Abstract
Objective
To investigate the dynamic characteristics of serological antibodies against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is of much current significance.
Methods
The dynamic changes and prevalence of immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies against SARS-CoV-2 were assessed from the time of symptom onset up to 210 days. Antibodies were detected using a chemiluminescence immunoassay.
Results
The average titers and IgG/IgM positivity rates reached a peak within 30 days of symptom onset and then began to decline continuously. Between 180 and 210 days following symptom onset, the titers of IgG and IgM were 43.1 ± 27.0 AU/mL and 4.4 ± 5.2 AU/mL, respectively, while the respective positivity rates were 84.3% and 12.0%. Further statistical analyses revealed that the dynamic changes and prevalence of the SARS-CoV-2 IgG/IgM antibodies were related to age and disease severity, but not to sex. The dynamic changes and the prevalence were similar for both the IgM and the IgG antibodies. Even so, there was a more rapid rate of decline for the IgM antibodies. It was found that an IgG level of 16.33 ± 3.15 AU/mL may represent a threshold value that should act as an alert, as it may indicate that the IgG level will become undetectable within the next 30–60 days.
Conclusion
The results provide important information concerning COVID-19 and may be of relevance for diagnosis, treatment, and vaccine development.
Keywords: COVID-19, IgG/IgM antibodies, Dynamic characteristics, Positivity rate, IgG antibody alert level
Introduction
The pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to significant morbidity and mortality worldwide. Although nucleic acid detection has been regarded as the gold standard of laboratory diagnosis, serological testing is valuable for rapid clinical diagnosis and for evaluating the efficacy of vaccines and coronavirus disease 2019 (COVID-19) treatments (Shen et al., 2020).
Neutralizing antibodies (NAbs) are produced during an immune response, and these play a vital role against SARS-CoV-2. Immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies are also produced that are specific to SARS‐CoV‐2, and they have been detected within as little as 2 weeks from symptom onset (Pérez-García et al., 2020). The IgM antibody is the key indicator of an ongoing or current infection, as it is the earliest to develop following exposure to the pathogen. The IgG antibody is a protective antibody and is a sign of recovery from the disease or of a past infection. It is the most abundant antibody in an immunological response and can protect against an infection by blocking viral entry into host cells. A variety of different factors affect the development of IgG/IgM antibodies. Some studies suggest that virus-specific IgG antibodies decrease rapidly within around 3 months following an infection (Wang et al., 2020, Wang et al., 2021), whereas others indicate that IgG antibodies have stable titers over several weeks or several months (Dan et al., 2021). Many studies have reported that age, sex, and disease severity have an effect on the dynamic characteristics of serological antibodies (Kong et al., 2020, O’Driscoll et al., 2020, Zeng et al., 2020); however, these studies have been based on restricted study populations, or the study durations have been relatively short.
This paper presents a retrospective analysis of the dynamic changes and prevalence of SARS-CoV-2 IgG/IgM antibodies from symptom onset to 210 days, and how these relate to various factors, such as age and disease severity.
Materials and methods
Patients
This retrospective study was conducted at the Yichang Central People’s Hospital, China. All of the patients were clinically diagnosed with laboratory-confirmed COVID-19 and discharged according to the national recommendations for the diagnosis and treatment of pneumonia caused by SARS-CoV-2. The criteria for the initial diagnosis and discharge of patients have been described in detail in a previous paper (Liu et al., 2020). These include imaging features, laboratory examinations, clinical characteristics, and an epidemiological survey. A positive nucleic acid test result confirmed the infection. It is relevant to specify that in the case of an asymptomatic infection, there were no signs or symptoms of clinical manifestations such as fever, a cough, a sore throat, or other symptoms that could be perceived or recognized clinically; however, the nucleic acid test result was positive for respiratory tract or other specimens. These asymptomatic patients were identified by screening the close contacts of confirmed cases or through community screening programs. In the case of a mild infection, the clinical symptoms were mild, and there were no clinical manifestations of pneumonia. For all of the patients, the antibodies were tested periodically, in accordance with requirements from the municipal prevention and control headquarters. Medical records were also reviewed to obtain detailed demographic and clinical information. The study was approved by the Ethics Committees of Yichang Central People’s Hospital. The patients in the study signed informed consent forms or provided verbal consent over the telephone. The IgG/IgM antibodies were tested at regular intervals. The dates of these tests were calculated in relation to the day of symptom onset (day 0). In this way, serum samples were collected between (1) day 0 and day 30, (2) day 30 and day 60, (3) day 60 and day 90, and so on.
