Abstract
Using a two-wave online experiment, we investigate whether COVID-19 exposure changes participants' threat-detection threshold. Threat reactivity was measured in a signal detection task among 277 British adults who also reported how vulnerable they felt to infectious diseases. Participants' data were then matched to the local number of confirmed COVID-19 cases announced by the NHS every day. We found that participants who perceive themselves as more likely to catch infectious diseases displayed higher threat reactivity in response to increased COVID-19 cases.
Keywords: Threat reactivity, Disease vulnerability, Pathogen threat, COVID-19, Error-management theory
1. Introduction
Having an accurate perception of potential risks and benefits is essential to optimal decision-making. Although all errors should ideally be avoided, some errors are costlier than others. False positives and false negatives indeed have asymmetric consequences for the individual because failing to detect a threat when there is one is more dangerous than believing that there is a threat when there is none (Haselton & Buss, 2000). Given this asymmetry, error-management theory predicts that individuals should be biased to over-detect negative events. In line with this idea, surveys consistently show that people over-perceive all kinds of threats, such as crime rates, terrorism or unemployment risk (e.g., Lieder et al., 2018).
To be adaptive however, threat reactivity must be sensitive to individual circumstances, such as physical condition or age (Nettle & Bateson, 2012). For example, people in poor physical condition can escape less easily from threats, which means that for them, more so than for people in better shape, having a lower threat detection threshold is particularly adaptive. In line with this idea, people in poor physical shape are more prone to the auditory looming effect, a bias that helps us get ready to move away from approaching objects by making us hear sounds that are coming towards us as closer than sounds going away from us (Neuhoff et al., 2012). Similarly, women who perceive themselves as more vulnerable to sexual coercion tend to stereotype out-group men as more threatening (McDonald et al., 2015). Such inter-individual variations demonstrate that threat over-perception is affected by various individual factors.
Among these factors, perceived vulnerability to diseases plays a particularly important role to calibrate threat perception. Individuals who perceive themselves as more sensitive to diseases have stronger reactions to disease cues (Navarrete & Fessler, 2006; Reid et al., 2012). Stahl and Metzger (2013) found that the implicit association between old age and pathogen cues is amplified among participants with a high perceived vulnerability to diseases. Similarly, better perceived health in children is also associated with lower fear and disgust of disease-relevant animals (Prokop et al., 2010). Finally, research suggests that some xenophobic attitudes are associated with a fear of out-group members propagating foreign pathogens and that this relationship is also moderated by inter-individual variation in perceived vulnerability to diseases (Faulkner et al., 2004).
Arguably, the COVID-19 pandemic represents a considerable health threat. In line with previous research on pathogen threats, reactions to the COVID-19 pandemic are modulated by individuals' level of perceived vulnerability to diseases. Research conducted since March 2021 revealed that perceived vulnerability to diseases predicts increased preventive behaviors such as washing hands, wearing masks or social distancing and more support for public health measures (Stangier et al., 2021; De Coninck et al., 2020). These results suggest that feeling vulnerable to diseases increases people's reactivity to disease threats. In addition to these internal factors, readiness to react to threats should also respond to local circumstances. For instance, individuals living in a highly threatening environment should be tuned to react more readily to threat cues than individuals living in a less threatening environment (Nettle & Bateson, 2012). In line with this idea, a large-scale comparative experiment showed that the Hadza hunter-gatherers, who are more exposed to pathogens than Europeans on average, express a stronger preference for facial symmetry, a characteristic associated with higher immune competence, than British participants (Little et al., 2007). This suggests that a higher level of pathogen threat increases the reaction to disease-relevant cues.
Overall, the literature shows that the extent to which individuals over-perceive threats is conditioned by individual and contextual variables. In fact, these two mechanisms work in tandem and interact to produce greater effects on threat over-perception. Multiple studies find that threatening environments are a better predictor of increased pathogens-related threat detection for individuals who perceive themselves as more vulnerable to diseases than for individuals who feel safer (Duncan & Schaller, 2009; Little et al., 2007). For example, Little et al. (2007) reported that the preference for face symmetry is even stronger in Hadza pregnant women, who are more vulnerable to disease.
