Summary
Using in vivo muscle stem cell (satellite cell)-specific extracellular vesicle (EV) tracking, satellite cell depletion, in vitro cell culture, and single-cell RNA sequencing, we show satellite cells communicate with other cells in skeletal muscle during mechanical overload. Early satellite cell EV communication primes the muscle milieu for proper long-term extracellular matrix (ECM) deposition and is sufficient to support sustained hypertrophy in adult mice, even in the absence of fusion to muscle fibers. Satellite cells modulate chemokine gene expression across cell types within the first few days of loading, and EV delivery of miR-206 to fibrogenic cells represses Wisp1 expression required for appropriate ECM remodeling. Late-stage communication from myogenic cells during loading is widespread but may be targeted toward endothelial cells. Satellite cells coordinate adaptation by influencing the phenotype of recipient cells, which extends our understanding of their role in muscle adaptation beyond regeneration and myonuclear donation.
Subject Areas: Cell Biology, Functional Aspects of Cell Biology
Graphical abstract

Highlights
-
•
Widespread myogenic cell communication in muscle during mechanical overload (MOV)
-
•
Extracellular vesicles from myogenic progenitors repress Wisp1 in fibrogenic cells
-
•
Fibrogenic fate and chemokines are influenced by satellite cells early during MOV
-
•
Prior to fusion, satellite cell communication regulates long-term hypertrophy
Cell Biology; Functional Aspects of Cell Biology
Introduction
Tissue remodeling is a multi-faceted, multi-phasic process involving the coordinated regulation of numerous cell types (Farup et al., 2015; Wosczyna and Rando, 2018). Cellular choreography early in response to stress, specifically with respect to extracellular matrix (ECM) deposition and inflammatory signaling, generally determines whether long-term homeostasis is properly restored (Adler et al., 2020). In skeletal muscle, Pax7+ satellite cells mediate myonuclear accretion during muscle fiber growth (Bachman et al., 2018; McCarthy et al., 2011) and in response to exercise irrespective of growth (Masschelein et al., 2020; Murach et al., 2020b). Satellite cells may also play an important role in muscle adaptation by regulating various cell types via secreted factors (Chazaud et al., 2003; Christov et al., 2007; Fry et al., 2014a; Liu et al., 2015; Murphy et al., 2011; Patsalos et al., 2017; Tatsumi et al., 2009; Verma et al., 2018). With mechanical loading-induced skeletal muscle hypertrophy, numerous lines of evidence across different species suggest that muscle fibers can adapt and grow appreciably without satellite cell fusion and myonuclear accretion (Murach et al., 2018a); however, long-term hypertrophy without satellite cells is attenuated concomitant with excess ECM accumulation (Fry et al., 2014a). Our laboratory recently showed that satellite cells may communicate with fibrogenic cells (Fry et al., 2017) and muscle fibers (Murach et al., 2020c) via miRNA-containing extracellular vesicles (EVs) to regulate the muscle fiber milieu during hypertrophy. Presently, it is unclear whether early satellite cell-mediated secretory signaling throughout muscle is sufficient for long-term adult muscle hypertrophy or whether myonuclear addition is required to sustain hypertrophy.
In the current investigation, we utilize fluorescence-activated cell sorting (FACS), single-cell RNA sequencing (scRNA-seq), cell fate prediction analyses, inducible satellite cell depletion (McCarthy et al., 2011), in vivo EV tracking (Murach et al., 2020c), long-term mechanical overload (MOV), and in vitro approaches to explore satellite cell communication throughout muscle during load-induced hypertrophy. We provide evidence that satellite cell communication to fibroadipogenic progenitor cells (FAPs) during the early stage of MOV is sufficient to regulate ECM deposition and promote long-term hypertrophy in adult mice, independent from satellite cell fusion. Satellite cell depletion influences FAP fate progression toward fibrogenic and osteogenic transcriptional programs early during overload in vivo, concomitant with global- and FAP-specific upregulation of chemokine gene expression. Through delivery of miR-206 packaged in EVs, we identify a mechanism whereby satellite cells regulate fibrosis-associated Wnt-inducible signaling pathway protein 1 (Wisp1, also known as Ccn4) in FAPs during hypertrophy, the absence of which is associated with aberrant fibrogenic cell fate progression. We also provide evidence that satellite cell EV communication after the first few weeks of MOV is widespread but may be accentuated in endothelial cells. Fusion-independent satellite cell communication is a powerful stimulus to promote adult muscle growth, and we provide support for a model where initial reparative events trigger a cascade of cellular synchronization that enables long-term tissue homeostasis (Adler et al., 2020).
