Abstract
This disproportionality analysis uses the World Health Organization pharmacovigilance database to explore the potential safety signal of facial paralysis after COVID-19 vaccination.
During the pivotal phase 3 clinical trials of mRNA COVID-19 vaccines, several cases of facial paralysis were observed in the vaccine groups (7 of 35 654) compared with 1 case among people who received placebo (1 of 35 611).1,2 Although a causal relationship could not be established from clinical trials, the US Food and Drug Administration recommended monitoring vaccine recipients for facial paralysis. We thus explored this potential safety signal through a disproportionality analysis using the World Health Organization pharmacovigilance database, VigiBase.
Methods
Disproportionality analyses are hypothesis-generating methods that aim to detect putative associations between drugs and adverse drug reactions. Such methods quantify the extent to which a drug–event combination occurs disproportionally compared with what would be expected in the absence of any association, but they do not provide risk quantification because the population actually exposed to the drugs is unknown. Several frequentist, multivariate, and Bayesian disproportionality methods have been developed to date. In this study, we generated disproportionality signals through the Bayesian neural network method, which was deemed significant if the lower boundary of the 95% credible interval of the information component (IC025) was greater than 0.3 The CECIC Rhône-Alpes-Auvergne, Clermont-Ferrand, IRB 5891 determined that institutional review board approval and informed consent were not necessary owing to the use of retrospective, deidentified data.
Briefly, we performed 4 analyses with 2 control groups (all other viral vaccines and restricted to influenza vaccines) and 2 facial paralysis definitions (broad and narrow). All analyses were adjusted for sex and age. The statistical analysis was performed with R, version 3.6.2 (R Foundation). Details on methods used are presented in the eMethods in the Supplement.
Results
On March 9, 2021, among the 133 883 cases of adverse drug reactions reported with mRNA COVID-19 vaccines in the World Health Organization pharmacovigilance database, we identified a total of 844 (0.6%) facial paralysis-related events, including 683 cases of facial paralysis, 168 cases of facial paresis, 25 cases of facial spasms, and 13 cases of facial nerve disorders (some adverse events were coreported in the same case). A total of 749 cases were reported with the Pfizer-BioNTech vaccine, and 95 cases were reported with the Moderna vaccine. Of the 844 patients, 572 were female (67.8%), and the median (interquartile range) age was 49 (39-63) years. The median (range) time to onset was 2 (0-79) days. Case characteristics are summarized in the Table. Moreover, we identified 5734 (0.5%) and 2087 (0.7%) cases of facial paralysis among the 1 265 182 cases of adverse drug reactions reported with other viral vaccines and the 314 980 cases reported with influenza vaccines, respectively. We did not detect any signal of disproportionality of facial paralysis for broad and narrow definitions vs other viral vaccines (IC025 = −0.01 and IC025 = −0.06) or influenza vaccines alone (IC025 = −1.36 and IC025 = −0.32) (Figure).
Table. Characteristics of mRNA COVID-19 Vaccine–Related Facial Paralysis Cases Reported in the WHO Pharmacovigilance Database.
| Characteristic | No. (%) |
|---|---|
| Sex | |
| Female | 572 (67.8) |
| Male | 260 (30.8) |
| Missing data | 12 (1.4) |
| Age, y | |
| ≤11 | 4 (0.5) |
| 12-17 | 0 |
| 18-44 | 308 (36.5) |
| 45-64 | 295 (35.0) |
| 65-74 | 67 (7.9) |
| ≥75 | 117 (13.9) |
| Missing data | 53 (6.3) |
| Most reporting countries | |
| US | 312 (37.0) |
| United Kingdom | 196 (23.2) |
| Italy | 73 (8.6) |
| France | 52 (6.2) |
| Spain | 32 (3.8) |
| Germany | 31 (3.7) |
| Drug (WHO drug trade name) | |
| Pfizer BioNTech COVID-19 Vaccine | 749 (88.7) |
| Moderna COVID-19 vaccine | 95 (11.3) |
| Seriousnessa | |
| Yes | 473 (56.0) |
| No | 371 (44.0) |
| Reported facial symptoms (MedDRA preferred terms) | |
| Paralysis | 698 (80.9) |
| Paresis | 168 (19.9) |
| Spasm | 25 (3.0) |
| Nerve disorder | 13 (1.5) |
| Most associated symptoms (coreported MedDRA preferred terms) | |
| Headache | 113 (13.4) |
| Hypoesthesia | 107 (12.7) |
| Paresthesia | 93 (11.0) |
| Fatigue | 63 (7.5) |
| Hypoesthesia, oral | 40 (4.7) |
| Evolution | |
| Recovered | 179 (19.8) |
| Recovered with sequelae | 3 (0.3) |
| Recovering | 120 (13.3) |
| Not recovered | 216 (23.9) |
| Death | 1 (0.1) |
| Unknown | 386 (42.7) |
Abbreviations: MedDRA, Medical Dictionary for Regulatory Activities; WHO, World Health Organization.
Fatal, life-threatening, requiring hospitalization, resulting in significant disability/incapacity, and other medically important conditions.
Figure. Forest Plot of the Information Component Values of mRNA COVID-19 Vaccine–Related Facial Paralysis vs All Other Viral Vaccines and Influenza Vaccines Alone.
