Abstract
The pyrogallol autoxidation method has been widely utilized to evaluate various antioxidants in antioxidative bioactivities. However, this method is generally not appropriate for estimating the .O2 − radical scavenging capacity of bioflavonoids, as it enables bioflavonoids to generate .O2 − radical in oxygen‐alkaline (pH 8.2) surroundings. In the present study, an improved DMSO (dimethyl sulfoxide) system (pH 7.25, versus pH 8.2 of the pyrogallol autoxidation) was successfully developed to evaluate the .O2 − radical scavenging capacity of bioflavonoids by EPR technique and using the spin trapping reagent DMPO (5,5‐dimethyl‐1‐pyrroline‐N‐oxide). The non‐protonic environment supplied by the system promotes the stabilization of the .O2 −radical and therefore ensures a much more accurate measurement of .O2 −radical scavenging capacity in bioflavonoids if compared to protonic solvents. The results demonstrated that the effects of scavenging .O2 −radicals in natural bioflavonoids follows the order: dihydromyricetin>myricetin>quercetin>kaempferol>baicalein>chrysin, which are well associated with numbers of hydroxyl groups attached to their molecular skeletons and/or active H of their configurations. Interestingly, the higher superoxide‐anion scavenging effect measured for dihydromyricetin with respect to myricetin is possibly attributed to the fact that dihydromyricetin can be transformed into myricetin in the presence of .O2 − radical, resulting from the homolysis of active H donated from C3−H bond of DMY via .O2 − radicals.
Keywords: bioflavonoids, pyrogallol autoxidation, DMSO systems, EPR, superoxide-scavenging effects
A method for the determination of superoxide‐scavenging activities has been developed. A DMSO system combined with EPR technique and with the help of DMPO as spin trapping reagent, was found to effectively evaluate the superoxide‐scavenging effect in bioflavonoids. In this system, the pH 7.25 supplied by dry DMSO reacted with a base that is not sensitive to bioflavonoids.
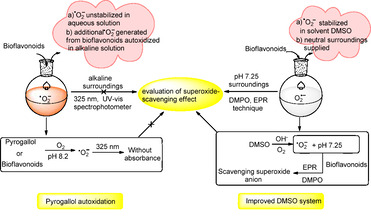
1. Introduction
Superoxide anion radicals (.O2 −) are reactive oxygen species[ 1 , 2 ] responsible for the promotion of oxidative stress under various pathophysiological conditions.[ 3 , 4 ] As a result of attacking cellar DNA, .O2 − is also primarily responsible for many pathological conditions, including cancer, neurological disorders, atherosclerosis, and hypertension.[ 5 , 6 , 7 , 8 ] There are some superoxide dismutases (SOD) existing inherently in the human body which act as potent antioxidants to scavenge .O2 − radicals. However, to scavenge further .O2 − radical or other free radicals, the human body needs to be supplied by natural antioxidants originated from fruits, vegetables and other natural resources[ 9 , 10 , 11 , 12 ] such as ellagic acid and bio‐flavonoids. These substances have a wide range of biological activities including antioxidative, [13] antimicrobials[ 14 , 15 , 16 ] and anti‐inflammatory properties. [17] Therefore, evaluating the effect of natural substances in terms of antioxidative activities with an appropriate and accurate method is still highly desirable.
Till now, several approaches have been reported to determine the superoxide scavenging activities (Scheme 1), which include cytochrome c reduction, [18] nitrotetrazolium blue chloride (NBT) reduction, [19] electron spin resonance (ESR), [18] high‐performance liquid chromatography (HPLC), [20] and pyrogallol autoxidation.[ 21 , 22 , 23 ] These approaches can be independently applied for the assessment of relatively low amounts of superoxide anions. It is notable that the pyrogallol autoxidation method, which is convenient to operate and does not require special instruments or expensive biological agents, has been widely applied for the determination of antioxidative activities. Initially, the method was designed by Marklund [1] specifically for SOD assayed in superoxide‐scavenging effect by detecting the absorbance value of wavelength centered at 420 nm, which was then utilized to determine other antioxidants. Afterward, the pyrogallol autoxidation method had been improved further by bio‐chemists[ 21 , 22 , 23 ] through optimizing some factors such as wavelength monitoring, pH value, and testing temperature. As a result, the pyrogallol autoxidation method enabled to accurately determine the concentration of superoxide anion by means of detecting absorbance value of wavelength at 325 nm and applied effectively to other antioxidants for determination of superoxide‐scavenging effect. To the best of our knowledge, the pyrogallol autoxidation can take place under alkaline conditions, while some of natural antioxidants are sensitive to basic surroundings, especially for bioflavonoids. Flavonoids are polyphenolic substances, and typically show weak acidity in solution. The molecular structures of flavonoids are altered in alkaline solution as previously observed under UV irradiation. [24] As an autoxidizing process of pyrogallol dissolves into alkaline solution in air, the pyrogallol autoxidation enables production of superoxide anion radicals, and relative semiquinones that could further react to form corresponding quinones and purpurogallin, regarded as oxidants.[ 25 , 26 ] In addition, the superoxide anion radical (.O2 −) is much sensitive to the aqueous solution, and can react rapidly with water to form peroxide (H2O2) and/or radical (HO2 .) in seconds.[ 27 , 28 ] Based on the above descriptions, the general applicability of the pyrogallol autoxidation method for antioxidants to evaluate their scavenging effect of superoxide‐anion radicals has been investigated (Scheme 1).
Scheme 1.
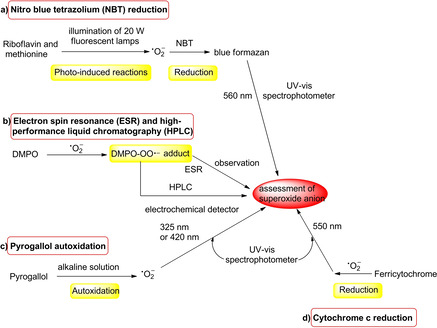
Potent approaches utilized to superoxide‐scavenging bio‐activity.
In the present study, an improved method of dimethyl sulfoxide (DMSO) autoxidation instead of the conventional pyrogallol autoxidation method has been developed by EPR technology. The improved method for determining superoxide scavenging effects has shown promise with regards to superoxide anions and potential antioxidants of bioflavonoids, becasuse they can be stabilized in testing solutions (Scheme 2). Therefore, the improved DMSO autoxidation method is much more appropriate to antioxidants that are sensitive to the alkaline environment, through which approaching neutral surroundings can be supplied and superoxide anions are preserved for a relatively long time.
Scheme 2.