Results
EV-mediated communication from myogenic to mononuclear cells following 14 days of mechanical overload
We report that there is a ∼275% increase in satellite cells concomitant with robust hypertrophy after 14 days of synergist ablation-induced MOV of the plantaris muscle (Kirby et al., 2016a; McCarthy et al., 2011). Using the Pax7CreER/+;R26tdTomato+/- reporter mouse (Pax7-tdT), we previously demonstrated tdTomato (tdT) protein and mRNA in myogenic progenitor cell (MPC)-derived EVs, as well as tdT transfer to recipient cells via MPC EVs in vitro (Murach et al., 2020c); Video S1 is a high-magnification video showing tdT+ MPC EVs. Packaging of cytoplasmic localized reporter mRNA and/or protein in EVs is consistent with prior investigations (Aswad et al., 2014; Cossetti et al., 2014; Flaherty et al., 2019; Forterre et al., 2014; Gao et al., 2020; Kanada et al., 2015). Based on these results, we used the Pax7-tdT model to explore in vivo EV tracking from myogenic cells (satellite cells and/or myofibers) to mononuclear cells throughout muscle during hypertrophic growth in response to 14 days of MOV (Figure 1A).
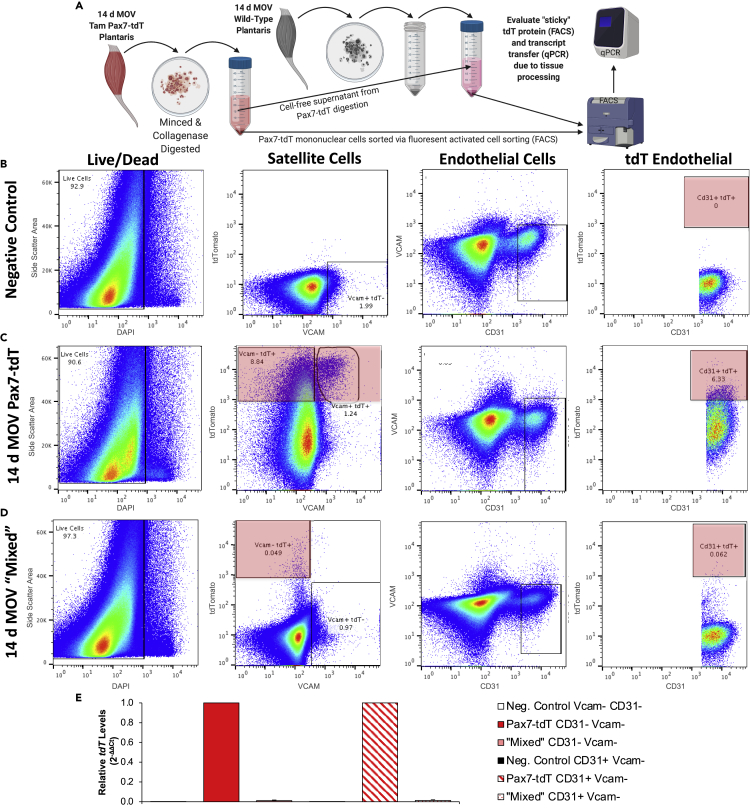
Figure 1.
Delivery of tdTomato (tdT) from myogenic cells to mononuclear cells throughout skeletal muscle during 14 days of mechanical overload (MOV) of the plantaris
(A) Experimental design schematic showing vehicle (Veh) or tamoxifen (Tam) administration for 5 consecutive days (5 d), washout period, and 14 day MOV in Pax7-tdT mice; n = 3 mice/6 plantaris muscles pooled for analysis captured at the same exposure and adjusted the same as the image in (B), scale bar, 20 μm.
(B) Flow cytometry plot illustrating Vcam+ tdT- satellite cells (SCs) of Veh-treated Pax7-tdT MOV plantaris muscle; SCs were also negative for CD31 (endothelial cell marker), CD45 (immune cell marker), and Sca1 (mesenchymal stem cell marker).
