Summary
Insulin-like growth factor-binding protein (IGFBP)-2 is a circulating biomarker of cardiometabolic health. Here, we report that circulating IGFBP-2 concentrations robustly increase after different bariatric procedures in humans, reaching higher levels after biliopancreatic diversion with duodenal switch (BPD-DS) than after Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG). This increase is closely associated with insulin sensitization. In mice and rats, BPD-DS and RYGB operations also increase circulating IGFBP-2 levels, which are not affected by SG or caloric restriction. In mice, Igfbp2 deficiency significantly impairs surgery-induced loss in adiposity and early improvement in insulin sensitivity but does not affect long-term enhancement in glucose homeostasis. This study demonstrates that the modulation of circulating IGFBP-2 may play a role in the early improvement of insulin sensitivity and loss of adiposity brought about by bariatric surgery.
Keywords: insulin-like growth factor, binding protein, bariatric surgery, type 2 diabetes, metabolism, BPD-DS, RYGB, sleeve gastrectomy, humans, patients, mice
Graphical abstract

Highlights
IGFBP-2 is modulated by different bariatric surgeries in both human and rodents
In humans, IGFBP-2 levels are closely related to insulin sensitization
In mice, IGFBP-2 partly mediates surgery-induced weight loss
IGFBP-2 is implicated in surgery-induced early improvements in glucose homeostasis
Faramia et al. show that the robust increase in IGFBP-2 levels is a common feature of bariatric procedures in humans and rodents. In patients, this increase closely correlates with insulin sensitization. In mice, IGFBP-2 deficiency partly impairs weight loss and early improvements in glucose homeostasis induced by Roux-en-Y surgery.
Introduction
The rising prevalence of obesity is a major health issue increasing the risk of metabolic diseases such as type 2 diabetes (T2D), hypertension, and dyslipidemia. Bariatric surgery provides a long-term solution for patients with severe obesity. Most patients undergoing bariatric surgery experience substantial weight loss as well as improvement and/or remission of T2D,1,2 especially in patients with a history of diabetes of <5 years.3 Biliopancreatic diversion with duodenal switch (BPD-DS) is currently the most effective treatment for sustained diabetes resolution compared with Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (SG).4 In most patients undergoing BPD-DS, diabetes resolution appears initially within days, whereas long-term recovery further relates to the extent of chronic weight loss.1,2,5, 6, 7 In rodents, RYGB and BPD-DS also have a rapid and lasting beneficial effect on glycemic control that is predominantly weight loss dependent, suggesting consistent mechanisms between these animal models and humans.8,9 Apart from glucose homeostasis, bariatric surgery also greatly improves lipid metabolism, often leading to the discontinuation of lipid-lowering therapy.10,11 In parallel, patients experience enhanced exercise capacity with higher peak heart rates, respiratory exchange ratio, and relative oxygen consumption.12 These surgery-induced improvements robustly reduce long-term cardiovascular risk,1 which is mostly weight loss-dependent and possibly linked to many endocrine changes, including increased glucagon-like peptide (GLP)-1,13 peptide YY,14 fibroblast growth factor (FGF)-19,15 FGF-21,16 as well as bile acid levels.17 However, experiments in animal models deficient for these pathways (using ligand or receptor knockout) have failed, at least partly, to provide evidence for the precise mechanisms mediating the impact of bariatric surgery on metabolism.18, 19, 20, 21, 22, 23, 24
Insulin-like growth factor (IGF) binding protein (IGFBP)-2 is a 36-kD circulating protein mainly produced by the liver. IGFBP-2 is implicated in glucose and lipid metabolism through its ability to regulate IGF bioavailability on the one hand, and by IGF-independent binding to heparin and integrin receptors to mediate intracellular signaling25, 26, 27, 28 on the other hand. In humans, low plasma levels of IGFBP-2 are strongly associated with obesity, dyslipidemia, and insulin resistance,27,29 which is similar to observations from genetic or nutritional rodent models of obesity.30 Consistently, the overexpression of IGFBP-2 in mice prevents age-induced insulin resistance and hypertension.31 Moreover, IGFBP-2 transgenic mice are resistant to diet-induced obesity (DIO) and fatty liver.31 In turn, injection of the heparin-binding domain (HBD) of IGFBP-2 in Igfbp2-deficient mice prevents age-induced weight gain, but does not affect glucose metabolism and food intake.32 In 2012, our group demonstrated in severely obese patients that BPD-DS induces a rapid, robust, and sustained rise in circulating IGFBP-2 levels, reaching concentrations at 1 year post-surgery that were at least 2-fold higher than those found in healthy individuals.33, 34, 35 More recent studies showed that RYGB and SG are also associated with increased plasma IGFBP-2 levels, independently of the variations in body composition induced by these surgeries.36,37 These findings suggest that IGFBP-2 is potentially implicated in the hormonal control of energy metabolism triggered by bariatric surgery.
To assess this hypothesis, we verified in human patients the impact of three of the most performed bariatric surgeries (BPD-DS, RYGB, and SG) on IGFBP-2 levels, and correlated this modulation with improvements in insulin sensitivity. Then, we confirmed that the increase in IGFBP-2 production is conserved in obese rats and mice after similar surgeries. Finally, hypothesizing that IGFBP-2 mediates (at least partly) the beneficial impact of bariatric procedures on energy metabolism, we compared the responses of high-fat-fed Igfbp2-deficient mice and their wild-type (WT) littermates to RYGB. Our findings strongly support the notion that the increase in IGFBP-2 observed after bariatric surgery contributes to weight loss and the associated improvements in metabolism.
Results
BPD-DS stimulates plasma IGFBP-2 levels in humans more strongly than RYGB and SG
We wished to confirm our previous observation33 of an increase in IGFBP-2 concentrations in severely obese patients with T2D undergoing BPD-DS. Plasma samples were obtained in fasting conditions before and after surgery in 20 individuals (all participants had preoperative T2D, other clinical characteristics in Figure S1A). As expected, baseline levels of IGFBP-2 were low (mean 164 ± SD 63 ng/mL, compared to a normal range of 300–500 ng/mL), but increased steadily after surgery, reaching a 6-fold augmentation (916 ± 324 ng/mL) 1 year post-operation (Figure 1A). These results were then compared to the changes in IGFBP-2 levels following the RYGB and SG procedures performed during the same period in samples of 20 patients matched for age and gender (Figure S1A). Pre-surgery IGFBP-2 levels in patients who had RYGB (150 ± 73 ng/mL) and SG (152 ± 67 ng/mL) operations were as low and not significantly different from those who had BPD-DS. Both RYGB and SG induced a 3-fold rise in IGFBP-2 levels 4 months post-operation, which then remained stable up to 1 year after the surgeries (500 ± 150 and 457 ± 284 ng/mL, respectively; Figure 1A). Changes in IGFBP-2 levels were statistically significant when adjusted for BMI (Figure 1B) or body weight loss (data not shown). Consequently, BPD-DS induced a significantly greater increase in circulating IGFBP-2 levels than RYGB and SG (p < 0.0001; Figure 1C) and occurred in all patients studied (Figure 1C). Interestingly, despite inter-individual variations, post-surgery IGFBP-2 levels reached a plateau 4 months after RYGB and SG that is within normal values in healthy individuals (∼300–500 ng/mL), whereas levels after BPD-DS exceeded that plateau in a rising manner (Figures 1D–1F).
Figure 1.
Upregulation of IGFBP-2 closely correlates with insulin sensitization after bariatric surgery in humans
(A) Fasting plasma IGFBP-2 concentrations in patients before and 4, 8, and 12 months after biliopancreatic diversion with duodenal switch (BPD-DS), Roux-en-Y gastric bypass (RYGB), and vertical sleeve gastrectomy (SG) (n = 18–20), ∗∗∗∗p < 0.0001 compared with RYGB and SG. Lines are means ± SEMs.
(B) Fasting plasma IGFBP-2 concentrations in patients as corrected by BMI over time; ∗∗∗∗p < 0.0001 compared with RYGB and SG. Lines are means ± SEMs.
(C–F) Increases in plasma IGFBP-2 levels at 12 months after surgery compared to baseline values (C). ∗∗∗∗p < 0.0001 compared with RYGB and SG. Individual data were also plotted according to surgery type: BDP-DS (D), RYGB (E), and SG (F). Each point represents 1 individual patient. Lines are means ± SEMs. ∗∗∗∗p < 0.0001 compared with pre-operation levels.
(G) Fasting plasma IGFBP-2 concentrations before and 3, 90, and 365 days after BPD-DS in 5 normoglycemic (filled circles) and 11 diabetic (empty circles) patients. Each point represents 1 individual patient, ∗∗∗∗p < 0.0001. Lines are means ± SEMs.
(H–L) In these individuals, IGFBP-2 levels were correlated with fasting glycemia (H), insulinemia (I), HOMA-IR (J), and ADIPO-IR (K) indexes of insulin resistance, as well as glucose infusion rate during euglycemic-hyperinsulinemic clamp (L) in patients studied before and at specific time points after the surgery. Correlation curves were analyzed by ANCOVA and multiple regression analyses to take repeated measurements over time into account. p values unadjusted, p∗ values adjusted for BMI; n.s., not significant.
