Abstract
OBJECTIVE
To compare the risk of mortality among men treated for benign prostatic hyperplasia (BPH) with 5 alpha-reductase inhibitors (5ARI) to those treated with alpha-blockers (AB) in community practice settings.
METHODS
We employed a retrospective matched cohort study in 4 regions of an integrated healthcare system. Men aged 50 years and older who initiated pharmaceutical treatment for BPH and/or lower urinary tract symptoms between 1992 and 2008 and had at least 3 consecutive prescriptions that were eligible and followed through 2010 (N = 174,895). Adjusted hazard ratios were used to estimate the risk of mortality due to all-causes associated with 5ARI use (with or without concomitant ABs) as compared to AB use.
RESULTS
In this large and diverse sample with 543,523 person-years of follow-up, 35,266 men died during the study period, 18.9% of the 5ARI users and 20.4% of the AB users. After adjustment for age, medication initiation year, race, region, prior AB history, Charlson score, and comorbidities, 5ARI use was not associated with an increased risk of mortality when compared to AB use (Adjusted hazard ratios: 0.64, 95% confidence interval: 0.62, 0.66).
CONCLUSION
Among men receiving medications for BPH in community practice settings, 5ARI use was not associated with an increased risk of mortality when compared to AB use. These data provide reassurance about the safety of using 5ARIs in general practice to manage BPH and/or lower urinary tract symptoms.
5 Alpha reductase inhibitors (5ARIs), used sequentially or concomitantly with alpha-blockers (ABs), are the standard approach to the pharmacotherapy of lower urinary tract symptoms associated with benign prostatic hyperplasia (BPH).1 Results from two clinical trials investigating the use of 5ARIs in the chemoprevention of prostate cancer suggested that 5ARIs were associated with a reduced risk of prostate cancer when compared to placebo.2,3 However, data from these trials and subsequent observational studies4–7 have found an increased risk of high-grade prostate cancers among men diagnosed in the 5ARI group. As a result, the use of 5ARIs as a chemo-preventive agent for prostate cancer remains controversial.8–10 The potential association between 5ARIs and prostate cancer mortality has been assessed in multiple observation studies; but to date, none of these studies have found that the increased risk of Gleason 8–10 prostate cancers among 5ARI users resulted in an increased risk of prostate cancer mortality.4–7
Results from the follow-up study of the Prostate Cancer Prevention Trial suggest that 5ARIs are also not associated with an increased risk of all-cause mortality.11 However, fully establishing the safety of using 5ARIs in the management of BPH and/or LUTS requires that studies assess their long-term use and overall safety in general practice settings, which move beyond randomized controlled trial and highly-selected clinic samples and include a real-world comparison group, in this case, ABs. Therefore, the goal of this study was to determine the safety of using 5ARIs in the management of BPH/LUTS by assessing the risk of mortality associated with 5ARIs use as compared to AB use for the treatment of BPH and/or LUTS (lower urinary tract symptoms) in a large population-based cohort of 214,272 men in community practice settings over a 19-year observation period.
MATERIAL AND METHODS
Study Population
The study population and methods have been described previously.7 Briefly, this study was conducted in 4 regions of Kaiser Permanente (KP) (Northern and Southern California, Colorado and Northwest), integrated healthcare systems that collectively provide comprehensive health care services to over 9.7 million members. The racial and socioeconomic diversity of the members closely reflects that of the region each serves.12 Comprehensive electronic health records along with a standardized data warehouse13 allow for the collection of demographic characteristics, health services utilization, disease diagnoses, and death records. In addition, the records include pharmacy data and inpatient, outpatient, and emergency department encounters, as well as care received outside of the system. This study was approved by the Institutional Review Boards at each site.
Male members ages 50 years and older who received a new prescription for a BPH and/or LUTS medication between 1992 and 2008 and were members for at least 1 year prior to first dispensing were eligible for inclusion (N = 281,034). As this analysis was part of a larger study focused on prostate cancer mortality, men with a diagnosis of prostate cancer prior to, or within 3 months after their first prescription (n = 20,170), those with <90 days of consecutive medication filled (n = 44516), and those treated with finasteride 1 mg for alopecia (n = 2023) were excluded, leaving 214,272 men eligible for matching.
Men who started a 5ARI were matched at the time of 5ARI initiation using risk-set sampling 1:6 to alpha-blocker users on age at matching (±2 years), race (African American vs Other), timing of BPH and/or LUTS medication initiation (within 2 years), prior history of AB use and health plan region. Of the 214,272 eligible men, 73% were successfully matched resulting in an analytical sample of 157,456 men with 174,895 records (18,321 men were matched as both 5ARI and AB user). Men were then followed via electronic health records through the end of 2010 for death due to all causes (Fig. 1).
Figure 1.
Analytic sample selection.
