Highlights
-
•
A report on the best treatment results on an osteosarcoma cohort.
-
•
There has been a lack of improvement in osteosarcoma treatment for the last four decades.
-
•
Mifamurtide is the only new drug against osteosarcoma licensed by the EMA but not by the FDA.
-
•
Oncologists have no consensus for mifamurtide use due to its expense.
Abbreviations: A/AP, adriamycin (doxorubicin)/adriamycin (doxorubicin) and cisplatin; AIC, Akaike information criterion; BIC, Bayesian information criterion; CI, confidence interval; CTCAE, common terminology criteria for adverse events; EFS, event free survival; EMA, European Medicines Agency; FDA, Food and Drug Administration; HR, hazard ratio; LY, lymphocytes; NEU, neutrophiles; M/F, male/female; MFS, metastatic free survival; MONO, monocytes; MTX, methotrexate; PFS, progression free survival; PLT, platelets; R0 and R1 resection, free margins and microscopic rest after resection respectively; SD, standard deviation
Keywords: Osteosarcoma, Mifamurtide, Survival, Single institution analysis, Comparative analysis
Abstract
Purpose
Conventional osteosarcoma is an orphan disease. Current treatment approaches include combining a three drug chemotherapy schedule and surgery. The 3- and 5-year event-free survival (EFS) in localized disease is roughly 65 and 60%, respectively. The registration study of mifamurtide reported survival benefit, but some methodological controversies have been insufficient for FDA market authorization in contrast to EMA.
Methods
prospective single centre survival analysis of a mifamurtide addition to conventional therapy in 23 patients over a 5.5 year enrolment period is reported and compared to a historical control of 26 patient with localized disease. Bias arising from observational methodology was addressed using Landmark analysis and time-dependent Cox models. Blood count dynamics were analysed during the treatment.
Results
The adverse event profile was as expected with no dose limiting toxicities. There were no local relapses observed, one patient died in the first complete remission due to doxorubicin cardiotoxicity, one patient had pulmonary metastatic relapse. The observed 3- and 5-year EFS was 87.4% (CI 72.4–100%) and 87.4% (CI 72.4–100%), progression free survival (PFS) was 92.9% (CI 80.3–100%) and 92.9% (CI 80.3–100%), overall survival was 94.1% (CI 83.6–100) and 80.7% (CI 58.3–100), respectively. Comparison to the historical control showed statistically significant better PFS for mifamurtide patients (Landmark analysis; p = 0.044). Risk of progression was 5-times lower for the mifamurtide group (Cox model; HR 0.21, p = 0.136). Only subtle differences in lymphocyte counts were observed across treatment.
Conclusion
the PFS benefit of mifamurtide is reported herein. The addition of mifamurtide could be considered as a best treatment option for localized osteosarcoma.
1. Introduction
Osteosarcoma is the most common malignant mesenchymal tumour of the bone in paediatric and adolescent populations. It can arise from any bone, but most often the localization of the tumour’s origin is in the metaphyseal area of the long bones. From a histological point of view, osteosarcoma can be subdivided into high grade forms, such as conventional, telangiectatic, chondroblastic and rare small cell subtype and into more indolent forms like parosteal or periosteal osteosarcoma. The incidence of osteosarcoma is 4.0 (3.5–4.6) for the range 0–14 years and 5.0 (4.6–5.6) for the range 0–19 years per year per million persons and it varies with age [1].
The clinical presentation of osteosarcoma is predominantly pain in the affected area which initially appears after physical activities and afterwards typically during sleep in “ON/OFF” mode. Up to 20% of patients are diagnosed with apparent metastatic disease (lungs, bones), but the majority of patients are diagnosed with a localized disease. Despite that, the majority of them already have microscopic metastases at the time of the diagnosis [2].
