therapy) |
| Conti 2007 | Not a randomized controlled trial. It was a matched‐controlled study |
| Cypel 2014 | The study population was different from the population included in this review. This study evaluated different interventions during the intraoperative period and our study evaluated interventions in participants who developed acute respiratory insufficiency in the postoperative period |
| Delclaux 2000 | The study population was different from the population included in this review. They evaluated adults admitted with acute respiratory failure secondary to pulmonary oedema |
| Denehy 2001 | The intervention studied was different from that proposed in this review. They compared periodic mask CPAP with physiotherapy after abdominal surgery |
| Foster 2005 | Not a randomized controlled trial. It was a comment on Squadrone 2005 |
| Frangos 2005 | Not a randomized controlled trial. It was a comment on Squadrone 2005 |
| Jaber 2005 | Not a randomized controlled trial. It was a case series |
| Kiil 2003 | The study population was different from the population included in this review. The aim of this study was to investigate the haemodynamic effects of CPAP in the late postoperative period in the general surgical ward |
| Kindgen‐Milles 2000 | Not a randomized controlled trial. It was a case series |
| Lee 2011 | Not a randomized controlled trial. It was a retrospective study |
| Michelet 2006 | The study intervention was different from the intervention included in this review. Participants underwent invasive mechanical ventilation |
| Souza 2012 | The study intervention was different from the intervention included in this review. The authors evaluated the use of CPAP therapy during the pre‐operative period |
| Tarraza 2001 | Not a randomized controlled trial. It was a case series |
| Zhang 2015 | The study intervention (PEEP) was different from the intervention included in this review CPAP or bilevel NPPV compared to oxygen therapy) |
CPAP: continuous positive airway pressure; NPPV: noninvasive positive pressure ventilation;
PEEP: positive end‐expiratory pressure.
Differences between protocol and review
We modified the title of our systematic review to make it clear what population the review will address.
We changed the term used in the protocol "incidence of tracheal intubation" to "rate of tracheal intubation" to facilitate understanding (Faria 2011).
We added the outcome "Changes in arterial blood gas levels" because it was part of our secondary objectives but was not included as an outcome in the protocol.
We defined how we would measure the primary outcome:
Hospital mortality (measured from the date of randomization to the time of discharge from hospital);
Rate of tracheal intubation (measured in number of events occurred from the date of randomization to the time of discharge from hospital).
We did not search the grey literature as proposed in the protocol.
Unit of analysis issues: we did not find any cross‐over trials; however, if we find some in future updates, we will use only the first period of intervention provided that complies with the criteria for the inclusion.
Contributions of authors
Conceiving the review: DF, FV, AA, ES.
Co‐ordinating the review: FV.
Undertaking manual searches: DF, FV.
Screening search results: DF, FV.
Organizing retrieval of papers: DF, FV.
Screening retrieved papers against inclusion criteria: DF, FV.
Appraising quality of papers: DF, FV.
Abstracting data from papers: DF, FV.
Writing to authors of papers for additional information: AA, ES, DF.
Providing additional data about papers: AA, ES, DF.
Obtaining and screening data on unpublished studies: DF, FV, ES.
Data management for the review: FV, ES, DF.
Entering data into Review Manager 5 (RevMan 2014): DF.
Review Manager 5 statistical data: DF, FV.
Other statistical analysis not using Review Manager 5: FV, ES.
Double entry of data: (data entered by person one: DF; data entered by person two: DF, FV).
Interpretation of data: DF, FV.
Statistical inferences: AA, ES.
Writing the review: DF.
Securing funding for the review: DF.
Performing previous work that was the foundation of the present study: DF.
Guarantor for the review (one author): DF.
Person responsible for reading and checking review before submission: DF, FV.
Sources of support
Internal sources
No sources of support supplied
External sources
Department of Emergency Medicine of Universidade Federal de São Paulo, UNIFESP, Brazil; Brasilian Cochrane Center, Brazil.
Declarations of interest
Debora de Almeida Silva Faria: none known.
Edina MK da Silva: none known.
Álvaro N Atallah: none known.
Flávia MR Vital: none known.
