Abstract
Novel mechanistic insights are discussed herein that link a single, nontoxic, low-dose radiotherapy (LDRT) treatment (0.5–1.0 Gy) to (1) beneficial subcellular effects mediated by the activation of nuclear factor erythroid 2-related transcription factor (Nrf2) and to (2) favorable clinical outcomes for COVID-19 pneumonia patients displaying symptoms of acute respiratory distress syndrome (ARDS). We posit that the favorable clinical outcomes following LDRT result from potent Nrf2-mediated antioxidant responses that rebalance the oxidatively skewed redox states of immunological cells, driving them toward anti-inflammatory phenotypes. Activation of Nrf2 by ionizing radiation is highly dose dependent and conforms to the features of a biphasic (hormetic) dose–response. At the cellular and subcellular levels, hormetic doses of <1.0 Gy induce polarization shifts in the predominant population of lung macrophages, from an M1 pro-inflammatory to an M2 anti-inflammatory phenotype. Together, the Nrf2-mediated antioxidant responses and the subsequent shifts to anti-inflammatory phenotypes have the capacity to suppress cytokine storms, resolve inflammation, promote tissue repair, and prevent COVID-19-related mortality. Given these mechanistic considerations—and the historical clinical success of LDRT early in the 20th century—we opine that LDRT should be regarded as safe and effective for use at almost any stage of COVID-19 infection. In theory, however, optimal life-saving potential is thought to occur when LDRT is applied prior to the cytokine storms and before the patients are placed on mechanical oxygen ventilators. The administration of LDRT either as an intervention of last resort or too early in the disease progression may be far less effective in saving the lives of ARDS patients.
Keywords: SARS-Cov 2, LDRT, Nrf2, Hormesis, Cytokine storm, COVID-19
The SARS CoV-2 virus that causes COVID-19 has proven to be problematic to effectively contain, treat, and manage given extant protocols and policies of the international medical and public health communities. The virus’s sudden occurrence, high infectivity, variant morbidity, lethality, potential mutability, and heretofore lack of safe and effective treatments or vaccines, have all contributed to, and exacerbated public-health challenges throughout the world. Although apparently effective vaccines are now available, there is ongoing consideration and concern about their provision and durability, as well as public willingness to accept them. Thus, until such broad distribution and utilization of the vaccine(s) are realized, there remains a continuing requirement for the safe and effective treatment of patients critically infected with—and dying of—COVID-19.
As an initial default strategy, current anti-viral and anti-inflammatory treatments have been identified that may be repurposed for use against COVID-19. One such treatment involves low-dose radiation therapy (LDRT), which was previously used (circa 1930–1950) prior to the “age of antibiotics” in the treatment of critically ill patients with bacterial and/or viral pneumonia [1], [2], [3], [4]. Based on the historically successful clinical use of LDRT, approximately 10 clinical trials are currently underway worldwide to test the safety and effectiveness of LDRT against COVID-19 [5]. Encouraging preliminary results have been reported in several clinical pilot studies [6], [7], [8], [9], [10], [11], [12]. Hess and co-workers have recently shown [7] that LDRT of whole lung with 1.5 Gy was effective in reducing intubation time for COVID-19 patients receiving dexamethasone and/or remdesevir. In an earlier trial of predominantly elderly COVID-19 pneumonia patients, the same LDRT treatment protocol [6] produced quicker recoveries in room air versus ventilatory support, and significant or trending improvements in delirium, radiographs, and (other) biomarkers, without signs of significant acute toxicity. Sharma et al. [8] reported a 90% response rate to single-fraction radiation dose of 0.7 Gy in COVID-19 patients having moderate to severe risk of disease. Similarly, Ameri et al. [9], [10], Moreno-Olmeado et al. [11], and Sanmamed et al. [12] also demonstrated beneficial outcomes in COVID-19 patients treated with LDRT.
Herein, we present a biomedical rationale for the clinical use of LDRT in the treatment of COVID-19 pneumonia and propose that the activation of nuclear factor erythroid 2-related transcription factor (Nrf2) and its mediation of anti-inflammatory effects are the primary mechanisms accounting for the beneficial outcomes of COVID-19 pneumonia patients to LDRT. The literature search strategy employed focused on Nrf2 activation via chemicals and ionizing radiation with application to the treatment of COVID-19. The key words employed involved Nrf2, COVID-19 and a series of chemicals that activate Nrf2 in experimental biological models in conjunction with the term COVID-19. The data bases searched included PubMed, Web of Sciences and Google Scholar. Once a relevant paper was obtained, all references citing that paper were evaluated, aswell as all relevant papers cited in the references.
The role of cytokines in SARS-COV-2 pneumonia and ARDS
When an antigen such as SARS-CoV2 enters the lungs of a healthy, immuno-competent individual, blood-borne monocytes are recruited to the alveoli where they differentiate to M1 macrophages and produce a host of pro-inflammatory cytokines (e.g., IL-1, IL-6, and IL-18). Such cytokines attract neutrophils that generate reactive oxygen species (ROS), phagocytose antigen(s) [13], and, under normal allostatic conditions, clear infection, resolve tissue damage, and facilitate recovery.
If, however, the individual is both immunocompromised (e.g., due to comorbidities, old age, and/or the activity of immunosuppressive agents) and infected with COVID-19, then a different - and much less desirable disease progression and outcome may occur. In such circumstances, COVID-19 may induce severe pulmonary inflammation that can lead to ARDS, respiratory failure, and ultimately death. This inflammatory reaction is characterized by mononuclear cell infiltration, fibrin exudates, proliferation of multinucleated giant cells, and thickening of the alveoli secondary to proliferating interstitial fibroblasts and type II pneumocyte hyperplasia. It is noteworthy that patients suffering advanced pulmonary diseases have been shown to have increased expressions of inflammatory markers, such as ferritin, C-reactive protein, elevated D dimers, and pro-inflammatory cytokines [13], [14], [15]. Such pulmonary hyper-inflammation has also been noted in COVID-19 patients, as well as patients infected with SARS-CoV and MERS-CoV. The so-called macrophage activation syndrome/secondary hemophagocytic lympho-histiocytosis (MAS/sHLH) is characterized by a “cytokine storm” with constituent elevations of interleukin 1 (IL-1), tumor necrosis factor 1 (TNF-1α), and interleukin 6 (IL-6) that are produced by M1 pro-inflammatory macrophages [15] and which may be fatal—even with the best of medical care.
