Abstract
Introduction
The longevity of antibody levels against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the duration of immunity are current topics of major scientific interest. Antibody kinetics during the acute phase are well studied, whereas the long-term kinetics are yet to be determined, with contradictory results from the studies to date. Here, we present a longitudinal analysis of the serological responses to a SARS-CoV-2 infection following convalescence and the association with post-COVID syndrome (PCS).
Materials and methods
A total of 237 serum samples were prospectively collected from 61 participants who had had a SARS-CoV-2 infection, which was confirmed using quantitative reverse-transcription polymerase chain reaction (qRT-PCR). For each participant, anti-nucleocapsid (N) and anti-spike subunit 1 receptor binding domain (RBD/S1) immunoglobulin (Ig) levels were regularly determined over a period of 8 months. COVID-19-associated symptoms were assessed using a standardized questionnaire at study entry and again after 6 months.
Results
Antibodies were detectable in 56 of the 61 participants. No substantial decline in anti-SARS-CoV-2 pan-Ig levels was observed for the duration of the follow-up period. Antibody levels correlated positively with the disease severity, body mass index, fever, and smoking status. It was found that 46.8% of the participants suffered from PCS, with olfactory and gustatory dysfunctions being the most commonly reported symptoms.
Conclusion
The results demonstrate stable anti-SARS-CoV-2 antibody titers and thus may indicate a long-lasting immunity. The results are in line with recently published data and provide further insight concerning asymptomatic to mildly-affected patients, the association with clinical features, and the frequency of PCS.
Abbreviations: ACE, angiotensin-converting enzyme; BMI, body mass index; CE, Communauté Européenne; COI, cut-off index; COVID-19, coronavirus disease 2019; ECLIA, electrochemiluminescence immunoassay; FDA, Food and Drug Administration; ICU, intensive care unit; Ig, immunoglobulin; ISO, International Organization for Standardization; N, nucleocapsid; PCS, post-COVID syndrome; qPCR, quantitative polymerase chain reaction; qRT-PCR, quantitative reverse-transcription polymerase chain reaction; RBD, receptor binding domain; REDCap, Research Electronic Data Capture; RNA, ribonucleic acid; S, spike protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization
Keywords: Antibody dynamics, Antibody kinetics, Anti-SARS-CoV-2 antibodies, Longitudinal assessment, Post-COVID syndrome, Serological immune response
Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified in December 2019 in Wuhan, China, as a pathogen causing severe respiratory infections termed coronavirus disease 2019 (COVID-19) (Zhou et al., 2020). Within a few months, SARS-CoV-2 had spread throughout China and finally resulted in a global pandemic (Ahn et al., 2020). As of March 2021, over 118 million confirmed cases and more than 2.5 million fatalities had been reported worldwide according to WHO statistics (WHO, 2021). Thus, there is a pressing need to understand the immune response induced by a SARS-CoV-2 infection as well as the duration of immunity (Figueiredo-Campos et al., 2020). This is of utmost importance for the future management of the COVID-19 pandemic, especially with regard to the development of sustainable vaccination strategies. In order to determine the efficacy and pattern of the immune response to vaccination, the seroprevalence, the rates of reinfection, and the risk factors for post-COVID syndrome (PCS), it is necessary to gain a deeper understanding of the longevity of immunity and its association with disease severity and the clinical presentation (Sasisekharan et al., 2021).
After infection with SARS-CoV-2, seroconversion usually occurs 7–14 days after diagnosis (Krajewski et al., 2020, Long et al., 2020a, Peeling et al., 2020, To et al., 2020), with a peak in antibody levels observed around 30–35 days following symptom onset (Crawford et al., 2020, Isho et al., 2020, Wang et al., 2020, Yao et al., 2020). The longevity of the antibody levels and the durability of immunity following a SARS-CoV-2 infection have not yet been fully elucidated due to the short time elapsed since this disease was first recognized, in contrast to the well-studied antibody kinetics during the acute phase. Moreover, the studies that have been conducted to assess the longevity of antibody levels are contradictory. Some report a rapid decline in anti-SARS-CoV-2-specific immunoglobulin (Ig) G antibodies up to 3 months following an infection (Beaudoin-Bussieres et al., 2020, Ibarrondo et al., 2020, Roltgen et al., 2020), whereas others report stable titers over several months, thus indicating long-lasting immunity (Gudbjartsson et al., 2020, Isho et al., 2020, Wang et al., 2020, Yao et al., 2020, Liu et al., 2021). Further studies are warranted to in order to gain a clearer understanding.
Here, we report the results of a prospective, longitudinal study of the serologic response to SARS-CoV-2 and the clinical presentation of 61 individuals. The disease severity ranged from asymptomatic cases to severe cases requiring admission to an intensive care unit (ICU), thus representing the full spectrum of SARS-CoV-2 infections. The serological response was assessed by measuring antibodies directed against the nucleocapsid (N) protein as well as the spike (S) subunit 1 receptor-binding domain (RBD) of SARS-CoV-2 (RBD/S1). Protective immunity mediated by neutralizing antibodies has been shown to correlate well with IgG antibodies targeting RBD/S1 (Okba et al., 2020, Robbiani et al., 2020, Suthar et al., 2020), and so RBD/S1 antibody levels are likely to reflect protective immunity. The main aims of this study were to (i) evaluate anti-SARS-CoV-2 antibody dynamics over a period of 8 months, and (ii) determine whether antibody levels correlate with disease severity, demographic factors, and the likelihood of developing PCS.