(C) Flow cytometry plot illustrating Vcam+ tdT+ SCs (population 1) in Tam-treated Pax7-tdT MOV plantaris muscle. SCs were also CD31-/CD45-/Sca1-.
(D) Flow cytometry plot illustrating endothelial, immune, and mesenchymal stem cells in Veh-treated Pax7-tdT MOV muscle, all identified via FITC-conjugated antibodies (population 2).
(E) Flow cytometry plot illustrating endothelial, immune, and mesenchymal cells that were tdT- (population 3) and tdT+ (population 4) in Tam-treated Pax7-tdT MOV muscle, all identified via FITC-conjugated antibodies. Another cloud of tdT+ cells (population 5, most likely fibrogenic cells) was also identified.
(F) Quantitative real-time PCR for tdT mRNA abundance in populations 1–5. Dotted line represents tdT- cells sorted from the tdTfl/fl parental strain (n = 3/group: Veh, Tam, and tdT parental mice, each pooled for sorting and RNA isolation), normalized to Gapdh and presented relative to parental strain cells using 2−ΔΔCt.
We conducted FACS on cell suspensions from vehicle- and tamoxifen-treated 14-day MOV Pax7-tdT plantaris muscles, MOV tamoxifen-treated tdT parental strain muscles, and resting tamoxifen-treated Pax7-tdT muscles (flow cytometry gating for these experiments is in Figures S1A–S1D). As expected, satellite cells from vehicle-treated 14-day MOV Pax7-tdT and parental muscles were tdT negative (tdT-, Figure 1B for vehicle, parental shown in Figure S1A), and most satellite cells from tamoxifen-treated MOV Pax7-tdT muscles were brightly tdT fluorescent (Figure 1C, population 1). In muscles from MOV vehicle-treated mice, non-satellite mononuclear cells (CD31 + endothelial cells, CD45 + immune cells, and/or Sca1+ mesenchymal cells) were tdT- (Figure 1D, population 2). This same population in muscles from tamoxifen-treated mice (Figure 1E, population 3) was expanded rightward, with some cells displaying clear tdT fluorescence (Figure 1E, population 4), and a second population that presumably contained a proportion of Sca1- FAPs/fibrogenic cells (Chapman et al., 2016) also appearing tdT+ (Figure 1E, population 5). Resting tamoxifen-treated Pax7-tdT muscle (collected from the forelimb and hindlimb) did not display appreciable tdT fluorescence other than in satellite cells, suggesting the appearance of tdT is mediated by activated satellite cells during MOV (Figure S1D). Based on the shift in tdT fluorescence observed via FACS in the tamoxifen condition, we posit that the appearance of tdT in non-satellite cells is due to spontaneous off-target Cre-mediated recombination because: 1) average fluorescence levels were appreciably lower than in satellite cells indicating the appearance of tdT was not due to constitutive expression, 2) there was negligible fluorescence in 14 day MOV tdTfl/fl parental strain plantaris muscles nor resting tamoxifen-treated Pax7-tdT muscle. Within cell populations 1–5, quantitative reverse-transcription PCR (RT-qPCR) revealed the highest relative tdT mRNA levels in tamoxifen-treated satellite cells (indicative of constitutive viral promoter-driven expression following recombination), moderate tdT mRNA in populations that had some tdT fluorescence, and low levels in all others (Figure 1F).