New
References
References to studies included in this review
Gao 2002 {published data only}
- Gao Y, Hong T, Wang XR, Hang YN. Comparison of noninvasive ventilation and oxygen mask in elderly patients with tachypnea after upper abdominal surgery [老年病人上腹部手术后无创通气与面罩吸氧的疗效比较]. Clinical Medical Journal of China 2002;9(5):570‐1. [CENTRAL: CN‐00450659] [Google Scholar]
Squadrone 2005 {published data only}
- Squadrone V, Coha M, Cerutti E, Schellino MM, Biolino P, Occella P, et al. Continuous positive airway pressure for treatment of postoperative hypoxemia: a randomized controlled trial. JAMA 2005;293(5):589‐95. [PUBMED: 15687314] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Benditt 2009 {published data only}
- Benditt JO. Novel uses of noninvasive ventilation. Respiratory Care 2009;54(2):212‐22. [PUBMED: 19173753] [PubMed] [Google Scholar]
Campbell 1986 {published data only}
- Campbell T, Ferguson N, McKinlay RGC. The use of a simple self‐administered method of positive expiratory pressure (PEP) in chest physiotherapy after abdominal surgery. Physiotherapy 1986;72(10):498‐500. [ISSN: 0031‐9406] [Google Scholar]
Conti 2007 {published data only}
- Conti G, Cavaliere F, Costa R, Craba A, Catarci S, Festa V, et al. Noninvasive positive‐pressure ventilation with different interfaces in patients with respiratory failure after abdominal surgery: a matched‐control study. Respiratory Care 2007;52(11):1463‐71. [PUBMED: 17971249] [PubMed] [Google Scholar]
Cypel 2014 {published data only}
- Cypel M, Fan E. Lung injury after abdominal and thoracic surgery. Lancet. Respiratory Medicine 2014;2(12):949‐50. [PUBMED: 25466340] [DOI] [PubMed] [Google Scholar]
Delclaux 2000 {published data only}
- Delclaux C, L'Her E, Alberti C, Mancebo J, Abroug F, Conti G, et all. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA 2000;284(18):2352‐60. [PUBMED: 11066186] [DOI] [PubMed] [Google Scholar]
Denehy 2001 {published data only}
- Denehy L, Carroll S, Ntoumenopoulos G, Jenkins S. A randomized controlled trial comparing periodic mask CPAP with physiotherapy after abdominal surgery. Physiotherapy Research International 2001;6(4):236‐50. [PUBMED: 11833245] [DOI] [PubMed] [Google Scholar]
Foster 2005 {published data only}
- Foster R, Scales DC. Can CPAP prevent the need for endotracheal intubation in patients with hypoxemia after abdominal surgery?. CMAJ: Canadian Medical Association Journal 2005;172(6):744. [PUBMED: 15772998] [DOI] [PMC free article] [PubMed] [Google Scholar]
Frangos 2005 {published data only}
- Frangos SG, Schwartz DR, Ranieri VM, Squadrone V. Continuous positive airway pressure and postoperative hypoxemia. JAMA 2005;293(22):2714‐5. [ISSN: 0098‐7484] [DOI] [PubMed] [Google Scholar]
Jaber 2005 {published data only}
- Jaber S, Delay JM, Chanques G, Sebbane M, Jacquet E, Souche B, et al. Outcomes of patients with acute respiratory failure after abdominal surgery treated with noninvasive positive pressure ventilation. Chest 2005;128(4):2688‐95. [PUBMED: 16236943] [DOI] [PubMed] [Google Scholar]
Kiil 2003 {published data only}
- Kiil C, Rosenberg J. Hemodynamic effects of nasal continuous positive airway pressure after abdominal surgery. Journal of Anesthesia 2003;17(2):136‐8. [PUBMED: 12903927] [DOI] [PubMed] [Google Scholar]
Kindgen‐Milles 2000 {published data only}
- Kindgen‐Milles D, Buhl R, Gabriel A, Bohner H, Muller E. Nasal continuous positive airway pressure: a method to avoid endotracheal reintubation in postoperative high‐risk patients with severe nonhypercapnic oxygenation failure. Chest 2000;117(4):1106‐11. [PUBMED: 10767248] [DOI] [PubMed] [Google Scholar]
Lee 2011 {published data only}
- Lee BC, Kyoung KH, Kim YH, Hong SK. Non‐invasive ventilation for surgical patients with acute respiratory failure. Journal of the Korean Surgical Society 2011;80(6):390‐6. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Michelet 2006 {published data only}
- Michelet P, D'Journo X, Roch A, Doddoli C, Marin V, Papazian L, et all. Protective ventilation influences systematic inflammation after esophagectomy: a randomized controlled study. Anesthesiology 2006;105(5):911‐19. [PUBMED: 17065884] [DOI] [PubMed] [Google Scholar]
Souza 2012 {published data only}