Total resection of clinically detectable tumours is a mainstay of long-term survival, even if the treatment approach for this disease needs to be multimodal. Relapse-free survival rates of localized extremity tumours are still approximately 15–17% in the case of surgical treatment only. A combination of surgery with intensive neoadjuvant and adjuvant multiagent chemotherapy controls the microscopic metastatic disease. The 3- and 5-year event-free survival (EFS) and overall –survival (OS) in localized disease is roughly 65 and 60%, 84 and 76%, respectively [11]. Nevertheless, in cases of apparent metastatic disease, for local progression or nonresectable primary tumours the success rate is still very poor with less than 20% survival five years after diagnosis [3]. Since the 1970’s to the present only four chemotherapeutic agents have been routinely used in systemic therapy. The backbone of conventional chemotherapy treatment comprises of doxorubicin, cisplatine and methotrexate. An alkylation agent, ifosfamide, is also effective in the treatment of osteosarcoma but the addition of ifosfamide to other agents was too toxic and did not improve treatment results. No new chemotherapy agents with proven efficacy have been added to the standard chemotherapy regimen so far and overall survival rates are stagnating. Thus, the treatment strategy for locoregional disease is still based on former schedules. Other additional and experimental treatments in metastatic or progressive disease also consist of targeting the bone microenvironment (bisfosfonates), tyrosine kinase receptors (e.g. sorafenib, pazopanib), intracellular signalling molecules (dasatinib) and of unspecific immune therapy (interferon).
Mifamurtide (Mepact) is synthetic lipophilic analogue of muramyl dipeptide, peptidoglycan contained in the bacterial wall, which can activate the innate immune system. Its tumoricidal effect on microscopic metastases is probably caused by the stimulation of monocytes and macrophages associated with an increasing level of proinflammatory cytokines. The rationale behind the use of mifamurtide in osteosarcoma treatment is to mimic a kind of infection that can help to eradicate residual micrometastases, which are not eliminated by systemic chemotherapy [2]. The view of the clinical community on the regular administration of mifamurtide with standard adjuvant chemotherapy is still not endorsed despite a registration study which showed the benefit of mifamurtide addition to adjuvant chemotherapy and significantly improved 6-year overall survival from 70% to 78%, event free survival was 61% [4].
2. Patients and methods
A comparative analysis was made of patients with localized osteosarcoma treated with or without an adjuvant mifamurtide regimen as a part of routine standard treatment. Patients or their legal guardians signed an informed consent about data handling for research purposes and analyses. Consecutive patients diagnosed between 2012 and 2018 and who received mifamurtide during adjuvant chemotherapy made up the mifamurtide group. Similarly, consecutive patients with localized disease diagnosed between 2004 and 2014 without mifamurtide as a part of adjuvant treatment made up the historical control group.
Mifamurtide was initiated during adjuvant treatment and administrated intravenously as a 1-hour infusion at a dose of 2 mg/m2 (maximum 4 mg single dose) according to the summary of product characteristics (SPC). Mifamurtide was administered twice weekly for 12 weeks followed by an additional 24 weeks of once weekly or until follow-up if not finished yet. This resulted in a maximum of 48 planned doses over a total of 36 weeks. Paracetamol 500–1000 mg as premedication was administered.
This report was approved by the institutional review board.
2.1. Statistical analysis
The outcome of the patients was analysed using survival analysis. Native, observational data-based Kaplan-Meier curves and estimates for 3- and 5-year survivals are given for descriptive purposes. In order to account for possible immortal time bias, Landmark [5] and time-dependent Cox models [6] analysis was used for comparison between the control and mifamurtide group. Both methods are described in the respective papers. Briefly, Landmark analysis uses an instrumental time point at which patients are divided into the treatment groups based on receiving time-dependent treatment at that time. Simply said it renders a quasi randomization effect at that time with respect to immortal time bias. On the other hand, time-dependent Cox models handle periods on and off treatment separately by correctly creating a risk table.