Exposure Assessment
The primary exposure of interest was a new prescription for a 5ARI or AB from 1992–2008 identified via prescription fills in electronic pharmacy records, as described previously.7 Combination users (men who were exposed to both an AB and 5ARI) were defined as 5ARI users for matching purposes and contributed person-time to both exposure groups. Cumulative exposure and dose were calculated from 5ARI initiation in men who were 5ARI users and corresponding matched index date among AB users.
Outcome Assessment
The primary outcome was mortality due to all-causes, which was identified via a comprehensive search across multiple sources for vital status. This included local (regional) KP death databases, state death records, a Social Security Death Index match on all eligible men and a National Death Index match for all men who were missing underlying cause of death information. This included 54,423 men who died prior to the end of the study period, including 10,651 who left KP alive but died prior to the end of the study period and were therefore censored at disenrollment. While our exposure time period included the years 1992–2008, men were followed an additional 2 years through 2010 to determine their vital status.
Covariates
Covariates were defined at the time of matching (5ARI initiation or corresponding index date among AB users). Demographics such as age at matching and race and/or ethnicity (Non-Hispanic White, African American, Asian, Hawaiian/Pacific Islander/Native Alaskan/American Indian, and Unknown) were collected from electronic demographic files. Aggregate median household income and education were calculated via geocoding using 2000 US census estimates at the block, block-group, tract, and ZIP level. Medical history was assessed using a modified Charlson comorbidity score14 as well as history of BPH, cardiovascular disease, high blood pressure, hyperlipidemia (all defined using ICD-9 diagnosis codes), and use of medications to treat erectile dysfunction (ED) or overactive bladder (OAB) were assessed in the year prior to matching.
Statistical Analysis
The distributions of demographic and clinical characteristics were compared across exposure and outcome status, using chi-square tests and two-sided Wilcoxon–Mann–Whitney tests when appropriate. Person-time at risk was calculated from the time of matching (5ARI medication initiation or index date in AB user) to death (event), loss to follow-up (disenrollment from health plan) or end of study period. Men who died after they were lost to follow-up were censored at the time they were lost to follow-up. Adjusted Cox models were used to estimate the relative risk of death due to all causes among 5ARI users as compared to AB users after adjustment for the matching factors (age, race, region, medication initiation year, and history of AB use), as well as Charlson score, history of cardiovascular disease, high blood pressure, hyperlipidemia, diabetes, other cancer and use of other medications to treat OAB or ED. Models were then stratified by increasing cumulative exposure, dose, and history of AB use quartiles. The proportional hazard assumption was examined using Schoenfeld residuals.15 Crude and covariate weighted Kaplan Meier plots were constructed comparing the probability of survival among 5ARI users compared to AB users, for 6, 10, and 20 years. All analyses were performed using SAS 9.3 -and a P value < .05 was considered statistically significant.
Sensitivity Analyses
We conducted a number of sensitivity analyses, to assess the potential influence of selection biases introduced via matching and the influence of changing exposures and comorbidities over time. These analyses allowed for time-varying exposures as well as covariates, which were collected at 2 time points, the first prescription initiation and at matching (5ARI initiation or corresponding index date among AB users). Person-time was calculated from time at which the participant initiated his BPH and/or LUTS medication up until the time of the event, censoring or end of study period for these analyses. As part of these time-varying analyses, we also evaluated using age as the time scale and the influence of employing different methods for handling missing covariate data (last value carried forward vs left missing) and compared those to our results in the matched sample. Finally, as the medical management of LUTS is a long-term treatment strategy, we also assessed the influence of restricting our cohort to men who were exposed to these drugs for at least 1 year. The results of these sensitivity analyses are presented in the Online Supplemental Material.
RESULTS
Table 1 displays the demographic and clinical characteristics of the cohort after matching by exposure status. Men who used 5ARIs were more likely to be Non-Hispanic White compared to men who used ABs (78.3 vs 76.5%) (P < .001). Median serum PSA levels at 5ARI initiation (or index) were higher among those who used 5ARIs (4.1 ng/mL) compared to those who used ABs (2.1 ng/mL) (P < .001). Men who used 5ARIs were less likely to have a Charlson score of 2 or greater compared to those who used ABs (P < .001). The median duration of AB use before matching was shorter among those who used 5ARIs compared to those who used ABs (3.2 vs 3.4 years) (P < .001). The median dose and duration were both greater among men who used 5ARI (both P < .001). The median follow-up time after matching was also greater among men who used 5ARIs (3.3 vs 2.3 years) (P < .001) (Table 1).
Table 1.
