Abstract
Background:
Desensitization protocols for HLA-incompatible living donor kidney transplantation (ILDKT) vary across centers. The impact of these, as well as other practice variations, on ILDKT outcomes remain unknown.
Methods:
We sought to quantify center-level variation in mortality and graft loss following ILDKT using a 25-center cohort of 1,358 ILDKT recipients with linkage to SRTR for accurate outcome ascertainment. We used multi-level Cox regression with shared frailty to determine the variation in post-ILDKT outcomes attributable to between-center differences, and to identify any center-level characteristics associated with improved post-ILDKT outcomes.
Results:
After adjusting for patient-level characteristics, only 6 centers (24%) had lower mortality and 1 (4%) had higher mortality than average. Similarly, only 5 centers (20%) had higher graft loss and 2 had lower graft loss than average. Only 4.7% of the differences in mortality (p<0.01) and 4.4% of the differences in graft loss (p<0.01) were attributable to between-center variation. These translated to a median hazard ratio of 1.36 for mortality and 1.34 of graft loss for similar candidates at different centers. Post-ILDKT outcomes were not associated with the following center-level characteristics: ILDKT volume and transplanting a higher proportion of highly sensitized, prior transplant, pre-emptive, or minority candidates.
Conclusion:
Unlike most aspects of transplantation where center-level variation and volume impact outcomes, we did not find substantial evidence for this in ILDKT. Our findings support the continued practice of ILDKT across these diverse centers.
Keywords: Mortality, graft loss, incompatible living donor kidney transplantation
INTRODUCTION
HLA-incompatible living donor kidney transplantation (ILDKT) allows for transplantation in the face of donor-specific antibody (DSA) through the use of various desensitization protocols.1,2 ILDKT has been used to facilitate transplantation for highly sensitized candidates, who potentially face prolonged waiting times for kidney paired donation or deceased donor kidney transplantation.3–5 Despite this widespread use, published reports of ILDKT have shown significant differences in graft survival across studies and centers, with 1-year survival ranging from 82% – 100% and 5-year survival ranging from 69.4% – 95.1%.6–17 The reasons for these wide variations in post-ILDKT outcomes have not been fully characterized.
To date, studies of ILDKT outcomes have focused on the role of patient-specific factors. For example, one study reported a 1.64-fold increased risk of graft loss among ILDKT recipients with a positive flow cytometric crossmatch compared to compatible living donor kidney transplant recipients, and a 5.01-fold increased risk for ILDKT recipients with a positive cytotoxic crossmatch.18 Another study of recipients with pre-transplant DSA found that having a pre-transplant DSA with a mean fluorescence intensity, a semi-quantitative measure of antibody strength based on Luminex bead testing, of >8000 was associated with a 23-fold increase in the risk of antibody-mediated rejection, an important predictor of graft loss for which ILDKT recipients are at particular risk.19–21 However, patient-specific factors alone may not explain the wide variation in post-ILDKT outcomes across studies.
Center-level factors could plausibly impact outcomes following ILDKT, but have thus far not been studied. For example, it is possible that centers that perform more ILDKT, and therefore likely have standardized pre- and post-transplant protocols, may have better outcomes. Additionally, centers that more commonly perform higher-risk ILDKT, such as transplanting a higher proportion of recipients with a positive cytotoxic crossmatch, might be more equipped to manage pre- and post-ILDKT DSA. An understanding of the center-level factors associated with post-ILDKT outcomes may identify possible interventions to reduce the wide differences in reported post-ILDKT outcomes. To investigate this further, we used a 25-center ILDKT cohort to provide a more granular assessment of center-level variation in outcomes after ILDKT. The goals of our study were to describe and quantify center-level variation in outcomes after ILDKT, and to identify center-level characteristics associated with high-performing or low-performing centers.
METHODS
Study population
The study population was drawn from a previously described existing cohort of adult (≥18 years of age) patients undergoing ILDKT at 25 centers across the United States between 1997 and 2016.2 ILDKT recipients were defined as patients who received a kidney transplant from an HLA-incompatible living donor requiring any method of desensitization therapy for DSA identified prior to transplantation.18 Each center was asked to categorize patients as having undergone ILDKT across a positive Luminex crossmatch, flow cytometric crossmatch, or cytotoxic crossmatch based on whatever information was available at the time of transplant.
Data Linkage
Data on ILDKT recipients (such as strength of pre-transplant DSA) provided by the participating transplant centers were linked to the SRTR for reliable ascertainment of mortality and graft loss. The SRTR supplements mortality and graft loss ascertainment through linkage to Medicare data and the Social Security Death Master File.
Data source
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere.22 The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. This study was approved by the Johns Hopkins University Institutional Review Board.
