Abstract
Purpose
To describe findings of multimodal imaging in non-proliferative and proliferative stages of MacTel 2 in a pediatric patient, and results of aflibercept use for treating neovascularization secondary to MacTel 2.
Methods
Retrospective case report.
Results
An 11-year-old girl with no history of systemic disease. BCVA was 20/200 and 20/40 in the right and left eyes, respectively. FFA, SS-OCT and SS-OCTA revealed proliferative and non-proliferative stages of MacTel 2 in the right and left eyes, respectively. Intravitreal aflibercept (2mg/0.05mL) was started (PRN) in the right eye. The patient received 5 injections that led to involution of macular neovascularization and improvement of BCVA by 5 lines. BCVA in the left eye remained stable.
Conclusion
MacTel 2 can develop in an earlier age than previously reported. SS-OCTA is an effective alternative to conventional imaging in diagnosis and follow-up especially in pediatric patients. Intravitreal aflibercept is effective in treating proliferative MacTel 2.
Keywords: MacTel 2 in a child, OCTA in MacTel 2, Aflibercept in proliferative MacTel 2, Multimodal imaging in MacTel 2
1. Introduction
Macular telangiectasia type 2 (MacTel 2) is a rare neurodegenerative disorder of the macula whose hallmark is microvascular alteration involving the superficial (SCP) and deep (DCP) retinal plexuses. MacTel 2 is notorious of its propensity to bilateral involvement and progressive course that eventually causes significant visual morbidity.1 Typically, MacTel 2 develops during the fifth to sixth decades of life though the literature reports MacTel 2 manifesting as early as the second decade of life.1,2 The pathogenetic cascade leading to MacTel 2 is triggered by primary degenerative changes involving Müller cells. Continuing depletion of Müller cells results in an array of retinal structural remodeling including glial, neuronal, and retinal pigment epithelium (RPE) elements, and secondary retinal vascular remodeling.3,4 Clinically, nascent MacTel 2 manifests as a non-proliferative disorder that shows grayish opacification of the temporal retina and progresses later on to telangiectasia and eventually foveal atrophy and scarring. Some patients progress to the proliferative stage of the disease that is marked by development of macular neovascularization and finally disciform scarring.5, 6, 7, 8, 9 Macular neovascularization develops secondary to boosted abundancy of free vascular endothelial growth factor (VEGF) within the retinal tissue as a sequel of Müller cells dysfunction and photoreceptors damage.4 To date there is no proven effective treatment to the non-proliferative stage of MacTel 2. Several studies reported the use of Anti-VEGF agents to treat macular neovascularization secondary to MacTel 2.10, 11, 12 Herein we report a case of MacTel 2 in an 11-year old girl, in which we used swept-source optical coherence tomography (SS-OCT) and optical coherence tomography angiography (SS-OCTA) to document the entire pathological spectrum of the disease from early non-proliferative stage with exudative telangiectasia through late proliferative stage with macular neovascularization and scarring. In addition, we present our experience in using aflibercept for macular neovascularization secondary to MacTel 2.
2. Case report
An 11-year-old girl with no prior history of systemic disease, presented to our clinic with complaints of diminution of vision that was more pronounced in the right eye. Best-corrected visual acuity (BCVA) was 20/200 and 20/40 in the right eye and left eye, respectively. Slit-lamp examination of the anterior segment was unremarkable bilaterally. Fundus examination in the right eye revealed irregular greenish-gray lesion occupying the macular area. Fundus fluorescein angiography (FFA); Topcon TRC 50DX fundus camera (Topcon Corporation, Tokyo, Japan) confirmed the diagnosis of subretinal neovascularization (SRN). SS-OCT; DRI OCT Triton machine version 10.11 (Topcon Corporation, Tokyo, Japan) demonstrated features suggestive of type 2 neovascular membrane. SS-OCTA [SS-OCTangio; OCTARA (Optical Coherence Tomography Angiography Ratio Analysis); Topcon Corporation, Tokyo, Japan] revealed vascular dragging of the temporal juxta-foveal SCP with abrupt ending and posterior dipping of larger vessels that could be traced to the DCP and further deep to the choriocapillaris. SS-OCTA of the outer retina slab showed hyperintense signal of active neovascular network. The fundus of the left eye revealed perifoveal grayish discoloration along with intra-retinal cyst-like cavities formation. FFA revealed dilated ectatic perifoveal capillaries that leaked in later phases of the angiogram. SS-OCT revealed intra-retinal cavitation and inner retinal schisis. SS-OCTA revealed telangiectasia in the form of budlike capillary dilatation involving the entire circumference of the perifoveal capillary network mainly located in the DCP. Fig. 1, Fig. 2, Fig. 3. The patient had no siblings. Her parents were asymptomatic. FFA, fundus autofluorescence (FAF); Spectralis laser