Abstract
Porcine bocavirus (PBoV) is a single-stranded DNA virus, belongs to the genus Bocaparvovirus of family Parvoviridae. It was discovered along with porcine circovirus 2 (PCV 2) and torque tenovirus (TTV) in the lymph nodes of pigs suffering from postweaning multisystemic wasting syndrome (PMWS) in Sweden in 2009. PBoV has been reported throughout the world, mostly in weaning piglets, and has a broad range of tissue tropism. Since PBoV is prevalent in healthy as well as clinically infected pigs and is mostly associated with coinfection with other viruses, the pathogenic nature of PBoV is still unclear. Currently, there are no cell lines available for the study of PBoV, and animal model experiments have not been described. This review summarizes the current state of knowledge about PBoV, including the epidemiology, evolution analysis, detection methods, pathogenesis and public health concerns.
Keywords: Porcine bocavirus (PBoV), Emerging pathogen, Epidemiology, Evolution, Detection, Pathogenesis, Coinfection, Public health concerns
Introduction
Bocaviruses (Bo: bovine; ca: canine) were first reported in the 1960s (Blomström et al. 2009; Zhou et al. 2014; Lanave et al. 2015) with possible recombination between bovine parvovirus and canine minute virus (Allander et al. 2005; Lau et al. 2011). Bocavirus is an emerging pathogen and has been reported in canines (Bodewes et al. 2014), bovines (Mitra et al. 2016), humans (Allander et al. 2005), pigs (Blomström et al. 2009), gorillas (Nze-Nkogue et al. 2017), chimpanzees (Brožová et al. 2016), California sea lion (Li et al. 2011), rabbits (Lanave et al. 2015), Himalayan marmots (Ao et al. 2017), camels (Woo et al. 2017), wild boars (Cadar et al. 2011), bats (Lau et al. 2017), minks (Wang et al. 2017), house shrews (Xiong et al. 2018) and rodents (Lau et al. 2016; Xiong et al. 2018; Zhang C et al. 2018).
Porcine bocavirus (PBoV), the new member of Bocaparvovirus genus, was discovered in Sweden in 2009 in pigs from finishing herds suffering from postweaning multisystemic wasting syndrome (PMWS) (Blomström et al. 2009). Within a year, it was identified in healthy pigs (Shan et al. 2011a), diarrheic pigs (Shan et al. 2011b) and the pigs suffering from respiratory problems (Cheung et al. 2010; Zhai et al. 2010). It has been reported in Asia (Zhai et al. 2010), Europe (Cadar et al. 2011), Africa (Blomstrom et al. 2013; Ndze et al. 2013) and North America (Schirtzinger et al. 2015). Although this virus has been found for 10 years, there still many questions remain unresolved. This review describes the genome structure, classification, detection methods, molecular epidemiology and public health importance of this virus.
Discovery and Epidemiology of PBoV
PBoV was first discovered in pigs in Sweden in 2009. Porcine circovirus 2 (PCV-2), torque tenovirus (TTV), and parvovirus (Boca-like virus) were identified in the lymph nodes of two pigs obtained at autopsy in a field study of naturally occurring PMWS in finishing herds. However, in the same study, in addition to samples from these two pigs, samples from two other pigs from the same farm were analyzed; one pig was healthy and negative for PCV-2, and the other suffered from PMWS. The healthy pig had TTV-1, TTV-2 and PCV-2 but had no detectable level of a boca-like virus. However, the pig that suffered from PMWS had TTV-1, TTV-2, PCV-2, and boca-like parvovirus (Blomström et al. 2009). The prevalence rate of porcine boca-like parvovirus was 46% in pigs without PMWS and 88% in pigs with PMWS. The prevalence was almost twice as high in clinically affected pigs as in unaffected pigs (Blomstrom et al. 2010). The complete genome of PBoV was first determined in China in 2010 (PBoV1/2CHN) from fecal samples (Cheng et al. 2010).
Later, PBoV was identified in archived samples (collected in 2006) from Croatia (gene accession number: KF206161.1, KF206162.1) (Cadar et al. 2013) and China (Cheng et al. 2010). Similarly, bocaviruses were also identified in samples from the wild boar population in Romania collected in 2006/2007 and 2010/2011. The prevalence rate of bocaviruses was found to increase with time [from 9.14% (43/470) in 2006/2007 to 17.74% (66/372) in 2010/2011] (Cadar et al. 2011). These studies show that bocaviruses circulated in the swine population independently before they were discovered in 2009 (Zhou et al. 2014).
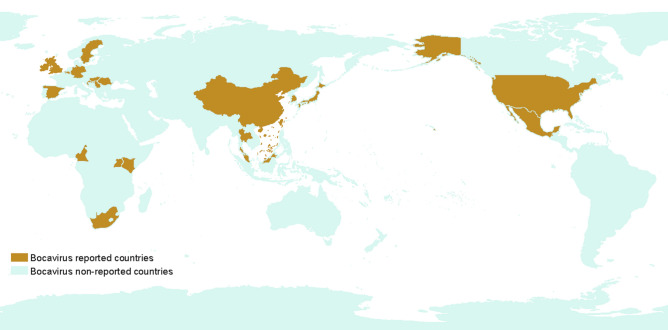
Since 2009, the virus has been reported throughout the world (Fig. 1), including Sweden (Blomström et al. 2009), China (Cheng et al. 2010; Zhai et al. 2010), the USA (Cheung et al. 2010; Shan et al. 2011b; Jiang et al. 2014), Ireland (McKillen et al. 2011; Gunn et al. 2015), Romania (Cadar et al. 2011), Hungary (Csagola et al. 2012), Croatia (Cadar et al. 2013; Keros et al. 2017), Cameroon (Ndze et al. 2013), the UK (McMenamy et al. 2013), Thailand (Saekhow and Ikeda 2014), Korea (Choi et al. 2014), the Czech Republic, Slovakia (Vlasakova et al. 2014), Mexico (Schirtzinger et al. 2015), Germany (Pfankuche et al. 2016), Japan (Zhang et al. 2016), Uganda, Kenya (Amimo et al. 2017), Slovenia (Keros et al. 2017), Belgium (Conceicao-Neto et al. 2017), and Malaysia (Jacob et al. 2018). Even though PBoV has been identified in many countries (Fig. 1), its prevalence may differ based on geographical location, pig age, and pig herd management.
Fig. 1.
Major countries representing the identification of porcine bocavirus.
In China, the prevalence rate of PBoV in stool samples of piglets was found to be 12.59% (Cheng et al. 2010). Similarly, the prevalence rate of bocaviruses in clinical samples from one-month-old piglets was reported to be 5.77% (9/156) (Meng et al. 2018). Another study reported that the prevalence rate of PBoV was 18.1% (84/463) (Zhou et al. 2018). Serological analysis of 2025 serum specimens, revealed that 856 (42.3%) of serum specimens were positive for anti-PBoV IgG antibodies (Shi et al. 2019). Comparative analysis of the prevalence rate of PBoV in the USA and China showed that the prevalence rate of PBoV in the USA (42%) was higher than that in China (11.4%) (Zhang et al. 2015).
In Croatia, the prevalence rate of PBoV was found to be 100% (10/10) in small commercial farms, 45.45% (5/11) in composite fecal samples after small backyard holdings, 42.1% (24/57) in small commercial pig farms and 27.9% (12/43) in small backyard holdings (Keros et al. 2017). In Malaysia, out of 103 samples from 17 pigs, 32 samples from 15 pigs were positive for PBoV, revealing a prevalence rate of 90.9% (Jacob et al. 2018). In Thailand, eighty tonsil samples from pigs were collected and analyzed from a slaughterhouse; the prevalence rate was 18% (23/80) (Saekhow and Ikeda 2014).
The occurrence of bocavirus infection was found to be higher in the spring (72.7%, 16/22) than in the summer (28.9%, 26/90), autumn (38.7%, 12/31) or winter (41.7%, 20/48). Nevertheless, there is no significant difference in PBoV infection rate between seasons (Zhai et al. 2010).