Center-level variation in mortality and graft loss after ILDKT
To quantify center-level variation in mortality and graft loss following ILDKT, we used a Cox proportional hazards model with shared frailty, which allows a random effect for each center.23 In other words, each center was allowed to have a different underlying hazard of mortality and graft loss. The term shared frailty in this context refers to the idea that recipients at each center would share the same risk specific to the center they were transplanted at (‘shared frailty’), and is distinct from the traditional clinical usage of the term frailty. One advantage of the shared frailty framework is that it allows for the use of all follow-up time, whereas other models that limit follow-up time (e.g. 1-year mortality or graft loss) cannot.23
We adjusted this shared frailty model for the following patient-level characteristics: donor and recipient age, recipient gender, recipient blood type, recipient race (white, black, or other), years on dialysis, cause of end-stage renal disease (glomerular vs. non-glomerular), panel reactive antibody (PRA), history of prior transplant, and DSA strength (positive Luminex crossmatch, positive flow crossmatch, or positive cytotoxic crossmatch). To determine whether there were center-level factors that were associated with mortality or graft loss beyond patient-level factors, we also explored the following center-level characteristics: percentage of ILDKT recipients with a positive cytotoxic crossmatch, total ILDKT volume, total living donor kidney transplant (LDKT) volume, percentage of total LDKT recipients with a history of prior transplant, percentage of total LDKT recipients that were transplanted pre-emptively, percentage of ILDKT recipients who were African-American, and percentage of total LDKT recipients with a PRA ≥ 80. In this framework, we were able to distinguish the impact of DSA strength (i.e. at the patient-level) and how frequently centers perform ILDKT across stronger DSA (i.e. at the center-level). In selecting our final model, we removed non-significant center-level characteristics for model parsimony, which helps improve model interpretability and stability at the expense of model precision, although this is minimized by removing only non-significant variables.
Using the final shared frailty model, we used the variance of the random effects (i.e. the variability between different center’s underlying hazard of death or graft failure) to calculate two measures of center-level variation in mortality and graft loss. First, we calculated the variance partition coefficient, which describes the percentage of variation in outcome that is due to center-level differences. This is obtained by taking the variance of the random effects (Ω), and solving for Ω/(Ω + 2).23 Second, we calculated the median hazard ratio (MHR), a quantitative measure of the magnitude of center-level differences. For gamma-distributed frailties, the MHR is calculated as the upper quantile of an F(√Ω−2, √Ω−2) distribution.24 The MHR can be interpreted as the median increase in the hazard of the outcome (mortality or graft loss) that would arise if a patient moved from a lower risk center to a higher risk center. We then estimated the hazard ratio associated with each center after adjusting for recipient characteristics by taking each center’s frailty, and generated a 95% confidence interval of this estimate by bootstrapping with replacement for 1000 replications, selecting the 2.5th percentile and 97.5th percentile ordered estimate as the lower and upper bounds of the 95% confidence interval, respectively.
Statistical analysis
There were no missing demographic data for any patient- or center-level variable used in our models. Based on each center’s underlying center-specific hazard of mortality or graft loss, we divided centers into two groups: higher-performing (center-specific frailty < 1.0) and lower-performing centers (center-specific frailty > 1.0). We compared baseline characteristics between higher-performing and lower-performing centers using the chi-squared test for categorical variables, student’s t-test for normally distributed continuous variables, and the Kruskal-Wallis test for non-normally distributed continuous variables. Confidence intervals are reported as per the method of Louis and Zeger.25 All analyses were performed using Stata 15.0/IC for Windows (College Station, Texas).
Sensitivity analysis
To understand whether our results were driven by transplants performed early in our study period, when clinical experience with ILDKT was still developing and Luminex testing was not routinely reported, we performed a sensitivity analysis restricting our study population to ILDKT recipients from 2009–2016. Results of this were consistent with our main analysis.
RESULTS
Study Population
We identified 1,358 recipients from 25 centers who were followed for a median of 9.0 years. To better understand between-center differences in recipient characteristics, we compared 829 recipients at 10 centers with worse unadjusted post-ILDKT outcomes than average to 529 recipients at 15 centers with better unadjusted post-ILDKT outcomes than average (Table 1). Compared to recipients at centers with worse unadjusted post-ILDKT outcomes than average, recipients at centers with better unadjusted post-ILDKT outcomes than average were less likely to be African-American (14.2% vs. 19.3%, p<0.001) and spent less time on dialysis (1.1 years vs. 1.8 years, p<0.001). However, recipients at centers with better unadjusted post-ILDKT outcomes than average were more likely to have had a prior transplant (59.9% vs. 53.1%, p=0.01), more likely to have DSA that reached only a positive Luminex crossmatch strength (40.8% vs. 13.5%, p<0.001), and more likely to have received induction (91.9% vs. 81.9%, p<0.001). Recipients at centers with better unadjusted post-ILDKT outcomes than average were of a similar age (45.5 years vs. 45.1 years, p=0.6), had donors of a similar age (41.4 years vs. 40.3 years, p=0.09), were equally likely to be female (56.3% vs. 56.5%, p=0.9), and had a similar PRA (57% vs. 66%, p=0.2) compared to recipients at centers with worse unadjusted post-ILDKT outcomes than average.
