Abstract
Objectives
The oral cavity is potentially high-risk transmitter of COVID-19. Antimicrobial mouthrinses are used in many clinical preprocedural situations for decreasing the risk of cross-contamination in the dental setting. It is important to investigate the efficacy of mouthwash solutions against salivary SARS-CoV-2 in order to reduce the exposure of the dental team during dental procedures.
Aims
The aim of this in vivo study was to evaluate the efficacy of 2 preprocedural mouthrinses in the reduction of salivary SARS-CoV-2 viral load and to compare the results of the mouthwashes to a control group.
Materials and Methods
In this randomized-controlled clinical trial, studied group comprised laboratory-confirmed COVID-19 positive patients through nasopharyngeal swabs. Participants were divided into 3 groups. For 30 s, the control group mouthrinsed with distilled water, the Chlorhexidine group mouthrinsed with 0.2% Chlorhexidine and the Povidone-iodine group gargled with 1% Povidone-iodine. Saliva samples were collected before and 5 min after mouthwash. SARS-CoV-2 rRT-PCR was then performed for each sample. Evaluation of the efficacy was based on difference in cycle threshold (Ct) value. The analysis of data was carried out using GraphPad Prism version 5 for Windows. Kristal wullis and Paired t-test were used. A probability value of less than 0.05 was regarded as statistically significant.
Results
Sixty-one compliant participants (36 female and 25 male) with a mean age 45.3 ± 16.7 years-old were enrolled. A significant difference was noted between the delta Ct of distilled water wash and each of the 2 solutions Chlorhexidine 0.2% (P = .0024) and 1% Povidone-iodine (P = .012). No significant difference was found between the delta Ct of patients using Chlorhexidine 0.2% and 1% Povidone-iodine solutions (P = .24). A significant mean Ct value difference (P < .0001) between the paired samples in Chlorhexidine group (n = 27) and also in Povidone-iodine group (n = 25) (P < .0001) was found. In contrast, no significant difference (P = .566) existed before and after the experiment in the control group (n = 9).
Conclusion
Chlorhexidine 0.2% and 1% Povidone-iodine oral solutions are effective preprocedural mouthwashes against salivary SARS-CoV-2 in dental treatments. Their use as a preventive strategy to reduce the spread of COVID-19 during dental practice should be considered.
KEYWORDS: Covid-19, Salivary SARS-CoV-2, Chlorhexidine 0.2% mouthrinse, 1% Povidone-iodine gargle, Dentistry, Prevention
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the novel member of the human coronaviruses from the Coronaviridae family and belongs to the Betacoronavirus genus.1 The subsequent corona virus disease-2019 (COVID-19), rapidly spreading worldwide,2 mostly causes respiratory disorders.3 , 4 The human-to-human transmission of SARS-CoV-2 essentially happens by inhalation of respiratory droplets spread by coughing or sneezing from an infected person, and by direct contact of contaminated surfaces followed by touching the nose, mouth and eyes.5 , 6 The virus can even survive on various surfaces for days.7 Transmission via ocular conjunctival route has been shown.8 The oral cavity is potentially high-risk transmitter of COVID-19. In fact, when operating dental treatments with the high-speed handpiece, it is fundamental to use a water coolant,9 generating consequently aerosols mixed with saliva or blood. These bioaerosols, generally contaminated with microorganisms including bacteria, fungi, and viruses, float in the air then settle on the surfaces and can be transmitted to the dentists or other patients by inhalation or contact.10 , 11 SARS-CoV-2 was identified in saliva of infected patients.12 Furthermore, it has been reported that the main cell receptor of SARS-CoV-2, angiotensin-converting enzyme II (ACE2), is extremely expressed on the mucosa of the oral cavity and particularly in the epithelial cells of the tongue.13 Therefore, it is crucial for dental practitioners to decrease the risk of contamination with SARS-CoV-2 by focusing not only on patient placement, hand hygiene, all personal protective equipment, caution in performing aerosol-generating procedures but also on patient's preprocedural antiseptic mouthrinse.14 , 15 In fact, antimicrobial mouthrinses are an important part of oral care. Such solutions are used in many clinical preprocedural situations for prophylactic purposes.16 , 17 Preprocedural oral solution is one of the most effective methods of reducing the amount of microorganisms in oral aerosols.18 , 19 In addition, gargling is also assumed to produce favorable effects through removal of oral and pharyngeal protease that helps viral replication.20 SARS-CoV-2 is an emergent rapidly spreading virus. Thus, an investigation for an effective mouthrinse against COVID-19 is urgently required for the control of oral and respiratory tract infection and for the exposure reduction during dental procedures.
