Abstract
Objective
To analyze the timeline, prevalence, and survival of REM sleep-behavior disorder in patients who developed alpha-synucleinopathies: Parkinson disease, dementia with Lewy bodies, and Parkinson disease dementia compared to age- and sex-matched controls in a population-based incident-cohort study.
Methods
We used a population-based, 1991–2010 incident-cohort study of alpha-synucleinopathies. A movement-disorder specialist reviewed medical records to confirm diagnoses. REM sleep-behavior disorder was diagnosed by reported dream-enactment symptoms or polysomnography. Probable RBD and polysomnography- confirmed RBD were analyzed singularly and combined.
Results
Among the 444 incident cases of alpha-synucleinopathy, 86 were clinically diagnosed with REM sleep-behavior disorder (19.8%) including 30 (35%) by polysomnography and 56(65%) as probable. Idiopathic REM sleep-behavior-disorder prevalence at alpha-synucleinopathy diagnosis was 3.4%, increasing to 23.8% after 15 years. Cumulative lifetime incidence was 53 times greater in alpha-synucleinopathies patients than controls (OR=53.1, 95% CI: 13.0– 217.2, p<.0001), higher in dementia with Lewy bodies than Parkinson’s disease, (OR=2.57, 95% CI: 1.50–4.40, p=.0004), and men than women with PD/DLB/PDD (OR = 3.70, 95% CI: 2.07–6.62, p<.0001) but did not increase mortality risk.
Interpretation
Our cohort had REM sleep-behavior disorder incidence of 3.4%. Overall REM sleep-behavior disorder increased to 23.8% after 15 years, with overall incidence of 2.5 cases/100 person years. With 53 times greater lifetime incidence in alpha-synucleinopathies patients than controls, REM sleep-behavior disorder was more likely to develop in dementia with Lewy bodies than Parkinson’s disease or Parkinson’s disease dementia, and in men than women but did not increase mortality risk within our cohort.
Introduction
Rapid eye movement (REM) sleep behavior disorder (RBD) is a parasomnia that has been strongly associated with alpha-synucleinopathies. RBD has a prevalence of ~1%,1 and a strong link exists between idiopathic/isolated RBD (iRBD) and Parkinson’s disease (PD), dementia with Lewy bodies (DLB), Parkinson disease dementia (PDD), and multiple system atrophy (MSA).2–11 The overall frequency of RBD in PD patients is between 30% and 50%, and at least 70% of DLB patients develop RBD.12–15 Accordingly, iRBD has been recognized as a prodromal feature of alpha-synucleinopathies. The risk of phenoconversion from iRBD to an overt alpha-synucleinopathy is approximately ~7–8% per year, approaching 35% at 5 years and between 41% to 91% risk following 6 to 15 years.9, 10, 14, 16–20 RBD may even identify a distinct phenotype of PD.21, 22
Despite the large number of studies examining RBD as a risk factor or prodromal synucleinopathy, few studies have examined the lifelong sequence of RBD, including both before and after the onset of synucleinopathies. In the current study, we aimed to characterize the timeline of development, prevalence, and survival of RBD in a lifelong incident cohort of individuals with alpha-synucleinopathies. We also aim to analyze the differences between individuals diagnosed with iRBD and secondary RBD and the differences between probable RBD vs polysomnography confirmed RBD.
Methods
Case ascertainment and exclusion criteria
To identify cases of potential alpha-synucleinopathies we used the unique infrastructure of the Rochester Epidemiology Project (REP) medical records linkage system.23 This system allows us to study a geographically defined population of Olmsted County, Minnesota, from January 1, 1991, to December 31, 2010. The REP has a population catchment rate of over 97% of individuals in Olmsted County, and allows us to study lifelong medical histories of consenting participants from any hospital, private practice, or nursing home within this county.24 We ascertained potential cases of alpha-synucleinopathies in two phases. During Phase 1, we searched the indexes of the REP for 38 diagnostic codes indicating potential parkinsonism, which have been reported elsewhere.25 In a previous study, these codes completely captured all cases of parkinsonism in this same population, and was designed to be highly sensitive at the cost of low specificity.26
During Phase 2 of case ascertainment, a movement disorders specialist (RS) reviewed the complete medical histories of all individuals who received at least one of the 38 diagnostic codes during the study period. The movement disorder specialist then defined the type of alpha-synucleinopathy and the approximate date of onset using specific diagnostic criteria.27–30 The date of parkinsonism onset was defined as the approximate date of which 1 of the 4 cardinal features of parkinsonism was first indicated in the medical histories of the patients. Parkinsonism was then defined as the presence of at least two of four cardinal signs: rest tremor, bradykinesia, rigidity, and impaired postural reflexes. Among the persons who fulfilled the criteria for parkinsonism, we applied the diagnostic criteria to classify the type of alpha-synucleinopathy.25 Further details about the case ascertainment of participants with alpha-synucleinopathies have been reported elsewhere.25 Please see Table 1,29, 30 which provides the diagnostic criteria used to define PD, DLB, and PDD. After these two phases, we identified a total of 448 cases of alpha-synucleinopathies through the REP medical records-linkage system. When possible, a board-certified neuropathologist reviewed each available autopsy report to confirm the clinical diagnoses of an alpha-synucleinopathy. The clinicopathological correspondence and sensitivity for the patients with a clinical diagnosis of PD was 100%31 and 94.1% for DLB.25 Additionally the positive predictive value of the clinicopathological correspondence in PD patients was 83.3% (20/24) and 88.9% (16/18) for DLB patients.31 Further details regarding the clinicopathological details of this cohort have been reported elsewhere.31
Table 1:
Diagnostic criteria for specific types of overt alpha-synucleinopathies
| Parkinsonism: a syndrome with any two out of four cardinal signs |
| • Resting tremor |
| • Bradykinesia |
| • Rigidity |
| • Impaired postural reflexes |
| Overt alpha-synucleinopathies |
| Parkinson’s disease: parkinsonism with all of the following |
| • No other neurological explanation (e.g., repeated head injury; history of encephalitis, brain tumor; hydrocephalus; neuroleptic treatment within 6 months before onset; repeated stroke with step-wise progression) |
| • No documentation of unresponsiveness to levodopa at doses of at least 1 gm/day in combination with carbidopa (Only applicable to patients who received treatment) |
| • No prominent or early signs (within 1 year of onset) of more extensive nervous system involvement not explained otherwise (e.g., dysautonomia) |
| Dementia with Lewy bodies: |
| • Essential |
| ○ Dementia present before or within 1 year of the onset of parkinsonism |
| • Core clinical features |
| ○ One or more spontaneous cardinal feature of parkinsonism defined above |
| ○ Fluctuating cognition in attention and alertness |
| ○ Recurrent visual hallucinations |
| ○ REM sleep behavior disorder |
| Parkinsons disease with dementia: |
| • Core Features |
| ○ Diagnosis of idiopathic Parkinsons disease |
| ○ A dementia syndrome with insidious onset and slow progression presenting at least 1 year after the onset of parkinsonism |
All cases were matched by age (±1 year) and sex to an individual living in Olmsted County, Minnesota, during the study period (1991–2010). Matched controls were free of parkinsonism prior to the index date (year of parkinsonism onset); however they were not excluded for having other neurologic diseases. Using the infrastructure of the REP, a list of all county residents who matched this criterion was created. Controls were then randomly drawn from this list. Study staff reviewed all potential controls and marked any possible cases of parkinsonism. These marked controls were then reviewed by a movement disorder specialist (RS) to exclude the diagnosis of any type of parkinsonism before the index date in the matched cases. Any case that had a parkinsonism diagnosis on or before the index date was then included as a case and was replaced by another randomly drawn control.
