Abstract
Antiphospholipid syndrome (APS) is an acquired thromboinflammatory disorder characterized by the presence of antiphospholipid antibodies as well as an increased frequency of venous or arterial thrombosis and/or obstetrical morbidity. The spectrum of disease varies from asymptomatic to a severe form characterized by widespread thrombosis and multiorgan failure, termed catastrophic APS (CAPS). CAPS affects only about ~1% of APS patients, often presents as a thrombotic microangiopathy and has a fulminant course with >40% mortality, despite the best available therapy. Animal models have implicated complement in the pathophysiology of thrombosis in APS, with more recent data from human studies confirming the interaction between the coagulation and complement pathways. Activation of the complement cascade via antiphospholipid antibodies can cause cellular injury and promote coagulation via multiple mechanisms. Finally, analogous to classic complement-mediated diseases such as atypical hemolytic uremic syndrome, a subset of patients with APS may be at increased risk for development of CAPS because of the presence of germline variants in genes crucial for complement regulation. Together, these data make complement inhibition an attractive and potentially lifesaving therapy to mitigate morbidity and mortality in severe thrombotic APS and CAPS.
Keywords: antiphospholipid syndrome, complement, thrombosis, eculizumab, genetics
1 ∣. INTRODUCTION
The antiphospholipid syndrome (APS) is a thromboinflammatory disorder characterized by thrombosis affecting the venous or arterial vascular systems and/or obstetrical morbidity along with the persistent presence of antiphospholipid antibodies (aPL), which include lupus anticoagulants that are detected using phospholipid dependent coagulation tests, and anti-beta-2-glycoprotein-I (β2GPI) and anticardiolipin antibodies that are detected by ELISA.1 Anti-β2GPI antibodies in particular are central to the pathogenesis of APS.2,3 Multiple pathogenic mechanisms contributing to APS-associated clinical events have been hypothesized, including inhibition of the natural anticoagulant and fibrinolytic systems4,5; activation of vascular cells including endothelial cells,6 platelets7 and monocytes8; procoagulant effects of extracellular vesicles9; and disruption of the anticoagulant annexin A5 shield on cellular surfaces.10 However, a unifying mechanism remains elusive, and the spectrum of phenotypic severity associated with aPL, ranging from the asymptomatic carrier state to catastrophic APS (CAPS) and its attendant morbidity and mortality, remains unexplained. Activation of complement is a potential unifying mechanism for aPL-related pathogenic effects based on the crosstalk between complement and coagulation pathways, and the critical role of complement-mediated inflammation in APS-related vascular activation and placental pathology.11,12 Over the past 2 decades, animal studies have established the critical role of complement in aPL-mediated thrombosis13-16 and obstetric17-19 complications, which was supported by limited clinical data. That targeted complement inhibitors against C5 are now available and numerous other complement inhibitors are in development has further increased interest in complement as a target for mechanistic and therapeutic investigation in APS. Our recent experiments established that complement activation is associated with thrombotic events in APS, and that patient derived aPL directly activate complement.20 Most intriguingly, CAPS was associated with the presence of variants in complement genes at a rate comparable to atypical HUS (aHUS), the prototypical complement mediated thrombotic microangiopathy, setting the stage for precision medicine for APS.
2 ∣. COMPLEMENTOPATHY AND COAGULATION - THE “ MULTIPLE-HIT” HYPOTHESIS
2.1 ∣. Complement pathways and regulation
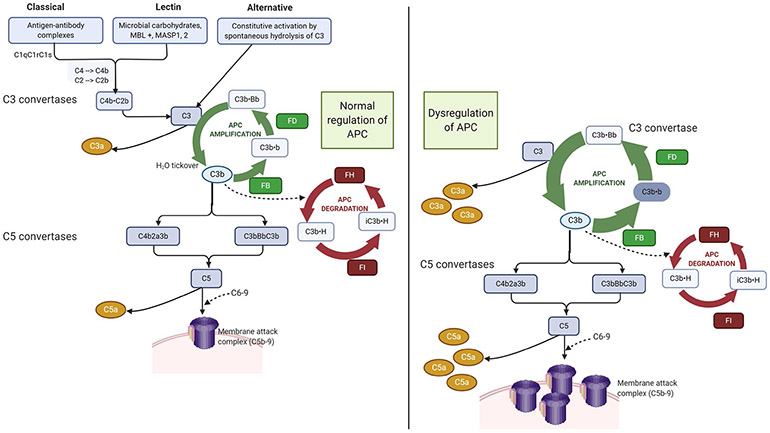
Complement is a part of the innate immune system comprising more than 50 soluble and membrane-bound proteins that mediate inflammatory responses and defend against microbes. Activation of the complement system occurs by three pathways: the classical pathway activated by antibody-antigen complexes, the lectin pathway initiated by mannose-binding lectins that recognize carbohydrate structures on the surfaces of microbes, and the alternative pathway that is in a continuous state of low-level activation through spontaneous hydrolysis of C3 and can be amplified on any cell surface. Critically, the alternative pathway also serves as an amplification loop for the lectin and classical pathways, accounting for the overwhelming majority of complement activation products (Figure 1). All three pathways converge at the level of complement component C3 and the terminal complement pathway that leads to generation of C5a, a potent pro-inflammatory molecule, and C5b-9 (the membrane attack complex). Complement pathways are under tight control by a system of fluid phase and membrane-bound regulators. Uncontrolled activation from failure of these regulation mechanisms leads to complement-mediated direct cellular injury and thrombosis.
FIGURE 1.
Complement regulation and dysregulation. Complement is activated by three pathways: (1) the lectin pathway, (2) the classical pathway, and (3) the alternative pathway. Importantly, the alternative pathway of complement (APC) also serves as an amplification loop for the lectin and classical pathways, accounting for the majority of complement activation products. The magnitude of APC activation depends on the amplification (green) and the degradation pathways (red). C3 tickover allows C3b to covalently bind to nearby cell surfaces. Once factor B binds to C3b, it is cleaved by factor D to generate the APC C3 convertase (C3bBb). C3bBb cleaves C3 to generate even more C3b setting off the amplification loop. Factor H (H) inhibits C3 amplification; it competes with B for binding to C3b, is a cofactor for factor I-catalyzed cleavage of C3b to iC3b (an opsonin), and accelerates decay of the C3 convertase (C3bBb). In the normal state, these pathways are in homeostasis. Dysregulation, for example from a loss of function mutation in CFH that reduces degradation (red loop), leads to an increase in the amplification loop because degradation of the C3 convertase (C3b.Bb) is blocked, resulting in increased production of complement effectors C3a, C5a and C5b-9 (the membrane attack complex). Additionally, complement amplifying triggers such as infection, surgery, or pregnancy can enhance the activation loop (green) and increase complement activation over the threshold required for clinical disease. (Figure was created using biorender.com.)