In the literature, it was reported that a preprocedural 0.12% Chlorhexidine mouth rinse can reduce the microbial load of saliva.21 A meta-analysis showed that the use of preprocedural mouth rinse, including Chlorhexidine, essential oils, and cetylpyridinium chloride, resulted in a mean reduction of 68.4% colony-forming units in dental aerosols.22 Although the effect of Chlorhexidine gluconate on human coronavirus is unknown but it is effective against many respiratory viruses, like herpes and HIV.23 On the other hand, Povidone-iodine is a broad-spectrum antimicrobial that has been used in infection control for over 60 years. It is available in various preparations for use as a disinfectant for the skin, hands, mucosal surfaces, as well as for wound treatment and eye applications.24 Povidone-iodine has well-established general antimicrobial activity, demonstrating in vitro efficacy against wide range of enveloped and non-enveloped viruses.25, 26, 27 Recent in vitro study has demonstrated rapid virucidal its products activity against MERS-CoV.28 , 29 The benefit of gargling with Povidone-iodine has also already been noted in Japanese clinical respiratory guidelines.30
This study, besides of being an additional research concerning the consistency of detection of SARS-CoV-2 in saliva from the Lebanese experience, aimed mainly to evaluate the virucidal efficacy of 2 preprocedural mouthrinses: 0.2% Chlorhexidine and 1% Povidone-iodine in the reduction of salivary SARS-CoV-2 viral load.
Materials and Methods
Ethical Approval
Ethical clear of this research was delivered from the Lebanese University Institutional Review Board (#CUER 13–2020). The study was conducted in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards, registered in Lebanon clinical trial registry (lbctr.moph.gov.lb) # LBCTR2021034768. All participants were informed about the study and gave their consent. It was conducted between June and September 2020 at the isolation ward of Rafik Hariri University Hospital (RHUH) of Beirut, Lebanon, for sampling and in the Laboratory of Cancer Biology and Cellular Immunology, COVID-19 Unit, Faculty of Sciences, Lebanese University, Lebanon, for viral PCR tests.
Trial Design
This study was a parallel group, blind, randomized, placebo-controlled clinical trial study. Patients, intervention supervisor, laboratory technicians and the outcome assessor were blinded. The groups were labelled as A, B and C. The codes of the intervention were only revealed at the end of study.
Participants
Participants were consecutively recruited. Studied group comprised, laboratory-confirmed COVID-19 positive patients, through nasopharyngeal swabs. The lapse of time between COVID-19 diagnosis and inclusion in the study ranged from zero to two days. Were excluded cases indicated for intubation or mechanical ventilation and patients who declined consent.
Solution Preparation
15 ml of each undiluted mouthwash solution were previously poured into a sterile cup within a Biosafety cabinet (Topair system) in the Laboratory of Cancer Biology and Cellular Immunology, Faculty of Sciences, Lebanese University with respect to the manufacturer recommendations for each solution. The containers were marked as A, B or C, and then delivered to RHUH to accomplish the sampling. Solution A referred to distilled water as a placebo treatment, B for 0.2% Chlorhexidine and C for 1% Povidone-iodine.
Sampling
Simple random sampling using Excel software was used to divide participants into the three groups. The allocation concealment was done using the SNOSE technique. The same trained operator explained, provided and supervised the sampling in patient's room with respect to COVID-19 infection control. Sample collection was performed by the patients themselves in the early morning on empty stomach and before brushing teeth. First, participants were asked to cough out saliva from throat (2 ml), into a first sterile container. Next, the control group (n = 11) was invited to mouthrinse for 30 s with solution A, the Chlorhexidine group (n = 33) to mouthrinse for 30 s with solution B, and the Povidone-iodine group (n = 33) were invited to gargle for 30 s with solution C, and then to spit the solution. Five minutes later, saliva collections were done again in a second sterile container. Each cup held patient's name and the date of saliva collection while contaminated waste was appropriately discarded. Each collected sample was then inserted into separated tubes containing 2 mL of the virus transport medium (VTM) and transported to the COVID-19 Unit Laboratory in the Lebanese university for PCR processing.
Outcome
The primary outcome in this trial is the change in cycle threshold (Ct) values of salivary SARS-CoV-2 (delta Ct) after mouthrinsing respectively with distilled water, 0.2% Chlorhexidine and 1% Povidone-iodine.
SARS-CoV-2 rRT-PCR
The presence of SARS-CoV-2 was confirmed by real-time reverse transcriptase polymerase chain reaction (RT-PCR). 200 μL of VTM was used for RNA purification. RNA was extracted from the clinical samples on Kingfisher flex purification system Thermo Fisher using MagMAX Viral/Pathogen Nucleic Acid Isolation Kit (thermos fisher). Reactions were performed in 20 μL final volume reaction containing 5 μL of extracted RNA, rRT-PCR was performed using CFX96 real-time PCR detection system (Bio-Rad, Hercules, CA, USA) and Bosphore Novel Coronavirus (2019-nCoV) PCR Detection Kit v4 (Anatolia, Turkey), which targeted the RdRP, N and E genes of SARS-CoV-2. In this assay, a RNase P gene region is used as an endogenous internal control for the analysis of biological samples. It is normally used to ensure the quality of the test, at extraction and PCR levels and to exclude the false negative results.
Thus, in order to evaluate possible variability in the amount of material retrieved from saliva specimen before and after mouth wash we utilized RNase P as reference gene to normalize the input data.