We then conducted a complete in-depth review of the full medical records of each case and control. The American Academy of Sleep Medicine International Classification of Sleep Disorders, 3rd edition, was used for diagnosis of RBD.32 In particular, the following clinical criterion was used: repeated episodes of sleep-related vocalization and/or complex motor behaviors. These behaviors must be documented by polysomnography (PSG) to occur during REM sleep, or based on a clinical history of dream enactment. PSG must demonstrate REM sleep without atonia, and the disturbance cannot be better explained by another sleep disorder, mental disorder, medication or substance abuse.32 If REM sleep without atonia can be explained by the aforementioned variables they were excluded from the study. If individuals had a clearly described history of sleep-related vocalizations and complex motor behaviors suggestive of probable dream enactment behavior, but did not receive a PSG, they were classified as probable RBD (pRBD). The Mayo Sleep Questionnaire (MSQ) was also used to classify pRBD when available, which has been shown to have adequate sensitivity and specificity for the diagnosis of RBD especially in an aging population.33 The date of RBD symptom onset was abstracted by using the approximate date of the first symptom of RBD by either using the report of symptom onset in the medical records, or if unavailable, the date of the first symptom in the medical charts. Patients were classified as iRBD if the onset and diagnosis of RBD preceded the diagnosis of an overt alpha-synucleinopathy and could not be further explained by medications or another medical condition. Finally, patients were classified to have symptomatic RBD if the clinical diagnosis of RBD followed the onset of other primary symptoms of an overt alpha-synucleinopathy (i.e., cognitive, motor, or autonomic symptoms occurred before dream enactment history). Of the 448 identified cases of alpha-synucleinopathy, 4 had case-matched controls that were missing information regarding sleep disorders. These 4 patients were removed from analysis, resulting in a study population of 444 alpha-synucleinopathy patients and 444 age- and sex-matched controls.
Statistical analysis
Continuous features were summarized with medians and interquartile ranges (IQRs); categorical features were summarized with frequency counts and percentages. Odds ratios were used to compare the occurrence of RBD between sub-groups. Incidence rates of RBD per 100 person-years were calculated using the number of incidence cases of RBD divided by the number of years at risk for the cohort during a given time period. Patients with partial follow-up within a period are counted proportionally.
The risk of mortality was assessed using two Cox proportional hazards models. The first model considered the full cohort of alpha-synucleinopathy patients and case-matched healthy controls, whereas the second model only used the parkinsonism patients. Both models included a time-dependent covariate for the diagnosis of RBD, created using the time from parkinsonism onset or control index date to the diagnosis of RBD, or last follow-up if RBD was never diagnosed. Patients diagnosed with RBD prior to the onset of parkinsonism or index date were considered to have RBD at time zero. Both models were adjusted for patient sex, age at onset of parkinsonism or index date, and parkinsonism type. Age at onset was scaled to 5-year increments.
Standard Protocol Approvals, Registrations, and Patient Consents
The Mayo Clinic and Olmsted Medical Center Institutional Review Boards approved this study. The participating patients (or their legally authorized representatives) provided informed written consent for the use of their medical information for research. Further detailed information about the Olmsted County population is reported elsewhere.23, 24, 34, 35 Information about the different types of overt alpha-synucleinopathy and the clinical and pathological characteristics of this cohort is also reported elsewhere.25, 31, 36
Data Availability Statement
All the relevant data have been shared and published in this article; data regarding case ascertainment of parkinsonism and methodology on case identification have been previously published.36
Results
Demographics, incidence, and prevalence
From January 1, 1991, to December 31, 2010, there were 444 incident cases of overt alpha-synucleinopathies. Specifically there were 308 cases of PD, 81 cases of DLB, and 55 cases of PDD. The median age at overt alpha-synucleinopathy onset was 75 years of age (75 years for men and 76 years for women). Among the cases diagnosed with an overt alpha-synucleinopathy, 368 (82.9%) died by the time of abstraction with a median age at death of 84 years (83 years for men and 86 years for women). Further details of patient demographics and characteristics can be found in Table 2.
