Abstract
Objective
To summarize data on atrial fibrillation (AF) detection rates and predictors across different rhythm monitoring strategies in patients with cryptogenic stroke (CS) or embolic stroke of undetermined source (ESUS).
Methods
MEDLINE, Embase, and Web of Science were searched to identify all published studies providing relevant data through July 6, 2020. Random-effects meta-analysis method was used to pool estimates.
Results
We included 47 studies reporting on a pooled population of 8,215 patients with CS or ESUS. Using implantable cardiac monitor (ICM), the pooled rate of AF was 12.2% (95% CI 9.4–15.0) at 3 months, 16.0% (95% CI 13.2–18.8) at 6 months, 18.7% (95% CI 15.7–21.7) at 12 months, 22.8% (95% CI 19.1–26.5) at 24 months, and 28.5% (95% CI 17.6–39.3) at 36 months. AF rates were significantly higher in patients with ESUS vs CS (22.0% vs 14.2%; p < 0.001) at 6 months, and in studies using Reveal LINQ vs Reveal XT ICM (19.1% vs 13.0%; p = 0.001) at 12 months. Using mobile cardiac outpatient telemetry (MCOT), the pooled rate of AF was 13.7% (95% CI 10.2–17.2) at 1 month. Predictors of AF detection with ICM included older age, CHA2DS2-VASc score, left atrial enlargement, P wave maximal duration and prolonged PR interval.
Conclusion
The yield of ICM increases with the duration of monitoring. More than a quarter of patients with CS or ESUS will be diagnosed with AF during follow-up. About one in seven patients had AF detected within a month of MCOT, suggesting that a non-invasive rhythm monitoring strategy should be considered before invasive monitoring.
Keywords: Atrial fibrillation, Cryptogenic stroke, Embolic stroke of undetermined source, Insertable cardiac monitor, Holter, Telemetry
1. Introduction
Ischemic stroke is a leading cause of mortality and disability globally [1], [2]. Identifying stroke etiology is crucial for effective secondary prevention. However, in about one third of ischemic strokes, the likely cause cannot be identified despite extensive investigations according to current protocols [3]. Cardioembolism due to atrial fibrillation (AF) is responsible for about one third of all ischemic strokes, and could be the underlying cause of a significant proportion of cryptogenic strokes (CS) [4]. Ischemic stroke due to AF is associated with higher mortality and disability compared to stroke of other etiology [3], [5]. Because AF-related strokes can largely be prevented by oral anticoagulation, an active search for underlying AF is essential in patients with CS, including those with the more restrictive definition of embolic stroke of undetermined source (ESUS) [4].
According to the American Heart Association/American Stroke Association (AHA/ASA) guidelines, the clinical benefit of prolonged cardiac monitoring to detect atrial fibrillation after acute ischemic stroke is uncertain (Class IIb; Level of Evidence C) and there is no consensus on the most appropriate modality and duration of monitoring [6]. The CRYSTAL AF trial was the first to evaluate the utility of prolonged continuous cardiac monitoring with implantable cardiac monitor (ICM) and demonstrated increased AF detection compared to standard-of-care monitoring in patients with CS (12.4% vs 2.0% at 12 months) [7]. Several other studies have reported variable AF detection rates with ICM in patients with CS, depending on the length of monitoring or other potential factors such as the duration of qualifying AF episodes, or patient characteristics [4]. The current systematic review and meta-analysis aimed to summarize data on AF detection rates across different rhythm monitoring strategies (non-invasive and ICM) at precise time points (e.g. 1 month, 12 months or 24 months) in patients with CS or ESUS, and to explore factors influencing these detection rates.
2. Methods
This review is reported in accordance with the Meta-analyses Of Observational Studies in Epidemiology guidelines [8]. It was registered with PROSPERO (CRD42020204206).
2.1. Literature search
PubMed/MEDLINE, Excerpta Medica Database (EMBASE), and Web of Science were searched to identify all cohort studies or randomized controlled trials (RCTs) reporting primary data on the rates and predictors of AF detection in patients with CS or ESUS, published by July 6, 2020 (date of the last search), without language restriction. The search strategy used a combination of the following terms or their synonyms “cryptogenic stroke”, “embolic stroke of undetermined source”, “atrial fibrillation”, “implantable cardiac monitor”, “holter” or “telemetry” (Supplementary Table 1). The reference lists of eligible articles were also scrutinized to identify potential additional data sources.
Table 1.
Pooled estimates of atrial fibrillation detection using invasive and non-invasive cardiac monitoring strategies.
