Abstract
Background
Recently, several novel scoring systems have been developed to evaluate the severity and outcomes of acute pancreatitis. This study aimed to compare the effectiveness of novel and conventional scoring systems in predicting the severity and outcomes of acute pancreatitis.
Methods
Patients treated between January 2003 and August 2020 were reviewed. The Ranson score (RS), Glasgow score (GS), bedside index of severity in acute pancreatitis (BISAP), pancreatic activity scoring system (PASS), and Chinese simple scoring system (CSSS) were determined within 48 h after admission. Multivariate logistic regression was used for severity, mortality, and organ failure prediction. Optimum cutoffs were identified using receiver operating characteristic curve analysis.
Results
A total of 1848 patients were included. The areas under the curve (AUCs) of RS, GS, BISAP, PASS, and CSSS for severity prediction were 0.861, 0.865, 0.829, 0.778, and 0.816, respectively. The corresponding AUCs for mortality prediction were 0.693, 0.736, 0.789, 0.858, and 0.759. The corresponding AUCs for acute respiratory distress syndrome prediction were 0.745, 0.784, 0.834, 0.936, and 0.820. Finally, the corresponding AUCs for acute renal failure prediction were 0.707, 0.734, 0.781, 0.868, and 0.816.
Conclusions
RS and GS predicted severity better than they predicted mortality and organ failure, while PASS predicted mortality and organ failure better. BISAP and CSSS performed equally well in severity and outcome predictions.
Keywords: Acute pancreatitis, Severity, Acute respiratory distress syndrome, Acute renal failure, Mortality, Scoring system, Predict, Retrospective
Background
Acute pancreatitis (AP) is an inflammatory disease of the pancreas with a worldwide incidence varying from 33.2/100,000 to 45/100,000 in the general population [1–3]. Approximately 10% ~ 20% of patients with AP have a severe clinical course, with significant morbidity and mortality due to local and systemic complications [3–6]. Acute respiratory distress syndrome (ARDS) and acute renal failure (ARF) are common complications of severe acute pancreatitis, and result in worse outcomes [7–9]. Therefore, the early detection of ARDS and ARF in patients with AP is indispensable.
Many studies have compared biochemical markers and various scoring systems in the early stage to predict disease course and outcomes in AP [10–13]. Conventional scoring systems, including the Ranson score (RS), Glasgow score (GS), and Acute Physiology, Chronic Health Evaluation (APACHE) II score, and bedside index of severity in acute pancreatitis (BISAP) have been used to assess the severity of AP. However, these scores are complicated and require multiple difficult clinical parameters for risk stratification. Although biomarkers are easy to obtain, their ability in predicting outcomes varies [14–17]. Recently, some novel scoring systems have been developed. A prospective cohort study [18] showed that the pancreatic activity scoring system (PASS; Table 1), which was first reported by the Southern California Pancreas Study Group in 2017 [19], could predict important clinical events at different points during the course of AP. Another new scoring system called the Chinese simple scoring system (CSSS; Table 2) was proposed in 2020 [20]. Both scoring systems are not yet widely used.
Table 1.
Pancreatic activity scoring system (PASS)
| Parameter | Weights |
|---|---|
| Organ failure | × 100 for each system |
| SIRS | × 25 for each criterion |
| Abdominal pain (0–10) | × 5 |
| Morphine equivalent dose (mg) | × 5 |
| Tolerating solid diet (yes = 0, no = 1) | × 40 |
SIRS Systemic inflammatory response syndrome; Organ failure definition: modified Marshall or SOFA score ≥ 2 points in any category
Table 2.
Chinese simple scoring system (CSSS)
| Variables | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Serum creatinine (μmol/L) | < 100 | >100 | |||
| Blood glucose (mmol/L) | < 12 | >12 | |||
| LDH (U/L) | < 380 | >380 | |||
| CRP (mmol/L) | < 65 | >65 | |||
| Heart rate (beats/min) | < 100 | >100 | |||
| Extent of pancreatic necrosis | 0 | < 30% | 30–50% | 50–70% | >70% |
LDH Lactate dehydrogenase, CRP C-reactive protein
The present study aimed to specifically determine the accuracy of these conventional and novel scoring systems as well as biomarkers in predicting severity, mortality, and organ failure in patients with AP.
Materials and methods
Study design and patient selection
A retrospective study was conducted. Records of patients with AP who were treated in The First Affiliated Hospital of Guangxi Medical University, between January 2003 and July 2020, were reviewed.
Patients were diagnosed with AP if they met at least two of the following three criteria: (1) abdominal pain consistent with AP, (2) serum lipase activity or amylase activity at least three times greater than the upper limit of normal, and (3) characteristic findings on abdominal imaging. Patients younger than 16 years, those known to have chronic pancreatitis, or those without sufficient data were excluded from the study.
Definitions of severity and organ failure
Severity of AP was evaluated based on the revised Atlanta classification [21]. Mild AP was defined as AP in the absence of organ failure and local/systemic complications. Severe AP was characterized by the presence of organ failure and/or local complications. Organ failure was defined according to the modified Marshall scoring system [22].
Biochemical markers, scoring systems, and their cutoffs
Biochemical markers measured within 48 h after admission were analysed. RS [23], GS [24], BISAP [25], PASS [19], and CSSS [20] were calculated for each patient within 48 h after admission. Scores were compared for their accuracy in the prediction of disease severity, mortality, and development of organ failure (ARDS and ARF).
Statistical analysis
SPSS v23.0 (IBM Corp., Armonk, NY) was used for statistical analyses. Continuous variables were displayed as mean ± standard deviation. The Student t-test was used for continuous variables. The chi-square test was used for categorical variables. Univariate and multivariate logistic regression analyses were carried out to identify risk factors. Potential risk factors with P < 0.05 in the univariate analyses were enrolled into the binary logistic backward stepwise regression analysis. The results are presented as odds ratios (OR) with 95% confidence intervals (CIs). ROC curves of the scores were used for the prediction of severe AP, mortality, ARDS, and ARF. Areas under the curve (AUCs) were used to evaluate the predictive accuracy of each scoring system. All optimum cutoffs were identified on the basis of the highest sensitivity and specificity values generated from the ROC curves. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. A P value of less than 0.05 was considered to indicate statistical significance.