Table 2:
Patient Characteristics by Alpha-Synucleinopathy
| Control (N = 444) | PD (N = 308) | DLB (N = 81) | PDD (N = 55) | Total (N = 888) | |
|---|---|---|---|---|---|
| Race | |||||
| Asian | 13 (2.9%) | 9 (2.2%) | 3 (3.7%) | 0 (0%) | 25 (2.8%) |
| Black or African American | 1 (0.2%) | 2 (0.6%) | 1 (1.2%) | 0 (0%) | 4 (0.5%) |
| Other/Mixed | 6 (1.4%) | 1 (0.3%) | 0 (0%) | 1 (1.8%) | 8 (0.9%) |
| White | 424 (95.5%) | 296 (96.1%) | 77 (95.1%) | 54 (98.2%) | 851 (95.8%) |
| Sex | |||||
| Male | 264 (59.5%) | 182 (59.1%) | 56 (69.1%) | 26 (47.3%) | 528 (59.5%) |
| Death | |||||
| Deceased | 255 (57.4%) | 232 (75.3%) | 81 (100%) | 55 (100%) | 623 (70.2%) |
| Age at Parkinsonism Onset | |||||
| Overall | 76 (67, 81) | 73 (65, 80) | 77 (72, 80) | 81 (76, 86) | 75 (67, 81) |
| Female | 76 (67, 83) | 74 (66, 81) | 78 (73, 81) | 81 (76, 87) | 76 (68, 83) |
| Male | 74 (66, 81) | 73 (65, 80) | 76 (72, 79) | 81 (76, 84) | 75 (67, 80) |
| Age at Death | |||||
| Overall | 88 (83, 92) | 86 (79, 90) | 83 (79, 87) | 84 (79, 90) | 84 (79, 89) |
| Female | 90 (85, 94) | 87 (81, 92) | 84 (80, 87) | 88 (80, 90) | 86 (80, 91) |
| Male | 87 (83, 91) | 84 (77, 89) | 83 (79, 86) | 83 (80, 88) | 83 (78, 88) |
| RBD Diagnosis | |||||
| Yes | 2 (0.5%) | 55 (17.9%) | 29 (35.8%) | 2 (3.6%) | 88 (9.9%) |
Note: Age at Parkinsonism Onset for controls refers to age at control index date
Among the controls, 255 (57.4%) died by the time of abstraction with a median age at death of 88 years (87 years for males and 90 years for females). Among the 444 incident cases of an alpha-synucleinopathy, 86 were clinically diagnosed with RBD. Figure 1 shows the incidence timeline of the 86 RBD cases stratified by parkinsonism diagnostic subtype from 1991–2019. Of these, 30 (35%) were confirmed by polysomnography and 56 (65%) were pRBD. There were 27 patients who fit the clinical criteria for pRBD who subsequently underwent polysomnography testing. Of these 27 patients 85.2% (24/27) were confirmed to have RBD. Additionally, 78/86 (90.7%) cases of RBD were either clinically diagnosed by a sleep medicine specialist or neurologist. The prevalence of iRBD at the time of overt diagnosis of alpha-synucleinopathy was 3.4%. The prevalence of symptomatic RBD after the diagnosis of an alpha-synucleinopathy in five years increments was 13.7% at 5 years, 17.8% at 10 years, and 23.8% at 15 years. The overall incidence of RBD in alpha-synucleinopathies was 2.5 cases per 100 patient-years compared to 0.04 cases per 100 patient-years in controls. Table 3 shows the prevalence and incidence of RBD divided into sex and subtype of alpha-synuclenopathy. Figure 2 also shows the prevalence of RBD through disease years.
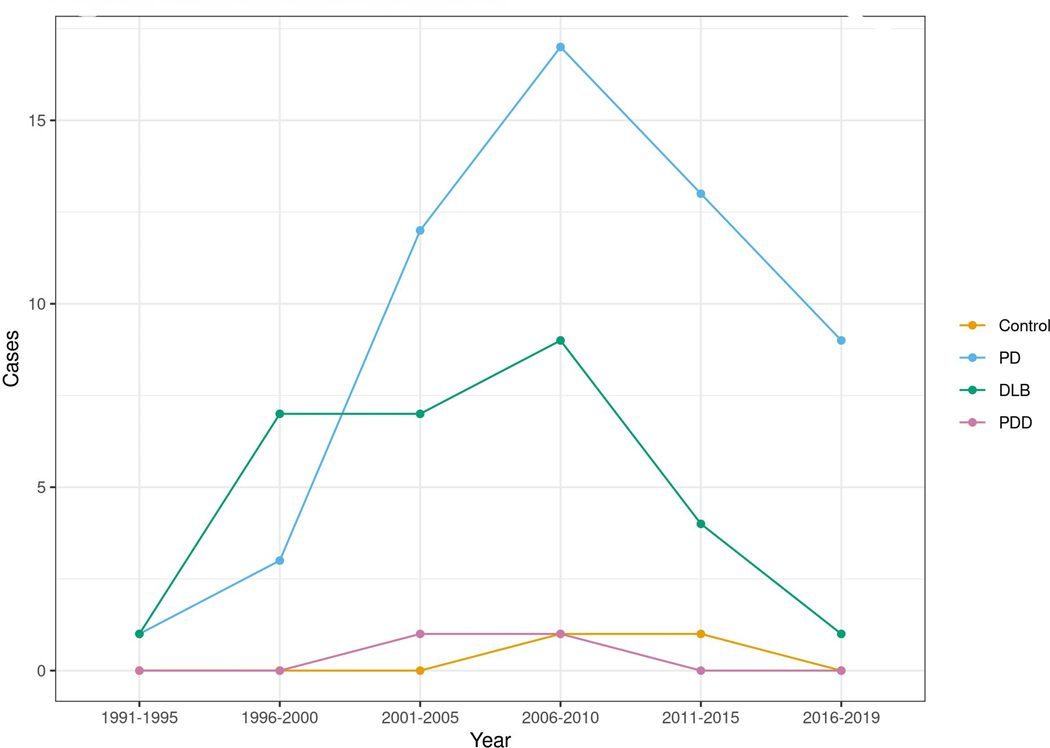
Figure 1:
The incident rate of RBD cases is reported for each diagnostic subtype of overt alpha-synucleinopathy and controls in five-year increments.