SARS‐CoV‐2 IgG/IgM antibody detection
Serum IgG/IgM antibodies were detected using a chemiluminescence immunoassay (Shenzhen Yafilong Biological Technology Co. Ltd., IgG/IgM kits, Shenzhen, China) in an iFlash 3000-A (Shenzhen Yafilong Biological Technology Co. Ltd., Shenzhen, China), as described in a previous paper (Zeng et al., 2020). The cutoff value of IgM and IgG recommended by the manufacturer is 10 AU/mL. The recombinant antigens contain the SARS-CoV-2 nucleoprotein and spike protein. All of the procedures were based on the manufacturer’s protocols.
Statistical analyses
The statistical analyses were performed using Prism 6.0 (GraphPad, San Diego, USA). The categorical variables were compared using a chi-squared test, and the SARS‐CoV‐2 IgG/IgM antibody titers were compared using a Mann–Whitney U test. A p-value less than 0.05 was considered to be statistically significant.
Results
The demographic characteristics of the patients in this study
As shown in Table 1 , of the 678 COVID‐19 patients, 39 cases were asymptomatic, 117 were mild, 401 were moderate, 95 were severe, and 26 were critical. The proportion of males was 48.38% (95% CI: 44.64–52.14) in the whole group, 43.59% (95% CI: 29.31–59.02) for the asymptomatic group, 52.99% (95% CI: 44–61.79) for the mild group, 47.63% (95% CI: 42.79–52.52) for the moderate group, 48.42% (95% CI: 38.63–58.33) for the severe group, and 46.15% (95% CI: 28.75–64.54) for the critical group. The average age was 54.53 ± 12.92 years (males: 53.01 ± 13.50 years; females: 56.05 ± 12.34 years) for the whole group, 43.15 ± 17.1 years (males: 42.12 ± 17.16 years; females: 44.18 ± 17.04 years) for the asymptomatic group, 51.49 ± 12.57 years (males: 52.55 ± 12.22 years; females: 50.43 ± 12.92 years) for the mild group, 50.13 ± 13.18 years (males: 49.16 ± 13.82 years; females: 51.02 ± 12.55 years) for the moderate group, 59.38 ± 12.24 years (males: 56.56 ± 14.37 years; females: 62.19 ± 10.11 years) for the severe group, and 68.54 ± 9.50 years (males: 64.64 ± 9.92 years; females: 72.43 ± 9.07 years) for the critical group. The average hospitalization time for the whole group of patients was 26.10 ± 6.30 days (see Table 1), and for the mild, moderate, severe, and critical groups, this was 20.55 ± 7.58 days, 22.36 ± 6.35 days, 29.00 ± 6.33 days, and 32.47 ± 4.65 days, respectively. It should be noted that for the asymptomatic patients, the hospitalization time was unavailable, because these patients were isolated in designated places instead of being hospitalized.
Table 1.
Baseline demographic characteristics of the patients in this study.