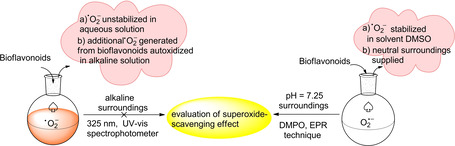
The improved DMSO system instead of the pyrogallol autoxidation for determining superoxide‐scavenging effect.
Experimental Section
Chemicals
Dihydromyricetin (DMY) was prepared in our laboratory from tender stem and leaves of Ampelsis grossedentata in >97 % purity. Pyrogallol(98 %), DMSO (97 %), vitamin C (97 %), myricetin (98 %), quercetin (98 %), kaempferol (98 %), chrysin (98 %), baicalein (98 %), DMPO reagent, Na2EDTA, and sodium phenoxide (solid) were obtained from Balinway Technology Co. LTD (Beijing, China). Tris‐HCl buffer solution (pH 8.2) was prepared by following the procedures of the product instruction. Water was double‐distilled. Analytical TLC was performed on silica gel GF254. Purification of the products was performed by chromatographic operation on silica gel Qingdao GF254 under normal pressure. All the reagents were of analytical grade, and organic solvents used were dried by standard methods when necessary.
Instrumentation
EPR spectra were recorded at room temperature with a Bruker A300 spectrometer for detection of superoxide anion, using a center magnetic field at 3500.00 G, sweep width at 150.00 G, microwave power at 3.99 mW, and modulation amplitude at 1.000 G with scanning field and switching time operating for 30 s and 40 ms respectively. The NMR spectra were recorded on a JEOL‐ECX 500 instrument (500 MHz for 1H, 125 MHz for 13C) using DMSO‐d6 as the solvent. Tetramethylsilane (δ=0) and DMSO‐d6 (δ=40.1) served as internal standards for 1H NMR and 13C NMR spectral experiments, respectively. The mass spectral analysis was performed on Waters XevoTQ‐XSMs Apparatus. The element analysis (FlashSmart, ThermoFisher, Germany) was used to identify compound composition. UV spectra were recorded at room temperature, using an UV‐Vis spectrophotometer (UV759S) equipped with the wavelength in the range of 200 nm to 800 nm (Techcomp, Shanghai, China). HPLC equipped with DAD detector was utilized to monitor the whole process of superoxide‐anion scavenging in the presence of bioflavonoids as potential scavengers (Shimadzu LC20AT, Japan).
Pyrogallol Autoxidation Method for the Evaluation of Antioxidants Scavenging Superoxide‐Anion Effect
The Control Group
A Tris‐HCl buffer (0.05 M, pH 8.2, 2.90 mL) solution was added to Na2EDTA (1 mM). Subsequently, 100 μL of pyrogallol solution (6 mg, in 1 mM HCl) was transferred to the buffer solution. After shaking vigorously for 30 min at room temperature, the reaction mixture taken by 1 mL was allowed to be scanned at range of from 200 to 800 nm by means of an UV/Vis spectrophotometer. The UV spectra were afforded and regarded as a control groups.
The Testing Group
Samples of flavonoids were respectively prepared to be various concentrations (0.01 mg mL−1, 0.05 mg mL−1, 0.10 mg mL−1, 0.15 mg mL−1, 0.20 mg mL−1, in water‐methanol (50 %) solution). Vc acted as a positive control sample was prepared to be 0.20 mg mL−1 in water. Then 100 μL of samples respectively was transferred to above control group (2.00 mg mL−1, 1.00 mL), shaking vigorously for 1 min. And then the reaction mixture was made as following above the same measurement of UV‐Vis spectrophotometer.
ΔA 325 nm, control: absorbance of control group at 325 nm
ΔA 325 nm, sample: absorbance of testing group at 325 nm
T: 25 °C
General Procedure for Flavonoids Autoxidized Under Air‐Saturated Alkaline Solution
Different concentrations of flavonoids were respectively dissolved into ethanol solution (V/V, 50 %) to be 0.01 mg mL−1, 0.05 mg mL−1, 0.10 mg mL−1, 0.15 mg mL−1 and 0.20 mg mL−1. A Tris‐HCl buffer solution (0.05 M, pH 8.2, 2.90 mL) containing Na2EDTA (1 mM) was added to 100 μL of flavonoids solution, respectively. After stirring for 1 min, the reaction mixture was scanned using an UV/Vis spectrophotometer. In addition, 100 μL of flavonoids at a concentration of 0.01 or 0.05 mg mL−1 was placed to Tris‐HCl buffer solution (0.05 M, pH 8.2, 2.90 mL) containing Na2EDTA (1 mM). The resulting mixture was repeatedly measured at 1 min, 3 min, 5 min, as following above measurement of UV/Vis spectrophotometer.
Superoxide Anion Generated by Pyrograllol Autoxidation (Air‐Saturated Alkaline Surroundings)
By using an air supplier equipment, 2.00 mg mL−1 concentration of pyrogallol was prepared in Tris‐HCl buffer solution (pH 8.2) containing Na2EDTA (1 mM) in an air‐saturated environment with continuous stirring for 1 min. To 100 μL of this solution, DMPO (100 mg L−1 in methanol, 10 μL) was added, the resulting mixture was transferred into a silica tube and then subjected to EPR analysis.
Superoxide Anion Generated by Dihydromyricetin or Other Flavonoids (Air‐Saturated Alkaline Surroundings)
By using an air supplier equipment, the air was continuously forced into the Tris‐HCl buffer solution (pH 8.2) containing Na2EDTA (1 mM) over a period of 10 min. Dihydromyricetin or other flavonoids was dissolved into methanol to obtain solutions in various concentrations of e. g., 0.001 mg mL−1, 0.005 mg mL−1 and 0.01 mg mL−1, respectively. Subsequently, those solutions (100 μL) were transferred to the above Tris‐HCl buffer solution (1 mL) with continuous stirring for 1 min. To 100uL of this solution was added DMPO (100 mg L−1 in methanol, 10 μL), the resulting mixture was transferred into a silica tube and then subjected to EPR analysis.
Superoxide Anion Generated by the DMSO System (Oxygen‐Saturated Neutral Surroundings)
By using air supplier equipment, dry DMSO (1 mL) was transferred to an experimental tube (5 mL) and oxygen was introduced to the system for 10 min at room temperature. Subsequently, sodium phenoxide (8 mmol L−1, 10 μL, in dry DMSO) was added to the above system and stirred slowly for 40 minutes at 37 °C. At this point pH of the solution was tested which was found to be appropriate acidity. To 100 μL of this solution was added DMPO (100 mg L−1 in methanol, 10 μL), the resulting mixture was transferred into a silica tube and then subjected to EPR analysis.