In the present paper, we further test this association of threat reactivity with internal and external variables by analyzing the effect of the COVID-19 epidemic on individuals' threat reactivity. The analysis of the COVID-19 pandemic allows to assess the effect of variations in diseases exposures within a population longitudinally. Using signal detection theory, we test the hypothesis that being surrounded by more COVID-19 cases, as measured by local COVID-19 prevalence, is associated with an increase in people's reactivity to threats. More specifically, we predict that this effect is modulated by individuals' perceived vulnerability to infectious diseases, with a larger effect among individuals who perceive themselves as highly vulnerable to diseases.
In order to test this hypothesis, we assessed subjective vulnerability to diseases and threat reactivity in a sample of participants at two time points: on March 24th and two weeks later, on April 7th. Local variations of the number of COVID-19 cases allow us to obtain quasi-experimental natural variations of threat exposure. For instance, on March 24th, the urban local tier of Wakefield reported only 13 cases, less than the mostly rural area of Wiltshire, which counted almost 3 times more cases. The evolution of the number of COVID-19 cases also varied from one region to the next. For instance, both Hounslow and Essex districts counted around 80 cases on March 24th but Essex district counted 938 cases two weeks later while Hounslow district counted only 279 more cases at that date. Similarly, while the district of Merton ranked 21st in the number of cases on Match 24th it fell to the 40th position on April 7th. Conversely, the district of Liverpool jumped from the 47th to 17th position in that same time interval. We thus exploited these local variations in order to assess the association between participants' reactivity to threat and the combination of exposure to disease threat and subjective vulnerability to diseases.
2. Methods
2.1. Ethics statement
Our study was approved by the local Ethical Committee (CERES n°201659; the same Ethical Committee number as a study conducted in January 2020 using a similar experimental design - information available at https://osf.io/g9chs - no data from our pilot study was used in the current paper, as both studies generated distinct datasets). Each participant received a description of the study and provided their informed consent before starting the experiment. All scripts and raw data are available in the OSF project (https://osf.io/5cexn/), together with a short report of the results of the 1st wave of the study, which we used as a pre-registration document (https://osf.io/wk54j/?show=revision, version 1).
2.2. Participants
352 UK participants recruited on Prolific Academic completed our study twice: once between March 24th at 2 PM and March 25th at 2 PM, and a second time between April 7th at 2 PM and April 8th at 2 PM. The 2 PM limit was chosen because daily figures of COVID19 cases are announced by the British government at that time. All participants received compensation for their time (£5 per hour), as well as a variable bonus depending on task performance (£0–0.50).
Participants were excluded based on the exact same criteria as previous studies that used the same experimental paradigm (Chevallier et al., 2016; Safra et al., 2021). In line with these pre-registered criteria (pre-registration available at: https://osf.io/wk54j/?show=revision, version 1), all trials with reaction times below 150 ms or above 2500 ms were excluded in both waves, 1 participant was removed for having mean reaction times at ±3 SD of the mean in the first wave and 4 were excluded in the second wave, 22 participants were then removed for having reaction times outside these ranges on more than 40% of the trials in the first wave and 8 were excluded based on this same criterion in the second wave. In addition, the presence of missing data in key independent variables resulted in the exclusion participants who had not answered the “Perceived Infectability and Germ Aversion” questionnaire (Duncan et al., 2009) (First wave: N = 7; Second wave: N = 27), 4 participants who had not provided their date of birth and 2 participants who provided an invalid postal code (and who thus could not be associated with a COVID-19 exposure score). In total, 75 participants were excluded. The final sample included 277 participants living in 112 geographical areas (158 Females, mean age: 38.43 ± 12.58 s.d. years).
2.3. Materials
The materials used for this task are adapted from Chevallier et al., 2016. The task was presented using Qualtrics. Monetary punishments were presented using text (“-5 pennies”), which represented the true amount that was subtracted from participants' total bonus payment at the end of the experiment. The punishments were provided in response to incorrect identifications of a line appearing in the center of a circle as being short (11.5 mm) or long (13 mm). Following the experimental task, participants completed questionnaires including standard socio-demographic questions (age, income, education, postal code of residence) as well as their perceived sensitivity to diseases.