tomography (Heidelberg engineering, Heidelberg, Germany), SS-OCT and SS-OCTA examination of the parents revealed normal findings. We decided to observe the left eye and to start intravitreal aflibercept (2mg/0.05mL) regimen in the right eye for treating the macular neovascularization. The patient received 2 injections 6 weeks apart that resulted in significant regression of the neovascular network. BCVA improved to 20/50 and we opted for follow-up on pro re nata (PRN) regimen. Three months later she presented to our office with complaints of recent diminution of vision in the right eye. Her BCVA was 20/100. SS-OCTA revealed reactivation of the previously regressed macular neovascularization. She received three more injections of intravitreal aflibercept 4 weeks apart that resulted in regression of the neovascular network and improvement of BCVA to 20/60. Fig. 4. Her BCVA in the right eye remained stable over the following year. BCVA of the left eye remained 20/40 despite progressive thinning of the fovea. FAF of the left eye acquired during follow-up visits revealed loss of the normal hypoautofluorescent center seen on blue-light FAF and patchy redistribution of macular pigment Fig. 5, Fig. 6.
Fig. 1.
A) Color photo and FFA of an 11-year-old girl with proliferative MacTel 2 in the right eye. Macular area showed a greenish-gray sub-retinal lesion approximately ½ DD in size. Two right-angled venules with tapered tips seemed emerging abruptly from the center of the lesion (black arrows). The lesion was surrounded by grayish opacified retina. On FFA, the lesion seen in the color photo revealed early hyperfluorescence due to filling of the anastomotic vessels within the neovascular complex. Note that the right-angled venule seen in color photo did not fill up with dye in the arterio-venous phase, which denoted its origin from the retinal circulation (white arrow). Late frames demonstrated profuse leakage of the dye obscuring the lesion boundaries. B) SS-OCT of the same eye in a 9-mm radial scan mode demonstrated sub-foveal hyperreflective dome-shaped lesion above the RPE with discrete hyporeflective foci. EZ could not be identified, and continuity of ELM and IS/OS layers were lost. Central macular thickness (CMT) was 387μ. Note the typical features of sub-retinal neovascularization as PED, CME, or NSD were lacking. C) Color photo and FFA of the same patient showed non-proliferative MacTel 2 in the left eye. Macular area showed grayish opacification in the peri-foveal area with intra-retinal multiple cyst-like spaces. On FFA, early frames showed budlike dilatation of the perifoveal capillaries (inset). The foveal avascular zone (FAZ) was irregular. Late frames demonstrated increasing hyperfluorescence in the parafoveal area due to minimal leakage from dilated perifoveal capillaries (inset). D) SS-OCT in a 9-mm radial scan mode demonstrated subfoveal hyporeflective cavities in the inner retina that were traversed by hyperreflective streaks. The internal limiting membrane (ILM) remained intact. The CMT was 208μ, which indicated that these hyporeflective cavities were in fact schitic cavities that developed due to degenerative erosion of the inner retina rather than inner retinal edema. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
A) Right-angled venules (black arrows) are shown in color photos (A) and (B) and compared to SS-OCTA depiction of different vascular layers of the right macula in a 3 × 3 mm area (C–G). The nasal vessels in the SCP and DCP layers showed characteristic vascular dragging and agglomeration in a V-shaped bend pointing centripetally and encroaching upon the FAZ. Note the course of the two larger venules seen in color photo. The venules lie adjacent to the area of vascular dragging and take a sharp posterior dip into deeper layers. These venules could be traced in successive slabs as deep as the choriocapillaris (white asterisks), which suggested RCA. Additional features in the SCP and DCP layers included vascular rarefaction with subsequent increase in the intercapillary spaces, and telangiectasia. The outer retina slab demonstrated hyperintense signal of neovascular network. The network showed dense arborization, looping and intervascular anastomosis denoting its activity. H) Volume-rendered image of the SCP seen from the inner retina side shows the abnormal course of the fore-mentioned 2 right-angled venules (white arrows). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
SS-OCTA depiction of different vascular layers of the left macula in a 3 × 3 mm area (A–E). In contrast to the SCP layer that appeared rather normal, the DCP layer showed extensive vascular rarefaction with widened intercapillary spaces. The perifoveal capillary arcade showed budlike capillary dilatation instead of the normal closed-knit configuration. These lesions appeared as hyperintense club-shaped clusters that were more revealed in the volume-rendered image of the DCP seen from the outer retina side (white arrows). The en face projection of the DCP layer clearly depicted inner retina cavitation.