To compare the paired samples before and after mouth wash, we calculated a Ct value modified according to the ratio of sample RNase P and mean RNase P Ct values.31
Statistical Analysis
All data analysis was performed with the GraphPad Prism 5 (GraphPad Software Inc., USA). Data were expressed as mean ± SEM. Normality test was performed using Kolmogorov–Smirnov test. Differences between means were explored using Kruskal-Wallis test followed by Dunn's multiple comparison post-hoc test. The Student paired t-test was used for comparison between two groups. Differences were considered to be significant at a level of P < .05.
Results
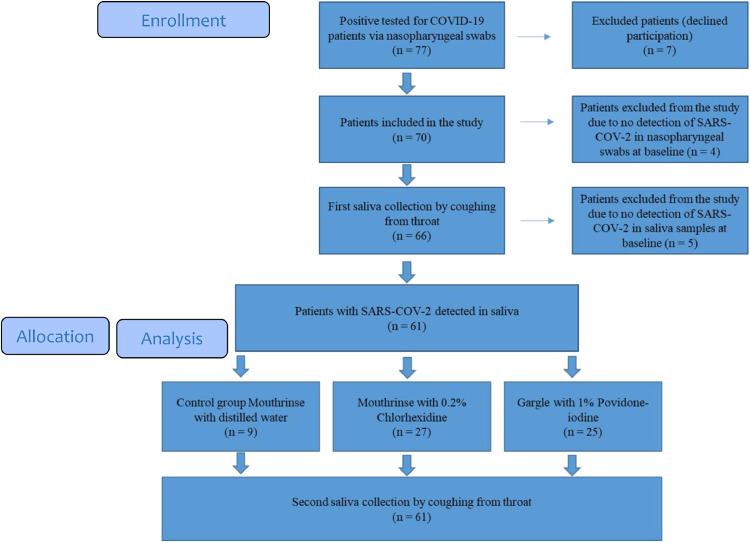
In total, after four months of recruitment, while 77 patients were eligible for the study, 16 were excluded resulting in 61 compliant participants (Figure 1 ). Among the final study group, 36 (59.1%) were female and 25 (40.9%) were male. The mean age of all patients was 45.3 ± 16.7 with an age range between 17 and 85 years old. The description of each group is mentioned in Table 1 . No adverse events were reported with any of the patients.
Figure 1.
Flow diagram showing the enrolment process of participants in the study.
Table 1.
Description of the study population.
| Participants | Age years |
Gender |
||
|---|---|---|---|---|
| Median (range) (years) | Mean ± SD (years) | Female n(%) | Male | |
| All patients n = 61 |
43 (17–85) | 45.3 ± 16.7 | 36 (59.1%) | 25 (40.9%) |
| Distilled water group n = 9 |
56 (21–85) | 57.2 ± 22.5 | 7 (77.8%) | 2 (22.2%) |
| Chlorhexidine 0.2% group n = 25 |
51 (25–84) | 47 ± 15.4 | 15 (60%) | 10 (40%) |
| Povidone-iodine 1% group n = 27 |
40 (17–63) | 39.9 ± 14.2 | 14 (51.8%) | 13 (48.2%) |
The mean Ct value of human RNaseP in saliva samples before mouthwash was 25.41 ± 2.5 [18.4–32.21]. Among the specimens tested, 72.4% had RNaseP Ct values below 27, 20.7% between 27 and 30, and 7.9% between 30 and 32.2. The mean Ct value of human RNaseP in saliva samples after mouthwash was 26 ± 2.72 [19.49–32.5]. No significant difference was found between the mean Ct values of human RNaseP in the 2 groups (P = .332).
The expression of the SARS-CoV-2 target genes (RdRp, E and N) used was approximately the same in each tested sample. To simplify our analysis, we presented the results with RdRp. For this gene the mean Ct value was 28.9 ± 5.5 (Median 29.9 [16.45–38.16]) in salivary pre-wash samples. After normalization, the SARS-CoV-2 mean Ct value was 28.3 ± 6.3.
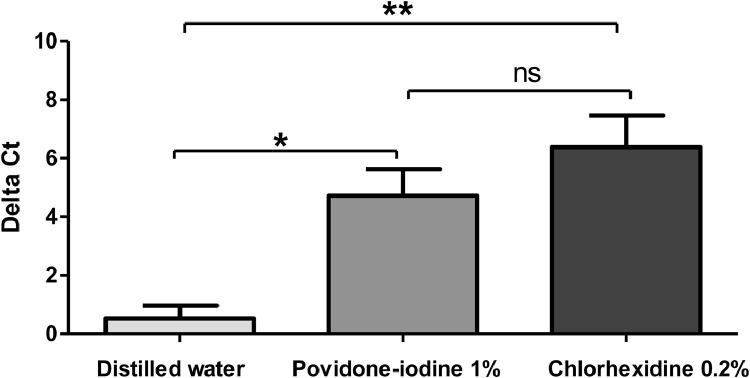
The comparison of the delta Ct using Kruskal Wallis test showed a significant difference between the means of the 3 groups. The post hoc test showed a significant difference between the delta Ct of patients using distilled water wash (0.519 ± 0.519) and each of the 2 solutions 1% Povidone iodine (4.72 ± 0.89) and Chlorhexidine 0.2% (6.37 ± 1.08) (P values 0.012 and 0.0024 respectively). No significant difference was found between the delta Ct of patients using Povidone iodine and Chlorhexidine 0.2% solutions (P value = 0.24) (Figure 2 ). We noted that Ct values are considered inversely related to viral load and may serve as an indirect method of arbitrarily quantifying the viral load in the sample.