The possible effect of mifamurtide on blood element counts was evaluated in longitudinal analysis using linear mixed-effect models [7]. All blood count data, available from archives, were merged to the patient data set, so that profiles of neutrophils (NEU), monocytes (MONO), lymphocytes (LY) and platelets (PLT) could be created for each patient and chemotherapy cycle. In order to make the analysis comparable within protocol cycles, periods with out-of-protocol treatment switches were excluded and only per-protocol cycles (A/AP & MTX) were considered. First, absolute counts of blood cell data were logarithmically transformed, and analyses were performed on the transformed data. Second, in order to capture the dynamics of the data and to handle the nonlinearity while enabling plasticity in the models, a spline or interval square linear mixed model with 4 knots every 7 days was fitted. Due to possible delays in subsequent cycles, data in the models were followed up to 5 weeks after the start of each chemotherapy cycle. All time- and time-squared terms and their first-order interactions with cycle type were included in this base model. Random effects for each patient within a specific week of the cycle were considered. These nested random effects enabled the possible bias arising from the time factor due to long time period of the whole treatment to be accounted for. Effect of mifamurtide was then analysed in the above base model. The biological behaviour of the data, hypothesis questions and AIC and BIC criteria were considered when model building. Final models with the types of chemotherapy cycle and mifamurtide were presented using spaghetti (case-profile) plots with model group means plotted.
Analyses were done using survival [8] and nlme [9] packages in R software 3.5.3 [10].
3. Results
A total of 23 patients in the mifamurtide group and 26 patients in the control group were analyzed. Two patients in the mifamurtide group received adjuvant mifamurtide as late as during the treatment of their first relapse. These patients were also included in the control group as their paired controls without mifamurtide during the primary treatment. There were two patients enrolled in an overlapped period. One patient in the control group from 2014 was in an outside hospital with periosteal osteosarcoma of the femur with a pathological fracture and was initially treated with chemotherapy and surgery and achieved complete remission. Later he had a locoregional relapse and was treated with surgery and adjuvant mifamurtide in 2018. Another patient diagnosed in year 2012 had periosteal osteosarcoma which was not indicated for mifamurtide.
Summary characteristics of the analysed groups are given in Table 1.
Table 1.
Baseline and treatment summary sample characteristics.
| Baseline characteristics | Control (n = 26) |
Mifamurtide (n = 23) |
p |
|---|---|---|---|
| Gender M/F (%) | 15 (58%)/11 (42%) | 15 (65%)/8 (35%) | 0.770 |
| Age mean ± SD | 14.1 ± 4.4 | 13.8 ± 4.3 | 0.824 |
| Histology (%) | ~1.0† | ||
| Conventional osteoblastic | 17 (65%) | 15 (65%) | |
| High-grade other | 7 (27%) | 7 (31%) | |
| Periosteal | 2 (8%) | 1 (4%) | |
| Site | 0.653† | ||
| Femur | 11 | 6 | |
| Tibia or fibula | 5 | 8 | |
| Lower limb – other | 2 | 0 | |
| Humerus | 2 | 5 | |
| Upper limb – other | 3 | 2 | |
| Skull/axial | 3 | 2 | |
| Lung unspecific nodules (%) | 7 (27%) | 11 (48%) | 0.151 |
| surgery | |||
| Resection‡ R0/R1 (%) | 19 (76%)/6 (24%) | 20 (87%)/3 (13%) | 0.466 |
| Limb‡ sparing/amputation (%) | 20 (80%)/5 (20%) | 23 (100%)/0 (0%) | 0.051 |
| Good/poor response* (%) | 14 (64%)/8 (36%) | 14 (70%)/6 (30%) | 0.750 |
| Follow-up | |||
| Median [months] | 73.2 | 42.7 | |
| Events (progression/toxicity/death) | 12/0/8 | 1/1/2 |
p: statistical significance.
4 or 3 patients underwent surgical resection before administration of chemotherapy thus their histologic response was not evaluable.
Categories were appropriately grouped to enable valid testing.
Surgery was not possible in one patient.
Only one out of six poor responders in the mifamurtide group later died of progressive metastatic disease.