Fig. 4.
Serial SS-OCTA images in a 3 × 3 mm area of the outer retina and the corresponding color-coded flow density maps and SS-OCT 9-mm radial scans (A-C, D-F, G-I, J-L, M − O) of the right macula showing neovascular network response to serial intravitreal injections of aflibercept over approximately 8 months period. Note the dramatic regression of neovascularization following the first 2 injections compared to baseline. Note that after successive injections the dense arborizing neovascular network seen initially was replaced with larger more mature type of vessels denoting decreased activity. Note that SS-OCT images showed a rather stationary neovascular network from inception through last follow-up, despite SS-OCTA revealing dynamic changes in the neovascular network through phases of involution and relapse. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
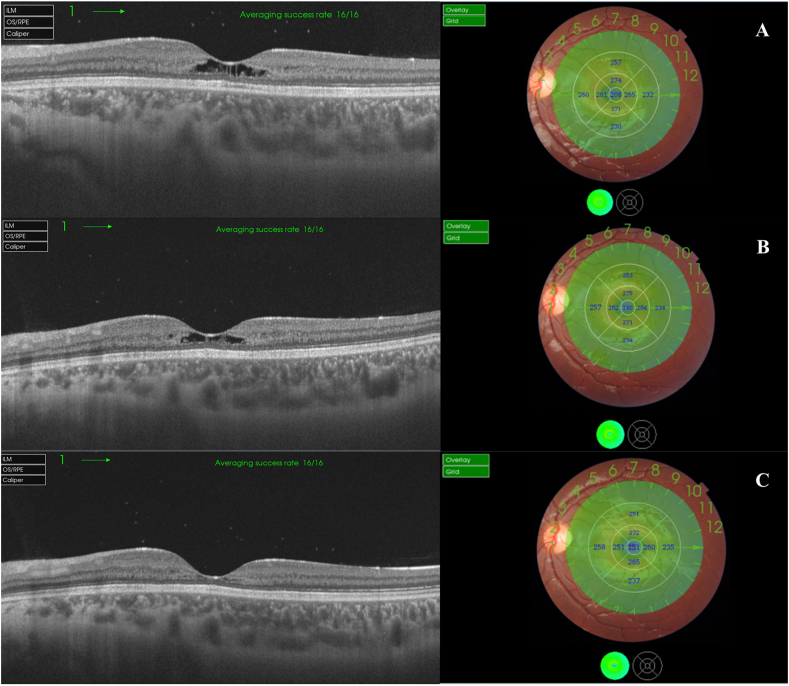
Fig. 5.
Serial SS-OCT scans in a 9-mm radial scan mode of the left eye and the corresponding retinal thickness maps and color-coded maps. Note progressive thinning of the fovea and the para-foveal area over time. CMT thickness measured 208μ, 190μ, and 151μ in visits A, B, & C respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 6.
FAF of the left macula shows loss of the normal central hypoautofluorescence due to loss of central macular pigment. The redistribution of macular pigment in the para-foveal area resulted in patchy autofluorescence in the form of hypoautofluorescent dot (empty white arrow), differential autofluorescence between the nasal and temporal areas of the para-fovea (full white arrows). Note the irregular hypoautofluorescence present in the temporal juxta-foveal area caused by RPE atrophy (white arrow-head). Numerous refractile dots are seen at a more superficial level relative to the previously-described lesions, and possibly represent crystal deposition in the retinal nerve fiber layer. A couple of these dots are highlighted (white circle). Note that these 2 dots lies superficial to the blood vessel underneath them.
The parents of the patient received thorough explanation about her disease condition, the nature of diagnostic tests performed and the outcome of intravitreal aflibercept injection, and provided their consent including procession of her data for research purposes in an anonymous manner that could not reveal her identity. The report acted in accordance of the tenets of the Declaration of Helsinki (2013 revision). All procedures described herein were undertaken by an experienced retina specialist (MM).