Results
Baseline characteristics
Among 1848 patients enrolled, 1164 (62.99%) had mild AP and 684 (37.01%) had severe AP. The mean age of the patients was 48.22 ± 16.21 years. The mean age of severe group was significantly higher in the severe AP group than in the mild AP group (P < 0.001). A male preponderance (68.19%) was found. ARF was more common in male patients than in female patients (P < 0.001). A higher body-mass index (BMI) was observed in the severe AP group than in the mild AP group (P < 0.001). The BMI of patients with ARDS/ARF was higher than those of patients without ARDS/ARF (P < 0.05; Table 3). Gallstones (38.47%) were the most common cause of AP, followed by hypertriglyceridemia (16.72%) and alcohol consumption (10.77%). Alcohol-associated pancreatitis was more common in the severe AP group, ARDS group, and ARF group (Table 3). Hyperlipidemia (14.88%) and type-2 diabetes mellitus (7.52%) were common comorbidities. A history of smoking and alcohol intake history was present in 541 (29.27%) and 591 (31.98%) patients, respectively. Alcohol consumption was more common in patients with severe AP (P < 0.001), ARDS (P = 0.002), and ARF (P < 0.001; Table 3). Longer hospital stay was observed in patients with severe AP than in patients with mild AP (P < 0.001). The mortality rate was much higher in the severe AP group than in the mild AP group (P < 0.001; Table 3).
Table 3.
Univariate analysis of factors associated with severity, mortality, ARDS, and ARF in AP
| Characteristic | Severity | Mortality | ARDS | ARF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild (n = 1164) |
Severe (n = 684) |
P | Survivor (n = 1782) |
Non-survivor (n = 66) |
P | No (n = 1735) |
Yes (n = 113) |
P | No (n = 1706) |
Yes (n = 142) |
P | |
| Age, y | 46.22 (15.40) | 51.62 (16.99) | < 0.001 | 48.07 (16.10) | 52.09 (18.69) | 0.048 | 48.12 (16.18) | 49.79 (16.66) | 0.288 | 48.12 (16.22) | 49.41 (16.12) | 0.363 |
| Male gender, n (%) | 783 (67.27) | 477 (69.74) | 0.271 | 1210 (67.90) | 50 (75.76) | 0.178 | 1175 (67.72) | 85 (75.22) | 0.097 | 1142 (66.94) | 118 (83.10) | < 0.001 |
| BMI, kg/m2 | 23.43 (4.26) | 24.73 (4.52) | < 0.001 | 23.99 (4.45) | 23.07 (2.92) | 0.293 | 23.85 (4.39) | 25.44 (4.44) | 0.009 | 23.85 (4.33) | 25.51 (5.16) | 0.006 |
| Comorbidities, n (%) | ||||||||||||
| Hyperlipidemia | 169 (14.52) | 106 (15.50) | 0.568 | 267 (14.98) | 8 (12.12) | 0.521 | 257 (14.81) | 18 (15.93) | 0.747 | 245 (14.36) | 30 (21.13) | 0.03 |
| T2DM | 85 (7.30) | 54 (7.89) | 0.641 | 134 (7.52) | 5 (7.58) | 0.986 | 130 (7.49) | 9 (7.96) | 0.854 | 135 (7.91) | 4 (2.82) | 0.013 |
| Etiology, n (%) | ||||||||||||
| Gallstones | 467 (40.12) | 244 (35.67) | 0.058 | 697 (39.11) | 14 (21.21) | 0.003 | 681 (39.25) | 30 (26.55) | 0.007 | 679 (39.80) | 32 (22.54) | < 0.001 |
| Alcoholism | 101 (8.68) | 98 (14.33) | < 0.001 | 191 (10.72) | 8 (12.12) | 0.718 | 178 (10.26) | 21 (18.58) | 0.006 | 169 (9.91) | 30 (21.13) | < 0.001 |
| Hypertriglyceridemia | 186 (15.98) | 123 (17.98) | 0.265 | 301 (16.89) | 11 (16.67) | 0.308 | 288 (16.60) | 21 (18.58) | 0.584 | 278 (16.30) | 31 (21.83) | 0.089 |
| Smoker, n (%) | 337 (28.95) | 204 (29.82) | 0.691 | 525 (29.46) | 16 (24.24) | 0.36 | 505 (29.11) | 36 (31.86) | 0.533 | 490 (28.72) | 51 (35.92) | 0.07 |
| Alcohol intake history, n (%) | 334 (28.69) | 257 (37.57) | < 0.001 | 568 (31.87) | 23 (34.85) | 0.611 | 540 (31.12) | 51 (45.13) | 0.002 | 520 (30.48) | 71 (50.00) | < 0.001 |
| Hospital stay, d | 12.35 (8.18) | 16.56 (11.95) | < 0.001 | 14.01 (9.68) | 10.08 (14.82) | 0.042 | 13.53 (8.85) | 19.38 (19.8) | 0.003 | 13.58 (9.02) | 17.52 (17.16) | 0.009 |
| Death, n (%) | 6 (0.52) | 60 (8.77) | < 0.001 | – | – | – | 29 (1.67) | 37 (32.74) | < 0.001 | 26 (1.52) | 40 (28.17) | < 0.001 |
| WBC (*109/L) | 10.46 (4.96) | 14.71 (6.37) | < 0.001 | 11.94 (5.77) | 14.58 (8.26) | 0.012 | 11.84 (5.81) | 14.89 (6.36) | < 0.001 | 11.79 (5.58) | 14.92 (8.28) | < 0.001 |
| Hemoglobin (g/L) | 127.61 (21.99) | 128.11 (30.90) | 0.713 | 128.26 (24.63) | 115.28 (43.57) | 0.019 | 127.78 (24.37) | 127.99 (40.63) | 0.957 | 127.78 (23.77) | 127.99 (42.19) | 0.955 |