| Group | No. | Sex |
Age ± SD | Male | Hospitalization time ± SD | |
|---|---|---|---|---|---|---|
| Male | Proportion % (95% CI) | Female | ||||
| Total population | 678 | 328 | 48.38 (44.64–52.14) | 54.53 ± 12.92 | 53.01 ± 13.50 | 26.10 ± 6.30 |
| 56.05 ± 12.34 | ||||||
| Asymptomatic | 39 | 17 | 43.59 (29.31–59.02) | 43.15 ± 17.1 | 42.12 ± 17.16 | |
| 44.18 ± 17.04 | ||||||
| Mild | 117 | 62 | 52.99 (44–61.79) | 51.49 ± 12.57 | 52.55 ± 12.22 | 20.55 ± 7.58 |
| 50.43 ± 12.92 | ||||||
| Moderate | 401 | 191 | 47.63 (42.79 t0 52.52) | 50.13 ± 13.18 | 49.16 ± 13.82 | 22.36 ± 6.35 |
| 51.02 ± 12.55 | ||||||
| Severe | 95 | 46 | 48.42 (38.63–58.33) | 59.38 ± 12.24 | 56.56 ± 14.37 | 29.00 ± 6.63 |
| 62.19 ± 10.11 | ||||||
| Critical | 26 | 12 | 46.15 (28.75–64.54) | 68.54 ± 9.50 | 64.64 ± 9.92 | 32.47 ± 4.65 |
| 72.43 ± 9.07 | ||||||
The relationship between SARS-CoV-2 IgG/IgM antibody levels and sex
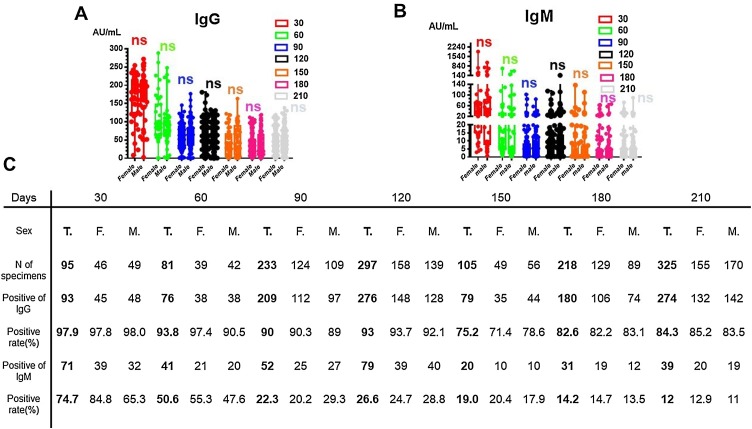
To examine whether dynamic changes in SARS‐CoV‐2 IgG/IgM levels and rates of positivity were related to sex, we determined the IgG/IgM titers and calculated the positivity rates for both male and female patients from the time of disease onset up to 210 days. As shown in Figure 1 A and B, the titers of IgG/IgM rose to a peak within 30 days following symptom onset, with average IgG antibody titers of 167.2 ± 49.3 AU/mL for female patients and 169.9 ± 47.8 AU/mL for male patients, and average IgM titers of 123.0 ± 145.8 AU/mL for female patients and 103.3 ± 118.9 AU/mL for male patients (see Table 2 ). The average IgG titers were then seen to decrease continuously. As shown in Table 2, the average IgG titers for both female and male patients declined down to 62.8% and 50.3%, respectively, of the peak value between day 30 and day 60, and was seen to remain relatively stable between day 150 and day 210, with levels at around 21.7%–29.7% of the peak. No significant differences were found between the male and female patients for the dynamic changes in SARS-CoV-2 IgG/IgM levels, as shown in Figure 1A and B. In Figure 1C, it can be seen that the IgG positivity rate reached a peak within 30 days, with 97.8% for females and 98.0% for males; this then declined slowly, with positivity rates of 85.2% for females and 83.5% for males between day 180 and day 210. For IgM, the titer was found to decline quickly, so that it was often undetectable by day 90. It was found that the IgM positivity rate peaked within 30 days, with rates of 84.8% for females and 65.3% for males; this then declined quickly and was found to be 12.9% for females and 11% for males between day 180 and day 210.
Figure 1.
Trends in the SARS-CoV-2 IgG and IgM antibody titers and positivity rates in females and males over time. (A, B) Distribution of SARS‐CoV‐2 IgG/IgM antibody titers in serum samples from males and females over time in days from symptom onset. (C) Positivity rates of SARS-CoV-2 IgG/IgM in males and females according to the number of days after symptom onset.
F, female patients; M, male patients; ns, no significant difference; T, all patients.
Table 2.