Cytokine storms are primarily driven by the production of IL-1β and require activation of pathogen-associated receptors (including toll-like receptors, TLRs), followed by engagement of inflammasomes in target cells [15]. Specifically, the NLRP3 inflammasome mediates caspase-1 activation, and the secretion of pro-inflammatory cytokines in response to infection and cellular damage. Activated NLRP3 recruits the apoptosis-associated speck-like protein (ASC) and pro-caspase-1 to form the NLRP3-inflammasome assembly, leading to the formation of IL-1β. IL-1β-activated monocytes/macrophages induce the production and release of IL-6 and other cytokines that increase vascular permeability, shrinking of cytosolic F-actin fibers, and collagen deposition, leading ultimately to tissue fibrosis. The persistence of inflammatory neutrophils in the alveoli and the increased concentrations of ROS and TNFα directly contribute to pulmonary injury [16].
During elimination of infection, the normal process entails reversing the macrophage population from predominantly an M1 “pro-inflammatory” to an M2 “anti-inflammatory” phenotype [17]. Although somewhat controversial, evidence indicates that reversal of the macrophage population to an M2 anti-inflammatory phenotype results from newly recruited monocytes being polarized to an M2 phenotype upon their differentiation into macrophages (rather than mature M1 pro-inflammatory macrophages being “re-polarized” to an M2 phenotype) [18]. In ARDS and some autoimmune disorders, the hyper-inflammatory state persists indefinitely, leading to both lung damage and multiple organ system failure.
SARS-CoV-encoded E, ORF3a, and ORF8b proteins can activate the NLRP3-inflammasome to produce a hyper-inflammatory syndrome that can produce increased levels of cytokines, with resultant cardiac and pulmonary involvement [19]. Inflammasome activation by COVID-19 plays a central role in increased levels of IL-1 or IL-6 [20], and appears to be instrumental to the occurrence of ventilator-induced lung injury [21]. Pharmacological treatments for SARS-CoV-2-induced MAS/sHLH syndrome are similar to those approved for autoimmune diseases (e.g., rheumatoid arthritis), and include administration of IL-6/IL-1 blockers and anti-inflammatory corticosteroids.
When seeking to identify pharmacological targets for the future development of treatments against SARS-CoV-2 pneumonia, preventing early activation of the NLRP3 inflammasome may prove to be a more effective strategy than directly suppressing the subsequent production of various cytokines (a notable example is the inhibition of NLRP3 by a small-molecule drug and the protection it affords against infection by influenza A virus [22], [23]).
Nrf2 activation via low-dose radiotherapy in SARS-CoV-2 pneumonia and ARDS
As previously noted, prior to the development and widespread use of antibiotics, LDRT was used as an effective intervention for numerous types of inflammatory and infectious diseases [2], [24], [25], [26], [27], [28]. Single LDRT administration ranging from 0.2 to 1.0 Gy proved to be highly effective in producing rapid and enduring clinical improvements in patients with either viral or bacterial pneumonia. Putative patterns and mechanisms related to these LDRT-induced beneficial outcomes have been investigated in animal studies. In a mouse model, LDRT proved highly effective against pneumonia caused by interstitial swine influenza [17]. However, after a relatively short time, and before a thorough understanding of mechanisms and effects could be achieved, LDRT was generally abandoned following WWII, when equally effective antibiotic and anti-inflammatory pharmacotherapeutics were becoming readily available, and when the “atomic-age” was increasing awareness and heightening concerns about radiation-induced cancer risks [29].
The documented historical success of LDR in the treatment of pulmonary infections reflects its effectiveness in modulating the immune system and stimulating an integrated, anti-inflammatory response (i.e. sufficiently robust and durable to mitigate or prevent the life-threatening effects of a “cytokine storm”). Recent evidence suggests that this anti-inflammatory response originates at the cellular level, with the polarization of macrophages toward an anti-inflammatory M2 phenotype [30]. At the molecular level, however, LDRT-induced polarization of M2 macrophages is thought to involve cytoplasmic activation of transcription factor Nrf2, its translocation to the nucleus, and its initiation of a cascade of antioxidant responses and enzymatic detoxification processes that suppress inflammation in the lungs as well as in other organs.
Nrf2 is a transcription factor of emerging interest that is encoded by the NFE2L2 gene and is a member of cap “n” collar (CNC) subfamily of basic region leucine zipper (bZip) transcription factors. Nrf2 has been called the “guardian of healthspan”, the “master regulator of cellular redox homeostasis”, and the “gatekeeper of species longevity” as it regulates redox balance, inflammation, and proteostasis. Under normal conditions, cellular Nrf2 levels are regulated in the cytoplasm by Kelch-like ECH-associated protein1 (Keap1), a redox-sensitive E3 ubiquitin ligase substrate adaptor that binds Nrf2, and regulates its constitutive degradation by proteosomes. Keap1 is therefore appropriately considered the principal negative regulator of Nrf2. In the presence of any exogenous or endogenous cell stressors, such as ionizing radiation or inflammatory ROS, the oxidative potential of the cell increases, Keap1 is oxidized, and the Keap1-mediated degradation of Nrf2 is halted, thereby liberating Nrf2 and allowing it to accumulate and translocate to the nucleus. In the nucleus, Nrf2 forms heterodimer complexes with transcription factors, such as small Maf proteins (G/F/K) and c-Jun, after which the Nrf2 complex binds to the antioxidant response element (ARE), a regulatory enhancer region within gene promoters. The binding of Nrf2 to ARE up-regulates cellular antioxidant and anti-inflammatory defense mechanisms by controlling the expression of more than 200 genes, including those that encode for proteins involved in metabolic balance, detoxification, redox homeostasis, and repair of macromolecular (eg., DNA and protein) damage [31], [32].
To be sure, Nrf2 triggers a prompt response against metabolic, oxidative, and inflammatory stressors. Targeting Nrf2 is therefore important to the treatment of diseases characterized by oxidative stress and inflammation, such as COVID-19-induced pneumonia and ARDS [33], [34], [35], [36]. A recent study of lung biopsies obtained from COVID-19 patients revealed that the virus mediates suppression of Nrf2 and limits the anti-inflammatory response of the host [37]. Therefore, the capacity for LDRT to actívate Nrf2 and mediate a potent anti-inflammatory response offers a viable opportunity to re-establish a safe and successful treatment for ARDS, and to develop and improve its clinical use and efficacy in the treatment of patients with COVID-19 and other diseases of inflammatory origin.
Nrf2 activation and its downstream mechanisms interact with other transcription factors (either positively or negatively) to modulate cellular adaptations associated with various antioxidant, cytoprotective, and anti-inflammatory responses. It has been reported that Nrf2 may regulate inflammation through two discrete mechanisms: (1) redox-dependent, and (2) redox-independent. The redox dependent pathway is thought to up-regulate cellular antioxidant defenses via ARE-driven transcription (suppression of ROS in antigen presenting cells, MAP kinase activation, NF-kB signaling, and epigenetic histone deacetylase activity) and, in the process, to polarize macrophages toward the anti-inflammatory M2 phenotype by cross-talk with NF-κB, mitogen-activated protein kinases (MAPKs), peroxisome proliferator-activated receptor γ (PPARγ), and autophagy. Conversely, the redox independent pathway is thought to involve the direct suppressive action of Nrf2 on the genes of pro-inflammatory cytokines, preventing their transcriptional upregulation [38], [39], [40].