The increase in IGFBP-2 is associated with insulin sensitization in humans
To document the relationships between the increase in IGFBP-2 levels and glucose homeostasis, we studied a second sample of 16 patients, 11 of whom had T2D (Figure S1B), who underwent BPD-DS with euglycemic-hyperinsulinemic clamps before, 3 days, 3 months, and 12 months after surgery (previously reported in Grenier-Larouche et al.11). As expected, BPD-DS induced a robust increase in IGFBP-2, which occurred irrespective of diabetic status (Figure 1G). Analyzed by multiple regression to take into account measures in the same patients over time, this increase in IGFBP-2 was negatively associated with glycemia (p = 0.03; Figure 1H), insulinemia (p < 0.001; Figure 1I), the homeostatic model assessment of insulin resistance (HOMA-IR) (p < 0.001; Figure 1J), and adipose tissue-IR (ADIPO-IR) indexes of insulin resistance (p = 0.02; Figure 1K). In these same patients, the progressive augmentation of IGFBP-2 after BPD-DS was strongly correlated in a logarithmic and time-dependent manner to the increase in insulin sensitivity (p < 0.001; Figure 1L). Apart from glycemia, the associations between IGFBP-2 and these parameters of glucose homeostasis remained significant when corrected for BMI (∗p; Figures 1H–1L). Moreover, in a predicted model of insulin sensitivity with plasma IGFBP-2 concentration, HOMA-IR, ADIPO-IR, plasma insulin, and glucose concentration as variables, IGFBP-2 was the most efficient parameter to predict insulin sensitivity (p < 0.001; Figures S1C–S1E). Thus, these results indicated that IGFBP-2 is robustly associated with insulin sensitization brought about by BPD-DS.
Bariatric surgeries targeting the intestinal tract consistently stimulate plasma IGFBP-2 levels in rats and mice
The strong clinical associations described above next prompted the hypothesis that the increase in IGFBP-2 mediates, at least in part, some of the metabolic improvements induced by bariatric surgery. To test this possibility, rodent models were developed. We characterized whether the robust increase in IGFBP-2 observed after different types of bariatric surgeries was also observed in Wistar DIO rats subjected to either SG, BPD-DS, or DS only (duodenal switch without SG or biliopancreatic diversion). Sham-operated DIO rats were divided into controls with ad libitum feeding, or weight matched (WM) by calorie restriction to BPD-DS rats to dissociate the impact of surgery from that of weight loss. Nine weeks post-surgery, circulating concentrations of IGFBP-2 were 2.5-fold higher in rats that had DS and BPD-DS (mean 69 ± SEM 14 and 59 ± 12 ng/mL, respectively) compared to those in sham-operated animals who had ad libitum access to food (26 ± 2 ng/mL; Figure 2A). In contrast, plasma IGFBP-2 levels were not changed in SG and WM groups (Figure 2A). These changes were mirrored by significantly higher mRNA levels of IGFBP-2 in the livers of DS and BPD-DS-operated rats than that of the other groups (Figure 2B). Interestingly, in a separate experiment, hepatic IGFBP-2 mRNA expression was also stimulated by BPD-DS in non-obese Wistar rats fed regular chow (1.91-fold, p < 0.05, data not shown). These findings suggest that the rearrangement of the intestinal tract upon duodenal switch is a potent inducer of IGFBP-2 expression that is potentially involved in the effects of BPD-DS.
Figure 2.
Modulation of hepatic production of IGFBP-2 after bariatric surgery in rodents
(A and B) Plasma protein (A) and hepatic mRNA (B) levels of IGFBP-2 were quantified in high-fat (HF)-fed obese rats (n = 6–11) 9 weeks after either sham, SG, DS, or hybrid (BPD-DS) bariatric surgeries, or in sham-operated rats weight matched (WM) to BPD-DS by food restriction. Each point represents 1 individual animal. Lines are means ± SEMs. ∗p < 0.05 compared with ad libitum (Ad Lib)-sham group; $p < 0.05 compared with WM sham group.
(C–E) Levels of IGFBP-2 in plasma (C and D) and liver (D and E) in HF-fed mice 21 weeks after undergoing sham or RYGB surgeries or in non-surgical mice weight matched to RYGB by caloric restriction (WM) (n = 4–10). Each point represents 1 individual animal. Lines are means ± SEMs. ∗∗∗p < 0.001 compared to Ad Lib-sham group; $$$p < 0.001 compared to WM group.
A similar conceptual approach was used in C57BL/6J DIO mice subjected to either sham or RYGB surgeries and compared to a non-operated DIO group calorie restricted to match the weight of RYGB animals. Twenty-one weeks post-operation, plasma IGFBP-2 levels were 2- to 3-fold higher in mice with RYGB (mean 504 ± SEM 34 ng/mL) compared to sham operation (149 ± 15 ng/mL) and weight-matching regimen (251 ± 67 ng/mL, not significantly different compared to sham) (Figure 2C). In the liver, these RYBG-induced changes were also observed at the protein (Figure 2D) and mRNA (Figure 2E) expression levels. Interestingly, although weight matching resulted in the higher hepatic expression of IGFBP-2 (both mRNA and protein), changes in the plasma levels of IGFBP-2 remained statistically unchanged in this group (Figures 2C–2E). These results indicate that, in contrast to simple calorie restriction, bariatric procedures that bypass a portion of the intestinal tract (duodenum and proximal jejunum), such as RYGB and BPD-DS, can trigger an increase in hepatic IGFBP-2 expression that is reflected in the circulation, in a manner that suggests consistent mechanisms from mice to humans.
IGFBP-2 is directly involved in the beneficial impact of bariatric surgery on body weight
To investigate the direct role of IGFBP-2 in the improvements in energy metabolism after bariatric surgery, whole-body Igfbp2 knockout (Igfbp2−/−) mice and Igfbp2+/+ littermates were fed a 2-choice diet consisting of 60% high-fat pellets and regular low-fat chow for 5 weeks and then subjected to RYGB or calorie restriction to match the loss of weight achieved by the surgery (WM). Irrespective of the regimen, Igfbp2−/− mice had no detectable IGFBP-2 protein in either liver or plasma measured at the termination of the study (Figure 3A). In Igfbp2+/+ mice, consistent with our previous data (Figures 2C–2E), RYGB but not WM groups were characterized by an increase in IGFBP-2 protein levels in the circulation, whereas both treatments stimulated liver expression (Figure 3A).
Figure 3.
Absence of IGFBP-2 partly impairs the impact of RYGB on body weight and adiposity in mice through changes in food intake
(A) Absence of IGFBP-2 was confirmed in plasma and liver by immunoblotting 21 weeks after undergoing sham or RYGB surgeries or weight matching by caloric restriction (WM). WT: Igfbp2+/+; knockout (KO): Igfbp2−/− mice.
(B–G): Effects of RYGB (green symbols), sham surgery (red symbols), and WM (blue symbols in D–G) in Igfbp2+/+ (open symbols) and Igfbp2−/− (filled symbols) mice on absolute body weight (B), percent change in body weight (C), fat mass (D), lean mass (E), adiposity index (fat mass/lean mass, F), and plasma leptin levels (G). Body weights of WM mice, not shown here for clarity since they are very close to those of RYGB mice, are displayed in Figures S2A and S2B. WT: SHAM (n = 13), RYGB (n = 11), WM (n = 5); KO: SHAM (n = 6), RYGB (n = 9), WM (n = 5). #, RYGB/Igfbp2−/− versus RYGB/Igfbp2+/+, p < 0.05; ˆ, WM/Igfbp2−/− versus WM/Igfbp2+/+, p < 0.05, based on Benjamini-Hochberg-corrected, pairwise t tests. Lines are means ± SEMs.
(H and I) In the same animals, intake of food was evaluated over time (H) and at specific periods (I) before and after sham surgery (red), RYGB surgery (green), and in WM (blue) obese Igfbp2+/+ and Igfbp2−/− mice. Data in (H) are means ± SEMs of 2-day averages. Lines in (I) are means ± SEMs.
(J–L) Energy expenditure (J), respiratory exchange ratio (K), and physical activity (L) were evaluated in metabolic chambers at thermoneutrality (29°C) 5 weeks after surgery (see gray area in B). WT: SHAM (n = 12), RYGB (n = 10), WM (n = 5); KO: SHAM (n = 6), RYGB (n = 9), WM (n = 5). Energy expenditure was adjusted for lean mass by ANCOVA. Lines are means ± SEMs.
All plot panels show individual data points over a box indicating means ± SEMs. Data that do not share the same letters are significantly different from each other (p < 0.05, pairwise t tests with Benjamini-Hochberg correction, false discovery rate [FDR] = 0.05, following ANOVA).
At the time of surgery, obese Igfbp2−/− and Igfbp2+/+ mice showed a similar body weight and body composition (Figures 3B–3F). All of the mice recovered well after RYGB or sham surgery and there were no complications associated with the surgery. Whereas all sham-operated animals continued to gain weight after the procedure independent of genotype, those that were subjected to RYGB experienced a robust reduction in body weight (Figures 3B–3C). In Igfbp2+/+ mice, RYGB resulted in rapid weight loss with a nadir of −32% at 5 weeks, with moderate regain of weight until termination of the study, whereas in Igfbp2−/− mice it produced less initial weight loss with a nadir of −18% at 3–5 weeks and return to pre-surgical levels at 12 weeks after surgery (Figure 3B), resulting in a 20% difference in body weight between genotypes at 20 weeks post-operation (Figure 3C).
The differential body weight response between Igfbp2−/− and Igfbp2+/+ RYGB-operated mice was first reflected in fat mass (Figure 3D). In both genotypes, fat mass was more potently affected in RYGB groups than in WM animals (Figure 3D). However, compared to their WT littermates, mice without IGFBP-2 were characterized by a lesser reduction in fat mass following surgery (Figure 3D). Consistently, compared to those of their Igfbp2+/+ counterparts, white adipose depots were heavier in Igfbp2−/− RYGB-operated mice (Figure S2). In contrast, the weights of brown adipose tissue (BAT), kidneys, heart, and liver were similar between genotypes after the surgery ( Figure S2). RYGB-operated Igfbp2+/+ mice also showed a reduction in lean mass, although to a lesser extent than in WM animals (Figure 3E). Surprisingly, lean mass appeared totally preserved in Igfbp2−/− after surgery (Figure 4E). Despite this, changes in body composition resulted in a higher adiposity index in Igfbp2−/− mice after RYBG (Figure 3F), which was mirrored by higher circulating leptin levels than those in Igfbp2+/+ mice (Figure 3G). Thus, these findings indicate that RYGB was more potent than weight matching to specifically target fat mass, and that these effects are less pronounced in the absence of IGFBP-2.