Table 3:
Incidence of RBD (100 Person-Years)
| Control (N = 444) | PD (N = 308) | DLB (N = 81) | PDD (N = 55) | α-Syn (N = 444) | |
|---|---|---|---|---|---|
| Sex | |||||
| Overall | 0.040 | 1.933 | 7.865 | 0.870 | 2.497 |
| Female | 0.050 | 1.151 | 1.279 | 0.000 | 1.069 |
| Male | 0.034 | 2.517 | 12.710 | 1.903 | 3.596 |
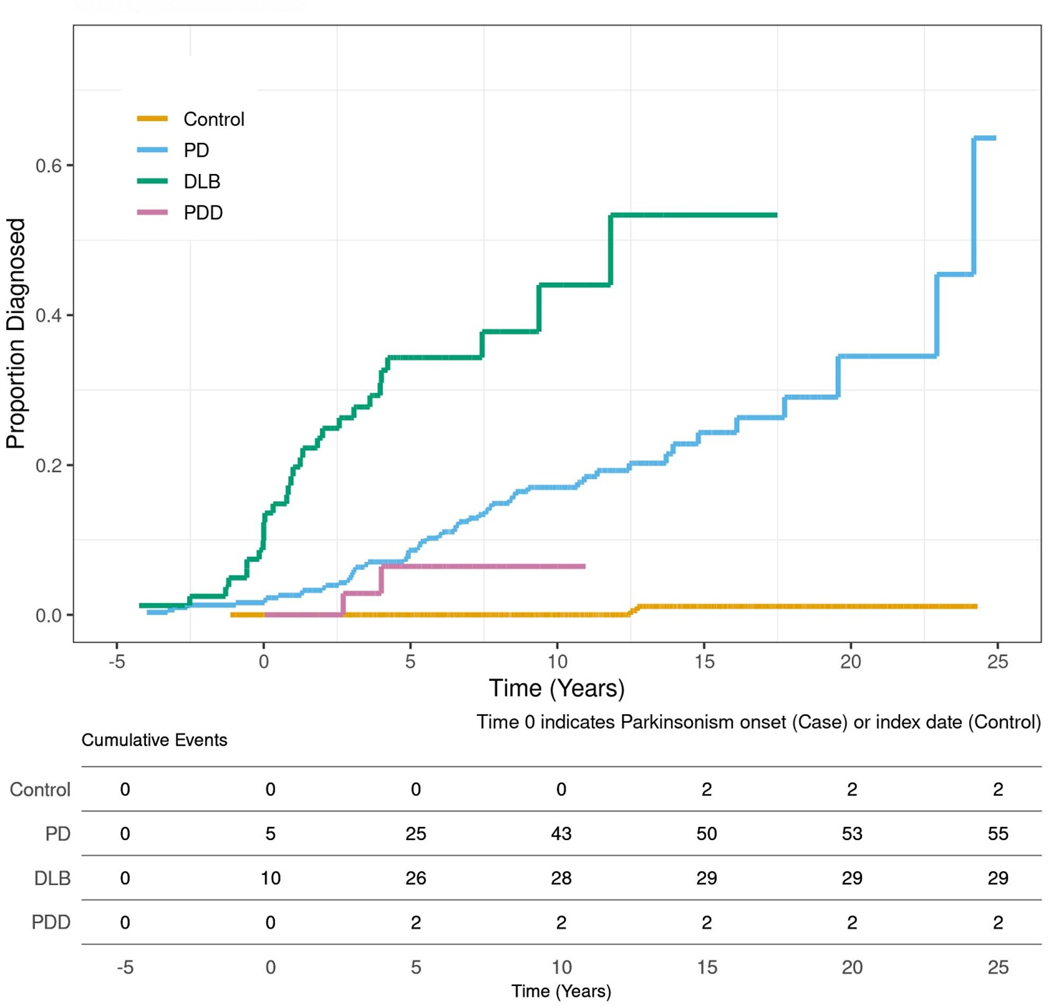
Figure 2:
RBD prevalence is reported for each individual overt alpha-synucleinopathy in five disease year increments. The diagnosis of overt alpha-synucleinopathy is indicated by time 0 on the x-axis. The bottom table within Figure 2 reports the individual RBD events per 5-year increments either before or after the onset of overt alpha-synucleinopathy.
Patients with an overt alpha-synucleinopathy were 53 times higher more likely to be diagnosed with RBD than controls (OR = 53.1, 95% CI: 13.0 – 217.2, p < 0.0001). When comparing alpha-synucleinopathies, the odds of a DLB patient being diagnosed with RBD were 157% greater than a PD patient who does not have dementia (OR = 2.57, 95% CI: 1.50 – 4.40, p = 0.0004). Conversely, the odds of a PDD patient being diagnosed with RBD were 82.6% lower than a PD patient who does not have dementia (OR = 0.17, 95% CI: 0.04 – 0.73, p = .008). Men with an alpha-synucleinopathy had 270% greater odds of a RBD diagnosis compared to women (OR = 3.70, 95% CI: 2.07 – 6.62, p < .0001).
Timeline of RBD
Table 4 shows RBD symptom onset by parkinsonism-disease year in both cases of isolated RBD and symptomatic RBD with the earliest case of RBD symptom onset starting over 30 years before the onset of an alpha-synucleinopathy. Figure 3 shows the median RBD progression by alpha-synucleinopathy disease year. Furthermore, Figure 4 shows each patient’s timeline of RBD symptom onset to diagnosis in respect to overt alpha-synucleinopathy diagnosis. Among the 55 PD patients with RBD, the median overall onset of RBD occurred 0.7 years following PD onset (IQR= −3.3 – 5.4). There was no difference between the start of symptom onset between PD cases confirmed by polysomnography and clinically diagnosed cases (p=.21). In those patients with PD-first diagnoses, the median years between PD diagnosis and subsequent RBD diagnosis was 5.4 years (IQR=2.9–8.5). The median delay from RBD symptom onset to clinical diagnosis of RBD was 4.8 years in the PD cohort (IQR=1.4–9.5). In comparison, for the 28 DLB patients diagnosed with RBD, the median RBD symptom onset occurred 3.8 years prior to the clinical diagnosis of DLB (IQR=−7.1 — −0.4). The median DLB disease year of RBD diagnosis was 1.0 year (IQR=0.0–3.2), with a median lag of 4.9 years (IQR= 2.9 – 8.6) between RBD symptom onset and diagnosis. When comparing these groups, DLB patients with RBD were 3.5 times more likely to have isolated RBD symptom onset relative to PD patients with RBD (OR=3.51, p=0.020). However, patients with symptom onset 10 or more years before parkinsonism diagnosis were equally likely to have PD or DLB (OR =1.49, p=0.53). The median lag between symptom onset and diagnosis of RBD was similar between men and women (4.6 years in males (IQR=2.0–8.9) and 4.8 in females (IQR=2.1–5.9)).