|
Subgroup |
Number of Studies | No. of patients |
Detection rate (95% CI) |
Heterogeneity |
Egger’s test (P value) |
||
|---|---|---|---|---|---|---|---|
| I2 | P value | ||||||
| Total | Cases | ||||||
| ICM monitoring | |||||||
| 3 months | |||||||
|
11 | 1 098 | 143 | 12.19 (9.36–15.01) | 52.8% | 0.0199 | 0.0692 |
|
|||||||
|
3 | 307 | 51 | 16.44 (10.99–21.90) | 41.2% | 0.1827 | 0.0297 |
|
8 | 791 | 92 | 10.68 (7.77–13.58) | 43.2% | 0.0905 | 0.3490 |
|
|||||||
|
3 | 521 | 74 | 11.06 (4.94–17.18) | 59.3% | 0.0857 | 0.3150 |
|
6 | 255 | 29 | 13.54 (9.43–17.66) | 49.5% | 0.0779 | 0.0651 |
|
|||||||
|
4 | 447 | 69 | 15.07 (11.76–18.38) | 0.0% | 0.4718 | 0.6839 |
|
4 | 340 | 35 | 9.69 (5.39–14.00) | 46.7% | 0.1311 | 0.1187 |
| 6 months | |||||||
|
18 | 3 223 | 476 | 16.00 (13.21–18.79) | 76.3% | <0.0001 | 0.0085 |
|
|||||||
|
4 | 408 | 91 | 22.05 (18.04–26.06) | 0.0% | 0.6027 | 0.2102 |
|
14 | 2 815 | 385 | 14.25 (11.41–17.09) | 73.8% | <0.0001 | 0.0864 |
|
|||||||
|
5 | 613 | 61 | 9.89 (6.12–13.66) | 59.1% | 0.0444 | 0.0555 |
|
11 | 2 288 | 360 | 19.22 (15.54–22.89) | 71.1% | 0.0001 | 0.0002 |
|
|||||||
|
7 | 2 132 | 293 | 14.72 (10.64–18.80) | 80.8% | <0.0001 | 0.2880 |
|
7 | 705 | 106 | 15.82 (10.67–20.96) | 72.8% | 0.0012 | 0.0098 |
|
2 | 175 | 45 | 25.62 (19.16–32.08) | 0.0% | 0.5515 | NE |
| 12 months | |||||||
|
16 | 3 310 | 603 | 18.71 (15.71–21.70) | 76.3% | <0.0001 | 0.1229 |
|
|||||||
|
4 | 372 | 86 | 22.08 (15.32–28.88) | 61.8% | 0.0492 | 0.5716 |
|
12 | 2 938 | 517 | 17.74% (14.45–21.03) | 77.9% | <0.0001 | 0.3224 |
|
|||||||
|
4 | 464 | 60 | 12.88 (9.84–15.93) | 0.0% | 0.9598 | 0.3096 |
|
9 | 2 379 | 461 | 22.21 (18.55–25.87) | 69.0% | 0.0011 | 0.0022 |
|
|||||||
|
8 | 2 460 | 448 | 19.14 (14.95–23.34) | 82.0% | <0.0001 | 0.2757 |
|
4 | 470 | 63 | 13.32 (10.25–16.39) | 0.0% | 0.9024 | 0.0333 |
|
3 | 240 | 60 | 23.86 (11.47–36.26) | 82.2% | 0.0036 | 0.3252 |
| 24 months | |||||||
|
13 | 2 901 | 661 | 22.78 (19.90–26.47) | 78.6% | <0.0001 | 0.4384 |
|
|||||||
|
3 | 312 | 79 | 24.79 (16.60–32.98) | 64.7% | 0.0586 | 0.8340 |
|
10 | 2 589 | 582 | 22.27 (18.02–26.52) | 81.6% | <0.0001 | 0.6144 |
|
|||||||
|
7 | 880 | 174 | 19.13 (13.84–24.42) | 75.3% | 0.0005 | 0.3194 |
|
5 | 1 926 | 459 | 26.98 (22.11–31.85) | 72.5% | 0.0057 | 0.0027 |
|
|||||||
|
7 | 2 262 | 530 | 25.70 (19.53–31.87) | 89.2% | <0.0001 | 0.2727 |
|
4 | 398 | 93 | 23.27 (16.00–30.54) | 65.2% | 0.0347 | 0.5355 |
| MCOT monitoring | |||||||
|
13 | 1745 | 218 | 11.76 (9.12–14.40) | 65.4% | 0.0005 | 0.1203 |
|
|||||||
|
6 | 643 | 70 | 9.49 (5.55–13.43) | 64.0% | 0.0164 | 0.6172 |
|
7 | 1 102 | 148 | 13.67 (10.16–17.18) | 63.8% | 0.0110 | 0.0659 |
ICM: implantable cardiac monitor; MCOT: mobile cardiac outpatient telemetry.
2.2. Study selection
We included: (1) cohort studies or RCTs, (2) with more than 30 participants, (3) reporting on rates and predictors of AF in patients with CS or ESUS, or studies with enough data to compute these estimates, (4) using either ICM or a non-invasive cardiac monitoring strategy. We excluded AF screening strategies such as 12-lead electrocardiography (ECG), 24-hour Holter or inpatient telemetry that are normally part of the minimum work-up for patients with acute ischemic stroke. For records/articles reporting data from the same group/cohort of patients, we included the single most comprehensive report with the largest sample size. Two investigators (JJN and JKT) independently screened records for eligibility based on titles and abstracts. Full texts of articles deemed potentially eligible were retrieved and screened independently by the same investigators for final inclusion. Disagreements were resolved via discussion and consensus.
2.3. Data extraction and management
Data were extracted using a standard data abstraction form by one investigator (JJN) and cross-checked by second investigator (JKT). We collected data on study characteristics, study population (CS or ESUS), sample size, etiological work-up, definition criteria for CS or ESUS, mean or median time from event to initiation of monitoring, minimal duration of qualifying AF episodes (e.g. 30 sec, 2 min or more), monitoring device, mean or median age, sex proportion, frequency of/prevalence of/proportion of patients with co-morbidities such as hypertension, diabetes, heart failure or previous thromboembolism (stroke or transient ischemic attack [TIA]), number of participants with AF detected at various time points (3 weeks, 1 month, 3 months, 6 months, 9 months, 12 months, 18 months, 24 months and 36 months), and risk estimate (hazard ratio, odds ratio or relative risk) with the 95% confidence interval (95% CI) for each variable assessed as a potential predictor of AF. For each study, the risk of bias was assessed using an adapted version of the tool developed by Hoy et al. [9].
2.4. Statistical analysis
Analyses were conducted with R statistical software (version 3.6.2, The R Foundation for statistical computing, Vienna, Austria). We performed random-effects meta-analysis of detection rates using the inverse variance method. Clinical conditions potentially contributing to the variance of the estimates were evaluated by meta-regression, with the proportion of categorical variables, such as diabetes, fitted on a continuous scale. Heterogeneity was assessed by the χ2 test on Cochrane's Q statistic [10], and was quantified by I2 values, assuming that I2 values of <25%, 50–75%, and >75% respectively represent low, medium, and high heterogeneity [11]. Heterogeneity across studies was further explored using the Leave-One-Out influencer analysis model, with the aim of detecting extreme effect sizes and assessing the influence of each study on the overall estimates. We assessed small-study effect by visual inspection of funnel plots and tests of funnel plot asymmetry (Egger’s linear regression test). Next, we performed univariable random-effects meta-analyses to assess the association between clinical variables and AF detection rates, reporting this in odds ratio (OR) and 95% confidence interval (95% CI). For continuous variables, we converted the reported mean and standard deviations (SD) to standardized mean difference and rescaled to OR (95% CI) per SD change in the variable [12]. All statistical tests were two-tailed and statistical significance defined as p-value ≤ 0.05.