2.4. Design and procedure
The design and procedure used for this task are adapted from Chevallier et al. (2016). The experiment was conducted online and lasted approximately 25 min. Participants were told that their task was to classify a line as either short or long by pressing the corresponding key and that feedback for incorrect responses would occur some of the time. The training phase consisted of 22 practice trials during which the difficulty of the task was progressively raised until real-game conditions were reached.
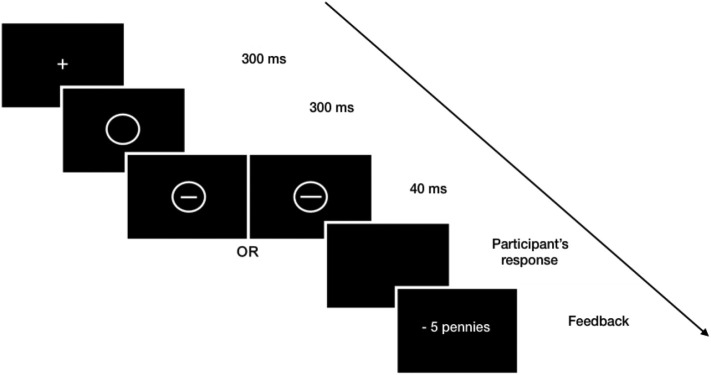
The experiment consisted of 300 trials separated in three 100-trial blocks. Each trial began with the presentation of a fixation cross (300 ms), followed by an empty circle (300 ms). The short or the long line was then flashed within the circle (40 ms) and disappeared to show a plain black screen during which participants could submit their response (Fig. 1 ). An equal number of short and long lines were presented within each block. Short and long lines were presented in a random order. Participants were given an infinite amount of time to indicate their response using ‘e’ or ‘p’ on the keyboard. Based on the reinforcing schedule, incorrect responses were followed by an 800 ms negative feedback screen with a probability of 75% or 25%, depending on the type of stimulus (see below). Otherwise, the next trial was immediately presented.
Fig. 1.
Schematic representation of the tasks.
A fixation cross appears for 300 ms, followed by an empty circle. A short or a long line is then flashed inside the circle for 40 ms. Participants have an infinite amount of time to respond before they receive a punishment for some of their incorrect responses.
An asymmetrical reinforcement ratio was introduced such that one type of line (i.e. short or long) was punished more often. The line type that was punished more frequently is referred to as the “harsh stimulus” and the line type that was punished less frequently is referred to as the “lenient stimulus”. Misidentifying the harsh line (i.e., if the harsh line is short, a misidentification is to respond “long”) was followed by a negative feedback with a probability of 75%. Misidentifying the lenient line (i.e., if the lenient line is long, a misidentification is to respond “short”) was followed by a negative feedback with a probability of 25%. The long line was randomly assigned to being the Harsh or the Lenient stimulus for each participant.
Preliminary analyses confirmed that the negative feedback was appropriately distributed across line types during the task. 75% of errors on the Harsh line and 26.2% of errors on the Lenient line were followed by a negative feedback (−5 pennies) on average in the first wave. Similar results were found in the second wave, where 74.5% of errors on the Harsh line and 25.9% of errors on the Lenient line were punished on average.
2.5. Threat reactivity
Following Nettle and Bateson (2012), threat reactivity was conceptualized as participants' bias to avoid threatening stimuli, i.e. stimuli associated with a higher probability punishment. In our task, this meant over-detecting the line for which misidentification was associated with more frequent punishment (i.e., the Harsh line). Indeed, by being associated with a higher probability of negative outcome – or punishment -, the Harsh stimulus is thus associated with a higher level of threat than the Lenient stimulus. Response bias towards the harsh line was computed using the standard signal detection measure:
with Harsh correct and Lenient correct corresponding to the proportion of correct identifications (hits and correct rejections) to the total number of harsh and lenient trials respectively, and Harsh incorrect and Lenient incorrect corresponding to the proportion of false identifications (misses and incorrect rejections) to the total number of harsh and lenient trials respectively. When accuracy was equal to 1 or 0, we followed the log linear correction procedure described by Hautus (1995).
