Abstract
Older adults have reduced vascular endothelial function, evidenced by attenuated nitric oxide (NO)-dependent cutaneous vasodilatation. Folic acid and its metabolite, 5-methyltetrahydrofolate (5-MTHF), are reported to improve vessel function. We hypothesized that (i) local 5-MTHF administration and (ii) chronic folic acid supplementation would improve cutaneous microvascular function in ageing through NO-dependent mechanisms. There were two separate studies in which there were 11 young (Y: 22 ± 1 years) and 11 older (O: 71 ± 3 years) participants. In both studies, two intradermal microdialysis fibres were placed in the forearm skin for local delivery of lactated Ringer’s solution with or without 5 mM 5-MTHF. Red cell flux was measured by laser-Doppler flowmetry. Cutaneous vascular conductance [CVC = red cell flux/mean arterial pressure] was normalized as percentage maximum CVC (%CVCmax) (28 mM sodium nitroprusside, local temperature 43 °C). In study 1 after CVC plateaued during local heating, 20 mM NG-nitro-l-arginine methyl ester (l-NAME) was perfused at each site to quantify NO-dependent vasodilatation. The local heating plateau (%CVCmax: O = 82 ± 3 vs Y = 96 ± 1, P = 0.002) and NO-dependent vasodilatation (%CVCmax: O = 26 ± 6% vs Y = 49 ± 5, P = 0.03) were attenuated in older participants. 5-MTHF augmented the overall (%CVCmax = 91 ± 2, P = 0.03) and NO-dependent (%CVCmax = 43 ± 9%, P = 0.04) vasodilatation in older but not young participants. In study 2 the participants ingested folic acid (5 mg/day) or placebo for 6 weeks in a randomized, double-blind, crossover design. A rise in oral temperature of 1°C was induced using a water-perfused suit, body temperature was held and 20 mM l-NAME was perfused at each site. Older participants had attenuated reflex (%CVCmax: O = 31 ± 8 vs Y = 44 ± 5, P = 0.001) and NO-dependent (%CVCmax: O = 9 ± 2 vs Y = 21 ± 2, P = 0.003) vasodilatation. Folic acid increased CVC (%CVCmax = 47 ± 5%, P = 0.001) and NO-dependent vasodilatation (20 ± 3%, P = 0.003) in the older but not the young participants. Both local perfusion of 5-MTHF and supplementation with folic acid increase vasodilatation in ageing individuals through NO-dependent mechanisms.
Keywords: ageing, cutaneous blood flow, folate supplementation, methyltetrahydrofolate, vascular dysfunction
INTRODUCTION
Even in the absence of overt cardiovascular disease, healthy older men and women demonstrate attenuated vascular endothelial function, as evidenced by a reduced cutaneous vasodilatory response to local skin heating and whole-body heat stress [1–3]. This microvascular dysfunction is attributable to an age-related decrease in vasodilator mechanisms, resulting in functionally absent co-factor-mediated vasodilatation accompanied by attenuated nitric oxide (NO)-dependent vasodilatation [1,3]. As such, older adults rely primarily on this attenuated NO-dependent vasodilatation to increase skin blood flow [1,2]. Consequently, the NO pathway is an important molecular target for potential pharmacological intervention strategies aimed at improving or maintaining vascular health and function throughout older adulthood.
Age-related impairments in NO-dependent vasodilatation are due, in part, to decreases in tetrahydrobiopterin (BH4) bioavailability [4,5], and increases in arginase activity [6,7] and oxidant stress [7,8]. BH4 is required to maintain the functional conformation of the nitric oxide synthase (NOS) dimer for NO production and is reduced with ageing as a result of decreased synthesis and increased oxidation by free radicals [9,10]. In the absence of adequate substrate or co-factor (BH4) availability, the NOS dimer becomes functionally uncoupled and produces superoxide rather than NO [11]. Increases in reactive oxygen species, including superoxide (O2•−), with advancing age, further contribute to reduced BH4 bioavailability by oxidizing BH4 to dihydrobiopterin (BH2), a competitive inhibitor that lacks the co-factor properties of BH4 [12]. Collectively, these age-related increases in oxidant stress and subsequent decreases in BH4 bioavailability contribute to endothelial dysfunction and attenuated NO-dependent vasodilatation in primary ageing.
Recently published data from our lab demonstrated that (i) local perfusion of BH4 directly into the dermal space and (ii) oral administration of sapropterin (pharmaceutical-grade BH4) acutely reverse vascular dysfunction in aged human skin through NO-dependent mechanisms [4,13]. However, the high cost and relative unavailability of oral formulations of BH4 underscore the need for a readily available, inexpensive, intervention strategy to effectively improve microvascular function in older adults.
Folic acid and its active metabolite, 5-methyltetrahydrofolate (5-MTHF), improve conduit vessel endothelial function in patients with overt cardiovascular and metabolic disease [14–16]. 5-MTHF increases vascular BH4 bioavailability by increasing production (BH2 recycling) and reducing oxidant stress. Therefore, improvements in vessel function with folic acid may be mediated though restoration of NO production [17,18]. The purpose of the present study was to determine whether exogenous folic acid supplementation could increase NO-dependent vasodilatation in aged cutaneous microvessels. Folic acid supplementation may be a viable intervention strategy to improve cutaneous microvascular function and increase NO-dependent thermoregulatory skin blood flow in older adults; however, few in vivo studies have examined the mechanistic role of folic acid in improved endothelial function in primary ageing. We hypothesized that acute, direct, local microperfusion of 5-MTHF into the dermal space would increase the cutaneous vasodilatory response to an endothelial-NOS (eNOS)-dependent stimulus (local heating) through NO-dependent mechanisms in older adults (study 1). We further hypothesized that a chronic, high-dose, oral folic acid supplementation (5 mg daily for 6 weeks) would increase the magnitude of reflex cutaneous vasodilatation through NO-dependent mechanisms in older adults during passive whole-body heating (study 2).
MATERIALS AND METHODS
Participants
Experimental protocols were approved by the institutional review board of the Pennsylvania State University and the Food and Drug Administration [FDA Investigational New Drug (IND) application: 103180]. Written and verbal consent were obtained voluntarily from all participants before participation according to the Declaration of Helsinki. Participants were screened for neurological, cardiovascular and dermatological diseases, and underwent a complete medical screening, including resting electrocardiogram (ECG), physical examination, lipid profile and blood chemistry (Quest Diagnostics). All participants were normally active, non-hypertensive, non-diabetic, healthy non-smokers who were not taking prescription medications with primary or secondary vascular effects (e.g. statins, antihypertensives, anticoagulants, antidepressants). Women taking hormonal birth control or hormone replacement therapy, or who had recently taken hormone replacement therapy, were excluded from the study. All premenopausal women were normally menstruating and were studied during the early follicular phase (days 1–7) of their menstrual cycle. All participants were asked to abstain from alcohol consumption for 24 h and caffeine for 12 h before participation.