Figure 2.
Comparison of Delta Ct mean between the mouth wash solutions. Delta Ct were calculated as follow: normalized Ct value post mouthwash minus normalized Ct value pre mouthwash. Groups were compared by using the Kruskal Wallis test. Peak values were reported as mean +/- SD. Significant differences between means are indicated by *(P < .05), ** (P < .01), ns indicates no significant difference (P > .05).
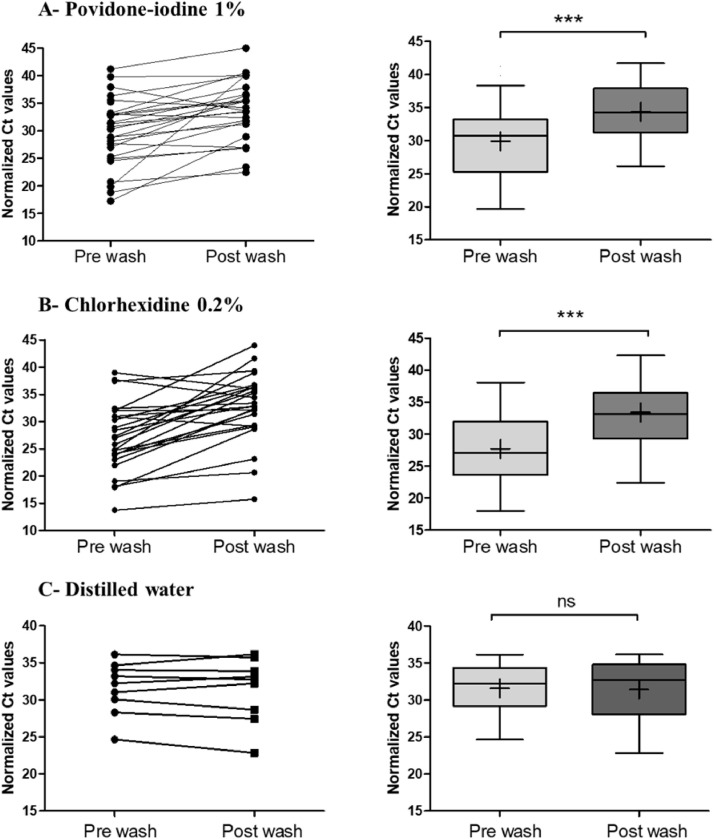
Our results showed a significant mean difference between the paired samples before (29.88 ± 6.2; median 30.75) and after mouthwash (34.36 ± 6.3; median 34.19) with 1% Povidone-iodine (P < .0001). After mouthwash, the difference between means was 4.45 (Figure. 3 A). In addition, a higher significant difference of means was found in paired samples using Chlorhexidine 0.2% (P < .0001). The mean Ct increased 5.69 after mouthwash. The mean Ct of pre and post mouthwash was respectively 27.69 ± 7.16 (median 27.11) and 33.9 ± 7.08 (median 33.13) (Figure 3B). In contrast, no significant difference was found in the control group using the distilled water as mouthwash solution as shown in Figure 3C (P = .566).
Figure 3.
Normalized threshold cycle (Ct) values for matched pre and post mouthwash salivary specimens. The Ct values of RdRp obtained with RT–PCR that were detected in salivary specimens before and after mouthwash and normalized to the internal control RNAseP endogenous human gene. (A) 1% Povidone-iodine (n = 25), (B) Chlorhexidine 0.2% (n = 27) and (C) distilled water (n = 9) were used as mouthwash solutions. The before-after graph shows the changes of Ct after mouth wash for each patient. The box plots show the medians (middle line) and the first and third quartiles (boxes). The mean is marked by a plus sign inside the box. Paired Groups were compared by using the Paired t-test. ***Indicates a P-value ≤ .0001, ns indicates no significant difference (P > .05).
Discussion
With the novel COVID-19 pandemic, dental care practitioners were in urge to develop quick infection control policies.32 So far, clinical implementation of new concepts was mainly based on recommendations without being evident based. Particularly, different preprocedural mouthwash solutions to minimize the SARS-CoV-2 transmission during dental treatment were recommended by some dental specialist societies.33, 34, 35 Despite lack of any clinical data supporting the virucidal effects of mouthwash solutions against SARS‐CoV‐2, many propositions were adopted in reviews discussing the COVID-19 preventive measurements in Dentistry.36, 37, 38, 39, 40, 41 A recent in vitro study tested the effect of the following mouth rinses on cell viability: hydrogen peroxide, povidone-iodine, chlorhexidine gluconate and essential oils with alcohol. The experiments found that mouth rinses can significantly reduce virus infectivity, suggesting a potential benefit for reducing SARS-CoV-2 spread. The study concluded that the clinical investigation of antiviral effects of mouth rinses is needed for proving their potential to reduce the virus spread.42
For our knowledge this current in vivo study is the first large scale controlled-clinical trial testing the efficacy of 0.2% Chlorhexidine oral mouthwash and 1% Povidone-iodine oral gargle on salivary SARS-CoV-2 virus of positive tested patients.