The average IgG/IgM antibody titers for female and male patients from symptom onset to day 210.
| Days | IgG AU/mL |
IgM AU/mL |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| All |
Female |
Male |
All | Female | Male | ||||
| ± SD | % | ± SD | % | ± SD | % | ± SD | ± SD | ± SD | |
| 30 | 168.5 ± 48.7 | 167.2 ± 49.3 | 169.9 ± 47.8 | 113.2 ± 131.8 | 123.0 ± 145.8 | 103.3 ± 118.9 | |||
| 60 | 91.2 ± 45.8 | 54.1 | 105 ± 52.6 | 62.8 | 85.4 ± 39.0 | 50.3 | 38.4 ± 45.2 | 39.7 ± 46.1 | 37.1 ± 44.3 |
| 90 | 61.7 ± 27.8 | 36.6 | 61 ± 29.2 | 36.5 | 63.6 ± 27.8 | 37.4 | 8.8 ± 11 | 9.7 ± 11.9 | 8.0 ± 7.9 |
| 120 | 63.7 ± 29.3 | 37.8 | 59.7 ± 27.2 | 35.7 | 66.3 ± 29.3 | 39.0 | 9.8 ± 11.1 | 9.6 ± 10.7 | 9.9 ± 11.6 |
| 150 | 46.6 ± 36.7 | 27.7 | 42.7 ± 29.8 | 25.5 | 50.5 ± 36.7 | 29.7 | 8.4 ± 8.6 | 8.2 ± 8.5 | 8.5 ± 8.8 |
| 180 | 38.9 ± 27.4 | 23.1 | 36.2 ± 24.0 | 21.7 | 41.6 ± 27.4 | 24.5 | 5.0 ± 6.5 | 5.2 ± 6.9 | 4.8 ± 6.04.3 |
| 210 | 43.1 ± 27.0 | 25.6 | 42.3 ± 23.0 | 25.3 | 43.9 ± 26.9 | 25.8 | 4.4 ± 5.2 | 4.5 ± 5.5 | 4.3 ± 5.0 |
Note: ‘All’ is used to indicate all of the patients, both female and male.
The relationship between SARS-CoV-2 IgG/IgM antibody levels and age
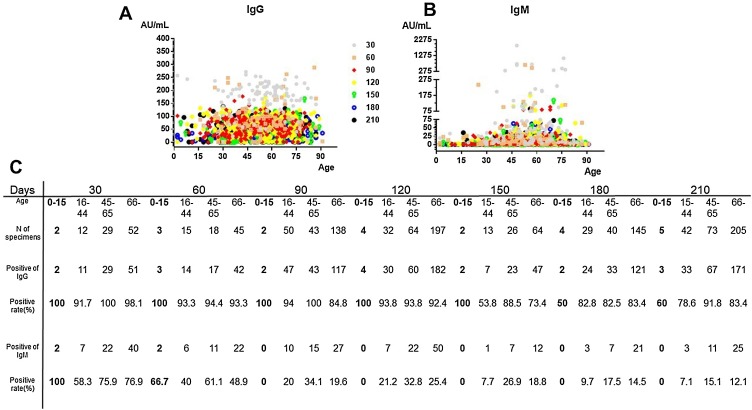
As shown in Figure 2 A and B, the titers of IgG/IgM rose to a peak within 30 days following symptom onset for the four different age groups: 0–15 years, 16–44 years, 45–65 years, and 66+ years. The average IgG antibody titers at this point were 250.6 ± 6.9, 158.4 ± 60.9, 187.3 ± 32.7, and 157.9 ± 50 AU/mL for the respective age groups. The average titers of IgG then decreased continuously. This decrease was faster in the younger age groups, and the titer of IgG was found to remain relatively stable from day 150 in all of the age groups, with percentage values of the peak as follows: 6.7%–11% for 0–15 years, 16.5%–26.8% for 16–44 years, 19.9%–27.7% for 45–65 years, and 25.8%–31.2% for 66+ years, as shown in Table 3 . There was no significant difference between the age groups in terms of the whole dynamic trend (p > 0.05). However, the average IgG antibody titers at the last time points were higher in the older age groups. As shown in Figure 2C, the rates of IgG positivity peaked within 30 days up to 100%, 91.7%, 100%, and 98.1% in the four different age groups; these then declined slowly to final rates of 91.8% and 83.4% in the two older age groups, but more quickly to final rates of 60% and 78.6% in the two younger age groups. There was a significant difference between the rates of positivity in the different age groups (p = 0.04).
Figure 2.
Trends in SARS-CoV-2 IgG/IgM antibodies based on age. (A, B) Distribution of SARS-CoV-2 IgG/IgM antibody concentrations. The x-axis shows the patient age in years, the y-axis shows the antibody concentration, and the different symbols show the number of days after symptom onset. (C) Positivity rates of SARS-CoV-2 IgG/IgM in the four different age groups according to the number of days after symptom onset.