Some known side effects were observed during administration of mifamurtide. These were fever and chills in the majority of patients. One of the patients had myalgia/lumbalgia after the first dose of mifamurtide and another one had nausea and vomiting repeated several times. The relevance of adverse events was predominantly grade I or grade II (according to CTCAE v3.0) and all of them were successfully resolved after symptomatic medication. In general, most of the patients tolerated administration of mifamurtide really well. No patients required discontinuation of mifamurtide because of side effects and no major departures from the scheduled plan of administration were observed.
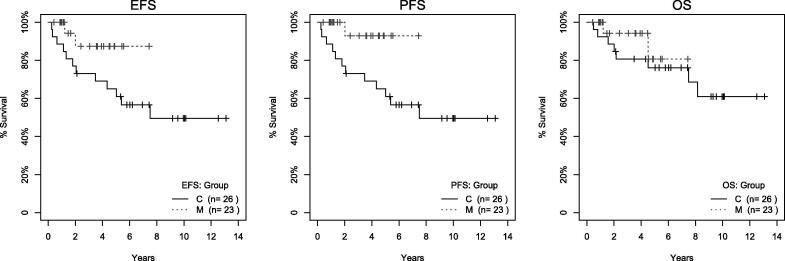
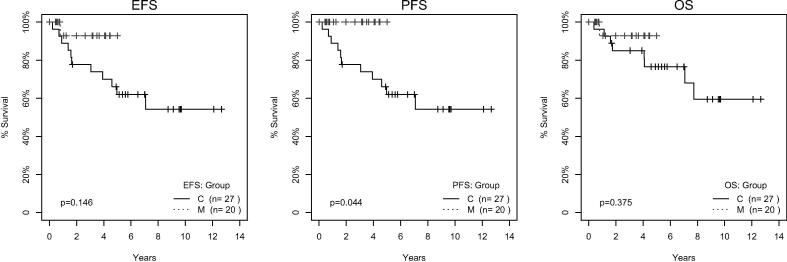
With a median follow-up period of 42.7 months in the mifamurtide group, 21 out of 23 patients are alive and in complete remission. One patient died of heart failure during a secondary myelodysplastic syndrome, one patient had metastatic progression to the lung and died eventually. None of the patients had loco-regional progression. The observed event free survival at 3 years was 87.4% (CI 72.4–100%), overall survival at 3 years was 94.1% (CI 83.6–100%), progression free survival at 3 years was 92.9% (CI 80.3–100%). These and 5-year survival estimates are shown in Table 2 and Fig. 1. Landmark analysis is shown in Fig. 2. The time point at twenty-two weeks after initiating chemotherapy was selected as the separation point because most (>85%) patient indicated for mifamurtide were already on the treatment and only 2 patients had to be excluded due to experiencing an event before that time point. Significantly better progression-free survival (log-rank test, p = 0.044) was identified between the control and mifamurtide group. Results from a time-dependent Cox model are summarized in Table 3. The risk of progression is 5-times lower in patients on mifamurtide than in controls (HR 0.21, p = 0.136). Neither event- nor over-all survival had a statistically significant difference between the two groups in either of the bias-adjusted models.
Table 2.
Kaplan-Meier estimates of 3- and 5-year survivals for control and mifamurtide group. Table shows survival estimates based on native observational data.
| Event | Survival | Control (95% CI) N = 26 |
Mifamurtide (95% CI) N = 23 |
|---|---|---|---|
| EFS | 3-year | 73.1 (57.9–92.3) % | 87.4 (72.4–100) % |
| 5-year | 65 (48.9–86.4) % | 87.4 (72.4–100) % | |
| PFS | 3-year | 73.1 (57.9–92.3) % | 92.9 (80.3–100) % |
| 5-year | 65 (48.9–86.4) % | 92.9 (80.3–100) % | |
| OS | 3-year | 80.6 (66.7–97.4) % | 94.1 (83.6–100) % |
| 5-year | 76.1 (61.1–94.9) % | 80.7 (58.3–100) % |
Fig. 1.