The participant characteristics are presented in Table 1. Older participants had a higher body mass index (BMI) and elevated low-density lipoprotein (LDL)-cholesterol, total cholesterol and HbA1c compared with the young participants. However, these values were all well within clinically normal ranges.
Table 1.
Characteristics of participants
| Age | Sex | BMI | LDL | HDL | Total cholesterol | SBP | DBP | MAP | ||
|---|---|---|---|---|---|---|---|---|---|---|
| (years) | (M:F) | (kg/m2) | (mg/dl) | (mg/dl) | (mg/dl) | HbA1c (%) | (mmHg) | (mmHg) | (mmHg) | |
| Young | 22 ± 1 | 5:6 | 22 ± 1 | 92 ± 10 | 63 ± 6 | 165 ± 11 | 5.3 ± 0.1 | 116 ± 3 | 71 ± 2 | 86 ± 2 |
| Older | 71 ± 3 | 5:6 | 25 ± 1* | 123 ± 4* | 65 ± 6 | 199 ± 8* | 5.6 ± 0.1* | 121 ± 3 | 74 ± 3 | 90 ± 2 |
Values are means ± S.E.M.s.
DBP, diastolic blood pressure; HDL, high-density lipoprotein; SBP, systolic blood pressure.
P < 0.05, significant difference from young participants.
Study 1: local heating
Two intradermal microdialysis fibres (10-mm, 20-kDa cutoff membrane, MD 2000, Bioanalytical Systems) were placed into the dermal layer of the ventral left forearm for the local delivery of pharmacological agents [19]. Pharmacological agents were mixed just before use, dissolved in lactated Ringer’s solution, sterilized using syringe microfilters (Acrodisc, Pall Corp.), and wrapped in foil to prevent degradation from light exposure. Microdialysis sites were randomly assigned to receive (i) 5 mM 5-MTHF for local delivery of the folic acid metabolite or (ii) lactated Ringer’s solution to serve as a control. The specific 5 mM concentration of 5-MTHF was chosen based on pilot testing at our laboratory, and was determined to be the highest effective dose that did not induce baseline vasodilatation. Site-specific pharmacological solutions were perfused through the microdialysis fibres at a rate of 2 μl/min (Bee Hive controller and Baby Bee microinfusion pumps, Bioanalytical Systems).
Hyperaemia was allowed to resolve for 60–90 min before a standard local heating protocol was initiated to induce eNOS-dependent vasodilatation [19,20]. The local heater temperature was increased from the baseline temperature of 33°C to a temperature of 42°C at a rate of 0.1°C/s, and then held at 42°C for the remainder of the heating protocol. After approximately 30–40 min, when the skin blood flow reached an established plateau, 20 mM NG-nitro-l-arginine methyl ester (l-NAME, Calbio-chem, Merck Millipore) was perfused at a rate of 4 μl/min to quantify NO-dependent vasodilatation at all sites. After infusion of l-NAME and subsequent stabilization of a post-l-NAME plateau in skin blood flow, 28 mM sodium nitroprusside (Nitropress, Abbott Laboratories) was perfused and the local temperature increased to 43°C to elicit maximal dilatation [21].
Cutaneous red blood cell flux was continually measured directly over each microdialysis site with an integrated laser-Doppler flowmetry (LDF) probe placed in a local heating unit (SH02, Moor Instruments). Cutaneous vascular conductance (CVC) was calculated as red blood cell flux divided by mean arterial pressure (MAP) and expressed as a percentage of the site-specific maximal vasodilatation (%CVCmax).
Study 2: whole-body heating
Participants ingested 5 mg of folic acid (Bio-Tech Pharmacal) or cellulose placebo once daily for 6 weeks in a randomized, double-blind, crossover study design. This treatment regimen was chosen based on previous reports and pilot testing in our laboratory which suggested that the dose and time would confer efficacy [15,22–24]. Pharmacokinetic studies in humans report that the elimination half-life of folic acid in the plasma is approximately 2.5–5 h [25–27]. Based on these data, 10 half-lives, which would clear approximately 99.9% of ingested treatment, would be 50 h. We selected 2 weeks washout between treatments to allow complete clearance, taking into account that some accumulation may have occurred during the treatment period. Participants did not ingest folic acid or the placebo for 24 h before experimental testing. On arrival at the laboratory, a blood sample was obtained for the measurement of plasma homocysteine (Quest Diagnostics), folic acid and 5-MTHF.
HPLC/MS-MS analysis of plasma folic acid and 5-MTHF
Plasma folic acid and 5-MTHF were measured at the Penn State Hershey College of Medicine Mass Spectrometry and Proteomics Core Facility. Stock solutions of folic acid and 5-MTHF were prepared in 1 M NaOH containing 10 mg of ascorbic acid. The standard stock solution was series diluted by 20 mM PBS containing 10 mg/ml of ascorbic acid, pH 6.8 to make the standard working solutions range from 13 ng/ml to 7000 ng/ml for folic acid and from 9.75 ng/ml to 5000 ng/ml for 5-MTHF. All the stock solutions and working solutions were kept at −20°C before use. Serum samples (60 μl) were mixed with 7 μl of 200 mM ascorbic acid. The samples were deproteinized by adding 120 μl methanol. After centrifugation at 4°C for 10 min at 14 000 rev./min, the supernatant was loaded on to a HPLC/MS-MS system for analysis.
Folic acid and 5-MTHF were determined by AB SCIEX 5600 TripleTOF mass spectrometry coupled with a Shimadzu Prominence UFLCXR separation system. A 1.7-μm ACQUITY UPLC BEH C8 analytical column (2.1×100 mm, Waters) was used to separate folic acid and its metabolite, 5-MTHF. The gradient elution was conducted using a flow rate of 0.3 ml/min with the following conditions: 1 min in 90% solvent A (0.1% formic acid in water) and 10% solvent B (0.1% formic acid in methanol), a linear gradient to 100% solvent B in 2 min, keeping the 100% solvent B for 3 min to flush the column. The autosampler was kept at 4°C and the column temperature was maintained at 30°C. The AB SCIEX 5600 TripleTOF mass spectrometer was equipped with an electrospray ionization probe operated in the positive mode. The multiple reaction monitoring mode (MRM) was used to analyse and quantify folic acid and 5-MTHF, with the transitions of m/z 442 > 295 for folic acid and m/z 460 > 313 for 5-MTHF. The ion source, gas 1 and gas 2, was 50 l/h and 60 l/h, respectively, whereas the curtain gas was 50 l/h for all analytes. The interface heater temperature was 150°C. All peaks were integrated and quantified using AB SCIEX Multiquant 2.1 software.