- Souza FSP, Silva BG, Echevarria LB, Antunes MAS, Pessoti E, Forti EMP. Respiratory physiotherapy associated with airway positive pressure in the postoperative bariatric surgery evolution [Fisioterapia respiratória associada à pressão positiva nas vias aéreas na evolução pós‐operatória da cirurgia bariátrica]. Fisioterapia e Pesquisa 2012;19(3):204‐9. [DOI: ] [Google Scholar]
Tarraza 2001 {published data only}
- Tarraza GB, Bernucci F, Apablaza F, Peña VS, Zuñiga P, Tomicic VF, et al. Noninvasive ventilation with positive pressure in patients with acute hypoxemic respiratory failure [Ventilación con presión positiva no invasiva, VPPNI, en pacientes con insuficiencia respiratoria aguda hipoxémica]. Revista Chilena de Medicina Intensiva 2001;16(1):36‐40. [Lilacs: 290200] [Google Scholar]
Zhang 2015 {published data only}
- Zhang X, Wang Q, Zhang S, Tan W, Wang Z, Li J. The use of a modified, oscillating positive expiratory pressure device reduced fever and length of hospital stay in patients after thoracic and upper abdominal surgery: a randomized trial. Journal of Physiotherapy 2015;61(1):16‐20. [DOI] [PubMed] [Google Scholar]
Additional references
Anand 2010
- Anand RJ. Prophylactic use of noninvasive positive pressure ventilation after video‐assisted thoracoscopic surgery (VATS). Journal Thoracic Disease 2010;2(4):194‐6. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arozullah 2000
- Arozullah AM, Daley J, Henderson WG, Khuri SF. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. Annals of Surgery 2000;232(2):242‐53. [PUBMED: 10903604] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brochard 2000
- Brochard L. What is really important to make noninvasive ventilation work?. Critical Care Medicine 2000;28(6):1785‐90. [PUBMED: 10890682] [DOI] [PubMed] [Google Scholar]
Brown 2005
- Brown SR, Tiernan J. Transverse verse midline incisions for abdominal surgery. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005199.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burns 2010
- Burns KEA, Meade MO, Premji A, Adhikari NKJ. Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD004127.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chatila 2002
- Chatila WM, Criner GJ. Complication of long‐term mechanical ventilation. Respiratory Care Clinics of North America 2002;8(4):631‐47. [PUBMED: 12602419] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by simple, graph test. BMJ 1997;315(7109):629‐34. [PUBMED: 9310563 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 2001
- Evans TW. International Consensus Conferences in Intensive Care Medicine: non‐invasive positive pressure ventilation in acute respiratory failure. Organised jointly by the American Thoracic Society, the European Respiratory Society, the European Society of Intensive Care Medicine, and the Société de Réanimation de Langue Française, and approved by the ATS Board of Directors, December 2000. Intensive Care Medicine 2001; Vol. 27, issue 1:166‐78. [PUBMED: 11280630] [DOI] [PubMed]
Fagevik 2002
- Fagevik MO, Wennberg E, Johnsson E, Josefson K, Lönroth H, Lundell L. Randomized clinical study of the prevention of pulmonary complications after thoracoabdominal resection by two different breathing techniques. British Journal of Surgery 2002;89(10):1228‐34. [PUBMED: 12296888] [DOI] [PubMed] [Google Scholar]
Ferreyra 2008
- Ferreyra GP, Baussano L, Squadrone V, Richiardi L, Marchiaro G, Sorbo LD, et al. Continuous positive airway pressure for treatment of respiratory complications after abdominal surgery: a systematic review and meta‐analysis. Annals of Surgery 2008;247(4):617‐26. [PUBMED: 18362624] [DOI] [PubMed] [Google Scholar]
GRADEpro 2011 [Computer program]
- Brozek JL, Exman A, Schünemann HJ. GRADEprofiler. GRADEprofiler, 2011.
Hedenstierna 1990
- Hedenstierna G. Gas exchange during anaesthesia. British Journal of Anaesthesia 1990;64(4):507‐14. [PUBMED: 2185816] [DOI] [PubMed] [Google Scholar]
Heyland 1999
- Heyland DK, Cook DJ, Griffith L, Keenan SP, Brun‐Buisson C. The attributable morbidity and mortality of ventilator‐associated pneumonia in the critically ill patient. The Canadian Critical Trials Group. American Journal of Respiratory and Critical Care Medicine 1999;159(4 Pt1):1249‐56. [PUBMED: 10194173] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org 2011.