2.2 ∣. The interplay between complement and thrombosis
Complement and coagulation were traditionally considered distinct, nonoverlapping pathways. However, there is considerable crosstalk between these evolutionarily related proteolytic cascades.21,22 Thrombosis is a cardinal feature of complement-mediated disorders such as paroxysmal nocturnal hemoglobinuria (PNH), cold agglutinin disease, and aHUS, and the thrombotic diathesis associated with these disorders resolves with treatment with complement inhibition.23,24
Complement activation leads to the generation of the anaphyl-atoxins, C3a and C5a, and the membrane attack complex (C5b-9). C3a and C5a cause release of pro-inflammatory and procoagulant cytokines such as tumor necrosis factor and interleukin-6 from monocytes and endothelial cells, and these induce tissue factor and adhesion molecule expression on the cell surface.25-28 C5a recruits neutrophils and induces neutrophil tissue factor-dependent procoagulant activity.29 C5b-9 also induces secretion of VWF, P-selectin, and pro-inflammatory cytokines by endothelial cells and platelets,30,31 as well as the release of procoagulant extracellular vesicles.32-35 Complement fixing antibodies can trigger C3-dependent thiol-disulfide exchange through cell-surface protein disulfide isomerase and C5b-C7 membrane insertion leading to procoagulant phosphatidylserine exposure and monocyte tissue factor activation. This was first shown in the context of antithymocyte globulin.36 β2GPI-dependent and β2GPI-independent aPL can also cause monocyte tissue factor expression independent of C5, through a C3 and protein disulfide isomerase-mediated pathway.37,38 Monocyte surface tissue factor expression and monocyte TF mRNA is increased in patients with APS compared with unaffected controls, particularly those with thrombotic APS.39 Assembly of terminal complement products on platelets enhances platelet prothrombinase activity40 and C3 activation contributes to platelet activation and fibrin formation in venous clots.41 C3-deficient mice display attenuated platelet aggregation responses to several agonists and delayed arteriolar thrombosis,42 and C3a receptor knockout mice are less prone to experimental stroke and myocardial infarction.43 The link between complement activation and coagulation is bidirectional, and thrombin generation and fibrinolysis promote complement activation through multiple processes, as comprehensively reviewed by Foley and Conway.21,22
2.3 ∣. Cardinal features of complementopathies - the multiple-hit hypothesis
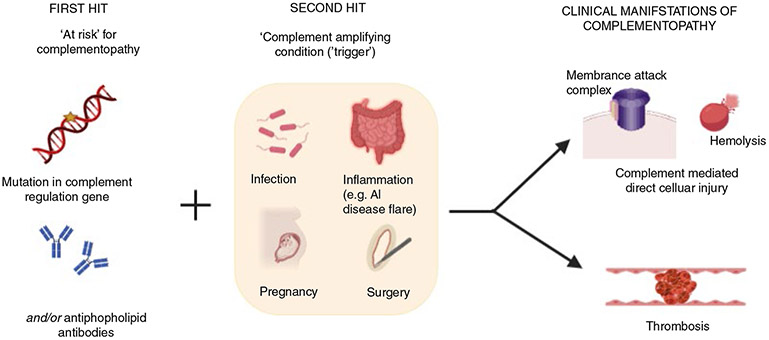
Complementopathies are disorders in which complement dysregulation drives disease pathogenesis, and complement inhibition has the potential to abate the disease course.44 They are frequently associated with thrombosis, often refractory to anticoagulation, and exacerbated by common triggers such as pregnancy, inflammation, and cancer. Examples include PNH, cold agglutinin disease, aHUS, and transplant-associated thrombotic microangiopathy. More recently, we demonstrated complement activation in the Hemolysis Elevated Liver Enzymes and Low Platelets (HELLP) syndrome of pregnancy45 and found that complement activation is associated with thrombosis in APS and CAPS.20 We also showed a high incidence of rare germline variants in complement regulation genes in patients with HELLP and CAPS,20 which is analogous to aHUS, the prototypical complement-mediated thrombotic microangiopathy. However, complement gene variants associated with these disorders exhibit incomplete penetrance and a “second hit” in the form of a complement amplifying trigger, such as pregnancy, surgery, or inflammation associated with an autoimmune disease, is commonly required for the manifestation of disease. Triggering events, commonly infections, are identified in more than 50% of patients with aHUS46,47 as well as CAPS48 in which the trigger may serve as the third hit in addition to genetic predisposition and antiphospholipid antibodies (Figure 2). Identifying the triggering event, if any, is critical because it may eventually help tailor the duration of complement inhibition therapy.
FIGURE 2.
Multiple “hits” contributing to complementopathy. Complement-mediated disorders such as aHUS, HELLP syndrome, and catastrophic APS share clinical features including thrombocytopenia, widespread small vessel thrombosis, and ischemic end-organ injury. These disorders are associated with inherited mutations in complement regulation genes, which confer a predisposition to disease. These mutations have incomplete penetrance and a complement amplifying ‘trigger’ is often required for the disorder to manifest. In addition to tissue injury from direct effects of complement, thrombosis is a hallmark of complementopathies, and the thrombotic risk is mitigated on treatment with terminal complement inhibitors. (Figure was created using biorender.com.)
3 ∣. ROLE OF COMPLEMENT IN APS
3.1 ∣. Animal data
Complement is critical for aPL-induced pregnancy loss or thrombosis in murine models of APS. Passive transfer of IgG from APS patients leads to fetal loss in pregnant mice.49 Holers et al showed that intraperitoneal injection of IgG from patients with APS into pregnant mice led to fetal resorption in 40% of pregnancies and a 35% fetal weight reduction compared with control mice.50 Inhibition of the complement cascade with the C3 convertase inhibitor complement receptor 1–related gene/protein y Ig prevented aPL-mediated fetal resorption. C3-deficient mice were also resistant to aPL-mediated fetal loss.50 Girardi et al later demonstrated that C5 deficiency or treatment of mice with anti-C5a monoclonal antibody protects against aPL-induced pregnancy loss and growth retardation.17 Placentae from the aPL IgG-treated mice showed human IgG deposition in the decidua, which demonstrated focal necrosis and apoptosis with neutrophil infiltrates.50 C5a recruits neutrophils expressing tissue factor, which potentiate neutrophil activation and the respiratory burst leading to trophoblastic injury and fetal loss.19,51 The absence of aPL-induced growth retardation and fetal resorption in mice deficient in C4 or C5 suggests that the classical pathway is involved in initiating these effects. However, factor B is necessary for aPL mediated fetal loss and its inhibition ameliorates these effects, supporting a role of the alternative pathway in amplifying complement activation.52 Taken together, these studies suggest that C3 and C5 activation is central to aPL-mediated fetal loss in this model, with neutrophils and tissue factor playing pro-inflammatory roles.