Table 4:
Incident Cases of RBD Symptom Onset by alpha-synucleinopathy Disease Year
| Control (N = 444) | PD (N = 308) | DLB (N = 81) | PDD (N = 55) | Total (N = 888) | |
|---|---|---|---|---|---|
| Isolated RBD | |||||
| >30 Years | 0 | 2 | 1 | 0 | 3 |
| 30–25 Years | 0 | 1 | 1 | 0 | 2 |
| 25–20 Years | 0 | 1 | 0 | 0 | 1 |
| 20–15 Years | 0 | 0 | 2 | 0 | 2 |
| 15–10 Years | 0 | 3 | 2 | 0 | 5 |
| 10–5 Years | 0 | 3 | 6 | 0 | 9 |
| 5–0 Years | 0 | 13 | 11 | 0 | 24 |
| Symptomatic RBD | |||||
| 0–5 Years | 0 | 16 | 5 | 2 | 23 |
| 5–10 Years | 1 | 12 | 1 | 0 | 14 |
| 10–15 Years | 1 | 3 | 0 | 0 | 4 |
| 15–20 Years | 0 | 0 | 0 | 0 | 0 |
| 20–25 Years | 0 | 1 | 0 | 0 | 1 |
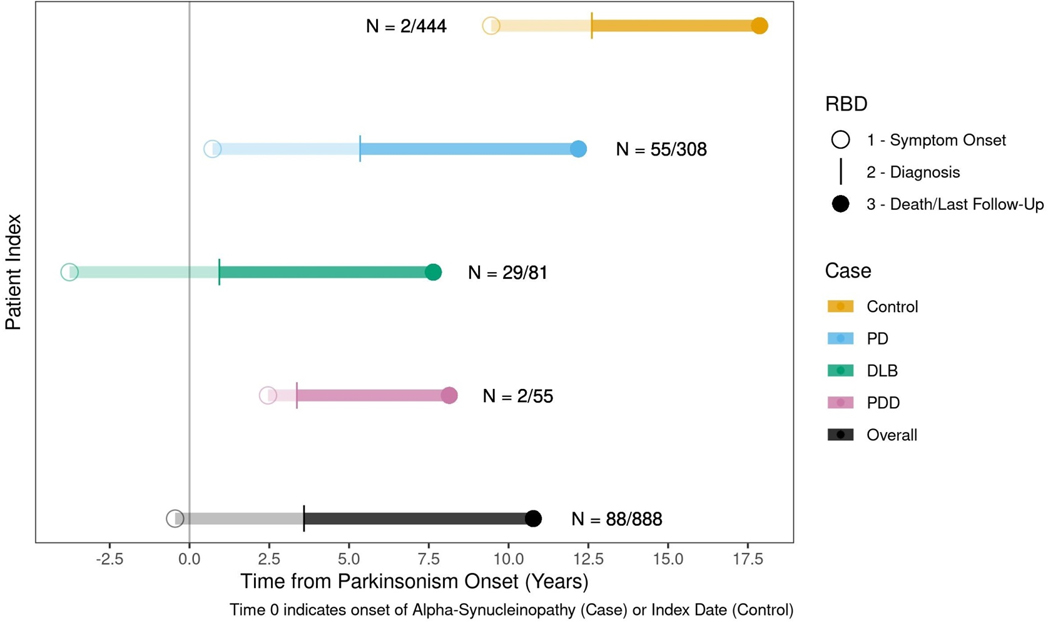
Figure 3:
The median lifelong timeline of RBD progression is represented in each overt alpha-synucleinopathy subtype (yellow=control, blue=PD, green=DLB, purple=PDD). The x-axis represents the years before and after the onset of overt alpha-synucleinopathy (onset is indicated by the number 0). The timeline of the progression of RBD is represented by the horizontal lines. The circle represents the median onset of RBD symptoms. The transparent line indicates the median latency from RBD symptom onset to diagnosis. The solid line indicates the median time from RBD diagnosis to the date of last follow-up or death.
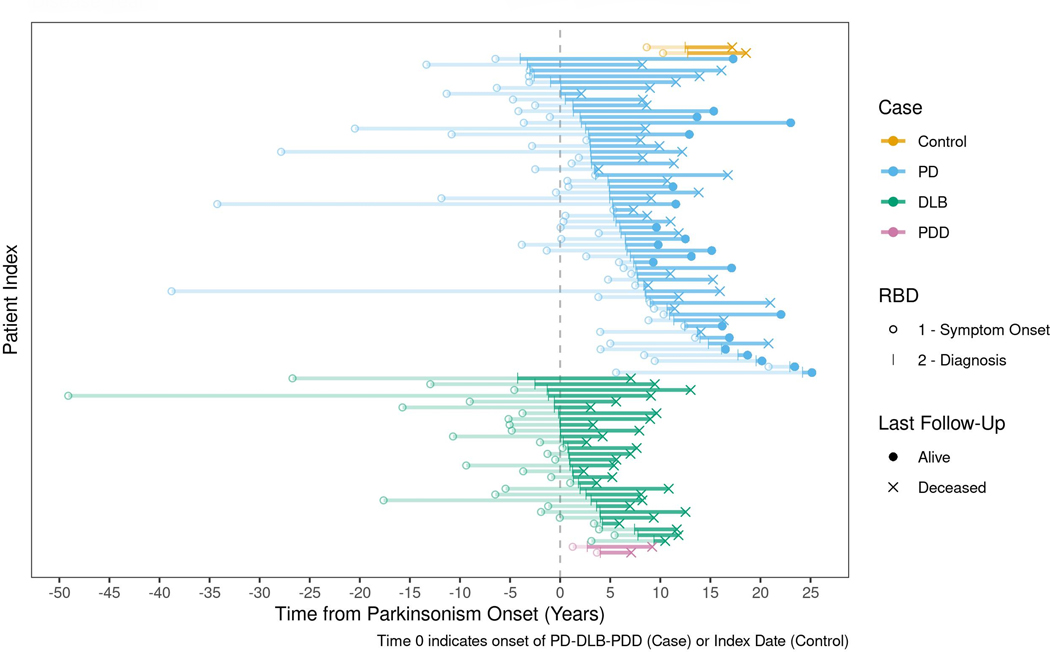
Figure 4:
The life-long timeline of RBD development is shown in each individual patient differentiated by disease subtype (yellow=control, blue=PD, green=DLB, purple=PDD). The x-axis represents the years before and after the onset of parkinsonism (onset is indicated by the number 0). For each subtype of alpha-synucleinopathy, the timeline of the development of RBD is represented by the horizontal lines. The circle represents each individual’s onset of RBD symptoms. The transparent line indicates each individual’s latency from RBD symptom onset to diagnosis. The solid line indicates each individual’s time from RBD diagnosis to the date of last follow-up or death.