Table 3.
The average IgG/IgM antibody titers for the different age groups from symptom onset to day 210.
| Days | IgG AU/mL |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0–15 years | % | 16–44 years | % | 45–65 years | % | 66+ years | % | |
| 30 | 250.6 ± 6.9 | 158.4 ± 60.9 | 187.3 ± 32.7 | 157.9 ± 50 | ||||
| 60 | 86.7 ± 0 | 24.6 | 98.3 ± 48.1 | 62.1 | 69.2 ± 22.5 | 36.9 | 103.0 ± 56.1 | 65.2 |
| 90 | 51.6 ± 20.0 | 20.6 | 62.0 ± 32.5 | 39.1 | 66.9 ± 22.6 | 35.7 | 60.4 ± 27.8 | 38.3 |
| 120 | 75.2 ± 31.8 | 30 | 63.8 ± 33.9 | 40.3 | 71.6 ± 28.6 | 38.2 | 60.9 ± 28.4 | 38.6 |
| 150 | 18.7 ± 18.4 | 7.5 | 26.2 ± 24.6 | 16.5 | 51.8 ± 29.3 | 27.7 | 49.2 ± 35.9 | 31.2 |
| 180 | 16.8 ± 9.8 | 6.7 | 32.0 ± 26.0 | 20.2 | 37.2 ± 27.8 | 19.9 | 40.8 ± 26.1 | 25.8 |
| 210 | 27.5 ± 30.1 | 11.0 | 42.1 ± 32.5 | 26.8 | 45.9 ± 22.7 | 24.5 | 42.8 ± 27.1 | 27.1 |
| IgM AU/mL | ||||||||
| 30 | 6.2 ± 3.2 | 39.8 ± 34.6 | 107.8 ± 102.8 | 136.1 ± 171.2 | ||||
| 60 | 20.9 ± 0 | 16.3 ± 15.8 | 92.1 ± 121.3 | 27.4 ± 28.1 | ||||
| 90 | 2.1 ± 1.5 | 2.4 ± 2.0 | 13.3 ± 15.0 | 8.6 ± 18.9 | ||||
| 120 | 4.4 ± 5.0 | 7.0 ± 6.2 | 14.5 ± 15.0 | 9.8 ± 10.6 | ||||
| 150 | 0.51 ± 0.3 | 8.1 ± 8.8 | 12.2 ± 12.9 | 7.4 ± 7.2 | ||||
| 180 | 0.6 ± 0.2 | 2.1 ± 2.4 | 6.3 ± 8.3 | 5.5 ± 7.0 | ||||
| 210 | 6.3 ± 9.7 | 3.4 ± 3.9 | 6.5 ± 7.5 | 4.0 ± 4.7 | ||||
For IgM, the titer level was found to peak within 30 days following the onset of symptoms (see Figure 2B and Table 3). The titer was then found to decline quickly, until it fell below the cutoff value by 90 days, with the exception of the group aged 45–65 years. The IgM positivity rates were found to be higher in the older age groups, as shown in Figure 2C.
The relationship between SARS‐CoV‐2 IgG/IgM antibody levels and disease severity
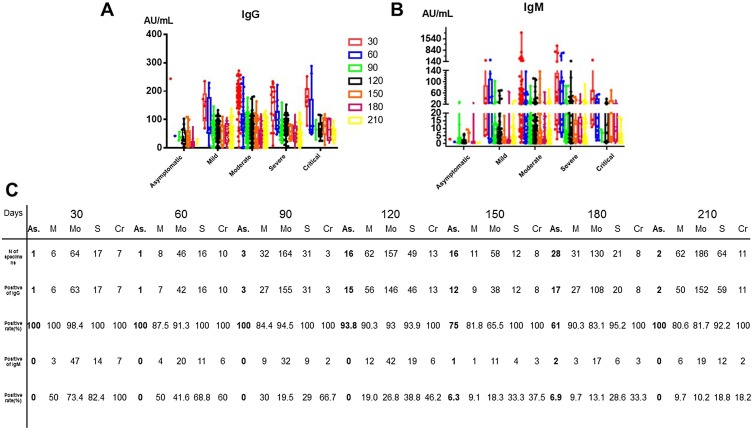
To examine the association between the disease severity and dynamic antibody changes, we plotted the whole antibody titer distribution for the asymptomatic, mild, moderate, severe, and critical groups at the different time points. As shown in Figure 3 A and B, the titers of IgG/IgM differ according to the disease severity. As described above, the titers of IgG/IgM rose to a peak within 30 days following symptom onset; then the average titers of IgG decreased continuously. Overall, the change in the IgG level varied according to the disease severity. In the critical group, the rate of IgG decrease was the lowest and the titer was the highest; in contrast, the asymptomatic group had the highest rate of IgG decrease. It can also be seen that the rates of IgG positivity were highest in the severe and critical groups. For IgM, the titer level peaked within 30 days following symptom onset (see Figure 3B and Table 4 ) and then declined quickly, becoming undetectable by 90 days, with the exception of the asymptomatic, severe and critical groups. The rate of IgM positivity was found to be higher in both the severe and critical groups, as shown in Figure 3C.