Fischetti et al showed that passive transfer of aPL IgG from patients with APS to mice primed with endotoxin led to thrombosis, whereas administration of control IgG did not. Intravascular microscopy showed thrombosis in mesenteric vessels, and IgG and C3 colocalized in the vessel wall.15 Thrombosis did not occur in C6-deficient rats or animal treated with a C5 inhibitor.15 These experiments more closely recapitulate the human model of APS compared with earlier studies that used direct vessel injury by pinching or chemical injury to induce thrombosis in mice treated with aPL.14,53 In all of these models, thrombosis was reduced in mice deficient in complement proteins C3, C5, or C6,14 or in the presence of a C5 inhibitor.13 In another set of experiments, a re-combinant single-chain fragment variable recognizing domain 1 of β2GPI induced thrombosis in male rats primed with lipopolysaccharide and pregnancy loss in female mice, but these effects were blocked in C6-deficient rats or C5-depleted mice.16 A CH2-deleted version of this antibody still recognized β2GPI but failed to fix complement and did not induce thrombosis or pregnancy loss. In these murine models of thrombotic APS, C9 is deposited on the vascular endothelium indicating the presence of the membrane attack complex.15,16 The membrane attack complex triggers the extrinsic pathway of coagulation by inducing tissue factor expression on the endothelial surface, a process that is mediated by release of interleukin 1 alpha.54 In all of these experiments, the need for mechanical injury or lipopolysaccharide in addition to aPL highlights the thromboinflammatory nature of APS, with a baseline inflammatory state on which is superimposed a second insult “hit” that precipitates thrombosis. This is supported by studies that show elevated levels of inflammatory cytokines in patients with APS.55-57
3.2 ∣. Clinical studies
Nearly 3 decades ago, Davis et al demonstrated higher levels of C5b-9 in sera of patients with aPL and stroke compared with non-APS-related stroke.58 Others have reported hypocomplementemia59 and higher levels of complement fragment Bb and C3a60,61 in patients with APS; however, the association with APS-related thrombotic events or serologic characteristics is inconsistent.59,61 Meroni and colleagues described the case of a woman with primary APS who developed venous and arterial thromboses, with limb-threatening recurrences of arterial thrombosis despite anticoagulation.62 Eculizumab was used to allow successful femoral-popliteal bypass without recurrent thrombosis. Increased plasma levels of C5a and C5b-9 were observed and on pathologic examination of the affected artery, deposits of C1q, C4, C3, and C5b-9 colocalized with β2GPI and IgG in the vessel, suggesting activation of complement via the classical pathway.62
In a recent prospective study, we evaluated complement activation in sera of patients with APS by measuring complement dependent cell killing in the modified Ham assay and cell-surface deposition of C5b-9 by flow cytometry.20 Complement activation (a positive modified Ham assay) was observed in 85.7% of CAPS, 35.6% of APS, and only 6.8% of systemic lupus erythematosus (SLE) sera. Complement activation was more likely to be detected near the time of a thrombotic event and 68.5% of APS samples collected within 1 year of thrombosis showed evidence of complement activation. A positive modified Ham assay, particularly >1 year after the index thrombotic event was associated strongly with recurrent thrombosis, even in patients on therapeutic anticoagulation. These findings were also corroborated by increased deposition of C5b-9 by flow cytometry induced by sera from patients with APS or CAPS. Patient-derived anti-β2GPI antibodies also induced C5b-9 deposition, which was blocked completely by an anti-C5 monoclonal antibody, but not by a factor D inhibitor, indicating that complement activation by anti-β2GPI antibodies occurs primarily through the classical or lectin pathway.20
4 ∣. CAPS AND COMPLEMENT GENE MUTATIONS
In addition to complement activation via an environmental stressor, patients with complement-mediated disease are thought to be predisposed to dysregulation of the immune system because of the presence of genetic alterations. This is supported by the discovery of a germline variant in alternative complement pathway regulatory genes in approximately 50% to 60% of patients with aHUS.47 Heterozygous variants in CFH are most common; however, variants in CFI, CFB, C3, and MCP have been reported in patients with aHUS.47 Variants in other genes important for complement function, such as THBD and the complement factor H–related proteins have also been identified. Incomplete penetrance is the rule rather than the exception, and the risk of developing disease for any given variant is difficult to predict.
Because the role of complement in pathogenesis of disease has expanded beyond aHUS, so has the search for predisposing genetic alterations. We previously found a high incidence of germline variants in complement genes in patients with HELLP, providing evidence for complement in the etiology if this disorder.45,63 More recently, we hypothesized that patients with APS and CAPS may harbor germline variants in complement genes and performed targeted sequencing of a cohort of patients with CAPS, APS, or SLE (without aPL) with comparison to patients with aHUS and healthy controls. This study was done using a broad gene panel that resulted in an increased frequency of variants identified in control patients. Despite this, the presence of a rare germline variant (minor allele frequency < 1%) was significantly higher in CAPS than in APS, SLE, and controls, and similar to that seen in aHUS.20 Though the total number of patients in each cohort was relatively small, variants were present in 60.0% (6 of 10) patients with CAPS and 51.5% (17 of 33) of aHUS, compared with 21.8% (12 of 55) patients with thrombotic APS, 28.6% (6 of 21) of SLE, and 23.3% (10 of 43) of unaffected individuals.20 This indicates that the subset of patients with APS who also harbor germline variants may be at risk to develop CAPS from deregulation of complement.