Evidence that the appearance of tdT in non-satellite cells during MOV is not due to off-target recombination or technical artifact from tissue processing
We evaluated recombination in genomic DNA of MPCs from vehicle- and tamoxifen-treated Pax7-tdT mice, as well as from the tdTfl/fl parental strain, and found that recombination of the stop cassette was only detectable in Pax7-tdT MPCs following tamoxifen treatment (Figures S2A and S2B). Analysis of DNA from pooled, non-satellite cell populations (CD31-/Vcam-, mesenchymal, fibrogenic, and immune cells), as well as CD31 + endothelial cells from tamoxifen-treated Pax7-tdT plantaris muscle following 14 days of MOV, showed the stop cassette was intact, indicating that fluorescence was not due to off-target recombination (Figures S2A and S2B). To determine if the tdT protein detected in non-satellite cells might have been the result of tdT protein from lysed cells “sticking” to mononuclear cells in the single-cell suspension, we digested tamoxifen-treated 14-day MOV Pax7-tdT plantaris muscles and then used the Pax7-tdT cell-free supernatant to digest wild-type (no tdT transgene in genome) MOV plantaris muscles (Figure 2A); primary cells from digested wild-type muscle were used as negative controls. FACS confirmed no tdT signal in controls (Figure 2B), tdT fluorescence in Pax7-tdT MOV muscle (Figure 2C), and minimal fluorescent labeling from “sticky” tdT protein and/or cellular debris as a consequence of the digestion process (Figure 2D). Furthermore, isolation of Vcam-/CD31- and Vcam-/CD31 + cells from wild-type muscles followed by qPCR showed that exposure to cell-free supernatant from overloaded wild-type muscles (“mixed” in Figure 2E) did not account for tdT transcript levels observed in those cell populations from tamoxifen-treated Pax7-tdT MOV muscles (Figure 2E). The results of these experiments collectively indicated that neither off-target recombination nor cellular disruption and non-specific labeling/uptake can explain the magnitude of the appearance of tdT protein and transcript in non-satellite cells of tamoxifen-treated MOV Pax7-tdT muscle. Our results are also consistent with similar experiments conducted in thymus tissue using two comparable inducible cell-specific reporter Cre models after observations of fluorescence transfer between cells in vivo (Wang et al., 2016).
Figure 2.
Presence of tdTomato (tdT) protein and mRNA in non-satellite cells (SCs) during mechanical overload (MOV) is not attributable to technical artifact from tissue processing
(A) Experimental design illustrating how cell-free supernatant from digested Tam-treated 14-day MOV Pax7-tdT muscles (n = 2 mice/4 plantaris) was used to digest wild-type (no tdT transgene in genome) 14 d MOV muscles (“mixed”, n = 3 mice/2 separate pools of 3 plantaris, processed as technical replicates), followed by FACS isolation of different populations for downstream analyses.
(B) Flow cytometry plots from muscle digest of unperturbed wild-type muscle (triceps and quadriceps), used as a negative control for tdT fluorescence; forward and side scatter area gating not shown.
(C) Flow cytometry plots from Tam-treated 14-day MOV Pax7-tdT muscles, confirming the appearance of tdT in satellite and non-satellite cell (endothelial and non-endothelial) populations; forward and side scatter area gating not shown.
(D) Flow cytometry plots from MOV wild-type control plantaris muscle digested with the cell-free supernatant from Tam-treated 14-day MOV Pax7-tdT plantaris muscle (“mixed”); forward and side scatter area gating not shown.