Materials and methods
Participant recruitment and sample collection
Participants were recruited prospectively from April 2020 to December 2020 at the University Medical Center in Mannheim, Germany, as part of the Immunitor Study (UMM, 2020). Individuals aged 18 and above with a SARS-CoV-2 infection that was confirmed using quantitative reverse-transcription polymerase chain reaction (qRT-PCR) were eligible for inclusion following convalescence and polymerase chain reaction (PCR) negativity. The study protocol was approved by the Institutional Review Board (2020-556N) and was conducted in accordance with the Declaration of Helsinki. Informed written consent was obtained from each subject prior to study inclusion, sample collection, and sample analysis.
Sociodemographic and medical data were collected using a standardized questionnaire on Research Electronic Data Capture (REDCap), a secure web platform for online databases and surveys supported by the University of Heidelberg (University V, 2021). The data included the date of PCR positivity for SARS-CoV-2, the clinical symptoms, the type of treatment (e.g., outpatient, hospitalized, ICU), demographic data, and medical comorbidities. The study participants completed the questionnaire at the time of enrollment and were asked about long-lasting COVID-19 symptoms after 6 months. All of the data were recorded in a pseudonymized form.
Sixty-one participants met the above-mentioned inclusion criteria and provided serial blood samples for the analysis of anti-SARS-CoV-2 antibody levels. Participants were asked to provide blood samples at five time points: the time of study enrollment, after 2 weeks, after 1 month, after 3 months, and after 6 months. At each time point, 7.5 ml of serum was collected. The serum samples were stored at room temperature for at least 1 h to ensure adequate clotting. The clotted samples were centrifuged at 2000 g for 10 min at 18 °C. The serum was aliquoted and stored at −80 °C until further use. A total of 237 serum samples were collected and used for anti-SARS-CoV-2 antibody detection. The following time intervals were combined for the statistical analyses: t0 = 0–50 days, t1 = 50–100 days, t2 = 100–150 days, t3 = 150–200 days, and t4 = 200–250 days.
Anti-SARS-CoV-2 antibody detection
Tests for detecting antibodies against SARS-CoV-2 N and RBD/S1 were run on all of the serum samples. The tests were performed as previously described (Haselmann et al., 2020). Briefly, the total anti-N Ig was analyzed using the Elecsys® anti-SARS-CoV-2 assay (Roche, Germany), a qualitative electrochemiluminescence immunoassay (ECLIA) that is FDA approved and CE marked. The total anti-RBD/S1 Ig was analyzed using the Elecsys® Anti-SARS-CoV-2 S (Roche, Germany), a quantitative FDA-approved, CE-marked ECLIA. The results were reported as a cut-off index (COI) for anti-N and in U/ml for anti-RBD/S1, with values above 1.0 and 0.8 considered to be positive, respectively. The tests were performed according to the manufacturer’s instructions and after internal verification in an accredited laboratory, in accordance with ISO 15189.
Data analysis
Clinical data were systematically collected via the REDCap platform. To assess whether the clinical parameters relate to the presence of antibodies against SARS-CoV-2, statistical analyses were run using SPSS version 26 (e.g., t-test for independent samples, Spearman’s rank correlation coefficient). For all of the statistical analyses, p-values less than 0.05 were considered to be statistically significant and were indicated using a single asterisk; p-values less than 0.01 were indicated using two asterisks. Graphs were plotted using GraphPad 7.0, SPSS, or R version 3.6.
Results
Demographics and baseline antibody levels
Sixty-one participants who had had a SARS-CoV-2 infection were prospectively enrolled following convalescence. Overall, 237 serum samples were collected between 18 days and up to 277 days following the positive qRT-PCR test, with a mean of 3.9 samples per patient. As displayed in Table 1 , the mean age was 46.4 years (range 18–76 years), the mean body mass index (BMI) was 25.4, and 59% of the participants were female. Among the participants, 90.2% had not required hospitalization, and only 3.3% were admitted to an ICU. The majority of the patients suffered from olfactory and gustatory dysfunctions at the time of diagnosis (70.1% and 73.7%, respectively), and a high proportion suffered from muscle pain (52.5%) and fever (49.2%).
Table 1.
Patient characteristics and antibody levels.
| Number of symptoms | 0 | 1–5 | >5 | Total |
|---|---|---|---|---|
| n | 4 | 40 | 17 | 61 |
| Sex (% female/male) | 75%/25% | 57.5%/42.5% | 58.8%/42.2% | 59%/41% |
| Age (years) | 44.5 ± 15.2 | 45.78 ± 17.6 | 48.41 ± 14.8 | 46.4 ± 16.5 |
| BMI | 24.7 ± 4.6 | 24.8 ± 4.3 | 27.1 ± 4.8 | 25.4 ± 4.5 |
| Smoker (n) | 0 | 4 | 0 | 4 |
| Severity | 0 | 1.7 ± 1.1 | 4.6 ± 0.9 | 3.0 ± 1.5 |
| Hospitalization (n/%) | 0/0% | 4/10% | 2/11.8% | 6/9.8% |
| Mean anti-RBD/S1 level (U/ml) | 64.2 ± 76.5 | 256.4 ± 479.8 | 539.7 ± 539.7 | 322.7 ± 498.3 |
| PCS (n/%) | 0/0% na: 2 | 15/37.5% na: 9/22.5% | 7/41.2% na: 3/17.6% | 22/36.1% na: 14/23% |
anti-RBD/S1, anti-spike subunit 1 receptor binding domain; BMI, body mass index; PCS, post-COVID syndrome; na, not answered.