2.5. Ethic committee approval
This is a systematic review using published data. An ethics approval is not required.
2.6. Patient and public involvement
This is not applicable to this systematic review that used published data.
3. Results
3.1. Study selection and characteristics
Database and bibliographic searches retrieved 1139 records and 47 articles were finally included (Supplementary Fig. 1). The list of included studies and their characteristics are presented in the appendix (Supplementary Tables 2–4). The included studies reported data from a pooled sample of 8,215 patients with CS or ESUS, were conducted between 2003 and 2019, and published between 2008 and 2020. Most studies were conducted in Europe (55.3%, n = 26) and Northern America (36.2%, n = 17), with the most represented country being the U.S. (34.0%, n = 16). Most studies (59.6%, n = 28) had low risk of bias (Supplementary Table 5).
3.2. AF detection by implantable cardiac monitors
3.2.1. Rates of AF by duration of monitoring
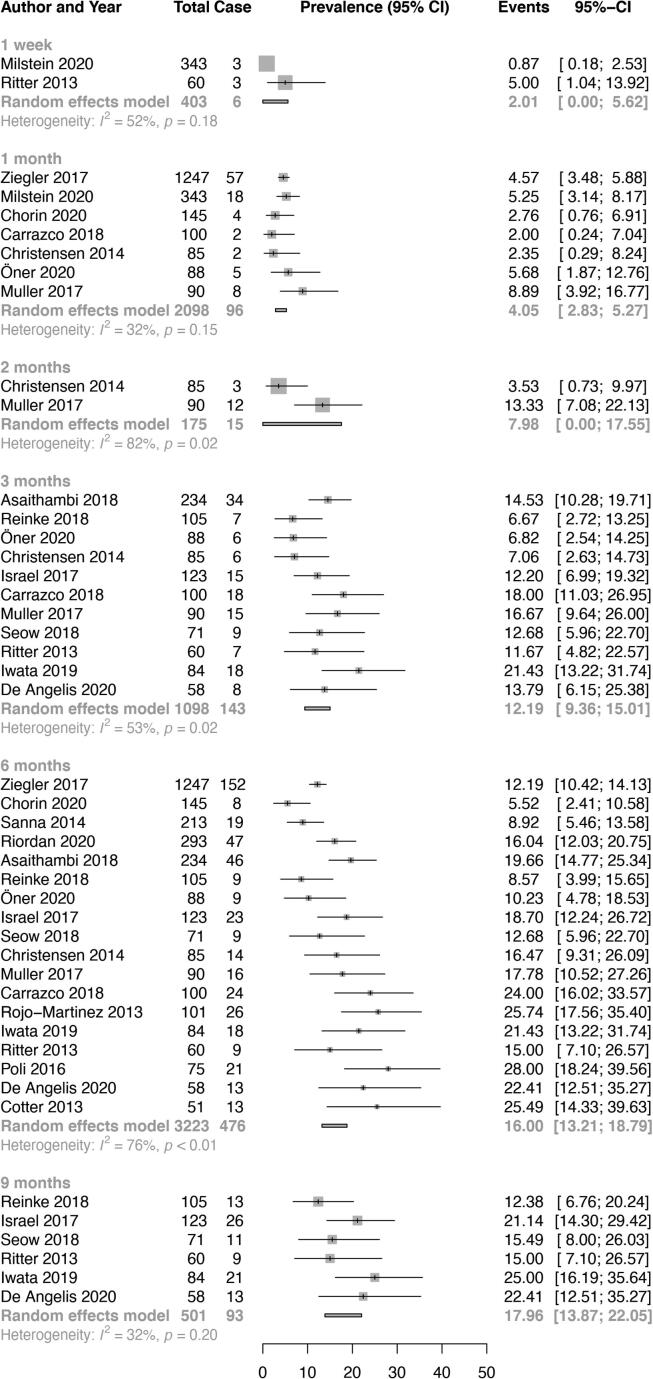
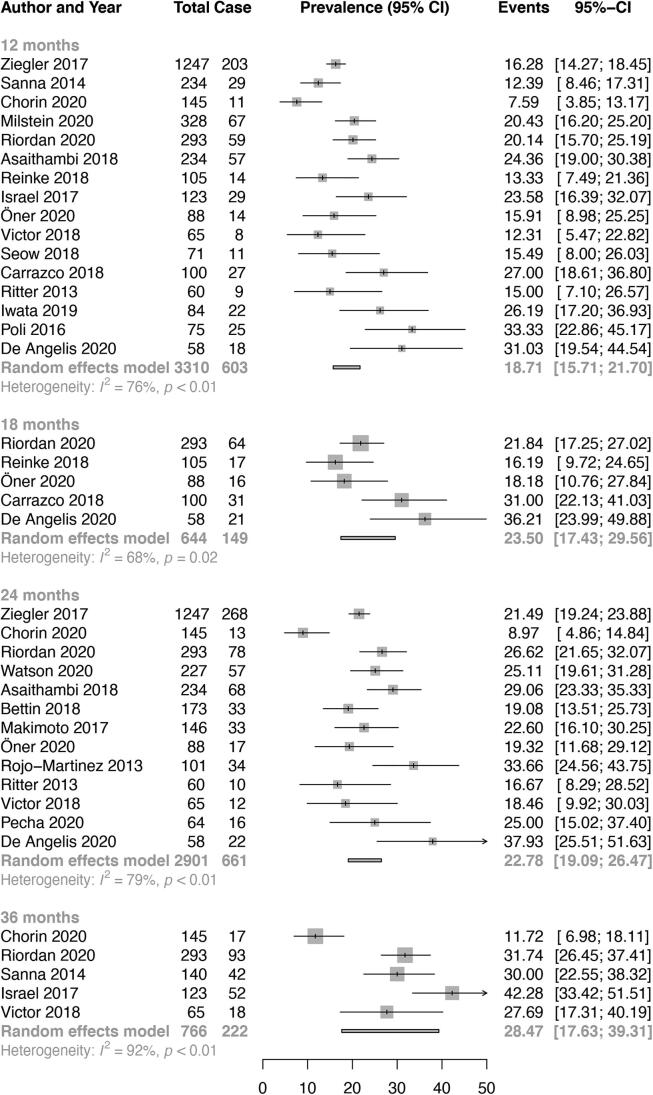
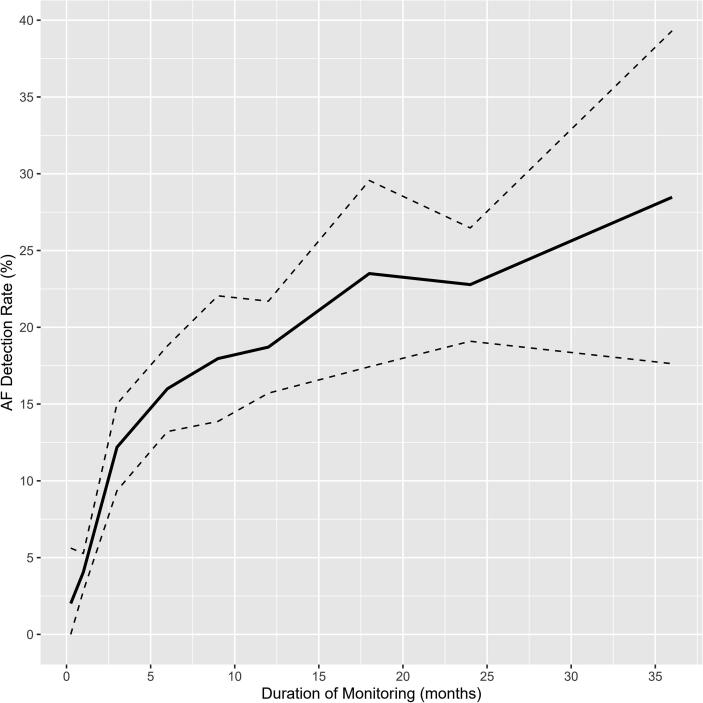
The pooled rate of AF was 2.0% (95% CI 0.0–5.6, I2 52%) at 1 week, 4.1% (95% CI 2.8–5.3, I2 32%) at 1 month, 8.0% (95% CI 0.0–17.6, I2 82%) at 2 months, 12.2% (95% CI 9.4–15.0, I2 53%) at 3 months, 16.0% (95% CI 13.2–18.8, I2 76%) at 6 months, 18.0% (95% CI 13.9–22.1, I2 32%) at 9 months, 18.7% (95% CI 15.7–21.7, I2 76%) at 12 months, 23.5% (95% CI 17.4–29.6, I2 68%) at 18 months, 22.8% (95% CI 19.1–26.5, I2 79%) at 24 months and 28.5% (95% CI 17.6–39.3, I2 92%) at 36 months (Table 1, Fig. 1A, Fig. 1B [Panels A and B]). Overall, AF detection rates significantly increased with duration of monitoring (p < 0.001, Fig. 2).
Fig. 1A.