(E) tdT mRNA levels in Vcam-/CD31- mononuclear cells and CD31+/Vcam- endothelial cells in resting control, Tam-treated 14-day MOV Pax7-tdT, and 14-day MOV wild-type control muscle “mixed” with cell-free supernatant from the Pax7-tdT muscle digestion, normalized to 18S rRNA and presented relative to the respective cell type in the Pax7-tdT condition using 2−ΔΔCt.
See also Figures S2 and S3.
scRNA-seq reveals the identity of tdT+ mononuclear cells after 14 days of MOV
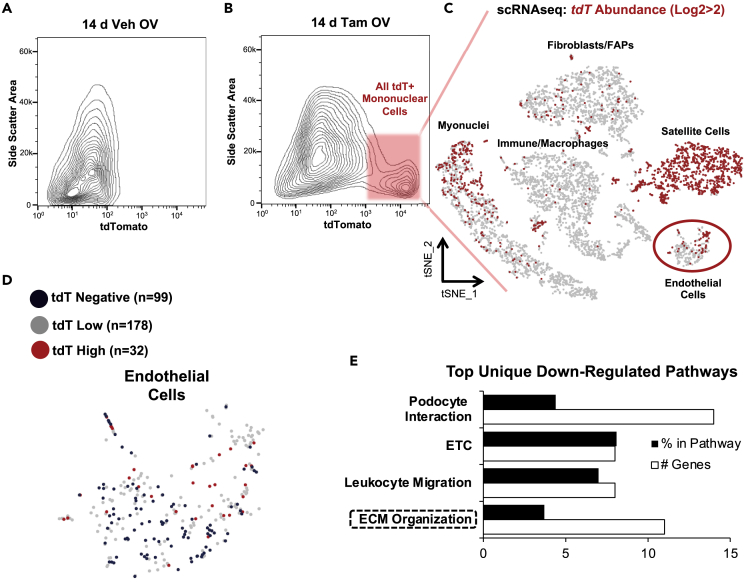
To further interrogate the identity of tdT+ mononuclear cells in muscle after 14 days of MOV, we conducted FACS and isolated all cells with tdT fluorescence in tamoxifen-treated 14-day MOV Pax7-tdT muscles (Figures 3A and 3B) and conducted droplet-based 10X Genomics Chromium scRNA-seq. The scRNA-seq preparation was enriched for satellite cells, as would be expected, but a variety of cell types according to global transcriptional profiles were also identified via scRNA-seq, including fibrogenic cells (fibroblasts and FAPs), immune cells/macrophages, and endothelial cells (Figure 3C). These data suggest that by 14 days of MOV, myogenic cells communicate with the major mononuclear cell populations throughout the muscle. Myonuclei also appeared in the preparation, identified by myosin heavy chain transcripts, consistent with recent reports (De Micheli et al., 2020; Oprescu et al., 2020). To interrogate tdT message within the scRNA-seq data set, we created a custom transcriptome where the tdT transgene (Madisen et al., 2010) was included in the mouse mm10 annotated transcriptome. At the transcript level, the highest tdT levels were in satellite cells and what were likely satellite cell-derived myonuclei (top of “myonuclei” cluster, Figure 3C). Myonuclei with very high tdT expression (log2tdT>3) had elevated levels of cell cycle regulatory genes Cdkn1a and Btg2, as well as myosin light chain 1 embryonic (Myl4) (false discovery rate [FDR] adjusted p < 0.05) and developmental myosins (Myh3 and Myh8), suggesting nascent myonuclei as a result of satellite cell fusion.
Figure 3.
Single-cell RNA sequencing (scRNA-seq) analysis of extracellular vesicle (EV)-mediated communication from myogenic cells during 14 days of mechanical overload (MOV)
(A) Flow cytometry plot illustrating a lack of tdTomato+ (tdT+) cells in Veh-treated 14 d MOV Pax7-tdT plantaris muscle (n = 1 mouse/2 plantaris muscles, used for establishing background fluorescence).
(B) Flow cytometry plot illustrating tdT+ cells in Tam-treated 14 d MOV plantaris muscle (n = 4 mice/8 plantaris muscles pooled for analysis).
(C) t-distributed stochastic neighbor embedding atlas representing appreciable (log2>2) tdT transcript detected in the Tam-treated Pax7-tdT MOV sample via scRNA-seq.
(D) Endothelial cell cluster from t-distributed stochastic neighbor embedding (t-SNE) atlas, illustrating tdT-negative cells (Log2tdT, tdT 5′, Pax7, Myod, and Myog = 0, n = 99 blue cells) and tdT high cells (Log2tdT > 2, tdT5′, Pax7, Myod, and Myog = 0, n = 32 red cells).
(E) Top unique downregulated pathways from KEGG, WikiPathways, and Reactome analyses using the top 100 differentially expressed genes in tdT high vs tdT-negative endothelial cells from the tdT+ FACS isolated cluster, organized in descending order according to p value (all were p < 0.05). ETC, electron transport chain, ECM, extracellular matrix.
See also Figure S4.