A General Procedure to Evaluate Superoxide‐Anion Scavenging Effect of Flavonoids
Having optimized the conditions for generating superoxide anion from DMSO system, flavonoids in different concentrations in dry DMSO (100 μL) were respectively transferred to the above DMSO system (1 mL) already enriched in superoxide anion. After the prescribed time, to 100 μL of this solution was added DMPO (100 mg L−1 in methanol, 10 μL), the resulting mixture was transferred into a silica tube and then subjected to EPR analysis.
: intensity of superoxide anion adduct of DMPO−.O2 −/.O2H in solution
: intensity of superoxide anion adduct of DMPO−.O2 −/.O2H in solution with the presence of superoxide‐anion scavenger
T: 37 °C
Statistical Analysis
Potential bioflavonoids were evaluated by the DMSO system equipped EPR technique to analyze the effect of scavenging superoxide‐anion. The data were recorded as the mean ±SD. The value of IC50 was defined as the concentration for 50 % effect of scavenging superoxide‐anion radical, calculated by linear regression, and analyzed using Origin 8.5 professional software. All the diagrams made using a professional software( Origin 8.5). The significant difference was determined by using T test (P<0.05).
2. Results and Discussion
Based on the improved method of pyrogallol autoxidation reported by Li, [21] the superoxide‐scavenging effects of some of natural bioflavonoids (e. g., dihydromyricetin, myricetin, quercetin, chrysin and baicalein) have been measured. To this aim, solutions of different concentrations of dihydromyricetin (DMY) (0.01 mg mL−1, 0.05 mg mL−1, 0.10 mg mL−1, 0.15 mg mL−1, 0.20 mg mL−1, in water‐methanol (50 %) solution, 100 μL) were firstly prepared and then transferred to the system. The improved method was generated from autoxidation of pyrogallol (2.00 mg mL−1, in Tris‐HCl buffer solution, pH 8.2, 1 mL) under air‐saturated conditions with continuous stirring for 30 min. The resulting mixture was immediately analyzed by UV/Vis spectrophotometer, scanning from 200 to 800 nm wavelength, acted as a control group. It was found that the absorbance of UV spectra of the testing group in the presence of DMY at a concentration of 0.01 mg mL−1 or 0.05 mg mL−1 is lower (P<0.05) than that of control group at 325 nm. Based on the ΔA 325 nm calculation, the effect of antioxidation of DMY was found to be 29.90±1.04 % and 48.42±0.91 % at the concentration of 0.01 mg mL−1 and 0.05 mg mL−1, respectively. Surprisingly, as the concentration of DMY continually increased from 0.05 mg mL−1 to 0.20 mg mL−1, the inhibitions were declined (P>0.05), performing negative effect (Figure 1 and 2a). Overall, above antioxidant data of DMY with concentrations ranging from 0.01 mg mL−1 to 0.20 mg mL−1 were significant (P<0.05) in autoxidation of pyrogallol (Figure S1 and Table S1 in the Supporting Information). To further observe the validation, other natural bioflavonoids such as myricetin, quercetin, chrysin and baicalein were also operated following the above procedure. The UV spectra of our developed protocol demonstrated that all of them enable to be on different intensities of absorbance at 325 nm, with their concentrations ranging from 0.01 mg mL−1 to 0.20 mg mL−1, indicating that all of myricetin, quercetin, chrysin and baicalein can respond positively to pyrogallol autoxidation method at certain range of concentrations. They were able to perform 50.22±0.50 %, 34.83±0.83 %, 16.94±0.88 %, and 25.53±0.89 % at maximum effect of antioxidation, respectively (Figure 2b), which were not significant (P>0.05) in effect. To our surprise, especially for myricetin and quercetin, their absorbance values at higher concentrations (more than 0.05 mg mL−1) at 325 nm were found to be practically higher (P <0.05, P>0.05) than those at their lower concentrations, indicating very poor superoxide‐anion scavenging activity under the experimental conditions (Figure S1 and Table S1 in the Supporting Information). Those results were opposite to our expectations on hunting for good potential antioxidants of natural bioflavonoids.
Figure 1.
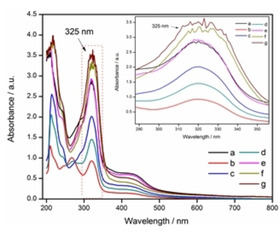
The effect of superoxide‐anion scavenging determined by UV/Vis spectrophotometer in the presence of DMY. Note: (a) control group: autoxidation of pyrogallol in air‐saturated alkaline solution. (b) autoxidation of pyrogallol+Vc (0.20 mg mL−1). (c) autoxidation of pyrogallol+DMY (0.01 mg mL−1). (d) autoxidation of pyrogallol+DMY (0.05 mg mL−1), (e) autoxidation of pyrogallol+DMY (0.10 mg mL−1), (f) autoxidation of pyrogallol+DMY (0.15 mg mL−1), and (g) autoxidation of pyrogallol+DMY (0.20 mg mL−1)
Figure 2.
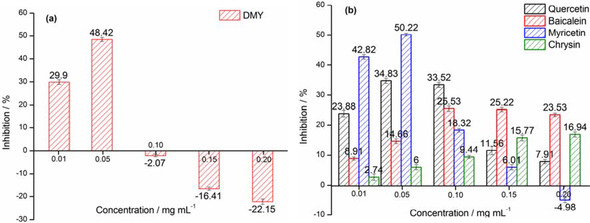
DMY and other flavonoids estimated by UV‐Vis spectrophotometer in superoxide‐anion scavenging effect, (means±SD, n=3).
Relying on above results of bioflavonoids of DMY, myricetin and quercetin at higher concentrations performing negative or lower effects in antioxidation, applied by the pyrogallol autoxidation method. We further investigated the behavior of bioflavonoids alone in the absence of pyrogallol under above experimental conditions (in Tris‐HCl buffer solution, pH 8.2), as determined by UV/Vis spectrophotometer. In UV spectrum of DMY, the absorbance emerged at 325 nm was obvious, with different intensities at various concentrations of 0.01 mg mL−1, 0.05 mg mL−1, 0.10 mg mL−1, 0.15 mg mL−1 and 0.20 mg mL−1, respectively (Figure 3a and b). Furthermore, DMY at a certain concentration of 0.05 mg mL−1 in Tris‐HCl buffer solution (pH 8.2), with proceeding for different times (1 min, 3 min and 5 min), was allowed to be scanned by UV/Vis spectrophotometer. The results indicated that the intensity of absorbance at 325 nm performs to be enhanced (Figure 3c and d). It was apparently implicated that DMY can be oxidized gradually with reacting time prolonged under air‐saturated alkaline solution, accompanying some its oxidative products formed absorbed at 325 nm. Therefore, the pyrogallol autoxidation method equipped monitoring wavelength of 325 nm could not reflect the accuracy of concentration of superoxide‐ anion radical.