Panel A. Overall pooled atrial fibrillation detection rates on implantable cardiac monitor in patients with cryptogenic stroke (one week to 9 months).
Fig. 1B.
Panel B. Overall pooled atrial fibrillation detection rates on implantable cardiac monitor in patients with cryptogenic stroke (12 months to 36 months).
Fig. 2.
Relationship of atrial fibrillation detection with duration of monitoring in patients with cryptogenic stroke. Legend: the black bold line and dot lines represent the curves of atrial fibrillation detection and the upper and lower limits of the 95% confidence interval.
3.2.2. Rates of AF in ESUS versus CS
The pooled AF detection rate in ESUS vs CS was 16.4% (95% CI 11.0–21.9, I2 41%) vs 10.7% (95% CI 7.8–13.6, I2 43%) at 3 months (p = 0.067), 22.0% (95% CI 18.0–26.1, I2 0%) vs 14.2% (95% CI 11.4–17.1, I2 74%) at 6 months (p < 0.001), 22.1% (95% CI 15.3–28.8, I2 62%) vs 17.7% (95% CI 14.4–21.0, I2 78%) at 12 months (p = 0.2579), 24.8% (95% CI 16.6–33.0, I2 65%) vs 22.3% (95% CI 18.0–26.5, I2 82%) at 24 months (p = 0.592) (Table 1, Supplementary Figs. 2–5.
3.2.3. Rates of AF by minimal duration of qualifying episodes
The rate of AF was lower in studies using a cut-off of 30 sec compared to those using a cut-off of 120 sec. The detection rate for the studies using a 30 sec cut-off vs studies using a 120 sec cut-off was 11.1% (95% CI 4.9–17.2, I2 59%) vs 13.5% (95% CI 9.4–17.6, I2 50%) at 3 months (p = 0.509), 9.9% (95% CI 6.1–13.7, I2 59%) vs 19.2% (95% CI 15.5–22.9, I2 71%) at 6 months (p = 0.001), 13.2% (95% CI 8.1–18.4, I2 0%) vs 20.7% (95% CI 16.4–25.0, I2 0%) at 9 months (p = 0.03), 12.9% (95% CI 9.8–15.9, I2 0%) vs 22.2% (95% CI 18.6–25.9, I2 69%) at 12 months (p < 0.001), 17.9% (95% CI 12.5–23.3, I2 70%) vs 27.0% (95% CI 22.1–31.9, I2 73%) at 24 months (p = 0.01) (Table 1, Supplementary Figs. 6–10).