Figure 3.
Trends in SARS-CoV-2 IgG/IgM antibodies based on disease severity. (A, B) Distribution of SARS-CoV-2 IgG/IgM antibody concentrations according to the disease severity. The different colors show the number of days after symptom onset. (C) Positivity rates of SARS-CoV-2 IgG/IgM in the different disease severity groups according to the number of days after symptom onset.
As, asymptomatic; M, mild; C, moderate; S, severe; Cr, critical.
Table 4.
The average IgG/IgM antibody titers according to the disease severity from symptom onset to day 210.
| Days | IgG AU/mL |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Asymptomatic | % | Mild | % | Moderate | % | Severe | % | Critical | % | |
| 30 | 243.7 ± 0 | 148.7 ± 41.6 | 172.7 ± 46.8 | 154.7 ± 59.1 | 170.8 ± 36.6 | |||||
| 60 | 24.2 ± 0 | 9.9 | 95.0 ± 62.7 | 63.9 | 92.6 ± 40.8 | 53.6 | 97.8 ± 38.2 | 63.2 | 109.6 ± 73.8 | 64.2 |
| 90 | 38.6 ± 27.3 | 15.8 | 60.7 ± 32.4 | 40.8 | 61.8 ± 30.0 | 35.8 | 62.8 ± 25.6 | 40.6 | 73.3 ± 33.3 | 43.1 |
| 120 | 39.9 ± 23.3 | 16.4 | 58.4 ± 32.6 | 39.3 | 65.1 ± 28.3 | 37.7 | 72.8 ± 27.6 | 47.1 | 62.4 ± 24.0 | 36.5 |
| 150 | 39.9 ± 10.7 | 16.4 | 51.0 ± 33.0 | 34.3 | 44.0 ± 36.8 | 25.5 | 51.6 ± 20.0 | 33.4 | 64.3 ± 23.5 | 37.6 |
| 180 | 17.7 ± 11.0 | 7.3 | 47.8 ± 28.8 | 32.1 | 24.7 ± 24.7 | 14.3 | 49.1 ± 24.8 | 31.7 | 64.3 ± 23.3 | 37.6 |
| 210 | 21.8 ± 9.1 | 15.1 | 44.5 ± 28.6 | 29.9 | 40.7 ± 24.3 | 23.6 | 48.5 ± 25.1 | 31.4 | 49.1 ± 19.5 | 28.7 |
| IgM AU/mL | ||||||||||
| 30 | 2.9 ± 0 | 50.1 ± 55.4 | 107.8 ± 21.2 | 184.5 ± 231.9 | 60.1 ± 50.1 | |||||
| 60 | 1.1 ± 0 | 59.0 ± 63.3 | 16.7 ± 15.3 | 110.0 ± 127.0 | 19.7 ± 15.9 | |||||
| 90 | 4.0 ± 4.1 | 3.8 ± 3.5 | 9.4 ± 12.3 | 10.7 ± 11.0 | 18.7 ± 18.5 | |||||
| 120 | 2.0 ± 1.6 | 7.8 ± 8.8 | 9.5 ± 9.6 | 18.8 ± 19.6 | 11.6 ± 10.0 | |||||
| 150 | 0.6 ± 0.2 | 10.4 ± 11.8 | 7.0 ± 6.9 | 14.4 ± 15.5 | 15.9 ± 3.0 | |||||
| 180 | 2.1 ± 2.8 | 6.8 ± 9.8 | 4.2 ± 5.2 | 9.5 ± 11.5 | 11.1 ± 8.6 | |||||
| 210 | 0.5 ± 0.1 | 2.9 ± 3.3 | 4.3 ± 5.1 | 6.3 ± 7.4 | 4.4 ± 4.8 | |||||
The IgG antibody alert level due to degradation
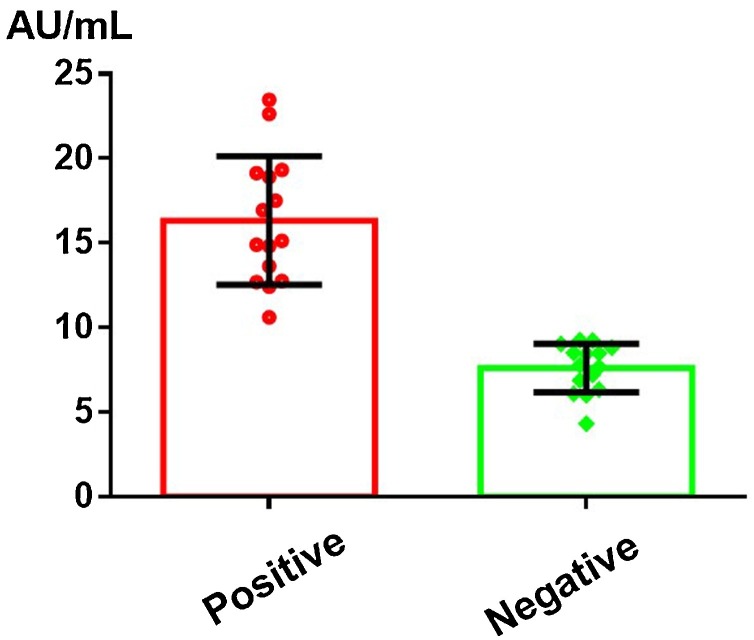