At the time of study inclusion, antibodies against SARS-CoV-2 N and RBD/S1 were detected in 55 and 56 of the 61 participants, respectively. This corresponds to a sensitivity of 90.2% and 91.8%, respectively. Only one discrepant result was observed between the SARS-CoV-2 N and RBD/S1 antibody analyses. Here, there was a weak positive result (COI = 3.12) for the anti-RBD/S1 and a negative result (COI = 0.43) for the anti-N. Remarkably, this was confirmed in four later assessments. In total, five participants had a negative test result for both of the pan-Ig antibody tests, corresponding to a negativity rate of 8.2%. Of these, four did not develop detectable antibodies over time; the other participant’s results could not be confirmed over time because they discontinued study participation.
Correlation between clinical features and baseline antibody levels
Correlation analyses were run between the clinical symptoms at the time of diagnosis, as assessed using a standardized questionnaire, and the anti-SARS-CoV-2 antibody levels. These were only run for anti-RBD/S1 exclusively, as only this test allowed a quantitative determination of anti-SARS-CoV-2 Ig. Significant positive correlations were observed between the mean anti-RBD/S1 antibody levels and the severity of COVID-19 (Spearman’s rho: 0.437; p < 0.001), the participants’ age (spearman’s rho: 0.300; p = 0.019), and the body mass index (BMI; Spearman’s rho: 0.292; p = 0.022). A significant positive correlation was also observed between the BMI and the disease severity (Spearman’s rho: 0.348; p = 0.006). A comparison between smokers (n = 4) and non-smokers (n = 57) showed significantly higher antibody levels in the latter group (mean anti-RBD/S1 antibody level in non-smokers: 339.2 U/ml; mean anti-RBD/S1 level in smokers: 88.4 U/ml; p = 0.045). There was also a trend towards higher antibody levels in men compared to women (mean anti-RBD/S1 level in men: 420.7 U/ml; mean anti-RBD/S1 level in women: 254.6 U/ml; p = 0.059). Table 1 summarizes the information concerning the participants’ age, sex, and total anti-SARS-CoV-2 IgG levels according to the number of clinical symptoms.
Anti-SARS-CoV-2 antibody dynamics
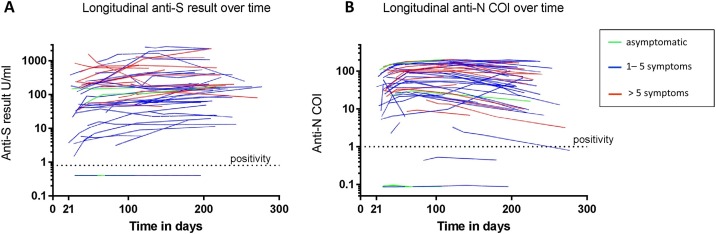
The antibody kinetics over the observation period (from day 18 to day 277 following diagnosis) are shown in Figure 1 . A total of 237 samples from 61 participants were serially analyzed for the presence and level of anti-N Ig and anti-RBD/S1 Ig, with an average of 3.9 sequential samples for each participant. Over the total observation period, 98.2% of the participants who were initially found to have seroconverted had detectable anti-N Ig and 100% had anti-RBD/S1 Ig at end of observation period (i.e., 6–8 months after study inclusion). It is relevant to note that no seroconversion was observed over time for those participants who had an initial COI <1 (anti-N Ig) or <0.8 U/ml (anti-RBD/S1 Ig).
Figure 1.
Anti-SARS-CoV-2 Ig dynamics.
The logarithmic plot shows the anti-N (A) and anti-RBD/S1 (B) Ig levels expressed in U/ml or as a cut-off index (COI; y-axis) over the observation period of up to 277 days post-diagnosis (x-axis). The dashed line highlights the threshold for assay positivity. Asymptomatic patients are displayed in green, patients with up to five clinical symptoms in blue, and patients with more than five clinical symptoms in red.