3.2.4. Rates of AF by device type
The pooled AF detection rate was higher in studies using Reveal LINQ compared to those using Reveal XT, although the difference was not significant at all timepoints. The detection rate for Reveal LINQ vs Reveal XT was 15.1% (95% CI 11.8–18.4, I2 0%) vs 9.7% (95% CI 5.4–14.0, I2 47%) at 3 months (p = 0.052), 14.7% (95% CI 10.6–18.8, I2 81%) vs 15.8% (95% CI 10.7–21.0, I2 73%) at 6 months (p = 0.744), 19.1% (95% CI 14.9–23.3, I2 82%) vs 13.0% (95% CI 9.7–16.3, I2 0%) at 12 months (p = 0.001), 25.7% (95% CI 19.5–31.9, I2 89%) vs 23.3% (95% CI 16.0–30.5, I2 65%) at 24 months (p = 0.618) (Table 1, Supplementary Figs. 11–14).
3.2.5. Publication bias, meta-regression, and sensitivity analysis
There was evidence of publication bias by funnel plot analysis and by Egger’s test (p < 0.01) only for studies reporting AF detection rates at 6 months (Table 1, Supplementary Figs. 15–18). We performed meta-regression to assess the source of heterogeneity in the estimation of AF rates (Supplementary Figs. 19–22). At 3 months of monitoring, history of hypertension accounted for most of the variation in AF detection rate (r2 = 53.3%), though it was not significant (p = 0.07). At 6 months of monitoring, no variable significantly explained the variance in AF detection. At 12 months of monitoring, mean age and history of hypertension explained 21.6% and 35.6% of the variation in AF detection rate, respectively, although it did not reach significance (p = 0.08 and 0.06, respectively). At 24 months of monitoring, history of dyslipidaemia explained most of the variance in the estimation (r2 44.8%, p = 0.15).
Influencer analysis to ascertain the contribution of each study to the overall heterogeneity showed that no study markedly reduced the overall heterogeneity when its estimate was removed from the overall analysis (Supplementary Figs. 23–26).
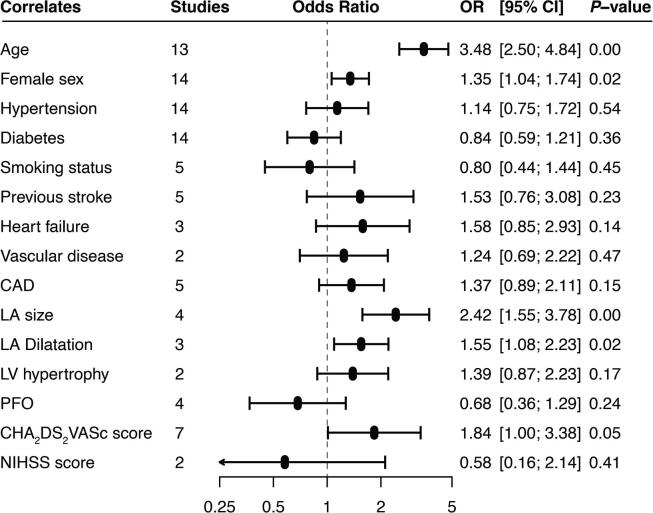
3.3. Predictors of AF detection
Data from 14 studies contributed to pooled univariable analysis of factors associated with AF detection (Fig. 3). Age (OR 3.48, 95% CI 2.50–4.84), female sex (OR 1.35, 95% CI 1.04–1.74), left atrial size (OR 2.42, 95% CI 1.55–3.78), left atrial dilatation (˃40 mm) (OR 1.55, 95% CI 1.08–2.23) and the CHA2DS2VASc score (includes age, sex, history of hypertension, diabetes, heart failure, vascular disease and stroke) (OR 1.84, 95% CI 1.00–3.38) were positively associated with AF detection.
Fig. 3.
Univariable correlates of atrial fibrillation detection on implantable cardiac monitors in patients with cryptogenic stroke.
Eleven studies reported multivariable estimates of potential predictors of AF detection (Supplementary Table 6). Due to high heterogeneity in these estimates, a meta-analysis was not performed. Age was consistently reported as a predictor of AF. In studies that used ICMs, other predictors included obesity, infarction in the posterior cerebral artery territory, total atrial conduction time assessed by tissue doppler imaging (PA-TDI Interval), left atrial enlargement, P wave maximal duration, prolonged PR interval and atrial runs (Supplementary Table 6).
3.4. Data on non-invasive cardiac monitoring strategies
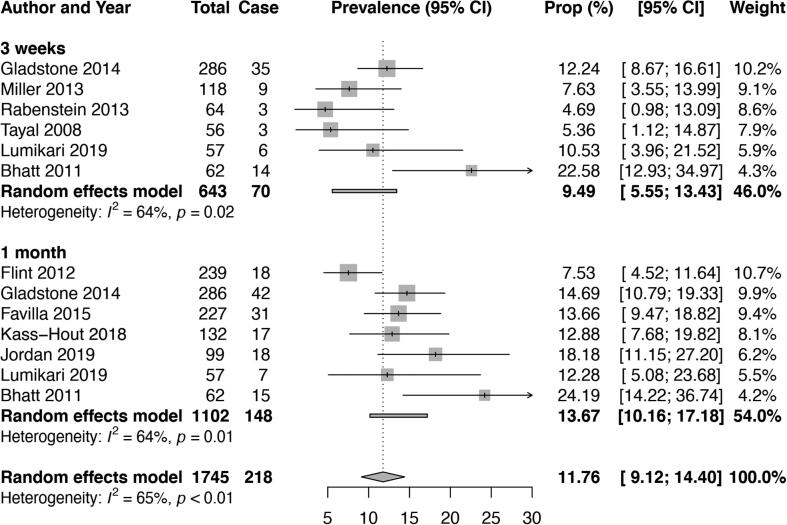
Atrial fibrillation detection rates from studies that used non-invasive cardiac monitoring are reported in Supplementary Table 7. A meta-analysis was only feasible for data on mobile cardiac outpatient telemetry (MCOT). The overall pooled AF detection rate (episode lasting at least 30 s) using MCOT was 9.5% (95% CI 5.6–13.4, I2 64%) at 3 weeks and 13.7% (95% CI 10.2–17.2, I2 64%) at 1 month (Table 1, Fig. 4).