This study comprised 61 compliant COVID-19 positive subjects. During sampling recruitment, the non-detection of SARS-CoV-2 in nasopharyngeal samples of four hospitalized positive patients could be explained by the fact that the nucleic acid test results of a significant proportion of patients are "false negative”.43 For ethical issues, mainly avoiding the patient subsequent discomfort, we preferred to exclude these participants instead of repeating the nasopharyngeal swab test for a clinical trial purpose.
In placebo-controlled clinical trials with “very ill” subjects it is unethical to assign equal subjects to each arm and it is preferable to have more subjects in test group compared to the control one. In such cases, sample size is adjusted if clear and clinically meaningful inputs on some points are available prior to working on sample size estimation.44 As COVID-19 is a recently emerging pandemic without previous related data in addition to its critical incompletely explored status, we considered that our sample size for the test and control groups were legible.
Saliva sampling was self-performed by the patients to reduce the risk of nosocomial SARS-CoV-2 transmission to health care providers.12 , 45 A lapse of time of 5 min between mouthrinsing or gargling and second saliva collection was chosen to conform the real procedure at dental clinic: during dental appointment, it usually takes few minutes between the patient's preprocedural mouthwash and the commencement of the treatment.
This study firstly revealed the consistency of the detection of SARS-CoV-2 in saliva since the virus was detected in the salivary samples of 61 out of 66 patients. This result is in accordance with other studies.12 , 46 , 47 In fact, using saliva specimens for the diagnosis of COVID-19 has many advantages like avoiding invasive procedures, contributing to the decrease of the risk of nosocomial COVID-19 transmission, usefulness for screening of a large number of individuals with less time-consuming and in situations in which nasopharyngeal specimen collection may be contraindicated.12 However, further studies comparing SARS-CoV-2 viral load between saliva and nasopharyngeal samples collected at the same time for each COVID-19 tested patient are required for a better assessment for the use of saliva as a diagnosis tool for COVID-19.
Moreover, our results showed that a 1% Povidone-iodine gargle reduces significantly the intraoral viral load in SARS-CoV-2-positive subjects. Our results are similar to another in-vivo study where the authors analyzed the impact of a mouthwash with Povidone-iodine on the salivary viral load of SARS-CoV-2 in 4 patients with COVID-19 and found that in 2 of the 4 participants, the gargle solution resulted in a significant drop in viral load, which remained for at least 3 h.48 Frank et al. (2020) found that Povidone-iodine can safely be used in the mouth at concentrations up to 2.5% for up to 5 months because it rapidly inactivates coronaviruses, including SARS and MERS, even when applied for as little as 15 s.49 The same authors were optimistic about the inactivation of SARS-CoV-2 by Povidone-iodine, and called for in vitro efficacy demonstration. Mady et al. (2020) proposed the use of oral/oropharyngeal wash with 10 mL of 0.5% aqueous Povidone-iodine solution in addition to nasal irrigation of 240 mL of 0.4% of the same antiseptic solution for patients and healthcare providers as a public health intervention for COVID-19.50 Suresh et al. (2020) proposed also a pioneer description in anesthesia practice on the use of preoperative Povidone-iodine gargles in COVID-19 cases to mitigate the chain of spread of COVID-19 through cross-infection among health care workers.51 In addition, Brida et al. (2020) investigated the in-vitro optimal contact time and concentration for virucidal activity of 0.5%, 1%, and 1.5% oral solution of povidone-iodine against SARS-CoV-2 and found that the efficacy was present at the lowest concentration of 0.5% Povidone-iodine and at the lowest contact time of 15 s and mentioned that, therefore, preprocedural rinsing with diluted Povidone-iodine in the range of 0.5% to 1.5% may be preferred over hydrogen peroxide during the COVID-19 pandemic.52 Pelletier et al. (2020) found that concentrations 1% to 5% of Povidone-iodine nasal antiseptics and oral rinse antiseptics completely inactivated the SARS-CoV-2 after 60-second exposure times on SARS-CoV-2 infected Vero 76 cell.53 On the other hand, Chorney et al. (2020) called for further research prior to strongly recommending Povidone-iodine use in preparation for nasal, oral or pharyngeal surgery in children.54
In addition, preprocedural mouthrinse with 0.2% Chlorhexidine showed in our study a significant efficacy against SARS-CoV-2. Our results are in accordance with those of Yoon et al. (2020) who found, in a clinical trial on 2 patients, that Chlorhexidine mouthwash was effective in reducing the SARS-CoV-2 viral load in the saliva for a short-term period.55 Meister et al. (2020) found while using Vero E6 cells that different SARS-CoV-2 strains can be efficiently inactivated with Chlorhexidine and other commercially available oral rinses and recommended further analysis during clinical studies to assess the in vivo effects of the oral solutions. Chlorhexidine has been suggested to reduce the viral transmission via aerosols.56 Although its action against this virus remains controversial but if the results are confirmed by other clinical trials, Chlorhexidine mouthrinse could help to prevent the spread of SARS-CoV-2.57
Although both solutions proved significant efficacy against salivary SARS-CoV-2, 0.2% Chlorhexidine showed non-significantly more efficiency on reducing the salivary viral load than 1% povidone iodine. Distilled water had no effect on viral load. The absence of placebo effect confirmed the effectiveness of the proposed disinfectant mouthwash solutions on salivary SARS-CoV-2.