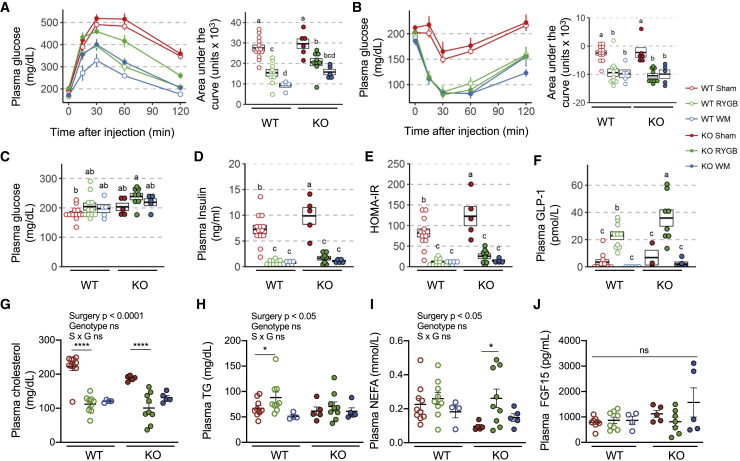
Figure 4.
Effects of RYGB on glucose and lipid metabolism in obese Igfbp2+/+ (WT) and Igfbp2−/− (KO) mice
(A and B) Excursion and area under the curve of glycemia after intraperitoneal glucose (A) or subcutaneous insulin injections (B) were determined at 3 and 10- weeks post-surgery, respectively, in obese Igfbp2+/+ and Igfbp2−/− mice subjected to sham surgery (red), RYGB surgery (green), or in WM mice (blue). WT: SHAM (n = 13), RYGB (n = 10–11), WM (n = 5); KO: SHAM (n = 6), RYGB (n = 8–9), WM (n = 5). Lines are means ± SEMs.
(C–E) Twenty-one weeks post-surgery, at sacrifice, blood was harvested after an overnight fast and used to quantify circulating levels of glucose (C), insulin (D), which were used to calculate HOMA index (E).
(F–J) Plasma GLP-1 (F), cholesterol (G), triglycerides (H), non-esterified fatty acid (NEFA) (I), and FGF-15 (J) levels were also quantified at sacrifice.
Time course data in (A) and (B) are means ± SEMs. All of the other panels show individual data points over a box indicating means ± SEMs. Data that do not share the same letters are significantly different from each other (p < 0.05; pairwise t tests with Benjamini-Hochberg correction; FDR = 0.05, following ANOVA).
To examine the mechanisms by which IGFBP-2 affected body weight in response to RYGB, the main components of energy balance were measured. Food intake before surgery was similar in all groups (Figures 3H and 3I) and similarly significantly suppressed in both genotypes during the first 10 days after RYGB compared to sham surgery (Figure 3I). However, during days 11–16 after RYGB, food intake was significantly higher in Igfbp2−/− compared to Igfbp2+/+ mice (Figure 3I). The amount of food necessary to match body weight to that of RYGB mice was significantly less than the food intake of RYGB mice for days 11–16 in both genotypes, with a similar trend for days 1–10 (Figure 3I), indicating lower feed efficiency in RYGB compared to calorie-restricted WM mice. The 2-choice diet allowed for the analysis of preference for chow over high-fat diet. As in previous studies,22 we found an increased preference for chow diet for the first 10 days after RYGB in WT mice, and this chow preference was similarly expressed between genotypes (Figure S3A). There was also a non-significant trend for this chow preference during days 11–16 (Figure S3B).
Total daily energy expenditure (EE), quantified by indirect calorimetry 5 weeks after surgery, adjusted for lean mass by analysis of covariance (ANCOVA) and measured at thermoneutrality (29°C) was not significantly different between RYGB and sham-operated mice for both genotypes (Figure 3J). At room temperature (23°C), it was significantly lower in RYGB compared with sham-operated mice for both genotypes, but there was no difference between Igfbp2+/+ animals and their Igfbp2−/− counterparts (Figure S3C). When adjusted for total body mass by ANCOVA, EE was significantly higher after RYGB compared with sham surgery when measured at thermoneutrality (29°C) (Figure S3F) and not significantly different when measured at room temperature (Figure S3G). Only when unadjusted and expressed on a per-mouse basis was EE significantly lower in RYGB groups compared with sham-operated animals (Figures S3H and S3I). In this setting, RYGB-operated Igfbp2−/− mice had higher EE than their Igfbp2+/+ controls (Figures S3H and S3I). Across all conditions and adjustment types, mice weight matched to RYGB animals generally had the lowest EE (Figures 3C, S4C, and S4F–S4I). Importantly, no matter what ambient temperature and how adjustment was made, there were no significant effects of genotype on total EE.
At 29°C, respiratory exchange rate (RER) was lower in WM mice compared to sham or RYGB-operated mice, but similar between genotypes (Figure 3K). Locomotor activity was not significantly different between any groups (Figure 3L). Similar statistically non-significant trends in respiratory exchange ratio (RER) and activity were observed between genotypes at 23°C (Figures S3D and S3E). These findings suggest that the absence of IGFBP-2 partly prevents RYGB-induced loss of weight in a manner that appears independent of relevant changes in EE but rather was due to modulation of food intake.
IGFBP-2 modulates the early changes in glucose metabolism in response to RYGB
Because IGFBP-2 affected changes in adiposity after RYGB, we evaluated whether its absence could consequently influence glucose and lipid metabolism. To this end, we conducted an IPGTT at 3 weeks, an insulin tolerance test at 10 weeks, and determined HOMA-IR from measurements of fasting glucose and insulin and measured plasma lipids at 21 weeks post-surgery. Again, the non-surgical, body weight-matched control group allowed us to distinguish weight loss-dependent from weight loss-independent effects of RYGB surgery. In Igfbp2+/+ animals, compared to sham operation, RYGB resulted in the expected improvements in glucose tolerance (Figure 4A), insulin sensitivity (Figure 4B), fasting insulin (Figure 4D), and HOMA-IR (Figure 4E). At 10 and 21 weeks post-operation, these improvements were fully accounted for by weight loss, as demonstrated by improvements of similar magnitude in the WM group. At 3 weeks post-surgery, RYBG-operated Igfbp2−/− mice showed significantly lower glucose tolerance compared to that of Igfbp2+/+ mice (Figure 4A). However, 10 weeks post-surgery, while differences in body weight and body fat were still robust between genotypes, Igfbp2−/− and Igfbp2+/+ RYGB-operated animals had similar insulin sensitivity (Figure 4B). At sacrifice, fasting glucose and insulin levels as well as HOMA-IR index were not significantly different between genotypes, although they tended to be higher in the absence of IGFBP-2 after RYGB (Figures 4C–4E). As expected, while plasma GLP-1 levels were barely above the detection limit in sham-operated and WM mice, they were several-fold higher after RYGB in both genotypes (Figure 4F). This response in GLP-1 levels was significantly stronger in Igfbp2−/− mice than in Igfbp2+/+ littermates.
Finally, whereas the absence of IGFBP-2 did not influence in an important manner the effects of RYGB on circulating levels of cholesterol (Figure 4G) or triglycerides (Figure 4H), Igfbp2−/− mice experienced an increase in free fatty acids upon RYGB (Figure 4I). However, plasma β-hydroxybutyrate (BOH) concentrations were not affected by surgery or genotype (Figure S4A), but acetoacetic acid levels were significantly higher in Igfbp2−/− mice than in Igfbp2+/+ littermates, irrespective of surgery status (Figure S4B). Plasma levels of FGF-15 were affected neither by surgery nor by the absence of IGFBP-2 (Figure 4J). Although hepatic expression of IGF-1 was higher in sham-operated Igfbp2−/− mice than in Igfbp2+/+ animals, no differences were observed between genotypes after RYGB or WM regimens (Figure S4C). These results suggest that IGFBP-2 modulates the early but not the later changes in glucose homeostasis after bariatric surgery, in a manner that is independent of effects on lipid levels and metabolism (also reflected by RER), GLP-1, and FGF-15.
Discussion
The biological mechanisms by which bariatric surgery influences body weight regulation and energy homeostasis are still not fully established. Previous findings showing a robust increase in IGFBP-2 after bariatric operations in humans suggested that this leptin-induced, insulin-sensitizing circulating protein may play a role in their beneficial effects. Here, we demonstrate that the hepatic expression and circulating levels of IGFBP-2 are robustly augmented by bariatric surgeries in mice, rats, and humans. In mice, IGFBP-2 appears in part necessary for the weight loss effect of RYBG, which predominantly affected adipose compartments. In addition, IGFBP-2 contributed to the early surgery-induced improvements in glucose tolerance, but not those in lipid metabolism. Consistently, in humans, the increase in IGFBP-2 was closely associated with improvements in insulin sensitivity and glucose homeostasis. Thus, we conclude that the systemic augmentation of IGFBP-2 levels after bariatric surgery partly contributes to weight loss and further propose that methods to increase circulating IGFBP-2 levels may mimic some of the beneficial metabolic effects of bariatric procedures.