Table 1.
Characteristics of participating transplant centers, stratified by unadjusted center-specific performance.
| Centers with worse outcomes than average | Centers with better outcomes than average | p-value | |
|---|---|---|---|
| Donor/Recipient characteristics | N = 829 | N = 529 | |
| Donor age, years ± sd* | 40.3 ± 11.9 | 41.4 ± 11.9 | 0.09 |
| Donor gender, % female | 56.5 | 56.3 | 0.9 |
| Recipient age, years ± sd* | 45.1 ± 13.4 | 45.5 ± 13.3 | 0.6 |
| Recipient gender, % female | 56.3 | 56.5 | 0.9 |
| Recipient race, % AA* | 19.3 | 14.2 | <0.001 |
| Recipient blood type, %O | 49.9 | 47.8 | 0.7 |
| Time on dialysis, median years (IQR*) | 1.8 (0.3 – 4.5) | 1.1 (0.1 – 2.9) | <0.001 |
| Panel reactive antibody, median (IQR*) | 66 (15 – 93) | 57 (17 – 89) | 0.2 |
| Cause of ESRD*, % due to diabetes | 11.8 | 17.8 | 0.002 |
| History of prior transplant, % | 53.1 | 59.9 | 0.01 |
| Strength of DSA*, % | <0.001 | ||
| Positive Luminex crossmatch | 13.5 | 40.8 | |
| Positive Flow crossmatch | 62.5 | 32.0 | |
| Positive Cytotoxic crossmatch | 24.0 | 27.2 | |
| Induction type | <0.001 | ||
| None | 18.1 | 8.1 | |
| Depleting | 5.6 | 15.2 | |
| Non-depleting | 76.3 | 76.7 | |
| Center characteristics | N = 10 | N = 15 | |
| Annual ILDKT* volume, median (IQR) | 3 (2 – 10.5) | 2 (1 – 6.5) | 0.3 |
| Annual LDKT* volume, median (IQR) | 43 (26 – 63) | 39 (13 – 83) | 0.8 |
| Percentage of pre-emptive LDKT recipients, median (IQR) | 30.5 (28 – 35) | 31 (26 – 39) | 0.9 |
| Proportion of ILDKT recipients with a positive Cytotoxic crossmatch, median (IQR) | 24.7 (0 – 64.9) | 17.9 (0 – 44.4) | 0.7 |
| Proportion of LDKT recipients with a prior transplant, median (IQR) | 13.5 (12 – 15) | 13 (11 – 20) | 1.0 |
| Proportion of LDKT recipients with a PRA* ≥ 80%, median (IQR) | 7 (7 – 9) | 8 (5 – 10) | 0.9 |
| Proportion of AA* ILDKT recipients, median (IQR) | 17 (3 – 33) | 15 (6 – 33) | 0.9 |
Higher-performing centers were defined as those with a frailty < 1.0, as derived from the Cox model with shared frailty for mortality. Lower-performing centers were those with a frailty > 1.0.
AA, African-American; ESRD, end-stage renal disease; ILDKT, incompatible living donor kidney transplantation; LDKT, living donor kidney transplantation; PRA, panel reactive antibody; sd, standard deviation; IQR, inter-quartile range
Compared to centers with worse unadjusted post-ILDKT outcomes than average, centers with better unadjusted post-ILDKT outcomes than average had similar median annual ILDKT (2 vs. 3, p=0.3) and LDKT volumes (39 vs. 43, p=0.8), and transplanted a similar percentage of pre-emptive LDKT recipients (31% vs. 30.5%, p=0.9), ILDKT recipients with a positive cytotoxic crossmatch (17.9% vs. 24.7%, p=0.7), LDKT recipients with a prior transplant (13% vs. 13.5%, p=1.0), LDKT recipients with a PRA ≥ 80% (8% vs. 7%, p=0.9), and African-American ILDKT recipients (15% vs. 17%, p=0.9).