In a study from China, the prevalence of PBoV was found to be higher in grower pigs than in pigs of other age groups (Zhang et al. 2013). In another study from Malaysia, the prevalence of bocavirus was higher in weaning and growing pigs than in newborn piglets (Jacob et al. 2018). Similarly, in samples from Romania, the prevalence of bocavirus infection was found to be higher in 6- to 12-month old animals (77.06%, 84/109) than in 12- to 36-month old animals (22.94%, 25/109). This result indicates that infection is more common in younger pigs than in older pigs (Cadar et al. 2011). In Korea, the Czech Republic and Slovakia, it has been reported that infection was more common in weaned piglets than in pigs of other age groups (Choi et al. 2014; Vlasakova et al. 2014). In piglets, maternal antibodies might play an important role in the prevention of bocavirus infection (Vlasakova et al. 2014). However, this hypothesis requires further investigation for confirmation.
Genomic Characteristics
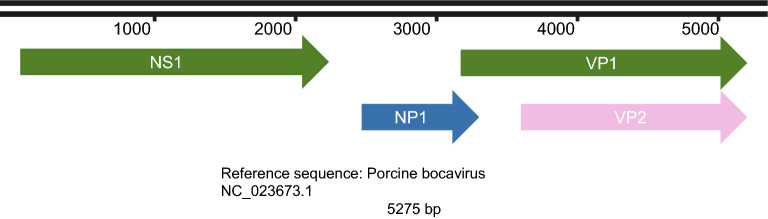
PBoV, which is a nonenveloped virus, exhibits icosahedral symmetry (Cheng et al. 2010; Zhou et al. 2017), has a diameter of 26–30 nm (Yang et al. 2012) and consists of linear, single-stranded DNA of 4–6 kb (Cheng et al. 2010; Zhou et al. 2017) of either positive or, most often, negative polarity, with most strains being negative-sense (Anne Christin Böhmer et al. 2009). As shown in Fig. 2, the genome of PBoV consists of three open reading frames (ORFs) (Zhou et al. 2018). ORF-1 encodes the nonstructural protein NS1, and ORF-2 encodes the structural capsid proteins VP1 and VP2 (Zhou et al. 2018). VP1 and VP2 overlap each other (Zhou et al. 2014). ORF-3 is regarded as the characteristic feature of bocavirus (Cheng et al. 2010; Shan et al. 2011b) and encodes the nonstructural protein NP1 (Zhou et al. 2018).
Fig. 2.
The genome structure of porcine bocavirus. The genome of porcine bocavirus contains three ORF regions that encode four proteins. The sequences for VP1 and VP2 overlap in the genome.
The ORF-1 region of PBoV resembles bovine parvovirus (BPV), and ORF-2 resembles canine minute virus (CMV) (Lau et al. 2011). ORF-1 encodes a protein associated with rolling circle replication, helicase, and ATPase activities. ORF-2 encodes a protein associated with phospholipase A2 motifs (the most conserved motif is YXGXG) required for parvovirus infectivity, calcium-binding loop, and catalytic residues (the most conserved motif is HDXXY) (Cheng et al. 2010; Zhou et al. 2017). The conserved motif YXGXF carried by porcine bocavirus is different from the YXGXG motif of other parvoviruses (Cheng et al. 2010; Zhou et al. 2017). However, in addition to the HDXXY motif and YXGXF motif other motifs have been reported in PBoV, including the HDQAY motif in strain GD6, the HDLAY motif in strain GD10, and the YLGPF motif in strains GD6, GD10, and GD23 (Zhou et al. 2017). The function of the protein encoded by ORF-3 is not clear, but in CMV, it has been associated with viral replication (Lau et al. 2011).
Episomes have been detected in PBoV, and replication takes place by a rolling circle mechanism with a head-to-tail structure. Hairpin-1 of PBoV has a different position and is conserved (Zhou et al. 2014).
Phylogenetics and Evolution of PBoV
According to the International Committee on Taxonomy of Viruses, PBoV was classed to the genera Bocaparvovirus (BoV), subfamily Parvovirinae and family Parvoviridae (Cotmore et al. 2013, 2019; Zhou et al. 2018). Previously, PBoV strains were designated as novel strains if they had < 95% homology of the nonstructural gene (NS1) with existing strain. Because of this high level of required homology, many current species consist of single isolates, creating confusion between taxa and viruses in the literature and hindering the taxonomic structure. According to a new proposal, bocavirus strains are designated as novel strains if they have less than 85% amino acid sequence identity of the NS1 protein. Based on this new criterion, most of the previously identified viruses belong to the same taxa, allowing individual species to contain more diverse viruses. Other existing criteria, such as host, antigenic properties, and genome characteristics, are still used. The PBoV has been placed under the Ungulate bocaparvovirus five type species with seven PBoV variants (Cotmore et al. 2013).
Originally, PBoV were classified into three (PBoV-likeV, PBoV1/PBoV2, and 6 V/7 V) phylogenetic clades based on nucleotide and amino acid alignments (Shan et al. 2011a). Further, some other researchers proposed classification methods based on ORF-2 encoding complete VP1 genes using the criteria of > 40% nucleotide differences considering members of different groups and > 10% difference from the VP1 nucleotide sequence considering members of different subgroups (Yang et al. 2012). In another study, PBoV from China and the USA were divided into six groups based on 400 bp VP1 fragment: PBoV1 (group 1), PBoV2 (group 2), PBoV3 (group 3), PBoV4 (group 4), PBoV5 (group 5), and PBoV6V7V (group 6). PBoV1 and PBoV2 were epidemic strains from 2006 to 2011 in China followed by PBoV3 subtypes epidemic from 2010 to 2012 in China and the USA. Further reported that PBoV3C, PBoV5, PBoV3/4 were epidemic viruses in China and the USA (Zhang et al. 2015). Based on phylogeographical analysis, it was reported that Hong Kong, Hebei Province, and Jiangsu Province were likely the geographical origins of PBoV in China, and that Minnesota and North Carolina were likely the geographical origins of PBoV in the USA (Zhang et al. 2015). In the recent study, PBoV were classified into three groups based on the complete VP1 gene; group 1 (HQ223038, HQ291308), group 2 (KX017193, HM053694), and group 3 (JF12472, KF025381). Group 3 PBoV were further subdivided into five subgroups (Zhou et al. 2017).
