Abstract
Prospective longitudinal studies of idiopathic autism spectrum disorder (ASD) have provided insights into early symptoms and predictors of ASD during infancy, well before ASD can be diagnosed at age 2–3 years. However, research on the emergence of ASD in disorders with a known genetic etiology, contextualized in a developmental framework, is currently lacking. Using a biobehavioral multi-method approach, we 1) determined the rate of ASD in N=51 preschoolers with FXS using a clinical best estimate (CBE) procedure with differential diagnoses of co-morbid psychiatric disorders and 2) investigated trajectories of ASD symptoms and physiological arousal across infancy as predictors of ASD in preschoolers with FXS. ASD was not diagnosed if intellectual ability or psychiatric disorders better accounted for the symptoms. Our results determined that 60.7% of preschoolers with FXS met DSM-5 criteria for ASD using the CBE procedure. In addition, 92% of these preschoolers presented with developmental delay and 45.4% also met criteria for psychiatric disorders, either anxiety, ADHD, or both. ASD diagnoses in preschoolers with FXS were predicted by elevated scores on traditional ASD screeners in addition to elevated autonomic arousal and avoidant eye contact from infancy.
Given the clinical heterogeneity within and across children with autism spectrum disorder (ASD), studies of identified genetic syndromes can advance the ASD field by identifying multiple causal pathways that lead to a high expression of ASD traits and likelihood of diagnosis. Fragile X syndrome (FXS) is a neurodevelopmental disorder that is the leading known monogenic cause of ASD (Simberlund & Veenstra-VanderWeele, 2019). The association of ASD with FXS was first documented over 30 years ago, sparking a debate that continues to date about whether ASD in FXS represents “true ASD” or if ASD symptoms are inherent to the FXS phenotype and whether a categorical diagnostic approach should be adopted in contrast to a symptom-based approach (Abbeduto, McDuffie, & Thurman, 2014; Hall, Lightbody, Hirt, Rezvani, & Reiss, 2010; Roberts et al., 2018). Contributing to this debate in the field of FXS, the present study adopts a deep phenotyping approach to characterize the profiles of preschoolers with FXS with a focus on ASD using a differential diagnostic approach that accounts for intellectual disability (ID), anxiety and attention deficit disorder. Despite different positions on the consideration of ASD in FXS, there is widespread agreement that research addressing the association of ASD in FXS is complex, multifaceted, and critical in advancing identification and treatment of these disorders.
This work also contributes to the ASD field as there is clear evidence that ASD is not a singular category but is a complex disorder with variation in terms of intellectual profiles, sex-specific factors and a high degree of psychiatric co-morbidity (Lundstrom et al., 2011). Given increasing recognition of the complexity of ASD, diagnostic practice has switched from viewing aspects of the disorder, such as intellectual impairment and anxiety symptoms, as part of ASD to being identified as co-morbid conditions (Gargaro, Rinehart, Bradshaw, Tonge, & Sheppard, 2011; Jacob et al., 2019). There is also consideration of diagnosing ASD as a primary versus secondary disorder as in the case of ID where ID might be considered the primary disorder and ASD as a secondary disorder (Thurm, Farmer, Salzman, Lord, & Bishop, 2019). Thus, the field of ASD is also grappling with how to best conceptualize the complexities associated with a diagnosis of ASD much as the field of FXS is.
While identification of multiple co-morbid disorders within ASD has increased, there are a number of challenges that have theoretical, clinical and research implications. From a theoretical standpoint, diagnoses can only be “valid” if the symptoms between multiple disorders are discernable and independent. For example, social-communication impairment (common among children with ID) must be greater than expected for the individual’s mental age in order to be considered diagnostic feature of ASD not better accounted for by ID (Thurm et al., 2019). There are also a number of clinical implications. Treatment of ASD with ID versus ASD without ID versus ID alone can vary considerably and is likely to affect outcomes. Refinement of early risk factors for ASD and comorbid psychiatric disorders, especially in individuals presenting with ID and/or known genetic syndromes, such as FXS, will lead to tailored treatment plans and contribute to individualized, precision medicine. Finally, research efforts are accelerated when multiple disorders or symptoms are identified as the higher level of specificity in participant characterization allows for more advanced interpretation of the individual studies themselves as well as improved translation of findings across studies.
Using a deep phenotyping approach, we identified the proportion of preschoolers with FXS who met DSM-5 criteria for ASD while accounting for comorbid diagnoses of ID, anxiety, and attention deficit hyperactivity disorder (ADHD). We also characterized the timing and expression of the emergence of ASD symptoms from infancy as they predicted a diagnosis of ASD in FXS. No studies to date have implemented a differential diagnostic approach to identification of ASD in FXS through consideration of multiple psychiatric disorders in preschool children. This line of research is critical to refine both the FXS and non-syndromic (nsASD) phenotypic profiles and to identify the shared and unique features across these two disorders. Understanding this relationship provides important clues about one potential cause of ASD and potential treatments. Thus, this work contributes to the nosology of ASD with important implications for causal mechanisms and behavioral and psychopharmacological treatment for nsASD, FXS, and other disorders that share features.
Given the importance of examining the association of ASD in FXS, a great deal of research has focused on examining the nature of this relationship. Differences in how ASD is conceptualized in the FXS field stem, in part, from recognition of the complexity of the FXS phenotype that includes intellectual impairment, anxiety, social avoidance, autonomic hyperarousal, and hyperactivity, all of which are commonly associated with ASD in non-syndromic cases. Some have argued that ASD in FXS is not a distinct disorder but represents part of the FXS phenotype (Hall et al., 2010) with suggestions that ASD in FXS is not “true ASD” but is a reflection of other symptoms and disorders (e.g., intellectual impairment and anxiety most notably). One of the challenges to this position is that ASD is diagnosed based on a set of discrete behavioral symptoms that can be determined independent of a known genetic diagnosis. In other words, if an individual’s behavioral presentation includes core symptoms of ASD then diagnostic criteria for ASD are met. Thus, the diagnosis of ASD in FXS is posited as a valid and discrete disorder. ASD is not diagnosed in all individuals with FXS, only in those with sufficiently elevated symptoms, which supports that a diagnosis of ASD conveys important phenotypic information that is highly relevant for clinical service eligibility and identification of appropriate treatment targets. In addition, this work refines the FXS phenotype and advances knowledge about genetic causes of ASD. However, even when an ASD diagnostic determination is viewed as valid and important in persons with FXS, some have argued that a symptom-based approach is the better alternative to a categorical diagnosis
A number of studies have supported that ASD is a distinct disorder that can be disassociated from other co-occurring disorders in FXS (Bailey, Raspa, Olmsted, & Holiday, 2008; Hagerman et al., 2018; Rogers, Wehner, & Hagerman, 2001). This work has shown that ASD is highly prevalent in FXS affecting 60–75% of males and 20–41% of females (Abbeduto et al., 2019; Kaufmann et al., 2017; Klusek, Martin, & Losh, 2014; Lee, Martin, Berry-Kravis, & Losh, 2016). And, while intellectual impairment, anxiety, and social avoidance may be elevated in individuals with FXS and ASD (FXS+ASD) contrasted to those with FXS who do not have ASD (FXSonly), these disorders are distinct.
While different positions on the consideration of ASD in FXS exist, it is clear that ASD is highly associated with FXS and that discoveries about the emergence, developmental course and expression of ASD in FXS are critical. The timing and targets of treatment, for example, are highly reliant on information regarding the association of ASD in FXS for applications to both FXS and nsASD populations. Behavioral treatment has clearly shown benefit for young children suspected or documented to have nsASD (Warren et al., 2011); however, application of these treatments to FXS has not yet been systematically employed. Likewise, a large number of pharmacological trials have focused on FXS given its fairly well-characterized genotype and potential to translate to individuals with nsASD (Berry-Kravis et al., 2012; Hagerman, Hoem, & Hagerman, 2010). The extent to which ASD in FXS represents the same or unique underlying mechanisms and symptom profiles as those with nsASD is critical to inform these efforts across both nsASD and FXS fields. This is particularly important given that individuals with FXS+ASD typically experience poorer overall adaptive, cognitive, and language skills and lower quality of life (Abbeduto et al., 2014; Bailey et al., 2008).
Studies regarding the association of ASD in FXS also contribute to important theoretical advances as well as to unpacking the neurobiology of different neurodevelopmental disorders. The field of developmental psychopathology has long recognized the importance of considering both equifinality, multiple causal pathways leading to similar outcomes, and multifinality, different outcomes resulting from similar or equivalent pre-existing vulnerabilities, in research addressing complex disorders (Beauchaine, Constantino, & Hayden, 2018; Cicchetti & Rogosch, 1996). Our study delineates unique developmental pathways for individuals with a shared genetic vulnerability that result in variation of final outcomes, including ASD, anxiety, and/or ADHD. Critically however, the degree to which these individuals share the same genetic vulnerability is arguable as there are important variations in genetic structure in FXS including methylation and size mosaicism along with activation ratio in females. Thus, even a single gene disorder that is considered a simple monogenic model for ASD is far from simple.
This work advancing knowledge on the association of ASD in FXS, however, has faced a number of barriers. First, there are several measurement issues. In the nsASD field, a clinical best estimate (CBE) procedure, a diagnostic approach in which gold standard diagnostic measures are utilized in tandem with clinician input, has been clearly articulated and is widely adopted. This diagnostic approach is described as particularly critical when diagnosing ASD in the context of intellectual impairment (Thurm et al., 2019). In contrast, the diagnostic determination of ASD in the FXS field is variable including informal parent report, global clinician ratings, screening measures, and reliance on diagnostic cutoff scores without consideration of clinical judgment in some cases (Abbeduto et al., 2014; Hazlett et al., 2012; Kaufmann et al., 2017; Lee et al., 2016; Wheeler et al., 2015). These discrepancies in ASD diagnostic procedures across the FXS and nsASD fields constrain efforts to integrate findings which has important implications for identification and treatment. Parent report of ASD diagnostic status in males with FXS, for example, appear to be largely discrepant from detailed research diagnoses (Klusek et al., 2014). Furthermore, reliance on score cut-offs to diagnose ASD in the absence of clinical judgment is made especially problematic by the fact that gold standard instruments [e.g., the Autism Diagnostic Observation Scale™-2, ADOS-2 (Lord, Rutter, et al., 2012) and Autism Diagnostic Interview™-Revised (ADI-R; Lord, Rutter, & Le Couteur, 1994)] have not been specifically validated or adapted for use in FXS. Also, the sensitivity and specificity of the ADOS-2 and ADI-R are reduced when applied to persons with a mental age of 18 months or lower and when co-occurring psychiatric disorders are present (Havdahl et al., 2016). Thus, the valid use of the ADOS-2 and ADI-R to detect ASD in FXS remains unknown highlighting the importance of clinical judgement given the overlap of features of intellectual impairment, psychiatric co-morbidity and ASD. Finally, the debate of the association of ASD in FXS has been largely based on DSM-IV TR conceptualization of ASD, which categorized ASD as multiple distinct disorders (e.g., autistic disorder, Asperger’s disorder). Reconceptualization of ASD in the DSM-5 has resulted in a single disorder with a spectrum of impairment. No studies to date have used DSM-5 criteria within the context of expert clinical judgement to diagnose ASD in preschoolers with FXS.
A second challenge to the study of ASD in FXS is a lack of or limited consideration of competing or complementary diagnoses when determining a diagnosis of ASD. A competing diagnostic approach is one in which ASD is diagnosed only if the features are not better attributed to another disorder (e.g., it is determined that the features are better accounted for by a diagnosis of ASD rather than of anxiety, criterion E of the DSM-5). A complementary diagnostic approach also considers multiple disorders; however, not only are other disorders ruled out, but other disorders are also ruled in (e.g., both ASD and anxiety are diagnosed if criteria for both disorders are present and distinguishable). The inclusion of competing or complementary ASD diagnostic approaches is becoming increasingly important given evidence that up to 90% of young children with ASD have at least one co-occurring psychiatric disorder (Salazar et al., 2015; Simonoff et al., 2008). The validity and importance of including co-morbid diagnoses is demonstrated by the fact that the DSM-5 now supports the co-diagnosis of ASD and ADHD. Anxiety and ADHD have received much of the focus on co-morbidities with 78.9% of young children with ASD diagnosed with at least one anxiety disorder and 59.1% diagnosed with ADHD (Salazar et al., 2015). Importantly, the presence of a co-morbid psychiatric disorder is associated with increased impairment and distress (Kerns, Newschaffer, & Berkowitz, 2015) and negatively affects the specificity of the primary ASD diagnostic tools (Havdahl et al., 2016).