We were uncertain whether transfer of tdT mRNA via satellite cell EVs to non-myogenic cells would be detectable using scRNA-seq given the relatively low sequencing depth obtained with this technique, as well as the potentially large amount of EV communication that would be required to occur. Recent evidence suggests that EV material transfer from wild-type cells of one lineage in vivo can be sufficient to restore the protein levels of a knocked-out gene in a distinct cell type (Crewe et al., 2018). The findings of Crewe et al. illustrate the robust and under-appreciated potential of EV-mediated cargo transfer, which made the detection of tdT transcript on a cell-by-cell basis in non-satellite cell populations via scRNA-seq seem worthwhile to explore. To first determine background levels of tdT mRNA, we conducted scRNA-seq on cells from tamoxifen-treated 14-day MOV tdTfl/fl parental strain plantaris muscles. Despite the absence of detectable fluorescence via FACS (see Figure S1), low levels of full-length tdT transcript were detected in some mononuclear cells via scRNA-seq, indicating occasional readthrough of the stop cassette (3x SV40 poly(A) signal) in the R26R-tdTfl/fl mouse (green box, Figure S3A). As expected, we also were able to detect abundant truncated tdT transcript (a short polyadenylated message generated by transcription up to the stop sequence, Figure S3A) providing evidence that the stop cassette was intact (Madisen et al., 2010) (Figure S3A). To account for the low level tdT expression, we generated a null distribution using the ratio of tdT full length to tdT truncated for each cell in the MOV tdT parental scRNA-seq data, where any full-length tdT transcript would be expected to be a consequence of readthrough (or perhaps spurious recombination). Using the non-parametric prediction interval and the probability for each sequenced cell, we determined whether its ratio was functionally indistinguishable from the null distribution of background readthrough and then corrected for multiple comparisons using FDR. With these criteria, very few cells in the parental MOV experiment had tdT expression above background levels (Figure S3B). Conversely, many cells had full-length tdT expression above the readthrough background levels in the Pax7-tdT MOV experiment (with varying degrees of confidence according to FDR, see Figure S3B). Furthermore, truncated tdT transcript levels can be used as a second method to assess off-target recombination, where high levels relative to a control (e.g. recombined satellite cells in tamoxifen-treated Pax7-tdT mice) indicate that the stop cassette effectively prevented constitutive expression of the downstream tdT coding sequence. Truncated transcript was virtually undetectable in Pax7+ and MyoD + cells (satellite cells) in the 14-day MOV Pax7-tdT experiment but was expressed at high levels in all other cell populations, providing further evidence of minimal off-target recombination in non-myogenic cells (Figures S3C and S3D).
We asked whether non-myogenic cells containing higher versus lower amounts of tdT mRNA in the FACS isolated tdT+ cells could be discriminated in the scRNA-seq data, suggesting functional consequences to potentially higher levels of EV-based communication in vivo. Besides satellite cells and myonuclei (Figures 3C), we noticed that a subset of endothelial cells had the highest absolute levels of full-length tdT transcript (Figure 3D). Although absolute endothelial cell numbers were small, we compared the transcriptional profiles of tdT high endothelial cells (log2tdT>2, not expressing Pax7, MyoD, and MyoG, and residing within the CD31/Pecam1-enriched endothelial cluster) to endothelial cells lacking tdT. Global pathway analysis of the top 100 genes with lower expression (using absolute as well as Log2 differences) revealed ECM organization as one of the top unique downregulated processes in tdT-high endothelial cells (Figures 3E and S4A). Furthermore, the ECM-remodeling genes Timp4 and Comp were trending to be lower in tdT-high endothelial cells (adj. p < 0.10). Some genes were lower in tdT-high endothelial cells as well, suggesting myogenic cells may influence angiogenic-related processes via EV communication (Figure S4B). In silico analysis using miRNAs abundant in MPC EVs (Murach et al., 2020c) predicted miRNA interactions consistent with changes in target gene expression in endothelial cells (Figure S4C). While this preliminary analysis was instructive, the presence of high tdT-expressing myonuclei after 14 days of MOV prevents us from drawing firm conclusions regarding the definitive source of tdT-containing EVs in vivo (i.e. satellite cells versus muscle fiber that experienced satellite cell fusion).
Satellite cell communication to mononuclear cells during the first 96 hr of MOV affects the ECM and chemokine gene expression
We performed a similar scRNA-seq experiment as above but at an earlier time point, prior to the fusion of satellite cells to myofibers, to determine the precise source of tdT in non-satellite cells after MOV in Pax7-tdT muscle. After subjecting tamoxifen-treated Pax7-tdT mice to MOV for 96 hr, we harvested the muscle, removed Vcam+ cells via FACS, and then conducted scRNA-seq (see Figure S5 for flow cytometry gating). We did not observe a prevalent population of tdT+ non-satellite cells via FACS (as in the 14-d MOV experiments), and utilizing the aforementioned full length to truncated tdT transcript ratio, background correction did not reveal bona fide tdT mRNA-containing cells in the preparation. It is conceivable that 96 hr of MOV was not a long enough duration for satellite cells to proliferate sufficiently and transfer enough tdT mRNA to mononuclear cells to be detectable by scRNA-seq.