Conclusion
0.2% Chlorhexidine and 1% Povidone-iodine oral solutions are effective preprocedural mouthwashes against SARS-CoV-2 in dental treatments. Their use might be a preventive strategy to reduce the spread of COVID-19 in dental clinics as in various health care services. Further studies including the length of their effectiveness over the time are required for an accurate prescription against SARS-CoV-2.
CRediT authorship contribution statement
Rola Elzein: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Fadi Abdel-Sater: Methodology, Software, Validation, Formal analysis, Resources, Data curation, Writing – review & editing. Soha Fakhreddine: Investigation. Pierre Abi Hanna: Methodology, Resources, Investigation. Rita Feghali: Methodology, Resources, Data curation. Hassan Hamad: Project administration. Fouad Ayoub: Conceptualization, Methodology, Supervision, Project administration, Validation, Visualization, Writing – review & editing, Funding acquisition.
Acknowledgments
Lebanese Ministry of Public Health.
Footnotes
Conflict of Interest: The authors have no actual or potential conflicts of interest.
Source of Funding: This work was supported by the Lebanese University
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus disease 2019 (COVID-19) Situation Reports 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 3.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30] Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han Y., Yang H. The transmission and diagnosis of 2019 novel coronavirus infection disease (COVID-19): a Chinese perspective. J Med Virol. 2020;92(6):639–644. doi: 10.1002/jmv.25749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents [published correction appears in J Hosp Infect. 2020 Jun 17;:] J Hosp Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu C.W., Liu X.F., Jia Z.F. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395(10224):e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farah R.I. Effect of cooling water temperature on the temperature changes in pulp chamber and at handpiece head during high-speed tooth preparation. Restor Dent Endod. 2018;44(1):e3. Published 2018 Dec 24. doi: 10.5395/rde.2019.44.e3. [DOI] [PMC free article] [PubMed]
- 10.Grenier D. Quantitative analysis of bacterial aerosols in two different dental clinic environments. Appl Environ Microbiol. 1995;61(8):3165–3168. doi: 10.1128/AEM.61.8.3165-3168.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones R.M., Brosseau L.M. Aerosol transmission of infectious disease. J Occup Environ Med. 2015;57(5):501–508. doi: 10.1097/JOM.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 12.To K.K., Tsang O.T., Yip C.C., et al. Consistent detection of 2019 Novel Coronavirus in Saliva. Clin Infect Dis. 2020;71(15):841–843. doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu H., Zhong L., Deng J., et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. Published 2020 Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li R.W., Leung K.W., Sun F.C., Samaranayake L.P. Severe acute respiratory syndrome (SARS) and the GDP. Part II: implications for GDPs. Br Dent J. 2004;197(3):130–134. doi: 10.1038/sj.bdj.4811522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge Z.Y., Yang L.M., Xia J.J., Fu X.H., Zhang Y.Z. Possible aerosol transmission of COVID-19 and special precautions in dentistry. J Zhejiang Univ Sci B. 2020;21(5):361–368. doi: 10.1631/jzus.B2010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrel S.K., Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc. 2004;135(4):429–437. doi: 10.14219/jada.archive.2004.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klyn S.L., Cummings D.E., Richardson B.W., Davis R.D. Reduction of bacteria-containing spray produced during ultrasonic scaling. Gen Dent. 2001;49(6):648–652. [PubMed] [Google Scholar]
- 18.Sawhney A., Venugopal S., Babu G.R., et al. Aerosols how dangerous they are in clinical practice. J Clin Diagn Res. 2015;9(4):ZC52–ZC57. doi: 10.7860/JCDR/2015/12038.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feres M., Figueiredo L.C., Faveri M., Stewart B., de Vizio W. The effectiveness of a preprocedural mouthrinse containing cetylpyridinium chloride in reducing bacteria in the dental office. J Am Dent Assoc. 2010;141(4):415–422. doi: 10.14219/jada.archive.2010.0193. [DOI] [PubMed] [Google Scholar]