Whole-body heating
All protocols were performed in a thermoneutral laboratory, with the participants in a semi-supine position and the experimental arm supported at heart level. All experiments took place at the same time of day to eliminate diurnal variation in blood flow responses [28]. Participants had two intradermal microdialysis fibres placed in the ventral forearm skin for the local delivery of pharmacological agents [29]. Sites were randomly assigned as (i) 5 mM 5-MTHF dissolved in Ringer’s solution for local delivery of the folic acid metabolite or (ii) lactated Ringer’s solution to serve as the control. During the hyperemia period (60–90 min), site-specific pharmacological agents were perfused through the fibres at a rate of 2 μl/min.
The skin temperature (Tsk) was controlled using a water-perfused suit that covered the entire body except for the head, hands, feet and forearms. Copper-constantan thermocouples were placed on the surface of the skin at six sites (calf, thigh, abdomen, chest, shoulder and back) for continuous measurement of Tsk. The heart rate was monitored continuously throughout the protocol (Cardiocap, GE Healthcare), and arterial blood pressure was measured by brachial auscultation every 5 min. The oral temperature (Tor) was measured as an index of changes in core temperature using a thermistor placed in the sublingual sulcus throughout the protocol. Proper placement of the thermistor was checked based on temperature readings and, once verified, was taped in place and closely monitored to ensure that it did not move throughout the protocol. The local Tsk over each microdialysis site was held at 33°C throughout baseline and whole-body heating (MoorLab, Temperature Monitor, SH02, Moor Instruments) to ensure that changes in skin blood flow were reflex in origin. The CVC was calculated and standardized as described above.
After microdialysis fibre placement, the hyperemia period and instrumentation, baseline data were collected (~20 min). Throughout the baseline, the mean Tsk was held at thermoneutral by perfusing 31°C water through the suit. After baseline data collection, warm water was perfused through the suit to hold the mean Tsk at 38°C and cause a gradual rise in Tor. At a 1°C rise in Tor, the mean body temperature was held by lowering the water temperature in the suit. After 5 min of steady LDF values, 20 mM l-NAME was perfused through the fibre at a rate of 4 μl/min to inhibit NOS production of NO and quantify NO-dependent vasodilatation within the site [4,13]. l-NAME perfusion was discontinued after LDF values decreased to a steady plateau (~40 min). At this time, whole-body heating was terminated and the water-perfused suit was perfused with 33°C water. After completion of the whole-body heating protocol, the fibre was perfused with 28 mM sodium nitroprusside at a rate of 4 μl/min. Simultaneously, the local Tsk over the experimental site was increased to 43°C to obtain the CVCmax.
Statistical analysis
A two-way, repeated-measure, mixed-model ANOVA was used to detect age and local treatment differences in the local heating plateau, NO-dependent vasodilatation and maximal CVC (using SAS, version 9.1.3, SAS Institute Inc.). To test for a possible carry-over effect of treatment, a three-way (age×treatment×sequence), repeated-measure ANOVA was used to test for possible effects of treatment sequence (sequence 1: placebo followed by folate; sequence 2: folate followed by placebo) on plasma folate, plasma 5-MTHF,%CVCmax and percentage NO-dependent vasodilatation in young and older participants. A four-way, repeated-measure, mixed-model ANOVA was used to detect age, temperature, oral treatment and local treatment differences in skin blood flow responses for every 0.1°C rise in Tor. Three-way, repeated-measure ANOVA was used to detect age, oral treatment and local treatment differences in NO-dependent vasodilatation and maximal CVC. Post-hoc comparisons with Bonferroni’s corrections were performed for specific planned comparisons. The level of significance was set at α = 0.05 for main effects. The results are presented as means ± S.E.M.s.
RESULTS
Figure 1 shows the skin blood flow (%CVCmax) at the local heating plateau (Figure 1A) and percentage NO-dependent vasodilatation (Figure 1B) in control and MTHF-treated microdialysis sites in young (Y) and older (O) participants. Older participants had a significantly attenuated heating plateau (%CVCmax: O = 84 ± 2 vs Y = 94 ± 2, P = 0.002) and NO-dependent vasodilatation (O = 26 ± 6% vs Y = 49 ± 5%, P = 0.03) in the control site compared with young participants. Local MTHF administration increased the magnitude of the local heating plateau compared with the control site in older (%CVCmax: MTHF = 93 ± 2 vs control = 84 ± 2, P = 0.03) but not young (%CVCmax: MTHF = 96 ± 1 vs control = 94 ± 2, P = 0.8) participants. Furthermore, local MTHF increased NOdependent vasodilatation compared with the control site in older (MTHF = 43 ± 4% vs control = 26 ± 6%, P = 0.04) but not young (MTHF = 43 ± 9% vs control = 49 ± 5%, P = 0.4) participants.
Figure 1. Vasodilatation response to local heating and percentage NO-dependent vasodilatation.