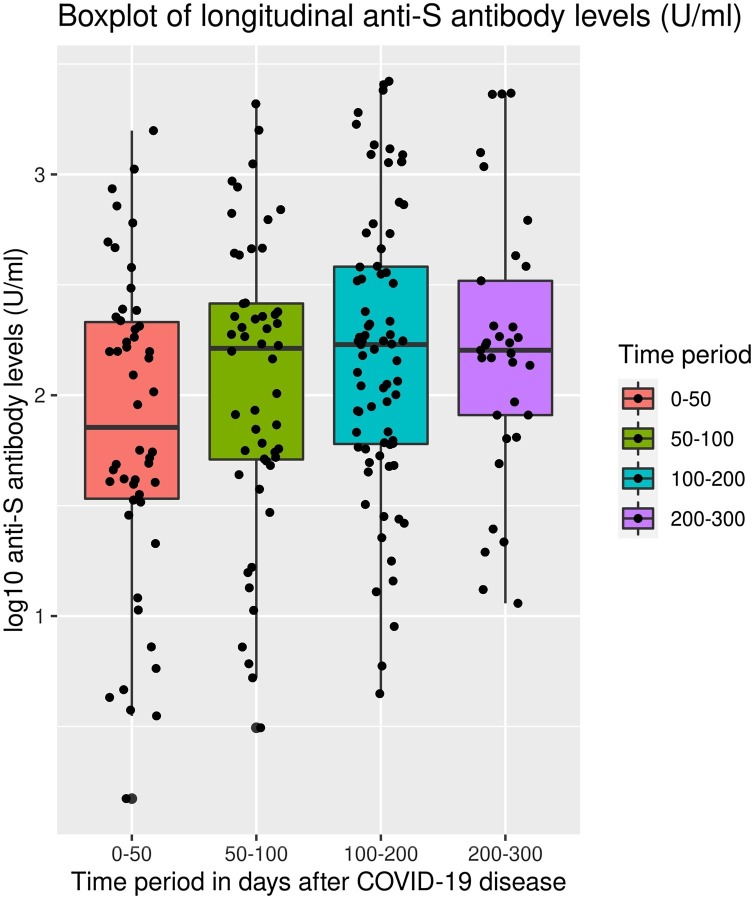
When comparing the time period t4 with t0/t1, anti-RBD/S1 Ig levels were found to remain constant. There was a slight increase between t0/t1 and t3 (p: 0.012) but no significant difference between t0/t1 and t2 and also t4 (p respectively 0.240 and 0.088) (Figure 2 ). Nevertheless, an absolute decrease in the antibody level was observed when comparing the first and the last measured values for 13 of the 56 participants who had anti-RBD/S1 Ig at the time of study inclusion. However, anti-RBD/S1 Ig levels did not fall below the threshold for seropositivity in any of the cases within the observation period of 8 months post diagnosis.
Figure 2.
Total anti-RBD/S1 Ig titer at the different time points.
Boxplots for anti-RBD/S1 (U/ml; y-axis) at different time points (t0/t1: 0–50 days after diagnosis; t2 = 50–100 days after diagnosis; t3 = 100–200 days after diagnosis, and t4 = 200–300 days after diagnosis of a SARS-CoV-2 infection; x-axis) are shown for the 61 participants who provided serial blood samples. The box and whisker plots show the interquartile range as the box, and the upper whisker is the maximum value of the data that is within 1.5 times the interquartile range over the 75th percentile. Lower whisker is the minimum value of the data within 1.5 times under 25% quartil. Furthermore some outliners are illustrated as dots beyond the whiskers and each dot represents the results obtained for a single participant.
For the anti-N Ig, antibodies were detectable at all of the time points for the seropositive participants (n = 55), with just one exception. In this case, a decrease was observed with results under positiviy cut-Off (COI ≤1.0).. In contrast to the anti-RBD/S1 Ig, a decrease in anti-N Ig was observed for half of the participants (n = 27). For 12 of these, anti-N Ig levels decreased twofold within the observation period, thus demonstrating a half-life of approximately 200 days for these participants.
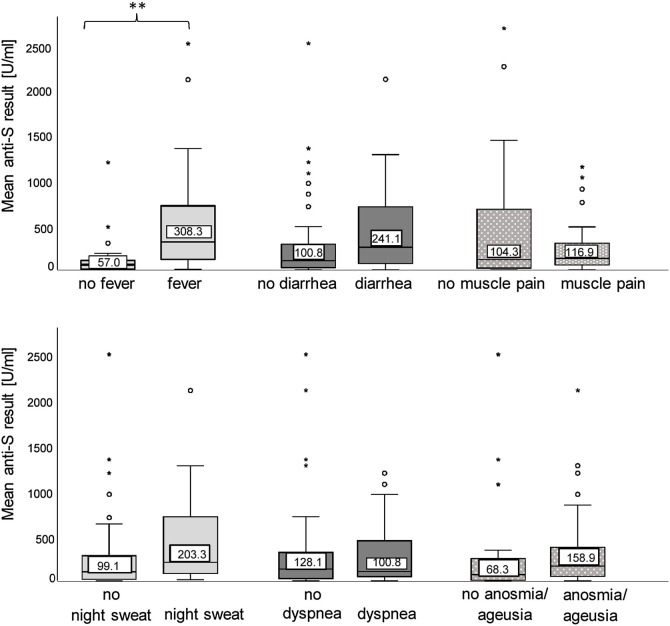
In line with the significant positive correlation between the disease severity and anti-RBD/S1 levels, the anti-N levels over time were higher on average in severely-affected individuals (mean anti-RBD level in patients with >5 clinical symptoms: 539.7 ± 539.7 U/ml, compared with a mean result of 256.4 ± 479.8 U/ml for participants with 1–5 clinical symptoms, and 64.2 ± 76.5 U/ml for asymptomatic individuals). However, no effect for the antibody kinetics was observed. With respect to the various clinical symptoms commonly associated with COVID-19, a significant positive association with anti-RBD/S1 levels over time and the presence of fever was shown (mean anti-RBD/S1 level without fever: 116.8 ± 221.1 U/ml ; mean anti-RBD/S1 level with fever: 535.5 ± 609.2 U/ml; p < 0.001). However, no significant differences were observed for the other symptoms on the questionnaire (Figure 3 ).
Figure 3.
Clinical symptoms and anti-SARS-CoV-2 antibody levels.