Raw Kaplan-Meier curves for control (C) and mifamurtide (M) group. The figure shows Kaplan-Meier curves for EFS (left), PFS (middle) and OS (right) for control (C, solid) and mifamurtide (M, dashed) using all native observational data.
Fig. 2.
Kaplan-Meier survival curves in Landmark analysis for control (C) and mifamurtide (M) group. The figure shows survival curves using the Landmark method which handles possible immortal time bias. Log-rank test comparing control and mifamurtide group is given.
Table 3.
Time dependent Cox models Table summarizes estimates of hazard ratios with corresponding p-values from time-dependent Cox models. Control was assumed as a reference group.
| Event | HR | P |
|---|---|---|
| EFS | 0.40 | 0.252 |
| PFS | 0.21 | 0.136 |
| OS | 0.57 | 0.489 |
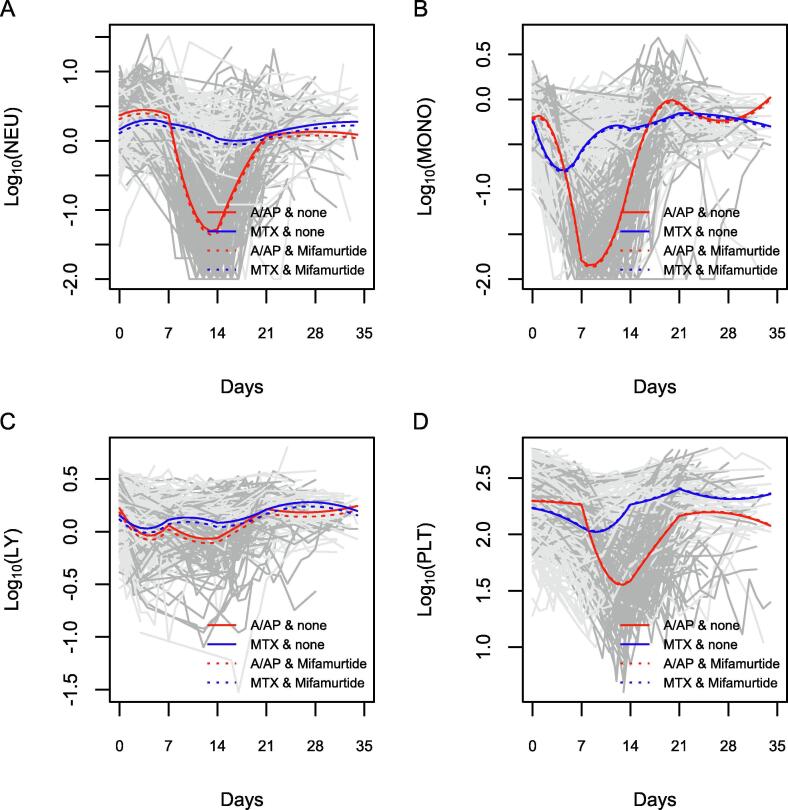
For the analysis of blood count dynamics, 19 patients from the mifamurtide and 23 patients from the control group with a total of 1492 and 1413, respectively, blood count data were available. Based on plotted data and fitted models (Fig. 3), different dynamics of the blood cells could be seen with a profound nadir of neutrophils, monocytes and platelets after A/AP compared to MTX cycles. The difference in the dynamics of lymphocytes was not so pronounced. Further, a nadir was observed earlier (median = 7 days) for monocytes than for neutrophils or platelets (median = 14 days). Although these “base” models were not the focus of our analysis, they helped verify the representativeness of the models which are in good agreement with reality.
Fig. 3.
Case-profile plots for blood count cells. The Fig. 3 shows profiles of neutrophils (A), monocytes (B), lymphocytes (C) and platelets (D) during A/P (red) and MTX (blue) cycles with (dotted) or without (solid) mifamurtide.