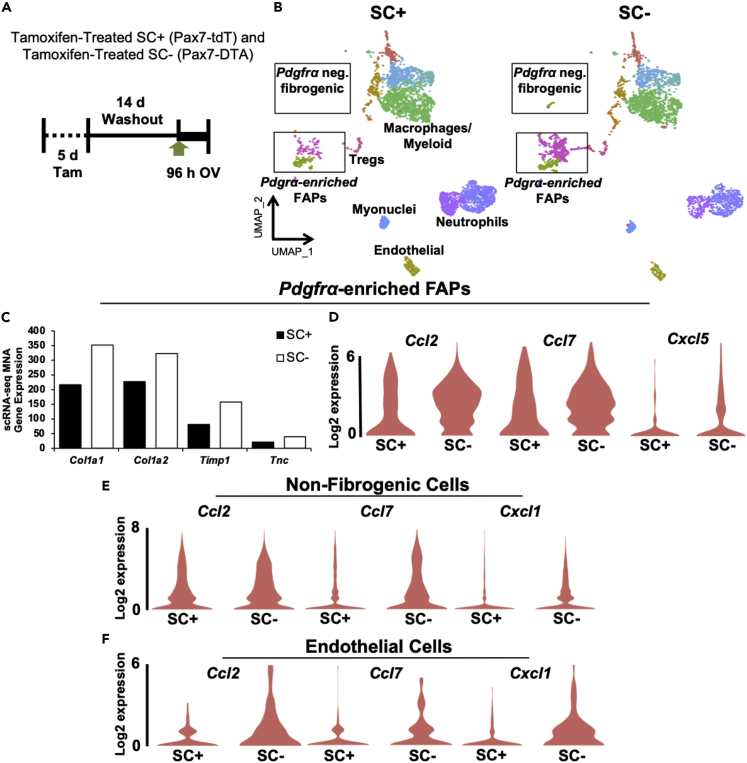
Given the technical challenges of blocking cell type-specific EV release (Gandham et al., 2020; Vechetti et al., 2020), and consistent with our previously published approach (Murach et al., 2020c), we employed our inducible in vivo satellite cell depletion model, the Pax7-DTA mouse (McCarthy et al., 2011; Murach et al., 2017), to gain insight into how satellite cells communicate throughout the muscle. We subjected tamoxifen-treated Pax7-DTA mice to MOV for 96 hr (Figure 4A), removed Vcam+ cells, conducted scRNA-seq, and then compared these results to the 96 hr MOV satellite cell replete experiment above (Figure 4B). In context with our prior work showing a secretory effect of satellite cells on fibrogenic cells during overload (Fry et al., 2014a, 2017), we first focused on differences in FAPs between satellite cell deplete and replete muscle. In the Pdgfrα-enriched FAP cluster, Col1a1 (the most differentially expressed gene in absolute terms) was 62% higher in the absence of satellite cells (adjusted p < 0.10, Figure 4C). Other highly expressed ECM genes such as Col1a2 (42% higher), Timp1 (93% higher, adj. p < 0.05), and Tnc (83% higher, adj. p = 0.07) were also higher in the absence of satellite cells during MOV (Figure 4C). Furthermore, FAPs from satellite cell-depleted muscle after 96 hr of MOV had higher Ccl2 (90%, adj. p < 0.05), Ccl7 (98%, adj. p < 0.05), and Cxcl5 (453%, adj. p < 0.05) (Figure 4D); this is noteworthy since upregulation of these specific immune markers distinguishes activated FAPs within 12 hr of regenerative injury (Oprescu et al., 2020). Satellite cells also affected immune gene expression outside of fibrogenic cells. Abundant chemokines such as Ccl2 (p = 0.12), Ccl7, and Cxcl1 were higher in mononuclear cells throughout the muscle in the absence of satellite cells (adj. p < 0.05, Figure 4E). Given the potential endothelial cell-specific effect of myogenic cell EV communication observed at 14 days of MOV, we interrogated endothelial cells at 96 hr and observed a similar induction in chemokine gene expression in the absence of satellite cells, which included higher Ccl2, Ccl7, and Cxcl1 (adj. p < 0.05, Figure 4F).
Figure 4.
scRNA-seq after 96 hr of mechanical overload (MOV) in the presence (SC+) and absence (SC-) of satellite cells
(A) Experimental design schematic demonstrating the Tam treatment strategy, washout period, and 96 hr synergist ablation-induced MOV in Pax7-tdT (SC+) and Pax7-DTA (SC-) mice, n = 4 mice/8 pooled plantaris per group.
(B) Uniform manifold approximation and projection (UMAP) atlas of scRNA-seq data in 96 hr MOV SC+ and SC- plantaris muscle. FAP, fibro/adipogenic progenitor cell, Treg, T-regulatory cell.