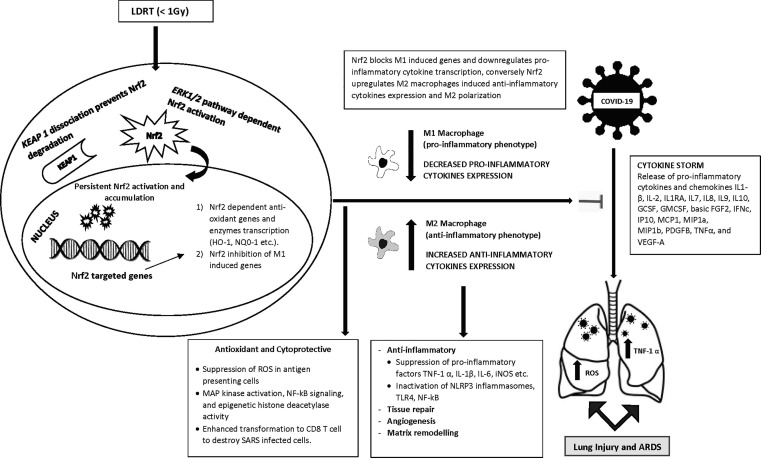
Wunderlich and co-workers [41] showed that LDRT reduced levels of pro-inflammatory cytokines TNF-α and IL-1β via the suppression of NF-kB in lipopolysaccharide (LPS)-activated macrophages. Other studies have shown that the production of the pro-inflammatory factors TNF-1α and IL-1β (as well as IL-6) by M1 macrophages was also significantly reduced following LDRT [30], [42], [43], [44], [45], [46]. Fig. 1 illustrates the activation of Nrf2 via LDRT and its subsequent effect on COVID-19-induced pneumopathy and ARDS.
Fig. 1.
Nrf2 activation by LDRT affecting COVID-19 induced pneumopathy and ARDS.
As well, Nrf2 activation has been shown to significantly suppress ROS in antigen-presenting dendritic cells and, as a result, enhanced their capacity to interact with and promote the transformation of naïve CD8 T cells into cytotoxic T lymphocytes (CTL); which, upon transformation, were then functionally equipped to destroy virally infected cells. In this case, the Nrf2 suppression of oxidative stress shifted the redox balance and elicited a series of molecular responses (including MAP kinase activation, NF-kB signaling, and epigenetic histone deacetylase activity) that enabled the dendritic cells to facilitate the CTL transformation [47]. The reported suppression of Nrf2 activity by COVID-19 [37] would likely enhance oxidative stress and reduce the CTL populations directed against COVID-19 antigens, exacerbating the infection.
A final example describes the coordinated interaction of Nrf2 with the transcription factor NF-E2 p45 and illustrates its role in regulating the maturation of megakaryocytes and the formation of blood platelets. As platelets form during the normal maturation of megakaryocytes, Nrf2, and NF-E2 p45, a regulator of megakaryopoiesis, compete for part of the same Nrf2 binding site on DNA encoding for stress-responsive antioxidant genes. During early stages of maturation, Nrf2 out-competes p45 and generates a less oxidative redox state that favors early stage maturation. However, during later stages of maturation, p45 out-competes Nrf2 and blocks expression of Nrf2 antioxidant genes, generating a more oxidative redox state that favors late-stage maturation and the formation of normal platelets [48]. Given that COVID-19 suppresses the Nrf2 pathway [37], the coordinated competitive balance between Nrf2 and p45 may be easily disrupted, resulting in a chronic highly oxidative state with potential to adversely affect megakaryopoiesis and normal platelet development, leading to possible microthromboses—as often reported to occur in COVID-19 pneumonia patients [49].
In light of the critical roles of oxidative stress and pro-inflammatory M1 macrophages to mediate “cytokine storms”, and of the countervailing potential of LDRT to activate Nrf2 and polarize macrophages to anti-inflammatory M2 phenotypes, we opine that LDRT is of value as a therapeutic intervention against SARS-Co-V2-induced pneumonia and ARDS.
Low doses of ionizing radiation typically induce M2 anti-inflammatory phenotypes in macrophages, while higher doses tend to induce M1 pro-inflammatory phenotypes [50]. In an extensive meta-analysis by Genard et al. [51], fifteen (15) studies of radiation-induced polarization of macrophages were reviewed that utilized a variety of biological models and radiation exposures, ranging from 0.01 to 60.00 Gy. It was found that macrophages are essentially polarized toward the M2 anti-inflammatory state at doses below 1.0 Gy, and toward the M1 proinflammatory state at doses above 1.0 Gy.
Recent publications demonstrated that radiation-induced polarization of macrophages into M2 and M1 phenotypes at lower (<1.0 Gy) and higher (>1.0 Gy) doses, respectively, are biphasic and hormetic [27], [30]. Further support for the induction of anti-inflammatory M2 phenotypes in macrophages by low dose radiation is provided by a study using an in vitro co-culture model of rheumatoid arthritis, wherein single low doses (0.5 Gy) of radiation reversed the imbalance in polarized macrophages from an M1 pro-inflammatory to an M2 anti-inflammatory phenotype in the presence of fibroblast-like synoviocytes [52]. Similarly, but not surprisingly, the activation of Nrf2 by ionizing radiation also displayed a typical hormetic-like biphasic dose response [53], [54], further implicating a role for Nrf2 in the biphasic mediation of M1 and M2 polarization responses in macrophages [27], [30].
Nrf2 activation protects against COVID-19 pneumonia, ARDS and other inflammatory diseases
Other agents are also known to activate Nrf2 and mediate a cascade of antioxidant and anti-inflammatory responses that are protective to multiple organs (including extrapulmonary sites, e.g., the heart), and conditions (e.g., diabetes) that are involved in the systemic effects of COVID-19. Such antioxidant/anti-inflammatory responses promote therapeutic benefit by mitigating predisposing pro-inflammatory conditions that have been shown to contribute to and/or exacerbate a number of chronic diseases (e.g., cancer, diabetes, heart disease, and arthritis). For example, it was shown that activating Nrf2 pathways that block NLRP3 inflammasomes and polarize macrophages to M2 anti-inflammatory phenotypes also mitigate myocardial ischemic-reperfusion injury via the downregulation of TXNIP and the inactivation of various proinflammatory factors, such as NLRP3 inflammasomes, TLR4, and NF-kB [55]. Another example is the activation of Nrf2 by the glucoregulatory (anti-diabetic) agent, metformin. Metformin-activated Nrf2 mediates both the AMPK/mTOR signaling pathways that prevent formation of NLRP3 inflammasomes and polarize macrophages to M2 anti-inflammatory phenotypes, resulting in the acceleration of wound healing [56]. These findings are similar to those of a number of other studies showing chemoprotective agents (experimentally conducted in standard and pre-conditioning protocols) that act in multiple organs to ameliorate a spectrum of diseases with predisposing and underlying inflammatory etiologies [21], [42], [57], [58], [59]. An assessment of the molecular cross-talk between Nrf2 and NF-kB (a key transcription factor involved in the upregulation of inflammatory responses) established that Nrf2 downregulates inflammasomes and suppresses their influence in numerous disease models, inclusive of inflammatory respiratory conditions [41], [60].