Polysomnography confirmed cases of RBD were diagnosed an average of 2.3 years after alpha-synucleinopathy onset. Conversely, pRBD diagnoses occurred on average 6.3 years after the diagnosis of an alpha-synucleinopathy. Polysomnography confirmed cases were diagnosed significantly closer to the alpha-synucleinopathy diagnosis (p = 0.0003). Patient age at the onset of RBD symptoms did not affect the time to onset of alpha-synucleinopathy (t=1.22, p=0.228).
The median age of death in individuals with an overt alpha-synucleinopathy and RBD was 83 (IQR= 77—87), and in those without RBD was 84 (IQR=79—89). The presence of RBD did not modify the survival of synucleinopathy. (SUPPLEMENTAL Figure).
Discussion
Of the 444 cases of alpha-synucleinopathies in our cohort, 86 (19.3%) were diagnosed with RBD during their lifespan. Within our cohort of alpha-synucleinopathies, the prevalence of isolated RBD prior to the onset of alpha-synucleinopathy was 3.4%. A retrospective study examining an incident cohort of patients with PD found that the prevalence of isolated RBD was around 1%.37 Furthermore, the results are similar to the reported overall prevalence of iRBD within the general population.1 The prevalence of symptomatic RBD five years after a diagnosis of alpha-synucleinopathy was 13.7%. When looking 10 years post alpha-synucleinopathy diagnosis, the prevalence increased to 17.8%, and to 23.8% 15 years post-diagnosis. The overall incidence of symptomatic RBD after alpha-synucleinopathies was 2.5 per 100 person years in our cohort. While to our knowledge there are no studies investigating the incidence or prevalence in overt alpha-synucleinopathies combined, there are reports examining RBD within the subsets of overt alpha-synucleinopathies, to which we can compare our results. A recent meta-analysis analyzing 28 studies found a combined prevalence of RBD within PD to be 42.3% with a 95% CI from 37.4–47.1.38 Further studies have shown the prevalence of RBD secondary to DLB to range from 46.7% to 83%.39–41 The overall prevalence of RBD in PD patients in our cohort was 17.9% and in DLB, 35.8%. In comparison, these studies find a greater prevalence of RBD in the subtypes of overt alpha-synucleinopathies compared to our cohort. Small discrepancies may be explained by differences in populations, study designs, and years of follow-up. However, larger differences between the prevalence of RBD within overt alpha-synucleinopathies may be better explained by the study periods used to examine these different cohorts. When examining the overall number of RBD cases within our cohort in 5-year increments from 1991 to 2010, we found a large jump in cases between the years 2000 and 2005. This could be explained by increased awareness of RBD among physicians due to the updated International Classification of Sleep Disorders Second edition, which was released in the year 2005 [ICDS-2]. The under reporting of RBD within overt alpha-synucleinopathies from 1991–2005 may account for the differences in the calculated prevalence. With a longer follow-up and an updated, more recent cohort, it is expected that the prevalence of RBD within overt alpha-synucleinopathies would increase. Importantly, our study examines the prevalence of both iRBD and symptomatic RBD within alpha-synucleinopathies. While iRBD has been shown to be a risk factor for alpha-synucleinopathies, it is important to examine the prevalence and risk of developing symptomatic RBD in alpha-synucleinopathies as it has been shown to significantly reduce the quality of life and increase the risk of developing dementia.9, 42
The median age of RBD symptom onset was 70 years of age with a median delay to diagnosis of 3.6 years. In our cohort symptoms of RBD preceded the diagnosis of an overt alpha-synucleinopathy by up to 30 years. Importantly, we observed that DLB patients were 3.5 times more likely to experience isolated symptoms of RBD compared to PD or PDD. However, if there is a 10-year or greater lag between RBD symptom onset and development of an alpha-synucleinopathy there is no significant difference in the odds of phenoconversion to PD, DLB, or PDD; thus patients with RBD symptoms for more than 10 years can equally develop one of the considered alpha-synucleinopathies. Having insight to the risk of phenoconversion may give treating physicians a tool in managing early symptoms of alpha-synucleinopathies and can be a valuable educational resource for patients. While iRBD has been shown to be a significant risk factor of phenoconversion to an alpha-synucleinopathy, the median time of RBD symptom onset only preceded the diagnosis among DLB patients. It is clinically significant to understand the timeline of RBD both before and after the onset of alpha-synucleinopathies. Within our cohort, RBD symptom onset succeeded the diagnosis of PD by a median of 0.72 years, and the diagnosis followed that of PD by 5.35 years. Similarly, the median start of RBD symptom onset followed the diagnosis of PDD by 2.46 years with the diagnosis of RBD to follow by a median of 3.36 years. Of great interest, we observed a difference between the onset of RBD in DLB compared to PDD. This is especially important as there might be overlap of clinical symptoms between these two disorders, which makes them difficult to differentiate. Data from a recent meta-analysis reported that up to 20% of individuals clinically diagnosed with DLB have received an incorrect diagnosis.43 Thus, the date of RBD symptom onset in conjunction with the onset of Parkinsonism may help clinicians to distinguish potential DLB from PDD; however, the overlap of the clinical features of DLB and PDD can be still a diagnostic challenge. Indeed, even the 1-year rule used to diagnose DLB,12 can be sometimes difficult to adopt given the unclear temporal relationship of the development of some of the symptoms. The Movement Disorders Society’s clinical diagnostic criteria on PD dispute the use of the 1-year rule whereas it is still adopted in the the clinical definition of DLB of the Fourth DLB Consortium consensus report.12,30 Our results highlight the importance of constant vigilance of possible symptoms of RBD throughout the patient’s timeline of care in order to manage comorbid diseases properly.