Demographic and clinical characteristics in the matched cohort by exposure status (N = 174,895).
| Overall (n = 174,895) |
5ARI Users (n = 25,388) |
Alpha–Blocker Users (n = 149,507) |
P Value | |
|---|---|---|---|---|
| Exposure and Follow-up | ||||
| Cumulative exposure time, years (mean (sd), median)1 | 1.5 (1.5),1.1 | 2.2 (2.1),1.5 | 1.4 (1.4), 1.1 | <0.0001 |
| Duration of follow-up time from matching time point, for loss of follow-up Pca deaths follow-up ends at disenrollment, years (mean [sd], median)1 | 3.1 (2.7), 2.4 | 4.0 (3.1),3.3 | 3.0 (2.6),2.3 | <0.0001 |
| At-matching characteristics | ||||
| Age | ||||
| mean (sd), median | 72.4 (9.2), 72.6 | 72.4 (9.3), 72.6 | 72.3 (9.2), 72.5 | 0.16 |
| <60 | 17884 (10.2) | 2546 (10.0) | 15338 (10.3) | 0.31 |
| 60–69 | 52980 (30.3) | 7639 (30.1) | 45341 (30.3) | |
| 70+ | 104031 (59.5) | 15203 (60.0) | 88828 (59.4) | |
| Race (%) | ||||
| Non-Hispanic White | 134220 (76.7) | 19889 (78.3) | 114331 (76.5) | <0.0001 |
| African American | 12711 (7.3) | 1885 (7.4) | 10826 (7.2) | |
| Asian | 14636 (8.4) | 2017 (7.9) | 12619 (8.4) | |
| HP, IN, MU, and UN | 13328 (7.6) | 1597 (6.3) | 11731(7.9) | |
| PSA | ||||
| Missing (%) | 65262 (37.3) | 6359 (25.1) | 58903 (39.4) | |
| mean (sd), median | 4.9 (42.6), 2.3 | 6.6 (30.2), 4.1 | 4.5 (44.8), 2.1 | <0.0001 |
| 0–2.5 | 56985 (32.6) | 6400 (25.2) | 50585 (33.8) | <0.0001 |
| 2.5–4 | 17497 (10.0) | 2964 (11.7) | 14533 (9.7) | |
| ≥4 | 35151 (20.1) | 9665 (38.1) | 25486 (17.1) | |
| SES | ||||
| Missing (%) | 3320 (1.9) | 377 (1.5) | 2943 (2.0) | |
| Median household income ($1000) (mean (sd), median) Education | 66.4 (28.3), 62.0 | 68.3 (29.7), 63.5 | 66.1 (28.0), 61.8 | <0.0001 |
| Less than 9th grade | 0.07 (0.09), 0.04 | 0.07 (0.09), 0.04 | 0.07 (0.09), 0.04 | <0.0001 |
| 9th-12th grade | 0.10 (0.07), 0.08 | 0.09 (0.07), 0.08 | 0.10 (0.0.7), 0.08 | <0.0001 |
| High school graduate | 0.21 (0.08), 0.21 | 0.20 (0.08), 0.20 | 0.21 (0.08), 0.21 | <0.0001 |
| Some college, no degree | 0.24 (0.07), 0.25 | 0.24 (0.07), 0.24 | 0.24 (0.07), 0.25 | <0.0001 |
| Associate degree | 0.08 (0.03), 0.08 | 0.08 (0.03), 0.07 | 0.08 (0.03), 0.08 | 0.007 |
| Bachelor degree | 0.19 (0.11), 0.19 | 0.20 (0.11), 0.20 | 0.19 (0.11), 0.18 | <0.0001 |
| Graduate or professional degree | 0.11 (0.09), 0.08 | 0.12 (0.10), 0.09 | 0.11 (0.09), 0.08 | <0.0001 |
| Alpha blocker history (years) (mean (sd), median) | 4.3 (4.1),3.4 | 4.2 (4.0),3.2 | 4.4 (4.1),3.4 | <0.0001 |
| BMI (kg/m2) (%) | ||||
| Missing (%) | 98215(56.2%) | 14244(56.1%) | 83971(56.2%) | <0.0001 |
| <25 kg/m2 | 29642(17.0%) | 4352(17.1%) | 25290(16.9%) | |
| 25–30 kg/m2 | 26590(15.2%) | 4076(16.1%) | 22514(15.1%) | |
| ≥30 kg/m2 | 20448(11.7%) | 2716(10.7%) | 17732(11.9%) | |
| Charlson comorbidity index (%) | <0.0001 | |||
| 0 | 75809(43.4%) | 11633(45.8%) | 64176(42.9%) | |
| 1 | 34670(19.8%) | 5017(19.8%) | 29653(19.8%) | |
| 2+ | 64416(36.8%) | 8738(34.4%) | 55678(37.2%) | |
| History of cardiovascular disease (Yes) | 59459(34.0%) | 8992(35.4%) | 50467(33.8%) | <0.0001 |
| History of high blood pressure (Yes) | 157553(90.1%) | 21289(83.9%) | 136264(91.1%) | <0.0001 |
| History of hyperlipidemia (Yes) | 117974(67.5%) | 16753(66.0%) | 101221(67.7%) | <0.0001 |
| History of diabetes (Yes) | 45273(25.9%) | 5554(21.9%) | 39719(26.6%) | <0.0001 |
| History of cancer (Yes) | 15877 (9.1%) | 2473(9.7%) | 13404(9.0%) | <0.0001 |
| Use of other medications to treat ED or OAB (Yes) | 23184(13.3%) | 3679(14.5%) | 19505(13.1%) | <0.0001 |
| Exposure and follow-up | ||||
| Cumulative exposure time, years (mean (sd), median)1 | 1.5 (1.5),1.1 | 2.2 (2.1),1.5 | 1.4 (1.4), 1.1 | <0.0001 |
| Cumulative dose (gram)* | 2.6 (3.9), 1.2 | 4.1 (4.0), 2.9 | 2.3 (3.8), 1.0 | <0.0001 |
| Duration of follow-up time, years (mean (sd), median)1 | 3.1 (2.7), 2.4 | 4.0 (3.1),3.3 | 3.0 (2.6),2.3 | <0.0001 |
Abbreviations: BMI, body mass index; ED, erectile dysfunction; OAB, overactive bladder,HP: Hawaiian/Pacific Islander, IN: Native American, MU: Mutiple Races, UN: Unknown Races; PSA: Prostate Specific Antigen.