The absence of Nrf2 resulted in the loss of both its downstream antioxidant responses and hepatoprotection in models of remote ischemic conditioning (RIC) against hemorrhagic shock [58]. This report concluded that the protective effects of RIC are a manifestation of hormesis, whereby mild (i.e.- subtoxic) ischemic reperfusion generates a low (subtoxic) dose of ROS (e.g., in one limb of an animal) to produce a systemic whole-body response that blocks a subsequent lethal (toxic) application of ischemic reperfusion in a remote vital organ [61], [62], [63], [64]. Such findings strongly suggest a central role for Nrf2 activation as part of an adaptive low-dose (i.e., hormetic) strategy to prevent and/or treat inflammatory diseases, or to protect against the deleterious effects of toxic exposures to particular chemical and physical agents [65].
In pulmonary SARS-CoV-2 infections, LDRT may act similarly to exert anti-inflammatory effects via engagement of Nrf2 and its mediation of antioxidant responses. When taken together, such antioxidant responses may alter redox status and reverse the sustained elevated imbalance of M1 pro-inflammatory macrophages in COVID-19 to favor the M2 anti-inflammatory phenotype, thereby reducing (1) the inflammatory response, (2) likelihood of cytokine storm, and (3) lethality.
We believe that not only the most critically ill, but most SARS-CoV-2 pneumonia patients can potentially benefit from LDRT at almost any time during the disease process. The literature indicates high general success rates of LDRT at administered doses between 0.5 and 1.0 Gy [2], [24], [27]. LDRT is most likely to be effective when cells are relatively healthy and possess sufficient energy to sustain interactive, complementary, and overlapping adaptive (hormetic) responses that synergistically promote a Nfr2-mediated anti-inflammatory state. Early activation of Nrf2—prior to occurrence of a cytokine storm—may prove most effective in preventing ARDS and saving lives. However, later activation of Nrf2—upon initiation of and/or during a cytokine storm—may be much less effective. With progression of the disease, the protective, metabolic, and bioenergetic capacities required to ward off virus, maintain vital functions, and repair damage become compromised, weakened, and eventually insufficient to sustain life—the accumulation of oxidative, inflammatory, and viral damage simply becomes too great to overcome. At some time during this progression, a point of diminishing returns will likely be reached with regard to the expected clinical effectiveness of LDRT, for example, when COVID-19 patients become hypoxic and are placed on mechanical ventilators to receive oxygen therapy and facilitate breathing.
At such a point of clinical intervention during disease progression, the alveolar epithelium and capillary endothelium have likely suffered severe viral-induced damage and, as a result, the diffusion of oxygen into blood has been slowed to a rate that no longer can sustain life. Administration of oxygen therapy to the patient at higher-than-ambient partial pressures (and up to 1 atm) elevates blood oxygen, enables cells to respire, and offers an immediate survival benefit to the patient. Sustained oxygen therapy (usually >24 h and at >0.6 atm), however, is known to induce oxidative stress (ROS) and hyperinflammation, culminating in oxygen toxicity and a host of untoward responses—such as edema, microthrombi, and fibrosis—that mimic responses associated with acute lung injury and ARDS [66]. Similar to oxygen-induced lung toxicity, COVID-19-induced ARDS also results from sustained oxidative stress (ROS) and hyperinflammation (cytokine storm). Since oxygen therapy produces a pathological profile similar to that of COVID-19, the therapeutic purpose of such oxygen therapy is obviously not to treat the underlying root causes of ARDS, but rather to manage an hypoxic crisis by providing bioenergetic support and preventing imminent death. Clearly, there is a tradeoff between short-term benefits (immediate survival) and possibly exacerbating longer-term pathological consequences of COVID-19 (e.g., pulmonary fibrosis), assuming, of course, that patients can survive the potentially exacerbating effects of oxygen therapy.
Hence, we argue that the best COVID-19 treatment would entail neutralizing the virally induced oxidative and inflammatory conditions with an antioxidative and anti-inflammatory therapy, and that LDRT is such a tool. To apply LDRT as a treatment of last resort to patients undergoing mechanical ventilation with oxygen, however, could jeopardize the likelihood of success (patient survival). Instead, it would be preferable to administer LDRT when the lungs are aberrantly inflamed but before the cytokine storm, and certainly before mechanical ventilation with oxygen. As oxygen therapy only increases the oxidative burden on the lungs and thus neutralizes the antioxidant effects of LDRT, it only undermines the very rationale for administering LDRT in the first place. On the other hand, administering LDRT too early in the course of a viral infection may also be counterproductive. If pro-inflammatory M1 states are required—and indeed normal—for early and effective immune responses against infective agents, then their inhibition by LDRT-induced antioxidant and anti-inflammatory responses may be unwise and ill-advised. To summarize, timing is likely to be important—if not essential—and administering LDRT at a time during the course of the disease that is either (1) too late (i.e., when patients are receiving oxygen therapy that tends to exacerbate already inflamed lungs) or (2) too early (i.e., when a pro-inflammatory state is necessary to kill viruses) may be self-defeating and likely to yield less-than-favorable outcomes, resulting in fewer lives saved.
The findings presented support recent demonstrations of positive clinical responses to LDRT in COVID-19 pneumonia patients [6], [7], [8], [9], [10], [11], [12]. However, animal studies also provide support for the effectiveness of Nrf2 in protecting various tissues from pro-inflammatory challenges. Nrf2-deficient mice displayed more extensive pulmonary inflammation and significantly enhanced susceptibility to ARDS compared to wild type mice [67] when both groups of mice were administered non-lethal, pro-inflammatory doses of lipopolysaccharide (LPS). LPS-treated, Nrf2-deficient mice had higher levels of inflammatory biomarkers as compared to controls. Other studies employing rat [68] and rabbit models [69] indicated that Nrf2-mediated and sulforaphane-induced mechanisms protected against acute oxidative lung injury. Such results indicate that Nrf2 deficiency enhances susceptibility to oxidative lung damage from ARDS, and that administration of Nrf2-activating agents prevents the occurrence of ARDS and ARDS-related oxidative damage. Interestingly, Nrf2 gene polymorphisms were found to enhance susceptibility to acute lung injury in humans [70]. Additionally, some Nrf2-activating agents, such as sulforaphane, have been shown to suppress the growth of respiratory influenza viruses [71], and other, structurally diverse chemical agents have been shown to activate Nrf2 and thus may also have the potential to yield clinical benefits for COVID-19 patients (Table 1 ).