Figure 3.
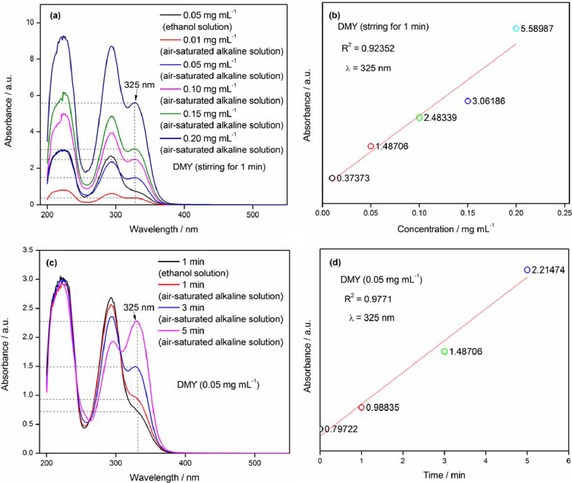
Autoxidation for DMY with various concentrations and stirring for different time observed by UV‐Vis spectrophotometer in air‐saturated alkaline solution.
Afterward, natural bioflavonoids of myricetin, quercetin, chrysin, and baicalein at a concentration of 0.01 mg mL−1 were respectively evaluated following the same procedure of DMY alone operated in air alkaline solution (in Tris‐HCl buffer solution, pH 8.2). The results of UV spectra demonstrated that their absorbance at 325 nm enable to happen absorption of bioflavonoids (myricetin, quercetin, chrysin, and baicalein) oxidized in air alkaline solution, with different intensities (Figure 4; Figure S2 and Table S2 in the Supporting Information). The absorbance of myricetin and quercetin with multiple phenolic hydroxyl groups attached to B ring of flavone skeleton are enhanced at the wavelength of 325 nm, whereas that of chrysin and baicalein are decreased, without OH groups at the B ring.
Figure 4.
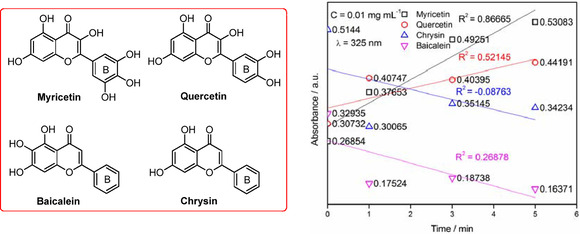
Other flavonoids autoxidized at the concentration of 0.01 mg mL−1 observed at 325 nm in air‐saturated alkaline solution.
Based on the above experimental results, it resulted that bioflavonoids dissolved into air alkaline solution can undergo oxidation. Moreover, their intensities are possibly involved with the number of hydroxyl moieties attached to the pristine molecule backbone and their configurations. It can be concluded that the pyrogallol autoxidation method utilized to evaluate natural bioflavonoids in superoxide‐anion scavenging effect cannot be compatible with the monitoring wavelength of 325 nm.
The phenomenon of natural bioflavonoids absorbed at 325 nm in air alkaline solution should nevertheless be clarified. In view of their molecular features, most of natural bioflavonoids belonged to electron‐enriching system, which facilitated to lose electron after deprotonation of their phenolic hydroxyl moieties. When the phenolate derived from phenolic hydroxyl moieties of bioflavonoids deprotonated by base came across an oxidant, it was more prone to initiate redox reaction. Herein, we noticed that during the period of natural bioflavonoids dissolved into alkaline solution (Tris‐HCl buffer solution, pH 8.2), the solution is prepared by double‐distilled water, containing oxygen molecule, which is regarded as a strong oxidizing agent. In other words, for natural bioflavonoids dissolved into air alkaline solution, the electron can be transferred to the oxygen molecule, forming corresponding oxygen radical. To verify our proposition involving oxygen radical generated, the DMY was taken as a representative example in air alkaline solution at varying concentrations, were conducted by EPR technique with the help of spin trapping reagent DMPO (5,5‐dimethyl‐1‐pyrroline‐N‐oxide). The results of our study demonstrated that the typical signals of superoxide anion adducts (DMPO−.O2 −/.O2H) are obviously observed, with the intensity ratio of 1 : 1 : 1 : 1 quadruplet peaks, which was shown in Figure 5 and 6. Simultaneously, the intensities of quadruplet peaks of DMPO−.O2 −/.O2H adducts exhibited to be enhanced with the increase in concentration of DMY (range of from 0.001 to 0.01 mg mL−1) (see Figure S3 in the Supporting Information).
Figure 5.
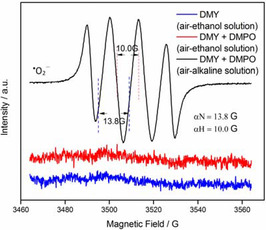
The typical signals of DMPO−.O2 −/.O2H adducts derived from DMY dissolved into air alkaline solution with DMPO.
Figure 6.
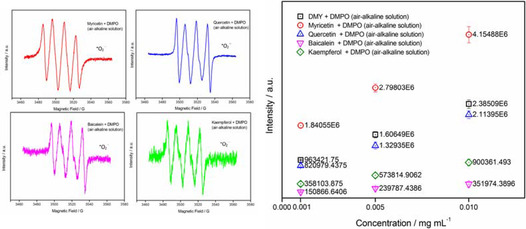
EPR analyses of analogues of bioflavonoids under air alkaline solution with DMPO.
Additionally, EPR analyses of analogues of bioflavonoids, such as myricetin, quercetin, chrysin and baicalein, were operated at a specific concentration of 0.001 mg mL−1 under air‐alkaline surroundings. The obtained EPR spectra demonstrated that all of these bioflavonoids can occur redox reaction to generate superoxide anion radical, with different intensities of quadruplet peaks of DMPO−.O2 −/.O2H adducts, as shown in Figure 6 (see Figure S3 in the Supporting Information). It was indicated that bioflavonoids in varied structures caused by locations and numbers of hydroxyl moiety(s) attached to parental molecular backbone or their configurations perform to be a difference in electronic density of molecule, which results in the intensity of quadruplet peaks of DMPO−.O2 −/.O2H adducts generated from myricetin bearing six phenolic hydroxyl moieties higher than that of quercetin with five phenolic hydroxyl moieties, baicalein in possession of three phenolic hydroxyl moieties, and chrysin having two phenolic hydroxyl moieties.