The functional consequences of the germline variants identified in individuals with CAPS remains an active area of investigation. Interestingly, multiple patients were found to harbor homozygous CFHR1-CFHR3 deletions. This is a relatively common genetic alteration that occurs in approximately 2% to 5% of the general population, but is significantly enriched in patients with aHUS.64 One reason for this is the known association of homozygous CFHR1-CFHR3 deletion with the presence of CFH antibodies, which can inhibit the function of CFH. However, other studies have suggested that the CFHR proteins have a direct role in complement regulation by competing for CFH binding sites or inhibiting C5 convertase activity.65,66 It is possible that the function of the CFHR proteins are not restricted to the alternative pathway, potentially explaining the presence of germline alterations in these genes in complement-mediated disease other than aHUS. This may also be the case for complement receptor 1 (CR1), which has known roles in both the alternative and classical pathways.67 Variants in this gene were also identified in multiple CAPS patients; however, currently they are characterized as variants of unknown significance. Genetic testing of larger cohorts of patients with CAPS, and functional analysis of germline variants, is required to determine pathogenicity.
5 ∣. THERAPEUTIC PERSPECTIVES
Long term-anticoagulation with a vitamin K antagonist remains the standard of care for thrombotic APS, and a combination of aspirin with low molecular weight heparin is the mainstay of therapy for obstetric APS. However, a number of patients with APS develop recurrent thrombotic events or pregnancy complications despite “adequate” therapies, indicating a need for other treatments.68 For example, in a study of high-risk (triple positive for the lupus anticoagulant, anti-β2GPI antibody, and anti-cardiolipin antibody) patients, 29.3% (36 of 123) of patients on anticoagulation and 51.4% of non-anticoagulated patients had recurrent thromboembolic events over 10 years.69 The evidence supporting a role for complement activation in the vascular and obstetric complications of APS is compelling and complement inhibition has emerged as an attractive clinical strategy. Complement inhibitors such as eculizumab and ravulizumab are widely used to treat aHUS and PNH, and their safety and efficacy are well established.70,71
5.1 ∣. Complement inhibition for thrombotic and catastrophic APS
Complement inhibitors are not currently approved for the treatment of APS or CAPS. A small phase 2 trial of eculizumab to allow renal transplantation in patients with CAPS enrolled three patients with APS (two with prior CAPS) who received eculizumab starting on the day before renal transplant surgery and continuing indefinitely post-transplant. All three patients underwent successful renal transplantation without recurrence of thrombosis or CAPS, and had functioning grafts at follow up of 4 months to 4 years.72 Anecdotal reports also support the use of eculizumab to treat thrombotic APS refractory to anticoagulation,62 and CAPS refractory to standard measures such as anticoagulation, plasma exchange, and immuno-suppression with corticosteroids, rituximab, or other agents.73-79 Patients in these reports received short courses of eculizumab and did not develop recurrent CAPS after stopping eculizumab, which is consistent with the multiple-hit hypothesis. It is likely that complement inhibitor therapy could be safely stopped once the thrombotic microangiopathy associated with CAPS has resolved, organ function has improved or plateaued at a new baseline, and the complement amplifying trigger is no longer active. This is also consistent with emerging reports that eculizumab can be safely stopped in the majority of patients with aHUS who meet similar criteria.80-83
5.2 ∣. Eculizumab for obstetric APS/HELLP
Women with APS are at particularly high risk for pregnancy complications and fetal loss, as well as a first or recurrent thrombosis during pregnancy. They are also at higher risk of hypertensive disorders of pregnancy such as preeclampsia and the HELLP syndrome, which clinically resembles a thrombotic microangiopathy,84 and may be complement mediated at least in a subset of patients.45,85,86 CAPS may also be precipitated by pregnancy and delivery and is difficult or impossible to distinguish from HELLP or aHUS associated with pregnancy. Eculizumab crosses the placenta minimally and has no adverse effects on the fetus.87 Eculizumab has been used to treat CAPS associated with pregnancy.78,79 Complement inhibition is a particularly attractive strategy for pregnancy-associated CAPS, HELLP that persists after delivery, and to salvage pregnancies with early onset of HELLP before fetal viability, which is being evaluated in a clinical trial (NCT04103489).
5.3 ∣. Challenges of clinical trials of complement inhibitors in APS
Complement inhibition as a therapeutic strategy for catastrophic, thrombotic, or obstetric APS needs to be tested in well-designed, prospective, clinical trials. However, this presents several challenges. Enrolling adequate numbers of patients with a rare disease is challenging, and this will be especially challenging for CAPS, and if patients at highest risk of recurrent VTE (who are also most likely to benefit from complement inhibition) are selected. Any adequately powered trial in APS will need to recruit from multiple centers, and possibly multiple countries. The second challenge will be to identify the patient population most likely to benefit from adjunctive therapies (in addition to anticoagulation). For example, patients at high risk of thrombosis (triple-positive aPL profile) or those with aPL and prior pregnancy loss despite aspirin and low molecular weight heparin may be the population of interest for thrombotic and obstetric APS, respectively. Ideally, complement-related biomarkers would be able to identify patients who are more likely to be refractory to standard therapy and those who would benefit from complement inhibition as an adjunct to anticoagulation and antiplatelet therapy. Unfortunately, standard serum complement assays such as C3, C4, C5b-9, or CH50 have not proved to be reliable biomarkers of disease activity for complement-mediated disorders such as aHUS, and have not been yet been shown to correlate with or predict the development of thrombosis. Although functional assays such as the modified Ham assay appear to correlate with disease activity and thrombotic risk, these are not available clinically and their prognostic utility in monitoring still needs to be established in larger cohorts. The third challenge is selecting appropriate endpoints. Although recurrent thrombosis may require 1 or more years of follow up, this is the most clinically meaningful endpoint. Surrogate endpoints such as suppression of thrombin generation and complement specific biomarker studies may be informative but cannot replace clinical outcomes.
Catastrophic APS presents the greatest opportunity for improving outcomes; however, recruiting patients with CAPS is exceptionally challenging because of the rarity of the condition and challenges with diagnosis. The clinical presentation of CAPS is very similar to, and may be indistinguishable from, other thrombotic microangiopathies or sepsis and disseminated intravascular coagulation unless the patient has a known diagnosis of APS and a high index of suspicion is maintained. Moreover, histologic confirmation of small vessel thrombosis, which is included in the diagnostic criteria for APS, is often not obtained because of critical illness and thrombocytopenia. Enrolling patients with CAPS in a randomized trial will also be challenging because many of these patients are critically ill, and patients and clinicians may not be amenable to randomization especially in the face of clinical worsening on standard therapy (versus off-label treatment with a complement inhibitor).