Mortality
After adjusting for recipient characteristics, 6 centers (24%) had significantly lower mortality than average, whereas 1 center (4%) had significantly higher mortality than average (Figure 1). However, we did not identify any center-level characteristics that were associated with higher mortality (Table 2). Post-transplant mortality was not associated with the following center-level characteristics: ILDKT volume (adjusted hazard ratio [aHR]: 0.981.011.04, p=0.7), LDKT volume (aHR: 0.991.001.01, p=0.9), or transplanting a higher proportion of highly sensitized (aHR: 0.961.001.04, p=0.9), prior transplant (aHR: 0.950.991.04, p=0.8), pre-emptive (aHR: 0.971.001.03, p=0.9), minority (aHR: 0.991.001.01, p=0.9), or positive cytotoxic crossmatch (aHR: 0.901.452.33, p=0.1) recipients.
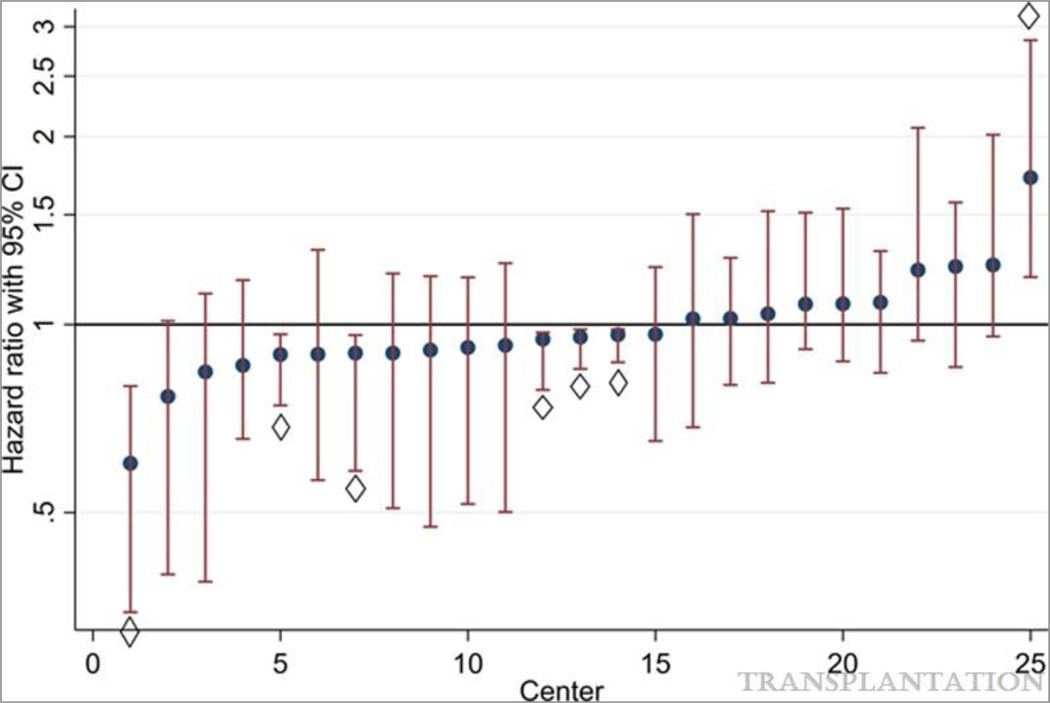
Figure 1. Center-specific hazard ratios for post-ILDKT mortality, adjusting for patient-level characteristics.
Although most centers had a post-ILDKT mortality risk that was indistinguishable from average, 6 (24%) centers had a significantly lower mortality risk than average, whereas 1 center (4%) had a significantly higher mortality risk than average, after adjusting for patient-level factors.
◊ Center whose post-ILDKT mortality risk was significantly different than average
Table 2.