| Hematocrit | 0.46 (1.52) | 0.41 (0.52) | 0.408 | 0.45 (1.26) | 0.33 (0.10) | 0.452 | 0.45 (1.28) | 0.39 (0.15) | 0.632 | 0.45 (1.29) | 0.40 (0.23) | 0.652 |
| BUN (mmol/L) | 4.70 (4.51) | 7.06 (6.74) | < 0.001 | 5.35 (5.16) | 12.37 (10.53) | < 0.001 | 5.27 (5.01) | 10.40 (9.88) | < 0.001 | 4.93 (4.32) | 13.50 (10.68) | < 0.001 |
| Creatinine (μmol/L) | 77.41 (35.51) | 109.02 (99.68) | < 0.001 | 85.73 (60.61) | 191.88 (159.04) | < 0.001 | 84.88 (58.86) | 156.95 (140.72) | < 0.001 | 76.51 (28.59) | 244.83 (163.11) | < 0.001 |
| Total bilirubin (μmol/L) | 38.25 (67.41) | 41.66 (52.51) | 0.277 | 38.41 (59.80) | 72.16 (107.79) | 0.023 | 39.54 (62.76) | 39.40 (53.76) | 0.983 | 39.07 (60.74) | 45.55 (79.37) | 0.274 |
| Albumin (g/L) | 37.78 (7.16) | 34.19 (7.33) | < 0.001 | 36.69 (7.31) | 28.97 (7.02) | < 0.001 | 36.6.69 (7.36) | 32.31 (7.32) | < 0.001 | 36.76 (7.24) | 32.04 (8.40) | < 0.001 |
| AST (IU/L) | 68.94 (113.56) | 104.17 (293.67) | 0.004 | 76.66 (121.03) | 244.29 (894.16) | 0.145 | 82.42 (206.45) | 76.95 (82.21) | 0.794 | 76.34 (120.21) | 158.44 (618.75) | 0.151 |
| Calcium (mmol/L) | 2.20 (0.24) | 2.00 (0.33) | < 0.001 | 2.14 (0.27) | 1.85 (0.63) | 0.001 | 2.14 (0.27) | 1.89 (0.48) | < 0.001 | 2.14 (0.27) | 1.90 (0.44) | < 0.001 |
| Blood glucose (mmol/L) | 7.41 (3.84) | 10.21 (5.37) | < 0.001 | 8.25 (4.47) | 13.33 (7.78) | 0.003 | 8.20 (4.40) | 11.54 (7.09) | 0.001 | 8.12 (4.26) | 12.59 (7.69) | < 0.001 |
| LDH (IU/L) | 234.9 (104.21) | 420.76 (235.22) | < 0.001 | 305.78 (186.17) | 476.00 (326.16) | 0.003 | 298.31 (177.79) | 493.86 (291.49) | < 0.001 | 298.65 (176.75) | 475.35 (300.05) | < 0.001 |
| Triglycerides (mmol/L) | 2.89 (4.37) | 4.5 (6.08) | < 0.001 | 3.51 (5.12) | 3.99 (6.52) | 0.558 | 3.38 (5.00) | 5.60 (6.88) | < 0.006 | 3.27 (4.79) | 6.7 (8.06) | < 0.001 |
| CRP (mg/L) | 78.53 (58.34) | 138.28 (60.29) | < 0.001 | 109.77 (66.56) | 128.77 (62.19) | 0.036 | 106.83 (65.32) | 143.21 (66.71) | < 0.001 | 107.57 (66.88) | 134.55 (58.07) | < 0.001 |
| Ranson score | 0.67 (0.77) | 2.57 (1.37) | < 0.001 | 1.34 (1.36) | 2.41 (1.58) | < 0.001 | 1.29 (1.33) | 2.64 (1.46) | < 0.001 | 1.29 (1.32) | 2.45 (1.61) | < 0.001 |
| Glasgow score | 0.48 (0.69) | 2.24 (1.25) | < 0.001 | 1.09 (1.23) | 2.39 (1.53) | < 0.001 | 1.05 (1.22) | 2.45 (1.20) | < 0.001 | 1.04 (1.20) | 2.28 (1.21) | < 0.001 |
| BISAP | 0.6 (0.72) | 1.95 (1.1) | < 0.001 | 1.05 (1.06) | 2.42 (1.25) | < 0.001 | 1.01 (1.04) | 2.49 (1.00) | < 0.001 | 1.00 (1.02) | 2.27 (1.20) | < 0.001 |
| PASS | 105.51 (52.27) | 172.05 (81.08) | < 0.001 | 125.56 (66.82) | 253.64 (94.44) | < 0.001 | 120.82 61.05) | 273.19 (76.21) | < 0.001 | 120.73 (61.42) | 243.11 (91.49) | < 0.001 |
| CSSS | 0.55 (0.78) | 2.12 (1.50) | < 0.001 | 1.08 (1.29) | 2.62 (1.69) | < 0.001 | 1.01 (1.22) | 2.98 (1.65) | < 0.001 | 0.99 (1.19) | 2.89 (1.66) | < 0.001 |
ARDS Acute respiratory distress syndrome, ARF Acute renal failure, AP Acute pancreatitis, BMI Body-mass index, T2DM type-2 diabetes mellitus; WBC White blood cell count, BUN Blood urea nitrogen, CRP C-reactive protein; AST: aspartate transaminase, LDH Lactate dehydrogenase, BISAP Bedside index of severity in acute pancreatitis, PASS Pancreatic activity scoring system, CSSS Chinese simple scoring system. P < 0.05 was accepted as statistically significant
Value of biomarkers in predicting severity, mortality, and organ failure
In the multivariate analysis, white blood cell count (WBC), serum albumin, lactate dehydrogenase (LDH), calcium, glucose, and C-reactive protein (CRP) predicted the severity of AP. Their ORs for predicting severe AP were 1.110 (95% CI, 1.040–1.184), 0.940 (95% CI, 0.894–0.989), 1.004 (95% CI, 1.002–1.006), 0.196 (95% CI, 0.065–0.592), 1.081 (95% CI, 1.016–1.150), and 1.007 (95% CI, 1.003–1.012), respectively. Serum total bilirubin was found to be an independent predictor of mortality (OR, 1.013; 95% CI, 1.004–1.023). For predicting organ failure, BMI, WBC and serum calcium were independent variables for ARDS, while blood urea nitrogen and serum triglyceride were independent variables for ARF. However, among them only serum calcium showed a better OR value than other variables (Table 4).