A primary important finding was the much larger increase in circulating IGFBP-2 levels after BPD-DS compared to the extent brought about by RYGB or SG in humans. A similar result was obtained in rats, in which BPD-DS (comprising SG) but not SG only significantly increased plasma IGFBP-2 levels. Moreover, in rats, simple DS (without SG) was sufficient to mimic the effects of BPD-DS. These results strongly suggest that the rearrangement of the digestive tract had a great influence on plasma IGFBP-2 levels, in a manner that suggests consistent mechanisms between mice, rats, and humans. Although the exact nature of the molecular inducer of plasma IGFBP-2 remains to be experimentally determined, our findings suggest two possible independent but possibly additive mechanisms. The analysis of liver expression of IGFBP-2 after surgery indicated that BPD-DS and RYGB robustly and chronically stimulated IGFBP-2 mRNA and protein expression levels in both mice and rats, suggesting again a conserved pathway converging on IGFBP-2 production. Because this increase in liver IGFBP-2 expression equally occurred upon caloric restriction in non-operated animals matched for body weight, it is likely that a nutrient-sensing transcriptional program participated in this effect. Interestingly, Kang et al.38 showed in 2016 that metformin treatment could induce IGFBP-2 expression through the downstream transactivation of the nuclear receptor peroxisome-proliferator-associated receptor (PPAR)-α. In fact, RYGB has been shown to enhance oleoylethanolamide (OEA) levels in the lower small intestine after a meal. Because OEA is an endogenous agonist of PPAR-α,39 it is possible that OEA activates a PPAR-α/IGFBP-2 pathway in the liver, as described in skeletal muscle.40 Another possible explanation of IGFBP-2 overproduction is the improvement of leptin sensitivity after surgery,36 despite that leptin levels are reduced greatly in this condition, as leptin has been demonstrated to enhance liver expression and circulating levels of IGFBP-2.41 Other surgery-induced or calorie restriction-associated signaling factors, such as bile acids, could be implicated in hepatic IGFBP expression, although this needs to be tested. Thus, the exact role of intestinal modifications, especially those involved in jejunal nutrient and leptin sensing,42 need to be investigated for their potential role in IGFBP-2 expression.38,39
A second important finding was that in both rats and mice, there was a clear dichotomy between, on the one hand, the increases in liver IGFBP-2 content at both mRNA and protein levels that were caused by weight loss (either induced by surgery or food restriction) and, on the other hand, the subsequent concentrations in IGFBP-2 observed in plasma, which were modified by surgery only. This second important finding suggests that bariatric surgeries, but not food restriction, could promote mechanisms involved in IGFBP-2 secretion, although this possibility may not explain the clear difference in IGFBP-2 levels between obese patients operated with BPD-DS and RYGB/SG. It is possible that IGFBP-2 production is enhanced in non-hepatic tissues such as the adipose or skeletal muscle, where it is also expressed, especially since the adrenergic system is upregulated by RYGB.43 It can also be postulated that bariatric surgeries specifically favor IGFBP-2 stabilization in circulation by robustly dampening factors that degrade IGFBP-2. Interestingly, previous studies have shown that IGFBP-2 fragmentation increases with aging, notably through complex changes in binding with plasma α-2-macroglobulin, which controls IGFBP-2 proteolysis.35,44 Although beyond the scope of the present study, the modulation of α-2-macroglobulin or other IGFBP-2-binding partners by bariatric surgery should be investigated. Thus, although the exact endogenous factors triggering the stimulation of circulating IGFBP-2 concentrations after bariatric surgeries remain unknown, these procedures appear associated with both robust and sustained augmentation in production and a possible diminution in degradation.
Previous studies have demonstrated the ability of IGFBP-2 to modulate cellular energy metabolism through IGF-independent mechanisms, affecting white adipocyte differentiation and peripheral insulin sensitivity,11,26,32,45,46 notably by inducing glucose transporter type 4 (GLUT-4) translocation and glucose uptake in adipocytes47 and inhibiting receptor protein tyrosine phosphatase (RPTP)-β activity.46 Consistent with this independent role, we observed similar changes in the hepatic expression of IGF-1, IGFBP-1, and IGFBP-3 mRNA between Igfbp-2+/+ and Igfbp-2−/− RYGB-operated mice (data not shown). Moreover, in Igfbp-2+/+animals, the 2-fold increase in IGF-1 brought about by the surgery was much lower than that of IGFBP-2. These data suggest that the impact of the RYGB-induced increase in IGFBP-2 goes beyond the stochiometric modulation of IGF-1 bioavailability, and that the metabolic improvement mediated by IGFBP-2 is possibly independent of the IGF/IGFBP axis. In turn, it is also possible that the lack of IGFBP-2 negated the RYGB-induced changes in lean mass due to a stimulatory effect on the IGF axis. These possibilities need to be explored further for skeletal muscles and bones, since IGFBP-2 was shown to negatively affect bone biology.48
The third important finding was that using an IGFBP-2-deficient mouse model, we demonstrated that IGFBP-2 drives, at least in part, the loss of weight induced by RYGB. Compared to Igfbp2+/+ mice, IGFBP-2-deficient animals displayed a markedly reduced response to RYGB, losing much less weight in the first 5 weeks and regaining less in the next 11 weeks post-surgery, resulting in a 20% body weight difference between genotypes. This contrasts with earlier findings using a similar RYGB surgery model, showing no significant differences in weight loss between GLP-1R, PYY/Y2R, GLP-1R+PYY/Y2R, FGF21, or TGR5-deficient mice and their respective WT controls.22,23,49, 50, 51 Thus, our finding that IGFBP-2 partially mediates the effect of this surgery on energy balance represents an important key discovery in the search for hormonal modulators of bariatric surgeries. Because EE was rather higher than lower in IGFBP-2-deficient mice compared to that of WT mice after RYGB, we propose that the modulation of energy intake is the main determinant of the effects of IGFBP-2 after RYGB. This hypothesis is supported by higher cumulative food intake in IGFBP-2-deficient mice in the first 2 weeks post-surgery. Of note, because we have not measured fecal loss, differences in fecal energy loss could be an additional factor contributing to overall higher calorie ingestion. Interestingly, previous groups have demonstrated an elevated expression of IGFBP-2 in the brain52,53 compared to other non-hepatic tissues, which suggest its possible influence on the CNS. A role for IGFBP-2 in the central control of food intake appears independent from pathways involving leptin and GLP-1, as both were higher in the plasma of IGFBP-2−/− mice than IGFBP-2+/+ mice, and counterintuitively associated with higher food intake. In addition, although leptin can increase IGFBP-2 levels peripherally,41 it is unlikely a downstream effector of hypothalamic leptin signaling on food intake, since leptin-deficient mice show no difference in food intake after RYBG,54 unlike what we observed in Igfbp-2−/− mice. Thus, the regulatory effect of IGFBP-2 on caloric intake should be thoroughly tested in various settings.
It is, however, possible to draw comparisons between the metabolic effects of RYGB in animals deficient in leptin or IGFBP-2. Both knockout models experience blunted efficacy of RYGB to induce a significant loss of weight and improvement in insulin sensitivity. These responses are related to adipose tissue changes, and the surgery-associated reduction in EE is blunted in both leptin- and Igfbp2-deficient mice. Moreover, leptin-deficient mice regain their body weight at 8 weeks post-surgery (20%),54 compared to 10 weeks for Igfbp-2−/− mice. In addition, although IGFBP-2 is not necessary for the glucose-lowering actions of leptin in non-operated mice,55 leptin-deficient mice display similar insulin sensitization in response to RYGB than did Igfbp2−/− mice54 early (3 weeks) but not chronically after surgery, demonstrating close relationships and the involvement of compensatory mechanisms. In that regard, we found higher GLP-1 levels in RYGB-operated Igfbp-2−/− mice, which could have contributed to stabilizing glucose homeostasis even in the presence of higher fat mass. This possibility could have been revealed by oral glucose tolerance test (GTT) or refeeding tests. Interestingly, enhanced circulating GLP-1 levels are increased in the long term in both Igfbp2−/− animals after RYGB and farnesoid X receptor (FXR)−/− animals after bile diversion, a surgery close to the RYGB model in mice and BPD in humans. FXR-deficient mice are partially refractory to weight loss after bile diversion,56 but whether FXR and IGFBP-2 are mechanistically linked remains unknown. The higher proportion of lean mass in RYGB-operated Igfbp2−/− mice could also have contributed to attenuate worsening in insulin sensitivity over time compared to their Igfbp2++ counterparts.
In summary, the present study demonstrated that the modulation of IGFBP-2 levels by bariatric surgery is robust, evolutionarily consistent, and associated with intestinal diversion. The augmentation in IGFBP-2 has a partial but direct implication in surgery-induced reduction in adiposity and early insulin sensitization. These findings were corroborated in humans, in whom IGFBP-2 was closely associated with surgery-induced improvement in glucose homeostasis. Thus, interventions elevating IGFBP-2 levels may have the potential to mimic some aspects of bariatric surgery for the development of non-chirurgical treatments against obesity and T2D.