Table 1.
Nrf2 Activating Agents Reported to Offer Clinical Benefits in COVID-19 Patients.
| No | Nrf2 Activating Agent Name | Reference |
|---|---|---|
| 1. | Dimethyl fumarate | [72] |
| 2. | Ozone-oxygen gas mixtures | [73], [74] |
| 3. | Methylene blue | [75], [76] |
| 4. | Low-level laser/photobiomodulation | [77], [78] |
| 5. | Azithromycin | [79], [80] |
| 6. | Chloroquine | [80], [81] |
| 7. | Dexamethasone | [14], [82] |
| 8. | Naringenin | [83], [84] |
| 9. | Quercitin | [85], [86] |
| 10. | Sulforaphane | https://www.dundee.ac.uk/stories/clinical-trial-potential-covid-19-treatment; [32] |
| 11. | Sulforadex (SFX-01) | [32] |
| 12. | Bardoxolone methyl | [32] |
| 13. | Omaveloxolone (RTA-408) | [32] |
Given that many Nrf2-activating agents, such as sulforaphane [82], are safe and commercially available, it may be that combinatory treatments could complement both the pharmacodynamic and pharmacokinetic strengths and limits of each specific agent. Although this approach has yet to be considered in the experimental and clinical literature with respect to Nrf2-activating agents, a sound mechanistic framework suggests that this approach may be worth further investigation. For example, since damage to the lungs, heart, and kidneys are major concerns in most cases of COVID-19, the optimal targeting of multiple organs with multiple Nrf2 pathways may require a cocktail of diverse Nrf2 activators. In theory, such a cocktail would be composed of a variety of chemical Nrf2 activators that had been preselected based on their differing but complementary pharmacokinetic and pharmacodynamic profiles, as well as on their differing but complementary (and/or synergistic) Nrf2 pathway profiles. When Nrf2 activators are administered in combination as a cocktail, treatment outcomes should then improve as a result of being able to induce optimal Nrf2 responses in multiple organs of the body.
Although the focus of this paper is on the treatment of COVID-19 with LDRT, it is clear that a variety of chemical and physical agents can activate Nrf2 and mediate a multitude of potent antioxidant and anti-inflammatory responses. Regardless of the Nrf2-activating agent, however, we consider – and herein purpose - Nrf2 activation per se to be a promising potential (and potent) therapeutic target. Upon its activation with low hormetic doses of virtually any activating agent, such as LDRT, Nrf2 may advance the treatment of many diverse and serious diseases with pro-inflammatory etiologies, including COVID-19 pneumonia as well as other types of viral and/or bacterial pneumonias.
Conclusion
The demonstrated clinical success of LDRT in saving lives of patients with COVID-19-induced pneumonia and ARDS is closely related to two sequentially and causally linked physiologic responses. First, Nrf2-mediated antioxidant responses are activated by LDRT to change cellular redox states from predominantly pro-oxidant to antioxidant. Second, in such an antioxidant environment, the dominant balance of macrophage phenotypes shifts from M1 pro-inflammatory to M2 anti-inflammatory, thereby reducing the likelihood of cytokine storms, ARDS, and lethality. We argue that such beneficial outcomes cannot – and should not - be overlooked and that further consideration of the clinical utility and value of the timely administration of LDRT against COVID-19—and possibly other inflammatory conditions—is warranted and recommended.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
EJC acknowledges long time support from the US Air Force (AFOSR FA9550-19-1-0413) and ExxonMobil Foundation (S18200000000256). JG’s work was supported in part by funding from the Henry Jackson Foundation for Military Medicine; Leadership Initiatives; Brain NeuroBio International; NeuroGen Corporation; Coburg University Distinguished Visiting Professorship in Integrative Health Promotions; and federal funds 2UL1TR001409-06 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through the Clinical and Translational Science Awards (CTSA) Program, a trademark of the US Department of Health and Human Services, part of the Roadmap Initiative, “Re-Engineering the Clinical Research Enterprise.” The U.S. Government is authorized to reproduce and distribute for governmental purposes notwithstanding any copyright notation thereon. The views and conclusions contained herein are those of the author and should not be interpreted as necessarily representing policies or endorsement, either expressed or implied. Sponsors had no involvement in study design, collection, analysis, interpretation, writing and decision to and where to submit for publication consideration.
References
- 1.Calabrese E.J. X-ray treatment of carbuncles and furuncles (boils): a historical assessment. Hum Exp Toxicol. 2013;32:817–827. doi: 10.1177/0960327112467046. [DOI] [PubMed] [Google Scholar]
- 2.Calabrese E.J., Dhawan G. How radiotherapy was historically used to treat pneumonia: Could it be useful today? Yale J Biol Med. 2013;86:555–570. [PMC free article] [PubMed] [Google Scholar]
- 3.Dhawan G., Kapoor R., Dhawan R., Dhawan G., Kapoor R., Calabrese E.J., et al. Low dose radiation therapy as a potential life saving treatment for COVID-19-induced acute respiratory distress syndrome (ARDS) Radiother Onocol. 2020;147:212–216. doi: 10.1016/j.radonc.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanekamp Y.N., Giordano J., Hanekamp J.C., Khan M.K., Limper M., Venema C.S., et al. Immunomodulation through low-dose radiation for severe COVID-9: lessons from the past and new developments. Dose Response. 2020;18 doi: 10.1177/1559325820956800. 1559325820956800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koosha F., Pourbagheri-Sigaroodi A., Bakhshandeh M., Davood B.D. Low-dose radiotherapy (LDRT) for COVID-19-induced pneumopathy: a worth considering approach. Internat J Rad Biol. 2021;97:302–312. doi: 10.1080/09553002.2021.1864049. [DOI] [PubMed] [Google Scholar]