Predictors of mortality after ILDKT, stratified by patient-level or center-level characteristics
| Hazard ratio | p-value | |
|---|---|---|
| Patient-level characteristics | ||
| Donor age | 0.991.001.01 | 0.9 |
| Recipient age | 1.031.041.05 | <0.001 |
| Recipient female gender | 0.720.901.13 | 0.4 |
| Recipient race | ||
| White | Ref | |
| Black | 0.670.911.24 | 0.6 |
| Other | 0.460.650.94 | 0.02 |
| Recipient blood type | ||
| O | Ref | |
| A | 0.710.871.10 | 0.3 |
| B | 0.610.841.16 | 0.3 |
| AB | 0.781.262.03 | 0.4 |
| Time on dialysis, per year | 1.011.041.05 | 0.001 |
| Panel reactive antibody, per % increase | 0.991.001.01 | 0.4 |
| Cause of ESRD*, diabetes | 0.390.500.64 | <0.001 |
| History of prior transplant | 0.881.121.43 | 0.3 |
| Strength of DSA* | ||
| Positive Luminex crossmatch | Ref | |
| Positive Flow crossmatch | 1.261.802.56 | 0.001 |
| Positive Cytotoxic crossmatch | 1.472.123.06 | <0.001 |
| Center-level characteristics | ||
| Median annual ILDKT* volume, per 1 transplant increase | 0.981.011.04 | 0.7 |
| Median annual LDKT* volume, per 1 transplant increase | 0.991.001.01 | 0.9 |
| Percentage of pre-emptive LDKT recipients, per 1 percent increase | 0.971.001.03 | 0.9 |
| Percentage of ILDKT recipients with a positive Cytotoxic crossmatch, per 1 percent increase | 0.901.452.33 | 0.1 |
| Percentage of LDKT recipients with a prior transplant, per 1 percent increase | 0.950.991.04 | 0.8 |
| Percentage of LDKT recipients with a PRA* ≥ 80%, per 1 percent increase | 0.961.001.04 | 0.9 |
| Percentage of AA* ILDKT recipients, per 1 percent increase | 0.991.001.01 | 0.9 |
Although certain patient-level characteristics were associated with higher post-ILDKT mortality, no center-level characteristics were. After adjusting for patient-level characteristics, only 4.7% of the variation in post-ILDKT mortality was explained by differences between centers.
ESRD, end-stage renal disease; ILDKT, incompatible living donor kidney transplantation; LDKT, living donor kidney transplantation; PRA, panel reactive antibody
After adjusting for patient-level characteristics, only 4.7% of the variation in post-ILDKT mortality was explained by center-level differences (p=0.001). This translated to a median hazard ratio (MHR) for the overall effect of the center on post-ILDKT mortality of 1.36. In other words, otherwise similar candidates across different centers have a 1.36-fold median difference in mortality. The magnitude of this effect was much less than the effect of having a flow cytometric positive crossmatch (aHR: 1.261.802.56 vs. a Luminex positive crossmatch) on post-ILDKT mortality.
Graft loss
After adjusting for recipient characteristics, 5 centers (20%) had significantly lower graft loss than average, whereas 2 centers (8%) had significantly higher graft loss than average (Figure 2). Four of the six centers with significantly lower mortality than average also had significantly lower graft loss than average. The one center with significantly higher mortality than average also had significantly higher graft loss than average. However, we did not identify any center-level characteristics that were associated with increased graft loss (Table 3).
Figure 2. Center-specific hazard ratios for post-ILDKT graft loss, adjusting for patient-level characteristics.
Although most centers had a post-ILDKT graft loss risk that was indistinguishable from average, 5 (20%) centers had a significantly lower graft loss risk than average, whereas 2 centers (8%) had a significantly higher graft loss risk than average, after adjusting for patient-level factors.
◊ Center whose post-ILDKT graft loss risk was significantly different than average
Table 3.
Predictors of graft loss after ILDKT, stratified by patient-level or center-level characteristics
| Hazard ratio | p-value | |
|---|---|---|
| Patient-level characteristics | ||
| Donor age | 0.991.011.01 | 0.07 |
| Recipient age | 0.991.001.01 | 0.5 |
| Recipient female gender | 0.820.971.16 | 0.7 |
| Recipient race | ||
| White | Ref | |
| Black | 0.921.161.46 | 0.2 |
| Other | 0.520.680.90 | 0.007 |
| Recipient blood type | ||
| O | Ref | |
| A | 0.680.810.97 | 0.02 |
| B | 0.750.961.23 | 0.7 |
| AB | 0.761.131.68 | 0.5 |
| Time on dialysis, per year | 1.011.021.04 | 0.005 |
| Panel reactive antibody, per % increase | 0.991.001.01 | 0.7 |
| Cause of ESRD*, diabetes | 0.520.650.81 | <0.001 |
| History of prior transplant | 0.881.061.28 | 0.5 |
| Strength of DSA* | ||
| Positive Luminex crossmatch | Ref | |
| Positive Flow crossmatch | 1.081.411.84 | 0.01 |
| Positive Cytotoxic crossmatch | 1.411.852.44 | <0.001 |
| Center-level characteristics | ||
| Median annual ILDKT* volume, per 1 transplant increase | 0.981.031.05 | 0.3 |
| Median annual LDKT* volume, per 1 transplant increase | 0.991.001.01 | 0.3 |
| Percentage of pre-emptive LDKT recipients, per 1 percent increase | 0.981.011.03 | 0.6 |
| Percentage of ILDKT recipients with a positive Cytotoxic crossmatch, per 1 percent increase | 0.861.301.97 | 0.2 |
| Percentage of LDKT recipients with a prior transplant, per 1 percent increase | 0.961.001.05 | 0.9 |
| Percentage of LDKT recipients with a PRA* ≥ 80%, per 1 percent increase | 0.950.991.03 | 0.6 |
| Percentage of AA* ILDKT recipients, per 1 percent increase | 0.991.001.01 | 0.9 |
Although certain patient-level characteristics were associated with higher post-ILDKT graft loss, no center-level characteristics were. After adjusting for patient-level characteristics, only 4.4% of the variation in post-ILDKT graft loss was explained by differences between centers.