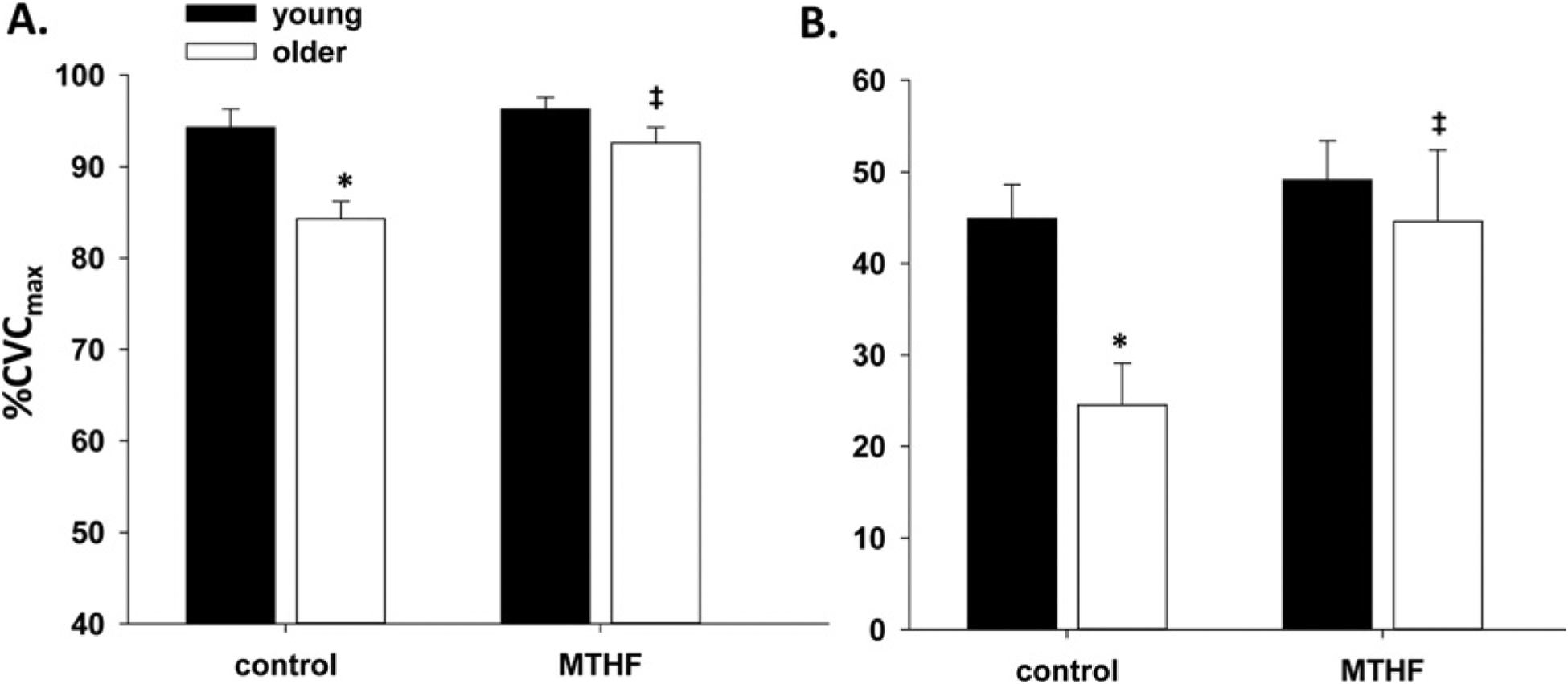
(A) The group vasodilatation response (%CVCmax) to local heating and (B) the percentage NO-dependent vasodilatation in control and MTHF-perfused microdialysis sites in young and older participants. Results are means ± S.E.M.s. *P < 0.05, significant difference compared with young controls; ‡P < 0.05, significant difference from older control.
Table 2 presents values for plasma folate, 5-MTHF and homocysteine after placebo and folic acid treatment in young and older participants. Folic acid oral supplementation significantly increased plasma folate (P = 0.02) and 5-MTHF (P < 0.001) in both young and older participants. Folic acid treatment had no effect on plasma homocysteine concentration in either group.
Table 2.
Values for plasma folate, 5-MTHF and homocysteine after placebo and folic acid treatment in young and older participants
| Plasma folate (ng/ml) | Plasma 5-MTHF (ng/ml) | Plasma homocysteine (μmol/l) | |
|---|---|---|---|
| Young | |||
| Placebo | 1.32 ± 0.24 | 28.56 ± 2.78 | 7.8 ± 0.6 |
| Folic acid supplemented | 28.36 ± 17.24* | 48.62 ± 3.96‡ | 7.3 ± 0.4 |
| Older | |||
| Placebo | 1.07 ± 0.14 | 25.81 ± 2.86 | 11.5 ± 0.8 |
| Folic acid supplemented | 26.11 ± 10.20* | 64.35 ± 5.83‡ | 10.9 ± 0.9 |
value are means ± S.E.M.s.
P = 0.02, significant effect of treatment on plasma folate;
P < 0.001, significant effect of treatment on plasma 5-MTHF.
There was no effect of treatment sequence on plasma folate, plasma 5-MTHF or %CVCmax response throughout whole-body heating, or percentage NO-dependent vasodilatation at a 1°C rise in Tor (all P values >0.1 for sequence and sequence×treatment interaction). Furthermore, therewere no differences between vascular responses in the older group when separated by sequence [after placebo (%CVCmax: sequence 1 = 31.1 ± 5 vs sequence 2 = 30.7 ± 3.5); after folate (%CVCmax: sequence 1 = 44.2 ± 7 vs sequence 2 = 49.3 ± 9)].
Folic acid treatment had no effect on systolic blood pressure (Y: folic acid = 112 ± 3 mmHg vs placebo = 110 ± 2 mmHg, P > 0.05; O: folic acid = 126 ± 4 mmHg vs placebo = 129 ± 2 mmHg, P > 0.05), diastolic blood pressure (Y: folic acid = 67 ± 2 mmHg vs placebo = 67 ± 2 mmHg, P > 0.05; O: folic acid = 71 ± 2 mmHg vs placebo = 73 ± 2 mmHg, P > 0.05) or MAP (Y: folic acid = 82 ± 2 mmHg vs placebo = 81 ± 2 mmHg, P > 0.05; O: folic acid = 89 ± 2 mmHg vs placebo = 92 ± 2 mmHg, P > 0.05) in either group.
Figure 2 depicts the skin blood flow (%CVCmax) response throughout whole-body heating at control (Ringer’s solution, Figure 2A) and MTHF-treated (Figure 2B) microdialysis sites in young and older participants after 6 weeks of placebo or folic acid oral supplementation. After placebo treatment, older participants had an attenuated reflex vasodilatation compared with the young participants at the control microdialysis site throughout heating. Folic acid supplementation increased vasodilatation in the control sites of older participants compared with placebo at oral temperatures exceeding a 0.5°C rise. There was no difference across the trials at the MTHF-treated sites in either young or older participants. Both oral folic acid and local 5-MTHF increased reflex vasodilatation in the older participants to values observed in young participants at temperatures above a 0.8°C rise.
Figure 2. Vasodilatation response to increasing oral temperature.