It was noted that 15 patients initially had positive antibody test results (from symptom onset up to 30 days) and that these turned negative at 30–60 days. In order to determine the level of IgG antibody that might become undetectable in patients within a certain period of time due to degradation, we statistically analyzed the IgG antibody results of these 15 patients for these two consecutive time periods. As shown in Figure 4 , the average initial IgG antibody level was 16.33 ± 3.15 AU/mL. This may therefore represent a key level, indicating that IgG may become undetectable within 30–60 days due to degradation. We therefore defined this level as an alert threshold.
Figure 4.
The IgG antibody alert level.
Discussion
A more comprehensive understanding of the characteristics of SARS-CoV-2 IgM and IgG antibodies, including the dynamics over time and the determinants, will have implications for the diagnosis and treatment of COVID-19.
The retrospective data presented in this study showed that females were more likely to be infected with SARS-CoV-2 than males, which is consistent with our previous report (Liu et al., 2020). The dynamic changes and the prevalence of SARS-CoV-2 IgG/IgM antibodies in males and females were almost the same. These changes are not nearly affected by sex. It was observed that the average titer of IgM antibodies was relatively higher in females from the time of symptom onset to 60 days. This could account for the higher levels of SARS‐CoV‐2 IgG antibodies found in female patients in the early phase of the disease, as seen in other studies (Zeng et al., 2020, Tian et al., 2020).
In line with previous studies, we found that elderly people were more susceptible to infection (Yang et al., 2020). Furthermore, we found that the SARS-CoV-2 IgM and IgG antibody titers significantly increased with age, which supports the view that elderly patients may have a stronger immune response against SARS-CoV-2 than young patients.
We demonstrated that the titers and positivity rates of SARS-CoV-2 IgM/IgG antibodies relate to the disease severity. The IgM/IgG titers and positivity rates in the severe and critical groups were found to be higher than in the asymptomatic and mild groups, which is in line with previous studies (Garcia-Beltran et al., 2021, Chen et al., 2020). In addition, the IgM/IgG titers and IgM positivity rates decreased more rapidly in the asymptomatic group. The IgG positivity rates in the severe and critical groups showed almost no decline from symptom onset to day 210.