Finally, mifamurtide treatment was analysed in the above base models a time-dependent variable. Including just the main effect of mifamurtide did not improve the models (according to AIC and BIC criteria) with only borderline or non-significant effects for log10(NEU) = −0.053 (p = 0.075), log10(MONO) = −0.022 (p = 0.384), log10(LY) = −0.041 (p = 0.019) and log10(PLT) = 0.01 (p = 0.869) corresponding to lower average values of NEU or LY by about 9–11% in the mifamurtide group as a maximum difference identified. More complex models including time interactions with mifamurtide did not turn out to be better and were rather indicative for overfitting.
4. Discussion
To the authors’ knowledge these are the best results published for event free and progression free survival during the treatment of locoregional osteosarcoma in a mifamurtide group. A highly experienced team of orthopaedic surgeons describes local disease control with no observed locoregional relapse while reducing rate of amputations in the mifamurtide group. Surgery is the mainstay of treatment for osteosarcoma. All but one patient in the control group with a huge, unresectable tumour in the iliac bone overlapping to the sacrum and lumbar vertebra underwent complete resection and thus achieved complete remission. The surgery margins did not differ significantly between groups. the paediatric oncologists, orthopaedic surgeons, pathologist and radiologists in our centre have set up a multidisciplinary board with regular meetings. All specialists discuss the disease management issues for each patient - the timing and type of surgery and coordination with chemotherapy or adjuvant radiotherapy – to minimize treatment protocol deviations.
Recent analysis of the Euramos-1 study, which was conducted worldwide and included 1810 patients with a localized disease, demonstrated a 3-year and 5-year since diagnosis event free survival of 65% and 60%, and overall survival of 84% and 76%, respectively [11]. This study confirmed previously reported results from other cooperative groups or institutional series.
For evaluation of mifamurtide efficacy, it is better to consider metastatic free survival as mifamurtide is primarily targeted at the immunocompetent cells in the lungs. Results show that both 3-year and 5-year progression free survival is 92.9% (CI 80.3–100%), which is equal to metastatic free survival (no local progression was observed in the mifamurtide arm). The chest CT scan of one patient, who progressed after administration of mifamurtide, was reviewed and she had had unspecified nodularities at diagnosis which later progressed and were thus confirmed as initial metastatic disease. This patient was left in the analysis group, because almost half of the study’s patients had similar initial findings, e.g. unspecified lung nodularities (Table 1.). If patients with proven non-metastatic disease are counted – e.g. those who never progressed with initially observed unspecific nodularities - then the PFS would be even higher.
Also one other small size study confirms the better treatment result with mifamurtide as the 3-year overall and event free survival intervals are 87.5% and 75.6%, respectively [12].
A large French study on 126 patients reports the survival benefit of mifamurtide as being an 18% improvement in 3-year event free survival (52 vs.70%, HR 0.55), even worse than in this analysis and with a small difference compared to the Euramos results without mifamurtide [13].
For patients with metastatic disease, the published results show a trend to longer overall and event free survival after the addition of mifamurtide, but the phase III sample size was small and the improvement did not achieve conventional statistical significance. The results of an expanded access trial suggest a decreased risk of subsequent recurrence and death with the inclusion of mifamurtide in the treatment strategy for metastatic high-risk patients too [14].
Mifamurtide did not show any obvious effect on the dynamics of blood count data. The only and subtle difference was identified in lymphocyte counts. Compared to the interindividual variability, this difference was not considered clinically important to alter patients’ well-being. On the other hand, statistically significant lower values of lymphocytes might be indicative for lowering some lymphocyte populations, namely T-regulatory cells which are known tumour microenvironment suppressors and their quantitative changes reflects mifamurtide immunomodulating characteristics. However, lacking flow cytometry data, this hypothesis could not be verified in this study. These subtle differencies were not addressed in large study on 205 patients with metastatic and recurrent osteosarcoma describing pharmakokinetic, pharmacodynamic and safety of mifamurtide given as monotherapy [15]. In this study thrombocytopenia and neutropenia were reported in 5% both which is incomparable to our results collected from patients on combination treatment with chemotherapy.