Following Pizzagalli et al. (2005) and Chevallier et al. (2016), reactivity to threat was computed by measuring the change in bias towards the Harsh line between the first block (in which the participant is naive about the value of the two lines) and the last block (in which the participant has experienced that the misidentification of one line is more frequently associated with punishments). Importantly, the difference in response bias between the first and the last block provides a combined measure of two components of threat reactivity: the degree to which individuals avoid the ‘high threat’ Harsh stimulus and the speed at which they develop this differential behavior towards the Harsh and the Lenient stimulus.
2.6. Perceived infectability
At the end of the experiment, participants' susceptibility to infectious diseases was assessed using the “Perceived Vulnerability to Disease” questionnaire (Duncan et al., 2009). This questionnaire is composed of two subscales: the “Perceived Infectability” subscale which measures the self-reported susceptibility to infectious diseases, and the “Germ Aversion” subscale which assesses the level of affective responses to situations of high risk of disease transmission. In our study, we used “Perceived Infectability” as our measure of interest as it indicates how much the individual feels threatened by infectious diseases, while “Germ Aversion” was used as a control measure for general affective reactions to diseases. Participants completed these scales twice, once in Wave 1 and a second time in Wave 2.
Our data shows strong consistency in participants' response to the perceived vulnerability to disease questionnaire. We found a strong inter-temporal correlation between the two waves for the global disgust score (r = 0.86 ± 0.01 s.e.m., p < .001), perceived infectability score (r = 0.86 ± 0.01 s.e.m., p < .001) and the germ aversion score (r = 0.84 ± 0.02 s.e.m., p < .001).
2.7. Perception accuracy of COVID-19 cases
In Wave 2, participants answered a free-text question: “Without looking it up, what is your estimate of the number of people in your country who are currently infected?”. In our analysis, the answer was then subtracted from the actual number of cases registered on April 7th 2020 in the UK (55,242 cases, figure obtained from https://www.worldometers.info/coronavirus/country/uk/). This value was included in our model either without any transformation (referred to as the signed difference) or after being transformed to its absolute value to indicate to the amplitude of the participants' errors.
2.8. Socio-demographic information
In Wave 1, participants provided information about their age, gender and level of education. Additional questionnaires were also included at the very end of Wave 1 and Wave 2 (see Supplementary information for details).
2.9. Local COVID-19 exposure
The number of local COVID-19 cases was retrieved from the NHS website based on participants' postal codes. In our analysis, we used the raw number of cases per local area. For residents of England, we retrieved COVID-19 cases statistics at the upper tier local authority level (UTLA) from the NHS website. UTLAs are an administrative subdivision below the level of the region. For residents of Scotland, COVID-19 figures were given at the level of health board areas from the Scotland NHS website (there are 14 such areas). Finally, COVID-19 cases statistics for Wales and Northern Ireland were not available for smaller subdivisions. We thus considered Wales and Northern Ireland as just other administrative sub-divisions.
2.10. Analyses
Threat reactivity in Wave 1 was first analyzed using robust mixed linear regressions taking the number of reported COVID-19 cases in the upper tier local authority, germ aversion and perceived infectability, as well as the interaction between local exposure to COVID-19 and these two dimensions of perceived vulnerability to diseases as predictors. All the predictors were transformed into z-scores to avoid issues due to scaling differences. To control for individual and local effects independent of the exposure to COVID-19 (such as living in a rural area or in a city), participant ID and the upper tier local authority was included as a random factor. In order to further assess the robustness of our results, we conducted additional models with age, gender and level of education as additional predictors.
Finally, in order to compare participants' behavior across the two waves, we analyzed Waves 1 and 2 simultaneously using similar robust mixed linear regressions as previously but taking “Wave” as additional predictor.