Table 4.
Multivariate analysis of factors predicting severity, mortality, ARDS, and ARF in AP
| Characteristic | Severity | Mortality | ARDS | ARF | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age | 0.994 (0.975–1.014) | 0.575 | 1.023 (0.97–-1.069) | 0.308 | – | – | – | – |
| Male gender | – | – | – | – | – | – | 0.731 (0.101–5.295) | 0.731 |
| BMI, kg/m2 | 0.985 (0.919–1.055) | 0.66 | – | – | 1.139 (1.022–1.271) | 0.019 | 1.125 (0.996–1.269) | 0.057 |
| Etiology | ||||||||
| Gallstones | 1.256 (0.635–2.487) | 0.512 | 0.255 (0.036–1.826) | 0.174 | 1.794 (0.620–5.193) | 0.281 | 0.974 (0.197–4.821) | 0.974 |
| Alcoholism | 1.416 (0.526–3.808) | 0.491 | – | – | 0.378 (0.074–1.923) | 0.241 | 0.844 (0.153–4.649) | 0.846 |
| Hypertriglyceridemia | 0.365 (0.065–2.036) | 0.25 | ||||||
| Smoker | 0.996 (0.285–3.488) | 0.995 | ||||||
| Alcohol intake history | 0.862 (0.467–1.590) | 0.634 | – | – | 1.956 (0.657–5.827) | 0.228 | 3.613 (0.810–16.122) | 0.092 |
| Comorbidities | ||||||||
| Hyperlipidemia | – | – | – | – | – | – | 1.501 (0.529–4.26) | 0.446 |
| T2DM | – | – | – | – | – | – | 0.999 (0.363–2.749) | 0.998 |
| WBC (*109/L) | 1.110 (1.040–1.184) | 0.002 | 0.946 (0.819–1.094) | 0.456 | 1.135 (1.048–1.23) | 0.002 | 0.946 (0.839–1.067) | 0.368 |
| Hemoglobin (g/L) | – | – | 1.023 (0.994–1.052) | 0.118 | – | – | – | – |
| BUN (mmol/L) | 1.124 (0.974–1.297) | 0.109 | 1.013 (0.914–1.122) | 0.808 | 0.99 (0.917–1.069) | 0.802 | 1.243 (1.097–1.408) | 0.001 |
| Creatinine (μmol/L) | 1.005 (0.996–1.015) | 0.268 | 1.006 (0.999–1.013) | 0.105 | 1.002 (0.996–1.009) | 0.484 | – | – |
| Total bilirubin (μmol/L) | – | – | 1.013 (1.004–1.023) | 0.007 | – | – | – | – |
| Albumin (g/L) | 0.940 (0.894–0.989) | 0.016 | 0.948 (0.833–1.079) | 0.418 | 1.035 (0.978–1.095) | 0.234 | 0.939 (0.854–1.032) | 0.191 |
| AST (IU/L) | 1.002 (0.999–1.006) | 0.18 | – | – | – | – | – | – |
| Calcium (mmol/L) | 0.196 (0.065–0.592) | 0.004 | 0.882 (0.089–8.692) | 0.914 | 0.042 (0.006–0.303) | 0.002 | 1.205 (0.313–4.639) | 0.786 |
| Blood glucose (mmol/L) | 1.081 (1.016–1.150) | 0.014 | 1.023 (0.916–1.143) | 0.686 | 1.021 (0.938–1.112) | 0.624 | 1.054 (0.956–1.162) | 0.294 |
| LDH (IU/L) | 1.004 (1.002–1.006) | < 0.001 | 1.003 (1.000–1.005) | 0.061 | 1.000 (0.998–1.002) | 0.785 | 1.000 (0.998–1.003) | 0.781 |
| Triglycerides (mmol/L) | 1.022 (0.961–1.086) | 0.486 | – | – | 0.943 (0.845–1.051) | 0.287 | 1.119 (1.012–1.239) | 0.029 |
| CRP (mg/L) | 1.007 (1.003–1.012) | 0.002 | 0.999 (0.988–1.011) | 0.844 | 1.002 (0.995–1.008) | 0.409 | 1.000 (0.993–1.008) | 0.926 |
ARDS Acute respiratory distress syndrome, ARF Acute renal failure, AP acute pancreatitis, BMI Body-mass index, T2DM Type-2 diabetes mellitus, CRP C-reactive protein, AST Aspartate transaminase, BUN Blood urea nitrogen, LDH Lactate dehydrogenase, BISAP Bedside index of severity in acute pancreatitis, PASS Pancreatic activity scoring system, CSSS Chinese simple scoring system, WBC White blood cell count, OR Odds ratio, CI Confidence interval. P < 0.05 was accepted as statistically significant
Value of scoring Systems in Predicting Severity, mortality, and organ failure
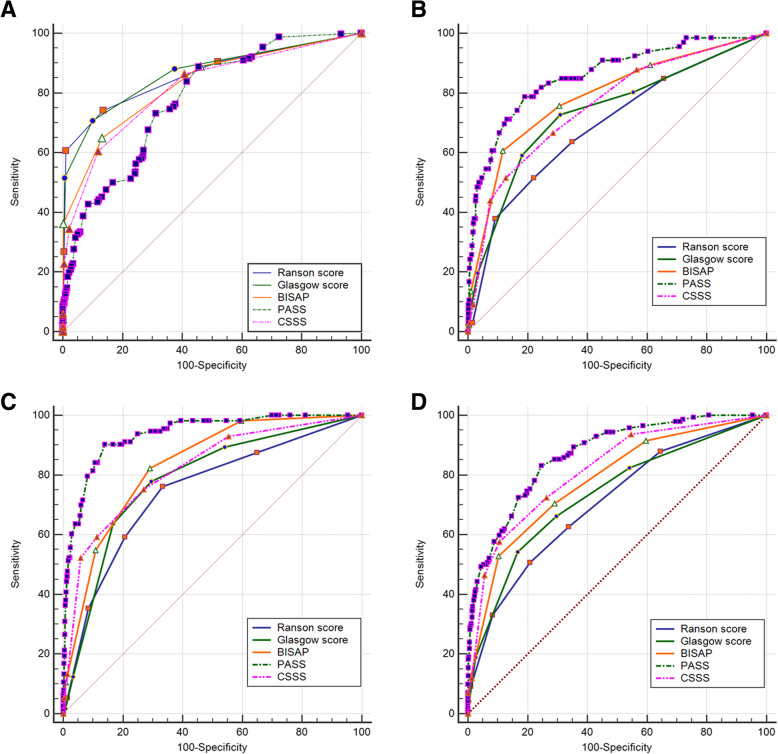
For severe AP prediction, the ROC curve indicated an AUC of 0.861 for RS, 0.865 for GS, 0.829 for BISAP, 0.778 for PASS, and 0.816 for CSSS. The cutoffs used were as follow: RS, at least 2; GS, at least 2; BISAP, at least 2; PASS, at least 90; and CSSS, at least 2 (Table 5, Fig. 1a). For mortality prediction, the AUCs of the scoring systems were as follow: 0.693 for RS, 0.736 for GS, 0.789 for BISAP, 0.858 for PASS, and 0.759 for CSSS. The cutoffs of the scoring systems for mortality prediction were as follow: RS, at least 3; GS, at least 2; BISAP, at least 3; PASS, at least 190; and