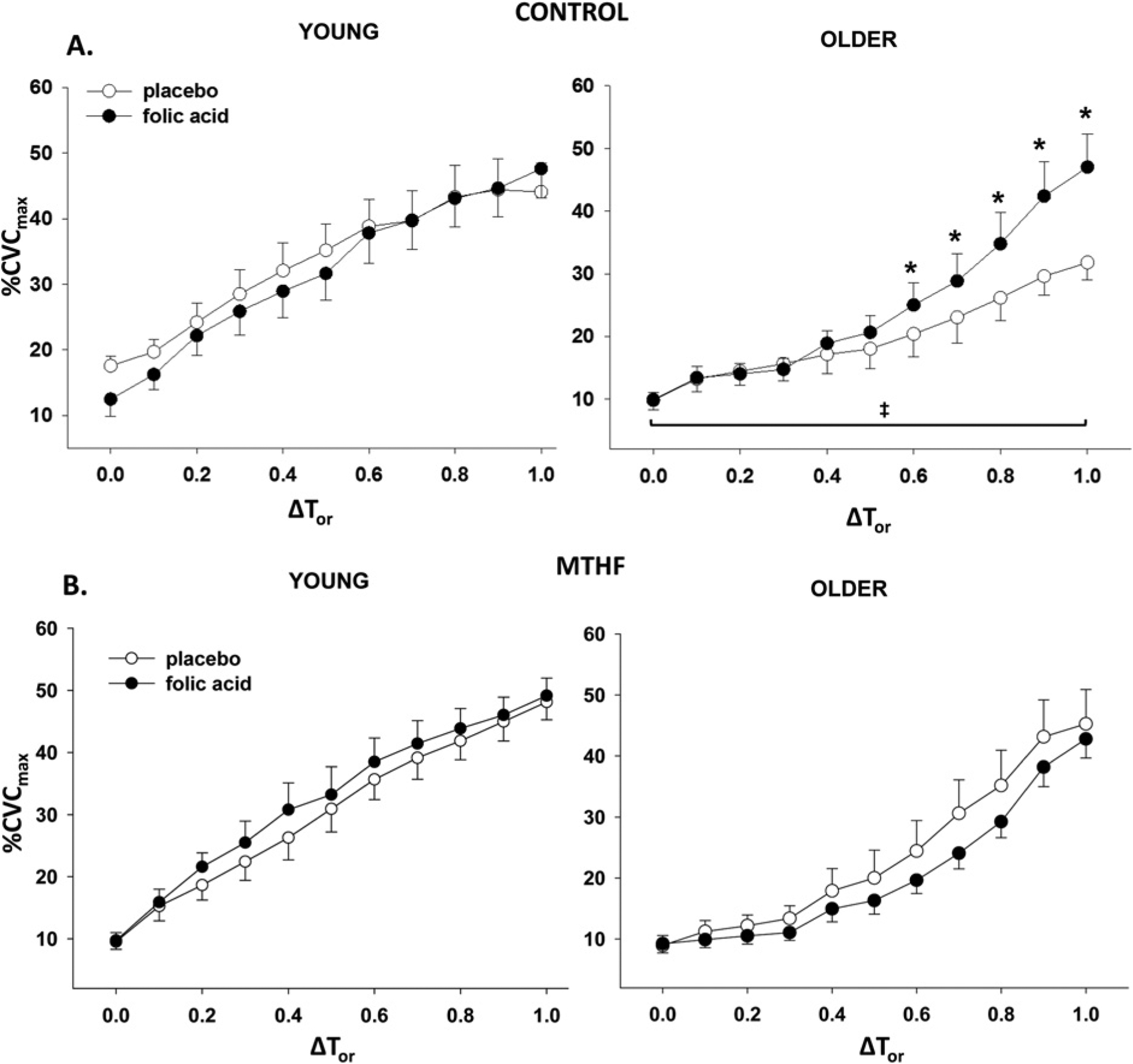
Group vasodilatation response (%CVCmax) to increasing oral temperature (ΔTor) in (A) control and (B) MTHF-perfused microdialysis sites in young and older participants after placebo and folic acid treatment. Results are means ± S.E.M.s. *P < 0.05, significant difference from older placebo; ‡P < 0.05, older placebo significantly different from young placebo.
Figure 3 shows the percentage NO-dependent vasodilatation response at a 1 °C rise in Tor at control (Ringer’s solution) and MTHF-treated microdialysis sites in young and older participants after 6 weeks of placebo and folic acid supplementation. After placebo treatment, older participants had an attenuated NO-dependent vasodilatation compared with young participants in the control microdialysis site (O = 9 ± 2% vs Y = 21 ± 1%, P = 0.003). Folic acid supplementation over 6 weeks increased NO-dependent vasodilatation at the control site of older (20 ± 3%, P = 0.02) but not young (24 ± 3%, P = 0.7) participants. There was no difference between groups or across supplement trials at the MTHF-treated sites (O: folic acid = 18 ± 2% vs placebo = 18 ± 4%, P > 0.05; Y: folic acid = 21 ± 2% vs placebo = 22 ± 3%, P > 0.05).
Figure 3. Percentage NO-dependent vasodilatation at 1 °C rise in Tor.
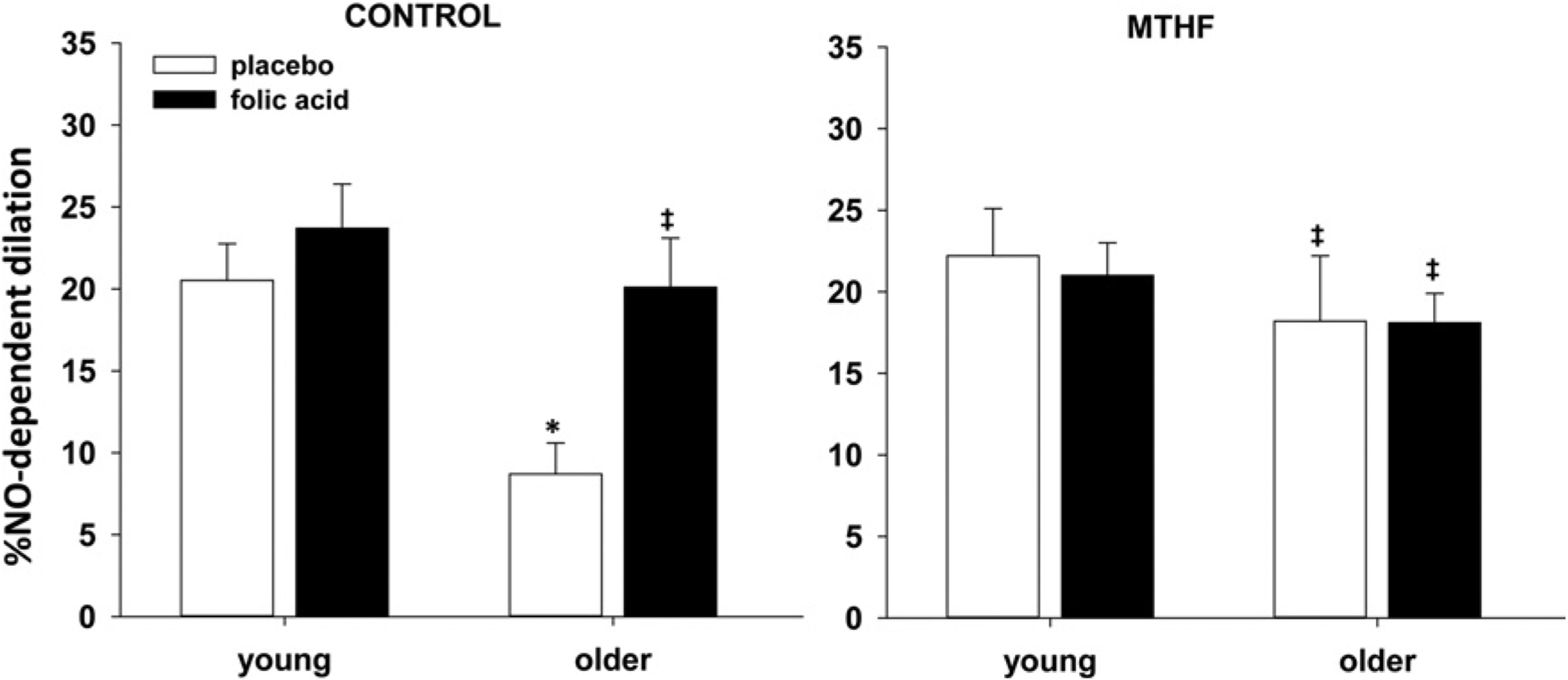
Group percentage NO-dependent vasodilatation at 1 °C rise in Tor in control and MTHF-perfused microdialysis sites in young and older participants after placebo and folic acid treatment. Results are means ± S.E.M.s. *P = 0.003, difference compared with young placebo control; ‡P < 0.05 difference compared with older placebo control.