3. Results
3.1. Stimulus identification
Our analyses show that participants identified the most punished line as predicted. Response bias towards the Harsh line - the tendency to mistakenly identify the Lenient line more than to mistakenly identify the Harsh line – increased throughout the 3 blocks (Wave 1: block 1: 0.20 ± 0.71 s.d., block 3: 0.43 ± 0.86 s.d.; Wave 2: block 1: 0.17 ± 0.71 s.d., block 3: 0.35 ± 0.83 s.d.). Importantly, the average difference between the response bias in the last and first block was significantly greater than 0 in both waves (Wave 1: mean difference = 0.24 ± 0.92 s.d., t(276) = 4.33, p < .001; Wave 1: mean difference = 0.18 ± 0.89 s.d., t(276) = 3.36, p < .001). These results show that participants learned to associate the harsh line with punishment throughout blocks and increasingly avoided the costliest error.
3.2. Perceived vulnerability to pathogens
Importantly, perceived vulnerability to pathogens was not significantly correlated with the local numbers of COVID-19 cases in Wave 1 (r = 0.01 ± 0.06 s.e.m., t(275) = 0.13, p > .250) or in Wave 2 (r = −0.01 ± 0.06 s.e.m., t(275) = −0.21, p > .250). Similarly, in both Waves, no significant correlation was found between the local number of COVID-19 cases and the subscales of Perceived Vulnerability to Disease: perceived infectability (Wave 1: r = −0.02 ± 0.06 s.e.m., t(275) = −0.37, p > .250; Wave 2: r = −0.04 ± 0.06 s.e.m., t(275) = −0.59, p > .250) and germ aversion (Wave 1: r = 0.03 ± 0.06 s.e.m., t(275) = 0.52, p > .250; Wave 2: r = 0.02 ± 0.06 s.e.m., t(275) = 0.26, p > .250).
3.3. Threat reactivity
No main effect of COVID-19 exposure was found on threat reactivity (b = −0.07 ± 0.06 s.e.m., z = −1.24, p = .215). However, there was a significant positive interaction between exposure to COVID-19 and perceived infectability (b = 0.15 ± 0.07 s.e.m., z = 2.27, p = .023) such that COVID-19 exposure predicted a greater threat reactivity level for participants who perceive themselves as more likely to catch infectious diseases than for participants with a lower perceived infectability score (Table 1 ). This interaction was robust to the inclusion of age, gender and education (interaction between perceived infectability and COVID-19 exposure: b = 0.18 ± 0.07 s.e.m., z = 2.48, p = .013; no other significant effect, Table 1) and was specific to perceived infectability as there was no significant effect of germ aversion (b = 0.08 ± 0.06 s.e.m., z = 1.31, p = .190; interaction: b = −0.00 ± 0.05 s.e.m., z = −0. 01, p > .250). The analysis of Waves 1 and 2 confirmed the positive interaction between exposure to COVID-19 and perceived infectability (b = 0.10 ± 0.05 s.e.m., z = 2.22, p = .026; Table 1). After controlling for demographic variables however, this interaction was only found as a trend (b = ±0.05 s.e.m., z = 1.85, p = .064; Table 1).
Table 1.
Coefficients of the mixed linear regressions on threat reactivity. Standardized regression coefficients are presented with the standard error to mean and the associated z value.