CSSS, at least 3 (Table 5, Fig. 1b). For ARDS prediction, the AUCs of scoring systems were as follow: 0.745 for RS, 0.784 for GS, 0.834 for BISAP, 0.936 for PASS, and 0.820 for CSSS. The cutoffs for RS, GS, BISAP, and CSSS were all at least 2, and the cutoff for PASS was at least 195 (Table 5, Fig. 1c). For ARF prediction, the AUCs of the scoring systems were as follow: 0.707 for RS, 0.734 for GS, 0.781 for BISAP, 0.868 for PASS, and 0.816 for CSSS. The cutoffs for RS, GS, BISAP, and CSSS were all at least 3, and the cutoff for PASS was at least 65 (Table 5, Fig. 1d).
Table 5.
Effectiveness of scoring systems for predicting severity, mortality, ARDS, and ARF in AP
| Cutoff | AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | |
|---|---|---|---|---|---|---|
| Severity | ||||||
| Ranson score | ≥2 | 0.861 (0.844–0.876) | 0.741 (0.707–0.774) | 0.864 (0.843–0.883) | 0.762 (0.728–0.794) | 0.850 (0.828–0.870) |
| Glasgow score | ≥ 2 | 0.865 (0.849–0.881) | 0.708 (0.672–0.742) | 0.900 (0.882–0.917) | 0.807 (0.773–0.838) | 0.840 (0.818–0.860) |
| BISAP | ≥ 2 | 0.829 (0.811–0.846) | 0.649 (0.612–0.685) | 0.869 (0.848–0.887) | 0.744 (0.707–0.778) | 0.808 (0.785–0.830) |
| PASS | ≥ 90 | 0.778 (0.759–0.797) | 0.889 (0.863–0.912) | 0.545 (0.516–0.574) | 0.534 (0.505–0.564) | 0.893 (0.868–0.915) |
| CSSS | ≥ 2 | 0.816 (0.797–0.833) | 0.605 (0.568–0.642) | 0.894 (0.876–0.910) | 0.750 (0.712–0.786) | 0.812 (0.791–0.832) |
| Mortality | ||||||
| Ranson score | ≥3 | 0.693 (0.671–0.714) | 0.515 (0.389–0.640) | 0.976 (0.967–0.983) | 0.500 (0.376–0.624) | 0.978 (0.968–0.985) |
| Glasgow score | ≥ 2 | 0.736 (0.715–0.756) | 0.727 (0.604–0.830) | 0.690 (0.668–0.712) | 0.080 (0.060–0.105) | 0.986 (0.977–0.991) |
| BISAP | ≥ 3 | 0.789 (0.770–0.807) | 0.606 (0.478–0.724) | 0.882 (0.866–0.897) | 0.160 (0.117–0.211) | 0.984 (0.976–0.989) |
| PASS | ≥ 190 | 0.858 (0.841–0.874) | 0.788 (0.670–0.879) | 0.809 (0.790–0.827) | 0.133 (0.101–0.170) | 0.990 (0.984–0.995) |
| CSSS | ≥ 3 | 0.759 (0.738–0.778) | 0.515 (0.389–0.640) | 0.872 (0.856–0.887) | 0.130 (0.092–0.177) | 0.980 (0.972–0.986) |
| ARDS | ||||||
| Ranson score | ≥2 | 0.745 (0.725–0.765) | 0.761 (0.672–0.836) | 0.666 (0.644–0.689) | 0.129 (0.105–0.157) | 0.977 (0.967–0.985) |
| Glasgow score | ≥2 | 0.784 (0.764–0.802) | 0.779 (0.691–0.851) | 0.705 (0.683–0.726) | 0.147 (0.119–0.178) | 0.980 (0.971–0.987) |
| BISAP | ≥2 | 0.834 (0.816–0.851) | 0.823 (0.740–0.888) | 0.710 (0.688–0.731) | 0.156 (0.127–0.187) | 0.984 (0.975–0.990) |
| PASS | ≥ 195 | 0.936 (0.924–0.946) | 0.903 (0.833–0.950) | 0.860 (0.843–0.876) | 0.296 (0.248–0.347) | 0.993 (0.987–0.996) |
| CSSS | ≥ 2 | 0.820 (0.802–0.838) | 0.752 (0.662–0.829) | 0.731 (0.709–0.752) | 0.154 (0.125–0.187) | 0.978 (0.969–0.986) |
| ARF | ||||||
| Ranson score | ≥ 3 | 0.707 (0.686–0.728) | 0.507 (0.422–0.592) | 0.792 (0.772–0.811) | 0.169 (0.134–0.208) | 0.951 (0.938–0.961) |
| Glasgow score | ≥ 3 | 0.734 (0.711–0.752) | 0.542 (0.457–0.626) | 0.857 (0.841–0.872) | 0.213 (0.172–0.259) | 0.963 (0.954–0.972) |
| BISAP | ≥ 3 | 0.781 (0.761–0.800) | 0.346 (0.283–0.413) | 0.897 (0.882–0.911) | 0.300 (0.244–0.361) | 0.915 (0.901–0.928) |
| PASS | ≥ 165 | 0.868 (0.852–0.883) | 0.831 (0.759–0.889) | 0.754 (0.733–0.774) | 0.219 (0.185–0.257) | 0.982 (0.973–0.988) |
| CSSS | ≥ 3 | 0.816 (0.798–0.834) | 0.578 (0.492–0.660) | 0.895 (0.879–0.909) | 0.313 (0.257–0.373) | 0.962 (0.952–0.971) |
ARDS Acute respiratory distress syndrome, ARF Acute renal failure, AP Acute pancreatitis, AUC Area under the curve, PPV Positive predictive value, NPV negative predictive value; BUN Blood urea nitrogen; BISAP Bedside index of severity in acute pancreatitis, PASS Pancreatic activity scoring system, CSSS Chinese simple scoring system, CI confidence interval
Fig. 1.