- 20.Kitamura T., Satomura K., Kawamura T., et al. Can we prevent influenza-like illnesses by gargling? Intern Med. 2007;46(18):1623–1624. doi: 10.2169/internalmedicine.46.0104. [DOI] [PubMed] [Google Scholar]
- 21.Marui V.C., Souto M.L.S., Rovai E.S., Romito G.A., Chambrone L., Pannuti C.M. Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol: a systematic review. J Am Dent Assoc. 2019;150(12):1015–1026. doi: 10.1016/j.adaj.2019.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Logothetis D.D., Martinez-Welles J.M. Reducing bacterial aerosol contamination with a chlorhexidine gluconate pre-rinse. J Am Dent Assoc. 1995;126(12):1634–1639. doi: 10.14219/jada.archive.1995.0111. [DOI] [PubMed] [Google Scholar]
- 23.Wood A., Payne D. The action of three antiseptics/disinfectants against enveloped and non-enveloped viruses. J Hosp Infect. 1998;38(4):283–295. doi: 10.1016/s0195-6701(98)90077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sneader W. Wiley; New York: 2005. Drug discovery: a History; p. 68. [Google Scholar]
- 25.Wutzler P., Sauerbrei A., Klöcking R., Brögmann B., Reimer K. Virucidal activity and cytotoxicity of the liposomal formulation of povidone-iodine. Antiviral Res. 2002;54(2):89–97. doi: 10.1016/s0166-3542(01)00213-3. [DOI] [PubMed] [Google Scholar]
- 26.Kawana R., Kitamura T., Nakagomi O., et al. Inactivation of human viruses by povidone-iodine in comparison with other antiseptics. Dermatology. 1997;195(2):29–35. doi: 10.1159/000246027. Suppl. [DOI] [PubMed] [Google Scholar]
- 27.Kariwa H., Fujii N., Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology. 2006;212(1):119–123. doi: 10.1159/000089211. Suppl 1Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eggers M., Eickmann M., Kowalski K., Zorn J., Reimer K. Povidone-iodine hand wash and hand rub products demonstrated excellent in vitro virucidal efficacy against Ebola virus and modified vaccinia virus Ankara, the new European test virus for enveloped viruses. BMC Infect Dis. 2015;15:375. doi: 10.1186/s12879-015-1111-9. Published 2015 Sep 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eggers M., Eickmann M., Zorn J. Rapid and Effective Virucidal Activity of Povidone-Iodine Products Against Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Modified Vaccinia Virus Ankara (MVA) Infect Dis Ther. 2015;4(4):491–501. doi: 10.1007/s40121-015-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Committee for the Japanese Respiratory Society Guidelines in Management of Respiratory. Prevention of hospital-acquired pneumonia (strategies for prevention of hospital-acquired infections) Respirology. 2004;9(1):S48–S50. doi: 10.1111/j.1440-1843.2003.00552.x. Suppl. [DOI] [PubMed] [Google Scholar]
- 31.Duchamp M.B., Casalegno J.S., Gillet Y., et al. Pandemic A(H1N1)2009 influenza virus detection by real time RT-PCR: is viral quantification useful? Clin Microbiol Infect. 2010;16(4):317–321. doi: 10.1111/j.1469-0691.2010.03169.x. [DOI] [PubMed] [Google Scholar]
- 32.Nicola M., O'Neill N., Sohrabi C., Khan M., Agha M., Agha R. Evidence based management guideline for the COVID-19 pandemic - Review article. Int J Surg. 2020;77:206–216. doi: 10.1016/j.ijsu.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Dental Association (ADA) ADA American Dental Association; 2020. ADA Adds Frequently Asked Questions from Dentists to Coronavirus Resources; p. 20.https://www.ada.org/en/publications/ada-news/2020-archive/march/ada-addsfrequently-asked-questions-from-dentists-to-coronavirus-resources Available at: [Google Scholar]
- 34.Institut der Deutschen Zahnärzte (IDZ) (2020) System Von Standardvorgehensweisen für Zahnarztpraxen Während Der Coronavirus-Pandemie. Available at: https://www.idz.institute/fileadmin/Content/Publikationen-PDF/Weitere_Dokumente/IDZ_SARS-CoV-2_Standardvorgehensweise_ZAP_2020-04-24.pdf.
- 35.Association Dentaire Française (2020). COVID-19 Guide pratique à Partir Des Recommandations D'experts Validées. Available at: https://www.fdiworlddental.org/sites/default/files/media/documents/covid-19_guide_pratique_a_partir_des_recommandations_dexperts_validees.pdf.