5.4 ∣. Thromboinflammation and antiphospholipid antibodies in the context of coronavirus disease 2019
The current pandemic of the severe acute respiratory coronavirus 2 (SARS-CoV-2), or coronavirus disease 2019 (COVID-19), has caused substantial morbidity and mortality. Thrombosis has emerged as a cardinal feature of COVID.88,89 The coagulopathy of COVID-19 is characterized by markedly elevated D-dimers, prolonged prothrombin time and partial thromboplastin time, consistent with a disseminated intravascular coagulation-like picture, but with unusually high fibrinogen levels and relatively preserved platelet count. Patients with COVID-19 demonstrate high rates of both arterial and venous thrombosis,89 and microvascular thrombosis is seen in pathology specimens from critically ill patients with COVID-19.90 Studies in murine models of SARS-CoV-1 highlight a critical role of complement in CoV-associated respiratory disease. For example, Gralinski et al demonstrated that SARS-CoV infection in mice resulted in induction of inflammatory cytokines and chemokines, and immune cell infiltration within the lung. Complement activation products (C3) were detected in SARS-CoV MA15-infected mice, but not in control mice,91 and C3-deficient mice were protected against CoV-induced disease.91 SARS-CoV can directly activate complement via the lectin pathway,92 and in patients with SARS-CoV-2, progression of severe pneumonia, acute lung injury, or acute respiratory distress syndrome is associated with C3c, a marker of complement activation.93 Based on these data, and clinical similarities of COVID-19 with thrombotic microangiopathy, complement inhibition has been proposed as a therapeutic strategy for severe COVID-19 respiratory disease. A recent case series from Italy reported rapid improvement in inflammatory markers, oxygenation, and recovery in four patients with COVID-19-associated severe pneumonia treated with eculizumab.94 Multiple clinical trials are evaluating inhibitors of C3 (NCT04395456) and C5 (NCT04288713, NCT04346797, NCT04390464, NCT04369469) to treat COVID-19.
Emerging reports suggest a high rate of antiphospholipid antibodies in patients with COVID-19. Zhang et al described three patients with COVID-19 complicated by coagulopathy, thrombocytopenia, and cerebral infarction, who had evidence of aCL IgA and anti-β2GPI IgG and IgA antibodies.95 It is unclear whether the LA/aPL was the cause of thrombosis, and patients in this series had preexisting cardiovascular disease that increases risk for arterial clots. Additionally, the significance of the IgA aPL is unclear because the clinical significance of these antibodies is not well established. Harzallah and colleagues evaluated 56 patients with confirmed or suspected COVID-19 for lupus anticoagulant and antiphospholipid antibodies.96 A lupus anticoagulant was detected in 25 (44.6%), and 5 (8.9%) had either of aCL or anti-β2GPI antibodies96; however, the persistence of aPL associated with COVID-19 have not been established. Transient antiphospholipid antibodies may be detected in the setting of acute infections, particularly viral infections, and these are generally not associated with the thrombotic or obstetric complications characteristic of the antiphospholipid syndrome.97-99 The discordance between the relatively high rates of positive lupus anticoagulants but low rate of aCL or anti-β2GPI antibodies is also intriguing, and raises the question of whether the positive lupus anticoagulant testing could be an inflammatory epiphenomena rather than a true antiphospholipid antibody. The in vitro prolongation of coagulation tests is due to lupus anticoagulant resulting from interference with the accumulation of coagulation factors and generation of clot on a negatively charged phospholipid surface. Elevated C-reactive protein levels in patients with an inflammatory syndrome can lead to false-positive lupus anticoagulant testing because C-reactive protein can bind to negatively charged phospholipids and interfere with coagulation test results for lupus anticoagulant in a dose-dependent manner.100 However, the association between positive aPL testing and COVID-19 is provocative and merits further exploration.
6 ∣. CONCLUSIONS
Experimental data indicate that complement activation plays a critical role in the pathogenesis of thrombosis and pregnancy complications in APS. The high rate of germline mutations in complement genes in individuals with CAPS suggests that CAPS is a form fruste of complement-mediated thrombotic microangiopathy. Complement inhibition may provide a useful adjunctive therapy for patients with APS refractory to standard therapies, which is supported by reports of successful use of complement inhibition in patients with CAPS and anticoagulation-refractory thrombotic APS. Ongoing research is evaluating the mechanisms and pathways of complement activation by aPL, which may guide the choice of optimal therapeutic agent. Several molecules targeting different complement pathways are in development or in clinical trials and may provide more targeted therapy depending on the specific pathways involved in APS.
ACKNOWLEDGMENTS
This work was supported by K99HL150594 and a HTRS Mentored Research Award (SC), K08HL138142 (EMB), and R01HL133113 (RAB).
Funding information
Hemostasis and Thrombosis Research Society, Grant/Award Number: Mentored Research Award; National Heart, Lung, and Blood Institute, Grant/Award Number: K08HL138142, K99HL150594 and R01HL133113
Footnotes
CONFLICT OF INTEREST
Dr. Chaturvedi reports grants from Shire (as a HTRS Mentored Research Award), participation on advisory boards for Alexion, and participation on advisory boards for Sanofi, outside the submitted work. Dr. Braunstein has no conflicts of interest to report. Dr. Brodsky reports grants and other from Alexion, outside the submitted work.