Follow-up time was calculated from matching time point (5ARI initiation or index date in AB user) to loss to follow-up, death or end of study period, which ever occurred first. For men who died after they lost to follow-up, their follow-up time ends at loss to follow-up.
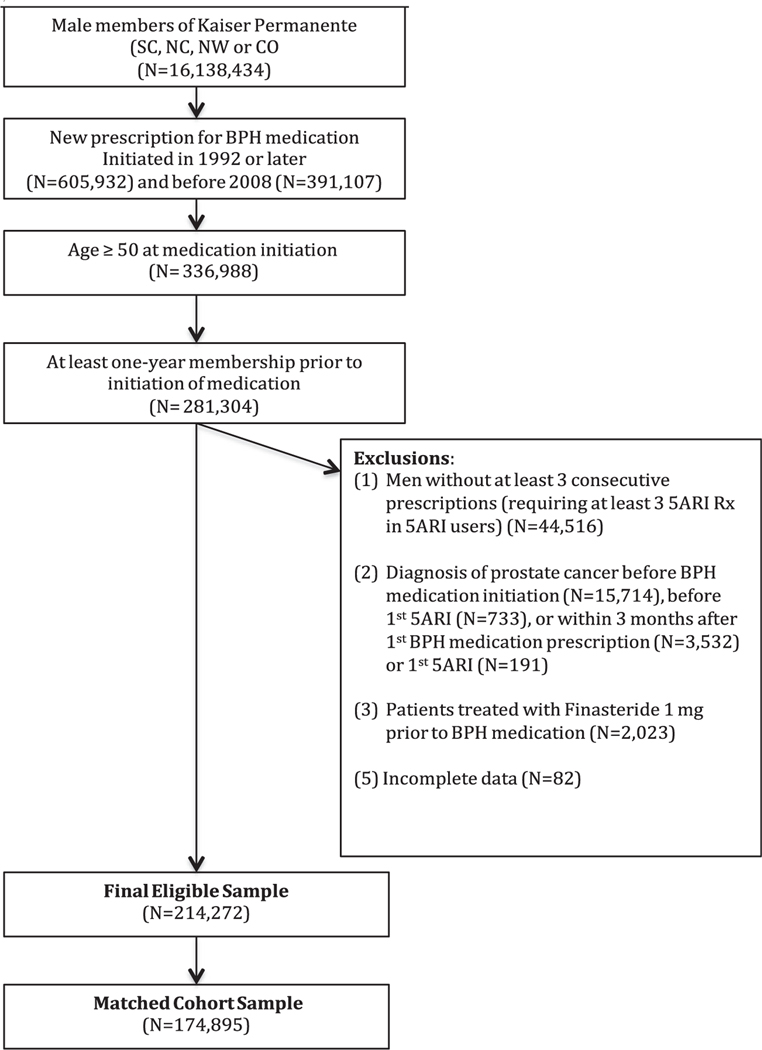
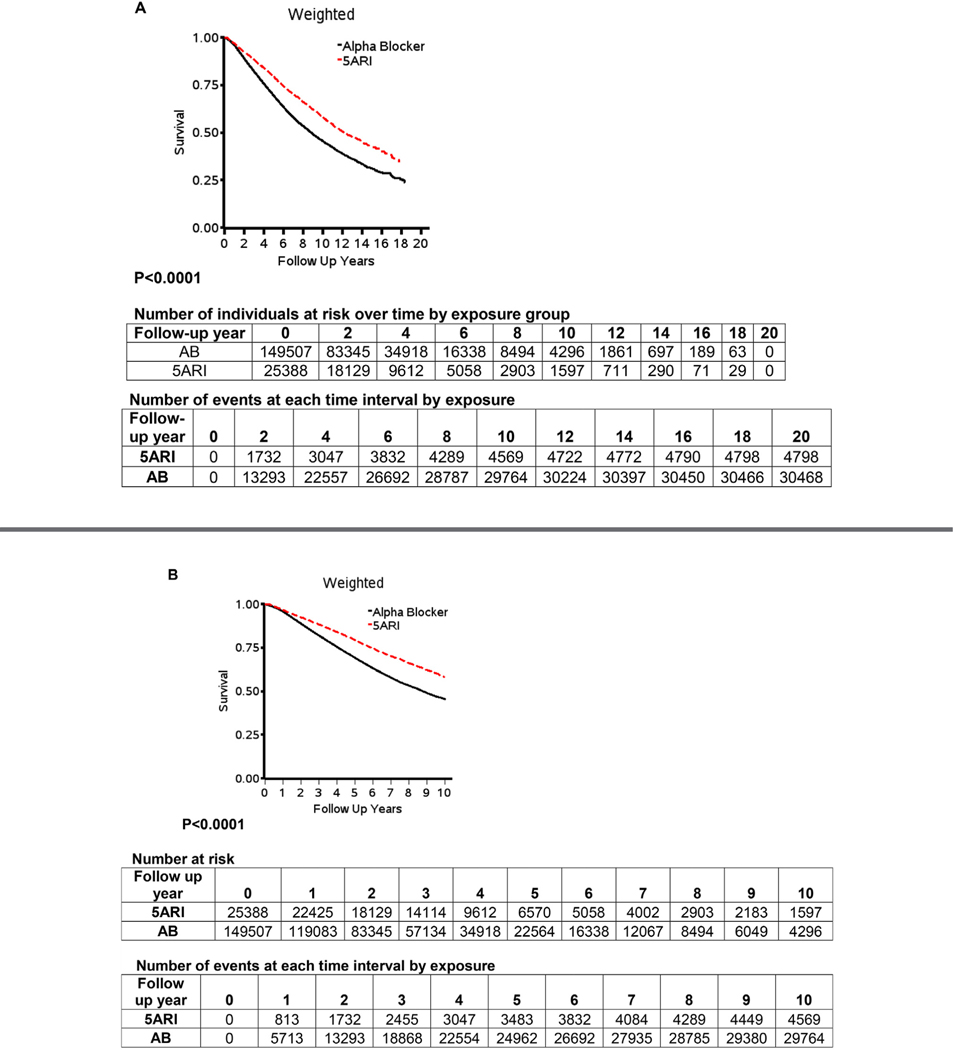
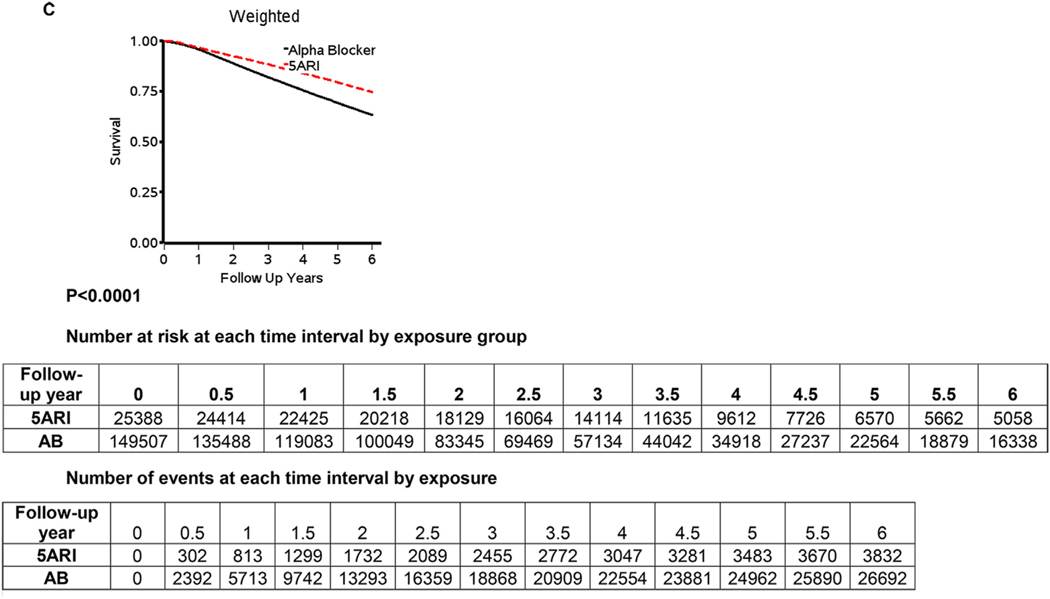
Figure 2 displays the Kaplan Meier curves for the full study period (2A), 10 years (2B) and 6 years (2C). The cumulative probability of survival across the study period was statistically significantly greater for 5ARI users when compared to AB users (all P < .0001). The difference was consistent across the study period (Fig. 2A), and in the first 6 (Fig. 2B) and 10 years (Fig. 2C).