- 6.Hess C.B., Buchwald Z.S., Stokes W., Nasti T.H., Switchenko J.M., Weinberg B.D., et al. Low-dose whole-ling radiation of COVID 19 pneumonia: planned Day 7 interim analysis of a registered clinical trial. Cancer. 2020;126:5109–5113. doi: 10.1002/cncr.33130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hess C.B., Nasti T.H., Dhere V.R., Kleber T.J., Switchenko J.M., Buchwald Z.S., et al. Immunomodulatory low-dose whole-lung radiation for patients with coronavirus disease 2019-related pneumonia. Int J Rad Oncol Biol Phys. 2021;109:867–879. doi: 10.1016/j.ijrobp.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma D.N., Guleria R., Wig N., Mohan A., Rath G.K., Subramani V., et al. Low dose radiation therapy for COVID-19 pneumonia: a pilot study. medRxiv. 2020 doi: 10.1101/2020.11.16.20231514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ameri A., Rahnama N., Bozorgmehr R., Mokhtari M., Farahbakhsh M., Nabavi M., et al. Low-dose whole-lung irradiation for COVID-19 pneumonia: short course results. Int J Rad Oncol Biol Phys. 2020;108:1134–1139. doi: 10.1016/i.jrobp.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ameri A., Ameri P., Rahnama N., Mokhtari M., Sedaghat M., Hadavand F., et al. Low-dose whole-lung irradiation for COVID-19 pneumonia: What is the optimal dose? Final results of a pilot study. Int J Rad Oncol Biol Phys. 2020;108:1134–1139. doi: 10.20944/preprints202009.0229.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreno-Olmedo E, Suárez-Gironzini V, Perez M. Early results of COVID-19 pneumonia cases treated with ultra-low doses of radiotherapy (ULTRA-COVID study) 2020; doi: 10.21203/rs.3.rs-51600/v1. [DOI] [PMC free article] [PubMed]
- 12.Sanmamed N., Alcantara P., Cerezo E., Gaztañaga M., Cabello N., Gómez S., et al. Low dose radiotherapy in the management of covid19 pneumonia (LOWRAD-Cov19). Preliminary report. Int J Rad Oncol Biol Phys. 2020;109:880–885. doi: 10.1016/j.ijrobp.2020.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdin S.M., Elgendy S.M., Alyammahi S.K., Alhamad D. Tackling the cytokine storm in COVID-19, challenges and hopes. Life Sci. 2020;257 doi: 10.1016/j.lfs.2020.118054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;102537 doi: 10.1016/j.autrev.2020.102537. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelley N., Jeltema D., Duan Y., He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aggarwal N.R., King L.S., D’Alessio F.R. Diverse macrophages populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol. 2014;306:L709–L725. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van den Bossche J., Baardman J., Otto N., der Velden S., Neele E., van den Berg S., et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Reports. 2016;17:684–696. doi: 10.1016/j.celrep.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Shi C.S., Nabar N.R., Huang N.N. SARS-Coronavirus open read reading frame-8b trigger intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019;5:101. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spel L., Martinon F. Inflammasomes contributing to inflammation in arthritis. Rev Immunol Rev. 2020;294:48–62. doi: 10.1111/imr.12839. [DOI] [PubMed] [Google Scholar]
- 21.Liu X.F., Zhou D.D., Xie T., Hao J.L., Malik T.H., Lu C.B., et al. The Nrf2 signaling in retinal ganglion cells under oxidative stress in ocular neurodegenerative diseases. Inter J Biol Sci. 2020;14:1090–1098. doi: 10.7150/ijbs.25996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coates B.M., Staricha K.L., Ravindran N., Koch C.M., Cheng Y., Davis J.M., et al. Inhibition of the NOD-like receptor protein 3 inflammasome is protective in juvenile influenza A virus infection. Front Immunol. 2017;8:782. doi: 10.3389/fimmu.2017.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tate M.D., Ong J.D., Dowling J.K., McAuley J.L., Robertson A.B., Latz E., et al. Reassessing the role of the NLRP3 inflammasome during pathogenic influenza A virus infection via temporal inhibition. Sci Reports. 2016;6:27912. doi: 10.1038/srep27912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calabrese E.J., Dhawan G. The historical use radiotherapy in the treatment of sinus infection. Dose-Response. 2013;11:484–494. doi: 10.2203/dose-response.13-004.Calabrese. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calabrese E.J., Dhawan G., Kapoor R. Use of X-rays to treat shoulder tendinitis/bursitis: a historical assessment. Arch Toxicol. 2014;88:1503–1517. doi: 10.1007/s00204-014-1295-6. [DOI] [PubMed] [Google Scholar]
- 26.Calabrese E.J., Dhawan G., Kapoor R. The use of X-rays in the treatment of bronchial asthma: a historical assessment. Rad Res. 2015;184:180–192. doi: 10.1667/rr14080.1. [DOI] [PubMed] [Google Scholar]
- 27.Calabrese E.J., Dhawan G., Kapoor K., Kozumbo W.J. Radiotherapy treatment of human inflammatory diseases and conditions: optimal Dose. Hum Exp Toxicol. 2019;38:888–898. doi: 10.1177/0960327119846925. [DOI] [PubMed] [Google Scholar]
- 28.Dhawan G., Kapoor R., Dhamija A., Singh R., Monga B., Calabrese E.J. Necrotizing fasciitis: low dose radiotherapy as a potential adjunct treatment. Dose-Response. 2019:1–6. doi: 10.1177/1559325819871757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calabrese E.J. The linear So-threshold (LNT) dose response model: a comprehensive assessment of its historical and scientific foundations. Chem-Biol Inter. 2019;301:6–25. doi: 10.1016/j.cbi.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Calabrese E.J., Giordano J.J., Kozumbo W.J., Leak R.K., Bhatia T.N. Hormesis mediates dose-sensitive shifts n macrophage activation patterns. Pharm Res. 2018;137:236–249. doi: 10.1016/j.phrs.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Petri S., Körner S., Kiaei M. Nrf2/ARE signaling pathway: key mediator in oxidative stress and potential therapeutic target in ALS. Neurology Research International. 2012;2012 doi: 10.1155/2012/878030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuadrado A., Pajares M., Benito C., Jimenez-Villegas J., Escoll M., Fernandez-Gines R., et al. Can activation of NRF2 be a strategy against COVID-19? Trends Pharmacol Sci. 2020;41:598–610. doi: 10.1016/j.tips.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCord J.M., Hybertson B.M., Cota-Gomez A., Geraci K.P., Gao B. Nrf2 Activator PB125® as a potential therapeutic agent against COVID-19. Antioxidants. 2020;9:518. doi: 10.3390/antiox9060518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robledinos-Antón N., Fernández-Ginés R., Manda G., Cuadrado A. Activators and inhibitors of NRF2: A review of their potential for clinical development. Oxid Med Cell Long. 2019;2019:9372182. doi: 10.1155/2019/9372182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidlin C.J., Dodson M.B., Madhavan L., Zhang D.D. Redox regulation by NRF2 in aging and disease. Free Rad Biol Med. 2019;134:702–707. doi: 10.1016/j.freeradbiomed.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis K.N., Mele J., Hayes J.D., Buffenstein R. Nrf2, a guardian of healthspan and gatekeeper of species longevity. Integ Comp Biol. 2010;50:829–843. doi: 10.1093/icb/icq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olagnier D., Farahani E., Thyrsted J., Blay-Cadanet J., Herengt A., Idorn M., et al. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat Comm. 2020;11:4938. doi: 10.1038/s41467-020-18764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobayashi E.H., Suzuki T., Funayama R., Nagashima T., Hayashi M., Sekine H., et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016;7:11624. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu H., Jia Z., Li Y.R. Nrf2 signaling in macrophages. React Oxyg Species (Apex) 2016;2:417–420. doi: 10.20455/ros.2016.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang YR, Zhang XN, Meng FG, Zeng T. Targeting macrophage polarization by Nrf2 agonists for treating various xenobiotics-induced toxic responses. Toxicol Mech Methods. 2021 Mar 7:1-9. doi: 10.1080/15376516.2021.1894624. Epub ahead of print. PMID: 33627030. [DOI] [PubMed]