The boxplots show the mean anti-RBD/S1 Ig levels (y-axis) in U/ml over time for patients suffering from a particular clinical symptom alongside the results of those unaffected by the same symptom (x-axis). The interquartile ranges are displayed as boxes, with the median highlighted by a line. The whiskers are the maximum and minimum value of the data that is within 1.5 times the interquartile range over the 75th percentile, respectively under the 25th percentile. Outliers are indicated by open circles and stars. The statistical significance of the comparison between the two groups is shown: fever (**p < 0.001), night sweat (p = 0.185), diarrhea (p = 0.267), cough (p = 0.537), dyspnea (p = 0.862), muscle pain (p = 0.147), and anosmia/ageusia (p = 0.698).
PCS association with clinical features and antibody dynamics
The participants were systematically asked about any long-lasting COVID-19 symptoms 6 months after their COVID-19 diagnosis. These long-term sequelae resulting from a SARS-CoV-2 infection are commonly referred to as PCS. The response rate to the questionnaire was 77.1% (47 out of 61, as 14 participants did not attend the last appointment). Of those who completed the questionnaire, 46.8% (22 out of 47) reported persistent symptoms. The most frequently reported clinical symptom was olfactory or gustatory dysfunction (59%), followed by fatigue (36%), and other sporadically reported symptoms such as loss of hair, a depressed mood, and poor concentration. In general, women (16/22) were more likely to have PCS compared to men (6/22). Interestingly, 28% of the participants who reported a loss of smell and/or taste at the time of study inclusion (77% of the total participants) still suffered from impaired olfactory and/or gustatory function after 6 months. A potential factor that could have an impact on the likelihood of developing PCS is the severity of the disease during the acute phase. To assess this, the participants’ symptom severity was quantified and assessed in relation to the occurrence of PCS. This analysis did not reveal a statistically significant difference between patients with PCS and those without symptom persistence (p = 0.554). Importantly, no significant differences were observed between the mean anti-RBD/S1 Ig levels over the observation period and the presence of long-term effects (mean anti-RBD/S1 level without long-term-effects: 354.6 ± 408.9 U/ml; mean anti-RBD/S1 level with long-term effects: 299.3 ± 548.7 U/ml; p = 0.7).
Discussion
Anti-SARS-CoV-2 serological testing is currently a topic of major scientific interest as it can be used to determine the seroprevalence, the efficacy and pattern of immune responses to vaccination, the re-infection risk, the duration of immunity following a SARS-CoV-2 infection, and for contact tracing (Sasisekharan et al., 2021). A detailed understanding is likely to have a major impact on sustainable vaccination strategies. However, peer-reviewed publications addressing the longevity of anti-SARS-CoV-2 antibody levels and the duration of immunity are limited, and the results are contradictory. Here, we provide a comprehensive description of the anti-SARS-CoV-2 Ig levels and the clinical presentation of a cohort of 61 participants who had convalesced from a qRT-PCR-confirmed SARS-CoV-2 infection. Antibody tests were run on blood samples obtained over a period of up to 8 months, and the long-term health outcomes were addressed.
The results showed that the majority of the participants (90.2%) were identified as seropositive using both of the pan-Ig antibody tests. However, one divergent result between the anti-N and anti-RBD/S1 testing was observed, and this was confirmed during the observation period. Such aberrant results have previously been described, with anti-N positivity alongside anti-S negativity in most cases (Brochot et al., 2020). There were five participants who had a negative test result for both assays at the time of study inclusion, and all of them had negative test results at the later time points. Therefore, either the qRT-PCR results were false positives or these participants never developed detectable antibody levels against N or RBD/S1 following a SARS-CoV-2 infection. Two of the five participants had an asymptomatic infection, for which the absence of seroconversion and thus undetectable antibody levels has been described previously (Milani et al., 2020). The other three participants were paucisymptomatic outpatients. Here, a weaker immune response has been described (Long et al., 2020b), and comparable results have been reported by Gudbjartsson et al. (2020), which could be explained by a predominantly antigen-specific B- and T-lymphocyte-driven immunity without the development of measurable antibody levels.
In our study, there was a significant positive correlation between the disease severity and the mean anti-RBD/S1 levels. These results are consistent with many other studies reporting higher antibody titers in individuals with severe COVID-19 (Crawford et al., 2020, Salazar et al., 2020, Zhao et al., 2020). The underlying mechanisms have not yet been fully elucidated, but they could be explained by a stronger immune response or an antibody-dependent enhancement that favors viral entry, thus triggering a stronger inflammatory response (Cao, 2020). In previous reports (Simonnet et al., 2020), the disease severity was found to be associated with a higher BMI, and in line with this, our study showed a positive correlation between BMI and anti-RBD/S1 Ig levels. Recently, the expression of the SARS-CoV-2 target receptor, human angiotensin-converting enzyme 2 (ACE2), on the surface of adipose tissue was identified as the pathophysiological mechanism that is likely to underlie this (Hussain et al., 2020). In addition to BMI, it was found that age, sex, and smoking status were associated with SARS-CoV-2 Ig levels. While older participants and men were found to have higher antibody levels, smokers had lower anti-SARS-CoV-2 Ig levels. These findings are consistent with recently published studies reporting a lower prevalence of smokers among hospitalized patients (Gudbjartsson et al., 2020). One possible contributing factor could be the tobacco mosaic virus, a non-pathogenic single-stranded RNA virus that infects tobacco leaves and has been detected in the respiratory tract of smokers. This viral infection could lead to immune system activation and thus act as a barrier preventing the entry of the SARS-CoV-2 virus (de Bernardis and Busà, 2020).