Holanda 2001
- Holanda MA, Oliveira CH, Rocha EM, Bandeira RM, Aguiar IV, Leal W, et al. Non‐invasive positive pressure ventilation in patients with acute respiratory failure: factors associated with failure or success [Ventilação não‐invasiva com pressão positiva em pacientes com insuficiência respiratória aguda: fatores associados à falha ou ao sucesso]. Brasilian Journal of Pulmonology 2001;27(6):301‐9. [Google Scholar]
Jansen 1990
- Jansen JE, Sorensen AI, Naesh O, Erichsen CJ, Pedersen A. Effect of doxapram on postoperative pulmonary complication after upper abdominal surgery in high‐risk patients. Lancet 1990;335(8695):936‐8. [PUBMED: 1970027] [DOI] [PubMed] [Google Scholar]
Joris 1997
- Joris JL, Sottiaux TM, Chiche JD, Desaive CJ, Lamy ML. Effect of bi‐level positive airway pressure (BiPAP) on the postoperative pulmonary restrictive syndrome in obese patients undergoing gastroplasty. Chest 1997;111(3):665‐70. [PUBMED: 9118706] [DOI] [PubMed] [Google Scholar]
Kramer 1995
- Kramer N, Meyer TJ, Meharg J, Cece RD, Hill NS. Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. American Journal of Respiratory and Critical Care Medicine 1995;151(6):1799‐806. [PUBMED: 7767523] [DOI] [PubMed] [Google Scholar]
Lawrence 1996
- Lawrence VA, Dhanda R, Hilsenbeck SG, Page CP. Risk of pulmonary complications after elective abdominal surgery. Chest 1996;110(3):744‐50. [PUBMED: 8797421] [DOI] [PubMed] [Google Scholar]
Lee 2014 [Computer program]
- www.lee.dante.br. Lee ‐ Laboratory of Epidemiology and Statistics [Lee ‐ Laboratório de Epidemiologia e Estatistica]. www.lee.dante.br, (accessed 20 March 2014).
Lång 2001
- Lång M, Niskanen M, Miettinen P, Alhava E, Takala J. Outcome and resource utilization in gastroenterological surgery. British Journal of Surgery 2001;88(7):1006‐14. [PUBMED: 11442536] [DOI] [PubMed] [Google Scholar]
Mal 2014
- Mal S, McLeod S, Iansavichene A, Dukelow A, Lewell M. Effect of out‐of‐hospital noninvasive positive‐pressure supports ventilation in adult patients with severe respiratory distress: a systematic review and meta‐analysis. Annals Emergency Medicine 2014; Vol. 63, issue 5:600‐7. [DOI: ] [DOI] [PubMed]
Mehta 1997
- Mehta S, Jay GD, Woolard RH, Hipona RA, Connolly EM, Cimini DM. Randomized, prospective trial of bilevel versus continuous positive airway pressure in acute pulmonary edema. Critical Care Medicine 1997;25(4):620‐8. [PUBMED: 9142026] [DOI] [PubMed] [Google Scholar]
Michelet 2009
- Michelet P, D'Journo XB, Seinaye F, Forel JM, Papazian L, Thomas P. Non‐invasive ventilation for treatment of postoperative respiratory failure after oesophagectomy. British Journal of Surgery 2009;96(1):54‐60. [PUBMED: 19108006] [DOI] [PubMed] [Google Scholar]
Michelet 2010
- Michelet P, Blayac D, Jaber S. Case scenario: management of postesophagectomy respiratoy failure with noninvasive ventilation. Anesthesiology 2010;113:454‐61. [DOI] [PubMed] [Google Scholar]
Nava 1993
- Nava S, Ambrosino N, Rubini F. Non‐invasive mechanical ventilation in the treatment of acute respiratory failure in chronic obstructive pulmonary disease. Monaldi Archives for Chest Disease 1993;48(2):144‐54. [PUBMED: 8518776] [PubMed] [Google Scholar]
Nourdine 1999
- Nourdine K, Combes P, Carton MJ, Beuret P, Cannamela A, Ducreux JC. Does noninvasive ventilation reduce the ICU nosocomial infection risk? A prospective clinical survey. Intensive Care Medicine 1999;25(6):567‐73. [PUBMED: 10416907] [DOI] [PubMed] [Google Scholar]
O'Donohue 1985