It was found that the relevant phenolate derived from phenolic hydroxyl moiety(s) of bioflavonoids deprotonated under alkaline surroundings, confronts free oxygen as an oxidant existed in the alkaline solution, which flexibly promotes the proceeding of redox reaction to generate superoxide‐anion radical. In summary, the pyrogallol autoxidation method could not be applied to evaluate superoxide‐anion scavenging effects of natural bioflavonoids, owing to the experimental evidences of absorption at 325 nm and superoxide anion radical generated from bioflavonoids which dissolved into air alkaline solution.
In addition, the pyrogallol autoxidation method described for determining superoxide‐scavenging effect was utilized widely to estimate numerous potential antioxidants by UV/Vis, on the basis of superoxide anion generated from pyrogallol autoxidized in air alkaline solution. It was meant that superoxide anion radical has absorption at wavelength of 325 nm, which is regarded as the typical absorbance of superoxide‐anion radical. In this study, superoxide anion generated from a system of dry DMSO reacted with strong base in the presence of oxygen molecule was permitted to be recorded by UV/Vis and investigated by EPR, with the aid of spin trapping reagent DMPO. The results explicitly indicated that the absorbance is not found at 325 nm in the UV spectra, and that the typical signals of DMPO−.O2 −/.O2H adducts in the EPR spectra is clearly observed (Figure 7a and b). Therefore, it was believable that the absorbance at 325 nm in pyrogallol autoxidation method is possibly not a typical signal of superoxide‐anion radical. Furthermore, some previous studies reported that the absorbance happened at 325 nm or 420 nm in pyrogallol autoxidation method should be quinones or purpurogallin generated from semiquinones oxidized by pyrogallol under oxygen alkaline conditions.[ 25 , 26 ]
Figure 7.
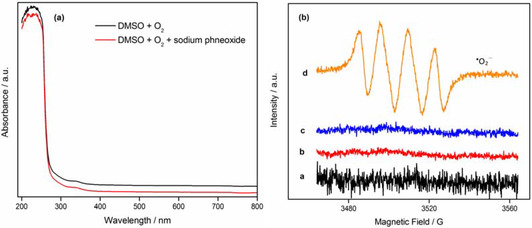
EPR and UV spectra of the DMSO system for generating superoxide anion. Note: (a) DMSO+O2+sodium phneoxide, (b) DMSO+sodium phneoxide+DMPO, (c) DMSO+O2+DMPO, and (d) DMSO+O2+sodium phneoxide+DMPO.
To make up for the weakness of the pyrogallol autoxidation method, we applied the DMSO system method to evaluate superoxide‐scavenging effect of natural bioflavonoids. DMSO as chemical reagent reacted with tiny strong base in the presence of oxygen molecule could undergo univalent reduction of oxygen molecule to produce superoxide anion and methanesulfinic acid/ methanesulfonate, which was documented by some literature.[ 29 , 30 , 31 ] Superoxide anion generated from the DMSO system was capable to be preserved stably in solvent DMSO, by which it enabled to ensure the accuracy of concentration of superoxide anion detected by EPR technology in the DMSO system method. Additionally, methanesulfinic acid/ methanesulfonate produced by reaction of DMSO with a strong base (e. g., sodium hydroxide / sodium phenoxide) could moderate the resulting mixture to be appropriate acidity, which played a pivotal role in helping stabilization of bioflavonoids, thus exhibiting superoxide anion scavenging ability with this system. So, we further investigated the relationship between the acidity of DMSO system and the reaction time in the presence of strong base in a small quantity (sodium phenoxide, 8 mmol L−1, 10 μL, in dry DMSO) under oxygen‐saturated conditions, which was convenient to ascertain an appropriate acidity of DMSO system for evaluating bioflavonoids in superoxide‐scavenging effect. In experiment, the acidity of DMSO system was determined by the method of acid‐base titration with the standard HCl solution after stirring for minutes. As shown in Figure 8a, both pH values at 25 °C and 37 °C perform respectively a decrease with good linear relationship (R2=0.9378 and 0.9651), but not necessarily linear, as prolonging reaction time at the range of 0–40 min. In aspect of chemical kinetics, the slope value at 37 °C is more than that at 25 °C, representing a faster reaction rate. The pH values at 25 °C and 37 °C are respectively much more significant (P<0.01). More importantly, it was obviously seen that after string for 40 min, the pH value of DMSO system at 37 °C is slightly lower (P>0.05) than that at 25 °C, which are 7.25 and 7.54, respectively. Of course, as continuously stirring for long time, the pH values of DMSO system at both of 25 °C and 37 °C are still on decline to be neutral. As well as considering the intensity of typical signals of DMPO−.O2 −/.O2H adducts in the EPR spectra to be the maximum value at 40 min, the pH value of 7.25 was selected to be the appropriate surroundings of DMSO system containing superoxide anion for evaluating the effect of scavenging superoxide anion in DMSO system (Figure 8b). It's worthy to be noted that structures of DMY and other bioflavonoids cannot be affected in the surroundings of pH 7.25, which were observed by UV/Vis.
Figure 8.
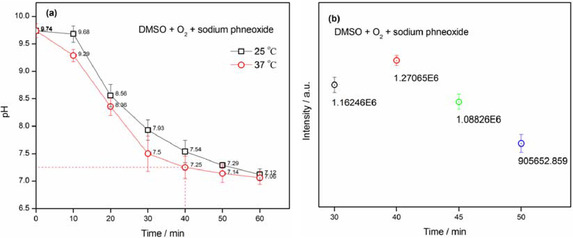
The relationship of reaction time with pH of DMSO system and intensity of DMPO−.O2 −/.O2H adducts. (means±SD, n=3).
Therefore, the optimized conditions of the improved DMSO system utilized to evaluate the potential superoxide‐scavenging effect of natural bioflavonoids were identified as below:
1 mL dry DMSO under oxygen‐saturated environment;
A solution of sodium phenoxide (8 mmol L−1, 10 μL, in dry DMSO);
Stirring slowly for 40 min at 37 °C.