a Receiver operating characteristic curves of scoring systems to predict severe AP. b with AP. c Receiver operating characteristic curves of scoring systems to predict ARDS in patients with AP. d Receiver operating characteristic curves of scoring systems to predict ARF in patients with AP
Discussion
In the present study, BMI was an independent factor for the development of ARDS in AP patients, which is consistent with the result of a meta-analysis, that demonstrated that obesity was an important risk factor for the development of ARDS [26]. Studies have shown that patients who are obese have higher levels of circulating neutrophils [27] and blood cytokines [28], and have low-grade chronic inflammation triggered by obesity [29]. Moreover, innate immune cell activation and endothelial injury in the pulmonary microvasculature are major contributors to increased cell permeability and pulmonary edema in obese patients [30, 31].
This study revealed that serum Ca2+ showed good ORs for severity and ARDS prediction. Abnormal regulation of Ca2+ signals acts as a crucial trigger in the pathogenesis of AP [32]. A study has shown that hypocalcemia is an independent risk factor of severe AP and for respiratory failure in AP [33]. According to the present study, the WBC predicted the development of severe AP and ARDS. Furthermore, serum albumin, glucose, LDH, and CRP were also predictive factors for severe AP. These biomarkers are commonly used factors to predict severe AP. In terms of mortality prediction, the multivariate analysis identified that an increase in serum total bilirubin was a risk factor. Although few studies have reported a definite relationship between total bilirubin and mortality in AP, some studies have found that the albumin-bilirubin score has a high predictive capacity for in-hospital mortality or prognosis in patients with critical diseases such as acute upper gastrointestinal bleeding due to liver cirrhosis [34], post-operative hepatic carcinoma [35, 36], and AP [37]. Moreover, the present study showed that the elevation of serum triglycerides was a risk factor for ARF in AP patients, which is consistent with the findings of a meta-analysis reported in 2018 [38].
RS, GS, and BISAP showed high accuracy in predicting the severity rather than the outcomes of AP in the present study. RS and GS predicted the severity and 3 outcomes of AP equally well, which was probably due to the similar parameters they share. Although simple, these scores are not repeatable. According to this study, BISAP was inferior to both RS and GS in predicting severity, which is consistent with the findings of other prospective studies [39, 40]. This is because the items in RS and GS cover more systems than those in BISAP. Nevertheless, BISAP was superior to RS and GS in predicting mortality in the present study. Hall et al. also found that RS and GS were not good indicators of mortality in AP [41]. BISAP was also better than RS and GS at predicting ARDS and ARF, possibly because it is based on 3 important items that are related to the renal and respiratory systems, namely, blood urea nitrogen (BUN), systemic inflammatory response syndrome (SIRS), and pleural effusion.
PASS is a system that assesses the activity of AP at any time during hospitalization. It contains not only objective items (organ failure and SIRS), but also subjective items (abdominal pain, morphine usage and ability to tolerate solid diet). The repeatable items make it available to be used at any time during hospitalization. A prospective study [18] demonstrated that a cutoff PASS score of > 140 on admission was associated with an AUC of 0.71 for predicting severe AP. The present study found a similar AUC for PASS for severe AP prediction. As the center in which this study was conducted rarely uses morphine to relieve abdominal pain in patients with AP, the cutoff for severity prediction was only 90. In the present study, PASS scores best predicted mortality and organ failure, especially ARDS prediction. This is because PASS contains organ failure items. However, its subjective items (such as abdominal pain, morphine usage and ability to tolerate solid diet) make it inferior to other scores in severity prediction. Thus, no study has reported the predictive ability of PASS for the outcomes of AP.
Four biomarkers, heart rate, and pancreatic imaging findings are included in CSSS. According to the present study, the AUCs of CSSS for severity and mortality prediction were 0.834 and 0.838, respectively. The cutoff points were 4 for severity and 6 for mortality. However, the AUCs and cutoff points in this study are smaller than those reported in the previous study [20], which is probably attributable to the larger sample size of the current study. In the present study, CSSS showed nearly the same ability in predicting the 4 outcomes of AP, and it shared nearly equal capacity with BISAP for predicting the outcomes of AP, which indicates that CSSS is a promising scoring system. However, no study evaluating CSSS was found. Hence, studies with larger sample size and prospective designed are required to verify the efficiency of this new scoring system.
Study strengths and limitations
The strengths of the present study are that it compared both conventional and novel scoring systems as well as biomarkers in a large sample of Chinese patients for the prediction of the severity and outcomes of AP.
This study does have some limitations. First, this was a retrospective single-center study. Second, there was diversity in the period between the onset of AP and admission. This probably resulted in heterogeneity in the timings of score calculations and biochemical marker measurements.