- 36.Ather A., Patel B., Ruparel N.B., Diogenes A., Hargreaves K.M. Coronavirus Disease 19 (COVID-19): implications for Clinical Dental Care. J Endod. 2020;46(5):584–595. doi: 10.1016/j.joen.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izzetti R., Nisi M., Gabriele M., Graziani F. COVID-19 Transmission in Dental Practice: brief Review of Preventive Measures in Italy. J Dent Res. 2020;99(9):1030–1038. doi: 10.1177/0022034520920580. [DOI] [PubMed] [Google Scholar]
- 38.Zimmermann M., Nkenke E. Approaches to the management of patients in oral and maxillofacial surgery during COVID-19 pandemic. J Craniomaxillofac Surg. 2020;48(5):521–526. doi: 10.1016/j.jcms.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meng L., Hua F. (COVID-19): emerging and Future Challenges for Dental and Oral Medicine. J Dent Res. 2020;99(5):481–487. doi: 10.1177/0022034520914246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diegritz C., Manhart J., Bücher K., et al. A detailed report on the measures taken in the Department of Conservative Dentistry and Periodontology in Munich at the beginning of the COVID-19 outbreak. Clin Oral Investig. 2020;24(8):2931–2941. doi: 10.1007/s00784-020-03440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jamal M., Shah M., Almarzooqi S.H., et al. Overview of transnational recommendations for COVID-19 transmission control in dental care settings [published online ahead of print, 2020 May 19] Oral Dis. 2020 doi: 10.1111/odi.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu C., Wang A., Hoskin E.R., et al. Differential effects of antiseptic mouth rinses on SARS-CoV-2 infectivity in vitro. bioRxiv. 2020 doi: 10.1101/2020.12.01.405662. Preprint. 2020.12.01.405662. Published 2020 Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y., Yao L., Li J., et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol. 2020;92(7):903–908. doi: 10.1002/jmv.25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakpal T.V. Sample size estimation in clinical trial. Perspect Clin Res. 2010;1(2):67–69. [PMC free article] [PubMed] [Google Scholar]
- 45.Yu C., Li L., Tuersun Y., et al. Oropharyngeal Secretion as Alternative for SARS-CoV-2 Detection. J Dent Res. 2020;99(10):1199–1205. doi: 10.1177/0022034520940292. [DOI] [PubMed] [Google Scholar]
- 46.Williams E., Bond K., Zhang B., Putland M., Williamson D.A. Saliva as a Noninvasive Specimen for Detection of SARS-CoV-2. J Clin Microbiol. 2020;58(8) doi: 10.1128/JCM.00776-20. e00776-20Published 2020 Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCormick-Baw C., Morgan K., Gaffney D., et al. Saliva as an Alternate Specimen Source for Detection of SARS-CoV-2 in Symptomatic Patients Using Cepheid Xpert Xpress SARS-CoV-2. J Clin Microbiol. 2020;58(8):e01109–e01120. doi: 10.1128/JCM.01109-20. Published 2020 Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martínez Lamas L., Diz Dios P., Pérez Rodríguez M.T., et al. Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests [published online ahead of print, 2020 Jul 2] Oral Dis. 2020 doi: 10.1111/odi.13526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frank S., Capriotti J., Brown S.M., Tessema B. Povidone-Iodine Use in Sinonasal and Oral Cavities: a Review of Safety in the COVID-19 Era. Ear Nose Throat J. 2020;99(9):586–593. doi: 10.1177/0145561320932318. [DOI] [PubMed] [Google Scholar]
- 50.Mady L.J., Kubik M.W., Baddour K., Snyderman C.H., Rowan N.R. Consideration of povidone-iodine as a public health intervention for COVID-19: utilization as "Personal Protective Equipment" for frontline providers exposed in high-risk head and neck and skull base oncology care. Oral Oncol. 2020;105 doi: 10.1016/j.oraloncology.2020.104724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suresh V., Sharma S., Aggarwal A. Preanesthetic Povidone-Iodine gargles for patients with COVID-19. J Clin Anesth. 2020;67 doi: 10.1016/j.jclinane.2020.110035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bidra A.S., Pelletier J.S., Westover J.B., Frank S., Brown S.M., Tessema B. Rapid In-Vitro Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Using Povidone-Iodine Oral Antiseptic Rinse. J Prosthodont. 2020;29(6):529–533. doi: 10.1111/jopr.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pelletier J.S., Tessema B., Frank S., Westover J.B., Brown S.M., Capriotti J.A. Efficacy of Povidone-Iodine Nasal and Oral Antiseptic Preparations Against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2) [published online ahead of print, 2020 Sep 21] [published correction appears in Ear Nose Throat J. 2020 Dec 8;:145561320977784] Ear Nose Throat J. 2020 doi: 10.1177/0145561320957237. [DOI] [PubMed] [Google Scholar]
- 54.Chorney S.R., Rizzi M.D., Dedhia K. Considerations for povidone-iodine antisepsis in pediatric nasal and pharyngeal surgery during the COVID-19 pandemic. Am J Otolaryngol. 2020;41(6) doi: 10.1016/j.amjoto.2020.102737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoon J.G., Yoon J., Song J.Y., et al. Clinical Significance of a High SARS-CoV-2 Viral Load in the Saliva. J Korean Med Sci. 2020;35(20):e195. doi: 10.3346/jkms.2020.35.e195. Published 2020 May 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meister T.L., Brüggemann Y., Todt D., et al. Virucidal Efficacy of Different Oral Rinses Against Severe Acute Respiratory Syndrome Coronavirus 2. J Infect Dis. 2020;222(8):1289–1292. doi: 10.1093/infdis/jiaa471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herrera D., Serrano J., Roldán S., Sanz M. Is the oral cavity relevant in SARS-CoV-2 pandemic? Clin Oral Investig. 2020;24(8):2925–2930. doi: 10.1007/s00784-020-03413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]