To date, few studies have longitudinally examined anti-SARS-CoV-2 antibody levels following convalescence from COVID-19 (Beaudoin-Bussieres et al., 2020, Crawford et al., 2020, den Hartog et al., 2021, Figueiredo-Campos et al., 2020, Gudbjartsson et al., 2020, Ibarrondo et al., 2020, Isho et al., 2020, L’Huillier et al., 2021, Lumley et al., 2021, Roltgen et al., 2020, Sasisekharan et al., 2021, Wang et al., 2020, Wheatley et al., 2021, Wu et al., 2020, Yamayoshi et al., 2021, Yao et al., 2020), and reports have yielded contradictory data concerning the longevity of the antibody response. Waning levels of antibodies against SARS-CoV-2 within several weeks have been reported, including anti-N, anti-S2 (C-terminal subunit of the spike protein), anti-S1 (N-terminal subunit of the spike protein), anti-RBD1 (receptor binding domain), and neutralizing antibodies (Beaudoin-Bussieres et al., 2020, Ibarrondo et al., 2020, Roltgen et al., 2020, Wheatley et al., 2021), with a more rapid decay of anti-N IgG levels compared to anti-S (Wheatley et al., 2021). These reports have raised concerns that the anti-SARS-CoV-2 humoral immunity may not be long-lasting. However, recently published studies have emphasized the sustainability of antibody titers of up to 3 (Wheatley et al., 2021), 4 (Crawford et al., 2020, Gudbjartsson et al., 2020, Isho et al., 2020, Sasisekharan et al., 2021), 6 (Figueiredo-Campos et al., 2020, L’Huillier et al., 2021, Wu et al., 2020), or 7 months (den Hartog et al., 2021), with some reporting an initial decay that decelerates after a few weeks (Figueiredo-Campos et al., 2020; 2021, Wu et al., 2020; Wu et al., 2020). These findings require rapid confirmation. Our results are consistent with these reports and even go beyond them by showing stable anti-N and anti-RBD/S1 levels up to 8 months. It is possible that the contradictory findings regarding the longevity of anti-SARS-CoV-2 antibodies may relate to the different types of serological assays. Our data were obtained using FDA-approved, CE-marked verified tests performed in an accredited setting.
Like Wheatley et al. (2021), we observed a decay in anti-N pan-Ig, with a twofold decrease seen in 12 participants within the observation period of 277 days. However, none of the participants became seronegative, with the exception of one case. For these participants, the anti-RBD/S1 results remained positive and a trend of a twofold decrease as seen for the N-antibodies could not be observed, thus demonstrating different antibody patterns during the follow-up. For the anti-RBD/S1, a slight increase was observed within the first 2–4 weeks. This initial increase is usually observed after a viral infection and is mediated by short-lived, antibody-producing plasmablasts. This is then followed by a rapid decline before reaching a stable plateau, which is maintained by long-living, antibody-producing plasma cells (Smith et al., 1996). However, our results were not able to demonstrate an early decline in antibody levels. This could be due to the fact that a pan-Ig antibody test was used, which does not distinguish between IgM and IgG levels. Additionally, participants were enrolled after convalescence from COVID-19, and in some cases this was several weeks after the infection. Therefore, early changes could have been missed.
Our study showed significantly higher anti-N and anti-RBD/S1 levels when there was a more severe infection, a higher number of clinical symptoms, and fever. This association with disease severity has previously been reported (den Hartog et al., 2021, L’Huillier et al., 2021, Yamayoshi et al., 2021). However, we were not able to confirm the correlation between disease severity and the decrease in antibody levels over time reported by Yamayoshi et al. (2021). Instead, our data indicate stable antibody levels and long-lasting immunity, irrespective of the disease severity.
The present study also examined whether the antibody dynamics, antibody levels, and clinical features were related to the likelihood of developing PCS. PCS is defined as the “signs and symptoms that develop during or after an infection consistent with COVID-19, continue more than 12 weeks and are not explained by an alternative diagnosis” (National Institute for Health and Care Excellence: Clinical Guidelines, 2020). Post-COVID symptoms were assessed 6 months after study inclusion. PCS was identified in 46.8% of the participants, a frequency that is comparable to that found in other recently-published reports (Moreno-Pérez et al., 2021, Pereira et al., 2021). However, as the participants were specifically asked about persistent symptoms and the symptoms previously reported, such as olfactory and gustatory dysfunctions and fatigue, the results are not objective, and overestimation is likely. Given that PCS is considered to have a large impact on the perception of one’s own health as well as on the public health situation, risk factors need to be identified. Our study confirmed that antibody levels do not affect the risk of developing PCS, and that the initial symptoms assessed by the questionnaire were also unrelated (Pereira et al., 2021). In line with the cohort study of Moreno-Pérez et al., the severity of the SARS-CoV-2 infection during the acute phase was not identified as an indicator of long-term sequelae. However, previous studies that focused on a severe COVID-19 cohort found that the occurrence of PCS (e.g., dyspnea, fatigue, weakness) was significantly associated with independent predictors, such as intensive care and invasive mechanical ventilation (Moreno-Pérez et al., 2021, Nalbandian et al., 2021). We were unable to confirm these findings, probably due to the fact that only two participants in our study were in intensive care, so that the numbers were too small to statistically identify relevant predictors.