DISCUSSION
The primary findings of this study are that (i) local perfusion of 5-MTHF directly into the dermal space increased local cutaneous vasodilatation in response to an eNOS-dependent stimulus, acting through NO-dependent mechanisms, and (ii) systemically increasing plasma folate through 6 weeks of high-dose oral folic acid supplementation increased NO-dependent reflex cutaneous vasodilatation in healthy, older adults. Older participants had a significantly attenuated cutaneous vasodilatation response to both local and whole-body heat stress, as previously demonstrated [1,3]. Local 5-MTHF administration, as well as chronic folic acid treatment, augmented vasodilator function in healthy aged skin through NO-dependent mechanisms. Furthermore, this augmentation reached values similar to those observed in young participants. Clinically, folic acid treatment may be a relevant intervention strategy for improved vascular endothelial function in older adulthood.
The human cutaneous circulation is an accessible and representative circulation for examining mechanisms of vascular dysfunction in vivo [30].There is a significant relationship between endothelial dysfunction measured in the skin and that measured invasively in the coronary and renal circulations, and intervention-induced improvements in vascular function are detectible in the cutaneous circulation before improvements in clinical outcome [31,32]. The use of whole-body thermal stimuli, coupled with systemic interventions and localized pharmacological manipulations (intradermal microdialysis), allows for the mechanistic examination of interventional efficacy in improved vessel function during an integrative cardiovascular challenge. In the present study, we have examined the efficacy of a high-dose folic acid intervention because folic acid and its active metabolite, 5-MTHF, have been shown to increase NO production in recombinant eNOS and cultured endothelial cells and, as such, may be capable of modulating NO synthesis in the vasculature of older adults [17]. Our results suggest that folic acid increases the magnitude of cutaneous vasodilatation in aged human skin probably through increasing NOS coupling and subsequent NO synthesis.
The mechanisms mediating cutaneous vasodilatation in response to local skin heating and whole-body heat stress are distinctly different. Local skin heating induces a biphasic increase in skin blood flow which is mediated by two independent mechanisms [20]. The initial rapid rise in skin blood flow is caused by a sensory axon reflex and, after a brief nadir, a secondary phase consists of a more slowly developing rise to a stable plateau that is approximately 60–70% reliant on eNOS-mediated NO production in healthy young individuals [19,20]. With ageing, there is a significant attenuation in the initial sensory axon reflex, as well as a reduction in the eNOS-dependent plateau. Although the underlying mechanisms mediating this decline are not fully defined in the ageing microvasculature, age-related changes in oxidant stress as well as substrate and co-factor availability contribute to endothelial dysfunction with ageing and are probably contributing factors [4,5,7]. As the skin blood flow response to local heating is predominantly eNOS dependent, local heating is a valuable method to non-invasively assess endothelium-dependent vasodilatation in vivo in the human vasculature.
In contrast to local heating, in reflex vasodilatation caused by whole-body heating approximately 30–40% of the total reflex vasodilatation response is mediated by NO signalling in healthy young individuals, with the remaining 60–70% relying on co-released neurotransmitter(s) and downstream vasodilatation mediated through other second messenger pathways, and activation of cyclo-oxygenase (COX) [33–35]. With ageing, the co-factor-mediated contribution to the overall expression of reflex vasodilatation is attenuated and COX-dependent signalling favours the production of vasoconstrictors [36]. Consequently, healthy aged adults rely predominately on a functionally compromised, NO-dependent vasodilatation to increase skin blood flow during hyperthermia [1].
The decreased NO bioavailability in aged skin results from a decrease in NO production by up-regulated vascular arginase and increased NOS uncoupling, with increased oxidant stress and reduced co-factor bioavailability [6,7,13,37]. Although the contributions and identities of the co-transmitters in human skin are not fully known, many of these co-transmitters converge on the NO pathway. Therefore interventions that target NO production and bioavailability may be capable of increasing reflex vasodilatation in aged human skin. In the present study, we utilized whole-body heating coupled with localized pharmacological manipulations to mechanistically examine the efficacy of folic acid supplementation in improving vascular function and NO-dependent vasodilatation during an integrative cardiovascular challenge.
We have chosen to utilize both local and whole-body heating to test the efficacy of 5-MTHF and folic acid in improved NO-dependent vasodilatation because both methods provide applied and mechanistic data about the role of NO in vascular endothelial function. Collectively, the data from studies 1 and 2 suggest that folic acid and its active metabolite, 5-MTHF, improve cutaneous vessel reactivity to vasodilator stimuli. In both studies we demonstrate that increasing 5-MTHF and/or folic acid augments both total and NO-dependent vasodilatation in aged adults.
In study 1 we examined the efficacy of local, microperfusion of 5-MTHF in the augmented NO-dependent vasodilatation response to an eNOS-specific stimulus [19]. 5-MTHF (i) increases vascular BH4 bioavailability by increasing production via BH2 recycling and reducing oxidant stress, and (ii) improves NO-dependent vasodilatation in ex vivo human vessels through eNOS coupling mechanisms [18]. In agreement with these data, our findings suggest that local delivery of 5-MTHF acutely improves NO-dependent vasodilatation in aged cutaneous vessels, to the level of that observed in our young participants.