Next, the improved DMSO system, equipped with EPR technique, was applied to evaluate potential natural superoxide‐anion scavenger of bioflavonoids armed multi‐hydroxyl moieties in superoxide‐scavenging activity. DMY as a representative example of bioflavonoids was firstly estimated by means of the improved DMSO system in superoxide‐anion scavenging effect. To the control solution of superoxide‐anion system generated from DMSO in oxygen‐saturated alkaline solution was added sample of DMY with varying concentrations (0.01 mg mL−1, 0.05 mg mL−1, 0.10 mg mL−1, 0.15 mg mL−1, 0.20 mg mL−1, 0.30 mg mL−1, 0.50 mg mL−1 and 1.00 mg mL−1). Furthermore, the pyrogallol autoxidation (pyrogallol in air‐saturated alkaline solution) armed with EPR technique was also utilized to evaluation of bioflavonoid DMY in scavenging activity of superoxide‐anion. As depicted in Figure 9a, the signal intensities of DMPO−.O2 −/.O2H adducts pertaining to both the control and the one involving DMY were determined by EPR. To our delight, signal intensity of DMPO−.O2 −/.O2H adducts in the test experiment with DMY was significant difference (P <0.05) compared to the control in the DMSO system. Using the appropriate formula of ΔI DMPO‐OO.‐ to calculate the extent to which DMY could act as a scavenger of superoxide anion generated from DMSO under the experimental conditions, it was observed that DMY exhibits fairly good scavenging potential (P<0.05) with the maximum of 66.91±1.32 % in effect at a concentration of 1.00 mg mL−1 (see Figure S5 and Table S4 in the Supporting Information). In comparison of the pyrogallol autoxidation via EPR, it found that all of results are being on negative effect (P<0.05) in the presence of DMY. That is to say, the signal intensity of DMPO−.O2 −/.O2H adducts in EPR spectra is much higher (P<0.05) than that of the control group in the presence of DMY (see Figure S4 and Table S3 in the Supporting Information). Hence, it deserved to believe that the improved DMSO system is actually and significantly a potent strategy for evaluating DMY scavenging ability. Herein, it must be emphasized that the surroundings of pyrogallol autoxidation method is being on mild alkaline solution (air‐saturated, Tris‐HCl buffer solution, pH 8.2). This phenomenon encouraged us to take further consideration of alkaline surroundings unsuitable for bioflavonoids having multi‐hydroxyl moieties in evaluating superoxide‐scavenging effect.
Figure 9.
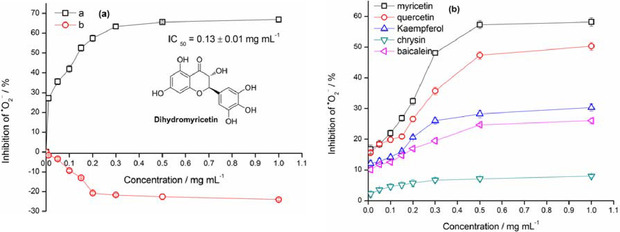
Scavenging effect of superoxide anion promoted by DMY and other bioflavonoids with the DMSO system and/or pyrogallol autoxidation method observed by EPR with DMPO. Note: (a) the pyrogallol autoxidation method for estimating superoxide‐anion scavenging effect of DMY armed with EPR with DMPO; (b) the DMSO system for estimating superoxide‐anion scavenging effect of DMY equipped with EPR with DMPO (means±SD, n=3).
Based on above experimental conditions, other bioflavonoids such as myricetin, quercetin, kaempferol, chrysin and baicalein were selected and permitted to follow above procedure of the DMSO system for evaluating their superoxide‐scavenging effect, due to the difference of hydroxyl moieties on their molecular skeletons in numbers. The EPR results demonstrated that these flavonoids can work in the DMSO system, as shown in Figure 9b, testing samples of flavonoids are totally and significantly in positive effect (P<0.01), but seemly related to numbers of hydroxyl groups on their molecular skeletons. Simultaneously, their scavenging effects are proportional to testing concentrations of flavonoids ranging from 0.01 mg mL−1 to 1.00 mg mL−1. Myricetin accompanying six −OH groups on the molecular backbone at a concentration of 0.50 mg mL−1 was able to show relatively better performance (P<0.01) in superoxide‐anion scavenging effect, as compared to other flavonoids, of course, except for dihydromyricetin (66.91±1.32 % in effect). Dihydromyricetin is an analogue of myricetin, incorporating two chiral carbons (2R, 3R) at 2,3‐positions of molecular backbone, with six −OH groups. Quercetin bearing five hydroxyl moieties on the molecular backbone as well as major conjugation effect on planar molecule exhibited 50.32±1.28 % in superoxide‐scavenging effect, at a concentration of 1.00 mg mL−1. Left three bioflavonoids with less than five −OH groups as well as planar skeletons such as kaempferol (four −OH groups), baicalein (three −OH groups) and chrysin (two −OH groups) presented apparently a decline (P<0.01) in superoxide‐anion scavenging effect, with 30.30±1.17 %, 26.03±0.87 % and 7.89±0.45 % at maximum, respectively. In addition, via viewing IC50 of investigated bioflavonoids in Figure 10, the IC50 value of dihydromyricetin performed to be 0.13±0.01 mg mL−1, less than that of myricetin (0.48±0.03 mg mL−1) and quercetin (0.96±0.05 mg mL−1), while the IC50 values of kaempferol, baicalein and chrysin could not be calculated (see Figure S5 and Table S4 in the Supporting Information). It was observed that the number of hydroxyl moieties and configuration are possibly correlated with the bioactivity of scavengers. This meant that dihydromyricetin, myricetin and quercetin as bioflavonoids can be natural bioactive agents. The DMSO system, which generates superoxide‐anion radicals, is a convenient choice to evaluate superoxide‐anion scavenging effect of some antioxidants, which are sensitive in alkaline environment.
Figure 10.
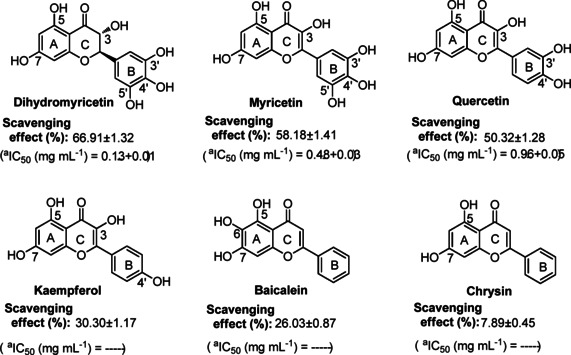
a IC50 value is defined as the concentration of 50 % superoxide radical inhibition and calculated by linear regression analysis and expressed as the mean±SD (n=3). The linear regression was analyzed by Origin 8.5 professional software. —, cannot be calculated.
In summary, assessment of superoxide‐scavenging capacity made by varying methods should further understand their applicable scope, resulting from testing environment enabling affect to superoxide‐scavenging capacity of antioxidants. Herein, we applied an improved and renew analysis system (the DMSO system) to evaluate superoxide‐scavenging effect of bioflavonoids and made a comparison with the pyrogallol autoxidation equipped with UV/Vis spectrophotometer or EPR, as shown in Table 1. Consequently, it was found that the improved system (the DMSO system) exhibits positive effect for bioflavonoids as scavengers in superoxide‐scavenging capacity, with the pyrogallol autoxidation equipped with UV/Vis spectrophotometer or EPR performing respectively to be invalid or negative effect.