This study has some limitations. First of all, the sample size is small with short follow-up for patients with mifamurtide. It would need one and half more years for robust 5-year survival estimates to conclude that 3-year estimate survivals will be maintained. Furthermore, comparative analysis with the historical control may always suffer from enthusiastic bias due to shorter follow-up in the latter group. Another major issue might arise from the observational nature of the study and the indication of mifamurtide in a later period of the treatment, which is the immortal time bias. This issue was, however, addressed using two methods – the Landmark [5] method for non-parametric comparison and enabling visualization, and using the time-dependent Cox model [6]. PFS did not reach statistical significance in the later analysis, though still showing the same trend. Small sample size, the imbalance between the two groups in the parametric analysis, may have influenced the results. It is thought, that the fact that EFS/OS did not reach statistical significance was, besides the small number of events, influenced by toxicity death in the mifamurtide group not related to mifamurtide and OS is, in general, a more conservative measure than PFS. As all models render congruent findings, it is concluded, that mifamurtide prolongs the time to progression.
Of note, osteosarcoma in children is a very rare disease and thus various design and analytical issues arise due to the small sample sizes. It is believed that observational data and comparison with historical control, utilizing repeated measurements, using suitable surrogates or even translational evidence, whilst being cautious of the limitations and addressing the bias issues appropriately, may offer beneficial support for clinical guidance in situations where classical, large, randomized control trials are both time-consuming and expensive.
5. Conclusion
It is concluded that a combination of chemotherapy, surgery and mifamurtide in adjuvant setting could be a better treatment option for young patients with osteosarcoma until novel therapies are defined. An experienced, multidisciplinary, advisory task board is essential in each centre, which cares for osteosarcoma patients. The expert team should make every effort at all times to avoid protocol deviations and keep treatment schedules as recommended. More prospective trials or metanalyses of published experiences is warranted to confirm the role of mifamurtide on survival benefit.
Funding
Supported by the European Regional Development Fund - Project ENOCH (No. CZ.02.1.01/0.0/0.0/16_019/0000868), project CZECRIN_4 PACIENTY (No. CZ.02.1.01/0.0/0.0/16_013/0001826) and project Large Research infrastructure CZECRIN (LM2018128); LQ1605 from the National Programme of Sustainability II (MEYS CR); MH CZ - DRO (FNBr, 65269705); MUNI/A/1409/2019.
CRediT authorship contribution statement
Peter Múdry: Conceptualization, Investigation, Methodology, Validation, Writing - original draft, Writing-review and editing. Michal Kýr: Formal analysis, Investigation, Methodology, Validation, Writing - original draft, Writing-review and editing. Ondřej Rohleder: Data curation, Validation, Writing-review and editing. Michal Mahdal: Data curation, Writing-review and editing. Iva Staniczková Zambo: Investigation. Marta Ježová: Investigation. Tomáš Tomáš: Funding acquisition. Jaroslav Štěrba: Conceptualization, Funding acquisition.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Other support: Takeda Pharmaceutical Company Limited supported post marketing analysis of usage and adverse events related to their drug mifamurtide, which in part was used in this analysis.