In study 2 we sought to determine the efficacy of a systemic, high-dose folic acid intervention in improving vascular endothelial function in aged human skin. In the past, our laboratory group has demonstrated that the effects of systemic treatments can be detected in the cutaneous vasculature [38,39]. Specifically, we have recently shown that acutely increasing BH4 bioavailability through oral administration of sapropterin restores NO-dependent vasodilatation in the cutaneous vessels of older adults [13]. Prior studies examining the use of folic acid for improved vascular function suggest that chronic folic acid treatment is capable of improving markers of vessel health in populations with overt cardiovascular disease [14,16,40]. In agreement with these findings, our results suggest that 5 mg of folic acid daily for 6 weeks augments reflex vasodilatation in aged cutaneous vessels throughout heating, and restores skin blood flow to the magnitude observed in young, healthy individuals at the highest temperatures. Furthermore, our results suggest that this increase is mediated through NO-dependent mechanisms.
Our results from study 2 demonstrate that local 5-MTHF perfusion through intradermal microdialysis similarly augments full and NO-dependent reflex vasodilatation in aged skin after placebo treatment. These results are in agreement with the findings from study 1, which suggest that local 5-MTHF increases NO synthesis in the aged cutaneous vasculature. However, this localized perfusion did not further increase the magnitude of the full or NO-dependent cutaneous vasodilatation response observed with folic acid treatment in study 2. In addition, there were no differences in full or NO-dependent reflex vasodilatation between the 5-MTHF-perfused microdialysis sites across oral treatments. These data could indicate that (i) the systemic folic acid treatment maximized activity through the NOS pathway such that the enzyme was working at or near its maximum velocity, Vmax, and/or (ii) we had reached a ceiling effect for the ability of the aged cutaneous vessels to vasodilate during hyperthermia. Taken together, these data suggest that systemic folic acid treatment increases plasma folate and/or 5-MTHF sufficiently to increase NO production through NOS.
In summary, folic acid and its metabolite, 5-MTHF, increase cutaneous vasodilatation in older people through NO-dependent mechanisms. Considering the putative role of NO in vascular health and vessel function, and the observed increase in the magnitude of cutaneous vasodilatation in the present study, folic acid administration may be a clinically relevant intervention strategy for improved cutaneous microvascular function and augmented thermoregulatory skin blood flow through NO-dependent vasodilatation in older adults.
CLINICAL PERSPECTIVES.
This study was conducted to determine if folic acid may be a viable intervention strategy for improved microvascular vasodilator function in older adults.
Our results suggest that folic acid and its metabolite, 5-MTHF, increase cutaneous vasodilatation in older adults through NO-dependent mechanisms.
Considering the protective role of NO in vascular health and the increase in NO-dependent vasodilatation observed in the present study, folic acid may be a viable intervention strategy for improved or maintained cutaneous microvascular function in older adults.
ACKNOWLEDGEMENTS
The authors are grateful to the participants for their time and effort and to Susan Slimak, RN, Jane Pierzga, MS and Jody Greaney, PhD for their assistance. We are also grateful to the Penn State Hershey College of Medicine Mass Spectrometry and Proteomics Core Facility, specifically Dr Bruce Stanley and Dr Dongxiao Sun, for their hard work and assistance with plasma folate and 5-MTHF measurements.
FUNDING
This research was supported by the National Institutes of Health [R01 AG-07004–23].
Abbreviations:
- 5-MTHF
5-methyltetrahydrofolate
- BH2
dihydrobiopterin
- BH4
tetrahydrobiopterin
- BMI
body mass index
- COX
cyclo-oxygenase
- CVC
cutaneous vascular conductance
- eNOS
endothelial nitric oxide synthase
- FDA
Food and Drug Administration
- LDF
laser/Doppler flowmetry
- l-NAME
NG-nitro-l-arginine methyl ester
- MAP
mean arterial pressure
- NO
nitric oxide
- NOS
nitric oxide synthase
REFERENCES
- 1.Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL and Minson CT (2003) Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am. J. Physiol. Heart Circ. Physiol 284, H1662–H1667 [DOI] [PubMed] [Google Scholar]
- 2.Kenney WL, Morgan AL, Farquhar WB, Brooks EM, Pierzga JM and Derr JA (1997) Decreased active vasodilator sensitivity in aged skin. Am J. Physiol 272, H1609–H1614 [DOI] [PubMed] [Google Scholar]
- 3.Minson CT, Holowatz LA, Wong BJ, Kenney WL and Wilkins BW (2002) Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J. Appl. Physiol 93, 1644–1649 [DOI] [PubMed] [Google Scholar]
- 4.Stanhewicz AE, Bruning RS, Smith CJ, Kenney WL and Holowatz LA (2012) Local tetrahydrobiopterin administration augments reflex cutaneous vasodilation through nitric oxide-dependent mechanisms in aged human skin. J. Appl. Physiol 112, 791–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delp MD, Behnke BJ, Spier SA, Wu G and uller-Delp JM (2008) Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J. Physiol 586, 1161–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holowatz LA, Thompson CS and Kenney WL (2006) L-Arginine supplementation or arginase inhibition augments reflex cutaneous vasodilatation in aged human skin. J. Physiol 574, 573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holowatz LA, Thompson CS and Kenney WL (2006) Acute ascorbate supplementation alone or combined with arginase inhibition augments reflex cutaneous vasodilation in aged human skin. Am. J. Physiol. Heart Circ. Physiol 291, H2965–H2970 [DOI] [PubMed] [Google Scholar]
- 8.Lu CY, Lee HC, Fahn HJ and Wei YH (1999) Oxidative damage elicited by imbalance of free radical scavenging enzymes is associated with large-scale mtDNA deletions in aging human skin. Mutat. Res 423, 11–21 [DOI] [PubMed] [Google Scholar]
- 9.Crabtree MJ and Channon KM (2011) Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide 25, 81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werner ER, Blau N and Thony B (2011) Tetrahydrobiopterin: biochemistry and pathophysiology. Biochem. J 438, 397–414 [DOI] [PubMed] [Google Scholar]