Fig. 4.
Overall pooled atrial fibrillation detection rates on mobile cardiac outpatient telemetry in patients with cryptogenic stroke.
4. Discussion
This study aimed to summarize data on the rates and predictors of AF across different rhythm monitoring strategies at precise time points in patients with CS or ESUS. We found 1) a steady increase of AF rates with duration of monitoring using ICM; 2) higher rates of AF when the cutoff of 120 s was used to define an episode of AF compared to 30 s; 3) higher rates of AF in patients with ESUS compared to those with CS; 4) association of older age, CHA2DS2-VASc score, PA-TDI Interval, left atrial enlargement, P wave maximal duration, prolonged PR interval and atrial runs with higher rates of AF detection.
Although there is still no specific recommendation on the optimal duration of prolonged cardiac monitoring [6], this study confirms that the longer the monitoring, the higher the AF detection rates in patients with CS. Therefore, it might be judicious to continue monitoring as long as possible until AF is detected. Most studies reported data on AF detection up to a maximum duration of ~36 months, probably because the current ICMs have about 3-year battery longevity. Although, as expected, the AF detection rate increases with the duration of monitoring, most cases of AF (about half) are detected in the first six months (Fig. 2). Moreover, it is uncertain whether AF detected much later, after two or three years of monitoring for instance, has any causative role in the index stroke. It is possible that AF detected long after the index stroke is of new onset, as a result of all the risk factors for AF that patients with stroke usually have, including older age and cardio-metabolic co-morbidities [4]. Furthermore, few studies have shown similar yield of new AF on ICM in patients with no stroke history and those with CS [4]. Altogether, these findings highlight the need to rethink the implications of prolonged continuous ECG monitoring after a stroke. Moreover, studies are needed to determine the adequate cutoff for AF burden that is significant and requires anticoagulation prophylaxis, as the current expert consensus to treat as significant any episode of AF ≥ 30 s detected by continuous monitoring after a stroke is not evidence-based [4].
The duration of cardiac monitoring to detect AF after an ischemic stroke might be individualized, based on a risk stratification score. The HAVOC score (abbreviation for Hypertension, Age, Valvular heart disease, peripheral Vascular disease, Obesity, Congestive heart failure, and Coronary artery disease) was developed based on seven clinical factors to stratify the risk of AF occurrence in patients with CS or TIA. The score showed good discrimination (c-statistic 0.77) of patients into three risk categories (low, medium, and high), with the potential of being used to select patients for prolonged monitoring [13]. Imaging parameters such as LA dimension and PA-TDI Interval, or electrocardiographic markers including PR interval and maximum P wave duration, could be combined with clinical risk factors to design an efficient risk score to predict the development of AF in patients with CS. Blood biomarkers such as cardiac natriuretic peptides may also be useful to refine AF risk stratification [14], [15].
We found that female sex was a correlate of AF detection in patients with CS. This finding contrasts with evidence of lower AF incidence rates in women in the general population [16]. In fact, in a large multi-country patient-level meta-analysis including 141,220 individuals, the screened-detected AF rates were consistently lower in women across all age strata [17]. The finding of higher AF detection rates in female patients with CS emerged from univariable analysis and is, therefore, not free from potential confounders. Furthermore, the sensitivity analysis revealed that this association between female sex and AF detection was driven by one study [18]. No association between female sex and AF detection was observed after excluding that study (Supplementary Fig. 27).
Atrial fibrillation detection rates using ICM were somewhat higher in patients with ESUS compared to those with CS across all timepoints, with a marked difference at 6 months. An ischemic stroke is considered cryptogenic when no definite cause is identified during the baseline etiological workup, whereas an ESUS is a clinical construct that refers to non-lacunar non-atherosclerotic ischemic strokes of presumable cardioembolic origin. The relative lower rate of AF in patients with CS highlights the greater heterogeneity of stroke etiologies in this subgroup while ESUS are pre-selected based on their likelihood of being embolic and, therefore, related to covert AF. Moreover, it is known that patients with ESUS who have an ipsilateral mild carotid stenosis are 2 times less likely to develop AF during follow-up [19], and there is evidence to suggest that the mild carotid stenosis instead of AF might be the actual cause of the stroke [20]. This further emphasizes the fact that a more comprehensive work-up and classification of stroke cases at baseline is important to increase the yield of ICM and may help optimize the cost-benefit ratio. Because advanced carotid and intracranial vascular imaging was not required for the definition of ESUS in the NAVIGATE-ESUS trial [21], it is possible that the residual heterogeneity of stroke etiologies contributed to the neutral results of the trial.
This review shows that higher rates of AF were reported by studies using a 2-minute cutoff to define an episode of AF on ICM than in those using a 30-second cutoff. This observation should not be considered as indicative that AF detection rates are higher with a cut-off of 2 min compared to 30-seconds. This is actually counterintuitive, as the lower the cut-off, the higher the yield. In fact, the two cut-offs were not compared in the same patients using the same devices. This unexpected finding can be partly explained by different device capabilities to detect AF. While the 30-seconds cutoff was established by consensus, the 2-minute cutoff is related to technology improvement in AF detection. Indeed, the most recent AF detection algorithms have a better episode detection accuracy than the older ones. For instance, the P-wave enhanced AF detection algorithm of Reveal LINQ is based on both R-R interval and a P-wave evidence score. The P-wave evidence score limits inappropriate AF detections in the original R-R interval pattern–based algorithm and leverages the evidence of a single P wave between two R waves using morphologic processing of the ECG signal [21]. The algorithm requires a 2-minute detection window. As a result, Reveal LINQ has better AF detection capabilities compared to the previous generation device Reveal XT [22]. Indeed, the detection rates in our study were higher with Reveal LINQ compared to the Reveal XT, although the difference was significant only at 6 months.