Although the risk of phenoconversion is an important tool in a physician’s plan of care and patient education, the risk of developing symptomatic RBD after an alpha-synucleinopathy is crucial to understand in order to provide the best patient-centered care. Within our cohort, we observed that individuals who have been diagnosed with an alpha-synucleiopathy were 53 times more likely to develop RBD compared to age and sex matched controls. Many studies have examined large differences in the prevalence between these two populations.1, 13–15 Furthermore, DLB patients had 157% greater odds of developing RBD compared to PD patients while PDD patients had 82.5% less odds of developing RBD compared to PD patients. Understanding the odds of developing RBD after an overt alpha-synucleinopathy can further assist physicians in providing counseling throughout the disease progression. It can also be used to increase awareness in patients and families to report RBD symptoms to healthcare providers. Importantly, we observed that the development of RBD did not increase the risk of mortality, regardless of when it develops in relation to an alpha-synucleinopathy. Future studies should explore the impact of an early diagnosis of RBD on patients and caregivers centered outcomes.
When examining sex differences, men had 270% greater odds of developing RBD compared to women, which is consistent to previous literature.3 We also found the incidence of RBD to be 3.60 in men compared to 1.07 in women with overt alpha-synucleinopathies. However, there was no difference in mortality between men and women. Additionally, no inter-group difference in the risk of mortality was observed between probable versus confirmed RBD, as well as isolated versus symptomatic RBD.
This study has several strengths, including the opportunity to explore the entire duration of symptoms of RBD before and after the onset of alpha-synucleinopathies, taking advantage of our accurate records-linkage system tool. On the other hand, it also has limitations. First, as a retrospective study relying on medical records, ascertainment bias is probable, since RBD symptoms were not uniformly sampled in clinical practice, and some dream-enactment symptoms were likely not reported by patients or identified by the clinician. This is a further concern within the diagnosis of RBD that symptoms may go unnoticed if minor or if the individual does not have a bed partner, which could lead to underestimated prevalence and incidence; also we did not have the full data on the medications exposure. However, to minimize this limitation, we used the medical histories throughout each individual’s entire life span during data abstraction. We also thoroughly reviewed all sleep questionnaires and sleep medicine specialist’s notes to ensure accurate identification of all possible recorded symptoms of RBD. Since polysomnographic confirmation of RBD is not always possible in clinical practice given its resource intensiveness and expense, we included cases of pRBD and examined inter-group differences between the polysomnography-confirmed RBD and pRBD patient subgroups. Also, RBD was not officially classified as a sleep disorder until 2005 in the International Classification of Sleep Disorders, 2nd revision; this may have led to an under-representation of the incidence in years prior to 2005. Our exploration of the count of RBD cases in five-year increments from 1991 to 2010 instead showed a decline in case frequency in the 2005 era and afterward, suggesting that under-ascertainment in earlier years was not a significant issue in our practice, which has long-standing experience with RBD following its original description in Minnesota by Schenck and Mahowald, and confirmation of the strong association between RBD and synucleinopathies at Mayo (Figure 2).44, 45
In addition, it is possible that some patients with mild cognitive symptoms went undiagnosed and unrecognized and also it is possible that patients with DLB may never develop parkinsonian symptoms; thus, we would be unable to identify them. However, only a minority of patient with DLB never develop any parkinsonian symptoms and our prolonged observation of about 20 years of medical records limit the possibility that patients are misdiagnosed or unrecognized. Furthermore, it is possible that some patients diagnosed with PD will later develop cognitive decline. However, our cohort is an incidence cohort that identified and diagnosed cases within the first years of the disease onset.25 While accounting for these possibilities, a previous study confirmed the accuracy of the clinicopathological correlation (PD 83.3% in PD and 88.9% in DLB patients).31
Conclusion
In our incident cohort spanning from 1991 to 2010, we observed the prevalence of iRBD to be 3.4%. RBD prevalence is 23.8% after 15 years of an alpha-synucleinopathy, and the overall incidence was 2.5 cases per 100 person years. We observed that DLB patients were more likely to develop RBD than PD patients; however, PD patients had greater odds of developing RBD than PDD patients. We also found that men have greater odds of developing RBD than women. The presence of RBD did not increase the risk of mortality within our cohort. Future prospective studies of population based cohorts using systematic ascertainment for RBD symptoms and polysomnography are needed to further elucidate the incidence and prevalence of RBD in patients with and without overt synucleinopathies.
Supplementary Material
Acknowledgments:
The authors thank Lea Dacy for proofreading and formatting assistance. This article was funded by the Mayo Clinic CTSA through grant number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH) and R01 AG034676 from the National Institute on Aging of the National Institutes of Health and by the Mayo Foundation for Medical Education and Research. The funder had no role in the conception or preparation of this study.
Footnotes
Potential Conflicts of Interest:
The authors have no conflicts of interest to report.