3. Discussion
In the present report we demonstrated the pathological spectrum of MacTel type 2 in both eyes of an 11-year-old patient. To our knowledge this is the first report to document development of the disease in pediatric age group. It is worthy of note that by the time the patient presented to our clinic, she already had macular neovascularization in one eye, which suggested that the disease process started even at an earlier age. In our experience, conventional FFA was not optimum in diagnosis and follow-up of MacTel 2. The reason was that the pathological features of MacTel 2 were mainly seated in the DCP, moreover, they included features as macular neovascularization and retino-choroidal anastomosis (RCA) that were located at different axial levels. The inherent drawbacks of FFA as profuse leakage of fluorescein dye from the choriocapillaris and scattering of light from retinal layers13 preclude detailed visualization of deeper layers and significantly impede detecting and monitoring the progression of those features. Moreover, the age group of our patient posed an additional challenge for repetitive dye injection. Similarly, SS-OCT was inconclusive in proliferative MacTel 2 detected in this report. The reason is that the proliferating neovascular network secondary to MacTel 2 starts in the retinal vascular plexuses and is comparably smaller and slowly-growing than type 2 neovascular membranes secondary to age-related macular degeneration, retinal angiomatous proliferation (RAP) or myopia. Moreover, it might not exhibit the clear-cut features of neovascular membranes as retinal pigment epithelial detachment (RPED), cystoid macular edema (CME) or neuro-sensory detachment (NSD),5 which renders it unamenable to accurate diagnosis, distinguishing from other non-proliferative lesions or monitoring response of neovascularization to therapy using SS-OCT.14 In our experience, SS-OCTA with integrated swept-source technology features as ultra-high-speed image acquisition, long wavelength laser scanning beam and reduced sensitivity roll-off, offered clear advantage over conventional FFA due to its non-invasive nature and ability for layer-to-layer segmentation of retinal vascular plexuses with unprecedented resolution.13 Moreover, it superseded SS-OCT due to its ability for detection of blood flow within the neovascular network and accurate depiction of macular neovascularization through different stages of evolution. It is worthy of note that loss of macular pigments occurs early in MacTel2 and is detectable by fundus autofluorescence (FAF) as increased transmitted blue light signal due to unmasking of the underlying RPE autofluorescence. Though, FAF is non-invasive and reproducible modality, it detects disease progression through topographic alteration of blue light signal while lacking ability to provide crucial information on the vascular profile of the disease.15,16 In our patient, intravitreal aflibercept resulted in involution of the macular neovascularization and improvement of BCVA by 5 lines of vision. Further visual recovery was hampered by failure of restoration of the ellipsoid zone (EZ), external limiting membrane (ELM), and inner segment/outer segment (IS/OS) layers. In the present report, we were able to reproduce the favorable outcome of intravitreal aflibercept reported previously,12 hence we advocate its use in proliferative MacTel 2 particularly in pediatric population due to its longer intravitreal half-life, thus fewer injections needed.
4. Conclusion
MacTel 2 can develop in an earlier age than previously reported. SS-OCTA is an effective alternative to conventional imaging in diagnosis and follow-up especially in pediatric patients. Intravitreal aflibercept is effective in treating proliferative MacTel 2.
Patient consent
Written consent to publish this case has been obtained from the parents of the patient. This report does not contain any personal identifying information.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
CRediT author statement
Magdy Moussa:conceptualization, supervision, methodology, visualization, investigation, validation, writing-reviewing and editing. Mahmoud Leila: conceptualization, methodology, visualization, investigation, validation, data curation, writing-original draft preparation. Omar Moussa: conceptualization, methodology, visualization, investigation, validation, data curation.
Declaration of competing interest
The following authors have no financial disclosures:
MM, ML, OM.
Acknowledgements
None.
Footnotes
Institution: The study was conducted at MEDIC Eye Center, Tanta, Egypt.
Contributor Information
Magdy Moussa, Email: magdymoussa@med.tanta.edu.eg.
Mahmoud Leila, Email: mahmoudleila@yahoo.com.
Omar Moussa, Email: omarmoussa_92@hotmail.com.