Conclusion
RS and GS predicted severity better than mortality and organ failure, while PASS predicted mortality and organ failure better. As a novel scoring system, PASS has potential, but some of its items are not that suitable for Chinese medical centers. BISAP and CSSS performed equally well in severity and outcome prediction.
Acknowledgements
Not applicable.
Abbreviations
- ARF
Acute renal failure
- ARDS
Acute respiratory distress syndrome
- RS
Ranson score
- GS
Glasgow score
- APACHE
Acute physiology and chronic health evaluation
- BISAP
Bedside index of severity in acute pancreatitis
- PASS
Pancreatic activity scoring system
- CSSS
Chinese simple scoring system
- AUC
Area under the curve
- ROC
Receiver operating characteristic
- BMI
Body-mass index
- T2DM
Type-2 diabetes mellitus
- WBC
White blood cell count
- BUN
Blood urea nitrogen
- SIRS
Systemic inflammatory response syndrome
- LDH
Lactate dehydrogenase
- CRP
C-reactive protein
- AST
Aspartate transaminase
Authors’ contributions
QW and JW contributed in the conception of the work, designing the study, collecting biochemical data and revising the draft. MQ and GDT contributed in the conception of the work and designing the study. HYY contributed in the conception of the work and collecting the biochemical data. ZHL contributed in the conception of the work, conducting the study and revising the draft. All authors approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (81970558) and the Natural Science Foundation of Guangxi Province (2018GXNSFBA281154).
Availability of data and materials
All data used in this study are available from the corresponding author.
Declarations
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of First Affiliated Hospital of Guangxi Medical University (No. 2020(KY-E-177).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no Competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qing Wu and Jie Wang are contributed equally to this work and should be considered co-first authors.
References
- 1.Frey CF, Zhou H, Harvey DJ, White RH. The incidence and case-fatality rates of acute biliary, alcoholic, and idiopathic pancreatitis in California, 1994–2001. Pancreas. 2006;33:336–344. doi: 10.1097/01.mpa.0000236727.16370.99. [DOI] [PubMed] [Google Scholar]
- 2.Satoh K, Shimosegawa T, Masamune A, Hirota M, Kikuta K, Kihara Y, et al. Nationwide epidemiological survey of acute pancreatitis in Japan. Pancreas. 2011;40:503–507. doi: 10.1097/MPA.0b013e318214812b. [DOI] [PubMed] [Google Scholar]
- 3.Shen HN, Lu CL, Li CY. Epidemiology of first-attack acute pancreatitis in Taiwan from 2000 through 2009: a nationwide population-based study. Pancreas. 2012;41:696–702. doi: 10.1097/MPA.0b013e31823db941. [DOI] [PubMed] [Google Scholar]
- 4.Hamada S, Masamune A, Kikuta K, Hirota M, Tsuji I, Shimosegawa T, et al. Nationwide epidemiological survey of acute pancreatitis in Japan. Pancreas. 2014;43:1244–1248. doi: 10.1097/MPA.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 5.Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143–152. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y, Pan X, Zeng H, He W, Xia L, Liu P, et al. A Study on the Etiology, Severity, and Mortality of 3260 Patients With Acute Pancreatitis According to the Revised Atlanta Classification in Jiangxi, China Over an 8-Year Period. Pancreas. 2017;46:504–509. doi: 10.1097/MPA.0000000000000776. [DOI] [PubMed] [Google Scholar]
- 7.Naqvi R. Acute Kidney Injury in association with Acute Pancreatitis. Pak J Med Sci. 2018;34:606–609. doi: 10.12669/pjms.343.14953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin HY, Lai JI, Lai YC, Lin PC, Chang SC, Tang GJ. Acute renal failure in severe pancreatitis: A population-based study. Ups J Med Sci. 2011;116:155–159. doi: 10.3109/03009734.2010.547636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manohar M, Verma AK, Venkateshaiah SU, Sanders NL, Mishra A. Chronic Pancreatitis Associated Acute Respiratory Failure. MOJ Immunol. 2017;5. 10.15406/moji.2017.05.00149. [DOI] [PMC free article] [PubMed]
- 10.Tan YHA, Rafi S, Tyebally Fang M, Hwang S, Lim EW, Ngu J, et al. Validation of the modified Ranson versus Glasgow score for pancreatitis in a Singaporean population. ANZ J Surg. 2017;87:700–703. doi: 10.1111/ans.13139. [DOI] [PubMed] [Google Scholar]
- 11.Jones MJ, Neal CP, Ngu WS, Dennison AR, Garcea G. Early warning score independently predicts adverse outcome and mortality in patients with acute pancreatitis. Langenbecks. Arch Surg. 2017;402(5):811–819. doi: 10.1007/s00423-017-1581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasudevan S, Goswami P, Sonika U, Thakur B, Sreenivas V, Saraya A. Comparison of Various Scoring Systems and Biochemical Markers in Predicting the Outcome in Acute Pancreatitis. Pancreas. 2018;47:65–71. doi: 10.1097/MPA.0000000000000957. [DOI] [PubMed] [Google Scholar]
- 13.Zheng J, Fan J, Huang C, Lu Y, Huang Z, Wang X, et al. Dynamic Detection of Monocyte Subsets in Peripheral Blood of Patients with Acute Hypertriglyceridemic Pancreatitis. Gastroenterol Res Pract. 2019;2019:5705782. doi: 10.1155/2019/5705782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho JH, Kim TN, Chung HH, Kim KH. Comparison of scoring systems in predicting the severity of acute pancreatitis. world. J Gastroenterol. 2015;21(8):2387–2394. doi: 10.3748/wjg.v21.i8.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiat TTJ, Gunasekaran SK, Junnarkar SP, Low JK, Woon W, Shelat VG. Are traditional scoring systems for severity stratification of acute pancreatitis sufficient? Ann Hepatobiliary Pancreat Surg. 2018;22:105–115. doi: 10.14701/ahbps.2018.22.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagjer S, Kumar N. Evaluation of the BISAP scoring system in prognostication of acute pancreatitis - A prospective observational study. Int J Surg. 2018;54:76–81. doi: 10.1016/j.ijsu.