Increasing awareness that symptoms of ASD cross diagnostic boundaries calls for careful consideration of potentially confounding factors and multiple competing or complementary diagnoses. Differential diagnostic determination is extremely challenging, however, and can only be done by administering an extensive battery of in-depth measures with interpretation by highly trained clinicians who have expertise in multiple fields and disorders. Without such a deep phenotyping approach, diagnostic determination that ASD in FXS is an independent or co-morbid condition that is not better accounted for by other factors including intellectual impairment, anxiety, and/or ADHD cannot be accomplished.
The final limitation is that research examining the association of ASD in FXS has typically lacked a developmentally informed approach. To date, there are only a handful of studies that have characterized ASD features in FXS across age, and most of this work has been done with school-age children or older individuals (Hernandez et al., 2009; Lee et al., 2016; McDuffie et al., 2010). In one of the few studies to report ASD diagnoses over time in preschool-aged children with FXS, results indicated that 30.2% of the FXS sample (16 of n=53) met criteria for ASD as defined by having scores from both the ADOS and ADI-R above the cutoff with a slight increase to 33.3% (13 of the n=39) for children who had a second assessment approximately two years later (Hazlett et al., 2012). Still, these studies used instrument cutoffs rather than a comprehensive clinical diagnostic approach, like CBE.
Data regarding trajectories of ASD features that emerge in the first few years of life and predict ASD diagnoses in FXS are also quite limited. Preliminary studies indicate that behavioral features of ASD are present and detectible in 53% of 12-month-old infants with FXS (8 out of n=15) and that motor and social-communication features appear to be salient features that signal risk for elevated ASD symptoms over the first 12 to 18 months (Brewe, Reisinger, Adlof, & Roberts, 2018; Hogan et al., 2017; Rague, Caravella, Tonnsen, Klusek, & Roberts, 2018; Roberts, Tonnsen, McCary, Caravella, & Shinkareva, 2016; Will, Bishop & Roberts, 2019). Social avoidance has also been documented in the first year of life in infants with FXS with a steady increase across early childhood (Roberts, Crawford, Hogan, et al., 2019). Of note, elevated social avoidance across the infant and toddler years differentially predicted increased severity of ASD symptoms, but not ADHD or anxiety symptoms, at preschool age (Roberts, Crawford, Will, et al., 2019).
Physiological hyperarousal has also been noted as an important feature of ASD in FXS (Roberts, Tonnsen, Robinson, & Shinkareva, 2012). The “hyperarousal hypothesis” in FXS refers to physiological dysregulation that reflects poor biological competence to address cognitive, behavioral and affective demands. To this end, several studies have examined autonomic function in infants and young children with FXS as indexed by cardiac function including respiratory sinus arrhythmia (RSA) and inter-beat-interval (IBI). Cross-sectional work with small samples has indicated that elevated baseline IBI and reduced RSA across the first years of life predicted more severe ASD features at preschool (Roberts et al., 2012) and was associated with elevated social fear in toddlers and preschoolers with FXS (Tonnsen, Shinkareva, Deal, Hatton, & Roberts, 2013). Also, elevated ASD features have been linked to less efficient heart rate deceleration during a visual attention task (Tonnsen, Richards, & Roberts, 2018).
In addition to the increasing number of behavioral and autonomic studies identifying early signs of ASD in FXS, are a series of studies characterizing the neurophenotype, i.e., unique patterns of brain development and nervous system organization, in young children with FXS. This work has documented two distinct clinically meaningful FXS neurophenotypes with one group demonstrating lower adaptive and developmental skills, higher ASD symptoms and larger brain volume (Bruno et al., 2017). Neurodevelopmental effects appear to emerge early with evidence of aberrant white matter pathways present by 6 months-of-age that are associated with lower developmental level (Swanson et al., 2018).
In summary, ASD is strongly associated with FXS. However, the nature of this relationship has been challenging to disentangle given the complexity of the FXS phenotype and multiple competing theoretical perspectives on the underlying mechanisms that account for elevated ASD in FXS. To date, there is a substantial gap in what is known about the prevalence of ASD in preschool children using a differential diagnostic approach considering complementary and competing psychiatric diagnoses via a CBE procedure. Likewise, no published work has reported the presence of both ASD-specific and more generalized broad-based early markers of ASD across infancy in FXS.
The over-arching aim of the present study is to report the proportion of preschoolers with FXS who meet diagnostic criteria for ASD and how early markers of ASD during infancy predict ASD diagnoses in preschool children with FXS implementing a CBE procedure with data across multiple levels of analysis. We address this aim by conducting a series of analyses that focus on different aspects of this aim. First, we examined the manifestation of ASD in preschoolers with FXS using a CBE procedure that rules out intellectual disability and psychiatric diagnoses as primary determinants of the ASD features. Related to this rigorous diagnostic process, we consider the degree of confidence in the differential diagnosis of ASD and determine (secondary/additional) psychiatric diagnoses. Second, we determined whether the initial level or rate of change in ASD symptomology and associated phenotypic features predicted an ASD outcome while controlling for intellectual ability (i.e., nonverbal cognition). Finally, we examined the arousal hypothesis by evaluating the relationship of initial level and trajectory of baseline IBI and RSA as a predictor of ASD diagnostic outcomes.
Methods
Participants
Participants included 54 children (15 females, 39 males) with fragile X syndrome (FXS). Data for these participants were collected as part of a large-scale longitudinal study of emergent ASD symptoms in infants and preschoolers in high-risk infants (R01MH90194, R01MH107573). Inclusion criteria included (a) full mutation FXS (≥ 200 CGG repeats on the FMR1 gene), confirmed by genetic report provided by the parent; (b) ≥ 36 weeks gestation; and (c) English as the primary language spoken in the home. Children with uncorrected vision/hearing impairments or other serious medical conditions (e.g., congenital heart disorders, birth-related trauma, brain injury) were excluded from the study. Of the larger sample, 51 children (14 females, 37 males) received a CBE outcome determination which typically occurred at 36-months of age or older. Consistent with the phenotype, the majority (92%) had a developmental delay. Table 1 outlines participant characteristics.
Table 1.
Characteristics of Children with CBE Outcome Data.
| All n = 51 |
FXS+ASD n = 31 |
FXSonly n = 20 |
|
|---|---|---|---|
| Sex (male), n(%) | 37 (72.55%) | 27 (87.10%) | 10 (50.00%) |
| Age at outcome1 (months), M(SD), range | 45.25 (10.79) | 46.14 (11.54) | 43.88 (9.66) |
| 25.36 – 76.20 | 25.36 – 76.20 | 34.50 – 69.00 | |
| NVDQ at outcome, M(SD), range | 57.76 (20.13) | 47.92 (16.39) | 72.52 (15.92) |
| 15.57 – 108.42 | 15.57 – 81.00 | 44.93 – 108.42 | |
| Comorbid developmental delay, n(%) | 47 (92%) | 30 (97%) | 17 (85%) |
| Ethnicity, n(%) | |||
| Hispanic/Latino | 2 (3.92%) | 1 (3.23%) | 1 (5.00%) |
| Not Hispanic/Latino | 45 (88.24%) | 27 (87.10%) | 18 (90.00%) |
| Unknown | 4 (7.84%) | 3 (9.68%) | 1 (5.00%) |
| Race | |||
| American Indian/Alaska Native | 2 | 1 | 1 |
| Black | 3 | 1 | 2 |
| Hispanic | 2 | 1 | 1 |
| White | 32 | 21 | 11 |
| More than One Race | 10 | 5 | 5 |
| Unknown | 2 | 2 | 0 |
Outcome = age at which participants were given a CBE diagnosis
Procedures
Participants were typically recruited into the study in infancy, although a small subset entered the study later in development due to later diagnosis of FXS. To reduce ascertainment bias towards elevated ASD features, the study was advertised as focused on early development with no mention of ASD in the recruitment materials. Standard assessment timepoints included 6 months, 9 months, 12 months, 18 months (parent-report questionnaires only), 24 months, 36 months, 48 months, 60 months, and 72 months. Participants were recruited nationally through social media and collaborations with existing research groups for a study focusing on broad development and not an ASD-specific focus. Assessments were conducted in participants’ homes or at the Neurodevelopmental Disorders Laboratory at the University of South Carolina, based on parent preference. Parents were reimbursed for their time and for any travel expenses incurred as part of their participation. Parents provided informed consent upon enrollment. All procedures were approved by the Institutional Review Board at the University of South Carolina.
Diagnostic Outcome Determination: Clinical Best Estimate Procedures
A primary diagnosis of ASD v. non-ASD outcomes, along with presence of absence of developmental delay, and secondary diagnoses of comorbid psychiatric disorders (e.g., anxiety, ADHD) were assigned via CBE procedures. Because ASD diagnosis has been shown to be more stable at 36 months or later (Ozonoff et al., 2015) and to address challenges associated with low mental age (Thurm et al., 2019), CBE diagnoses in the present study were included for the first timepoint at or after 36 months of age. One participant did not have CBE data from a timepoint at or after 36 months of age, so his CBE diagnosis from his 24-month timepoint is included. The CBE was led by a licensed psychologist (last author) with expertise in differential diagnosis of ASD and anxiety disorders in young children (e.g., she is at the trainer level for the ADOS-2).
Primary (ASD) Diagnosis.
A CBE diagnosis of ASD, Subthreshold ASD, Non-ASD Developmental Delay, or No Clinical Features was assigned via extensive case review. The CBE diagnostic procedure was adapted from standard procedures (Lord, Petkova, et al., 2012; Lord et al., 2006) and has been used by our team (Hogan et al., 2017; Will, Bishop, & Roberts, 2019). CBE diagnoses were determined by a multidisciplinary team (3 to 5 members) with training in clinical-community psychology, school psychology, applied developmental science and communication science. The CBE team was led by a licensed psychologist who was masked to the FX diagnostic status of the child as well as individuals who conducted the assessments who were not masked. While the lead psychologist was masked to the group status and was not informed that the child had FXS (the CBE process includes multiple groups of children including those with typical development, Down syndrome, and the FMR1 premutation), it is assumed that group status could be ascertained by the facial and other phenotypic features of the target child. All team members were research reliable on the ADOS-2 (Lord, Rutter, et al., 2012).
Using a multi-method and comprehensive approach, the following information was used to inform the CBE diagnosis: cognitive abilities as measured by the Mullen Scales of Early Learning (MSEL) (Mullen, 1995); adaptive functioning as measured by the Vineland Adaptive Behavior Scale-2nd Edition (VABS-2) (Sparrow, Cicchetti, & Balla, 2005); and ASD symptom severity as measured by the ADOS-2 (Lord, Rutter, et al., 2012), ADI-R (Lord et al., 1994), and Childhood Autism Rating Scale – Second Edition (CARS-2; Schopler, Van Bourgondien, Wellman, & Love, 2010). Details on psychiatric features (anxiety, ADHD) were also reviewed as detailed below. Only concurrent information was used for the CBE diagnoses, the CBE team did not have access to data from earlier or later assessments.
To receive an ASD diagnosis, a child was required to meet DSM-5 criteria for ASD (American Psychiatric Association, 2013). Also, consistent with the DSM-5, the diagnosis of ASD was only applied when the features of ASD were not better accounted for by developmental delay or other psychiatric diagnoses such as anxiety or ADHD. Children who did not meet diagnostic criteria for ASD were assigned to one of the following categories: (a) Subthreshold ASD, defined as having at least two DSM-5 features of ASD not better accounted for by developmental delay, but not meeting full DSM-5 diagnostic criteria for ASD; (b) Non-ASD Developmental Delay, defined as exhibiting fewer than two DSM-5 features of ASD and at least two MSEL domain t-scores of ≤ 35 (i.e., ≥ 1.5 standard deviations (SDs) below the normed means); or (c) No Clinical Features of ASD or developmental delay, defined as fewer than two DSM-5 features of ASD and fewer than two Mullen domain t-scores of ≤ 35. A child classified with ASD or Subthreshold ASD could also be diagnosed with Developmental Delay if they also had at least two MSEL domain t-scores of ≤ 35. Diagnostic certainty was indicated as a dichotomous variable with two options: “High degree of certainty” and “reduced certainty”. See Table 1 for participant characteristics at the CBE outcome timepoint.
Secondary (Psychiatric) Diagnoses.
In addition to the CBE of ASD, the following psychiatric diagnoses were also assigned when appropriate: attention deficit/hyperactivity disorder (ADHD), generalized anxiety disorder (GAD), social anxiety, specific phobia, and separation anxiety. Symptoms related to these comorbid psychiatric disorders were assessed via the Preschool Age Psychiatric Assessment (PAPA; Egger et al., 2006), a parent-interview designed for diagnosis of psychiatric disorders in children 2–8 years of age. If the PAPA indicated elevated symptoms for one or more comorbid psychiatric disorders, symptoms were discussed by the CBE team and diagnoses were assigned based on DSM-5 criteria. A child could receive as many comorbid psychiatric diagnoses as deemed appropriate by clinical presentation. For example, a child could receive diagnoses of ADHD, generalized anxiety disorder, and social anxiety if s/he met diagnostic criteria for all of these disorders. PAPA data was available for 44 of the 51 participants. Intellectual disability was not considered a psychiatric diagnosis; however, we report the proportion of participants who meet criteria for an intellectual disability (referred to as developmental delay in this study given the young age), and both ASD and psychiatric diagnoses were only assigned if the symptoms were not better accounted for by developmental delay.