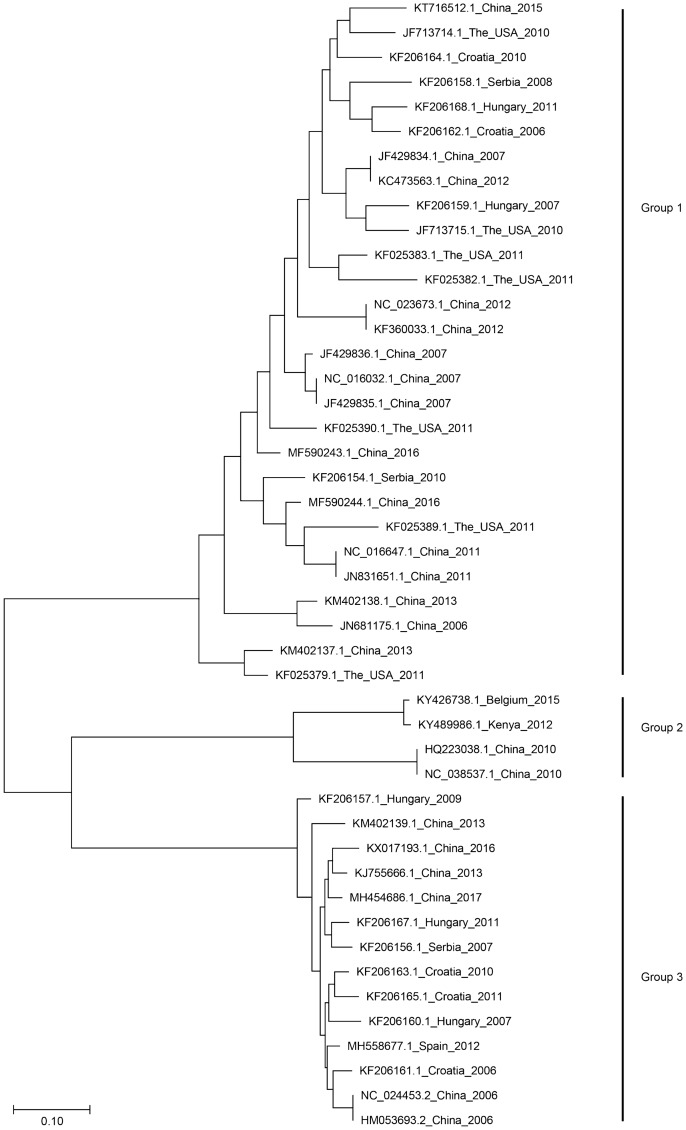
The relationships among PBoV strains isolated from different countries can be observed from the phylogenetic tree based on the full length sequence (Fig. 3). The result demonstrate that there are three major groups. Group 2 and 3 forms distinct monophyletic groups in comparison to group 1 which forms a paraphyletic group with many monophyletic groups. Group 2 contains PBoV isolated from China, Kenya and Belgium. The nucleotide sequence of these PBoV were truncated in the NS1 gene. Group 3 contains PBoV isolated from China and European countries. Group 1 contains PBoV isolated from Asia, Europe and the USA. It can be observed that PBoV isolated throughout the world are diverse and tends to cluster with other strains isolated from different countries (Fig. 3).
Fig. 3.
Phylogenetic analysis of bocaviruses. The phylogenetic tree was generated using full length nucleotide sequences from bocaviruses in GenBank using the MEGA 7 software package (neighbor joining method with 1000 bootstrap replicates). The relationship among the porcine bocaviruses from different countries can be observed from the tree. Detailed information related to certain PBoV strains is listed in Table 1.
Recombination is considered an important event for emerging viruses and has been described in human and porcine bocaviruses (Lau et al. 2011; Zhou et al. 2014, 2017). Recombination has been described in the NS1 gene, NP1 gene or VP1/VP2 gene. A recombination event between two recombination breakpoints near sites 1170 and 1780 of the VP1 sequence was demonstrated in a fecal sample (SH14F/12) containing PBoV4 (Lau et al. 2011). Similarly, a natural recombination breakpoint was identified in the NS1 gene (nucleotides 925 to 1959), but no genetic recombination was detected (Zhou et al. 2017). Also no recombination signal was reported for PBoV1 or PBoV2 (Cheng et al. 2010). Parvoviruses are known to have a high mutation rate of the capsid gene, with 10–4 nucleotide substitutions per site per year (Karuppannan and Opriessnig 2018). Recombination events as well as a high mutation rate may lead to better adaptation of bocaviruses as well as host escape.
Bocavirus isolated from other host species show near relationship with porcine bocavirus. The bocavirus isolated from minks shared 87% nucleotide similarity with porcine bocavirus (strain: PBoV-KU14; HQ223038, HQ291308, KJ622366) and formed separate clade with porcine bocavirus (Wang et al. 2017). Similarly, five full length genomes of PBoV (HD.44, HD.52, MM.57, YN.27, and YN.22) were constructed based on sequences generated from murine rodents and formed separate clade with group four porcine bocavirus (Xiong et al. 2018). Additionally, bocavirus isolated from bats, rodents and Himalayan marmonts are closely related with porcine bocavirus. The bocavirus isolated from Himalayan marmonts were closely related to porcine bocavirus (PBoV-SX/1-2) (Ao et al. 2017). The bocavirus isolated from bats were closely related to porcine bocavirus (NC_024453.2, NC_016032.1, HQ223038.1, HQ291308.1) (Lau et al. 2017). Similarly, the bocavirus isolated from rodents species were related with porcine bocavirus isolate PBoV-KU14 with 92.1%–92.9% amino acid identities in NS1 protein (Zhang C et al. 2018). Therefore these studies clearly reflects that interspecies transmission must have occurred from minks, bats, Himalayan marmonts and rodents to the porcine. However no any experimental evidence of interspecies transmission of bocavirus have been reported till date. Such studies can reflect the wide range of host adaptation of bocavirus as well as genetic variation in the porcine bocavirus.
Detection Methods of PBoV
The random amplification and high-throughput sequencing approach was implemented in Sweden (Blomström et al. 2009) to discover the porcine boca-like virus. These methods are gaining popularity for the discovery of novel viruses and to address metagenomic issues (Blomström et al. 2009; Li et al. 2011; Shan et al. 2011b; Yang et al. 2012; Xiong et al. 2018).
PCR-based detection methods have been developed to rapidly detect PBoV. Conventional multiplex PCR can be used to detect the three groups of PBoV. The sensitivity of single PCR assays is equal to that of the multiplex PCR assay and is 1.0 × 103 genomic copies/µL for PBoV group 1, 4.5 × 103 copies/µL for PBoV group 2 and 3.8 × 103 copies/µL for PBoV group 3 (Zheng et al. 2016a). Loop-mediated isothermal amplification (LAMP) was developed for rapid, specific, and sensitive detection of porcine boca-like virus. It has been reported that this method is 100 times more sensitive than conventional PCR and has a detection limit of approximately ten copies per reaction. Moreover, no crossreactivity has been reported with common circulating swine viruses such as porcine reproductive and respiratory syndrome virus (PRRSV), porcine circovirus type 2 (PCV-2), porcine parvovirus (PPV), and classical swine fever virus (CSFV) (Li et al. 2013). TaqMan-based real-time PCR (qPCR) has been used for the detection and quantitation of ungulate bocavirus 2 by targeting the VP1 gene. This method is highly sensitive and specific and can detect the real-time quantification of viral load without post-PCR detection procedures (Zhou et al. 2018). An EvaGreen-based multiplex real-time PCR method was developed for simultaneous detection and grouping of the three bocavirus groups (groups 1, 2, and 3) in a single step (Zheng et al. 2016b). Duplex nano PCR has been used for the simultaneous amplification and quantification of PBoV and pseudorabies virus (Luo et al. 2015). SYBR Green-based duplex real-time PCR has been used for detection of porcine epidemic diarrhea virus (PEDV) as well as PBoV genotypes (3, 4, 5), with detection limits of 10 copies/μL for both viruses (Zheng et al. 2020). Sequence-independent single primer amplification (SISPA) is a primer-initiated method that uses a single primer to amplify nucleic acids of an unknown sequence by sequence-independent PCR. This method has been used to amplify PBoV (Cheng et al. 2010). Nested polymerase chain reaction has been used for the identification of PBoV from throat swab, fecal, and serum samples by using two nested PCR primers (Xiong et al. 2018; Zhou et al. 2018). In the primer walking method, each specific primer is used along with a degenerate primer to hybridize to all known bocavirus sequences (Zeng et al. 2011). For this method, other sequences should be available to design specific primers. Nucleic acid sequence-based amplification (NASBA) has been used to determine the polarity of the human bocavirus genome and to analyze the transcription of uncharacterized genes (Anne Christin Böhmer et al. 2009).
Immunological methods, such as indirect immunofluorescence assays, have been developed to detect unidentified isolated PBoV in primary porcine kidney cell lines. In infected cell cultures, staining is observed in the nucleus and cytoplasm (McKillen et al. 2011). Monoclonal antibodies against PBoV 3 and 4 have also been produced for antigen-detecting ELISA (McNair et al. 2011). An indirect enzyme-linked immunosorbent assay based on the recombinant form of nucleoprotein 1 (NP1) has been developed to investigate the seroprevalence of PBoV in China. Cross reactivity is not observed with antiserum against PEDV, transmissible gastroenteritis virus (TGEV), PCV-2, (PPV), or PRRSV (Shi et al. 2019).