Limitations of study
This study has several limitations. The findings in human patients should be interpreted as associative. Clear causality between IGFBP-2 and insulin sensitization after bariatric surgery needs further confirmation in independent and larger cohorts. In mice, although our data show that EE is not affected by genotype in RYGB-operated animals, the absence of pair-fed control groups limits definite conclusions about the control of energy intake as the main determinant by which IGFBP-2 deficiency impairs body weight loss after bariatric surgery. Finally, concomitant GTTs, insulin sensitivity tests, and measurements of fasting glucose and insulin levels performed at different time points before and after bariatric surgery could provide more precise information on the role of IGFBP-2 in mediating the early versus later improvements in glucose homeostasis.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse IGFBP-2 (C-18) | Santa Cruz Biotechnology | Cat# sc-6001; RRID: AB_648837 |
| Anti-Actin Antibody, clone C4 | Millipore Sigma | Cat# MAB1501; RRID: AB_2223041 |
| Biological samples | ||
| Biobanked human plasma samples | Quebec Heart and Lung Institute, Québec, Canada | N/A |
| Biobanked human plasma samples | Sherbrooke University Hospital, Sherbrooke, Canada | N/A |
| Critical commercial assays | ||
| Mouse and rat plasma IGFBP-2 levels ELISA | ALPCO, Canada | #22-BP2MS-E01 |
| Human plasma IGFBP-2 levels ELISA | ALPCO, Canada | #22-BP2HU-E01 |
| Mouse total GLP-1 assay kit | Millipore Sigma | Cat# EZGLPHS-35K; RRID: AB_2884907 |
| Mouse insulin ELISA kit | Milliplex, Millipore, St. Charles, MO | MADKMAG-71K |
| Mouse FGF15 ELISA kit | Cedarlane, Canada | LS-F11446-1 |
| Mouse Ketone bodies (BOH and AcAc) | BioAssay Systems | EKBD-100 |
| Experimental models: organisms/strains | ||
| Male Wistar rats | 57 | N/A |
| Male Mouse: B6;129S5-Igfbp2Gt(OST365171)Lex/Mmucd | Mutant Mouse Regional Resource Center | Cat# 011721-UCD; RRID: MMRRC_011721-UCD |
| Oligonucleotides | ||
| mouse IGFBP-2 forward GCGCCAGCCCGGAGCAGGTT | Invitrogen | N/A |
| mouse IGFBP-2 reverse CCGGAAGGCGCATGGTGGAGAT | Invitrogen | N/A |
| mouse β2M forward ATGGGAAGCCGAACATACTG | Invitrogen | N/A |
| mouse β2M reverse CAGTCTCAGTGGGGGTGAAT | Invitrogen | N/A |
| mouse IGF-1 forward GGACCGAGGGGCTTTTACTTCAAC | Invitrogen | N/A |
| mouse IGF-1 reverse TGGCGCTGGGCACGGATAG | Invitrogen | N/A |
| rat IGFBP-2 forward AGCATGGCCTGTACAACCTC | Invitrogen | N/A |
| rat IGFBP-2 reverse ATCATTCTCCTGCTGCTCGT | Invitrogen | N/A |
| rat L27 forward CTGCTCGCTGTCGAAATG | Invitrogen | N/A |
| rat L27 reverse CCTTGCGTTTCAGTGCTG | Invitrogen | N/A |
| Software and algorithms | ||
| GraphPad Prism 8.4.2 for macOS | GraphPad Software, La Jolla, USA | https://www.graphpad.com |
| JMP® Version 14 for macOS | SAS Institute Inc., Cary, USA | https://www.jmp.com/en_us/home.geo.html |
| SAS Institute Inc. | SAS Campus Drive, Cary, North Carolina 27513, USA | https://www.sas.com/en_us/home.geo.html |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed and will be fulfilled by the Lead Contact, Frédéric Picard (Frederic.Picard@criucpq.ulaval.ca).
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate or analyze datasets or codes.
Experimental models and subject details
Bariatric surgery in humans
All participants to the studies described herein were 18 years old or older with a medical indication for bariatric surgery (BMI ³ 40 kg/m2 or BMI ³ 35 kg/m2 with at least one diagnosed comorbidity such as diabetes, hypertension or dyslipidemia). Patients underwent BPD-DS, RYGB or SG bariatric surgery (20 patients per type) at the Institut universitaire de cardiologie et de pneumologie de Québec (IUCPQ). The type of surgery was determined after careful consideration of the advantages and risks according to the medical and surgical evaluation and after discussion with the patient, and performed as described previously.58, 59, 60 All patients provided written informed consent before their inclusion in the study. The experimental protocol was approved by the ethics committee of the IUCPQ. Characteristics of the patients at baseline (before surgery) are available in the Supplementary Tables pertaining to each Figure.
Bariatric surgery in rats
Liver samples were obtained from a previously published study performed in male Wistar rats.57 Briefly, rats were fed a high-fat diet (D12492, Research Diets, USA) for 6 weeks and then assigned to four types of surgery (BPD-DS, SG, DS and sham surgery). Nine days after surgery, sham-operated rats were divided into two groups diverging in their food intake: ad libitum feeding (sham ad lib) or food-restricted to match the body weight (sham weight-matched, WM) of rats operated with BPD-DS. Nine weeks post-surgery, blood and tissues were collected after an overnight fast and processed as described below.
Bariatric surgery in mice
The mouse strain used for this research project, B6;129S5-Igfbp2Gt(OST365171)Lex/Mmucd, identification number 011721-UCD, was obtained from the Mutant Mouse Regional Resource Center, a NIH-funded strain repository, and was donated to the MMRRC by the NINDS-funded GENSAT BAC transgenic project (Lexicon Genetics Incorporated). Animals were backcrossed into a C57BL/6J genetic background for at least 5 generations before use. Homozygous mutant mice, named Igfbp2 −/− mice, were compared to Igfbp2 +/+ wild-type littermates in all experiments. Mice were fed a high-fat diet (D12492, Research Diets, USA) for 15 weeks to induce obesity. Then, RYGB surgery was performed under anesthesia exactly as described.22,61,62 Briefly, in a jejuno-gastric anastomosis, the cut end of the mid jejunum was connected with a very small gastric pouch and the other end of the cut jejunum was anastomosed to the lower jejunum, resulting in a 5-6 cm long Roux limb, a 9-11 cm long biliopancreatic limb, and a 20-25 cm long common limb. Sham surgery consisted of laparotomy only, without transection of jejunum and stomach. A group of un-operated mice (weight-matched, WM) were food-restricted to match the body weight obtained through RYGB. After surgery, mice were exposed to a two-choice diet (D12492, Research Diets, USA) and low-fat regular laboratory chow (Diet 5001, Purina LabDiet, USA) for the duration of the experiment, except for periods in the metabolic chamber when they were exposed to only high-fat diet. The rationale for the two-choice diet was twofold, first, it better mimics the human situation, and second, we found that mice eat relatively more chow immediately after RYGB. Mice were kept individually on corncob bedding except for the periods of food intake measurements, when they were on grid floors. All experimental procedures in animals were approved by the institutional animal care committees of Université Laval and Louisiana State University.
Method details
Human studies
Plasma biochemistry
Blood samples were collected after overnight fasting before and at indicated time-points after the surgery. Plasma IGFBP-2 levels were measured by ELISA (Alpco kit #22-BP2HU-E01, Canada) according to the manufacturer’s instructions. The detection limit was 0.2 ng/mL; the inter-assay coefficient of variability was below 10%. Glucose, insulin and triglyceride levels were quantified as previously described.11 The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from fasting glucose and insulin values.11 The adipose tissue insulin resistance index (Adipo-IR) was calculated from fasting glucose and fasting free fatty acids (FFA) concentrations.11
Insulin sensitivity: euglycemic-hyperinsulinemic clamp
Before and at 3, 90, and 365 days after surgery, a subgroup of sixteen obese participants were subjected to euglycemic-hyperinsulinemic clamps performed as previously described.11,63 Insulin sensitivity was calculated as dL glucose ⋅ kg lean mass-1 ⋅ min-1 ⋅ mU insulin-1 ⋅ 10−3.
Animal studies
Metabolic studies in mice
Body weight, food intake, body composition (DEXA) were recorded before and after the surgeries exactly as described previously.22,61,62 Energy expenditure was measured at two ambient temperatures, normal room temperature at 23°C and near thermoneutrality at 29°C for 3 days under each condition. Mice were adapted for at least one day to each condition before taking measurements. Energy expenditure was evaluated by indirect calorimetry (Phenomaster/ Labmaster, TSE Systems, Germany), and herein reported as kcal/mouse both unadjusted and adjusted for lean and total body mass using ANCOVA correction. Locomotor activity was measured in numbers of beam breaks in the X and Y planes (7mm spatial resolution, 10ms temporal resolution). Before and after the surgery, mice had access to chow or high-fat diets, and preferences for high-fat versus chow diets were probed as described previously.22,61,62 21-weeks post-surgery, mice were sacrificed after 3-5 hours food deprivation, and blood and tissues were collected for subsequent analyses.
Quantification of IGFBP-2 in plasma and tissues
Tissue RNA was isolated by using Illustra™ RNAspin Mini kit (GE Healthcar, Canada) according to the manufacturer’s instructions. Liver IGFBP-2 expression was determined by qPCR in the 7900HT Fast real-time PCR system (Applied Biosystem) by using Supergreen qPCR mastermix with high Rox (Wisent, Canada). The following primers were designed using Primer BLAST: mouse IGFBP-2 forward 5′-GCGCCAGCCCGGAGCAGGTT-3′, reverse 5′-CCGGAAGGCGCATGGTGGAGAT-3′; mouse IGF-1 forward 5′- GGACCGAGGGGCTTTTACTTCAAC-3′, reverse 5′- TGGCGCTGGGCACGGATAG-3′; mouse β2M forward 5′-ATGGGAAGCCGAACATACTG-3′, reverse 5′CAGTCTCAGTGGGGGTGAAT-3; rat IGFBP-2 forward 5′-AGCATGGCCTGTACAACCTC-3′, reverse 5′-ATCATTCTCCTGCTGCTCGT-3′; rat L27 forward 5′-CTGCTCGCTGTCGAAATG-3′; reverse 5′-CCTTGCGTTTCAGTGCTG-3′.