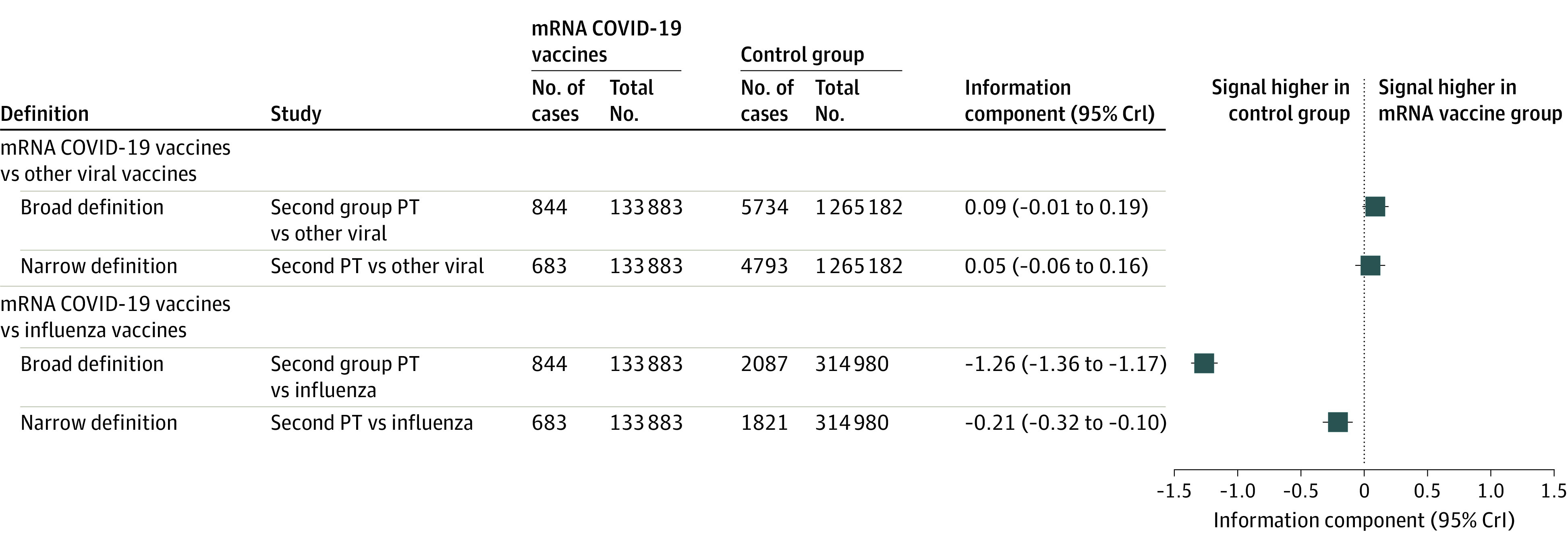
Number of facial paralysis cases (No. of cases) and total number of adverse drug reaction cases (Total No.) reported in the World Health Organization pharmacovigilance database are described. Broad definitions of facial paralysis correspond to the following Medical Dictionary for Regulatory Activities (MedDRA) preferred terms (PTs): facial nerve disorder, facial paralysis, facial paresis, facial spasm, oculofacial paralysis, VIIth nerve injury; narrow definition, only to the facial paralysis PT. CrI indicates credible interval.
Discussion
Facial paralysis can be observed in the context of many conditions, such as viral infections, traumatic injury, cancers, or hormonal changes during pregnancy. Idiopathic causes, also known as Bell palsy, are unilateral, generally reverse spontaneously, and cause partial or complete acute weaknesses of the face.4 Isolated facial paralysis after vaccination has been reported as case reports for decades with almost all viral vaccines, and it is thought to be immune mediated or induced by viral reactivations (eg, reactivation of a herpes virus infection).5 However, to date, pharmacoepidemiological studies have failed to identify a higher risk of facial paralysis after vaccination.5,6
When compared with other viral vaccines, mRNA COVID-19 vaccines did not display a signal of facial paralysis. As of March 9, 2021, more than 320 million COVID-19 vaccine doses had been administered worldwide. Therefore, despite selective reporting and a potential delay in reporting and transferring cases among pharmacovigilance databases, the reporting rate of facial paralysis after mRNA COVID-19 vaccination found in the present study is not higher than that observed with other viral vaccines. Although we adjusted for sex and age, residual confounding and reporting bias may influence the results. To conclude, if an association between facial paralysis and mRNA COVID-19 vaccines exists, the risk is likely very low, as with other viral vaccines.
eMethods.
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bate A, Lindquist M, Edwards IR, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998;54(4):315-321. doi: 10.1007/s002280050466 [DOI] [PubMed] [Google Scholar]
- 4.Holland NJ, Bernstein JM. Bell’s palsy. BMJ Clin Evid. 2014;2014:1204. [PMC free article] [PubMed] [Google Scholar]
- 5.Kamath A, Maity N, Nayak MA. Facial paralysis following influenza vaccination: a disproportionality analysis using the Vaccine Adverse Event Reporting System Database. Clin Drug Investig. 2020;40(9):883-889. doi: 10.1007/s40261-020-00952-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowhani-Rahbar A, Klein NP, Lewis N, et al. Immunization and Bell’s palsy in children: a case-centered analysis. Am J Epidemiol. 2012;175(9):878-885. doi: 10.1093/aje/kws011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.