Table 1.
Comparison of superoxide‐anion scavenging methods.
|
Method |
Reactive oxygen species |
Detector |
Typical signals |
Testing environment (pH) |
Scavenger |
Superoxide‐scavenging capacity |
|---|---|---|---|---|---|---|
|
The pyrogallol autoxidation |
.O2 − |
UV/Vis |
Absorbance at 325 nm |
Aqueous solution (pH 8.2) |
Bioflavonoids |
Invalid |
|
The pyrogallol autoxidationa |
.O2 − |
EPR |
Quadruplet |
Aqueous solution (pH 8.2) |
Bioflavonoids |
Negative effect |
|
The DMSO systema |
.O2 − |
EPR |
Quadruplet |
DMSO (pH 7.25) |
Bioflavonoids |
Positive effect |
Note: a The method work with spin trapping reagent DMPO.
Regarding the mechanism of bioflavonoids scavenging superoxide‐anion radical, DMY was selected to be an example. During the period of scavenging superoxide anion in the presence of DMY, interestingly, we noticed a prominent change in color of DMY test solution from colorless to bright yellow. To further get insight into the superoxide anion scavenging mechanism by DMY in DMSO system, after quenched with 1 % HCl aqueous solution, we transferred an aliquot of liquid in a testing vial for HPLC analysis. The interpretation of chromatographic result suggested the presence of an unknown compound whose peak intensity gradually increased as a function of reaction time. Of course, as reaction time continuously prolonged, the peak area of unknown component was observed to become low slowly (see Figure S6 in the Supporting Information). Subsequently, the component was isolated from the sample by column chromatography using petroleum ether/ethyl acetate (10/1 to 3/1) as an eluent. The resulting yellow solid was characterized by ESI‐MS, Elemental analysis, and 1H‐ and 13C‐NMR (see Figure S7 in the Supporting Information). Spectral data were consistent with the structure of myricetin (MY) documented in earlier reports.[ 32 , 33 ]
Having confirmed the structure of myricetin, it could be concluded that dihydromyricetin acting as a scavenger in participation of superoxide‐scavenging effect should make a transformation of dihydromyricetin oxidized into myricetin in the presence of oxygen radical (.O2 −). This might be a cause of dihydromyricetin performing much better than myricetin in bioactivity of scavenging superoxide anion. Additionally, according to the report regarding superoxide‐anion radical scavenged via a “donating hydrogen (.H)” approach,[ 21 , 34 ] acting as potential scavenger of bioflavonoids, it was unquestionable that the generation of .H from homolysis of R/ArO−H bond becomes much easier, as compared to R/Ar−H bond, under the same conditions. Therefore, the number of hydroxyl groups attached to the molecular backbone seemly enables the estimation of the compound antioxidant capacity. However, in case of DMY scavenging .O2 − radical, trace amount of myricetin component in the process of .O2 − radical scavenged by DMY was detected by HPLC, and identified by ESI‐MS, Elemental analysis, and 1H‐ and 13C‐NMR. Based on these results, active H donated from C3−H bond of DMY could be a unique origin of .H via homolysis. Therefore, the proposed mechanism of .O2 − radical scavenged by DMY could be explained by the process shown in Scheme 3.
Scheme 3.
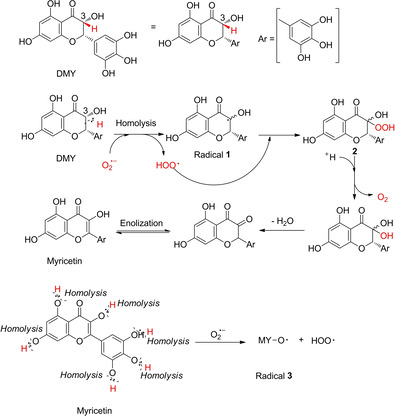
Unconventional pathways of dihydromyricetin scavenging superoxide anion.
Initially, the formation of radical 1 through the abstraction of the C3‐H atom of DMY by .O2 − radical via homolysis was authorized to be proceeded, with hydroperoxide anion (HOO.) generated. Subsequently, the radical 1 combining with peroxyl radical HOO. to form relative peroxide 2. Then, under acidic conditions, the peroxide 2 was flexible to be decomposed with releasing oxygen molecule. Undergoing removing H2O and enolization, product of myricetin could be afforded. Similarly, six hydroxyl groups of myricetin underwent homolysis in the presence of .O2 − radical could effectively scavenge superoxide anion to produce corresponding radical 3 and hydroperoxide anion (HOO.). Nevertheless, in term of transformation of DMY into MY in the process of scavenging .O2 − radical, there was not enough evidence to reveal the transformation at present, because of the complexity of performing the free radical reaction. Finally, it must be emphasized that the IC50 value of DMY in DMSO system is lower than that of MY we found. It was found that DMY is a superior system than MY in the evaluation of the effect of scavenging .O2 − radicals. This result is in agreement with our proposed mechanism.
3. Conclusions
In earlier studies, the pyrogallol autoxidation method coupled with UV/Vis analysis has been widely applied to estimate antioxidant bioactivity, because of its convenience and no requirement of special or expensive instruments and biological agents. Pyrogallol enables the proceeding of the univalent reduction of oxygen molecule to produce superoxide anion under air alkaline conditions. The method is also utilized to evaluate bio‐active agents in .O2 − radical scavenging capacity at monitoring wavelength of 325 nm. We found that the pyrogallol autoxidation method is limited to some potential antioxidants of natural bioflavonoids, which were sensitive to alkaline surroundings due to bioflavonoids having multi‐ hydroxyl moieties to absorb the wavelength of 325 nm under air alkaline surroundings and .O2 − radical, thus resulting in no absorbance at 325 nm. It was found that DMSO reacting with sodium phenoxide in the presence of oxygen is able to produce .O2 − radicals and provide agreeable environment under optimal conditions. A neutral environment ensures the stabilization of bioflavonoids in the reaction system. Simultaneously, .O2 − radical can be preserved in a stable form for several minutes in DMSO solvent, as identified by EPR with the aid of DMPO reagent, which is beneficial for evaluating accurately .O2 − radical scavenging capacity of potential compounds. Moreover, in our testing results, dihydromyricetin, a natural bioflavonoid, was found during the period of scavenging .O2 − radical, a component of myricetin that was detected and identified by HPLC, ESI‐MS, elemental analysis, and 1H‐ and 13C‐NMR. For the mechanism of DMY scavenging .O2 − radical, we proposed a rational pathway involving the transformation of DMY into myricetin. The reaction pathway initially undergoes removal of the active H originated from C−H bond at 3‐carbon of the molecular backbone by .O2 − radical. And then, via complicated free radical reaction, myricetin is formed. Afterward, myricetin is able to donate hydroxyl moieties to proceed homolysis with .O2 − radical to generate hydroperoxide anion (HOO−) and corresponding radicals 3. This could be the cause of DMY performing better than other flavonoids in .O2 − radical scavenging capacity.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We are grateful for the financial support received from Natural Science Foundations of Education Ministry of Guizhou Province (Grant No. KY [2018]033).