Liver proteins were extracted using lysis buffer (HEPES 500 mM pH 7.5, NaCl 4 M, EDTA 500 mM, NaVO4 200 mM, NaF 1 M, NaP 83.3 mM, Na-β G 500 mM, TX100 1%, protease inhibitor 1‰) by sonication. Proteins were quantified colorimetrically (Biorad, Canada), and 50 μg were prepared with 5X loading blue buffer (0.4 M Tris pH 6.8, 4% SDS, 2.5 mM EDTA, 20% glycerol, 0.1 M DTT, 1% bromophenol blue), then denatured at 95°C for 10 minutes. In turn, 1 μL of plasma was prepared with 4X Laemmli blue buffer (1 M Tris pH 6.8, SDS 8%, 40% glycerol, 0.01% bromophenol blue, 10% β-mercaptoethanol) and lysis buffer described above. Liver and plasma IGFBP-2 levels in mice were determined first by western immunobloting using antibodies against actin (EMD Millipore) and IGFBP-2 (C-18, Santa Cruz Biotechnology). Rats and mice plasma IGFBP-2 levels were also quantified by ELISA (ALPCO kit #22-BP2MS-E01, Canada) according to the manufacturer’s instructions and as described previously.30 Detection limit was 0.01 ng/mL. Inter-assay coefficient of variability was below 10%.
Leptin, GLP-1, FGF15, lipid profiles, insulin, glucose measurements
Glucose tolerance was assessed at 3 weeks (20 ± 4 days) after surgery by injecting α-D-glucose (1.5 mg/, 30% w/v in sterile saline, i.p.) and measuring blood glucose from the tail vein before and at 15, 30, 60, and 120 minutes after injection, with glucose strips and a glucometer (Onetouch Ultra Strips and Onetouch Ultra Glucometer, LifeScan INC, Milpitas, CA). Glucose tolerance tests were conducted between 09:00 and noon, after 3-5 h of food deprivation. Insulin tolerance was assessed at 70 ± 4 days (referred to as 10 weeks) after surgery by injecting insulin (0.6 U/kg in sterile saline, i.p., Novolin R, Novo Nordisk, Bagsvaerd, Denmark) and measuring blood glucose as above.
At 19 weeks after surgery, 3-5 hr food-deprived mice were administered 1.5g/kg of 40 % sterile Dextrose (Hospira Inc, Lake Forest, IL) in sterile water through oral gavage. At 5 ± 1 minutes after administration, 100 μL of whole blood were collected using heparinized capillary tubes (Fisherbrand Microhematocrit Capillary Tubes, Thermo Fisher Scientific, Waltham, MA) into centrifuge tubes containing 4.5 μL of protease inhibitor cocktail (1.5 μL of each of the following: Protease Inhibitor, Sigma, St. Louis, MO; DDP-IV Inhibitor, EMD Millipore, St. Charles, MO; Pefabloc SC, Roche, Indianapolis, IN) and immediately centrifuged at 4°C and 3000 rpm for 10 min to separate the plasma. Plasma GLP-1 levels were determined using the high sensitivity Total GLP-1 assay kit (Millipore Sigma, EZGLPHS-35K) on 50 μL plasma diluted 1:2 with buffer.
At 140 ± 4 days after surgery mice were food deprived for 3-5 hours and euthanized by decapitation. A few drops of trunk blood were collected, and blood glucose was immediately tested using glucose strips as above. An additional 500 μL of trunk blood was collected, treated with 83.5 μL EDTA (Sigma, St. Louis, MO) and a protease inhibitor cocktail (1.5 μL of each of the following: Protease inhibitor, Sigma, St. Louis, MO; DPP-IV inhibitor, EMD Millipore, St. Charles, MO; Prefabloc SC, Roche, Indianapolis, IN), and immediately centrifuged at 4°C and 3000 RPM for 10 min to separate the plasma from the whole blood. Plasma aliquots were frozen in liquid nitrogen and stored at - 80°C prior to processing.
Plasma was subjected to ELISA for measurement of insulin (MADKMAG-71K Milliplex, Millipore, St. Charles, MO) and FGF15 (LifeSpan Biosciences, USA). Plasma levels of triglycerides, NEFA and cholesterol levels were quantified colorimetrically using enzymatic reagents from Randox (Crumlin, UK). Acetoacetic acid (AcAc) and 3-hydroxybityric acid (BOH) were quantified colorimetrically using reagents from BioAssay Systems (CA) according to the manufacturer’s instructions.
Quantification and statistical analysis
Data in figures were expressed as means ± SEM. Statistical analyses were performed with GraphPad Prism version 8.4.2 for macOS (GraphPad Software, La Jolla, USA) and JMP®, Version 14. (SAS Institute Inc., Cary, USA), using ANOVA followed by Bonferroni-correction for multiple comparisons. For energy expenditure, ANCOVA was used. SAS was used to analyze BMI-independent associations between IGFBP-2 and parameters of insulin sensitivity. Differences were considered as being significant at p < 0.05.
Acknowledgments
We would like to thank Audrey Chabot for technical assistance. This work was supported by operating grants from the Canadian Institutes of Health Research (CIHR) (PJT-148550 to F.P. and MOP-97947 to A.C.C.), the Canadian Diabetes Association (grant no. OG-3-14-4507-AC to A.C.C.), the National Institutes of Health (NIH) (grant nos. DK047348 to H.-R.B. and P30 GM118430, behavioral and metabolic core), the Fondation de l’IUCPQ, a CIHR Team grant on bariatric care (TB2-138776 to A.T.), and an investigator-initiated study grant from Johnson & Johnson Medical Companies (grant no. ETH-14-610). The co-investigators and collaborators of the REMISSION study (Reaching Enduring Metabolic Improvements by Selecting Surgical Interventions in Obese Individuals) are (alphabetical order): L. Biertho, M. Bouvier, S. Biron, P. Cani, A. Carpentier, A. Dagher, F. Dubé, A. Fergusson, S. Fulton, F.S. Hould, F. Julien, T. Kieffer, B. Laferrère, A. Lafortune, S. Lebel, O. Lescelleur, E. Levy, A. Marette, S. Marceau, A. Michaud, F. Picard, P. Poirier, D. Richard, J. Schertzer, A. Tchernof, and M.C. Vohl. The research work of C.D.M. (DK081563) and H.M. (DK092287) was supported by the NIH. A.C.C. is the Canada Research Chair in Molecular Imaging of Diabetes. J.F. was supported by a studentship from Fonds d’Enseignement et de Recherche (FER) of the Faculty of Pharmacy of Université Laval. A.-M.C. was supported by a Diabetes Canada post-doctoral award and an FRQS clinician-investigator training award. Funding sources for the trial had no role in the design, conduct, or management of the study; in data collection, analysis, or interpretation of data; or in the preparation of the present manuscript and decision to publish.
Author contributions
Conceived and designed research project, F.P., A.T., D.R., and H.-R.B. Performed the experiments, J.F., Z.H., S.M., A.C.C., F.F., T.G.-L., C.N., A.-M.C., L.B., S.M., F.-S.H., S.L., R.L.T., and E.-D.B. IGFBP-2 quantification, J.F. and M.L. Interpreted the experimental results, J.F., H.-R.B., M.N., M.B.M., C.D.M., and H.M. Prepared the figures, J.F., M.B.M., and R.L.T. Drafted the manuscript, J.F., F.P., and H.-R.B. Revised the manuscript, H.-R.B., C.D.M., H.M., A.T., A.C.C., and A.-M.C. All of the authors approved the final version of the manuscript.
Declaration of interests
A.C.C. has received consultant fees from Janssen (2017 and 2018), Novartis (2018), Novo Nordisk (2018), HLS Therapeutics (2019), and Eli Lilly (2020). A.-M.C. has received consultant fees from Pfizer (2017). A.T. received consulting fees from Novo Nordisk and Bausch Health. A.T. and L.B. are the recipients of research grant support from Johnson & Johnson Medical Companies and Medtronic for studies on bariatric surgery and the Research Chair in Bariatric and Metabolic Surgery, respectively, at l’Institut Universitaire de Cardiologie et de Pneumologie de Québec (IUCPQ) and Laval University. All of the other authors reported no competing financial interests in relation to the work described herein.
Published: April 20, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2021.100248.
Contributor Information
Hans-Rudolf Berthoud, Email: berthohr@pbrc.edu.
Frédéric Picard, Email: frederic.picard@criucpq.ulaval.ca.