In this study, we observed the persistence of IgG for at least 7 months. As shown in Figure 4, an IgG level that decreases down to 16.33 ± 3.15 AU/mL should act as an alert, as the IgG level may become undetectable within 30–60 days. This could act as a reference value when monitoring effective vaccine-related antibody levels. There was one patient who did not show SARS-CoV-2 IgG or IgM positivity from symptom onset to day 210. Such findings raise concern that immune responses against SARS-CoV-2 may fail in certain cases. The mean IgM positivity rate in our study was 12% at day 210, which was slightly higher in the severe and critical groups. It remains to be determined whether the IgM antibody can indicate the potential risk of a viral infection. Our current observations and data do not suggest that this antibody will not protect from a further COVID-19 outbreak.
There are some shortcomings in this study that were inevitable. Firstly, continuously monitoring the antibody titers in the same patients from the time of symptom onset to day 210 could disproportionately increase the power of any findings. In addition, some of the groups in the study were small.
In summary, our study showed that the dynamic changes and prevalence of SARS‐CoV‐2 IgG/IgM antibodies are mainly affected by age and disease severity, not sex. In addition, the study identified a threshold value of the IgG level that should act as an alert, as it may indicate that the antibodies may become undetectable within 30–60 days. These results could provide important information concerning COVID-19 diagnosis, treatment, and vaccine development.
Ethical approval
The study was approved by the Ethics Committees of Yichang Central People’s Hospital.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The work was financially supported by the National Natural Science Foundation of China [grant number 81902168].
References
- Chen W., Zhang J., Qin X., Wang W., Xu M., Wang L.F., et al. SARS-CoV-2 neutralizing antibody levels are correlated with severity of COVID-19 pneumonia. Biomed Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Beltran W.F., Lam E.C., Astudillo M.G., Yang D., Miller T.E., Feldman J., et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184 doi: 10.1016/j.cell.2020.12.015. 476–488.e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W.H., Zhao R., Zhou J.B., Wang F., Kong D.G., Sun J.B., et al. Serologic response to SARS-CoV-2 in COVID-19 patients with different severity. Virol Sin. 2020;35:752–757. doi: 10.1007/s12250-020-00270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Li Q., Zhou J., Ai W., Zheng X., Zeng J., et al. Value of swab types and collection time on SARS-COV-2 detection using RT-PCR assay. J Virol Methods. 2020;286 doi: 10.1016/j.jviromet.2020.113974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Driscoll M., Ribeiro Dos Santos G., Wang L., Cummings D.A.T., Azman A.S., Paireau J., et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2020;590:140–145. doi: 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]
- Pérez-García F., Pérez-Tanoira R., Romanyk J., Arroyo T., Gómez-Herruz P., Cuadros-González J. Alltest rapid lateral flow immunoassays is reliable in diagnosing SARS-CoV-2 infection from 14 days after symptom onset: a prospective single-center study. J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M., Zhou Y., Ye J., Abdullah Al-Maskri A.A., Kang Y., Zeng S., et al. Recent advances and perspectives of nucleic acid detection for coronavirus. J Pharm Anal. 2020;10:97–101. doi: 10.1016/j.jpha.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian R., Wu W., Wang C., Pang H., Zhang Z., Xu H., et al. Clinical characteristics and survival analysis in critical and non-critical patients with COVID-19 in Wuhan, China: a single-center retrospective case control study. Sci Rep. 2020;10:17524. doi: 10.1038/s41598-020-74465-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Long Q.X., Deng H.J., Hu J., Gao Q.Z., Zhang G.J., et al. Longitudinal dynamics of the neutralizing antibody response to SARS-CoV-2 infection. Clin Infect Dis. 2020;579:270–279. doi: 10.1093/cid/ciaa1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li J., Li H., Lei P., Shen G., Yang C. Persistence of SARS-CoV-2-specific antibodies in COVID-19 patients. Int Immunopharmacol. 2021;90 doi: 10.1016/j.intimp.2020.107271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F., Dai C., Cai P., Wang J., Xu L., Li J., et al. A comparison study of SARS‐CoV‐2 IgG antibody between male and female COVID‐19 patients: a possible reason underlying different outcome between sex. J Med Virol. 2020;92:2050–2054. doi: 10.1002/jmv.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]