| Wave 1 |
Waves 1 & 2 |
|||
|---|---|---|---|---|
| Reduced model | Full model | Reduced model | Full model | |
| Intercept | 0.23 ± 0.06⁎⁎⁎z = 4.09 | 0.28 ± 0.08⁎⁎⁎z = 3.70 | 0.21 ± 0.06⁎⁎⁎z = 3.49 | 0.27 ± 0.07⁎⁎⁎z = 3.77 |
| COVID-19 exposure | −0.07 ± 0.06 z = −1.24 |
−0.09 ± 0.09 z = −0.96 |
−0.05 ± 0.05 z = −1.05 |
−0.04 ± 0.07 z = −0.54 |
| Perceived infectability | Main effect | |||
| −0.09 ± 0.06 z = −1.63 |
−0.10 ± 0.06 z = −1.66 |
−0.05 ± 0.04 z = −1.25 |
−0.06 ± 0.04 z = −1.53 |
|
| Interaction with COVID-19 exposure | ||||
|
0.15 ± 0.07⁎ z = 2.27 |
0.18 ± 0.07⁎ z = 2.48 |
0.10 ± 0.05⁎ z = 2.22 |
0.10 ± 0.06° z = 1.85 |
|
| Germ aversion | Main effect | |||
| 0.08 ± 0.06 z = 1.48 |
0.08 ± 0.06 z = 1.31 |
0.06 ± 0.04 z = 1.48 |
0.06 ± 0.04 z = 1.47 |
|
| Interaction with COVID-19 exposure | ||||
| 1.01 ± 0.05 z = 0.17 |
−0.00 ± 0.05 z = −0.01 |
−0.04 ± 0.03 z = −1.30 |
−0.05 ± 0.03 z = −1.39 |
|
| Age | Main effect | |||
| −0.02 ± 0.06 z = −0.36 |
0.04 ± 0.04 z = 1.10 |
|||
| Interaction with COVID-19 exposure | ||||
| −0.05 ± 0.08 z = −0.63 |
−0.04 ± 0.05 z = −0.72 |
|||
| Gender | Main effect | |||
| −0.12 ± 0.12 z = −1.06 |
−0.12 ± 0.08 z = −1.49 |
|||
| Interaction with COVID-19 exposure | ||||
| 0.05 ± 0.12 z = 0.43 |
−0.00 ± 0.09 z = −0.02 |
|||
| Education level | Main effect | |||
| 1.01 ± 0.06 z = 0.18 |
−0.01 ± 0.04 z = −0.15 |
|||
| Interaction with COVID-19 exposure | ||||
| −0.07 ± 0.08 z = −0.86 |
−0.04 ± 0.05 z = −0.72 |
|||
| Wave | −0.01 ± 0.09 z = −0.09 |
−0.03 ± 0.09 z = −0.36 |
||
The effect of interest (the interaction between Perceived infectability and COVID-19 exposure) is presented in bold.
Indicates a p-value inferior to 0.100.
A p-value inferior to 0.050.
A p-value inferior to 0.001.
To sum up, differences in the number of COVID-19 cases was not statistically associated with participants' threat reactivity but participants with a high sensitivity to diseases had a lower threat reactivity threshold when they lived in an area with many COVID-19 cases than when those who lived in an area with few COVID-19 cases. This suggests that threat reactivity may increase in response to higher levels of disease threat among those who perceived themselves as vulnerable to diseases.
One possible interpretation of our findings is that participants with a higher perceived sensitivity to diseases are also those who are more informed. This interpretation was not confirmed since perceived infectability was not correlated with participants' accuracy when they estimated the number of COVID-19 cases in the UK (r = 0.04 ± 0.06 s.e.m., t(275) = 0.66, p > .250; non-signed error: r = −0.04, t(275) = −0.63 ± 0.06 s.e.m., p > .250; measured in Wave 2). This suggests that participants with a high level of perceived infectability do not have a better or a more catastrophic image of the situation than those with a low level of perceived infectability. Moreover, the interaction between the number of COVID-19 cases and perceived infectability remained close to the 95% significance threshold after adjusting for participants' of the epidemics (after controlling for signed error: b = 0.09 ± 0.05 s.e.m., z = p = .049; after controlling for non-signed error: b = 0.09 ± 0.05 s.e.m., z = 1.89, p = .059), suggesting that differences in participants' knowledge of the pandemic does not fully account for the association between participants' threat reactivity, their perceived infectability and their exposure to COVID-19.
4. Discussion
In line with our hypothesis, our results revealed that variations in threat reactivity was associated to a combination of local threat level (as measured by the local prevalence of COVID-19) and individual sensitivity to threat: the more individuals perceived themselves as susceptible to infectious diseases, the more COVID-19 exposure increased their reactivity to threats. This association was present at the two time-points of the epidemics: on the day following the official lockdown enforcement in the UK and two weeks later. These results provide evidence that individuals react to the presence of survival threats by decreasing their threshold for responding to threats (Nettle & Bateson, 2012). These results are in line with previous findings by Makhanova and Shepherd (2020) suggesting that perceived infectability to diseases is associated with increased vigilance to health and disease-related issues in the context of the COVID-19 pandemic.