Coinfection of PBoV with Typical Swine Enteric Viruses
Many researchers have reported PBoV as a potential emerging pathogen (Blomström et al. 2009; Zhou et al. 2018) of the respiratory tract (Zhai et al. 2010) or the gastrointestinal tract (Amimo et al. 2017). Diversity of host species is assumed to be the specific feature of emerging viruses (Zhang C et al. 2018). To date, PBoV has not been reported as an enteric pathogen in the literature. However, the co-occurrence of this virus with circulating viruses has been reported since its discovery (Table 1) (Zhou et al. 2017; Zhang J et al. 2018). The most common circulating viruses are PEDV (Zhang et al. 2013; Zheng et al. 2020), GARV (Zhang et al. 2013), PRRSV (Blomström et al. 2009; Vlasakova et al. 2014), PCV-2 (Blomström et al. 2009; Blomstrom et al. 2010; Vlasakova et al. 2014; Zhang J et al. 2018), CSFV (Zhang et al. 2011; Zhou et al. 2018), porcine parvovirus (Zhang J et al. 2018), porcine pseudorabies virus (Luo et al. 2015) and porcine kobuvirus (Zhang et al. 2013). Clinically, the incidence of coinfection of PBoV with PCV-2 has been reported to be 83.8%, suggesting that PCV-2 enhances the infectivity of PBoV (Zhang J et al. 2018). Thus, PBoV may not be directly associated with disease and might function as a helper virus for triggering other infectious agents (McKillen et al. 2011).
Table 1.
General information of documented porcine bocavirus cases.
| Order | Year | Country | Gene access number | Co-infection | References |
|---|---|---|---|---|---|
| 1 | 2009 | Sweden | FJ872544 | PCV-2, TTV | Blomström et al. (2009) |
| 2 | 2010 | China | GU556573 to GU556591 | PCV-2, TTV-1, TTV-2, CSFV | Zhai et al. (2010) |
| 3 | 2010 | China | HM053693 and HM053694 | PKV, PCV, Frog virus | Cheng et al. (2010) |
| 4 | 2010 | USA | GQ387500 and GQ387499 | PCV-2 | Cheung et al. (2010) |
| 5 | 2010 | Ireland | JF512472 and JF512473 | Porcine adenovirus, Enteroviruses, Reoviruses, Circovirus, Porcine parvovirus | McKillen et al. (2011) |
| 6 | 2011 | Ireland | JF512472 and JF512473 | ND | McNair et al. (2011) |
| 7 | 2011 | China | JF429834, JF429836 | ND | Lau et al. (2011) |
| 8 | 2011 | China | HQ223038 | ND | Zeng et al. (2011) |
| 9 | 2011 | China | GU902971 | ND | Shan et al. (2011a, b) |
| 10 | 2011 | Romania | JF721404–JF721421 | PCV-2 (data not shown) | Cadar et al. (2011) |
| 11 | 2012 | China | NA | PEDV, PKV, RVA, TGEV | Zhang et al. (2013) |
| 12 | 2012 | Hungary | JN400850 to JN400879 | PPV2, PPV3, PPV4, PBoV1, PBoV2, 6 V and 7 V and PCV-2 | Csagola et al. (2012) |
| 13 | 2013 | Croatia | KC701291–KC701314, KC687097– KC687100, KC701315–KC701332, KC701333– KC701356, KC767891 | ND | Cadar et al. (2013) |
| 14 | 2013 | Cameroon | JX869077 to JX869100 | Mixed infection of different groups of porcine parvovirus | Ndze et al. (2013) |
| 15 | 2013 | Uganda | JX854557 | TTSuV1 and TTSuV2 | Blomstrom et al. (2013) |
| 16 | 2013 | Korea | Co-infection between group1, 2 and 3 | Choi et al. (2014) | |
| 17 | 2014 | Slovakia and Czech Republic | NA | PCV-2, TTSuV1, TTSuV2, PBoV, PRRSV, PTV | Vlasakova et al. (2014) |
| 18 | 2015 | North America | KR709262 to KR709268 | PRRSV | Schirtzinger et al. (2015) |
| 19 | 2015 | Thailand | AB973315-AB973334, AB973335-AB973354 | PPV1,2,3,4 | Saekhow and Ikeda (2014) |
| 20 | 2015 | Japan | LC090199 | ND | Zhang et al. (2016) |
| 21 | 2016 | Germany | KU311698 | Mycoplasma hyorhinis | Pfankuche et al. (2016) |
| 22 | 2017 | Belgium | KY426738 to KY426752 | PAstV, PEDV, Porcine enterovirus, Porcine picobirnavirus | Conceicao-Neto et al. (2017) |
| 23 | 2018 | Malaysia | KX686996 to KX686700 | PCV-2/PMWS | Jacob et al. (2018) |
Analysis of 12 fecal samples from diarrheic piglets and 24 fecal samples from healthy piglets by Shan et al. revealed that bocavirus (5/12 vs. 3/24) and coronavirus (11/12 vs. 13/24) are more prevalent in diarrheic than in healthy piglets (Shan et al. 2011b). The researchers concluded that the high rate of coinfection seen in high-density farms provides favorable conditions for recombination and accelerates viral evolution. In Uganda and Kenya, bocavirus was isolated from piglets with no signs of clinical diarrhea (Amimo et al. 2017). During a diarrhea outbreak in fattening pigs from Belgium, PEDV was detected along with PBoV, recombinant enterovirus-toroviruses, astroviruses, enteroviruses, and picobirnaviruses. The presence of PEVD (Cq > 30) in low loads along with high loads of PBoV and several enteroviruses suggests that the cause of viral diarrhea was a virus other than PEVD and that bocavirus was the most likely cause (Conceicao-Neto et al. 2017).
Pathogenesis of PBoV
The pathogenesis of PBoV has not been determined to date. It is thought that, as PBoV is highly prevalent in the swine population and has high genetic diversity, its pathogenesis could be determined by direct evidence from clinical disease (Zhou et al. 2017). The detection of bocavirus in different tissues represents a wide range of tissue tropism (Lau et al. 2011). PBoV was detected for the first time in the lymph nodes of weaning pigs suffering from PMWS (Blomström et al. 2009). Soon after that, it was detected in fecal samples from piglets (Cheng et al. 2010) as well as the respiratory tract (Zhai et al. 2010). In diarrheal disease, histopathological changes, including microscopic lesions and villous atrophy, are mainly detected in the jejunum, ileum, and duodenum (Zhang et al. 2013). Bocavirus has been isolated from piglets with encephalomyelitis, demonstrating the pathological role of this virus (Pfankuche et al. 2016). In a study novel porcine parvovirus, PPV4, similar to porcine bocavirus was identified from the lung lavage of a diseased pig coinfected with PCV-2. Tissue homogenates consisting of lung, lymph node, spleen, and heart were prepared and inoculated into two colostrum-deprived pig oronasally and monitored clinically. Both pigs developed respiratory disease and were euthanized. However, due to the coinfecting virus PCV-2, it was not clear if it caused disease on its own or contributed to the clinical disease (Cheung et al. 2010). Information related to the isolation of PBoV from different tissues is presented in Table 2. Lymph nodes, spleen, and tonsil have the highest detection rate suggesting that these organs are the sites of active replication and the pathogenesis of PBoV infections may involve the lymphoid tissues and gastrointestinal tract (Jacob et al. 2018). Besides tissues, bocavirus has been detected from the saliva (Choi et al. 2014) and serum (Csagola et al. 2012; Choi et al. 2014; Meng et al. 2018). In brief, bocavirus has been isolated from different tissues of the pig, but it is still not clear which cells in pigs are responsible for its multiplication and dissemination throughout the body.
Table 2.