References:
- 1.Haba-Rubio J, Frauscher B, Marques-Vidal P, et al. Prevalence and determinants of rapid eye movement sleep behavior disorder in the general population. Sleep 2018;41. [DOI] [PubMed] [Google Scholar]
- 2.Boeve BF. Rem sleep behavior disorder: Updated review of the core features, the rem sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci 2010;1184:15–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boeve BF, Silber MH, Ferman TJ, et al. Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep Med 2013;14:754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boeve BF, Silber MH, Parisi JE, et al. Synucleinopathy pathology and rem sleep behavior disorder plus dementia or parkinsonism. Neurology 2003;61:40–45. [DOI] [PubMed] [Google Scholar]
- 5.Iranzo A, Santamaria J, Tolosa E. Idiopathic rapid eye movement sleep behaviour disorder: Diagnosis, management, and the need for neuroprotective interventions. Lancet Neurol 2016;15:405–419. [DOI] [PubMed] [Google Scholar]
- 6.Mahlknecht P, Seppi K, Poewe W. The concept of prodromal parkinson’s disease. J Parkinsons Dis 2015;5:681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarter SJ, St Louis EK, Boeve BF. Rem sleep behavior disorder and rem sleep without atonia as an early manifestation of degenerative neurological disease. Curr Neurol Neurosci Rep 2012;12:182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKenna D, Peever J. Degeneration of rapid eye movement sleep circuitry underlies rapid eye movement sleep behavior disorder. Mov Disord 2017;32:636–644. [DOI] [PubMed] [Google Scholar]
- 9.Postuma RB, Iranzo A, Hu M, et al. Risk and predictors of dementia and parkinsonism in idiopathic rem sleep behaviour disorder: A multicentre study. Brain 2019;142:744–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: A 16-year update on a previously reported series. Sleep Med 2013;14:744–748. [DOI] [PubMed] [Google Scholar]
- 11.Tachibana N. [historical overview of rem sleep behavior disorder in relation to its pathophysiology]. Brain Nerve 2009;61:558–568. [PubMed] [Google Scholar]
- 12.McKeith IG, Ferman TJ, Thomas AJ, et al. Research criteria for the diagnosis of prodromal dementia with lewy bodies. Neurology 2020;94:743–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nomura T, Inoue Y, Kagimura T, Nakashima K. Clinical significance of rem sleep behavior disorder in parkinson’s disease. Sleep Med 2013;14:131–135. [DOI] [PubMed] [Google Scholar]
- 14.Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: Demographic, clinical and laboratory findings in 93 cases. Brain 2000; 123 ( Pt 2):331–339. [DOI] [PubMed] [Google Scholar]
- 15.Schenck CH, Mahowald MW. Rem sleep behavior disorder: Clinical, developmental, and neuroscience perspectives 16 years after its formal identification in sleep. Sleep 2002;25:120–138. [DOI] [PubMed] [Google Scholar]
- 16.Claassen DO, Josephs KA, Ahlskog JE, et al. Rem sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2010;75:494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iranzo A, Fernandez-Arcos A, Tolosa E, et al. Neurodegenerative disorder risk in idiopathic rem sleep behavior disorder: Study in 174 patients. PLoS One 2014;9:e89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: An observational cohort study. Lancet Neurol 2013;12:443–453. [DOI] [PubMed] [Google Scholar]
- 19.McCarter SJ, Sandness DJ, McCarter AR, et al. Rem sleep muscle activity in idiopathic rem sleep behavior disorder predicts phenoconversion. Neurology 2019;93:e1171–e1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology 1996;46:388–393. [DOI] [PubMed] [Google Scholar]
- 21.Lai YY, Hsieh KC, Nguyen D, et al. Neurotoxic lesions at the ventral mesopontine junction change sleep time and muscle activity during sleep: An animal model of motor disorders in sleep. Neuroscience 2008;154:431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai YY, Siegel JM. Physiological and anatomical link between parkinson-like disease and rem sleep behavior disorder. Mol Neurobiol 2003;27:137–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St Sauver JL, Grossardt BR, Yawn BP, et al. Use of a medical records linkage system to enumerate a dynamic population over time: The rochester epidemiology project. Am J Epidemiol 2011;173:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocca WA, Grossardt BR, Brue SM, et al. Data resource profile: Expansion of the rochester epidemiology project medical records-linkage system (e-rep). Int J Epidemiol 2018;47:368–368j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savica R, Grossardt BR, Bower JH, et al. Incidence of dementia with lewy bodies and parkinson disease dementia. JAMA Neurol 2013;70:1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence and distribution of parkinsonism in olmsted county, minnesota, 1976–1990. Neurology 1999;52:1214–1220. [DOI] [PubMed] [Google Scholar]
- 27.Jellinger KA. Formation and development of lewy pathology: A critical update. J Neurol 2009;256 Suppl 3:270–279. [DOI] [PubMed] [Google Scholar]
- 28.Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci 2005;25:6016–6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with lewy bodies: Fourth consensus report of the dlb consortium. Neurology 2017;89:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Postuma RB, Berg D, Stern M, et al. Mds clinical diagnostic criteria for parkinson’s disease. Mov Disord 2015;30:1591–1601. [DOI] [PubMed] [Google Scholar]
- 31.Turcano P, Mielke MM, Josephs KA, et al. Clinicopathologic discrepancies in a population-based incidence study of parkinsonism in olmsted county: 1991–2010. Mov Disord 2017;32:1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.International classification of sleep disorders. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 33.Boeve BF, Molano JR, Ferman TJ, et al. Validation of the mayo sleep questionnaire to screen for rem sleep behavior disorder in a community-based sample. J Clin Sleep Med 2013;9:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.St Sauver JL, Grossardt BR, Leibson CL, et al. Generalizability of epidemiological findings and public health decisions: An illustration from the rochester epidemiology project. Mayo Clin Proc 2012;87:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: The rochester epidemiology project (rep) medical records-linkage system. Int J Epidemiol 2012;41:1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savica R, Grossardt BR, Bower JH, et al. Incidence and pathology of synucleinopathies and tauopathies related to parkinsonism. JAMA Neurol 2013;70:859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schrag A, Horsfall L, Walters K, et al. Prediagnostic presentations of parkinson’s disease in primary care: A case-control study. Lancet Neurol 2015;14:57–64. [DOI] [PubMed] [Google Scholar]
- 38.Zhang ZX, Roman GC. Worldwide occurrence of parkinson’s disease: An updated review. Neuroepidemiology 1993;12:195–208. [DOI] [PubMed] [Google Scholar]
- 39.Guarnieri B, Adorni F, Musicco M, et al. Prevalence of sleep disturbances in mild cognitive impairment and dementing disorders: A multicenter italian clinical cross-sectional study on 431 patients. Dement Geriatr Cogn Disord 2012;33:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray ME, Ferman TJ, Boeve BF, et al. Mri and pathology of rem sleep behavior disorder in dementia with lewy bodies. Neurology 2013;81:1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pao WC, Boeve BF, Ferman TJ, et al. Polysomnographic findings in dementia with lewy bodies. Neurologist 2013;19:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rolinski M, Szewczyk-Krolikowski K, Tomlinson PR, et al. Rem sleep behaviour disorder is associated with worse quality of life and other non-motor features in early parkinson’s disease. J Neurol Neurosurg Psychiatry 2014;85:560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rizzo G, Arcuti S, Copetti M, et al. Accuracy of clinical diagnosis of dementia with lewy bodies: A systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2018;89:358–366. [DOI] [PubMed] [Google Scholar]
- 44.Boeve BF, Silber MH, Ferman TJ, et al. Rem sleep behavior disorder and degenerative dementia: An association likely reflecting lewy body disease. Neurology 1998;51:363–370. [DOI] [PubMed] [Google Scholar]
- 45.Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human rem sleep: A new category of parasomnia. Sleep 1986;9:293–308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the relevant data have been shared and published in this article; data regarding case ascertainment of parkinsonism and methodology on case identification have been previously published.36