- O'Donohue WJ Jr. National survey of the usage of lung expansion modalities for the prevention and treatment of postoperative atelectasis following abdominal and thoracic surgery. Chest 1985;87:76‐80. [PUBMED: 3880695 ] [DOI] [PubMed] [Google Scholar]
Pasquina 2006
- Pasquina P, Tramèr MR, Granier JM, Walder B. Respiratory physiotherapy to prevent pulmonary complications after abdominal surgery: a systematic review. Chest 2006;130(6):1887‐99. [PUBMED: 17167013] [DOI] [PubMed] [Google Scholar]
Penuelas 2007
- Penuelas O, Frutos‐Vivar F, Esteban A. Noninvasive positive‐pressure ventilation in acute respiratory failure. CMAJ: Canadian Medical Association Journal 2007;177(10):1211‐8. [PUBMED: 17984471] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pingleton 1988
- Pingleton S. Complications of acute respiratory failure. American Review of Respiratory Disease 1988;137(6):1463‐93. [PUBMED: 3059862] [DOI] [PubMed] [Google Scholar]
Rathgeber 1997
- Rathgeber J, Schorn B, Falk V, Kazmaier S, Spiegel T, Burchardi H. The influence of controlled mandatory ventilation (CMV), intermittent mandatory ventilation (IMV) and biphasic intermittent positive airway pressure (BIPAP) on duration of intubation and consumption of analgesics and sedatives. A prospective analysis in 596 patients following adult cardiac surgery. European Journal of Anaesthesiology 1997;14(6):576‐82. [PUBMED: 9466092] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rusca 2003
- Rusca M, Proietti S, Schnyder P, Frascarolo P, Hedenstierna G, Spahn DR, et al. Prevention of atelectasis formation during induction of general anesthesia. Anesthesia and Analgesia 2003;97(6):1835‐9. [PUBMED: 14633570] [DOI] [PubMed] [Google Scholar]
Siafakas 1999
- Siafakas NM, Mitrouska I, Argiana E, Bouros D. Effects of surgery on the function of the respiratory muscles. Monaldi Archives for Chest Disease 1999;54(6):526‐31. [PUBMED: 10695325] [PubMed] [Google Scholar]
Stock 1985
- Stock MC, Downs JB, Gauer PK, Alster JM, Imrey PB. Prevention of postoperative pulmonary complications with CPAP, incentive spirometry, and conservative therapy. Chest 1985;87(2):151‐7. [PUBMED: 3881226] [DOI] [PubMed] [Google Scholar]
Stoltzfus 2006
- Stoltzfus S. The role of noninvasive ventilation: CPAP and BiPAP in the treatment of congestive heart failure. Dimensions Of Critical Care Nursing 2006;25(2):66‐70. [PUBMED: 16552275] [DOI] [PubMed] [Google Scholar]
Struik 2014
Sun 2014
- Sun T, Wan Y, Kan Q, Yang F, Yao H, Guan F, et al. Efficacy of noninvasive ventilation on in‐hospital mortality in patients with acute cardiogenic pulmonary edema: a meta‐analysis. Zhonghua Xin Xue Guan Bing Za Zhi 2014; Vol. 42, issue 2:161‐8. [PUBMED: 24735630] [PubMed]
Thompson 2003
- Thompson JS, Baxter BT, Allison JG, Johnson FE, Lee KK, Park WY. Temporal patterns of postoperative complications. Archives of Surgery 2003;138(6):596‐602. [PUBMED: 12799329] [DOI] [PubMed] [Google Scholar]
Vitacca 2001
- Vitacca M, Ambrosino N, Clini E, Porta R, Rampulla C, Lanini B, et al. Physiological response to pressure support ventilation delivered before and after extubation in patients not capable of totally spontaneous autonomous breathing. American Journal of Respiratory and Critical Care Medicine 2001;164(4):638‐41. [PUBMED: 11520729] [DOI] [PubMed] [Google Scholar]
Vital 2013
- Vital FMR, Ladeira MT, Atallah ÁN. Non‐invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD005351.pub3] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Faria 2011
- Faria DDAS, Silva EMK, Atallah ÁN, Vital FMR. Noninvasive positive pressure ventilation for upper abdominal surgery. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD009134] [DOI] [PMC free article] [PubMed] [Google Scholar]