Figure 2.
(A) Covariate-weighted Kaplan–Meier plots, adjusted for age, BPH and/or LUTS initiation year, race, region, prior AB history, Charlson score, and comorbidities over the entire study period, (B) the first 10 years, and (C) the first 6 years. N = 174,895. AB, alpha-blockers; BPH, benign prostatic hyperplasia; LUTS, lower urinary tract symptoms. Note: Red color for dotted 5ARI line on graphs. (Color version available online.)
Table 2 displays the multivariable adjusted associations between 5ARI use and all-cause mortality when compared to AB use. 5ARI use was not associated with an increased risk of mortality when compared to AB use (Adjusted hazard ratio [HR]: 0.64, 95% confidence interval [CI]: 0.62, 0.66). When stratified by increasing cumulative exposure, there was a statistically significant trend of a lower risk of mortality with increasing cumulative exposure to 5ARIs. Among men treated for greater than 2 years, 5ARI use was associated with a 46% reduction in the risk of mortality compared to AB users (Adjusted HR: 0.54; 95% CI: 0.51, 0.57). Men in the highest dose quartile were also less likely to die of any cause if they were treated with a 5ARI as compared to an AB (Adjusted HR: 0.55; 95% CI: 0.52, 0.58) (Table 2).
Table 2.
Multivariable-Adjusted All-Cause Mortality Rates, and Hazard Ratios for 5ARI Users Compared to Alpha-Blocker Users Overall and Stratified by Duration of Cumulative Exposure and Cumulative Dose in the Matched Sample (N = 174,895).
| Mortality rate/ 1000 p-y |
Crude Hazard Ratio (95% CI) | Age-Adjusted Hazard Ratio (95% CI) | Multivariable Hazard ratio1 (95% CI) | ||
|---|---|---|---|---|---|
| Multivariable Adjusted | 5ARI | AB | |||
| Overall (n = 174,895, 35,266 deaths) | 118.34 | 176.65 | 0.65 (0.63,0.67)** | 0.63 (0.61,0.65)** | 0.64 (0.62,0.66)** |
| Cumulative exposure | |||||
| 0–3 months (n = 21,173, 3996 deaths) | 551.16 | 252.81 | 1.31(1.02,1.67)** | 1.15 (0.90,1.47) | 1.01 (0.79,1.29) |
| 3 months-1 year (n = 59,865, 12,951 deaths) | 130.37 | 113.27 | 0.88 (0.84,0.92)** | 0.83 (0.79,0.87)** | 0.83 (0.79,0.87)** |
| 1 year-2 years (n = 45,498, 9593 deaths) | 47.89 | 57.03 | 0.72(0.67,0.76)** | 0.70 (0.66,0.75)** | 0.75(0.70,0.80)** |
| 2+ years (n = 48,359, 8726 deaths) | 17.64 | 23.87 | 0.54 (0.52,0.57)** | 0.54 (0.51,0.57)** | 0.54 (0.51,0.57)** |
| Cumulative dose (gram) | |||||
| 1st quartile (n = 43,705, 8913 deaths) | 244.30 | 267.29 | 0.74 (0.58,0.94)** | 0.89 (0.70,1.13) | 0.97 (0.76,1.24) |
| 2nd quartile (n = 42,735, 9852 deaths) | 151.17 | 98.57 | 0.85 (0.81,0.90)** | 0.87 (0.82,0.92)** | 0.88 (0.83,0.93)** |
| 3rd quartile (n = 44,702, 9025 deaths) | 83.41 | 69.25 | 0.98 (0.92,1.03) | 0.97 (0.92,1.03) | 1.01 (0.95,1.07) |
| 4th quartile (n = 43,753, 7476 deaths) | 18.84 | 30.94 | 0.58 (0.55,0.61)** | 0.54 (0.51,0.57)** | 0.55 (0.52,0.58)** |
Results are from negative binomial regression, with the exception of the hazard ratios which were estimated using proportional hazard regression.
P< .05
Adjusted for age, BPH/LUTS initiation year, race, region, prior AB history, Charlson score, and comorbidities.
Supplemental Table 1e displays the multivariable adjusted association between 5ARI use and mortality stratified by race, and comorbidities. The reduced risk of mortality among 5ARI users when compared to AB users was homogenous across race categories. When stratified by Charlson score, cardiovascular disease, high blood pressure, hyperlipidemia, and history of cancers other than prostate, very little variation in the association between 5ARI use and mortality was seen. (Supplemental Table 1e)
The Online Supplemental Material displays the results of multiple sensitivity analyses completed to assess the robustness of our findings. When adjusting for the time-varying nature of the exposure and covariates (collected at BPH and/or LUTS medication initiation and matching or index date) over time in the matched cohort sample, 5ARIs were again not associated with an increased risk of mortality compared to AB (Adjusted HR: 0.96, 95% CI: 0.93, 0.99). Similar results, albeit closer to the null, were seen when we assessed the association between 5ARI use and all-cause mortality in the unmatched eligible cohort sample, taking into account the time-varying exposure and only adjusting for the covariates at baseline (Adjusted HR: 0.89, 95% CI: 0.87, 0.92). When age was used as the time scale in the eligible cohort, the results were comparable to those in the matched sample (HR: 0.76, 95%CI: 0.75, 0.78). Finally, when the minimum length of exposure time was restricted to at least 1 year, the results were again similar (Adjusted HR: 0.51, 95%CI: 0.49, 0.54) (Supplemental Table 2e).