- 41.Wunderlich R., Ernst A., Rodel F., Fietkau R., Ott O., Lauber K., et al. Low and moderate doses of ionizing radiation up to 2 Gy modulate transmigration and chemotaxis of activated macrophage, provoke an anti-inflammatory cytokines milieu, but do not impact upon viability and phagocytic function. Clin Exp Immunol. 2015;179:50–61. doi: 10.1111/cei.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J., Chen W., Zhang Y., Wang Y., Wang Y., Zhou W., et al. Zinc, sulphate preconditioning alleviates rat myocardial hypoxia reoxygenation injury in vitro: activation of the Nrf2/ARE signaling pathway. Intern J Clin Med. 2017;10:8004–8010. [Google Scholar]
- 43.Nakatsukasa H., Tsukimoto M., Tokunaga A., Kojima S. Repeated gamma irradiation attenuates collagen-induced arthritis via upregulation of regulatory T cells but not by damaging lymphocytes directly. Rad Res. 2010;174:313–324. doi: 10.1667/RR2121.1. [DOI] [PubMed] [Google Scholar]
- 44.Hildebrandt G., Loppnow G., Jahns J., Hindemith M., Anderegg U., Saalbach A., et al. Inhibition of the iNOS pathway in inflammatory macrophages by low-dose X-irradiation in vitro. Is there a time dependence? Strahlenther Onkol. 2003;179:158–166. doi: 10.1007/s00066-003-1044-x. [DOI] [PubMed] [Google Scholar]
- 45.Frey B., Hehlgans S., Rodel F., Gaipi U.S. Modulation of inflammation by low and high doses of ionizing radiation: implications for benign and malignant diseases. Cancer Lett. 2015;368:230–237. doi: 10.1016/j.canlet.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Calabrese E.J., Calabrese V. Reduction of arthritic symptoms by low dose radiation therapy (LD-RT) is associated with an anti-inflammatory phenotype. Int J Rad Biol. 2013;89:278–286. doi: 10.3109/09553002.2013.752594. [DOI] [PubMed] [Google Scholar]
- 47.Yeang H.X.A., Hamdam J.M., Al-Huseini L.M.A., Sethu S., Djouhri L., Walsh J., et al. Loss of transcription factor nuclear factor-erythroid 2 (NF-E2) p45-related factor-2 (Nrf2) leads to dysregulation of immune functions, redox homeostasis, and intracellular signaling in dendritic cells. JBC. 2012;287:10556–10564. doi: 10.1074/jbc.M111.322420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Motohashi H., Kimura M., Fujita R., Inoue A., Pan X., Takayama M., et al. NF-E2 domination over Nrf2 promotes ROS accumulation and megakaryocytic maturation. Blood. 2010;115:677–686. doi: 10.1182/blood-2009-05-223107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. NEJM. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Q., Allouch A., Martins I., Modjtahedi N., Deutsch E., Perfettini J.-L. Macrophage biology plays a central role during ionizing radiation-elicited tumor response. Biomed J. 2017;40:200–211. doi: 10.1016/j.bj.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Genard G., Lucas S., Michiels C. Reprogramming of tumor-associated macrophages with anticancer therapies: radiotherapy versus chemo- and immunotherapies. Front Immun. 2017;8:828. doi: 10.3389/fimmu.2017.00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X., Gong Y., Li D., Xiang L., Ou Y.H., Jiang L., et al. Low-dose radiation therapy promotes radiation pneumonitis by activating NLRP3 inflammasome. Int J Rad Oncol Biol Phys. 2020;107:804–814. doi: 10.1016/j.ijrobp.2020.02.643. [DOI] [PubMed] [Google Scholar]
- 53.Lee E.K., Kim J.A., Park S.J., Kim J.K., Heo K., Yang K.M., et al. Low-dose radiation activates Nrf1/2 through reactive species and the Ca2+/ERK1/2 signaling pathway in human skin fibroblast cells. BMB Reports. 2013;46:258–263. doi: 10.5483/BMBRep.2013.46.5.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen W., Jiang T., Wang H., Tao S., Lau S., Fang D., et al. Does Nrf2 contribute to p53-mediated control of cell survival and death? Antiox Redox Signal. 2012;17:1670–1675. doi: 10.1089/ars.2012.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dai Y., Wang S., Chang S., Ren D.Y., Shali S., Li C.G., et al. M2 Macrophage-derived exosomes carry microRNA-148a to alleviate myocardial ischemia/reperfusion injury via inhibiting TXNIP and the TLR4/NF-κ B/NLRP3 inflammasome signaling pathway. Mol Cell Cardiol. 2020;142:65–79. doi: 10.1016/j.yjmcc.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 56.Quing L., Fu J., Wu P., Zhou Z., Yu F., Tang J. Metformin induces the M2 macrophage polarization to accelerate the wound healing via regulating AMPK/mTOR/NLRP3 inflammasome singling pathway. Am J Transl Res. 2019;11:655–668. [PMC free article] [PubMed] [Google Scholar]
- 57.Garrido-Pascual P., Alonso-Varona A., Castro B., Buron M., Palomares T. H202-preconditioned human adipose-derived stem cells (HCO16) increase their resistance to oxidative stress by overexpressing Nrf2 and bioenergetic adaptation. Stem Cell Res Ther. 2020;11:335. doi: 10.1186/s13287-020-01851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leung C.H., Caldarone C.A., Guan R., Wen X.Y., Ailenberg M., Kapus A., et al. Nuclear factor (erythroid-derived 2)-like 2 regulates the hepatoprotective effects of remote ischemic conditioning in hemorrhagic shock. Antioxid Redox Signal. 2019;30:1760–1773. doi: 10.1089/ars.2018.7541. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Z.X., Jizhang Y., Xu X., Kwiechien T.D., Li N., Zhang Y., et al. Protective effects of remote ischemic conditioning against ischemia/reperfusion-induced retinal injury in rats. Vis Neurosci. 2014;31:245–252. doi: 10.1017/S0952523814000121. [DOI] [PubMed] [Google Scholar]
- 60.Hennig P., Garstkiewicz M., Grossi S., Di Filippo M., French L.E., Beer H.D. The crosstalk between Nrf2 and inflammasomes. Int J Mol Sci. 2018;19:562. doi: 10.3390/ijms19020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calabrese E.J. Why it is important to toxicology and toxicologists. Environ Toxicol Chem. 2008;27:1451–1474. doi: 10.1897/07-541. [DOI] [PubMed] [Google Scholar]