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 17.de Grooth HJ, Geenen IL, Girbes AR, Vincent JL, Parienti JJ, Oudemans-van Straaten HM. SOFA and mortality endpoints in randomized controlled trials: a systematic review and meta-regression analysis. Crit Care. 2017;21:38. doi: 10.1186/s13054-017-1609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buxbaum J, Quezada M, Chong B, Gupta N, Yu CY, Lane C, da B, Leung K, Shulman I, Pandol S, Wu B. The pancreatitis activity scoring system predicts clinical outcomes in acute pancreatitis: findings from a prospective cohort study. am. J Gastroenterol. 2018;113(5):755–764. doi: 10.1038/s41395-018-0048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu BU, Batech M, Quezada M, Lew D, Fujikawa K, Kung J, Jamil LH, Chen W, Afghani E, Reicher S, Buxbaum J, Pandol SJ. Dynamic measurement of disease activity in acute pancreatitis: the pancreatitis activity scoring system. am. J Gastroenterol. 2017;112(7):1144–1152. doi: 10.1038/ajg.2017.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Zeng YB, Chen JY, Luo Q, Wang R, Zhang R, et al. A simple new scoring system for predicting the mortality of severe acute pancreatitis: A retrospective clinical study. Medicine (Baltimore) 2020;99:e20646. doi: 10.1097/MD.0000000000020646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 22.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet. 1974;139:69–81. [PubMed] [Google Scholar]
- 24.Blamey SL, Imrie CW, O'Neill J, Gilmour WH, Carter DC. Prognostic factors in acute pancreatitis. Gut. 1984;25:1340–1346. doi: 10.1136/gut.25.12.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut. 2008;57:1698–1703. doi: 10.1136/gut.2008.152702. [DOI] [PubMed] [Google Scholar]
- 26.Zhi G, Xin W, Ying W, Guohong X, Shuying L. “Obesity Paradox” in Acute Respiratory Distress Syndrome: Asystematic Review and Meta-Analysis. PLoS One. 2016;11:e0163677. doi: 10.1371/journal.pone.0163677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JA, Park HS. White blood cell count and abdominal fat distribution in female obese adolescents. Metabolism. 2008;57:1375–1379. doi: 10.1016/j.metabol.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Ramos EJ, Xu Y, Romanova I, Middleton F, Chen C, Quinn R, et al. Is obesity an inflammatory disease? Surgery. 2003;134:329–335. doi: 10.1067/msy.2003.267. [DOI] [PubMed] [Google Scholar]
- 29.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pontiroli AE, Frige F, Paganelli M, Folli F. In morbid obesity, metabolic abnormalities and adhesion molecules correlate with visceral fat, not with subcutaneous fat: effect of weight loss through surgery. Obes Surg. 2009;19:745–750. doi: 10.1007/s11695-008-9626-4. [DOI] [PubMed] [Google Scholar]
- 31.Cottam DR, Schaefer PA, Shaftan GW, Velcu L, Angus LD. Effect of surgically-induced weight loss on leukocyte indicators of chronic inflammation in morbid obesity. Obes Surg. 2002;12:335–342. doi: 10.1381/096089202321088101. [DOI] [PubMed] [Google Scholar]
- 32.Frick TW. The role of calcium in acute pancreatitis. Surgery. 2012;152:S157–S163. doi: 10.1016/j.surg.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Peng T, Peng X, Huang M, Cui J, Zhang Y, Wu H, et al. Serum calcium as an indicator of persistent organ failure in acute pancreatitis. Am J Emerg Med. 2017;35:978–982. doi: 10.1016/j.ajem.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Zou D, Qi X, Zhu C, Ning Z, Hou F, Zhao J, Peng Y, Li J, Deng H, Guo X. Albumin-bilirubin score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis: a retrospective study. Turk. J Gastroenterol. 2016;27(2):180–186. doi: 10.5152/tjg.2016.15502. [DOI] [PubMed] [Google Scholar]
- 35.Mohammed MAA, Khalaf MH, Liang T, Wang DS, Lungren MP, Rosenberg J, et al. Albumin-Bilirubin Score: An Accurate Predictor of Hepatic Decompensation in High-Risk Patients Undergoing Transarterial Chemoembolization for Hepatocellular Carcinoma. J Vasc Interv Radiol. 2018;29:1527–34 e1. doi: 10.1016/j.jvir.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 36.Ye L, Liang R, Zhang J, Chen C, Chen X, Zhang Y, et al. Postoperative albumin-bilirubin grade and albumin-bilirubin change predict the outcomes of hepatocellular carcinoma after hepatectomy. Ann Transl Med. 2019;7:367. doi: 10.21037/atm.2019.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi L, Zhang D, Zhang J. Albumin-bilirubin score is associated with in-hospital mortality in critically ill patients with acute pancreatitis. Eur J Gastroenterol Hepatol. 2020. 10.1097/MEG.0000000000001753. [DOI] [PubMed]
- 38.Kiss L, Fur G, Matrai P, Hegyi P, Ivany E, Cazacu IM, et al. The effect of serum triglyceride concentration on the outcome of acute pancreatitis: systematic review and meta-analysis. Sci Rep. 2018;8:14096. doi: 10.1038/s41598-018-32337-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mounzer R, Langmead CJ, Wu BU, Evans AC, Bishehsari F, Muddana V, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology. 2012;142:1476–1482. doi: 10.1053/j.gastro.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Papachristou GI, Muddana V, Yadav D, O'Connell M, Sanders MK, Slivka A, et al. Comparison of BISAP, Ranson’s, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol. 2010;105:435–441. doi: 10.1038/ajg.2009.622. [DOI] [PubMed] [Google Scholar]
- 41.Hall TC, Stephenson JS, Jones MJ, Ngu WS, Horsfield MA, Rajesh A, et al. Is Abdominal Fat Distribution Measured by Axial CT Imaging an Indicator of Complications and Mortality in Acute Pancreatitis? J Gastrointest Surg. 2015;19:2126–2131. doi: 10.1007/s11605-015-2972-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this study are available from the corresponding author.