Tongren Science and Technology Bureau (Grant No. TSKY2019‐3); Guizhou Science and Technology Department (Grant No. QKHPTRC[2020]5009).
Y. Yao, S. Chen, H. Li, ChemistryOpen 2021, 10, 503.
Contributor Information
Prof. Yuanyong Yao, Email: chyyyy@gztrc.edu.cn.
Dr. Hu Li, Email: hli13@gzu.edu.cn.
References
- 1. Marklund S., Marklund G., Eur. J. Biochem. 1974, 47, 469–474. [DOI] [PubMed] [Google Scholar]
- 2. Nimse S. B., Pal D., RSC Adv. 2015, 35, 27986–28006. [Google Scholar]
- 3. Sawyer D. T., Gibian M. J., Tetrahedron. 1979, 35, 1471–1481. [Google Scholar]
- 4. Simon F., Varela D., Cabello-Verrugio C., Cell. Signalling 2013, 25, 1614–1624. [DOI] [PubMed] [Google Scholar]
- 5. Ikwegbue P. C., Masamba P., Oyinloye B. E., Kappo A. P., Pharmaceuticals. 2017, 11, 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khandrika L., Kumar B., Koul S., Maroni P., Koul H. K., Cancer Lett. 2009, 282, 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Essick E. E., Sam F., Oxid. Med. Cell. Longev. 2010, 3, 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yusuf M., Khan M., Robaian M. A., Khan R. A., Biol. Chem. 2018, 399, 305–319. [DOI] [PubMed] [Google Scholar]
- 9. Kuras M. J., Zielińska-Pisklak M., Duszyńska J., Jabłońska J., J. Food Sci. Technol. 2020, 6, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schiassi M. C. E. V., Souza V. R., Lago A., Campos L. G., Queiroz F., Food Chem. 2017, 245, 305–311. [DOI] [PubMed] [Google Scholar]
- 11. Zhou J., Ma Y., Jia Y., Pang M., Cheng G., Cai S., Food Chem. 2019, 288, 68–77. [DOI] [PubMed] [Google Scholar]
- 12. Nardini M., Garaguso I., Food Chem. 2020, 305, 125437–125447. [DOI] [PubMed] [Google Scholar]
- 13. Tošović J., Bren U., Antioxidants. 2020, 9, 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Biharee A., Sharma A., Kumar A., Jaitak V., Fitoterapia. 2020, 146, 104720–104741. [DOI] [PubMed] [Google Scholar]
- 15. Gatto M. T., Falcocchio S., Grippa E., Mazzanti G., Battinelli L., Nicolosi G., Lambusta D., Saso L., Bioorg. Med. Chem. 2002, 10, 269–272. [DOI] [PubMed] [Google Scholar]
- 16. Galvão S. S. L., Monteiro A. S., Siqueira E. P., Bomfim M. R. Q., Dias-Souza M. V., Ferreira G. F., Denadai A. M. L., Santos A. R. C., Santos V. L., Souza-Fagundes E. M., Fernandes E. S., Monteiro-Neto V., Front. Microbiol. 2016, 7, 2053–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ma Q., Jiang J. G., Yuan X., Qiu K., Zhu W., Food Chem. Toxicol. 2019, 125, 422–429. [DOI] [PubMed] [Google Scholar]
- 18. Barbacanne M. A., Souchard J. P., Darblade B., Iliou J. P., Nepveu F., Pipy B., Bayard F., Arna J. F., Free Radical Biol. Med. 2000, 29, 388–396. [DOI] [PubMed] [Google Scholar]
- 19. Siddhuraju P., LWT-Food Sci. Technol. 2007, 40, 982–990. [Google Scholar]
- 20. Pritsos C. A., Constantinides P. P., Tritton T. R., Heimbrook D. C., Sartorelli A. C., Anal. Biochem. 1985, 150, 294–299. [DOI] [PubMed] [Google Scholar]
- 21. Li X. C., J. Agric. Food Chem. 2012, 60, 6418–6424. [DOI] [PubMed] [Google Scholar]
- 22. Xu S., Hang H., Li Y., Chemistry. 2001, 64, 516–519. [Google Scholar]
- 23. Han S. H., Zhu J. B., Wang Y. Y., China. Brewing. 2009, 41, 155–157. (in Chinese). [Google Scholar]
- 24. Jurasekova Z., Domingo C., Garcia-Ramos J. V., Sanchez-Cortes S., Phys. Chem. Chem. Phys. 2014, 16, 12802–12813. [DOI] [PubMed] [Google Scholar]
- 25. Tauber H., J. Biol. Chem. 1953, 205, 395–400. [PubMed] [Google Scholar]
- 26. Yuan Z. B., Gao R. M., Chem. J. Chinese. U. 1997, 18, 1438–1441. [Google Scholar]
- 27. Tewari R. K., Sharma K. P. N., Planta. 2006, 223, 1145–1153. [DOI] [PubMed] [Google Scholar]
- 28. Lardinois O. M., Free Radical Res. Commun. 1995, 22, 251–274. [DOI] [PubMed] [Google Scholar]
- 29. Scaduto R. C., Free Radical Biol. Med. 1995, 18, 271–277. [DOI] [PubMed] [Google Scholar]
- 30. Babbs C. F., Griffin D. W., Free Radical Biol. Med. 1989, 6, 493–503. [DOI] [PubMed] [Google Scholar]
- 31. Yurkova I. L., Schuchmann H. P., Von-Sonntag C., J. Chem. Soc. Perkin Trans. 2 1999, 10, 2049–2052. [Google Scholar]
- 32. Liu T. F., Yu H. Z., Chen Y. M., Wang T., Chinses Journal of Synthetic Chemistry 2015, 23, 441–444 (in Chinese). [Google Scholar]
- 33. Yao Y. Y., Shi B. G., Chen S. X., Xing M. M., Zhang M. Q., Lu Z. Y., Journal of Molecular Science 2019, 35, 72–79 (in Chinese). [Google Scholar]
- 34.Y. Z. Fang, R. L. Zheng, Reactive oxygen species, Chapter 2, In Therory and Application of Free Radical Biology, 2nd ed., Science Press: Beijing, China, 2002, pp: 26–27.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