References
- 1.Ottaviani G., Jaffe N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 2.Kager L., Pötschger U., Bielack S. Review of mifamurtide in the treatment of patients with osteosarcoma. Ther. Clin. Risk Manage. 2010;6:279–286. doi: 10.2147/tcrm.s5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kansara M., Teng M.W., Smyth M.J., Thomas D.M. Transitional biology of Osteosarcoma. Nat. Rev. 2014;14:722–735. doi: 10.1038/nrc383. [DOI] [PubMed] [Google Scholar]
- 4.Chou A.J., Kleinerman E.S., Krailo M.D., Chen Z., Betcher D.L., Healey J.H., 3rd Conrad E.U., Nieder M.L., Weiner M.A., Wells R.J., Womer R.B., Meyers P.A. Children's Oncology Group. Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: a report from the Children's Oncology Group. Cancer. 2009;115:5339–5348. doi: 10.1002/cncr.24566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher L.D., Lin D.Y. Time-dependent covariates in the Cox proportional-hazards regression model. Annu. Rev. Public Health. 1999;20:145–157. doi: 10.1146/annurev.publhealth.20.1.145. [DOI] [PubMed] [Google Scholar]
- 6.Anderson J.R., Cain K.C., Gelber R.D. Analysis of survival by tumor response. J. Clin. Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 7.Singer J.D., Willett J.B. Oxford University Press; 2003. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. [Google Scholar]
- 8.T. Therneau. A Package for Survival Analysis in S_. version 2.38, https://CRAN.R-project.org/package=survival. 2015.
- 9.J. Pinheiro, D. Bates, S. DebRoy, D. Sarkar and R Core Team. _nlme: Linear and Nonlinear Mixed Effects Models_. R package version 3.1-139, https://CRAN.R-project.org/package=nlme. 2019.
- 10.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org. 2008.
- 11.Smeland S., Bielack S.S., Whelan J., Bernstein M., Hogendoorn P., Krailo M.D., Gorlick R., Janeway K.A., Ingleby F.C., Anninga J., Antal I., Arndt C., Brown K.L.B., Butterfass-Bahloul T., Calaminus G., Capra M., Dhooge C., Eriksson M., Flanagan A.M., Friedel G., Gebhardt M.C., Gelderblom H., Goldsby R., Grier H.E., Grimer R., Hawkins D.S., Hecker-Nolting S., Hall K.S., Isakoff M.S., Jovic G., Kühne T., Kager L., von Kalle T., Kabickova E., Lang S., Lau C.C., Leavey P.J., Lessnick S.L., Mascarenhas L., Mayer-Steinacker R., Meyers P.A., Nagarajan R., Randall R.L., Reichardt P., Renard M., Rechnitzer C., Schwartz C.L., Strauss S., Teot L., Timmermann B., Sydes M.R., Marina N., Survival and prognosis with osteosarcoma: outcomes in more than patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur. J. Cancer. 2019;109:36–50. doi: 10.1016/j.ejca.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tacyildiz N., Incesoy O.S., Unal E., Berber M., Dincaslan H., Yavus G. The Efficiency and toxicity of mifamurtide in childhood osteosarcoma. J. Pediatr. Hematol. Oncol. 2018;40:e373–e376. doi: 10.1097/mph.0000000000001236. [DOI] [PubMed] [Google Scholar]
- 13.Brard C., Piperno-Neumann S., Delaye J. Sarcome-13/OS2016 trial protocol: a multicentre, randomised, open-label, phase II trial of mifamurtide combined with postoperative chemotherapy for patients with newly diagnosed high-risk osteosarcoma. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-025877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyers P.A., Chou A.J. Muramyl tripeptide-phosphatidyl ethanolamine encapsulated in liposomes (L-MTP-PE) in the treatment of osteosarcoma. Adv. Exp. Med. Biol. 2014;804:307–321. doi: 10.1007/978-3-319-04843-7_17. [DOI] [PubMed] [Google Scholar]
- 15.Anderson P.M., Meyers P., Kleinerman E., Venkatakrishnan K., Hughes D.P., Herzog C., Huh W., Sutphin R., Vyas Y.M., Shen V., Warwick A., Yeager N., Oliva C., Wang B., Liu Y., Chou A. Mifamurtide in metastatic and recurrent osteosarcoma: A patient access study with pharmacokinetic, pharmacodynamic, and safety assessments. Pediatr. Blood Cancer. 2014;61:238–244. doi: 10.1002/pbc.24686. [DOI] [PMC free article] [PubMed] [Google Scholar]