Post-transplant graft loss was not associated with the following center-level characteristics: ILDKT volume (aHR: 0.981.031.05, p=0.3), LDKT volume (aHR: 0.991.001.01, p=0.3), or transplanting a higher proportion of highly sensitized (aHR: 0.950.991.03, p=0.6), prior transplant (aHR: 0.961.001.05, p=0.9), pre-emptive (aHR: 0.981.011.03, p=0.6), minority (aHR: 0.991.001.01, p=0.9), or positive cytotoxic crossmatch (aHR: 0.861.301.97, p=0.2) recipients.
After adjusting for recipient characteristics, only 4.4% of the variation in post-ILDKT graft loss was explained by center-level differences (p<0.001). This translated to a MHR for the overall effect of the center on post-ILDKT graft loss of 1.34. In other words, otherwise similar candidates across different centers have a 1.34-fold median difference in graft loss. The magnitude of this effect was less than the effect of having a flow cytometric positive crossmatch (aHR: 1.081.411.84 vs. a Luminex positive crossmatch) on post-ILDKT graft loss.
DISCUSSION
In this multi-center, observational cohort study of 1,358 ILDKT recipients from 25 centers, we have shown that <5% of the variation in post-ILDKT outcomes can be attributed to the center-specific factors. The majority of centers (76%) had outcomes that were indistinguishable from average, and the magnitude of between-center differences was only 1.36 for mortality and 1.34 for graft loss. For perspective, the median center-level effect was lower than the effect of having a flow cytometric positive crossmatch for mortality (1.80-fold increased mortality risk vs. Luminex positive crossmatch) and graft loss (1.41-fold increased risk of graft loss vs. Luminex positive crossmatch). Although there was some center-level variation in post-ILDKT outcomes, the vast majority of between-center differences in post-ILDKT mortality and graft loss can be attributed to differences in recipient composition, rather than between-center differences in performance.
Many aspects of transplantation have been shown to be influenced by center-specific factors, such as the development of delayed graft function, choice of induction therapy, and even post-transplant mortality and graft loss.26–29 Given the challenges of desensitization and post-transplant management of DSA and antibody-mediated rejection, one might also expect to see relatively high center-level variation in post-ILDKT outcomes.5,30,31 However, our results suggest that the center has a relatively small impact, and that patient-level factors largely drive post-ILDKT outcomes, and we did not find any evidence of a center-volume effect. Our results are consistent with a number of studies showing that transplant recipients can have substantially different risks of mortality, graft loss, and antibody-mediated rejection, depending on the strength of their DSA, and serve as an important perspective that the impact of DSA strength (>5-fold) alone far outweighed that of center-level differences (1.36-fold for mortality, 1.34-fold for graft loss).18–21 Our results also extend prior single-center reports on post-ILDKT outcomes by using a 25-center cohort and analytic framework that accounts for between-center differences in recipient composition that make it impossible to compare single-center publications side-by-side. In light of our findings, it might be that the vast majority of between-center differences in reported post-ILDKT survival are driven by differences in recipient composition (i.e. patient-level factors) rather than between-center differences in performance (i.e. center-level factors).
The major strength of this study is the use of a large, multi-center cohort of ILDKT recipients, augmented by linkage to SRTR for accurate outcome ascertainment, to study center-level variation in post-ILDKT outcomes, which would not otherwise be possible. However, our study has several limitations worth considering. First, we use a cohort of 25 centers who decided to participate in a multi-center study of ILDKT, and as such our results may not generalize to centers outside this study (or we might have missed some centers); for example, it is possible that centers who felt their post-ILDKT outcomes were excellent were more likely to participate than those who did not. Nevertheless, the 25 centers in our study represent a diverse group of centers, with a broad range of hospital size, ILDKT volume, and LDKT volume, and so are likely to be representative of other transplant centers performing ILDKT. Second, we do not have comprehensive data about desensitization protocols, flow cytometry interpretation, DSA monitoring, biopsy strategies (e.g. protocol biopsies), or center practices in treatment of identified antibody-mediated rejection in this study. Certainly, these vary across centers, and might affect post-ILDKT outcomes. However, our analytic design allows us to contextualize the effect of these protocols. Even if these accounted for all between-center variation in outcomes, they would still only be responsible for at most 4.7% (mortality) or 4.4% (graft loss) of the total variation in post-ILDKT outcomes. In other words, despite all of these between-center factors that might impact outcomes, ≥95% of differences in ILDKT outcomes are attributable to patient-level factors. Additionally, it is possible that there are between-center differences in recipient characteristics that are unaccounted for in the OPTN data. However, the effect of this would be to further lower the amount of variation attributable to between-center differences (since more variation is now being attributed to between-recipient differences), which only underscores the extent to which patient-level factors drive post-ILDKT outcomes.