Measures
Nonverbal cognitive ability.
A nonverbal developmental quotient (NVDQ) was computed from the MSEL at the CBE outcome timepoint and as a predictor in all models (Bishop, Guthrie, Coffing, & Lord, 2011; Shumway et al., 2012). The NVDQ was computed as .
ASD Measures.
ASD symptoms were assessed from multiple sources across development. In infancy, the Autism Observation Scale for Infants (AOSI; Bryson, Zwaigenbaum, McDermott, Rombough, & Brian, 2008) Total Score was used to measure early behavioral signs of ASD. The AOSI is a semi-structured play observation designed to identify signs of ASD in infants between the ages of 6 and 18 months. The Total Score is computed by summing 16 item scores and can range from 0–50, with higher scores indicating more severe ASD-related behaviors. A Total Score of 9 or higher indicates elevated ASD risk (Bryson et al., 2008; Zwaigenbaum et al., 2005). The Modified Checklist for Autism in Toddlers (M-CHAT; Robins, Fein, Barton, & Green, 2001) was used to assess parent perceptions of ASD symptoms at 18 and 24 months. The M-CHAT contains 23 items that are scored “absent” or “present” by parents, and a Total Score that ranges from 0–23 is computed by counting the number of items rated “present”.
The ADOS-2 (Lord, Rutter, et al., 2012) was administered as a direct assessment of ASD symptoms to children 24 months and older. The toddler module was used for children who were 24-months old and modules 1 and 2 were used for the remaining participants in compliance with guidelines detailed in the manual. The ADOS-2 is a semi-structured, standardized assessment of communication, social interaction, play, and restricted/repetitive behaviors. In order to compare scores across different modules, calibrated severity scores (CSS) were computed for the Social Affect and Restricted & Repetitive Behaviors domains (Gotham, Pickles, & Lord, 2009). As stated earlier, the ADOS-2 from the 36-month assessment was targeted for use in the CBE with only 1 child not having an assessment at that age. In the predictive longitudinal analyses, however, the 24-month assessments were included to determine if the trajectory of ADOS-2 scores predicted the CBE diagnosis of ASD.
The ADI-R (Rutter, LeCouteur, & Lord, 2003) was administered for children who were 36 months and older. The ADI-R is a standardized caregiver interview that collects information about both past and current symptoms of ASD. The ADI-R scores based on current symptoms were used as part of the CBE as information on past symptoms is not available for children this age. The ADI-R was not included in the longitudinal predictive analyses as there was only a single ADI-R per participant and it was concurrent with the age of the CBE determination.
The CARS-2 (Schopler et al., 2010) was administered as a global evaluation of ASD symptoms across the course of the entire assessment visit at 24 months and later. The CARS-2 consists of 15 items and can be used with children as young as 24 months of age. Scoring of the CARS-2 is based on a clinician’s overall observations of ASD-related behaviors over the course of an assessment as well as information from caregiver reports. Scores range from 15–60, with higher scores indicating more severe ASD symptoms.
Repetitive/Restricted Behaviors and Sensory Features.
Repetitive and restricted behaviors were measured using the Repetitive Behavior Scale – Revised (RBS-R; Bodfish, Symons, Parker, & Lewis, 2000), a 43-item parent-report questionnaire. Each item is scored on a four-point rating scale ranging from 0 (behavior does not occur) to 3 (behavior occurs and is a severe problem). The RBS-R Total Score and five factor scores (Bishop et al., 2013; Moskowitz, Will, Black & Roberts, 2020) were included as predictors in analyses. The Total Score is a sum of all item raw scores. The factor scores were computed using previously published standards (Bishop et al., 2013) and included (a) Sensory Motor, (b) Restricted Interests, (c) Self-injury, (d) Compulsive, and (e) Ritualistic/Sameness.
The Sensory Experiences Questionnaire (SEQ; Baranek, David, Poe, Stone, & Watson, 2006) version 1.0 was also utilized to obtain information on sensory issues specifically. The SEQ is a 21-item parent-report questionnaire designed to assess behavioral responses to sensory experiences (e.g., stares at lights/objects, ignores loud sounds). Items are scored on 5‐point Likert scale based on the frequency of occurrence of a sensory experience, ranging from 0 (Almost Never) to 4 (Almost Always). An overall mean score ranging from 0–4 is derived by averaging all item scores.
Social Avoidance/Eye Contact.
The Social Avoidance Scale (SAS) Eye Contact subscale (Roberts, Crawford, Hogan, et al., 2019; Roberts, Crawford, Will, et al., 2019; Roberts, Weisenfeld, Hatton, Heath, & Kaufmann, 2007) was used to measure eye contact avoidance at the end of each assessment visit. For the rating, the research team observes and records the participant’s response to their interactions across the final hour of interaction once that child has become familiar with them (details on reliability, validity, and procedure are in Roberts, Crawford, Hogan, et al., 2019). The eye contact subscale ranges from 0 “age appropriate eye contact” to 5 “no eye contact at all”; thus, higher ratings indicate more avoidant behavior.
Physiological Indices.
Heart activity was recorded during a 3-minute baseline period during which the child sat quietly and watched a video. Electrocardiogram (i.e., ECG) data were recorded using a telemetry-based system (Alive Technologies, Copyright 2005–2009; Actiwave Cardio Monitor, CamNtech Ltd., Cambridge, UK). Mean values for respiratory sinus arrhythmia (RSA) and interbeat interval (IBI) were extracted using the CardioBatch software (Brain-Body Center, University of Illinois at Chicago; Porges & Bohrer, 1990). IBI is defined as the mean time elapsed (in seconds) between the R-peaks of each heartbeat waveform (i.e., R-R interval). Respiratory sinus arrhythmia (RSA) reflects the influence of respiration on heart rate variability associated with the rest and restorative functions of the parasympathetic system. Longer IBI and elevated RSA have been associated with optimal emotional regulation and social engagement (e.g., Heilman et al., 2008; Patriquin, Hartwig, Friedman, Porges, & Scarpa, 2019).
Analytic Approach
Descriptive analyses were used to determine the rate of ASD and comorbid psychiatric diagnoses across the sample. For all analyses, we used R Version 3.6.1 (R Core Team, 2019) and Mplus Version 8 (Muthen & Muthen, 2017). We used two statistical models to test our hypotheses. In order to identify predictors of ASD in preschoolers with FXS, we tested the effect of initial levels (i.e. intercepts) and linear trajectory of ASD symptoms over time (i.e. slope of symptom on age) on whether or not children were later diagnosed with ASD. This modeling approach was most appropriate when data were available across three or more timepoints. In these models (i.e., trajectory models), we used a 2-level random slope and intercept model with a bivariate outcome (see Figure 1 for the analytic model). This model is separated into a within (i.e. separate observations within child across time) and between (i.e. child-level variables) parts. In the within part, we estimated a latent slope and intercept of each ASD Symptom variable regressed on Age in Months at the time of observation. The slope (black circle with S) in the within portion of the model represents the expected unit increase in ASD Symptom for every month increase in age. The intercept in the within portion of the model represents the expected value of the ASD Symptom at the first observation age, thus producing an estimate of initial level of ASD Symptom. Bayesian estimation was used for all parameters. All symptom variables (in the within model) were modeled as continuous outcomes and the ASD diagnosis variable was modeled as a bivariate outcome using a probit link function. The same analysis was run for heart activity variables (i.e., IBI and RSA). Data for the ADOS-2 and M-CHAT were available at fewer than three timepoints due to participant ages at which these were administered. For these models, we used a simple random intercept multilevel model to estimate whether the prior level (i.e., intercept) of symptomology predicted ASD diagnosis as a bivariate outcome. In addition, because the M-CHAT is intended as a pass/fail screening measure, we also used descriptive analyses to characterize the proportion of participants in each group meeting clinical cutoffs at each age (18 and 24 months).
Figure 1.
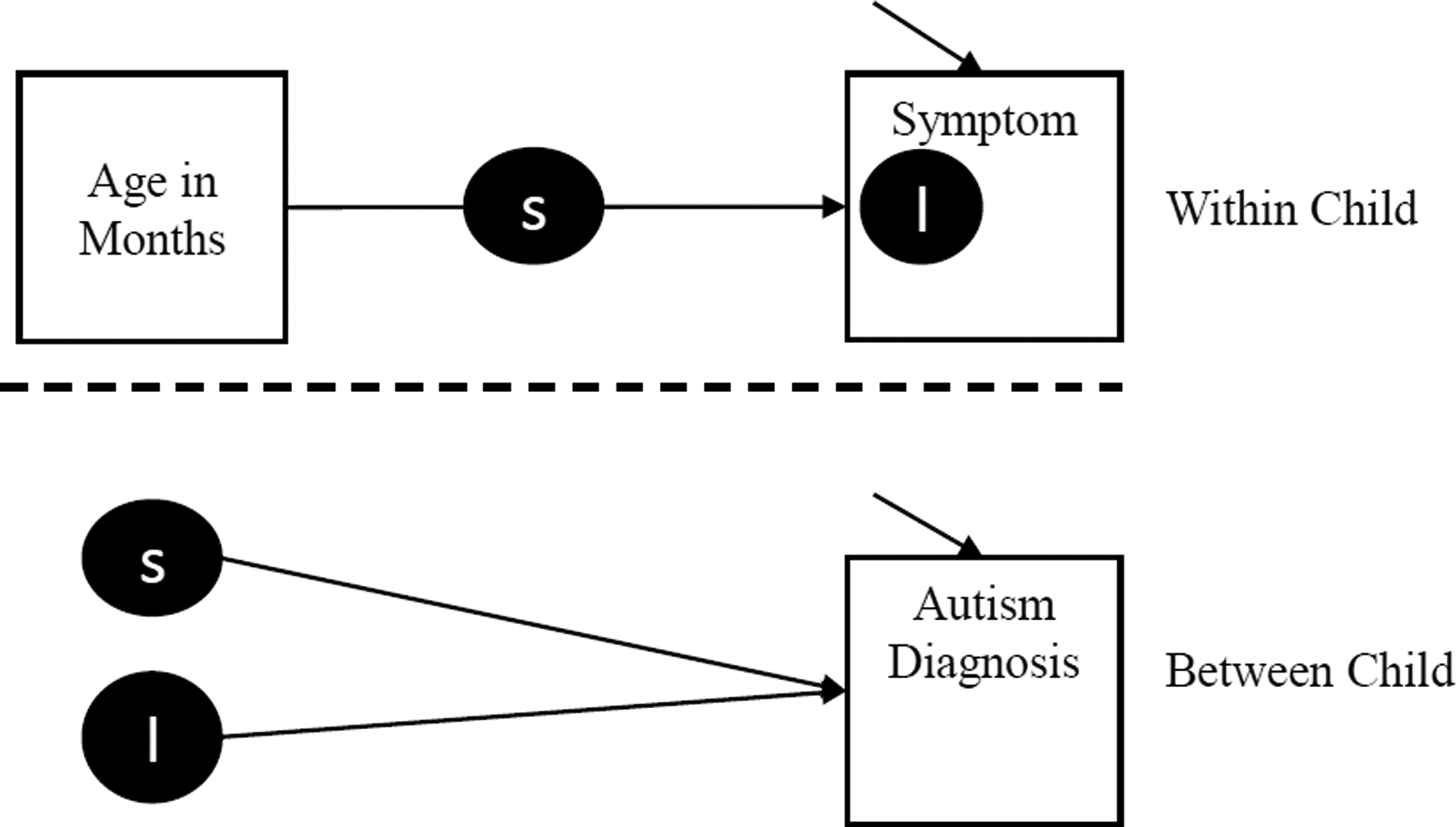
Analytic Model for Trajectory Models
Results
Rate of ASD in FXS
Rate of ASD.
According to CBE diagnostic procedures, 31 of the 51 participants with FXS received a diagnosis of ASD (FXS+ASD), for an overall FXS+ASD rate of 60.7%. Of the 14 females with FXS in the study, 4 (29%) were diagnosed with ASD and 10 (71%) were characterized as FXS-only. Of the males with FXS in the study 27 (73%) were diagnosed with ASD and 10 (27%) were characterized as FXS-only. In addition, the vast majority of participants presented with developmental delay (92%). Within the ASD+FXS group the rate of developmental delay was 97% (30/31), comparable to 85% (17/20) of the FXS-only group.
Certainty of ASD Diagnoses.