Supplemental information
References
- 1.Poirier P., Cornier M.A., Mazzone T., Stiles S., Cummings S., Klein S., McCullough P.A., Ren Fielding C., Franklin B.A., American Heart Association Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism Bariatric surgery and cardiovascular risk factors: a scientific statement from the American Heart Association. Circulation. 2011;123:1683–1701. doi: 10.1161/CIR.0b013e3182149099. [DOI] [PubMed] [Google Scholar]
- 2.Rubino F. Bariatric surgery: effects on glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9:497–507. doi: 10.1097/01.mco.0000232914.14978.c5. [DOI] [PubMed] [Google Scholar]
- 3.Abarca-Gómez L., Abdeen Z.A., Hamid Z.A., Abu-Rmeileh N.M., Acosta-Cazares B., Acuin C., Adams R.J., Aekplakorn W., Afsana K., Aguilar-Salinas C.A., NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris L.A., Kayser B.D., Cefalo C., Marini L., Watrous J.D., Ding J., Jain M., McDonald J.G., Thompson B.M., Fabbrini E. Biliopancreatic Diversion Induces Greater Metabolic Improvement Than Roux-en-Y Gastric Bypass. Cell Metab. 2019;30:855–864.e3. doi: 10.1016/j.cmet.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas S., Schauer P. Bariatric surgery and the gut hormone response. Nutr. Clin. Pract. 2010;25:175–182. doi: 10.1177/0884533610361739. [DOI] [PubMed] [Google Scholar]
- 6.Marceau P., Biron S., Hould F.-S., Lebel S., Marceau S., Lescelleur O., Biertho L., Simard S. Duodenal switch improved standard biliopancreatic diversion: a retrospective study. Surg. Obes. Relat. Dis. 2009;5:43–47. doi: 10.1016/j.soard.2008.03.244. [DOI] [PubMed] [Google Scholar]
- 7.Sjöström L., Lindroos A.-K., Peltonen M., Torgerson J., Bouchard C., Carlsson B., Dahlgren S., Larsson B., Narbro K., Sjöström C.D., Swedish Obese Subjects Study Scientific Group Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 8.Hao Z., Townsend R.L., Mumphrey M.B., Morrison C.D., Münzberg H., Berthoud H.R. RYGB Produces more Sustained Body Weight Loss and Improvement of Glycemic Control Compared with VSG in the Diet-Induced Obese Mouse Model. Obes. Surg. 2017;27:2424–2433. doi: 10.1007/s11695-017-2660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubino F., Forgione A., Cummings D.E., Vix M., Gnuli D., Mingrone G., Castagneto M., Marescaux J. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann. Surg. 2006;244:741–749. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heffron S.P., Parikh A., Volodarskiy A., Ren-Fielding C., Schwartzbard A., Nicholson J., Bangalore S. Changes in Lipid Profile of Obese Patients Following Contemporary Bariatric Surgery: A Meta-Analysis. Am. J. Med. 2016;129:952–959. doi: 10.1016/j.amjmed.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grenier-Larouche T., Carreau A.-M., Geloën A., Frisch F., Biertho L., Marceau S., Lebel S., Hould F.-S., Richard D., Tchernof A., Carpentier A.C. Fatty Acid Metabolic Remodeling During Type 2 Diabetes Remission After Bariatric Surgery. Diabetes. 2017;66:2743–2755. doi: 10.2337/db17-0414. [DOI] [PubMed] [Google Scholar]
- 12.Serés L., Lopez-Ayerbe J., Coll R., Rodriguez O., Vila J., Formiguera X., Alastrue A., Rull M., Valle V. Increased exercise capacity after surgically induced weight loss in morbid obesity. Obesity (Silver Spring) 2006;14:273–279. doi: 10.1038/oby.2006.35. [DOI] [PubMed] [Google Scholar]
- 13.Laferrère B., Teixeira J., McGinty J., Tran H., Egger J.R., Colarusso A., Kovack B., Bawa B., Koshy N., Lee H. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2008;93:2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bose M., Machineni S., Oliván B., Teixeira J., McGinty J.J., Bawa B., Koshy N., Colarusso A., Laferrère B. Superior appetite hormone profile after equivalent weight loss by gastric bypass compared to gastric banding. Obesity (Silver Spring) 2010;18:1085–1091. doi: 10.1038/oby.2009.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachdev S., Wang Q., Billington C., Connett J., Ahmed L., Inabnet W., Chua S., Ikramuddin S., Korner J. FGF 19 and Bile Acids Increase Following Roux-en-Y Gastric Bypass but Not After Medical Management in Patients with Type 2 Diabetes. Obes. Surg. 2016;26:957–965. doi: 10.1007/s11695-015-1834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gómez-Ambrosi J., Gallego-Escuredo J.M., Catalán V., Rodríguez A., Domingo P., Moncada R., Valentí V., Salvador J., Giralt M., Villarroya F., Frühbeck G. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clin. Nutr. 2017;36:861–868. doi: 10.1016/j.clnu.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 17.Flynn C.R., Albaugh V.L., Abumrad N.N. Metabolic Effects of Bile Acids: Potential Role in Bariatric Surgery. Cell. Mol. Gastroenterol. Hepatol. 2019;8:235–246. doi: 10.1016/j.jcmgh.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mokadem M., Zechner J.F., Margolskee R.F., Drucker D.J., Aguirre V. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol. Metab. 2013;3:191–201. doi: 10.1016/j.molmet.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson-Pérez H.E., Chambers A.P., Ryan K.K., Li B., Sandoval D.A., Stoffers D., Drucker D.J., Pérez-Tilve D., Seeley R.J. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like Peptide 1 receptor deficiency. Diabetes. 2013;62:2380–2385. doi: 10.2337/db12-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramracheya R.D., McCulloch L.J., Clark A., Wiggins D., Johannessen H., Olsen M.K., Cai X., Zhao C.-M., Chen D., Rorsman P. PYY-Dependent Restoration of Impaired Insulin and Glucagon Secretion in Type 2 Diabetes following Roux-En-Y Gastric Bypass Surgery. Cell Rep. 2016;15:944–950. doi: 10.1016/j.celrep.2016.03.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan K.K., Tremaroli V., Clemmensen C., Kovatcheva-Datchary P., Myronovych A., Karns R., Wilson-Pérez H.E., Sandoval D.A., Kohli R., Bäckhed F., Seeley R.J. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison C.D., Hao Z., Mumphrey M.B., Townsend R.L., Münzberg H., Ye J., Berthoud H.-R. Roux-en-Y gastric bypass surgery is effective in fibroblast growth factor-21 deficient mice. Mol. Metab. 2016;5:1006–1014. doi: 10.1016/j.molmet.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boland B., Mumphrey M.B., Hao Z., Gill B., Townsend R.L., Yu S., Münzberg H., Morrison C.D., Trevaskis J.L., Berthoud H.R. The PYY/Y2R-Deficient Mouse Responds Normally to High-Fat Diet and Gastric Bypass Surgery. Nutrients. 2019;11:585. doi: 10.3390/nu11030585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jørgensen N.B., Dirksen C., Bojsen-Møller K.N., Kristiansen V.B., Wulff B.S., Rainteau D., Humbert L., Rehfeld J.F., Holst J.J., Madsbad S., Clausen T.R. Improvements in glucose metabolism early after gastric bypass surgery are not explained by increases in total bile acids and fibroblast growth factor 19 concentrations. J. Clin. Endocrinol. Metab. 2015;100:E396–E406. doi: 10.1210/jc.2014-1658. [DOI] [PubMed] [Google Scholar]
- 25.Wheatcroft S.B., Kearney M.T. IGF-dependent and IGF-independent actions of IGF-binding protein-1 and -2: implications for metabolic homeostasis. Trends Endocrinol. Metab. 2009;20:153–162. doi: 10.1016/j.tem.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Assefa B., Mahmoud A.M., Pfeiffer A.F.H., Birkenfeld A.L., Spranger J., Arafat A.M. Insulin-Like Growth Factor (IGF) Binding Protein-2, Independently of IGF-1, Induces GLUT-4 Translocation and Glucose Uptake in 3T3-L1 Adipocytes. Oxid. Med. Cell. Longev. 2017;2017:3035184. doi: 10.1155/2017/3035184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter S., Li Z., Lemieux I., Alméras N., Tremblay A., Bergeron J., Poirier P., Deshaies Y., Després J.-P., Picard F. Circulating IGFBP-2 levels are incrementally linked to correlates of the metabolic syndrome and independently associated with VLDL triglycerides. Atherosclerosis. 2014;237:645–651. doi: 10.1016/j.atherosclerosis.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 28.Russo V.C., Azar W.J., Yau S.W., Sabin M.A., Werther G.A. IGFBP-2: the dark horse in metabolism and cancer. Cytokine Growth Factor Rev. 2015;26:329–346. doi: 10.1016/j.cytogfr.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Heald A.H., Kaushal K., Siddals K.W., Rudenski A.S., Anderson S.G., Gibson J.M. Insulin-like growth factor binding protein-2 (IGFBP-2) is a marker for the metabolic syndrome. Exp. Clin. Endocrinol. Diabetes. 2006;114:371–376. doi: 10.1055/s-2006-924320. [DOI] [PubMed] [Google Scholar]
- 30.Li Z., Picard F. Modulation of IGFBP2 mRNA expression in white adipose tissue upon aging and obesity. Horm. Metab. Res. 2010;42:787–791. doi: 10.1055/s-0030-1262854. [DOI] [PubMed] [Google Scholar]
- 31.Wheatcroft S.B., Kearney M.T., Shah A.M., Ezzat V.A., Miell J.R., Modo M., Williams S.C., Cawthorn W.P., Medina-Gomez G., Vidal-Puig A. IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes. 2007;56:285–294. doi: 10.2337/db06-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xi G., Solum M.A., Wai C., Maile L.A., Rosen C.J., Clemmons D.R. The heparin-binding domains of IGFBP-2 mediate its inhibitory effect on preadipocyte differentiation and fat development in male mice. Endocrinology. 2013;154:4146–4157. doi: 10.1210/en.2013-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z., Martin J., Poirier P., Caron-Cantin S.-M., Hould F.-S., Marceau S., Marceau P., Picard F. Upregulation of plasma insulin-like growth factor binding protein 2 levels after biliopancreatic diversion in humans. Obesity (Silver Spring) 2012;20:1469–1473. doi: 10.1038/oby.2012.90. [DOI] [PubMed] [Google Scholar]