In conclusion, we have shown that most of the variation in post-ILDKT outcomes can be attributed to patient-level characteristics, rather than center-level differences in performance. Unlike many aspects of transplantation which have been shown to be influenced by center-specific factors, there was very little center-level variation in post-ILDKT outcomes. Importantly, we found no center volume effect. Our findings support the continued practice of ILDKT across these diverse centers.
ACKNOWLEDGEMENTS
We would like to thank the co-investigators, coordinators, and research staff who contributed to this study.
This work was supported by grant numbers F32DK113719 (Jackson), K01DK101677 (Massie), RO1DK098431 (Segev), K24DK101828 (Segev), and K23DK115908 (Garonzik-Wang) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Dr. Garonzik-Wang is supported by a Clinician Scientist Development Award from the Doris Duke Charitable Foundation. The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
ABBREVIATIONS
- aHR
adjusted hazard ratio
- DSA
donor-specific antibody
- HLA
human leukocyte antigen
- ILDKT
incompatible living donor kidney transplantation
- LDKT
living donor kidney transplant
- MHR
median hazard ratio
- OPTN
Organ Procurement and Transplantation Network
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
DISCLOSURES
The authors of this manuscript have no conflicts of interest to disclose as described by Transplantation.
REFERENCES
- 1.Montgomery RA, Lonze BE, King KE, et al. Desensitization in HLA-Incompatible Kidney Recipients and Survival. New England Journal of Medicine. 2011;365(4): 318–326. [DOI] [PubMed] [Google Scholar]
- 2.Orandi BJ, Luo X, Massie AB, et al. Survival Benefit with Kidney Transplants from HLA-Incompatible Live Donors. New England Journal of Medicine. 2016;374(10): 940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montgomery RA, Lonze BE, Jackson AM. Using donor exchange paradigms with desensitization to enhance transplant rates among highly sensitized patients. Curr Opin Organ Transplant. 2011;16(4): 439–443. [DOI] [PubMed] [Google Scholar]
- 4.Montgomery RA. Renal Transplantation Across HLA and ABO Antibody Barriers: Integrating Paired Donation into Desensitization Protocols. American Journal of Transplantation. 2010;10(3): 449–457. [DOI] [PubMed] [Google Scholar]
- 5.Jordan SC, Choi J, Vo A. Kidney transplantation in highly sensitized patients. Br Med Bull. 2015;114(1): 113–125. [DOI] [PubMed] [Google Scholar]
- 6.Stegall MD, Gloor J, Winters JL, Moore SB, Degoey S. A comparison of plasmapheresis versus high-dose IVIG desensitization in renal allograft recipients with high levels of donor specific alloantibody. Am J Transplant. 2006;6(2): 346–351. [DOI] [PubMed] [Google Scholar]
- 7.Higgins R, Hathaway M, Lowe D, et al. Blood levels of donor-specific human leukocyte antigen antibodies after renal transplantation: resolution of rejection in the presence of circulating donor-specific antibody. Transplantation. 2007;84(7): 876–884. [DOI] [PubMed] [Google Scholar]
- 8.West-Thielke P, Herren H, Thielke J, et al. Results of positive cross-match transplantation in African American renal transplant recipients. Am J Transplant. 2008;8(2): 348–354. [DOI] [PubMed] [Google Scholar]
- 9.Jordan SC, Vo A, Bunnapradist S, et al. Intravenous immune globulin treatment inhibits crossmatch positivity and allows for successful transplantation of incompatible organs in living-donor and cadaver recipients. Transplantation. 2003;76(4): 631–636. [DOI] [PubMed] [Google Scholar]
- 10.Vo AA, Lukovsky M, Toyoda M, et al. Rituximab and Intravenous Immune Globulin for Desensitization during Renal Transplantation. New England Journal of Medicine. 2008;359: 242–251. [DOI] [PubMed] [Google Scholar]
- 11.Vo AA, Wechsler EA, Wang J, et al. Analysis of subcutaneous (SQ) alemtuzumab induction therapy in highly sensitized patients desensitized with IVIG and rituximab. Am J Transplant. 2008;8(1): 144–149. [DOI] [PubMed] [Google Scholar]