Out of the 31 participants diagnosed with ASD, there was reduced diagnostic certainty for presence of ASD in seven cases. For these seven cases of reduced certainty, five had no other secondary psychiatric diagnoses, one was diagnosed with both ADHD and multiple anxiety disorders and the final case was the single child diagnosed at 24-months-old who also did not have a PAPA assessment so psychiatric comorbidity is unknown. Of the 20 participants who were not diagnosed with ASD, 13 were determined to have a non-ASD developmental delay, 3 had subthreshold ASD, and 4 had no-clinical features. There was reduced diagnostic certainty for nine of these non-ASD cases: six had non-ASD developmental delay and the remaining three had subthreshold ASD (all of these three were female). None of the 3 subthreshold ASD cases carried additional psychiatric diagnoses. Of the six non-ASD developmental delay cases: one had no other psychiatric diagnoses, two were diagnosed with more than one anxiety disorder (one of these two was also diagnosed with ADHD), and three were diagnosed with one anxiety disorder (one of these three was also diagnosed with ADHD).
Co-morbid Psychiatric Diagnoses.
Data on psychiatric comorbidities was available for 44 of the 51 participants (N=24 for FXS+ASD and N=20 for FXS-only) for ADHD and 46 of the 51 participants for anxiety disorders (N=26 for FXS+ASD and N=20 for FXS-only). Among children with FXS+ASD, 35% were diagnosed with at least one anxiety disorder and 25% were diagnosed with ADHD. Within the FXS-nonASD group 45% were diagnosed with at least one anxiety disorder and 10% received an ADHD diagnosis. Table 2 provides a break-down of anxiety disorder subtypes across groups.
Table 2.
Percentage of Children with FXS Meeting Criteria for Anxiety Disorders
| Gender | Autism Status | |||||
|---|---|---|---|---|---|---|
| Anxiety Type | Total N=461 |
Female N=14 |
Male N=32 |
FXS-Only N=20 |
FXS+ASD N=26+ |
|
| More than one Anxiety Disorder | 9% | 0% | 13% | 10% | 8% | |
| One Anxiety Disorder | 30% | 29% | 31% | 35% | 27% | |
| Separation anxiety | 9% | 0% | 13% | 10% | 8% | |
| Social Phobia | 13% | 7% | 16% | 15% | 12% | |
| Specific Phobia | 26% | 14% | 31% | 23% | 30% | |
| GAD | 4% | 7% | 3% | 5% | 4% | |
5 participants are missing the PAPA measure.
Early Predictors of ASD Outcomes in FXS
ASD Screeners.
We tested models across three ASD screening measures – the AOSI, the M-CHAT, and the CARS, to determine whether these significantly predicted later ASD diagnostic outcomes. The first model examined whether the initial level (intercept) and linear change (slope) of AOSI total scores predicted ASD diagnosis. These results indicated that initial level of AOSI total scores significantly predicted ASD outcomes (z=3.49(1.01); p<.001; 95% CI = 1.16, 4.96) controlling for sex and cognitive functioning, whereas the linear change over age (i.e., slope) did not (see Table 3). Descriptive analyses from the M-CHAT revealed that across children with FXS+ASD at outcome (males and females), 100% with 18-month data met clinical cutoffs, whereas 50% met cutoffs at 24-months. Out of the males in the FXS+ASD group with M-CHAT data (n=18), a high proportion met clinical cutoffs at 18 and 24 months – 71% and 50% respectively. Three females with FXS+ASD at outcome had M-CHAT data available – one at 18-months, and two at 24-months. Accordingly, 100% met clinical cutoffs at 18 months, and 50% met cutoffs at 24-months. For males with FXS-only at outcome (n=8), six had data at 18 months, and 83% met clinical cutoffs at this age. A total of eight had data at 24 months, and 25% met clinical cutoffs at this age. For females in the FXS-only group at outcome, nine had data at 18 months, and seven had data at 24 months. Of these, a low proportion met clinical cutoffs at each age – 11% and 0% respectively. Results from the inferential M-CHAT analyses indicated that higher total M-CHAT scores significantly predicted later ASD outcomes (z=1.90(1.22); p<.001; 95% CI=0.31, 5.08), controlling for sex and cognitive level. Results from the model testing whether total CARS scores predicted ASD outcomes indicated that initial level of the CARS total score significantly predicted later ASD diagnosis (z=2.06(0.52); p<.001; 95% CI=0.82, 2.73), whereas change in CARS scores over age (i.e., slope) did not (see Table 3). Across all three ASD screeners, significant effects were such that higher initial scores were predictive of later ASD diagnosis (see Figure 2).
Table 3.
Predictors of ASD: Autism Screeners and Diagnostic Measures
| Autism Screeners | |||||
|---|---|---|---|---|---|
| AOSI (61 Observations) | 95% CI | ||||
| z | SE | p | Lower | Upper | |
| Linear Change (Slope) | −0.51 | 2.17 | .405 | −4.77 | 3.73 |
| Initial Level (Intercept) | 3.49 | 1.01 | <.001 | 1.16 | 4.96 |
| Sex | 0.16 | 2.10 | .469 | −3.94 | 4.28 |
| Cognitive Ability | −1.01 | 0.31 | <.001 | −1.61 | −0.41 |
| MCHAT (57 Observations) | 95% CI | ||||
| z | SE | p | Lower | Upper | |
| M-Chat Total Score | 1.90 | 1.22 | <.001 | 0.31 | 5.10 |
| Sex | 0.57 | 1.47 | .351 | −2.25 | 4.43 |
| Cognitive Ability | −0.22 | 0.13 | <.001 | −0.56 | −0.07 |
| CARS (87 Observations) | 95% CI | ||||
| z | SE | p | Lower | Upper | |
| Linear Change (Slope) | −0.01 | 2.56 | .498 | −4.42 | 4.46 |
| Initial Level (Intercept) | 2.06 | 0.52 | <.001 | 0.82 | 2.73 |
| Sex | −0.30 | 1.90 | .434 | −4.05 | 3.32 |
| Cognitive Ability | −0.73 | 0.20 | <.001 | −1.07 | −0.31 |
| Autism Diagnostic Measure | |||||
| ADOS (91 Observations) | 95% CI | ||||
| z | SE | p | Lower | Upper | |
| Overall CSS score | 1.81 | 0.73 | <.001 | 0.73 | 3.23 |
| Sex | 1.40 | 1.38 | .108 | −1.03 | 4.69 |
| Cognitive Ability | −0.16 | 0.08 | <.001 | −0.29 | −0.04 |
| Social Affect CSS | 1.15 | 0.19 | <.001 | 0.72 | 1.53 |
| Sex | 0.70 | 0.93 | .280 | −0.97 | 2.48 |
| Cognitive Ability | −0.10 | 0.02 | <.001 | −0.15 | −0.05 |
| Restricted Behavior CSS | 1.12 | 0.43 | <.001 | 0.42 | 2.14 |
| Sex | 0.14 | 0.93 | .437 | −1.76 | 2.02 |
| Cognitive Ability | −0.09 | 0.04 | .001 | −0.21 | −0.04 |
Figure 2.
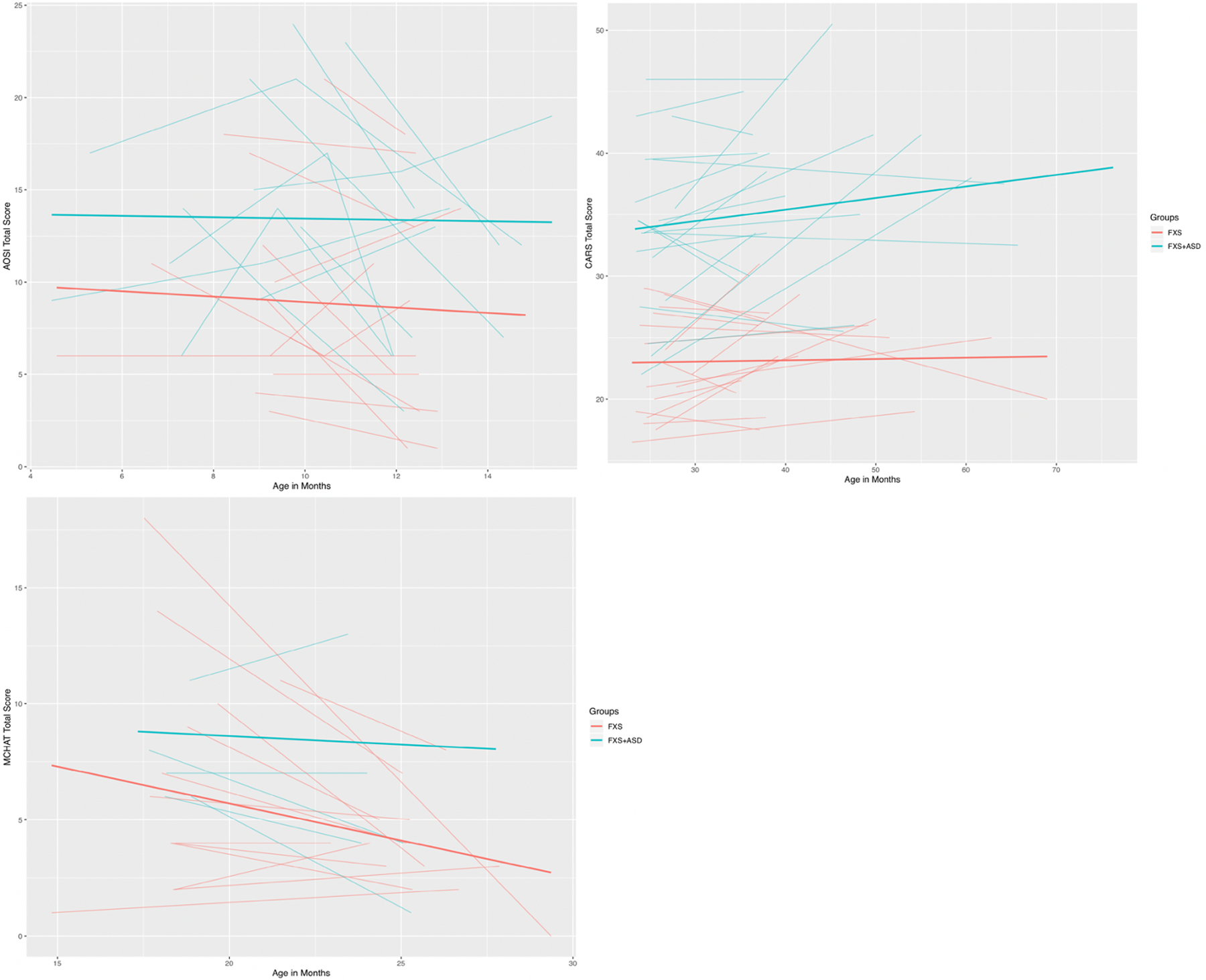
Trajectories of Autism Symptoms
ASD Diagnostic Measure.
The ADOS Overall CSS scores significantly predicted ASD outcomes (z=1.81(0.73); p<.001; 95% CI = 0.73, 3.23), such that higher ADOS Overall CSS scores were associated with ASD diagnosis. This effect was also significant controlling for cognitive level and sex and is expected given ADOS-2 scores are one of the measures used in clinical best estimate process. We computed post hoc models to determine whether Social Affect or Repetitive Behaviors severity scores, more specifically, were predictive of ASD outcomes. Results from these models suggested that both prior Social Affect scores (z=1.15(0.19); p<.001; 95% CI 0.72, 1.53) and Repetitive Behavior scores (z=1.12(0.43); p<.001; 95% CI 0.42, 2.14) each significantly predicted ASD outcomes, controlling for sex and cognitive functioning. These were relatively comparable in their effect size estimates and, as with the Overall CSS score, these effects were also such that higher scores were indicative of later ASD diagnosis (see Table 3). On a more macro-level, all 31 children diagnosed with ASD through the CBE process had ADOS-2 algorithm scores at or above the ASD (28/31) or spectrum cutoff (3/31). Nine of the children determined not to have ASD through the CBE process had ADOS-2 algorithm scores above the ASD (3/20) or spectrum (6/20) cutoff.
Repetitive Behaviors and Sensory Responses.