DISCUSSION
In this large, population-based study in community practice settings, the use of 5ARIs to treat BPH and/or LUTS is not associated with an increased risk of mortality when compared to AB use. Instead, our results suggest that 5ARI use may be associated with a reduced risk of mortality, with a greater reduction in mortality risk with increasing cumulative exposure.
Our findings build upon the follow-up results from the Prostate Cancer Prevention Trial (PCPT) that suggested men treated with finasteride were not more likely to die of all causes compared to men randomized to placebo.11 Our results are also similar in magnitude to those found by Azoulay and colleagues among men diagnosed with prostate cancer in the UK, albeit these results did not attain statistical significance.4 It should be noted however, that the comparison groups in these studies were not AB users, but rather placebo and no 5ARI use. This difference, along with differences in the study populations, may have contributed to the different survival estimates in our study and the PCPT trial. Our study utilized a diverse, aging cohort in general practice settings whereas the PCPT involved a highly-selected population who were younger and healthier. This most likely resulted in lower survival numbers in this cohort compared to the PCPT.
Our results provide further evidence of the overall safety of 5ARIs for the treatment of BPH and/or LUTS. As 5ARIs are commonly used along with ABs to manage lower urinary tract symptoms associated with BPH and/or LUTS,16,17 our findings that 5ARIs did not increase the risk of mortality, and may instead be associated with a reduced risk when compared to ABs, are reassuring. 5ARIs inhibit the conversion of testosterone to dihydrotestosterone, resulting in a reduction in prostate size and a subsequent alleviation of urinary symptoms.18 It remains debatable however, why 5ARIs reduce the risk of low-grade prostate cancer, but result in an increased risk of high-grade cancers, which previous studies, including or own, indicate is not associated with an increased risk of prostate cancer death.4,6,7 It is plausible that the increase in high-grade prostate cancer among men exposed to 5ARIs may be due to a detection bias, from the preferential detection of high-grade cancers in men receiving 5ARIs.9,10,19 Alternatively, it could be that ABs increase mortality risk. Prior studies have noted that the use of ABs is associated with fluid retention, congestive heart failure, orthostatic hypertension, and subsequent falls.20,21 Elucidating the mechanisms by which 5ARIs may reduce the risk of mortality or AB increase the risk of mortality warrants further investigation.
While this study was conducted in a large, diverse population in community practice settings which included 19 years of observational data, there are potential limitations that should be considered. As described previously,7 cohort studies comparing two treatment modalities are subject to immortal time bias.22 To limit the potential influence of this bias, we matched on exposure status so that the follow-up time in both treatment groups began at the same time point (5ARI initiation or index date) rather than date of diagnosis or first dispense in controls and employed risk set sampling of AB users.23 It is possible however, that our complex matching approach introduced some selection bias. We evaluated the potential impact of this bias on our results by performing multiple sensitivity analyses in the eligible cohort prior to matching, which also suggested that 5ARIs were not associated with an increased risk of mortality. Because the 5ARI group was comprised of both 5ARI only and combination therapy users, it is possible that there is some residual confounding due to differences in combination versus monotherapy users. However, we employed multiple strategies to minimize the impact of this confounding, including matching on prior history of AB use and controlling for characteristics found to differ between the groups in our multivariable analyses. The changing nature of both the exposure and covariates over time may have influenced our results. However, the time-varying sensitivity analyses in the eligible cohort that accounted for these changes over time also found no evidence that 5ARIs were associated with an increased risk of dying. While the results from the time-varying analyses were more conservative (closer to the null) than the matched analyses, this is most likely due to differences in the calculations of person-time used in these approaches. The average follow-up time after medication initiation in this population and cumulative duration of use was short, mostly due to the advanced age of the cohort and 5ARIs not becoming widely used until later in the study period. However, our study included over 200,000 men, with over 543,523 person-years of follow-up.
CONCLUSION
Treatment with 5ARIs was not associated with an increased risk of mortality when compared to treatment with ABs in men undergoing pharmaceutical management of BPH and/or LUTS. While the data displayed a consistent decrease in mortality risk for men treated with 5ARIs, the mechanisms by which 5ARIs may lower the risk of overall mortality are not clear and warrant further investigation.
Supplementary Material
Acknowledgment.
Drs. Wallner and Jacobsen had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This study was supported by a research grant from GlaxoSmithKline.