REFERENCES
- 1.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295–306. [DOI] [PubMed] [Google Scholar]
- 2.Galli M, Borrelli G, Jacobsen EM, et al. Clinical significance of different antiphospholipid antibodies in the WAPS (warfarin in the antiphospholipid syndrome) study. Blood. 2007;110(4):1178–1183. [DOI] [PubMed] [Google Scholar]
- 3.McNeil HP, Simpson RJ, Chesterman CN, Krilis SA. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: beta 2-glycoprotein I (apolipoprotein H). Proc Natl Acad Sci USA. 1990;87(11):4120–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marciniak E, Romond EH. Impaired catalytic function of activated protein C: a new in vitro manifestation of lupus anticoagulant. Blood. 1989;74(7):2426–2432. [PubMed] [Google Scholar]
- 5.Liestol S, Sandset PM, Jacobsen EM, Mowinckel M-C, Wisløff F. Decreased anticoagulant response to tissue factor pathway inhibitor type 1 in plasmas from patients with lupus anticoagulants. Br J Haematol. 2007;136(1):131–137. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, McCrae KR. Annexin A2 mediates endothelial cell activation by antiphospholipid/anti-beta2 glycoprotein I antibodies. Blood. 2005;105(5):1964–1969. [DOI] [PubMed] [Google Scholar]
- 7.Shi T, Giannakopoulos B, Yan X, et al. Anti-beta2-glycoprotein I antibodies in complex with beta2-glycoprotein I can activate platelets in a dysregulated manner via glycoprotein Ib-IX-V. Arthritis Rheum. 2006;54(8):2558–2567. [DOI] [PubMed] [Google Scholar]
- 8.Sorice M, Longo A, Capozzi A, et al. Anti-beta2-glycoprotein I antibodies induce monocyte release of tumor necrosis factor alpha and tissue factor by signal transduction pathways involving lipid rafts. Arthritis Rheum. 2007;56(8):2687–2697. [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi S, Alluri R, McCrae KR. Extracellular vesicles in the anti-phospholipid syndrome. Semin Thromb Hemost. 2018;44(5):493–504. [DOI] [PubMed] [Google Scholar]
- 10.Rand JH, Wu X-X, Guller S, et al. Reduction of annexin-V (placental anticoagulant protein-1) on placental villi of women with anti-phospholipid antibodies and recurrent spontaneous abortion. Am J Obstet Gynecol. 1994;171(6):1566–1572. [DOI] [PubMed] [Google Scholar]
- 11.de Groot PG, Urbanus RT. Antiphospholipid syndrome-not a non-inflammatory disease. Semin Thromb Hemost. 2015;41(6):607–614. [DOI] [PubMed] [Google Scholar]
- 12.Salmon JE, Girardi G, Lockshin MD. The antiphospholipid syndrome as a disorder initiated by inflammation: implications for the therapy of pregnant patients. Nat Clin Pract Rheumatol. 2007;3(3):140–147. [DOI] [PubMed] [Google Scholar]
- 13.Pierangeli SS, Girardi G, Vega-Ostertag M, Liu X, Espinola RG, Salmon J. Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. Arthritis Rheum. 2005;52(7):2120–2124. [DOI] [PubMed] [Google Scholar]
- 14.Carrera-Marin A, Romay-Penabad Z, Papalardo E, et al. C6 knockout mice are protected from thrombophilia mediated by antiphospholipid antibodies. Lupus. 2012;21(14):1497–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischetti F, Durigutto P, Pellis V, et al. Thrombus formation induced by antibodies to beta2-glycoprotein I is complement dependent and requires a priming factor. Blood. 2005;106(7):2340–2346. [DOI] [PubMed] [Google Scholar]
- 16.Agostinis C, Durigutto P, Sblattero D, et al. A non-complement-fixing antibody to beta2 glycoprotein I as a novel therapy for anti-phospholipid syndrome. Blood. 2014;123(22):3478–3487. [DOI] [PubMed] [Google Scholar]
- 17.Girardi G, Berman J, Redecha P, et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2003;112(11):1644–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med. 2004;10(11):1222–1226. [DOI] [PubMed] [Google Scholar]
- 19.Redecha P, Tilley R, Tencati M, et al. Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood. 2007;110(7):2423–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaturvedi S, Braunstein EM, Yuan X, et al. Complement activity and complement regulatory gene mutations are associated with thrombosis in APS and CAPS. Blood. 2020;135(4):239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conway EM. Complement-coagulation connections. Blood Coagul Fibrinolysis. 2018;29(3):243–251. [DOI] [PubMed] [Google Scholar]
- 22.Foley JH, Conway EM. Cross talk pathways between coagulation and inflammation. Circ Res. 2016;118(9):1392–1408. [DOI] [PubMed] [Google Scholar]
- 23.Gruppo RA, Rother RP. Eculizumab for congenital atypical hemolytic-uremic syndrome. N Engl J Med. 2009;360(5):544–546. [DOI] [PubMed] [Google Scholar]
- 24.Hill A, Kelly RJ, Hillmen P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood. 2013;121(25):4985–4996.quiz 5105. [DOI] [PubMed] [Google Scholar]
- 25.Facciabene A, De Sanctis F, Pierini S, et al. Local endothelial complement activation reverses endothelial quiescence, enabling T-cell homing, and tumor control during T-cell immunotherapy. Oncoimmunology. 2017;6(9):e1326442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riedl M, Noone DG, Khan M A, et al. Complement activation induces neutrophil adhesion and neutrophil-platelet aggregate formation on vascular endothelial cells. Kidney Int Rep. 2017;2(l):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noone DG, Riedl M, Pluthero FG, et al. Von Willebrand factor regulates complement on endothelial cells. Kidney Int. 2016;90(1):123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oikonomopoulou K, Ricklin D, Ward PA, Lambris JD. Interactions between coagulation and complement-their role in inflammation. Semin Immunopathol. 2012;34(1):151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritis K, Doumas M, Mastellos D, et al. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177(7):4794–4802. [DOI] [PubMed] [Google Scholar]
- 30.Hattori R, Hamilton KK, McEver RP, Sims PJ. Complement proteins C5b–9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J Biol Chem. 1989;264(15):9053–9060. [PubMed] [Google Scholar]
- 31.Ando B, et al. Complement proteins C5b–9 initiate secretion of platelet storage granules without increased binding of fibrinogen or von Willebrand factor to newly expressed cell surface GPIIb-IIIa. J Biol Chem. 1988;263(24):11907–11914. [PubMed] [Google Scholar]