The next set of models tested whether repetitive behavior, measured using the RBS-R, or sensory responses significantly predicted ASD outcomes while controlling for sex and cognitive level. Results indicated that the initial level of total raw scores from the RBS-R, using the Bishop factor structure (Bishop et al., 2013), significantly predicted later ASD outcomes (z=0.53(0.22); p<.001; 95% CI = 0.14, 0.90), whereas the linear change in repetitive behavior over time (i.e., slope) did not (see Table 4, Figure 3). We computed additional post hoc models for each of the five Bishop factor scales to determine which of these were specifically predictive of ASD outcomes. Model results demonstrated that initial level of sensory-motor behavior (z=1.41(0.64; p<.001; 95% CI = 0.38, 2.73), restricted interests (z=3.42(2.23); p<.001; 95% CI=1.00, 5.57), self-injury (z=1.66(0.94); p=.009; 95% CI=0.15, 3.29), and compulsive behavior (z=1.19(0.66); p=.016; 95% CI=0.062, 2.40) all significantly predicted ASD outcomes when controlling for cognitive level and sex. Initial level of ritualistic/sameness behavior was identified as a marginally significant predictor of later ASD diagnosis (z=0.47(0.28); p=.050; 95% CI = −0.13, 1.03). Across all of these models, linear change over age (i.e., slope) was not a significant predictor of later ASD outcomes. In regard to sensory responses, as measured by the SEQ, neither initial level (i.e., intercept), nor linear change over age (i.e., slope) were significant predictors of later ASD diagnosis (see Table 4, Figure 3).
Table 4.
Predictors of ASD: Sensory & Repetitive Behavior
| Sensory Impairment | |||||
|---|---|---|---|---|---|
| SEQ (133 Observations) | 95% CI | ||||
| z | SE | p | Lower | Upper | |
| Initial Level (Intercept) | 1.30 | 1.14 | .070 | −0.32 | 4.33 |
| Linear Change (Slope) | 0.84 | 2.30 | .383 | −3.80 | 5.20 |
| Sex | 0.63 | 0.58 | .107 | −0.38 | 1.92 |
| Cognitive Ability | −0.06 | 0.03 | <.001 | −0.12 | −0.02 |
| Repetitive Behaviors | |||||
| RBS (103 Observations) | 95% CI | ||||
| Total Score | z | SE | p | Lower | Upper |
| Initial Level (Intercept) | 0.53 | 0.22 | <.001 | 0.14 | 0.90 |
| Linear Change (Slope) | 0.14 | 2.23 | .478 | −4.49 | 4.34 |
| Sex | 0.46 | 1.39 | .362 | −2.72 | 3.04 |
| Cognitive Ability | −0.29 | 0.11 | <.001 | −0.47 | −0.09 |
| Sensory Motor | |||||
| Initial Level (Intercept) | 1.41 | 0.64 | <.001 | 0.38 | 2.73 |
| Linear Change (Slope) | −0.003 | 2.26 | .499 | −4.24 | 4.32 |
| Sex | −0.002 | 1.19 | .499 | −2.34 | 2.17 |
| Cognitive Ability | −0.20 | 0.08 | <.001 | −0.37 | −0.07 |
| Restricted Interests | |||||
| Initial Level (Intercept) | 3.41 | 1.25 | <.001 | 1.00 | 5.57 |
| Linear Change (Slope) | 0.22 | 2.23 | .472 | −4.16 | 4.49 |
| Sex | 1.18 | 1.01 | .121 | −0.76 | 3.13 |
| Cognitive Ability | −0.13 | 0.04 | <.001 | −0.22 | −0.06 |
| Self-Injury | |||||
| Initial Level (Intercept) | 1.66 | 0.94 | .009 | 0.15 | 3.29 |
| Linear Change (Slope) | −0.06 | 2.22 | .488 | −4.62 | 3.92 |
| Sex | 0.52 | 1.25 | .319 | −2.02 | 3.19 |
| Cognitive Ability | −0.19 | 0.10 | <.001 | −0.39 | −0.05 |
| Compulsive Behavior | |||||
| Initial Level (Intercept) | 1.19 | 0.66 | .016 | 0.06 | 2.40 |
| Linear Change (Slope) | −0.03 | 2.28 | .495 | −4.39 | 4.42 |
| Sex | 0.70 | 1.09 | .231 | −1.66 | 2.75 |
| Cognitive Ability | −0.15 | 0.08 | <.001 | −0.29 | −0.04 |
| Ritualistic/Sameness | |||||
| Initial Level (Intercept) | 0.47 | 0.28 | .050 | −0.13 | 1.03 |
| Linear Change (Slope) | −0.08 | 1.99 | .475 | −4.01 | 3.72 |
| Sex | 0.64 | 0.69 | .155 | −0.72 | 2.16 |
| Cognitive Ability | −0.07 | 0.03 | <.001 | −0.13 | −0.03 |
Figure 3.
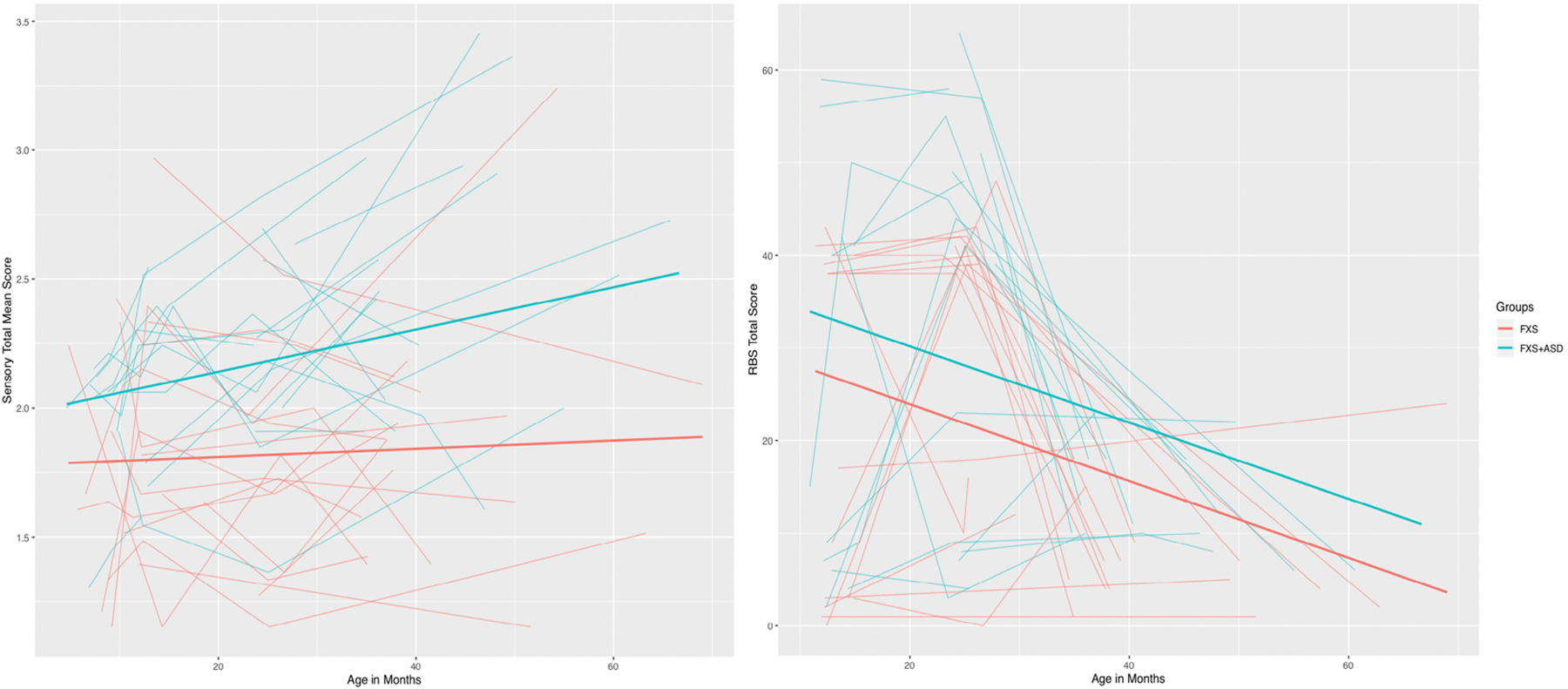
Trajectories of associated features.
Social Avoidance.
We examined whether initial level (i.e., intercept) or change in level over age (i.e., slope) of social avoidance (i.e., diminished eye contact) during the first minute or last hour of interaction during assessment predicted ASD outcomes. Results from these models indicated that neither initial level nor change over age in eye contact during the first minute of interaction predicted later ASD diagnosis (see Table 5). Initial level of eye contact during the last hour of interaction was identified as a marginally significant predictor of later ASD diagnostic outcomes (z=2.33(1.33); p=.055; 95% CI= −0.31, 4.95). Change in eye contact during the last hour of interaction over age (i.e., slope) did not significantly predict later ASD diagnostic outcomes (see Table 5).
Table 5.
Predictors of ASD: Heart Activity and Social Avoidance
| Physiological Measures | |||||
|---|---|---|---|---|---|
| Heart Activity (100 Observations) | 95% CI | ||||
| IBI | z | SE | p | Lower | Upper |
| Initial Level (Intercept) | 0.03 | 0.02 | .038 | −0.001 | .072 |
| Linear Change (Slope) | −0.99 | 1.10 | .173 | −3.14 | 1.35 |
| Sex | 0.63 | 1.30 | .265 | −1.64 | 4.03 |
| Cognitive Ability | −0.14 | 0.08 | .002 | −0.32 | −0.04 |
| RSA | |||||
| Initial Level (Intercept) | 0.31 | 0.63 | .308 | −0.82 | 1.72 |
| Linear Change (Slope) | −0.54 | 2.26 | .409 | −4.80 | 3.75 |
| Sex | 0.47 | 0.69 | .2441 | −0.95 | 1.81 |
| Cognitive Ability | −0.06 | 0.02 | <.001 | −0.12 | −0.02 |
| Social Avoidance | |||||
| SAS (140 Observations) | 95% CI | ||||
| Eye Contact Last Hour | |||||
| Initial Level (Intercept) | 2.33 | 1.33 | .055 | −0.31 | 4.59 |
| Linear Change (Slope) | 1.08 | 2.19 | .308 | −3.34 | 5.38 |
| Sex | 0.76 | 0.76 | .120 | −0.40 | 2.52 |
| Cognitive Ability | −0.06 | 0.02 | <.001 | −0.10 | −0.02 |
Physiological Indices.
We computed models to evaluate the initial level and change over time in heart activity as potential predictors of ASD outcomes controlling for cognitive level and sex. In the RSA model, initial level and change in RSA over age did not significantly predict later ASD outcomes (see Table 5, Figure 4). In the IBI model, change in IBI over age was not a significant predictor of ASD outcomes; however, the effect for initial level was significant (z=0.03(0.02); p=.038; 95% CI= −.001, .072; see Table 5, Figure 4).
Figure 4.

Trajectories of heart activity.
Cognitive functioning.
It is important to note that regardless of the model, cognitive functioning, measured concurrently with diagnostic outcome, was identified as a significant predictor of ASD diagnosis in every model. This suggests that intellectual functioning is a strong predictor of ASD diagnosis in FXS, even when accounting for sex and other symptomatology.
Discussion
Diagnostic Outcome Determination: Primary ASD Diagnosis
Using a clinical best estimate procedure, results indicate that 60.7% of preschoolers with FXS met DSM-5 diagnostic criteria for ASD (31 of n=51). Our diagnostic procedure included consideration of developmental delay along with differential diagnoses of psychiatric diagnoses of anxiety and/or ADHD. Consistent with the DSM-5 and clinical best practice, the diagnosis of ASD was only applied when the features of ASD were not better accounted for by developmental delay or other psychiatric diagnoses such as anxiety or ADHD. There was a high degree of confidence associated with the diagnosis of ASD with nearly 80% of cases rated with a high degree of diagnostic certainty. Contrasting ASD diagnoses made with high certainty versus those with reduced certainty did not suggest any readily apparent distinctions. In other words, cases with reduced certainty did not appear to be driven by increased rates of co-morbid psychiatric diagnoses or developmental delay.
Interestingly, 55% of the cases where ASD was ruled out were considered to have a high degree of diagnostic certainty. While preliminary, it does appear that the non-ASD cases rated with lower diagnostic certainty were ones with a more complicated clinical profile, including the presence of subthreshold ASD and multiple psychiatric diagnoses (e.g., multiple anxiety disorders or both anxiety and ADHD). Of note, nine out of the 20 preschoolers not diagnosed with ASD had ADOS-2 scores at or above the cutoff. Three of these nine were designated to have subthreshold ASD (all females), six of the nine had a developmental delay. If we had used the total score cutoffs in isolation without CBE procedures, 78.4% of our sample fell in the ASD range and these nine children would have been misclassified as having ASD.