Going further, the present study suggests that psychological adjustments following an increase of threat in the environment acts at the global level of individuals' psychology. More precisely, it appears that disease threat, such as COVID-19 exposure, is associated to differences threat reactivity across domains, even if the threat is not linked to health, as it is the case with the financial punishments used in our experiment.
Crucially, the significant interaction between perceived infectability and local COVID-19 exposure suggests that this response is conditional on the level of perceived threat and not on the absolute level of threat in the environment. Therefore, it appears that this effect is genuinely mediated by individuals' perceived risks and benefits of adopting different strategies in the response to an environmental change. Importantly, this effect was independent from individuals' knowledge of the epidemic as well as their age, gender and level of education.
Further research is needed to refine our results. Firstly, we measured local exposure to COVID-19 using actual and not perceived numbers of COVID-19 cases in the United Kingdom. Indeed, administrative data on COVID-19 was readily available, reliable and straightforward to interpret. While perceived numbers of COVID-19 cases may influence behavior more than actual numbers, their measurement is not without ambiguity. In particular, simply asking participants about their perceived number of COVID-19 cases leaves unclear whether they perceive these numbers as benign or overwhelming. Instead, further research should directly ask participants how much they feel COVID-19 is prevalent around them, without asking for specific numbers, and compare the impacts of perceived and actual COVID-19 prevalence.
Secondly, as opposed to previous research, we do not observe a significant main effect of contextual cues and individual factors on threat reactivity, but only an interaction. This finding fits the objective characteristics of the COVID-19 epidemic: individuals with underlying conditions that make them more vulnerable to diseases in general are more likely to suffer from severe forms of COVID-19-related illness or death. To the extent that individuals are accurate in defining their perceived vulnerability to disease, it is not surprising that increased exposure to COVID-19 mostly increases the threat reactivity of those who feel most vulnerable.
Lastly, our study leaves unclear the extent to which the evidenced interaction of perceived infectability and exposure to COVID-19 is due to a confounding variable. Perceived infectability may result from an underlying stable personality trait, such as neuroticism. As we did not measure such domain-general stable traits, we are unable to determine whether increased threat reactivity results from a domain-specific mechanism that detects vulnerability to disease or a domain-general mechanism that reflects a general propensity for anxiety. Future research investigating similar questions would benefit from including stable psychological traits likely to correlate with threat detection in their analysis.
In a nutshell, our study provides evidence that individuals react to the presence of threats in their environment by decreasing their threshold for reacting to threats. Moreover, our results suggest that adaptation of low-level psychological variables is conditional on how much the threat that is present in the environment corresponds to a threat subjectively perceived as such by the individual. This underlines the importance of taking inter- individual differences into account when designing public policies in response to large scale public threats.
5. Pre-registration statement
We applied the same analyses as those pre-registered following the collection of the first wave (https://osf.io/wk54j/?show=revision, version 1). The results presented in the paper were conducted on the final sample, in other words on the participants who completed both waves. In addition, after the pre-registration, we automatically coded the correspondence between postal codes and upper tier local areas, which corrected issues due to the initial manual coding. The interaction between participants' level of education and exposure to COVID-19 initially reported in the pre-registration was no longer significant after the correction of the coding issues (see Table 1). Therefore, in the manuscript, education is only taken as a control variable.
The following is the supplementary data related to this article.
Additional questionnaires included at the end of Wave 1 or at the end of Wave 2.
CRediT authorship contribution statement
L. Safra: Validation, Investigation, Data curation, Writing – review & editing. A. Sijilmassi: Software, Investigation, Data curation, Writing – review & editing. C. Chevallier: Conceptualization, Supervision, Methodology, Resources, Writing – review & editing.
Acknowledgments
This study was supported by the EUR FrontCog grant ANR-17-EURE-0017.