- 12.Al Meshari K, Pall A, Chaballout A, et al. Outcome of desensitization in human leukocyte antigen- and ABO-incompatible living donor kidney transplantation: a single-center experience in more than 100 patients. Transplant Proc. 2013;45(4): 1423–1426. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez C, Calvo M, Leite N, et al. Kidney transplantation from HLA-incompatible live donors: Efficiency and outcome of 32 patients after desensitisation. Nefrologia. 2017;37(6): 638–645. [DOI] [PubMed] [Google Scholar]
- 14.Jin MK, Cho JH, Kwon O, et al. Successful kidney transplantation after desensitization using plasmapheresis, low-dose intravenous immunoglobulin, and rituximab in highly sensitized patients: a single-center experience. Transplant Proc. 2012;44(1): 200–203. [DOI] [PubMed] [Google Scholar]
- 15.Vo AA, Toyoda M, Peng A, Bunnapradist S, Lukovsky M, Jordan SC. Effect of induction therapy protocols on transplant outcomes in crossmatch positive renal allograft recipients desensitized with IVIG. American Journal of Transplantation. 2006;6: 2384–2390. [DOI] [PubMed] [Google Scholar]
- 16.Haririan A, Nogueira J, Kukuruga D, et al. Positive cross-match living donor kidney transplantation: longer-term outcomes. Am J Transplant. 2009;9(3): 536–542. [DOI] [PubMed] [Google Scholar]
- 17.Ushigome H, Harada S, Nakao M, et al. Living-donor kidney transplantation with existing anti-donor specific antibodies at a Japanese single center. Transplant Proc. 2015;47(3): 612–616. [DOI] [PubMed] [Google Scholar]
- 18.Orandi BJ, Garonzik-Wang JM, Massie AB, et al. Quantifying the risk of incompatible kidney transplantation: a multicenter study. Am J Transplant. 2014;14(7): 1573–1580. [DOI] [PubMed] [Google Scholar]
- 19.Fidler SJ, Irish AB, Lim W, Ferrari P, Witt CS, Christiansen FT. Pre-transplant donor specific anti-HLA antibody is associated with antibody-mediated rejection, progressive graft dysfunction and patient death. Transpl Immunol. 2013;28(4): 148–153. [DOI] [PubMed] [Google Scholar]
- 20.Lefaucheur C, Loupy A, Hill GS, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21(8): 1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zecher D, Bach C, Staudner C, et al. Characteristics of donor-specific anti-HLA antibodies and outcome in renal transplant patients treated with a standardized induction regimen. Nephrol Dial Transplant. 2017;32(4): 730–737. [DOI] [PubMed] [Google Scholar]
- 22.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14(8): 1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin PC. A Tutorial on Multilevel Survival Analysis: Methods, Models and Applications. Int Stat Rev. 2017;85(2): 185–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Austin PC, Wagner P, Merlo J. The median hazard ratio: a useful measure of variance and general contextual effects in multilevel survival analysis. Stat Med. 2017;36(6): 928–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics. 2009(1): 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orandi BJ, James NT, Hall EC, et al. Center-level variation in the development of delayed graft function after deceased donor kidney transplantation. Transplantation. 2015;99(5): 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dharnidharka VR, Naik AS, Axelrod DA, et al. Center practice drives variation in choice of US kidney transplant induction therapy: a retrospective analysis of contemporary practice. Transpl Int. 2018;31(2): 198–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsampalieros A, Knoll GA, Fergusson N, Bennett A, Taljaard M, Fergusson D. Center Variation and the Effect of Center and Provider Characteristics on Clinical Outcomes in Kidney Transplantation: A Systematic Review of the Evidence. Can J Kidney Health Dis. 2017;4: 2054358117735523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schold JD, Harman JS, Chumbler NR, Duncan RP, Meier-Kriesche HU. The Pivotal Impact of Center Characteristics on Survival of Candidates Listed for Deceased Donor Kidney Transplantation. Med Care. 2009;47: 146–153. [DOI] [PubMed] [Google Scholar]
- 30.Sethi S, Choi J, Toyoda M, Vo A, Peng A, Jordan SC. Desensitization: Overcoming the Immunologic Barriers to Transplantation. J Immunol Res. 2017;2017: 6804678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blume OR, Yost SE, Kaplan B. Antibody-mediated rejection: pathogenesis, prevention, treatment, and outcomes. J Transplant. 2012;2012: 201754. [DOI] [PMC free article] [PubMed] [Google Scholar]