Our finding that 60.7% preschoolers with FXS met ASD diagnostic criteria aligns with previously reported rates of ASD in 50–75% of older-aged children, adolescents and adults with FXS (Abbedutto et al., 2019; Kaufmann et al., 2017; Lee et al., 2016). However, it is substantially higher than several studies of ASD in toddlers and preschoolers with FXS that report 30.2% to 39.0% (Hazlett et al., 2012; Rogers et al., 2001). Overall, comparing prevalence estimates across studies that likely vary in ascertainment strategies and diagnostic characterization procedures is problematic (Fombonne, 2018) and poses a challenge to make clear conclusions about prevalence rates. Still, it is critical to note that none of these existing preschool studies used DSM-5 criteria and a clinical best estimate procedure. Rather, they either used DSM-IV criteria (Rogers et al., 2001) or ADOS and ADI-R score cutoffs without clinical review and a somewhat younger sample (Hazlett et al., 2012). It is possible that the higher rate in our sample may reflect changes in diagnostic criteria and the DSM-5 conceptualizations of ASD as a wider spectrum of impairment with only two core domains. Given the frequent report that ~90% of children with FXS exhibit at least one ASD feature (Merenstein et al., 1996; Roberts, Crawford, Will, et al., 2019), coupled with the greater spectrum of impairment inherent in the DSM-5, differences in our rate versus others may reflect our use of the DSM-5 for diagnostic determination which has been suggested in a recent study with adolescents and young adults with FXS (Abbeduto et al., 2019). It is also possible that the increase in prevalence of ASD in FXS is associated with the national increase in prevalence of nsASD (Christensen et al., 2019).
As anticipated, a higher proportion of preschool males with FXS were diagnosed with ASD than females (73% versus 28.6% respectively). Our rate of ASD in preschool males aligns with rates reported for older males with FXS that range from to 54.6% to 80.6% (Harris et al., 2008; Lee et al., 2016; McDuffie et al., 2010). Investigations of ASD in females with FXS is far less advanced as most of the work has focused on males and ruled out females or included a very small number of females. Our rate of 28.6% in preschool females with FXS is consistent with rates of the handful of studies with older-aged children and adolescent females that show 29.4% to 41.2% meet criteria using direct assessments (Klusek et al., 2014; Lee et al., 2016). There are no published studies reporting the rate of ASD in preschool-aged females with FXS so our study is the first to do so. However, our sample of females is relatively small (14 out of n=51) so caution is warranted and replication is needed. Of note, 21.4% (3 out of n=14) were classified as subthreshold ASD (2 or more features of ASD but do not meet full DSM-5 criteria) while no males received the diagnosis of subthreshold ASD, suggesting that the presence of ASD features may be more subtle or nuanced in females than males with FXS as is the case with nsASD (Carter et al., 2007; Hiller, Young, & Weber, 2014; Kirkovski, Enticott, & Fitzgerald, 2013).
Diagnostic Outcome Determination: Secondary Psychiatric Diagnosis
In terms of psychiatric co-morbidity, 45.5% of the total sample (20 of n=44) was diagnosed with either anxiety, ADHD, or both. When considering anxiety disorders specifically, 39% of the overall sample was diagnosed with at least one anxiety disorder. For those with FXS+ASD, the rate was 35% contrasted to 45% for those in the FXS-only group. Our findings are fairly consistent with existing reports in a similarly aged sample using parental or clinical report (Kaufmann et al., 2017). However, our results reflect lower rates than reported in two studies of older individuals with FXS that included direct in-depth assessments of both anxiety and ASD. These studies report that 51.6% of males aged 16 to 24 years (Ezell et al., 2019) and 82.5% of males and females aged 5 to 26 years (Cordeiro, Ballinger, Hagerman, & Hessl, 2011) met diagnostic criteria for any anxiety disorder. The higher rate of anxiety disorders in the older FXS samples is consistent with the fact that anxiety disorders typically emerge in middle to late childhood and the severity of anxiety symptoms in FXS has been shown to increase with age (Cordeiro et al., 2011) as is the case with neurotypical individuals (Bosquet & Egeland, 2006; Copeland, Angold, Shanahan, & Costello, 2014).
When focusing on individual anxiety disorders, specific phobia was the most commonly diagnosed anxiety disorder, occurring in 30% of the FXS+ASD group and 23% of the FXS-only group. This pattern is consistent with the neurotypical and nsASD literature as specific phobia is one of the most common anxiety disorders during the preschool developmental period (Paulus, Backes, Sander, Weber, & von Gontard, 2015; Salazar et al., 2015). Given the greater overlap of ASD features with social phobia compared to other anxiety disorders, it was surprising to find that social phobia was relatively low in both groups with 12% in FXS+ASD and 15% in FXS-only. However, we did not ascertain “other social fear” as has been shown to be relevant in nsASD (Kerns et al., 2014) so our rates could change if we employed a conceptualization of social phobia that was more aligned with ASD-linked idiosyncrasies. Generalized anxiety disorder and separation anxiety disorder were also relatively uncommon across the groups with and without ASD (4% vs. 5% and 8% vs. 10% respectively). Consistent with the lower incidence of any anxiety disorder, our incidence of specific anxiety disorders is lower than studies published with older children and adults with FXS (Cordeiro et al., 2011; Ezell et al., 2019). However, two striking areas of consistency across the current and existing studies with older samples is that specific phobia is the most prevalent anxiety disorder, and ASD status or severity of symptoms is not clearly associated with differential rates of comorbid anxiety diagnoses in FXS. Given that ASD diagnoses in the present study were determined in the context of anxiety disorders (ruled out or diagnosed as co-morbid) and evidence that anxiety disorders were fairly evenly distributed across children with FXS+ASD and those with FXSonly, our results do not support the hypothesis that anxiety features are at the root of the elevated rate of ASD in FXS and that ASD in FXS represents a misdiagnosis of ASD based on elevated anxiety features.
The rate and profile of anxiety disorders in the current study differs a fair bit from work in nsASD. Our anxiety diagnosis rate of 27% of those with FXS+ASD is far lower than the rate of 78.9% reported in a sample of 101 males and females with nsASD (Salazar et al., 2015). Differences could be due to age as their sample was 6.7 years while ours is 3.10 years, and they did report that anxiety diagnoses increased with age (Salazar et al., 2015). Interestingly, the Salazar study found that higher IQ (>70) was associated with increased likelihood of an anxiety disorder which does help to reconcile the difference in rates as the majority of participants in our study had a developmental delay. The profile of anxiety disorders also differed in this study contrasted to ours with GAD as the most frequent (66.5%) followed by Specific Phobia (52.7%). Interestingly, the rates of Social Phobia were fairly consistent with 15.1% in their study contrasted to 13% in ours. One potential explanation for the differences in rates across studies could be due to the lower chronological and mental age and, specifically, low language skills in our sample. Limited communication skills would clearly affect a child’s ability to express fear and worry which would impact a parent’s ability to report these symptoms across most anxiety disorders with GAD and Social Phobia as particularly sensitive to this factor. This hypothesis is partially supported in the Salazar (2015) study as they report that older age is associated with increased likelihood of several anxiety disorders, and an IQ of >70 is associated with a higher prevalence of “any anxiety” but not with any single anxiety disorder. While we cannot test this relationship in our own data given that nearly 100% of the sample had a developmental delay, we did consider intellectual ability in conducting the CBE diagnoses by determining that a symptom was not better accounted for by intellectual impairment. Nonetheless, our rates of psychiatric co-morbidity reflect the young age and low developmental skills of our group.
In terms of ADHD diagnoses, the proportion of preschool children with FXS meeting diagnostic criteria for ADHD was quite different between those with and without ASD. The rate was 18.1% for the overall sample which broke out into 25% for those with FXS+ASD contrasted to 10% for the FXS-only group. Our finding that more than twice the number of children with FXS+ASD also met criteria for ADHD than those without ASD was consistent with our hypothesis given evidence that increased ADHD symptoms and diagnoses are associated with elevated ASD symptom severity in children with FXS (Sullivan et al., 2006). The elevated rate of ADHD in the FXS+ASD group is also consistent with elevated rates of ADHD in nsASD; however, the rate of ADHD in nsASD (59.1%) is higher than our results (Salazar et al., 2015). Results from our study provide partial support that elevated ADHD is associated with ASD in FXS as it was more than 2.5 times higher in those with FXS+ASD than those with FXS-only.
Predictors of ASD Across Infancy
The second aim of this study was to examine how early ASD-specific symptoms and associated phenotypic features predicted a diagnosis of ASD in preschoolers with FXS. Higher levels of ASD symptomology as indicated by both clinician-administered diagnostic tools (the AOSI, ADOS, and CARS) and parent-report measures (MCHAT) significantly predicted diagnosis of ASD. Additionally, ASD symptoms, measured by the AOSI, were largely stable between 6–14 months of age for all participants. In other words, infants with FXS+ASD exhibited elevated symptoms of ASD early on that remained stable across the first year of life. Higher scores on a parent measure of restricted interests and repetitive behaviors (RBS-R) were also associated with ASD outcome. This is notable given that 92% of this sample of preschoolers with FXS had developmental delay, which is often associated with increased repetitive behaviors. Most subscales of the RBS-R exhibited significant change over time, but this change was similar across all participants. Together, these results suggest that commonly used research and clinical measures are informative in the first two years of life for predicting diagnosis of ASD in infants with FXS. Use of clinician and parent administered screeners, including the CARS and the MCHAT, may useful in helping to identify infants with FXS at risk for developing ASD.
Measures that were not clear predictors of ASD outcome included sensory responsiveness and avoidant eye contact. Sensory responsiveness difficulties are highly prevalent in preschoolers with ASD and preschoolers with intellectual disability with ASD. Baranek et al., (2006) found that children with ASD and developmental delays had significantly greater sensory impairments compared to those with developmental delays alone. Pilot data from our group suggests that young children with FXS+ASD had the same level of sensory impairments as an age-matched control sample with nsASD, which was higher than a children with FXSonly (Knott, Will, & Roberts, 2019). Thus, it is not clear if sensory impairments across infancy are associated with increased risk of ASD in FXS, and this is an area for future research. In terms of avoidant eye contact, our previous work has shown that avoidant eye contact during infancy does predict the severity of ASD features at preschool age using ASD-features across a continuum rather than as a categorical outcome using diagnostic determination (Roberts, Crawford, Will, et al., 2019). Thus, our finding that avoidant eye contact across infancy does not predict diagnoses of ASD in this study likely reflects reduced power associated with categorical outcomes and small samples.
It is important to note that nonverbal cognitive outcome was statistically significant in all analytic models, suggesting that lower nonverbal cognitive skills are significantly associated with ASD in preschoolers with FXS. Descriptively, this is in line with our finding that a somewhat higher proportion of preschoolers with FXS+ASD also presented with developmental delay (97%) compared to preschoolers with FXS-only (85%). Also, when considering the level of intellectual disability, more preschoolers with FXS+ASD were in the moderate to severe range with a NVDQ ≤ 45 (61%) contrasted to those in the FXS-only group (10%). Critically, our findings suggest that early ASD features in infancy predict ASD in the preschool years above and beyond nonverbal cognitive functioning and that nonverbal cognitive ability does not solely account for the high classification rate of ASD in this sample. Collectively, our results indicate that lower developmental level is strongly associated with ASD in FXS; however, it is not deterministic as 85% of the FXS-only group had a developmental delay.
Our data suggest that faster initial heart rate (shorter IBI) was associated with a diagnosis of ASD while initial RSA level and trajectories of RSA and IBI were not. Evidence from recent studies with small samples of infants later diagnosed with ASD indicate that baseline RSA does not differ between nsASD and typical controls, but that infants with ASD exhibit lower RSA during interactions with a stranger (McCormick et al., 2018) and a smaller increase in RSA from infancy (18 months) through preschool ages (Sheinkopf et al., 2019). The majority of participants in these studies, however, were prenatally exposed to substances, and analyses did not control for mental age or include measures of heart rate or IBI. Interestingly, our earlier work reported a non-linear relationship between heart rate and elevated severity of ASD features in a cross-sectional study of infants with FXS 8 to 40 months of age (Roberts et al., 2012). This study indicated that ASD severity at preschool was predicted by slower heart rate (longer IBI) in infants younger than 10 months which transitioned to a relationship of faster heart rate (shorter IBI) in those 37 months and older. Reduced RSA also predicted elevated ASD severity outcomes but only starting at 22 months (Roberts et al., 2012). Another cross-sectional study from our group also reported a non-linear relationship between heart activity and social fear with faster heart rate (longer IBI) in infants <29 months that transitioned to slower heart rate (shorter IBI) that occurred at >51 months (Tonnsen et al., 2013). Both of these earlier studies reported a strong relationship between heart activity and cognitive ability. Given that the present study is a longitudinal study that controls for nonverbal cognitive ability, the finding that faster heart rate (shorter IBI) across the infant and toddler years predicted diagnoses of ASD at preschool is an important extension and confirmation of earlier work. Thus, the hypothesis that hyperarousal is associated with ASD in FXS is at least partially supported by our results. This suggests that elevated heart rate might be an underlying mechanism associated with ASD in FXS which supports its use as a potential biomarker to signal elevated risk for ASD in FXS.