There was a relatively high detection rate with 1-month MCOT, highlighting the importance of prolonged non-invasive cardiac monitoring before ICM. This is in keeping with AHA/ASA guidelines for secondary prevention of stroke which considers that cardiac rhythm monitoring for about a month is reasonable in the first six months following CS [6]. Although it is striking to see that the detection rate with 1-month MCOT (14.4%) was higher than the detection rate with ICM at the same timepoint (4.1%), these rates are not comparable due to difference in populations’ characteristics. In fact, patients who had an ICM were likely a more selected population who had extensive non-invasive monitoring before implantation and therefore, with a lower likelihood of AF detection compared to patients undergoing MCOT.
This study has some limitations. There were differences across studies, in terms of participants’ clinical characteristics and extent of etiologic investigations done before reaching the diagnosis of cryptogenic stroke. Even within the same study, the etiologic work-up was not always standardized. For instance, not all stroke units have inpatient telemetry and when available, only a small proportion of patients receives it [23]. The time between the index stroke and the start of monitoring, ICM for instance, was not always reported or when reported, it was done very differently across studies (Supplementary Table 3), making its contribution in interpreting the AF detection rates very limited. Moreover, many studies did not provide the criteria used to define ESUS. Therefore, it was not possible for us to make sure that appropriate criteria were applied. Furthermore, we could not appropriately investigate the predictors of AF detection. A multivariable meta-analysis was not possible due to insufficient data and inconsistent reporting across studies. Nevertheless, our review has important strengths. It has added value compared to previous systematic reviews on AF detection in the broader population of patients with ischemic stroke [24], [25]. Our study provides the most up-to-date estimates of AF detection rates and predictors from various rhythm monitoring strategies, specifically in patients with CS. We included only fully published peer-reviewed articles, not conference abstracts, to ensure that we have data with the highest possible quality and avoid including duplicates. All analyses were performed at precise timepoints (e.g. 3 months, 6 months, 12 months, 24 months), providing a better appreciation of the trends of AF rates over time.
5. Conclusion
This study shows that more than one quarter of patients with CS or ESUS will be diagnosed with AF during follow-up and that the yield of ICM increases with the duration of monitoring. About one in seven patients had AF detected within a month of MCOT, suggesting that a non-invasive monitoring strategy should be considered before invasive monitoring. Predictors of AF detection during monitoring include older age, CHA2DS2-VASc score, PA-TDI Interval, left atrial enlargement, P wave maximal duration, prolonged PR interval and atrial runs. Such factors combined in a score might help in risk stratification and selection of patients for extended ICM monitoring.
Declaration of Competing Interest
Dr. Sanders reports having served on the advisory board of Medtronic, Abbott Medical, Boston Scientific, CathRx and PaceMate. Dr. Sanders reports that the University of Adelaide has received on his behalf research funding, lecture and/or consulting fees from Medtronic, Abbott Medical, Boston Scientific and Microport. All other authors report no disclosures.
Acknowledgments
Acknowledgements
Availability of data and material.
All data generated or analyzed in this study are included in the published article and the supplementary files.
Funding.
This study received no funding.
Financial disclosures.
Dr. Noubiap and Fitzgerald are supported by a Postgraduate Scholarship from the University of Adelaide. Dr. Agbaedeng is supported by a Postdoctoral Fellowship from the University of Adelaide. Dr. Kamtchum-Tatuene is supported by a Faculty of Medicine and Dentistry Motyl Graduate Studentship in Cardiac Sciences and an Alberta Innovates Graduate Scholarship. Dr. Sanders is supported by a Practitioner Fellowships from the National Health and Medical Research Council of Australia and by the National Heart Foundation of Australia.
Authors’ contributions.
Conception and Design: JJN, JKT. Search strategy: JJN. Studies selection: JJN, JKT. Data extraction: JJN, JKT. Data analysis and synthesis: JJN, TAA. Data interpretation: JJN, TAA, JKT. Manuscript drafting: JJN, TAA. Manuscript revision: JJN, TAA, JKT, TK, JLF, MEM, PS. Approval of the final manuscript: JJN, TAA, JKT, TK, JLF, MEM, PS. Guarantor of review: JJN.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100780.
Contributor Information
Jean Jacques Noubiap, Email: jeanjacques.noubiapnzeale@adelaide.edu.au.
Thomas A. Agbaedeng, Email: thomas.agbaedeng@adelaide.edu.au.
Joseph Kamtchum-Tatuene, Email: kamtchum@ualberta.ca.
John L. Fitzgerald, Email: john.fitzgerald@adelaide.edu.au.
Melissa E. Middeldorp, Email: melissa.middeldorp@adelaide.edu.au.
Timothy Kleinig, Email: Timothy.Kleinig@sa.gov.au.