The limitations of this study include the small cohort size and the fact that the participants were recruited from a single study site. However, this study is one of, if not the largest study to date in terms of the number of participants continuously followed up for a period of more than 6 months, and whose antibody titers were determined in an accredited diagnostic setting. Previous studies have reported data for numerous patients and determined antibody levels at different time points after a SAR-CoV-2 infection, but they did not conduct a longitudinal follow-up of patients. Another limitation of this study is that neither neutralizing antibodies, the best surrogate of immunity, nor T- and B-lymphocyte responses were assessed. Therefore, the persistence of anti-RBD/S1 levels does not necessarily equate with immunity, although it has previously been shown that neutralizing antibody levels correlate well with anti-S1 levels (Figueiredo-Campos et al., 2020, Wu et al., 2020).
In conclusion, our results support a sustained humoral immunity in patients who had convalesced from COVID-19, regardless of the disease severity, as stable anti-N and anti-RBD/S1 pan-Ig levels were detected up to 8 months following symptom onset. Together with recent findings of a long-term cellular immune response driven by memory T-cells, this provides hope for long-lasting immunity and the success of vaccination programs in our fight against the COVID-19 pandemic. Our study also found that a substantial number of participants suffered from PCS. The results need to be confirmed in further studies that assess the stability of antibody levels over the long term, particularly in vaccinated individuals.
Contribution
Michael Neumaier and Verena Haselmann designed the study. Catharina Gerhards, Margot Thiaucourt, Maximilian Kittel, and Verena Haselmann were responsible for the data collection and management. Catharina Gerhards, Margot Thiaucourt, and Maximilian Kittel enrolled the patients and collected the blood samples. Catharina Gerhards and Verena Haselmann performed the assays. Michael Hetjens structured the survey on the REDCap platform. Margot Thiaucourt, Catharina Gerhards, and Volker Ast were responsible for the biostatistical analyses. Margot Thiaucourt and Catharina Gerhards were responsible for interpreting the data. Margot Thiaucourt, Volker Ast, and Catharina Gerhards prepared the tables and figures. Margot Thiaucourt, Catharina Gerhards, and Verena Haselmann drafted the manuscript. All of the authors contributed to the revision of the manuscript and approved it for submission.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors have declared no conflicts of interest.
Acknowledgements
We thank Ameli Götz, Angelika Duda, Marina Talamini, Sihem Aida, Romy Eichner, and Laura Mirbach for organizing the study and aliquoting the samples. We thank Ingrid Brechtel and Cornelia Keup for managing the appointments and follow-up of the patients. Finally, we thank all of the study participants.
References
- Ahn D.G., Shin H.J., Kim M.H., Lee S., Kim H.S., Myoung J., et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J Microbiol Biotechnol. 2020;30(3):313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin-Bussieres G., Laumaea A., Anand S.P., Prevost J., Gasser R., Goyette G., et al. Decline of humoral responses against SARS-CoV-2 spike in convalescent individuals. mBio. 2020;11(5) doi: 10.1128/mBio.02590-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochot E., Demey B., Touzé A., Belouzard S., Dubuisson J., Schmit J.L., et al. Anti-spike, anti-nucleocapsid and neutralizing antibodies in SARS-CoV-2 inpatients and asymptomatic individuals. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.584251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 rapid guideline: managing the long-term effects of COVID-19. National Institute for Health and Care Excellence: Clinical Guidelines. London. 2020. [PubMed]
- Crawford K.H.D., Dingens A.S., Eguia R., Wolf C.R., Wilcox N., Logue J.K., et al. Dynamics of neutralizing antibody titers in the months after SARS-CoV-2 infection. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bernardis E., Busà L. A putative role for the tobacco mosaic virus in smokers’ resistance to COVID-19. Med Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hartog G., Vos E.R.A., van den Hoogen L.L., van Boven M., Schepp R.M., Smits G., et al. Persistence of antibodies to SARS-CoV-2 in relation to symptoms in a nationwide prospective study. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo-Campos P., Blankenhaus B., Mota C., Gomes A., Serrano M., Ariotti S., et al. Seroprevalence of anti-SARS-CoV-2 antibodies in COVID-19 patients and healthy volunteers up to 6 months post disease onset. Eur J Immunol. 2020;50(12):2025–2040. doi: 10.1002/eji.202048970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudbjartsson D.F., Norddahl G.L., Melsted P., Gunnarsdottir K., Holm H., Eythorsson E., et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383(18):1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselmann V., Kittel M., Gerhards C., Thiaucourt M., Eichner R., Costina V., et al. Comparison of test performance of commercial anti-SARS-CoV-2 immunoassays in serum and plasma samples. Clin Chim Acta. 2020;510:73–78. doi: 10.1016/j.cca.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A., Mahawar K., Xia Z., Yang W., El-Hasani S. Obesity and mortality of COVID-19. Meta-analysis. Obes Res Clin Pract. 2020;14(4):295–300. doi: 10.1016/j.orcp.