References
- Chevallier C., et al. Measuring social motivation using signal detection and reward responsiveness. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0167024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coninck D., d'Haenens L., Matthijs K. Perceived vulnerability to disease and attitudes towards public health measures: COVID-19 in Flanders, Belgium. Personality and Individual Differences. 2020;166:110220. doi: 10.1016/j.paid.2020.110220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan L.A., Schaller M. Prejudicial attitudes toward older adults may be exaggerated when people feel vulnerable to infectious disease: Evidence and implications. Analyses of Social Issues and Public Policy. 2009;9(1):97–115. [Google Scholar]
- Duncan L.A., Schaller M., Park J.H. Perceived vulnerability to disease: Development and validation of a 15-item self-report instrument. Personality and Individual Differences. 2009;47(6):541–546. [Google Scholar]
- Faulkner J., et al. Evolved disease-avoidance mechanisms and contemporary xenophobic attitudes. Group Processes & Intergroup Relations. 2004;7(4):333–353. [Google Scholar]
- Haselton M.G., Buss D.M. Error management theory : A new perspective on biases in cross-sex mind reading. Journal of Personality and Social Psychology. 2000;78(1):81–91. doi: 10.1037//0022-3514.78.1.81. [DOI] [PubMed] [Google Scholar]
- Hautus M.J. Corrections for extreme proportions and their biasing effects on estimated values of d. Behavior Research Methods, Instruments, & Computers. 1995;27(1):46–51. [Google Scholar]
- Lieder F., Griffiths T.L., Hsu M. Overrepresentation of extreme events in decision making reflects rational use of cognitive resources. Psychological Review. 2018;125(1):1. doi: 10.1037/rev0000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little A.C., Apicella C.L., Marlowe F.W. Preferences for symmetry in human faces in two cultures: Data from the UK and the Hadza, an isolated group of hunter-gatherers. Proceedings of the Royal Society B: Biological Sciences. 2007;274(1629):3113–3117. doi: 10.1098/rspb.2007.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhanova A., Shepherd M.A. 2020. Behavioral immune system linked to responses to the threat COVID-19 Personality and Individual Differences, 167, 110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald M.M., Donnellan M.B., Cesario J., Navarrete C.D. Mate choice preferences in an intergroup context : Evidence for a sexual coercion threat- management system among women. Evolution and Human Behavior. 2015;36(6):438–445. [Google Scholar]
- Navarrete C.D., Fessler D.M.T. Disease avoidance and ethnocentrism : The effects of disease vulnerability and disgust sensitivity on intergroup attitudes. Evolution and Human Behavior. 2006;27(4):270–282. [Google Scholar]
- Nettle D., Bateson M. The evolutionary origins of mood and its disorders. Current Biology. 2012;22(17):R712–R721. doi: 10.1016/j.cub.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Neuhoff J.G., Long K.L., Worthington R.C. Strength and physical fitness predict the perception of looming sounds. Evolution and Human Behavior. 2012;33(4):318–322. [Google Scholar]
- Pizzagalli D.A., Jahn A.L., O’Shea J.P. Toward an objective characterization of an anhedonic phenotype : A signal-detection approach. Biological Psychiatry. 2005;57(4):319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop P., Usak M., Fančovičová J. Health and the avoidance of macroparasites: A preliminary cross-cultural study. Journal of Ethology. 2010;28(2):345–351. [Google Scholar]
- Reid S.A., et al. Parasite primes make foreign-accented English sound more distant to people who are disgusted by pathogens (but not by sex or morality) Evolution and Human Behavior. 2012;33(5):471–478. [Google Scholar]
- Safra L., Chevallier C., Sijilmassi A. 2021. Poverty and threat reactivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl S.T., Metzger A. College students’ ageist behavior: The role of aging knowledge and perceived vulnerability to disease. Gerontology & Geriatrics Education. 2013;34(2):197–211. doi: 10.1080/02701960.2012.718009. [DOI] [PubMed] [Google Scholar]
- Stangier U., Kananian S., Schüller J. Perceived vulnerability to disease, knowledge about COVID-19, and changes in preventive behavior during lockdown in a German convenience sample. Current Psychology. 2021:1–9. doi: 10.1007/s12144-021-01456-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional questionnaires included at the end of Wave 1 or at the end of Wave 2.