Prashanthan Sanders, Email: prash.sanders@adelaide.edu.au.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 390(10100) (2017) 1260–344. [DOI] [PMC free article] [PubMed]
- 2.Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 390(10100) (2017) 1151–210. [DOI] [PMC free article] [PubMed]
- 3.Li L., Yiin G.S., Geraghty O.C., Schulz U.G., Kuker W., Mehta Z., Rothwell P.M. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol. 2015;14(9):903–913. doi: 10.1016/S1474-4422(15)00132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schnabel R.B., Haeusler K.G., Healey J.S., Freedman B., Boriani G., Brachmann J. Searching for Atrial Fibrillation Poststroke A White Paper of the AF-SCREEN International Collaboration. Circulation. 2019;140(22):1834–1850. doi: 10.1161/CIRCULATIONAHA.119.040267. [DOI] [PubMed] [Google Scholar]
- 5.Kaarisalo M.M., Immonen-Räihä P., Marttila R.J., Salomaa V., Kaarsalo E., Salmi K. Atrial fibrillation and stroke. Mortality and causes of death after the first acute ischemic stroke. Stroke. 1997;28(2):311–315. doi: 10.1161/01.str.28.2.311. [DOI] [PubMed] [Google Scholar]
- 6.Powers W.J., Rabinstein A.A., Ackerson T., Adeoye O.M., Bambakidis N.C., Becker K., Biller J., Brown M., Demaerschalk B.M., Hoh B., Jauch E.C., Kidwell C.S., Leslie-Mazwi T.M., Ovbiagele B., Scott P.A., Sheth K.N., Southerland A.M., Summers D.V., Tirschwell D.L. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12) doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 7.Sanna T., Diener H.-C., Passman R.S., Di Lazzaro V., Bernstein R.A., Morillo C.A., Rymer M.M., Thijs V., Rogers T., Beckers F., Lindborg K., Brachmann J. Cryptogenic stroke and underlying atrial fibrillation. N. Engl. J. Med. 2014;370(26):2478–2486. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 8.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 9.Hoy D., Brooks P., Woolf A., Blyth F., March L., Bain C., Baker P., Smith E., Buchbinder R. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012;65(9):934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Cochran W.G. The Combination of Estimates from Different Experiments. Biometrics. 1954;10(1):101–129. [Google Scholar]
- 11.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 12.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat. Med. 2000;19(22):3127–3131. doi: 10.1002/1097-0258(20001130)19:22<3127::aid-sim784>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 13.Kwong C., Ling A.Y., Crawford M.H., Zhao S.X., Shah N.H. A Clinical Score for Predicting Atrial Fibrillation in Patients with Cryptogenic Stroke or Transient Ischemic Attack. Cardiology. 2017;138(3):133–140. doi: 10.1159/000476030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamtchum-Tatuene J., Jickling G.C. Blood Biomarkers for Stroke Diagnosis and Management. NeuroMol. Med. 2019;21(4):344–368. doi: 10.1007/s12017-019-08530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noubiap J.J., Sanders P., Nattel S., Lau D.H. Biomarkers in Atrial Fibrillation: Pathogenesis and Clinical Implications. Card Electrophysiol. Clin. 2021;13(1):221–233. doi: 10.1016/j.ccep.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Magnussen C., Niiranen T.J., Ojeda F.M., Gianfagna F., Blankenberg S., Njølstad I. Sex Differences and Similarities in Atrial Fibrillation Epidemiology, Risk Factors, and Mortality in Community Cohorts: Results From the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe) Circulation. 2017;136(17):1588–1597. doi: 10.1161/CIRCULATIONAHA.117.028981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowres N., Olivier J., Chao T.F., Chen S.A., Chen Y., Diederichsen A. Estimated stroke risk, yield, and number needed to screen for atrial fibrillation detected through single time screening: a multicountry patient-level meta-analysis of 141,220 screened individuals. PLoS Med. 2019;16(9) doi: 10.1371/journal.pmed.1002903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asaithambi G., Monita J.E., Annamalai M.R., Ho B.M., Marino E.H., Hanson S.K. Prevalence of atrial fibrillation with insertable cardiac monitors in cryptogenic stroke: A single-center experience. J. Electrocardiol. 2018;51(6):973–976. doi: 10.1016/j.jelectrocard.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 19.Ntaios G., Perlepe K., Sirimarco G., Strambo D., Eskandari A., Karagkiozi E., Vemmou A., Koroboki E., Manios E., Makaritsis K., Michel P., Vemmos K. Carotid plaques and detection of atrial fibrillation in embolic stroke of undetermined source. Neurology. 2019;92(23):e2644–e2652. doi: 10.1212/WNL.0000000000007611. [DOI] [PubMed] [Google Scholar]
- 20.Kamtchum-Tatuene J., Wilman A., Saqqur M., Shuaib A., Jickling G.C. Carotid Plaque With High-Risk Features in Embolic Stroke of Undetermined Source: Systematic Review and Meta-Analysis. Stroke. 2020;51(1):311–314. doi: 10.1161/STROKEAHA.119.027272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart R.G., Sharma M., Mundl H., Kasner S.E., Bangdiwala S.I., Berkowitz S.D. Rivaroxaban for Stroke Prevention after Embolic Stroke of Undetermined Source. New Engl. J. Med. 2018;378(23):2191–2201. doi: 10.1056/NEJMoa1802686. [DOI] [PubMed] [Google Scholar]
- 22.Sanders P., Pürerfellner H., Pokushalov E., Sarkar S., Di Bacco M., Maus B., Dekker L.R.C. Performance of a new atrial fibrillation detection algorithm in a miniaturized insertable cardiac monitor: Results from the Reveal LINQ Usability Study. Heart Rhythm. 2016;13(7):1425–1430. doi: 10.1016/j.hrthm.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Edwards J.D., Kapral M.K., Fang J., Saposnik G., Gladstone D.J. Underutilization of Ambulatory ECG Monitoring After Stroke and Transient Ischemic Attack: Missed Opportunities for Atrial Fibrillation Detection. Stroke. 2016;47(8):1982–1989. doi: 10.1161/STROKEAHA.115.012195. [DOI] [PubMed] [Google Scholar]
- 24.Tsivgoulis G., Katsanos A.H., Köhrmann M., Caso V., Perren F., Palaiodimou L., Deftereos S., Giannopoulos S., Ellul J., Krogias C., Mavridis D., Triantafyllou S., Alexandrov A.W., Schellinger P.D., Alexandrov A.V. Duration of Implantable Cardiac Monitoring and Detection of Atrial Fibrillation in Ischemic Stroke Patients: A Systematic Review and Meta-Analysis. J. Stroke. 2019;21(3):302–311. doi: 10.5853/jos.2019.01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sposato L.A., Cipriano L.E., Saposnik G., Vargas E.R., Riccio P.M., Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2015;14(4):377–387. doi: 10.1016/S1474-4422(15)70027-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.