Tissue tropism of porcine bocavirus.
| Tissue | Key findings | References |
|---|---|---|
| Lymph nodes | The infection rates of PBoV in the PMWS-affected pigs were twice higher than in the non-PMWS affected pigs. The co-infection of PBoV along with the TTSV and PCV-2 might have facilitate the development of PMWS | Blomström et al. (2009) |
| The co-existence of two bocavirus strain within the same fecal sample revealing inter and intra host genetic diversity | Lau et al. (2011) | |
| The genetic diversity of the circulating bocavirus strains in Xinjiang belong to three subgroups of three different genetic groups | Meng et al. (2018) | |
| Gastrointestinal tract | The prevalence rate of PBoV was higher in stool samples and these viruses multiply in the intestinal tract of piglets | Cheng et al. (2010) |
| Respiratory tract | The first evidence of infection of weaning piglets with respiratory tract symptoms representing an emerging virus for swine respiratory tract diseases | Zhai et al. (2010) |
| Nasopharyngeal sample | PBoV were higher in nasopharyngeal samples in deceased pigs than in healthy pigs | Lau et al. (2011) |
| Mesenteric lymph nodes | The mesenteric lymph node had the highest detection rate suggesting the pathogenesis of PBoV infection involves the lymphoid tissues | Jacob et al. (2018) |
| Inguinal lymph nodes | 25% of the organ tested were positive for PBoV | Jacob et al. (2018) |
| Spleen | 23.5% of the spleen tested were positive for PBoV | Jacob et al. (2018) |
| Tonsil | Out of 80 tonsil samples, 23 samples were positive for the PBoV | Saekhow and Ikeda (2014) |
| The tonsil had the second highest detection rate suggesting the pathogenesis of PBoV infection involves the lymphoid tissues | Jacob et al. (2018) | |
| Lung | Porcine parvovirus 4 was similar to PBoV. After inoculation of tissue homogenate in the colostrum deprived piglets, clinical symptoms were observed. But due to coinfection with PCV-2. It was not clear whether PPV4 can cause disease on its own or contributed to the disease phenomenon | Cheung et al. (2010) |
| One lungs tissue sample was positive for the PBoV without coinfection of the PCV-2, suggesting PBoV as not the risk factor the Hungarian pigs | Csagola et al. (2012) | |
| The first description of the prevalence of PBoV in Korean swine herds with the mean positive rate of 34.9% | Choi et al. (2014) | |
| 33.3% of lungs tissues were positive for PBoV | Jacob et al. (2018) | |
| Kidney | PBoV was detected from kidney tissues of two pigs suggesting the ability of virus to replicate within kidney cells causing renal pathology | Jacob et al. (2018) |
| Cerebral tissue | By using fluorescent in situ hybridization for histologic detection of encephalomyelitis assigns a potential role of PBoV in provoking CNS lesions | Pfankuche et al. (2016) |
| Liver | 25% of liver tissue were positive for PBoV | Jacob et al. (2018) |
However, in a study conducted in Thailand in dogs, it was reported that most of the viruses belonging to the Parvoviridae family replicate in mitotically active cells such as intestinal crypt epithelial cells. Analysis of tissue samples from the small intestine revealed nuclear signals for canine bocavirus 2 in enterocytes, mainly those located at the villus tips and crypts. Transmission electron microscopy showed numerous electron-dense icosahedral viral particles measuring approximately 20 nm in diameter that aggregated to form large intranuclear inclusion bodies within apical small intestinal enterocytes (Piewbang et al. 2018). This study provides novel insights into the pathogenicity of canine bocavirus infections, which could also apply to other bocaviruses isolated from different animals. Rats naturally infected with parvovirus are mostly healthy. However, in some cases, growth retardation and fetal loss is observed in pregnant rats experimentally inoculated with parvovirus (Lau et al. 2016).
Cells lines for the isolation of bocavirus have not yet been reported. Different cells such as porcine kidney cells (PK-15), swine testicular cells, porcine alveolar macrophages, monkey kidney cells (MARC-145), and human embryonic kidney epithelial cells (HEK293T) have been used for the propagation of PBoV. However, no success has been reported (Zeng et al. 2011). During the diarrheal disease outbreak in Belgium, an attempt was made to isolate bocavirus and recombinant enterovirus-torovirus from primary porcine kidney epithelial cells and swine testicular cells. Although a cytopathic effect was observed in porcine kidney epithelial cells after two days, no virus could be detected in passaged cells (Conceicao-Neto et al. 2017). In another study in Northern Ireland, fecal suspensions and homogenates of the small intestines of 6-week-old piglets exhibited cytopathic effect after four passages in cultured primary porcine kidney cells. PCV1, PCV-2, PPV, porcine enterovirus types 1, 2, and 3, porcine adenovirus, and porcine reovirus were not detected by PCR or RT-PCR; thus, this study was the first to describe the growth of bocavirus in a primary cell line (McKillen et al. 2011). Because this virus cannot be cultured in cell lines and since it commonly coexists with other circulating swine enteric viruses, the exact role of this virus in disease progression is unclear.
Due to the association of bocavirus with other circulating pathogenic enteric viruses, its nature has always been confusing. Due to the involvement of bocavirus in respiratory tract infection as well as gastrointestinal tract infection, it is still unclear whether bocaviruses are respiratory pathogens or enteric pathogens, especially in humans, pigs and other animals. Due to the lack of an experimental animal model, the pathogenic nature of PBoV has not been investigated.
Public Health Concerns
As for other enteric pathogens, the fecal–oral route may be the main transmission route of PBoV. Diarrheal feces and/or vomitus and other contaminated fomites, such as transport vehicles and feeds, may be significant transmission sources. Since PBoV is a nonenveloped virus, it can survive harsh environment and is also responsible for the widespread distribution of various parvoviruses (Saekhow and Ikeda 2014). In 2007, human bocaviruses were isolated from 17 of 21 (81%) raw sewage samples from different states in the USA (Blinkova et al. 2009). This finding reflects the robust nature of bocaviruses. Once they enter swine herds, they can cause subclinical infection of pigs and circulate in the swine population. However, whether aerosolized PBoV is infectious should be investigated.
Human bocavirus, human parvovirus 4 and parvovirus B19 of the family Parvoviridae are known to be associated with human disease (Martin et al. 2009; Karuppannan and Opriessnig 2018). Human bocaviruses have been reported worldwide (Rikhotso et al. 2018). The first case of human bocavirus was reported in the respiratory tract of a child from Sweden in 2005, four years before the discovery of bocavirus in swines (Allander et al. 2005). Bocaviruses have been isolated from the respiratory tract (Vicente et al. 2007; Arthur et al. 2009; Martin et al. 2009) and gastrointestinal tract (Zhao et al. 2012), and from cancer patients (Schildgen et al. 2013). They have also been isolated from the respiratory and gastrointestinal tracts of healthy and clinically infected children. Therefore, in humans, it is still unclear whether bocaviruses are real pathogens, opportunistic pathogens or indwelling flora.
In a case study from Iran published in 2018, PBoV was detected in a three-year-old child with an acute respiratory tract infection (Safamanesh et al. 2018), implying that PBoV may be evolving as a human pathogen.
The xenotransplantation of pig organs is gaining greater attention (Karuppannan and Opriessnig 2018). With the increasing concern of the pathogenic role of PBoV, screening, monitoring and risk analysis of ssDNA viruses is recommended to eliminate the risk of transmission during xenotransplantation of pig organs.
In southern China, brown rats from markets or restaurants were infected with the rat bocavirus. Further investigations are required to study the potential for interspecies transmission from rats to humans (Lau et al. 2016).
Zoonotic diseases have caused devastating effects in human beings. There is increasing concern regarding the broad host range, wide tissue tropism and increasing pathogenic potential of bocaviruses. These studies indicate that bocaviruses have important public health implications.
Prospects of PBoV
Bocavirus is an emerging pathogen with a broad host range and wide tissue tropism. In the past decade, many researchers have tried to isolate this virus in cell culture, but success has not been achieved. Thus, an organ coculture system might be beneficial for its propagation. Studies in such a system could be used to determine whether this virus favors the respiratory tract, the gastrointestinal tract or other tissue systems for its propagation.