- 62.Calabrese E.J. Preconditioning is hormesis. Part I: Documentation, dose-response features and mechanistic foundations. Pharm Res. 2016;110:242–264. doi: 10.1016/j.phrs.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 63.Calabrese E.J. Precondition is hormesis. Part II. How the conditioning dose mediates protection: dose optimization within temporal and mechanistic frameworks. Pharm Res. 2016;110:265–275. doi: 10.1016/j.phrs.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 64.Calabrese E.J., Blain R.B. The hormesis database: the occurrence of hormetic dose responses in the toxicological literature. Reg Toxicol Pharm. 2011;61:73–81. doi: 10.1016/j.yrtph.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Leak R.K., Calabrese E.J., Kozumbo W.J., Gidday J.M., Johnson T.E., Mitchel J.R., et al. Enhancing and extending biological performance and resilience. Dose-Response. 2018;16 doi: 10.1177/1559325818784501. 1559325818784501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mach W.J., Thimmesch A.R., Pierce J.T., Pierce J.D. Consequences of hyperoxia and the toxicity of oxygen in the lung. Nursing Res Pract. 2011;2011 doi: 10.1155/2011/260482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chan K., Kan Y.W. Is essential for protection against pulmonary injury in mice. PNAS. 1999;96:12731–12736. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao B., Gao W., Gao X., Leng Y., Liu M., Hou J., et al. Sulforaphane attenuates acute lung injury by inhibiting oxidative stress via Nrf2/HO-1 pathway in a rat sepsis model. J Clin Pathol. 2017;10:9021–9028. [PMC free article] [PubMed] [Google Scholar]
- 69.Sun Z., Niu Z., Wu S., Shan S. Protective mechanism of sulforaphane in Nrf2 and anti-lung injury in ARDS rabbits. Exper Ther Med. 2018;15:491–495. doi: 10.3892/etm.2018.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marzec J.M., Christie J.D., Reddy S.P., Jedlicka A.E., Vuong H., Lanken P.N., et al. Functional polymorphisms in the transcription factor Nrf2 in humans increase the risk of acute lung injury. FASEB. 2007;21:2237–2246. doi: 10.1096/fj.06-7759com. [DOI] [PubMed] [Google Scholar]
- 71.Kesic M.J., Simmons S.O., Bauer R., Jaspers I. Nrf2 expression modifies influenza A entry and replication in nasal epithelial cells. Free Rad Biol Med. 2011;51:444–453. doi: 10.1016/j.freeradbiomed.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mantero V., Abate L., Basilico P., Salmaggi A., Nourbakhsh B., Cordano C. COVID-19 in dimethyl fumarate-treated patients with multiple sclerosis. J Neurol. 2020 doi: 10.1007/s00415-020-10015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martinez-Sanchez G., Schwartz A., DiDonna V. Potential cytoprotective activity of ozone therapy in SARS-CoV-2/COVID-19. Antioxidant. 2020;9:389. doi: 10.3390/antiox9050389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hernandez A., Vinals M., Isidoro T., Vilas F. Potential role of oxygen therapy in treatment of COVID-19 pneumonia. Amer J Case Rep. 2020;21 doi: 10.12659/AJCR.925849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alamdari D.H., Moghaddam A.B., Amini S., Keramati M.R., Zarmehri A.M., Alamdari A.H., et al. Application of methylene blue-vitamin C-N-acetyl cysteine for treatment of critically ill COVID-19 patients, reports of a phase-1 clinical trial. Europ J Pharmacol. 2020;885 doi: 10.1016/j.ejphar.2020.173494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stack C., Jainuddin S., Elipenahli C., Gerges M., Starkova N., Starkov A.A., et al. Methylene blue upregulates Nrf2/ARE genes and prevents tau-related neurotoxicity. Hum Mol Gen. 2014;23:3716–3732. doi: 10.1093/hmg/ddu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sigman S.A., Mokmell S., Monici M., Vetrici M.A. A 57-year old African American man with severe COVID-19 pneumonia who responded to supportive photobiomoulation therapy (PPBMT): first use of PBMT in COVID-19. Amer J Case Reports. 2020;21 doi: 10.12659/AJCR.926779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Migliario M., Sabbatini M., Maortellaro C., Reno F. Near infrared low-level laser light therapy and cell proliferation: the emerging role of redox sensitive signal transfusction pathways. J Biophotonics. 2018;11 doi: 10.1002/jbio.201800025. [DOI] [PubMed] [Google Scholar]
- 79.Cuevas S, Yang Y, Amando I, Jose PA. Mechanisms involved in the antioxidant properties of azithromycin in lung epithelial cell stimulated with cigarette extract. FASAB 2016; 30(1): (Abstract only).
- 80.Borba M., Val F., Sampaio V., Alexandre M., Melo G., Brito M., et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS0Cov-2) infection: a randomized clinical trial. JAMA Network Open. 2020;3:3208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shen H., Wu N., Wang Y., Zhao H., Zhang L., Li T., et al. Chloroquine attenuates paraquat-induced lung injury in mice by altering inflammation, oxidative stress and fibrosis. Intern Immunopharm. 2017;46:16–22. doi: 10.1016/j.intimp.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 82.Staurengo-Ferrari L., Badaro-Garcia S., Hohmann M.S.N., Manchope M.F., Zaninelli T.H., Casagrande R., et al. Contribution of Nrf2 modulation to the mechanisms of action of analgesic and anti-inflammatory drugs I pre-clinical and clinical stages. Front Pharm. 2019;9:1536. doi: 10.3389/fphar.2018.01536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tutunchi H., Naeini F., Ostadrahimi A., Hosseinzadeh-Attar M.J. Naringenin, a flavanone with antiviral and anti-inflammatory effects: a promising treatment strategy against COVID-19. Phytother Res. 2020:1–11. doi: 10.1002/ptr.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramprasath T., Senthamizharasi M., Vasudevan V., Sasikumar S., Yuvaraj S., Selvam G.S. Naringenin confers protection against oxidative stress through upregulation of Nrf2 target genes in cardiomyoblast cells. J Physiol Biochem. 2014;70:407–415. doi: 10.1007/s13105-014-0318-3. [DOI] [PubMed] [Google Scholar]
- 85.Derosa G., Maffioli P., D’Angelo A., Di Pierro F. A role for quercetin in coronavirus disease 2019 (COVID-19) Phytother Res. 2020;35:1230–1236. doi: 10.1002/ptr.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fuentes J., Arias-Sante M.F., Atala E., Pastene E., Kogan M.J., Speisk H. Low nanomolar concentrations of a quercetin oxidation product, which naturally occurs in onion peel, protect cells against oxidative damage. Food Chem. 2020 doi: 10.1016/j.foodchem.2020.126166. [DOI] [PubMed] [Google Scholar]