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ibarrondo F.J., Fulcher J.A., Goodman-Meza D., Elliott J., Hofmann C., Hausner M.A., et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med. 2020;383(11):1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isho B., Abe K.T., Zuo M., Jamal A.J., Rathod B., Wang J.H., et al. Mucosal versus systemic antibody responses to SARS-CoV-2 antigens in COVID-19 patients. medRxiv. 2020;5(52) doi: 10.1126/sciimmunol.abe5511. 2020.08.01.20166553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewski R., Golebiowska J., Makuch S., Mazur G., Agrawal S. Update on serologic testing in COVID-19. Clin Chim Acta. 2020;510:746–750. doi: 10.1016/j.cca.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Huillier A.G., Meyer B., Andrey D.O., Arm-Vernez I., Baggio S., Didierlaurent A., et al. Antibody persistence in the first 6 months following SARS-CoV-2 infection among hospital workers: a prospective longitudinal study. Clin Microbiol Infect. 2021;27(5):784.e1–784.e8. doi: 10.1016/j.cmi.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Yu X., Gao C., Zhang L., Zhai H., Hu Y., et al. Characterization of antibody responses to SARS-CoV-2 in convalescent COVID-19 patients. J Med Virol. 2021;93(4):2227–2233. doi: 10.1002/jmv.26646. [DOI] [PubMed] [Google Scholar]
- Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- Lumley S.F., Wei J., O’Donnell D., Stoesser N.E., Matthews P.C., Howarth A., et al. The duration, dynamics and determinants of SARS-CoV-2 antibody responses in individual healthcare workers. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani G.P., Dioni L., Favero C., Cantone L., Macchi C., Delbue S., et al. Serological follow-up of SARS-CoV-2 asymptomatic subjects. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-77125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Pérez O., Merino E., Leon-Ramirez J.M., Andres M., Ramos J.M., Arenas-Jiménez J., et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;82(3):378–383. doi: 10.1016/j.jinf.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okba N.M.A., Muller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M., et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease 2019 patients. Emerg Infect Dis. 2020;26(7):1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeling R.W., Wedderburn C.J., Garcia P.J., Boeras D., Fongwen N., Nkengasong J., et al. Serology testing in the COVID-19 pandemic response. Lancet Infect Dis. 2020;20(9):e245–e249. doi: 10.1016/S1473-3099(20)30517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira C., Harris B.H.L., Di Giovannantonio M., Rosadas C., Short C.E., Quinlan R., et al. Antibody response to SARS-CoV-2 infection is not associated with Post-COVID-19 Syndrome in healthcare workers. J Infect Dis. 2021 doi: 10.1093/infdis/jiab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roltgen K., Powell A.E., Wirz O.F., Stevens B.A., Hogan C.A., Najeeb J., et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol. 2020;5(54) doi: 10.1126/sciimmunol.abe0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar E., Kuchipudi S.V., Christensen P.A., Eagar T.N., Yi X., Zhao P., et al. Relationship between anti-spike protein antibody titers and SARS-CoV-2 in vitro virus neutralization in convalescent plasma. bioRxiv. 2020 2020.06.08.138990. [Google Scholar]
- Sasisekharan V., Pentakota N., Jayaraman A., Tharakaraman K., Wogan G.N., Narayanasami U. Orthogonal immunoassays for IgG antibodies to SARS-CoV-2 antigens reveal that immune response lasts beyond 4 mo post illness onset. Proc Natl Acad Sci U S A. 2021;118(5) doi: 10.1073/pnas.2021615118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.G., Hewitson T.D., Nossal G.J., Tarlinton D.M. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur J Immunol. 1996;26(2):444–448. doi: 10.1002/eji.1830260226. [DOI] [PubMed] [Google Scholar]
- Suthar M.S., Zimmerman M.G., Kauffman R.C., Mantus G., Linderman S.L., Hudson W.H., et al. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep Med. 2020;1(3) doi: 10.1016/j.xcrm.2020.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMM . 2020. UMCM. IMMUNITOR. Available from: https://www.umm.de/institut-fuer-klinische-chemie/immunitor-studie/. [Accessed 4 June 2020] [Google Scholar]
- University V. REDCap. Available from: https://redcap.umm.uni-heidelberg.de/redcap/. [Accessed 7 September 2020].
- Wang K., Long Q.X., Deng H.J., Hu J., Gao Q.Z., Zhang G.J., et al. Longitudinal dynamics of the neutralizing antibody response to SARS-CoV-2 infection. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley A.K., Juno J.A., Wang J.J., Selva K.J., Reynaldi A., Tan H.X., et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat Commun. 2021;12(1) doi: 10.1038/s41467-021-21444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2021. WHO health emergency dashboard. Available from: https://covid19.who.int/. [Accessed 11 February 2021] [Google Scholar]
- Wu F., WA, Liu M., Wang Q., Chen J., Xia S., et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. 2020 2020.03.30.20047365. [Google Scholar]
- Yamayoshi S., Yasuhara A., Ito M., Akasaka O., Nakamura M., Nakachi I., et al. Antibody titers against SARS-CoV-2 decline, but do not disappear for several months. EClinicalMedicine. 2021;32 doi: 10.1016/j.eclinm.2021.100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X.-Y., Liu W., Li Z.-Y., Xiong H.-L., Su Y.-Y., Li T.-D., et al. Neutralizing and binding antibody kinetics of COVID-19 patients during hospital and convalescent phases. Nat Med. 2020;26:845–848. [Google Scholar]
- Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020;71(16):2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]