Conflict of Interest Disclosures: Drs. Wallner, Jacobsen, Abell, Van Den Eeden, D’Agsotino Jr., DiBello, Loo, and Horwitz report funding from GlaxoSmithKline for this study. Dr. Dibello is an employee of GlaxoSmithKline and reports stock holdings. Dr. Van Den Eeden report funding from Takeda not related to the topic of this study. Drs. Aaronson, Li, Weinmann, Ritzwoller, and Richert-Boe have no additional financial disclosures to report.
Footnotes
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.urology.2018.05.033.
References
- 1.McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–1803. [DOI] [PubMed] [Google Scholar]
- 2.Andriole GL, Bostwick DG, Brawley OW, et al. Effect of dutasteride on the risk of prostate cancer. New Engl J Med. 2010;362:1192–1202. [DOI] [PubMed] [Google Scholar]
- 3.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. [DOI] [PubMed] [Google Scholar]
- 4.Azoulay L, Eberg M, Benayoun S, Pollak M. 5A-reductase inhibitors and the risk of cancer-related mortality in men with prostate cancer. JAMA Oncol. 2015;1:314–320. [DOI] [PubMed] [Google Scholar]
- 5.Murtola TJ, Karppa EK, Taari K, Talala K, Tammela TL, Auvinen A. 5-alpha reductase inhibitor use and prostate cancer survival in the Finnish Prostate Cancer Screening Trial. Int J Cancer. 2016;138:2820–2828. [DOI] [PubMed] [Google Scholar]
- 6.Preston MA, Wilson KM, Markt SC, et al. 5alpha-Reductase inhibitors and risk of high-grade or lethal prostate cancer. JAMA Intern Med. 2014;174:1301–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallner LP, DiBello JR, Li BH, et al. 5-Alpha Reductase inhibitors and the risk of prostate cancer mortality in men treated for benign prostatic hyperplasia. In: Paper Presented at: Mayo Clinic Proceedings. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andriole GL, Humphrey PA, Serfling RJ, Grubb RL. High-grade prostate cancer in the Prostate Cancer Prevention Trial: fact or artifact. J Natl Cancer Inst. 2007;99:1355–1356. [DOI] [PubMed] [Google Scholar]
- 9.Figg WD, Thompson IM. Effect of 5a-reductase inhibitor use on mortality from prostate cancer. JAMA Oncol. 2015;1:321–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LeFevre M A role for finasteride in the prevention of prostate cancer. New Engl J Med. 2013;369:670–671. [DOI] [PubMed] [Google Scholar]
- 11.Thompson IM Jr., Goodman PJ, Tangen CM, et al. Long-term survival of participants in the prostate cancer prevention trial. N Engl J Med. 2013;369:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross TR, Ng D, Brown JS, et al. The HMO research network virtual data warehouse: a public data model to support collaboration. EGEMS (Washington, DC). 2014;2:1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 15.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 16.Kaplan SA, Lee JY, Meehan AG, Kusek JW. Medical therapy of prostatic symptoms research g. long-term treatment with finasteride resulted in a significant improvement relative to placebo in clinical progression of benign prostatic hyperplasia (BPH) in men with enlarged prostates (30 mL), but not in those with smaller prostates (<30 mL): data from the medical therapy of prostatic symptoms (MTOPS) Trial. J Urol. 2011;185:1369–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan SA. Update on the American Urological Association Guidelines for the treatment of benign prostatic hyperplasia. Rev Urol. 2006;8(Suppl 4):S10–S17. [PMC free article] [PubMed] [Google Scholar]
- 18.Goldenberg L, So A, Fleshner N, Rendon R, Drachenberg D, Elhilali M. The role of 5-alpha reductase inhibitors in prostate pathophysiology: is there an additional advantage to inhibition of type 1 isoenzyme. Can Urol Assoc J. 2009;3(3 Suppl 2):S109–S114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman PJ, Thompson IM Jr., Tangen CM, Crowley JJ, Ford LG, Coltman CA Jr. The prostate cancer prevention trial: design, biases and interpretation of study results. J Urol. 2006; 175:2234–2242. [DOI] [PubMed] [Google Scholar]
- 20.Gillenwater JY, Conn RL, Chrysant SG, et al. Doxazosin for the treatment of benign prostatic hyperplasia in patients with mild to moderate essential hypertension: a double-blind, placebo-controlled, dose-response multicenter study. J Urol. 1995;154:110–115. [PubMed] [Google Scholar]
- 21.Welk B, McArthur E, Fraser LA, et al. The risk of fall and fracture with the initiation of a prostate-selective alpha antagonist: a population based cohort study. BMJ. 2015;351:h5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suissa S Immortal time bias in pharmacoepidemiology. Am J Epidemiol. 2007;167:492–499. [DOI] [PubMed] [Google Scholar]
- 23.Suissa S Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf. 2007;16:241–249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.