Many studies from various countries have reported that PBoV is associated with other coinfecting pathogens of swine, including PEDV, PRRSV, CSFV, and rotavirus A. While PBoV was discovered ten years ago, we still do not know if it is a real pathogen or an opportunistic pathogen. Therefore, single virus isolation could be used for the study of animal infection models. Additionally, direct evidence of the pathogenesis of this virus is needed.
Phylogenetic analysis of PBoV isolated from minks and mice has revealed complex evolutionary mechanisms, interspecies transmission and recombination points. Further studies are needed to elucidate interspecies transmission between different host species.
Bocavirus poses a significant public health concern. The clinical case from Iran (Safamanesh et al. 2018) is a reminder that it is necessary to understand emerging pathogens such as PBoV because they might have devastating effects in the future. As mentioned above, our significant contribution to the understanding of PBoV will facilitate the prevention and control of this emerging virus and reduce its economic impact.
Acknowledgements
The author would like to acknowledge the support from the Organization for Women in Science for the Developing World (OWSD) and the Swedish International Development. This work was financially supported by the National Key R&D Program of China (2016YFD0500103), and partly by China Central Public-interest Scientific Institution Basal Research Fund (1610312020020).
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no competing interests.
Animal and Human Rights Statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Allander T, Tammi M, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B (2005) Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A 102:12891–12896 [DOI] [PMC free article] [PubMed]
- Amimo JO, Njuguna J, Machuka E, Okoth E, Djikeng A. First complete genome sequences of porcine bocavirus strains from East Africa. Genome Announc. 2017;5:e00093–e117. doi: 10.1128/genomeA.00093-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anne Christin Böhmer V, Lüsebrink J, Ziegler S, Tillmann RL, Kleines M, Schildgen O. Novel application for isothermal nucleic acid sequence-based amplification (NASBA) J Virol Methods. 2009;158:199–201. doi: 10.1016/j.jviromet.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Ao Y, Li X, Li L, Xie X, Jin D, Yu J, Lu S, Duan Z. Two novel bocaparvovirus species identified in wild Himalayan marmots. Sci China Life Sci. 2017;60:1348–1356. doi: 10.1007/s11427-017-9231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur JL, Higgins GD, Davidson GP, Givney RC, Ratcliff RM. A novel bocavirus associated with acute gastroenteritis in Australian children. PLoS Pathog. 2009;5:e1000391. doi: 10.1371/journal.ppat.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinkova O, Rosario K, Li L, Kapoor A, Slikas B, Bernardin F, Breitbart M, Delwart E. Frequent detection of highly diverse variants of cardiovirus, cosavirus, bocavirus, and circovirus in sewage samples collected in the United States. J Clin Microbiol. 2009;47:3507–3513. doi: 10.1128/JCM.01062-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomström A-L, Belák S, Fossum C, McKillen J, Allan G, Wallgren P, Berg M. Detection of a novel porcine boca-like virus in the background of porcine circovirus type 2 induced postweaning multisystemic wasting syndrome. Virus Res. 2009;146:125–129. doi: 10.1016/j.virusres.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Blomstrom AL, Belak S, Fossum C, Fuxler L, Wallgren P, Berg M. Studies of porcine circovirus type 2, porcine boca-like virus and torque teno virus indicate the presence of multiple viral infections in postweaning multisystemic wasting syndrome pigs. Virus Res. 2010;152:59–64. doi: 10.1016/j.virusres.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Blomstrom AL, Stahl K, Okurut AR, Masembe C, Berg M. Genetic characterisation of a porcine bocavirus detected in domestic pigs in Uganda. Virus Genes. 2013;47:370–373. doi: 10.1007/s11262-012-0855-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodewes R, Lapp S, Hahn K, Habierski A, Förster C, König M, Wohlsein P, Osterhaus A, Baumgärtner W. Novel canine bocavirus strain associated with severe enteritis in a dog litter. Vet Microbiol. 2014;174:1–8. doi: 10.1016/j.vetmic.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brožová K, Hrazdilová K, Slaninková E, Modrý D, Černý J, Celer V. Genetic and phylogenetic characterization of novel bocaparvovirus infecting chimpanzee. Infect Genet Evol. 2016;37:231–236. doi: 10.1016/j.meegid.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Cadar D, Cságola A, Lőrincz M, Tombácz K, Kiss T, Spînu M, Tuboly T. Genetic detection and analysis of porcine bocavirus type 1 (PoBoV1) in European wild boar (Sus scrofa) Virus Genes. 2011;43:376–379. doi: 10.1007/s11262-011-0650-4. [DOI] [PubMed] [Google Scholar]
- Cadar D, Lőrincz M, Kiss T, Novosel D, Podgorska K, Becskei Z, Tuboly A, Cságola AC. Emerging novel porcine parvoviruses in Europe: origin, evolution, phylodynamics and phylogeography. J Gen Virol. 2013;94:2330–2337. doi: 10.1099/vir.0.055129-0. [DOI] [PubMed] [Google Scholar]
- Cheng WX, Li JS, Huang CP, Yao DP, Liu N, Cui SX, Jin Y, Duan ZJ. Identification and nearly full-length genome characterization of novel porcine bocaviruses. PLoS ONE. 2010;5:e13583. doi: 10.1371/journal.pone.0013583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AK, Wu G, Wang D, Bayles DO, Lager KM, Vincent AL. Identification and molecular cloning of a novel porcine parvovirus. Arch Virol. 2010;155:801–806. doi: 10.1007/s00705-010-0646-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi MG, Park SJ, Nguyen VG, Chung HC, Kim AR, Park BK. Molecular detection and genetic analysis of porcine bocavirus in Korean domestic swine herds. Arch Virol. 2014;159:1487–1492. doi: 10.1007/s00705-013-1944-8. [DOI] [PubMed] [Google Scholar]
- Conceicao-Neto N, Theuns S, Cui T, Zeller M, Yinda CK, Christiaens I, Heylen E, Van Ranst M, Carpentier S, Nauwynck HJ, Matthijnssens J. Identification of an enterovirus recombinant with a torovirus-like gene insertion during a diarrhea outbreak in fattening pigs. Virus Evol. 2017;3:24. doi: 10.1093/ve/vex024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore SF, Agbandje-McKenna M, Chiorini JA, Mukha DV, Pintel DJ, Qiu J, Soderlund-Venermo M, Tattersall P, Tijssen P, Gatherer D, Davison AJ. The family parvoviridae. Arch Virol. 2013;159:1239–1247. doi: 10.1007/s00705-013-1914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore SF, Agbandje-McKenna M, Canuti M, Chiorini JA, Eis-Hubinger AM, Hughes J, Mietzsch M, Modha S, Ogliastro M, Penzes JJ, Pintel DJ, Qiu J, Soderlund-Venermo M, Tattersall P, Tijssen P, Ictv Report C. ICTV virus taxonomy profile: parvoviridae. J Gen Virol. 2019;100:367–368. doi: 10.1099/jgv.0.001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csagola A, Lorincz M, Cadar D, Tombacz K, Biksi I, Tuboly T. Detection, prevalence and analysis of emerging porcine parvovirus infections. Arch Virol. 2012;157:1003–1010. doi: 10.1007/s00705-012-1257-3. [DOI] [PubMed] [Google Scholar]