- 32.Yin W, Ghebrehiwet B, Peerschke El. Expression of complement components and inhibitors on platelet microparticles. Platelets. 2008;19(3):225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owens AP 3rd, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108(10):1284–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Combes V, Simon A-C, Grau G-E, et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest. 1999;104(1):93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willemze R, Bradford RL, Mooberry MJ, Roubey RAS, Key NS. Plasma microparticle tissue factor activity in patients with anti-phospholipid antibodies with and without clinical complications. Thromb Res. 2014;133(2):187–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langer F, Spath B, Fischer C, et al. Rapid activation of monocyte tissue factor by antithymocyte globulin is dependent on complement and protein disulfide isomerase. Blood. 2013;121(12):2324–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller-Calleja N, Hollerbach A, Ritter S, et al. Tissue factor pathway inhibitor primes monocytes for antiphospholipid antibody-induced thrombosis. Blood. 2019;134(14):1119–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muller-Calleja N, Ritter S, Hollerbach A, Falter T, Lackner KJ, Ruf W. Complement C5 but not C3 is expendable for tissue factor activation by cofactor-independent antiphospholipid antibodies. Blood Adv. 2018;2(9):979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez-Pedrera C, Buendía P, Cuadrado MJ, et al. Antiphospholipid antibodies from patients with the antiphospholipid syndrome induce monocyte tissue factor expression through the simultaneous activation of NF-kappaB/Rel proteins via the p38 mitogen-activated protein kinase pathway, and of the MEK-1/ERK pathway. Arthritis Rheum. 2006;54(1):301–311. [DOI] [PubMed] [Google Scholar]
- 40.Wiedmer T, Esmon CT, Sims PJ. On the mechanism by which complement proteins C5b–9 increase platelet prothrombinase activity. J Biol Chem. 1986;261(31):14587–14592. [PubMed] [Google Scholar]
- 41.Subramaniam S, Jurk K, Hobohm L, et al. Distinct contributions of complement factors to platelet activation and fibrin formation in venous thrombus development. Blood. 2017;129(16):2291–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gushiken FC, Han H, Li J, Rumbaut RE, Afshar-kharghan V. Abnormal platelet function in C3-deficient mice. J Thromb Haemost. 2009;7(5):865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sauter RJ, Sauter M, Reis ES, et al. Functional relevance of the anaphylatoxin receptor C3aR for platelet function and arterial thrombus formation marks an intersection point between innate immunity and thrombosis. Circulation. 2018;138(16):1720–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baines AC, Brodsky RA. Complementopathies. Blood Rev. 2017;31(4):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaught AJ, Braunstein EM, Jasem J, et al. Germline mutations in the alternative pathway of complement predispose to HELLP syndrome. JCI Insight. 2018;3(6):e99128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fremeaux-Bacchi V, Fakhouri F, Garnier A, et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8(4):554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noris M, Caprioli J, Bresin E, et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5(10):1844–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cervera R, Rodríguez-Pintó I, Colafrancesco S, et al. 14th international congress on antiphospholipid antibodies task force report on catastrophic antiphospholipid syndrome. Autoimmun Rev. 2014;13(7):699–707. [DOI] [PubMed] [Google Scholar]
- 49.Branch DW, Dudley DJ, Mitchell MD, et al. Immunoglobulin G fractions from patients with antiphospholipid antibodies cause fetal death in BALB/c mice: a model for autoimmune fetal loss. Am J Obstet Gynecol. 1990;163(1 Pt 1):210–216. [DOI] [PubMed] [Google Scholar]
- 50.Holers VM, Girardi G, Mo L, et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp Med. 2002;195(2):211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Redecha P, Franzke C-W, Ruf W, Mackman N, Girardi G. Neutrophil activation by the tissue factor/Factor VIIa/PAR2 axis mediates fetal death in a mouse model of antiphospholipid syndrome. J Clin Invest. 2008;118(10):3453–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thurman JM, Kraus DM, Girardi G, et al. A novel inhibitor of the alternative complement pathway prevents antiphospholipid antibody-induced pregnancy loss in mice. Mol Immunol. 2005;42(l):87–97. [DOI] [PubMed] [Google Scholar]
- 53.Pierangeli SS, Vega-ostertag M, Liu X, Girardi G. Complement activation: a novel pathogenic mechanism in the antiphospholipid syndrome. Ann N Y Acad Sci. 2005;1051:413–420. [DOI] [PubMed] [Google Scholar]
- 54.Saadi S, Holzknecht RA, Patte CP, Stern DM, Platt JL. Complement-mediated regulation of tissue factor activity in endothelium. J Exp Med. 1995;182(6):1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Forastiero RR, Martinuzzo ME, de Larranaga GF. Circulating levels of tissue factor and proinflammatory cytokines in patients with primary antiphospholipid syndrome or leprosy related antiphospholipid antibodies. Lupus. 2005;14(2):129–136. [DOI] [PubMed] [Google Scholar]
- 56.Bertolaccini ML, Atsumi T, Lanchbury JS, et al. Plasma tumor necrosis factor alpha levels and the −238*A promoter polymorphism in patients with antiphospholipid syndrome. Thromb Haemost. 2001;85(2):198–203. [PubMed] [Google Scholar]
- 57.Ahmed K, Vianna JL, Khamashta MA, Hughes GR. IL-2, IL-6 and TNF levels in primary antiphospholipid syndrome. Clin Exp Rheumatol. 1992;10(5):503. [PubMed] [Google Scholar]
- 58.Davis WD, Brey RL. Antiphospholipid antibodies and complement activation in patients with cerebral ischemia. Clin Exp Rheumatol. 1992;10(5):455–460. [PubMed] [Google Scholar]
- 59.Oku K, Atsumi T, Bohgaki M, et al. Complement activation in patients with primary antiphospholipid syndrome. Ann Rheum Dis. 2009;68(6):1030–1035. [DOI] [PubMed] [Google Scholar]
- 60.Breen KA, Seed P, Parmar K, et al. Complement activation in patients with isolated antiphospholipid antibodies or primary anti-phospholipid syndrome. Thromb Haemost. 2012;107(3):423–429. [DOI] [PubMed] [Google Scholar]
- 61.Devreese KM, Hoylaerts MF. Is there an association between complement activation and antiphospholipid antibody-related thrombosis? Thromb Haemost. 2010;104(6):1279–1281. [DOI] [PubMed] [Google Scholar]
- 62.Meroni PL, Macor P, Durigutto P, et al. Complement activation in antiphospholipid syndrome and its inhibition to prevent rethrombosis after arterial surgery. Blood. 2016;127(3):365–367. [DOI] [PubMed] [Google Scholar]
- 63.Vaught AJ, Gavriilaki E, Hueppchen N, et al. Direct evidence of complement activation in HELLP syndrome: a link to atypical hemolytic uremic syndrome. Exp Hematol. 2016;44(5):390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dragon-Durey MA, Blanc C, Marliot F, et al. The high frequency of complement factor H related CFHR1 gene deletion is restricted to specific subgroups of patients with atypical haemolytic uraemic syndrome. J Med Genet. 2009;46(7):447–450. [DOI] [PubMed] [Google Scholar]
- 65.Heinen S, Hartmann A, Lauer N, et al. Factor H-related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood. 2009;114(12):2439–2447. [DOI] [PubMed] [Google Scholar]