References
- 1.Yannuzzi L.A., Chew E.Y. Macular telangiectasia. In: Schachat A.P., editor. Ryan's Retina. sixth ed. Elsevier; Netherlands: 2018. pp. 1180–1187. [Google Scholar]
- 2.Ronquillo C.C., Wegner K., Calvo C.M., Bernstein P.S. Genetic penetrance of macular telangiectasia type 2. JAMA Ophthalmol. 2018;136(10):1158–1163. doi: 10.1001/jamaophthalmol.2018.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bringmann A., Pannicke T., Grosche J. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Spaide R.F., Marco R.D., Yannuzzi L.A. Vascular distortion and dragging related to apparent tissue contraction in macular telangiectasis type 2. Retina. 2018;38:S51–S60. doi: 10.1097/IAE.0000000000001694. [DOI] [PubMed] [Google Scholar]
- 5.Spaide R.F., Yannuzzi L.A., Maloca P.M. Retinal-choroidal anastomosis in macular telangiectasia type 2. Retina. 2018;38:1920–1929. doi: 10.1097/IAE.0000000000002289. [DOI] [PubMed] [Google Scholar]
- 6.Yannuzzi L.A., Bardal A.M., Freund K.B., Chen K.J., Eandi C.M., Blodi B. Idiopathic macular telangiectasia. Arch Ophthalmol. 2006;124:450–460. doi: 10.1001/archopht.124.4.450. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q., Wang R.K., Chen C.L. Swept source optical coherence tomography angiography of neovascular macular telangiectasia type 2. Retina. 2015;35:2285–2299. doi: 10.1097/IAE.0000000000000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaudric A., Krivosic V., Tadayoni R. Outer retina capillary invasion and ellipsoid zone loss in macular telangiectasia type 2 imaged by optical coherence tomography angiography. Retina. 2015;35:2300–2306. doi: 10.1097/IAE.0000000000000799. [DOI] [PubMed] [Google Scholar]
- 9.Balaratnasingam C., Yannuzzi L.A., Spaide R.F. Possible choroidal neovascularization in macular telangiectasia type 2. Retina. 2015;35:2317–2322. doi: 10.1097/IAE.0000000000000887. [DOI] [PubMed] [Google Scholar]
- 10.Matt G., Sacu S., Ahlers C. Thirty-month follow-up after intravitreal bevacizumab in progressive idiopathic macular telangiectasia type 2. Eye. 2010;24:1535–1542. doi: 10.1038/eye.2010.113. [DOI] [PubMed] [Google Scholar]
- 11.Narayanan R., Chhablani J., Sinha M. Efficacy of anti-vascular endothelial growth factor therapy in subretinal neovascularization secondary to macular telangiectasia type 2. Retina. 2012;32:2001–2005. doi: 10.1097/IAE.0b013e3182625c1d. [DOI] [PubMed] [Google Scholar]
- 12.Karasu B., Gunay B.O. Comparison of anatomical and visual outcomes following different anti-vascular endothelial growth factor treatments in subretinal neovascular membrane secondary to type 2 proliferative macular telangiectasia. Graefes Arch Clin Exp Ophthalmol. 2019 doi: 10.1007/s00417-019-04520-x. ([Epub ahead of print]) [DOI] [PubMed] [Google Scholar]
- 13.Bonnin S., Mané V., Couturier A. New insight into the macular deep vascular plexus imaged by optical coherence tomography angiography. Retina. 2015;35:2347–2352. doi: 10.1097/IAE.0000000000000839. [DOI] [PubMed] [Google Scholar]
- 14.Moussa M., Leila M., Khalid H., Lolah M. Detection of silent type I choroidal neovascular membrane in chronic central serous chorioretinopathy using en face swept-source optical coherence tomography angiography. J Ophthalmol. 2017 doi: 10.1155/2017/6913980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmitz-Valckenberg S., Holz F.G., Bird A.C., Spaide R.F. Fundus autofluorescence imaging: review and perspectives. Retina. 2008;28(3):385–409. doi: 10.1097/IAE.0b013e318164a907. [DOI] [PubMed] [Google Scholar]
- 16.Wong W.T., Forooghian F., Majumdar Z., Bonner R.F., Cunningham D., Chew E.Y. Fundus autofluorescence in type 2 idiopathic macular telangiectasia: correlation with optical coherence tomography and microperimetry. Am J Ophthalmol. 2009;148(4):573–583. doi: 10.1016/j.ajo.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]