- Gunn L, Collins PJ, Fanning S, McKillen J, Morgan J, Staines A, O'Shea H. Detection and characterisation of novel bocavirus (genus Bocaparvovirus) and gastroenteritis viruses from asymptomatic pigs in Ireland. Infect Ecol Epidemiol. 2015;5:27270. doi: 10.3402/iee.v5.27270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob DM, Lee CY, Arshad SS, Selvarajah GT, Bande F, Ong BL, Ooi PT. First molecular detection of porcine bocavirus in Malaysia. Trop Anim Health Prod. 2018;50:733–739. doi: 10.1007/s11250-017-1489-z. [DOI] [PubMed] [Google Scholar]
- Jiang YH, Xiao CT, Yin SH, Gerber PF, Halbur PG, Opriessnig T. High prevalence and genetic diversity of porcine bocaviruses in pigs in the USA, and identification of multiple novel porcine bocaviruses. J Gen Virol. 2014;95:453–465. doi: 10.1099/vir.0.057042-0. [DOI] [PubMed] [Google Scholar]
- Karuppannan AK, Opriessnig T. Possible risks posed by single-stranded DNA viruses of pigs associated with xenotransplantation. Xenotransplantation. 2018;25:e12453. doi: 10.1111/xen.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keros T, Jemersic L, Toplak I, Prpic J. The silent spread of porcine bocavirus in croatian pigs: Should we be concerned? Acta Vet Hung. 2017;65:565–573. doi: 10.1556/004.2017.055. [DOI] [PubMed] [Google Scholar]
- Lanave G, Martella V, Farkas SL, Marton S, Fehér E, Bodna L, Lavazza A, Decaro N, Buonavoglia C, Bányai K. Novel bocaparvoviruses in rabbits. Vet J. 2015;206:131–135. doi: 10.1016/j.tvjl.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Lau SK, Woo PC, Yip CC, Li KS, Fu CT, Huang Y, Chan KH, Yuen KY. Co-existence of multiple strains of two novel porcine bocaviruses in the same pig, a previously undescribed phenomenon in members of the family Parvoviridae, and evidence for inter- and intra-host genetic diversity and recombination. J Gen Virol. 2011;92:2047–2059. doi: 10.1099/vir.0.033688-0. [DOI] [PubMed] [Google Scholar]
- Lau S, Yeung H, Li K, Lam C, Juice C, Yuen M-C, Wang M, Zheng B, Woo P, Yuen K-Y. Identification and genomic characterization of a novel rat bocavirus from brown rats in China. Infecti Genet Evol. 2016;47:68–76. doi: 10.1016/j.meegid.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Lau SKP, Syed SA, Hoi-Wah T, Hazel CY, Kenneth SML, Rachel YYF, Pyrear SHZ, Candy CCL, Carol SFL, Kelvin KFC, Ben CHC, Jian-Piao C, Samson SYW, Honglin C, Hai-Lin Z, Libiao Z, Ming W, Woo PCY, Yuen K-Y. Bats host diverse parvoviruses as possible origin of mammalian dependoparvoviruses and source for bat–swine interspecies transmission. J Gen Virol. 2017;98:3046–3059. doi: 10.1099/jgv.0.000969. [DOI] [PubMed] [Google Scholar]
- Li L, Shan T, Wang C, Cote C, Kolman J, Onions D, Gulland FMD, Delwart E. The fecal viral flora of California sea lions. J Virol. 2011;85:9909–9917. doi: 10.1128/JVI.05026-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Ma JJ, Xiao SB, Zhang XH, Wen LB, Mao L, Ni YX, Guo RL, Zhou JM, Lv LX, He KW. Development of a loop-mediated isothermal amplification method for rapid detection of porcine boca-like virus. JVirol Methods. 2013;179:390–395. doi: 10.1016/j.jviromet.2011.11.027. [DOI] [PubMed] [Google Scholar]
- Luo Y, Liang L, Zhou L, Zhao K, Cui S. Concurrent infections of pseudorabies virus and porcine bocavirus in China detected by duplex nanoPCR. J Virol Methods. 2015;219:46–50. doi: 10.1016/j.jviromet.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Martin ET, Taylor J, Kuypers J, Magaret A, Wald A, Zerr D, Englund JA. Detection of bocavirus in saliva of children with and without respiratory illness. J Clin Microbiol. 2009;47:4131–4132. doi: 10.1128/JCM.01508-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKillen J, McNeilly F, Duffy C, McMenamy M, McNair I, Hjertner B, Millar A, McKay K, Lagan P, Adair B, Allan G. Isolation in cell cultures and initial characterisation of two novel bocavirus species from swine in Northern Ireland. Vet Microbiol. 2011;152:39–45. doi: 10.1016/j.vetmic.2011.04.013. [DOI] [PubMed] [Google Scholar]
- McMenamy M, McKillen J, McNair I, Duffy C, Blomström A-L, Charreyre C, Welsh M, Allan G. Detection of a porcine boca-like virus in combination with porcine circovirus type 2 genotypes and Torque teno sus virus in pigs from postweaning multisystemic wasting syndrome (PMWS)-affected and non-PMWS-affected farms in archival samples from Great Britain. Vet Microbiol. 2013;164:293–298. doi: 10.1016/j.vetmic.2013.03.009. [DOI] [PubMed] [Google Scholar]
- McNair I, McNeilly F, Duffy C, McKillen J, McMenamy M, Welsh M, Allan G. Production, characterisation and applications of monoclonal antibodies to two novel porcine bocaviruses from swine in Northern Ireland. Arch Virol. 2011;156:2157–2162. doi: 10.1007/s00705-011-1107-8. [DOI] [PubMed] [Google Scholar]
- Meng Q, Qiao M, Gong S, Tian L, Li C, Qiao J, Meng D, Wu Y, Cai K, Zhang Z, Cai X. Molecular detection and genetic diversity of porcine bocavirus in piglets in China. Acta Virol. 2018;62:343–349. doi: 10.4149/av_2018_401. [DOI] [PubMed] [Google Scholar]
- Mitra N, Cernicchiaro N, Torres S, Li F, Hause BM. Metagenomic characterization of the virome associated with bovine respiratory disease in feedlot cattle identified novel viruses and suggests an etiologic role for influenza D virus. J Gen Virol. 2016;97:1771–1784. doi: 10.1099/jgv.0.000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndze VN, Cadar D, Csagola A, Kisfali P, Kovacs E, Farkas S, Ngu AF, Esona MD, Dan A, Tuboly T, Banyai K. Detection of novel porcine bocaviruses in fecal samples of asymptomatic pigs in Cameroon. Infect Genet Evol. 2013;17:277–282. doi: 10.1016/j.meegid.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Nze-Nkogue C, Horie M, Fujita S, Inoue E, Akomo-Okoue EF, Ozawa M, Ngomanda A, Yamagiwa J, Tsukiyama-Kohara K. Identification and molecular characterization of novel primate bocaparvoviruses from wild western lowland gorillas of Moukalaba-Doudou National Park, Gabon. Infect Genet Evol. 2017;53:30–37. doi: 10.1016/j.meegid.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Pfankuche VM, Bodewes R, Hahn K, Puff C, Beineke A, Habierski A, Osterhaus ADME, Baumgärtner W. Porcine bocavirus infection associated with encephalomyelitis in a pig, Germany. Emerg Infect Dis. 2016;22:1310–1312. doi: 10.3201/eid2207.152049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piewbang C, Jo WK, Puff C, Ludlow M, van der Vries E, Banlunara W, Rungsipipat A, Kruppa J, Jung K, Techangamsuwan S, Baumgärtner W. Canine bocavirus type 2 infection associated with intestinal lesions. Vet Pathol. 2018;55:434–447. doi: 10.1177/0300985818755253. [DOI] [PubMed] [Google Scholar]
- Rikhotso MC, Kabue JP, Ledwaba SE, Traore AN, Potgieter N. Prevalence of human bocavirus in africa and other developing countries between 2005 and 2016: a potential emerging viral pathogen for diarrhea. J Trop Med. 2018;2018:7875482. doi: 10.1155/2018/7875482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saekhow P, Ikeda H. Prevalence and genomic characterization of porcine parvoviruses detected in Chiangmai area of Thailand in 2011. Microbiol Immunol. 2014;59:82–88. doi: 10.1111/1348-0421.12218. [DOI] [PubMed] [Google Scholar]