- 66.Goicoechea de Jorge E, Caesar JJE, Malik TH, et al. Dimerization of complement factor H-related proteins modulates complement activation in vivo. Proc Natl Acad Sci USA. 2013;110(12):4685–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Java A, Liszewski MK, Hourcade DE, Zhang F, Atkinson JP. Role of complement receptor 1 (CR1; CD35) on epithelial cells: a model for understanding complement-mediated damage in the kidney. Mol Immunol. 2015;67(2):584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cervera R, Serrano R, Pons-Estel GJ, et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis. 2015;74(6):1011–1018. [DOI] [PubMed] [Google Scholar]
- 69.Pengo V, Ruffatti A, Legnani C, et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J Thromb Haemost. 2010;8(2):237–242. [DOI] [PubMed] [Google Scholar]
- 70.Lee JW, Sicre de Fontbrune F, Wong Lee Lee L, et al. Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: the 301 study. Blood. 2019;133(6):530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hillmen P, Muus P, Röth A, et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2013;162(l):62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lonze BE, Zachary AA, Magro CM, et al. Eculizumab prevents recurrent antiphospholipid antibody syndrome and enables successful renal transplantation. Am J Transplant. 2014;14(2):459–465. [DOI] [PubMed] [Google Scholar]
- 73.Skoczynska M, Crowther MA, Chowaniec M, et al. Thrombotic microangiopathy in the course of catastrophic antiphospholipid syndrome successfully treated with eculizumab: case report and systematic review of the literature. Lupus. 2020;29(6):631–639. [DOI] [PubMed] [Google Scholar]
- 74.Shapira I, Andrade D, Allen SL, et al. Brief report: induction of sustained remission in recurrent catastrophic antiphospholipid syndrome via inhibition of terminal complement with eculizumab. Arthritis Rheum. 2012;64(8):2719–2723. [DOI] [PubMed] [Google Scholar]
- 75.Wig S, Chan M, Thachil J, et al. A case of relapsing and refractory catastrophic anti-phospholipid syndrome successfully managed with eculizumab, a complement 5 inhibitor. Rheumatology (Oxford). 2016;55(2):382–384. [DOI] [PubMed] [Google Scholar]
- 76.Zikos TA, Sokolove J, Ahuja N, Berube C. Eculizumab induces sustained remission in a patient with refractory primary catastrophic antiphospholipid syndrome. J Clin Rheumatol. 2015;21(6):311–313. [DOI] [PubMed] [Google Scholar]
- 77.Strakhan M, Hurtado-Sbordoni M, Galeas N, et al. 36-year-old female with catastrophic antiphospholipid syndrome treated with eculizumab: a case report and review of literature. Case Rep Hematol. 2014;2014:704371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rovere-Querini P, Canti V, Erra R, et al. Eculizumab in a pregnant patient with laboratory onset of catastrophic antiphospholipid syndrome: a case report. Medicine (Baltimore). 2018;97(40):e12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gustavsen A, Skattum L, Bergseth G, et al. Effect on mother and child of eculizumab given before caesarean section in a patient with severe antiphospholipid syndrome: a case report. Medicine (Baltimore). 2017;96(11):e6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sheerin NS, Kavanagh D, Goodship T, et al. A national specialized service in England for atypical haemolytic uraemic syndrome-the first year's experience. QJM. 2016;109(l):27–33. [DOI] [PubMed] [Google Scholar]
- 81.Ardissino G, Testa S, Possenti I, et al. Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome: a report of 10 cases. Am J Kidney Dis. 2014;64(4):633–637. [DOI] [PubMed] [Google Scholar]
- 82.Wetzels JF, van de Kar NC. Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome. Am J Kidney Dis. 2015;65(2):342. [DOI] [PubMed] [Google Scholar]
- 83.Merrill SA, Brittingham ZD, Yuan X, Moliterno AR, Sperati CJ, Brodsky RA. Eculizumab cessation in atypical hemolytic uremic syndrome. Blood. 2017;130(3):368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Appenzeller S, Souza FHC, Wagner Silva de Souza A, Shoenfeld Y, de Carvalho JF. HELLP syndrome and its relationship with antiphospholipid syndrome and antiphospholipid antibodies. Semin Arthritis Rheum. 2011;41(3):517–523. [DOI] [PubMed] [Google Scholar]
- 85.Burwick RM, Feinberg BB. Eculizumab for the treatment of pre-eclampsia/HELLP syndrome. Placenta. 2013;34(2):201–203. [DOI] [PubMed] [Google Scholar]
- 86.Burwick RM, Fichorova RN, Dawood HY, et al. Urinary excretion of C5b–9 in severe preeclampsia: tipping the balance of complement activation in pregnancy. Hypertension. 2013;62(6):1040–1045. [DOI] [PubMed] [Google Scholar]
- 87.Hallstensen RF, Bergseth G, Foss S, et al. Eculizumab treatment during pregnancy does not affect the complement system activity of the newborn. Immunobiology. 2015;220(4):452–459. [DOI] [PubMed] [Google Scholar]
- 88.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lodigiani C, lapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tian S, Hu W, Niu LI, et al. Pulmonary pathology of early-phase 2019 Novel Coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15(5):700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gralinski LE, Hu W, Niu LI, et al. Complement activation contributes to severe acute respiratory syndrome Coronavirus pathogenesis. MBio. 2018;9(5):e01753–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ip WK, Chan KH, Law HKW, et al. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J Infect Dis. 2005;191(10):1697–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pang RT, Poon TCW, Chan KCA, et al. Serum proteomic finger-prints of adult patients with severe acute respiratory syndrome. Clin Chem. 2006;52(3):421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Diurno F, Numis FG, Porta G,et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci. 2020;24(7):4040–4047. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harzallah I, Debliquis A, Drenou B. Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemost. 2020. 10.1111/jth.14980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Uthman IW, Gharavi AE. Viral infections and antiphospholipid antibodies. Semin Arthritis Rheum. 2002;31(4):256–263. [DOI] [PubMed] [Google Scholar]
- 98.Avcin T, Toplak N. Antiphospholipid antibodies in response to infection. Curr Rheumatol Rep. 2007;9(3):212–218. [DOI] [PubMed] [Google Scholar]
- 99.Asherson RA, Cervera R. Antiphospholipid antibodies and infections. Ann Rheum Dis. 2003;62(5):388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schouwers SM, Delanghe JR, Devreese KM. Lupus anticoagulant (LAC) testing in patients with inflammatory status: does C-reactive protein interfere with LAC test results? Thromb Res. 2010;125(1):102–104. [DOI] [PubMed] [Google Scholar]