This is the first study to predict ASD from infancy in a sample of preschoolers with FXS using a differential diagnosis model based on DSM-5 criteria and implementing a CBE approach. In light of these strengths, there are several limitations to note. First, the sample size is relatively small and some of our statistical models were likely underpowered to detect true effects. While behavioral manifestation of ASD is robust during preschool, anxiety disorders and ADHD may continue to unfold in the early childhood years. And, our study covered a relatively short period of time in development and though our slopes are not significantly different between groups in this study, differences may emerge beyond 36 months of age. Thus, it will be important to continue to follow these clinical groups as psychopathology may continue emerge. The present study would be strengthened with the inclusion of a nsASD group to determine whether nsASD and FXS+ASD groups share similar developmental pathways and outcomes, despite unique genetic risk factors. This research would help us understand more precisely the multiple pathways that lead to ASD and whether FXS as a distinct genetic etiology produces distinct phenotypic outcome, or whether FXS+ASD represents a phenotype indistinguishable and just as heterogenous as nsASD, as this study suggests.
Summary and Implications
We report that 60.7% of preschoolers with FXS met DSM-5 criteria for ASD using a comprehensive clinical best estimate procedure that considered differential diagnoses of developmental delay, anxiety and ADHD diagnoses. There was a moderate to high degree of psychiatric comorbidity overall (i.e., 45.4%) and the rate of anxiety disorders were relatively similar across FXS+ASD and FXS-only while ADHD was more frequent in FXS+ASD. Despite the high prevalence of psychiatric diagnoses and developmental delay in those with FXS+ASD, there was a high degree of confidence in these diagnostic determinations. ASD diagnoses were predicted by a number of ASD-specific measures obtained during infancy including ASD screeners and indices of restrictive and repetitive behaviors with sensory symptoms implicated albeit to a lesser degree. Elevated autonomic arousal during infancy were also associated with ASD diagnoses at preschool. These findings highlight how multicausal pathways (e.g., FXS as a monogenic syndrome and ASD of unknown cause), result in ASD as a single behavioral outcome. Our findings also indicate that preschoolers with FXS experience varied outcomes despite having the same neurobiological vulnerability (i.e., FMR1 gene dysfunction). The range of outcomes in this study include ASD, anxiety, ADHD, hyperarousal and intellectual impairment which we found occur independently or in tandem. The co-occurrence of these outcomes likely reflects interactions with the environment and family genetic background.
Collectively, our results indicate that ASD is highly prevalent in preschoolers with FXS and can be predicted by both ASD and associated indices that are present and measurable during infancy. Our findings provide support that developmental delay, autonomic hyperarousal and ADHD are highly associated with ASD in young children with FXS with anxiety implicated in a more nuanced and complicated manner. This study contributes to the ongoing debate regarding the association of ASD to FXS which is longstanding and complex. Our data suggest that ASD can be differentially diagnosed in young children with FXS with a high degree of confidence using a CBE approach. We also found that the majority of ASD-specific measures predicted ASD as is the case in nsASD. Similar to nsASD, our sample also had a high rate of co-morbid psychiatric disorders including anxiety and ADHD in addition to autonomic dysfunction. Thus, our results support that ASD is a distinct disorder within FXS. Also, while not directly tested with a nsASD group, the profiles in our group with FXS+ASD shows a high degree of similarity to what is reported in nsASD studies. These points support that ASD in FXS may be “true ASD”. In addition, the high prevalence of ASD in our sample and the lack of differentiation of the slopes across the FXS-only and FXS+ASD groups indicate that ASD is expressed in the majority of preschoolers with FXS. Thus, while not all children with FXS will meet criteria for ASD, FXS is clearly a high-risk population for ASD with autistic features being a prominent aspect of the FXS phenotype, emerging as early as the infant and preschool years.
Acknowledgments:
We would like to thank the families who participated in this research. We are also grateful to Leonard Abbeduto, Somer Bishop, Heather Hazlett, Joseph Piven, and Jason Wolff for their thoughtful feedback on previous drafts of this manuscript. This work was supported by the National Institutes of Health through the following grants: 2R01MH90194, R01MH107573 (PI: Roberts); 1F32HD097877-01 (PI: Will); and K23MH120476 (PI: Bradshaw).
Footnotes
Conflicts of Interest: None
References
- Abbeduto L, McDuffie A, & Thurman AJ (2014). The fragile X syndrome-autism comorbidity: What do we really know? Frontiers in Genetics, 5, 1–10. 10.3389/fgene.2014.00355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbeduto L, Thurman AJ, McDuffie A, Klusek J, Feigles RT, Ted Brown W, … Roberts JE (2019). ASD Comorbidity in Fragile X Syndrome: Symptom Profile and Predictors of Symptom Severity in Adolescent and Young Adult Males. Journal of Autism and Developmental Disorders. 10.1007/s10803-018-3796-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). The Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Washington, D.C.: American Psychiatric Association. [Google Scholar]
- Bailey DBJ, Raspa M, Olmsted M, & Holiday DB (2008). Co-occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. American Journal of Medical Genetics Part A, 146A(16), 2060–2069. 10.1002/ajmg.a.32439 [DOI] [PubMed] [Google Scholar]
- Baranek GT, David FJ, Poe MD, Stone WL, & Watson LR (2006). Sensory Experiences Questionnaire: discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry, 47(6), 591–601. 10.1111/j.1469-7610.2005.01546.x [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Constantino JN, & Hayden EP (2018). Psychiatry and developmental psychopathology: Unifying themes and future directions. Comprehensive Psychiatry, 87, 143–152. 10.1016/j.comppsych.2018.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis EM, Hessl D, Rathmell B, Zarevics P, Cherubini M, Walton-Bowen K, … Hagerman RJ (2012). Effects of STX209 (Arbaclofen) on Neurobehavioral Function in Children and Adults with Fragile X Syndrome: A Randomized, Controlled, Phase 2 Trial. Science Translational Medicine, 4(152). [DOI] [PubMed] [Google Scholar]
- Bishop SL, Guthrie W, Coffing M, & Lord C (2011). Convergent validity of the Mullen Scales of Early Learning and the Differential Ability Scales in children with autism spectrum disorders. American Journal on Intellectual and Developmental Disabilities, 116(5), 331–343. 10.1352/1944-7558-116.5.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SL, Hus V, Duncan A, Huerta M, Gotham K, Pickles A, … Lord C (2013). Subcategories of restricted and repetitive behaviors in children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 43(6), 1287–1297. 10.1007/s10803-012-1671-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, & Lewis MH (2000). Varieties of repetitive behavior in autism: Comparisons to mental retardation. Journal of Autism and Developmental Disorders, 30(3), 237–243. 10.1023/A:1005596502855 [DOI] [PubMed] [Google Scholar]
- Bosquet M, & Egeland B (2006). The development and maintenance of anxiety symptoms from infancy through adolescence in a longitudinal sample. Development and Psychopathology, 18(02). 10.1017/S0954579406060275 [DOI] [PubMed] [Google Scholar]
- Brewe AM, Reisinger DL, Adlof SM, & Roberts JE (2018). Initiating joint attention use in infants at high-risk for autism spectrum disorder. Journal of Intellectual Disability Research, 62(10), 842–853. 10.1111/jir.12539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno JL, Romano D, Mazaika P, Lightbody AA, Hazlett HC, Piven J, & Reiss AL (2017). Longitudinal identification of clinically distinct neurophenotypes in young children with fragile X syndrome. Proceedings of the National Academy of Sciences of the United States of America, 114(40), 10767–10772. 10.1073/pnas.1620994114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson SE, Zwaigenbaum L, McDermott C, Rombough V, & Brian J (2008). The autism observation scale for infants: Scale development and reliability data. Journal of Autism and Developmental Disorders, 38(4), 731–738. 10.1007/s10803-007-0440-y [DOI] [PubMed] [Google Scholar]
- Carter AS, Black DO, Tewani S, Connolly CE, Kadlec MB, & Tager-Flusberg H (2007). Sex differences in toddlers with autism spectrum disorders. Journal of Autism and Developmental Disorders, 37(1), 86–97. 10.1007/s10803-006-0331-7 [DOI] [PubMed] [Google Scholar]
- Christensen DL, Maenner MJ, Bilder D, Constantino JN, Daniels J, Durkin MS, … Dietz P (2019). Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 4 Years — Early Autism and Developmental Disabilities Monitoring Network, Seven Sites, United States, 2010, 2012, and 2014. MMWR. Surveillance Summaries, 68(2), 1–19. 10.15585/mmwr.ss6802a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, & Rogosch FA (1996). Equifinality and multifinality in developmental psychopathology. Development and Psychopathology, 8(4), 597–600. 10.1017/S0954579400007318 [DOI] [Google Scholar]
- Cohen I (1995). A Theoretical Analysis of the Role of Hyperarousal in the Learning and Behavior of Fragile X Males. Mental Retardation and Developmental Disabilities Research Reviews, 1, 286–291. [Google Scholar]
- Copeland WE, Angold A, Shanahan L, & Costello EJ (2014). Longitudinal Patterns of Anxiety From Childhood to Adulthood: The Great Smoky Mountains Study. Journal of the American Academy of Child & Adolescent Psychiatry, 53(1), 21–33. 10.1016/j.jaac.2013.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro L, Ballinger E, Hagerman RJ, & Hessl D (2011). Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: Prevalence and characterization. Journal of Neurodevelopmental Disorders, 3(1), 57–67. 10.1007/s11689-010-9067-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, & Angold A (2006). Test-retest reliability of the Preschool Age Psychiatric Assessment (PAPA). Journal of the American Academy of Child and Adolescent Psychiatry, 45(5), 538–549. 10.1097/01.chi.0000205705.71194.b8 [DOI] [PubMed] [Google Scholar]
- Ezell J, Hogan A, Fairchild A, Hills K, Klusek J, Abbeduto L, & Roberts JE (2019). Prevalence and Predictors of Anxiety Disorders in Adolescent and Adult Males with Autism Spectrum Disorder and Fragile X Syndrome. Journal of Autism and Developmental Disorders, 49(3), 1131–1141. 10.1007/s10803-018-3804-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fombonne E (2018). Editorial: The rising prevalence of autism. Journal of Child Psychology and Psychiatry, 59(7), 717–720. 10.1111/jcpp.12941 [DOI] [PubMed] [Google Scholar]
- Gargaro BA, Rinehart NJ, Bradshaw JL, Tonge BJ, & Sheppard DM (2011, April). Autism and ADHD: How far have we come in the comorbidity debate? Neuroscience and Biobehavioral Reviews, Vol. 35, pp. 1081–1088. 10.1016/j.neubiorev.2010.11.002 [DOI] [PubMed] [Google Scholar]
- Gotham K, Pickles A, & Lord C (2009). Standardizing ADOS Scores for a Measure of Severity in Autism Spectrum Disorders. Journal of Autism and Developmental Disorders, 39(5), 693–705. 10.1007/s10803-008-0674-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Hoem G, & Hagerman P (2010). Fragile X and autism: Intertwined at the molecular level leading to targeted treatments. Mol Autism, 1(1), 12. 10.1186/2040-2392-1-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Protic D, Rajaratnam A, Salcedo-Arellano MJ, Aydin EY, & Schneider A (2018). Fragile X-Associated Neuropsychiatric Disorders (FXAND). Frontiers in Psychiatry, 9, 1–9. 10.3389/fpsyt.2018.00564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Hirt M, Rezvani A, & Reiss AL (2010). Autism in fragile X syndrome: A category mistake? Journal of the American Academy of Child and Adolescent Psychiatry, 49(9), 921–933. 10.1016/j.jaac.2010.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, … Hagerman RJ (2008). Autism profiles of males with fragile X syndrome. American Journal on Mental Retardation, 113(6), 427–438. 10.1352/2008.113:427-438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havdahl KA, Hus Bal V, Huerta M, Pickles A, Øyen AS, Stoltenberg C, … Bishop SL (2016). Multidimensional Influences on Autism Symptom Measures: Implications for Use in Etiological Research. Journal of the American Academy of Child and Adolescent Psychiatry, 55(12), 1054–1063.e3. 10.1016/j.jaac.2016.09.490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Lightbody AA, Styner M, MacFall JR, Reiss AL, & Piven J (2012). Trajectories of early brain volume development in fragile X syndrome and autism. Journal of the American Academy of Child and Adolescent Psychiatry, 51(9), 921–933. 10.1016/j.jaac.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KJ, Bal E, Bazhenova OV, Sorokin Y, Perlman SB, Hanley MC, & Porges SW (2008). Physiological responses to social and physical challenges in children: Quantifying mechanisms supporting social engagement and mobilization behaviors. Developmental Psychobiology, 50(2), 171–182. [DOI] [PubMed] [Google Scholar]