- Safamanesh S, Azimian A, Shakeri A, Ghazvini K, Amel Jamehdar S, Khosrojerdi M, Youssefi M. Detection of porcine bocavirus from a child with acute respiratory tract infection. Pediatric Infect Dis J. 2018;37:1. doi: 10.1097/INF.0000000000002003. [DOI] [PubMed] [Google Scholar]
- Schildgen V, Malecki M, Tillmann R-L, Brockmann M, Schildgen O. The human bocavirus is associated with some lung and colorectal cancers and persists in solid tumors. PLoS ONE. 2013;8:e68020. doi: 10.1371/journal.pone.0068020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirtzinger EE, Suddith AW, Hause BM, Hesse RA. First identification of porcine parvovirus 6 in North America by viral metagenomic sequencing of serum from pigs infected with porcine reproductive and respiratory syndrome virus. Virol J. 2015;12:170. doi: 10.1186/s12985-015-0401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan T, Lan D, Li L, Wang C, Cui L, Zhang W, Hua X, Zhu C, Zhao W, Delwart E. Genomic characterization and high prevalence of bocaviruses in swine. PLoS ONE. 2011;6:e17292. doi: 10.1371/journal.pone.0017292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan T, Li L, Simmonds P, Wang C, Moeser A, Delwart E. The fecal virome of pigs on a high-density farm. J Virol. 2011;85:11697–11708. doi: 10.1128/JVI.05217-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi QK, Zhang JL, Gu WY, Hou LS, Yuan GF, Chen SJ, Fan JH, Zuo YZ. Seroprevalence of porcine bocavirus in pigs in north-central China using a recombinant-NP1-protein-based indirect ELISA. Arch Virol. 2019;164:2351–2354. doi: 10.1007/s00705-019-04325-7. [DOI] [PubMed] [Google Scholar]
- Vicente D, Cilla G, Montes M, Pérez-Yarza EG, Pérez-Trallero E. Human bocavirus, a respiratory and enteric virus. Emerg Infect Dis. 2007;13:636–637. doi: 10.3201/eid1304.061501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasakova M, Leskova V, Sliz I, Jackova A, Vilcek S. The presence of six potentially pathogenic viruses in pigs suffering from post-weaning multisystemic wasting syndrome. BMC Vet Res. 2014;10:221. doi: 10.1186/s12917-014-0221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao J, Zheng M, Liu Z, Yuan J, Zhao J, Shen Q, Fan Z, Jiang L, Yang S. Genome sequence of a porcine bocavirus detected in feces of domestic minks in China. Genome Announc. 2017;5:e01170. doi: 10.1128/genomeA.01170-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo PCY, Lau SKP, Tsoi HW, Patteril NG, Yeung HC, Joseph S, Wong EYM, Muhammed R, Chow FWN, Wernery U, Yuen K-Y. Two novel dromedary camel bocaparvoviruses from dromedaries in the Middle East with unique genomic features. J Gen Virol. 2017;98:1349–1359. doi: 10.1099/jgv.0.000775. [DOI] [PubMed] [Google Scholar]
- Xiong YQ, You FF, Chen XJ, Chen YX, Wen YQ, Chen Q. Detection and phylogenetic analysis of porcine bocaviruses carried by murine rodents and house shrews in China. Transbound Emerg Dis. 2018;66:259–267. doi: 10.1111/tbed.13011. [DOI] [PubMed] [Google Scholar]
- Yang WZ, Yu JM, Li JS, Cheng WX, Huang CP, Duan ZJ. Genome characterization of a novel porcine bocavirus. Arch Virol. 2012;157:2125–2132. doi: 10.1007/s00705-012-1407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng S, Wang D, Fang L, Ma J, Song T, Zhang R, Chen H, Xiao S. Complete coding sequences and phylogenetic analysis of porcine bocavirus. J Gen Virol. 2011;92:784–788. doi: 10.1099/vir.0.028340-0. [DOI] [PubMed] [Google Scholar]
- Zhai S, Yue C, Wei Z, Long J, Ran D, Lin T, Deng Y, Huang L, Sun L, Zheng H, Gao F, Zheng H, Chen S, Yuan S. High prevalence of a novel porcine bocavirus in weanling piglets with respiratory tract symptoms in China. Arch Virol. 2010;155:1313–1317. doi: 10.1007/s00705-010-0698-9. [DOI] [PubMed] [Google Scholar]
- Zhang HB, Huang L, Liu YJ, Lin T, Sun CQ, Deng Y, Wei ZZ, Cheung AK, Long JX, Yuan SS. Porcine bocaviruses: genetic analysis and prevalence in Chinese swine population. Epidemiol Infect. 2011;139:1581–1586. doi: 10.1017/S0950268811000847. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Hu R, Tang X, Wu C, He Q, Zhao Z, Chen H, Wu B. Occurrence and investigation of enteric viral infections in pigs with diarrhea in China. Arch Virol. 2013;158:1631–1636. doi: 10.1007/s00705-013-1659-x. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhang C, Gao M, He X, Diao Y, Goyal SM, Mor SK, Huang J. Evolutionary, epidemiological, demographical, and geographical dissection of porcine bocavirus in China and America. Virus Res. 2015;195:13–24. doi: 10.1016/j.virusres.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Zhang W, Sano N, Kataoka M, Ami Y, Suzaki Y, Wakita T, Ikeda H, Li TC. Virus-like particles of porcine bocavirus generated by recombinant baculoviruses can be applied to sero-epidemic studies. Virus Res. 2016;217:85–91. doi: 10.1016/j.virusres.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Zhang C, Song F, Xiu L, Liu Y, Yang J, Yao L, Peng J. Identification and characterization of a novel rodent bocavirus from different rodent species in China. Emerg Microbes Infect. 2018;7:48. doi: 10.1038/s41426-018-0052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lu Y, Li S, Ku X, Liu X, Memon AM, He Q, Bi D, Meng X. Co-infection with porcine bocavirus and porcine circovirus 2 affects inflammatory cytokine production and tight junctions of IPEC-J2 cells. Virus Genes. 2018;54:684–693. doi: 10.1007/s11262-018-1596-6. [DOI] [PubMed] [Google Scholar]
- Zhao H, Zhao L, Sun Y, Qian Y, Liu L, Jia L, Zhang Y, Dong H. Detection of a bocavirus circular genome in fecal specimens from children with acute diarrhea in Beijing, China. PLoS ONE. 2012;7:e48980. doi: 10.1371/journal.pone.0048980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Liu G, Opriessnig T, Wang Z, Yang Z, Jiang Y. Development and validation of a multiplex conventional PCR assay for simultaneous detection and grouping of porcine bocaviruses. J Virol Methods. 2016;236:164–169. doi: 10.1016/j.jviromet.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Zheng X, Liu G, Opriessnig T, Wang Z, Yang Z, Jiang Y. Rapid detection and grouping of porcine bocaviruses by an EvaGreen((R)) based multiplex real-time PCR assay using melting curve analysis. Mol Cell Probes. 2016;30:195–204. doi: 10.1016/j.mcp.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Zheng LL, Cui JT, Han HY, Hou HL, Wang L, Liu F, Chen HY. Development of a duplex SYBR Green based real-time PCR assay for detection of porcine epidemic diarrhea virus and porcine bocavirus3/4/5. Mol Cell Probes. 2020;51:101544. doi: 10.1016/j.mcp.2020.101544. [DOI] [PubMed] [Google Scholar]
- Zhou F, Sun H, Wang Y. Porcine bocavirus: achievements in the past five years. Viruses. 2014;6:4946–4960. doi: 10.3390/v6124946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Xu J, Zhu SK, Meng QF, Lin ZX, Chen R, Qian AD. Genetic analysis of three porcine bocaparvoviruses and identification of a natural recombinant breakpoint in NS1. Arch Virol. 2017;163:707–712. doi: 10.1007/s00705-017-3606-8. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Xu J, Wang WL, Song SW, Zhu SK, Meng QF, Yu F, Li CP, Liu N, Luan WM. A TaqMan-based real-time PCR assay for the detection of ungulate bocaparvovirus 2. J Virol Methods. 2018;261:17–21. doi: 10.1016/j.jviromet.2018.07.013. [DOI] [PubMed] [Google Scholar]