- 34.Mattsson A., Svensson D., Schuett B., Osterziel K.J., Ranke M.B. Multidimensional reference regions for IGF-I, IGFBP-2 and IGFBP-3 concentrations in serum of healthy adults. Growth Horm. IGF Res. 2008;18:506–516. doi: 10.1016/j.ghir.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Sunderić M., Mihailović N., Nedić O. Protein molecular forms of insulin-like growth factor binding protein-2 change with aging. Exp. Gerontol. 2014;58:154–158. doi: 10.1016/j.exger.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Ceccarini G., Pelosini C., Ferrari F., Magno S., Vitti J., Salvetti G., Moretto C., Marioni A., Buccianti P., Piaggi P. Serum IGF-binding protein 2 (IGFBP-2) concentrations change early after gastric bypass bariatric surgery revealing a possible marker of leptin sensitivity in obese subjects. Endocrine. 2019;65:86–93. doi: 10.1007/s12020-019-01915-y. [DOI] [PubMed] [Google Scholar]
- 37.Shah R.V., Hwang S.J., Yeri A., Tanriverdi K., Pico A.R., Yao C., Murthy V., Ho J., Vitseva O., Demarco D. Proteins Altered by Surgical Weight Loss Highlight Biomarkers of Insulin Resistance in the Community. Arterioscler. Thromb. Vasc. Biol. 2019;39:107–115. doi: 10.1161/ATVBAHA.118.311928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang H.S., Cho H.C., Lee J.H., Oh G.T., Koo S.H., Park B.H., Lee I.K., Choi H.S., Song D.K., Im S.S. Metformin stimulates IGFBP-2 gene expression through PPARalpha in diabetic states. Sci. Rep. 2016;6:23665. doi: 10.1038/srep23665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hankir M.K., Seyfried F., Hintschich C.A., Diep T.A., Kleberg K., Kranz M., Deuther-Conrad W., Tellez L.A., Rullmann M., Patt M. Gastric Bypass Surgery Recruits a Gut PPAR-α-Striatal D1R Pathway to Reduce Fat Appetite in Obese Rats. Cell Metab. 2017;25:335–344. doi: 10.1016/j.cmet.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Lim J.H., Gerhart-Hines Z., Dominy J.E., Lee Y., Kim S., Tabata M., Xiang Y.K., Puigserver P. Oleic acid stimulates complete oxidation of fatty acids through protein kinase A-dependent activation of SIRT1-PGC1α complex. J. Biol. Chem. 2013;288:7117–7126. doi: 10.1074/jbc.M112.415729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hedbacker K., Birsoy K., Wysocki R.W., Asilmaz E., Ahima R.S., Farooqi I.S., Friedman J.M. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab. 2010;11:11–22. doi: 10.1016/j.cmet.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Breen D.M., Rasmussen B.A., Kokorovic A., Wang R., Cheung G.W., Lam T.K. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat. Med. 2012;18:950–955. doi: 10.1038/nm.2745. [DOI] [PubMed] [Google Scholar]
- 43.Ye Y., Abu El Haija M., Morgan D.A., Guo D., Song Y., Frank A., Tian L., Riedl R.A., Burnett C.M.L., Gao Z. Endocannabinoid Receptor-1 and Sympathetic Nervous System Mediate the Beneficial Metabolic Effects of Gastric Bypass. Cell Rep. 2020;33:108270. doi: 10.1016/j.celrep.2020.108270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Šunderić M., Miljuš G., Nedić O. Interaction of insulin-like growth factor-binding protein 2 with α2-macroglobulin in the circulation. Protein J. 2013;32:138–142. doi: 10.1007/s10930-013-9471-8. [DOI] [PubMed] [Google Scholar]
- 45.Reyer A., Schindler N., Ohde D., Walz C., Kunze M., Tuchscherer A., Wirthgen E., Brenmoehl J., Hoeflich A. The RGD sequence present in IGFBP-2 is required for reduced glucose clearance after oral glucose administration in female transgenic mice. Am. J. Physiol. Endocrinol. Metab. 2015;309:E409–E417. doi: 10.1152/ajpendo.00168.2015. [DOI] [PubMed] [Google Scholar]
- 46.Shen X., Xi G., Wai C., Clemmons D.R. The coordinate cellular response to insulin-like growth factor-I (IGF-I) and insulin-like growth factor-binding protein-2 (IGFBP-2) is regulated through vimentin binding to receptor tyrosine phosphatase β (RPTPβ) J. Biol. Chem. 2015;290:11578–11590. doi: 10.1074/jbc.M114.620237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z., Miard S., Laplante M., Sonenberg N., Picard F. Insulin stimulates IGFBP-2 expression in 3T3-L1 adipocytes through the PI3K/mTOR pathway. Mol. Cell. Endocrinol. 2012;358:63–68. doi: 10.1016/j.mce.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 48.Hoeflich A., Wu M., Mohan S., Föll J., Wanke R., Froehlich T., Arnold G.J., Lahm H., Kolb H.J., Wolf E. Overexpression of insulin-like growth factor-binding protein-2 in transgenic mice reduces postnatal body weight gain. Endocrinology. 1999;140:5488–5496. doi: 10.1210/endo.140.12.7169. [DOI] [PubMed] [Google Scholar]
- 49.Boland B.B., Mumphrey M.B., Hao Z., Townsend R.L., Gill B., Oldham S., Will S., Morrison C.D., Yu S., Münzberg H. Combined loss of GLP-1R and Y2R does not alter progression of high-fat diet-induced obesity or response to RYGB surgery in mice. Mol. Metab. 2019;25:64–72. doi: 10.1016/j.molmet.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hao Z., Leigh Townsend R., Mumphrey M.B., Gettys T.W., Yu S., Münzberg H., Morrison C.D., Berthoud H.-R. Roux-en-Y Gastric Bypass Surgery-Induced Weight Loss and Metabolic Improvements Are Similar in TGR5-Deficient and Wildtype Mice. Obes. Surg. 2018;28:3227–3236. doi: 10.1007/s11695-018-3297-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye J., Hao Z., Mumphrey M.B., Townsend R.L., Patterson L.M., Stylopoulos N., Münzberg H., Morrison C.D., Drucker D.J., Berthoud H.-R. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;306:R352–R362. doi: 10.1152/ajpregu.00491.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan S. IGFBP-2 Signaling in the Brain: From Brain Development to Higher Order Brain Functions. Front. Endocrinol. (Lausanne) 2019;10:822. doi: 10.3389/fendo.2019.00822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fletcher L., Isgor E., Sprague S., Williams L.H., Alajajian B.B., Jimenez D.F., Digicaylioglu M. Spatial distribution of insulin-like growth factor binding protein-2 following hypoxic-ischemic injury. BMC Neurosci. 2013;14:158. doi: 10.1186/1471-2202-14-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hao Z., Münzberg H., Rezai-Zadeh K., Keenan M., Coulon D., Lu H., Berthoud H.R., Ye J. Leptin deficient ob/ob mice and diet-induced obese mice responded differently to Roux-en-Y bypass surgery. Int. J. Obes. 2015;39:798–805. doi: 10.1038/ijo.2014.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neumann U.H., Chen S., Tam Y.Y., Baker R.K., Covey S.D., Cullis P.R., Kieffer T.J. IGFBP2 is neither sufficient nor necessary for the physiological actions of leptin on glucose homeostasis in male ob/ob mice. Endocrinology. 2014;155:716–725. doi: 10.1210/en.2013-1622. [DOI] [PubMed] [Google Scholar]
- 56.Albaugh V.L., Banan B., Antoun J., Xiong Y., Guo Y., Ping J., Alikhan M., Clements B.A., Abumrad N.N., Flynn C.R. Role of Bile Acids and GLP-1 in Mediating the Metabolic Improvements of Bariatric Surgery. Gastroenterology. 2019;156:1041–1051.e4. doi: 10.1053/j.gastro.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baraboi E.D., Li W., Labbé S.M., Roy M.C., Samson P., Hould F.S., Lebel S., Marceau S., Biertho L., Richard D. Metabolic changes induced by the biliopancreatic diversion in diet-induced obesity in male rats: the contributions of sleeve gastrectomy and duodenal switch. Endocrinology. 2015;156:1316–1329. doi: 10.1210/en.2014-1785. [DOI] [PubMed] [Google Scholar]
- 58.Piché M.E., Auclair A., Harvey J., Marceau S., Poirier P. How to choose and use bariatric surgery in 2015. Can. J. Cardiol. 2015;31:153–166. doi: 10.1016/j.cjca.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 59.Kim W.W., Gagner M., Biertho L., Waage A., Jacob B. Taking posterior rectus sheath laparoscopically to reinforce the gastrojejunostomy in laparoscopic Roux-en-Y gastric bypass. Obes. Surg. 2003;13:258–262. doi: 10.1381/096089203764467171. [DOI] [PubMed] [Google Scholar]
- 60.Marceau P., Biron S., Marceau S., Hould F.S., Lebel S., Lescelleur O., Biertho L., Kral J.G. Biliopancreatic diversion-duodenal switch: independent contributions of sleeve resection and duodenal exclusion. Obes. Surg. 2014;24:1843–1849. doi: 10.1007/s11695-014-1284-0. [DOI] [PubMed] [Google Scholar]
- 61.Hao Z., Mumphrey M.B., Townsend R.L., Morrison C.D., Münzberg H., Ye J., Berthoud H.R. Body Composition, Food Intake, and Energy Expenditure in a Murine Model of Roux-en-Y Gastric Bypass Surgery. Obes. Surg. 2016;26:2173–2182. doi: 10.1007/s11695-016-2062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hao Z., Zhao Z., Berthoud H.R., Ye J. Development and verification of a mouse model for Roux-en-Y gastric bypass surgery with a small gastric pouch. PLoS ONE. 2013;8:e52922. doi: 10.1371/journal.pone.0052922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carpentier A., Patterson B.W., Uffelman K.D., Giacca A., Vranic M., Cattral M.S., Lewis G.F. The effect of systemic versus portal insulin delivery in pancreas transplantation on insulin action and VLDL metabolism. Diabetes. 2001;50:1402–1413. doi: 10.2337/diabetes.50.6.1402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate or analyze datasets or codes.