- Hernandez RN, Feinberg RL, Vaurio R, Passanante NM, Thompson RE, & Kaufmann WE (2009). Autism spectrum disorder in fragile X syndrome: A longitudinal evaluation. American Journal of Medical Genetics Part A, 149A(6), 1125–1137. 10.1002/ajmg.a.32848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller RM, Young RL, & Weber N (2014). Sex Differences in Autism Spectrum Disorder based on DSM-5 Criteria: Evidence from Clinician and Teacher Reporting. Journal of Abnormal Child Psychology, 42(8), 1381–1393. 10.1007/s10802-014-9881-x [DOI] [PubMed] [Google Scholar]
- Hogan AL, Caravella KE, Ezell J, Rague L, Hills K, & Roberts JE (2017). Autism Spectrum Disorder Symptoms in Infants with Fragile X Syndrome: A Prospective Case Series. Journal of Autism and Developmental Disorders, 47(6), 1628–1644. 10.1007/s10803-017-3081-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S, Wolff JJ, Steinbach MS, Doyle CB, Kumar V, & Elison JT (2019, December 1). Neurodevelopmental heterogeneity and computational approaches for understanding autism. Translational Psychiatry, Vol. 9, pp. 1–12. 10.1038/s41398-019-0390-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WE, Kidd SA, Andrews HF, Budimirovic DB, Esler A, Haas-Givler B, … Berry-Kravis E (2017). Autism spectrum disorder in fragile X syndrome: Cooccurring conditions and current treatment. Pediatrics, 139, S194–S206. 10.1542/peds.2016-1159F [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns CM, Kendall PC, Berry L, Souders MC, Franklin ME, Schultz RT, … Herrington J (2014). Traditional and atypical presentations of anxiety in youth with autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(11), 2851–2861. 10.1007/s10803-014-2141-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns CM, Newschaffer CJ, & Berkowitz SJ (2015). Traumatic Childhood Events and Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 45(11), 3475–3486. 10.1007/s10803-015-2392-y [DOI] [PubMed] [Google Scholar]
- Kirkovski M, Enticott PG, & Fitzgerald PB (2013). A Review of the Role of Female Gender in Autism Spectrum Disorders. J Autism Dev Disord. 10.1007/s10803-013-1811-1 [DOI] [PubMed] [Google Scholar]
- Klusek J, Martin GE, & Losh M (2014). Consistency between research and clinical diagnoses of autism among boys and girls with fragile X syndrome. Journal of Intellectual Disability Research. 10.1111/jir.12121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott C, Will EA, & Roberts JE (2019). Sensory Processing in Relation to Sleep Disturbances in Young Children with Autism Spectrum Disorder and Fragile X Syndrome with and without Comorbid Autism Spectrum Disorder. Annual Meeting of the International Society for Autism Research. Montreal, Quebec. [Google Scholar]
- Lee M, Martin GE, Berry-Kravis E, & Losh M (2016). A developmental, longitudinal investigation of autism phenotypic profiles in fragile X syndrome. Journal of Neurodevelopmental Disorders, 8, 47. 10.1186/s11689-016-9179-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Petkova E, Hus V, Gan W, Lu F, Martin DM, … Risi S (2012). A multisite study of the clinical diagnosis of different autism spectrum disorders. Archives of General Psychiatry, 69(3), 306–313. 10.1001/archgenpsychiatry.2011.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, & Pickles A (2006). Autism from 2 to 9 years of age. Archives of General Psychiatry. 10.1001/archpsyc.63.6.694 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, & Bishop S (2012). Autism Diagnostic Observation Schedule, Second Edition: ADOS-2. Torrance, CA: Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. [DOI] [PubMed] [Google Scholar]
- Lundstrom S, Chang Z, Kerekes N, Gumpert CH, Rastam M, Gillberg C, … Anckarsater H (2011). Autistic-like traits and their association with mental health problems in two nationwide twin cohorts of children and adults. Psychological Medicine, 00(00), 1–11. [DOI] [PubMed] [Google Scholar]
- McCormick CEB, Sheinkopf SJ, Levine TP, LaGasse LL, Tronick E, & Lester BL (2018). Diminished respiratory sinus arrhythmia response in infants later diagnosed with autism spectrum disorder. Autism Research. 10.1002/aur.1929 [DOI] [PubMed] [Google Scholar]
- McDuffie A, Abbeduto L, Lewis P, Kover S, Kim JS, Weber A, & Brown WT (2010). Autism spectrum disorder in children and adolescents with fragile X syndrome: Within-syndrome differences and age-related changes. American Journal on Intellectual and Developmental Disabilities, 115(4), 307–326. 10.1352/1944-7558-115.4.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merenstein SA, Sobesky WE, Taylor AK, Riddle JE, Tran HX, & Hagerman RJ (1996). Molecular-clinical correlations in males with an expanded FMR1 mutation. American Journal of Medical Genetics, 64(2), 388–394. [DOI] [PubMed] [Google Scholar]
- Mullen E (1995). Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service. [Google Scholar]
- Muthen LK, & Muthen BO (2017). Mplus User’s Guide. Los Angeles, CA: Muthen & Muthen. [Google Scholar]
- Ozonoff S, Young GS, Landa RJ, Brian J, Bryson S, Charman T, … Iosif A-M (2015). Diagnostic stability in young children at risk for autism spectrum disorder: a baby siblings research consortium study. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 56(9), 988–998. 10.1111/jcpp.12421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriquin MA, Hartwig EM, Friedman BH, Porges SW, & Scarpa A (2019). Autonomic response in autism spectrum disorder: Relationship to social and cognitive functioning. Biological Psychology, 145, 185–197. 10.1016/j.biopsycho.2019.05.004 [DOI] [PubMed] [Google Scholar]
- Paulus FW, Backes A, Sander CS, Weber M, & von Gontard A (2015). Anxiety Disorders and Behavioral Inhibition in Preschool Children: A Population-Based Study. Child Psychiatry & Human Development, 46(1), 150–157. 10.1007/s10578-014-0460-8 [DOI] [PubMed] [Google Scholar]
- Porges SW, & Bohrer RE (1990). Analyses of periodic processes in psychophysiological research (Cacioppo JT & Tassinary LG, Eds.). New York: Cambridge University Press. [Google Scholar]
- R Core Team. (2019). R: A language and environment for statistical compting.
- Rague L, Caravella K, Tonnsen BL, Klusek J, & Roberts JE (2018). Early gesture use in fragile X syndrome. Journal of Intellectual Disability Research, 62(7), 625–636. 10.1111/jir.12498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Crawford H, Hogan AL, Fairchild A, Tonnsen BL, Brewe A, … Abbeduto L (2019). Social Avoidance Emerges in Infancy and Persists into Adulthood in Fragile X Syndrome. Journal of Autism and Developmental Disorders. 10.1007/s10803-019-04051-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Crawford H, Will EA, Hogan AL, McQuillin S, Tonnsen BL, … Brewe AM (2019). Infant social avoidance predicts autism but not anxiety in fragile X syndrome. Frontiers in Psychiatry, 10(May). 10.3389/fpsyt.2019.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Ezell JE, Fairchild AJ, Klusek J, Thurman AJ, McDuffie A, & Abbeduto L (2018). Biobehavioral composite of social aspects of anxiety in young adults with fragile X syndrome contrasted to autism spectrum disorder. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics, 177(7), 665–675. 10.1002/ajmg.b.32674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Tonnsen BL, McCary LM, Caravella KE, & Shinkareva SV (2016). Brief Report: Autism Symptoms in Infants with Fragile X Syndrome. Journal of Autism and Developmental Disorders, 46(12), 3830–3837. 10.1007/s10803-016-2903-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Tonnsen BL, Robinson A, & Shinkareva SV (2012). Heart activity and autistic behavior in infants and toddlers with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities, 117(2), 90–102. 10.1352/1944-7558-117.2.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Weisenfeld LH, Hatton D, Heath M, & Kaufmann WE (2007). Social approach and autistic behavior in children with fragile X syndrome. Journal of Autism and Developmental Disorders, 37, 1748–1760. [DOI] [PubMed] [Google Scholar]
- Robins DL, Fein D, Barton ML, & Green JA (2001). The Modified Checklist for Autism in Toddlers: An Initial Study Investigating the Early Detection of Autism and Pervasive Developmental Disorders. In Journal of Autism and Developmental Disorders (Vol. 31). [DOI] [PubMed] [Google Scholar]
- Rogers S, Wehner E, & Hagerman RJ (2001). The Behavioral Phenotype in Fragile X: Symptoms of Autism in Very Young Children with Fragile X Syndrome, Idiopathic Autism, and Other Developmental Disorders. Journal of Developmental & Behavioral Pediatrics, 22(6), 409–417. [DOI] [PubMed] [Google Scholar]
- Rutter M, LeCouteur A, & Lord C (2003). Autism Diagnostic Interview – Revised (ADI–R) Manual. Los Angeles: Western Psychological Services. [Google Scholar]
- Salazar F, Baird G, Chandler S, Tseng E, O’sullivan T, Howlin P, … Simonoff E (2015). Co-occurring Psychiatric Disorders in Preschool and Elementary School-Aged Children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 45(8), 2283–2294. 10.1007/s10803-015-2361-5 [DOI] [PubMed] [Google Scholar]
- Schopler E, Van Bourgondien M, Wellman J, & Love S (2010). Childhood autism rating scale—Second edition (CARS-2). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Sheinkopf SJ, Levine TP, McCormick CEB, Puggioni G, Conradt E, Lagasse LL, & Lester BM (2019). Developmental trajectories of autonomic functioning in autism from birth to early childhood. Biological Psychology, 142, 13–18. 10.1016/j.biopsycho.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumway S, Farmer C, Thurm A, Joseph L, Black D, & Golden C (2012). The ADOS Calibrated Severity Score: Relationship to Phenotypic Variables and Stability over Time. Autism Research, 5(4), 267–276. 10.1002/aur.1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simberlund J, & Veenstra-VanderWeele J (2019). Fragile X: Autism in the Setting of a Known Genetic Syndrome. In Pediatric Neuropsychiatry (pp. 67–74). 10.1007/978-3-319-94998-7_7 [DOI] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, & Baird G (2008). Psychiatric Disorders in Children With Autism Spectrum Disorders: Prevalence, Comorbidity, and Associated Factors in a Population-Derived Sample. Journal of the American Academy of Child & Adolescent Psychiatry, 47(8), 921–929. 10.1097/CHI.0b013e318179964f [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, & Balla DA (2005). Vineland Adaptive Behavior Scales, Second Edition (Vineland II) (2nd ed.). San Antonio, TX: Pearson. [Google Scholar]
- Sullivan K, Hatton D, Hammer J, Sideris J, Hooper S, Ornstein P, & Bailey D (2006). ADHD symptoms in children with FXS. American Journal of Medical Genetics Part A, 140A(21), 2275–2288. 10.1002/ajmg.a.31388 [DOI] [PubMed] [Google Scholar]
- Swanson MR, Wolff JJ, Shen MD, Styner M, Estes A, Gerig G, … Hazlett HC (2018). Development of white matter circuitry in infants with fragile x syndrome. JAMA Psychiatry, 75(5), 505–513. 10.1001/jamapsychiatry.2018.0180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurm A, Farmer C, Salzman E, Lord C, & Bishop S (2019). State of the Field: Differentiating Intellectual Disability From Autism Spectrum Disorder. Frontiers in Psychiatry, 10. 10.3389/fpsyt.2019.00526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnsen BL, Richards JE, & Roberts JE (2018). Heart rate-defined sustained attention in infants at risk for autism. Journal of Neurodevelopmental Disorders, 10(1), 7. 10.1186/s11689-018-9224-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnsen BL, Shinkareva SV, Deal SC, Hatton DD, & Roberts JE (2013). Biobehavioral indicators of social fear in young children with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities, 118(6), 447–459. 10.1352/1944-7558-118.6.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren Z, McPheeters ML, Sathe N, Foss-Feig JH, Glasser A, & Veenstra-VanderWeele J (2011). A Systematic Review of Early Intensive Intervention for Autism Spectrum Disorders. Pediatrics, 127(5), e1303–e1311. 10.1542/peds.2011-0426 [DOI] [PubMed] [Google Scholar]
- Wheeler AC, Mussey J, Villagomez A, Bishop E, Raspa M, Edwards A, … Bailey DB (2015). DSM-5 Changes and the Prevalence of Parent-Reported Autism Spectrum Symptoms in Fragile X Syndrome. Journal of Autism and Developmental Disorders, 45(3), 816–829. 10.1007/s10803-014-2246-z [DOI] [PubMed] [Google Scholar]
- Will EA, Bishop SL, & Roberts JE (2019). Developmental divergence: Motor trajectories in children with fragile X syndrome with and without co-occurring autism. Journal of Neurodevelopmental Disorders, 11(1), 1–10. 10.1186/s11689-019-9281-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, & Szatmari P (2005). Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience, 23(2–3), 143–152. 10.1016/j.ijdevneu.2004.05.001 [DOI] [PubMed] [Google Scholar]