- 11.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P and Pritchard KA Jr. (1998) Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc. Natl. Acad. Sci. U.S.A 95, 9220–9225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugiyama T, Levy BD and Michel T (2009) Tetrahydrobiopterin recycling, a key determinant of endothelial nitric-oxide synthase-dependent signaling pathways in cultured vascular endothelial cells. J. Biol. Chem 284, 12691–12700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanhewicz AE, Alexander LM and Kenney WL (2013) Oral sapropterin acutely augments reflex vasodilation in aged human skin through nitric oxide-dependent mechanisms. J. Appl. Physiol 115, 972–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alian Z, Hashemipour M, Dehkordi EH, Hovsepian S, Amini M, Moadab MH and Javanmard SH (2012) The effects of folic acid on markers of endothelial function in patients with type 1 diabetes mellitus. Medicinski Arhiv 66, 12–15 [DOI] [PubMed] [Google Scholar]
- 15.Doshi SN, McDowell IF, Moat SJ, Lang D, Newcombe RG, Kredan MB, Lewis MJ and Goodfellow J (2001) Folate improves endothelial function in coronary artery disease: an effect mediated by reduction of intracellular superoxide? Arterioscler. Thromb. Vasc. Biol 21, 1196–1202 [DOI] [PubMed] [Google Scholar]
- 16.Verhaar MC, Wever RM, Kastelein JJ, van Dam T, Koomans HA and Rabelink TJ (1998) 5-methyltetrahydrofolate, the active form of folic acid, restores endothelial function in familial hypercholesterolemia. Circulation 97, 237–241 [DOI] [PubMed] [Google Scholar]
- 17.Stroes ES, van Faassen EE, Yo M, Martasek P, Boer P, Govers R and Rabelink TJ (2000) Folic acid reverts dysfunction of endothelial nitric oxide synthase. Circ. Res 86, 1129–1134 [DOI] [PubMed] [Google Scholar]
- 18.Antoniades C, Shirodaria C, Warrick N, Cai S, de Bono J, Lee J, Leeson P, Neubauer S, Ratnatunga C, Pillai R et al. (2006) 5-methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation 114, 1193–1201 [DOI] [PubMed] [Google Scholar]
- 19.Bruning RS, Santhanam L, Stanhewicz AE, Smith CJ, Berkowitz DE, Kenney WL and Holowatz LA (2012) Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin. J. Appl. Physiol 112, 2019–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minson CT, Berry LT and Joyner MJ (2001) Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J. Appl. Physiol 91, 1619–1626 [DOI] [PubMed] [Google Scholar]
- 21.Johnson JM, O’Leary DS, Taylor WF and Kosiba W (1986) Effect of local warming on forearm reactive hyperaemia. Clin. Physiol 6, 337–346 [DOI] [PubMed] [Google Scholar]
- 22.Doshi SN, McDowell IF, Moat SJ, Payne N, Durrant HJ, Lewis MJ and Goodfellow J (2002) Folic acid improves endothelial function in coronary artery disease via mechanisms largely independent of homocysteine lowering. Circulation 105, 22–26 [DOI] [PubMed] [Google Scholar]
- 23.Bellamy MF, McDowell IF, Ramsey MW, Brownlee M, Newcombe RG and Lewis MJ (1999) Oral folate enhances endothelial function in hyperhomocysteinaemic subjects. Eur. J. Clin. Invest 29, 659–662 [DOI] [PubMed] [Google Scholar]
- 24.Chao CL, Chien KL and Lee YT (1999) Effect of short-term vitamin (folic acid, vitamins B6 and B12) administration on endothelial dysfunction induced by post-methionine load hyperhomocysteinemia. Am. J. Cardiol 84, 1359–1361, A1358 [DOI] [PubMed] [Google Scholar]
- 25.Loew D, Eberhardt A, Heseker H and Kubler W (1987) [Plasma kinetics and elimination of folic acid]. Klin. Wochenschr 65, 520–524 [DOI] [PubMed] [Google Scholar]
- 26.Willems FF, Boers GH, Blom HJ, Aengevaeren WR and Verheugt FW (2004) Pharmacokinetic study on the utilisation of 5-methyltetrahydrofolate and folic acid in patients with coronary artery disease. Br. J. Pharmacol 141, 825–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stern SJ, Matok I, Kapur B and Koren G (2011) A comparison of folic acid pharmacokinetics in obese and nonobese women of childbearing age. Therapeut. Drug Monit 33, 336–340 [DOI] [PubMed] [Google Scholar]
- 28.Aoki K, Kondo N, Shibasaki M, Takano S and Katsuura T (1997) Circadian variation in skin blood flow responses to passive heat stress. Physiol. Behav 63, 1–5 [DOI] [PubMed] [Google Scholar]
- 29.Stanhewicz A, Alexander LM and Kenney WL (2013) Oral sapropterin acutely augments reflex vasodilation in aged human skin through nitric oxide-dependent mechanisms. J. Appl. Physiol 15, 972–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holowatz LA, Thompson-Torgerson CS and Kenney WL (2008) The human cutaneous circulation as a model of generalized microvascular function. J. Appl. Physiol 105, 370–372 [DOI] [PubMed] [Google Scholar]
- 31.RG IJ, de Jongh RT, Beijk MA, van Weissenbruch MM, Delemarre-van de Waal HA, Serne EH and Stehouwer CD (2003) Individuals at increased coronary heart disease risk are characterized by an impaired microvascular function in skin. Eur. J. Clin. Invest 33, 536–542 [DOI] [PubMed] [Google Scholar]
- 32.Abularrage CJ, Sidawy AN, Aidinian G, Singh N, Weiswasser JM and Arora S (2005) Evaluation of the microcirculation in vascular disease. J. Vasc. Surg 42, 574–581 [DOI] [PubMed] [Google Scholar]
- 33.McCord GR, Cracowski JL and Minson CT (2006) Prostanoids contribute to cutaneous active vasodilation in humans. Am. J. Physiology. Regul. Integr. Comp. Physiol 291, R596–R602 [DOI] [PubMed] [Google Scholar]
- 34.Wong BJ, Wilkins BW and Minson CT (2004) H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J. Physiol 560, 941–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennett LA, Johnson JM, Stephens DP, Saad AR and Kellogg DL Jr (2003) Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature of humans. J. Physiol 552, 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holowatz LA, Jennings JD, Lang JA and Kenney WL (2009) Ketorolac alters blood flow during normothermia but not during hyperthermia in middle-aged human skin. J. Appl. Physiol 107, 1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanhewicz AE, Bruning RS, Smith CJ, Kenney WL and Holowatz LA (2012) Local tetrahydrobiopterin administration augments reflex cutaneous vasodilation through nitric oxide-dependent mechanisms in aged human skin. J. Appl. Physiol 112, 791–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holowatz LA, Santhanam L, Webb A, Berkowitz DE and Kenney WL (2011) Oral atorvastatin therapy restores cutaneous microvascular function by decreasing arginase activity in hypercholesterolaemic humans. J. Physiol 589, 2093–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruning RS, Dahmus JD, Kenney WL and Alexander LM (2013) Aspirin and clopidogrel alter core temperature and skin blood flow during heat stress. Med. Sci. Sports Exerc 45, 674–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lekakis JP, Papamichael CM, Papaioannou TG, Dagre AG, Stamatelopoulos KS, Tryfonopoulos D, Protogerou AD, Stamatelopoulos SF and Mavrikakis M (2004) Oral folic acid enhances endothelial function in patients with hypercholesterolaemia receiving statins. Eur. J. Cardiovasc. Prevent. Rehab 11, 416–420 [DOI] [PubMed] [Google Scholar]