(C) Expression of extracellular matrix-related genes in Pdgfrα-enriched FAPs during MOV in the presence (SC+) and absence (SC-) of SCs.
(D–F) Expression of chemokine genes in the presence or absence of SCs in (D) Pdgfrα-enriched FAP clusters, (E) non-fibrogenic cells, and (F) endothelial cells. MNA, median normalized average.
See also Figure S5.
Satellite cell communication during the first 96 hr of MOV involves an EV-mediated miR-206/Wisp1 axis
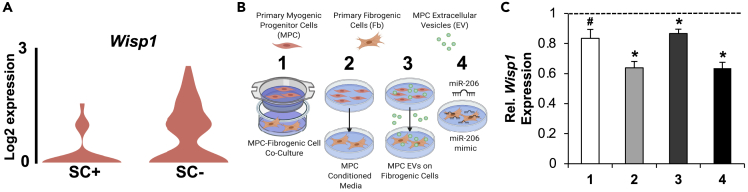
Wisp1 is a secreted matricellular protein that was recently shown to be enriched in FAPs (Lukjanenko et al., 2019; Oprescu et al., 2020) and is generally associated with fibrogenic cell proliferation and fibrosis (Berschneider et al., 2014; Berschneider and Königshoff, 2011; Colston et al., 2007; Ono et al., 2018; Venkatachalam et al., 2009). Immune gene-enriched FAPs transition to ECM gene-enriched Wisp1+ FAPs between 3.5 and 5 days after injury, indicative of cell state progression toward a more fibrogenic phenotype (Oprescu et al., 2020). FAPs in the absence of satellite cells during MOV had higher Wisp1 (93%, adj. p < 0.05), which points to accelerated progression of a fibrogenic program in FAPs when satellite cells are absent; this was especially apparent in the FAP cluster most enriched with Pdgfrα (Figure 5A). In MPC EVs, miR-206 is the most abundant miRNA (Fry et al., 2017; Murach et al., 2020c), and several bioinformatic analyses in the context of cancer (Seifi-Alan et al., 2018), as well as murine RNA hybrid analysis (ΔG −21.7 for binding) (Krüger and Rehmsmeier, 2006), indicated that Wisp1 could be targeted by miR-206. We reasoned that EV delivery of miR-206 could explain lower Wisp1 in FAPs during MOV in the presence of satellite cells in our scRNA-seq experiment. Incubation of primary fibrogenic cells (equivalent to FAPs) (Contreras et al., 2019) with MPC-conditioned media, co-culture with MPCs, and treatment with MPC EVs (Figure 5B) all lowered Wisp1 in fibrogenic cells (p < 0.05, Figure 5C). Transfection of miR-206 into fibrogenic cells similarly reduced Wisp1 (p < 0.05, Figure 5C). Other miRNAs abundant in MPC EVs (Murach et al., 2020c), such as miR-92a (Berschneider et al., 2014), may also contribute to satellite cell-mediated repression of Wisp1 in FAPs during MOV. Nevertheless, in context with our prior work showing MPC EV-mediated miR-206 repression of Rrbp1 in fibrogenic cells (Fry et al., 2017), these in vitro experiments provide evidence for EV delivery of miR-206 as an explanation for Wisp1 repression in FAPs by satellite cells.
Figure 5.
Wisp1 in primary fibrogenic cells (Fbs) is regulated by primary myogenic progenitor cells (MPCs) via an EV-mediated miR-206 delivery mechanism
(A) Wisp1 levels in Pdgfrα-high FAPs after 96 hr of MOV in the presence and absence of satellite cells (SCs), measured by scRNA-seq.
(B) Experimental design schematic illustrating (1) MPC-Fb co-culture, (2) MPC conditioned media incubated with Fbs, (3) MPC EVs incubated with Fbs, and (4) miR-206 mimic or scrambled oligonucleotide transfected into Fbs for 24 hr.
(C) Wisp1 gene expression in Fbs in conditions 1–4, relative to their respective control condition (dotted line), normalized to the geomean of 18S rRNA and Gapdh and presented as 2−ΔΔCt. #p = 0.05, ∗p < 0.05, data are presented as mean ± SEM.
Trajectory inference analyses revealed altered cell state progression in the absence of satellite cells during the first 96 hr of MOV
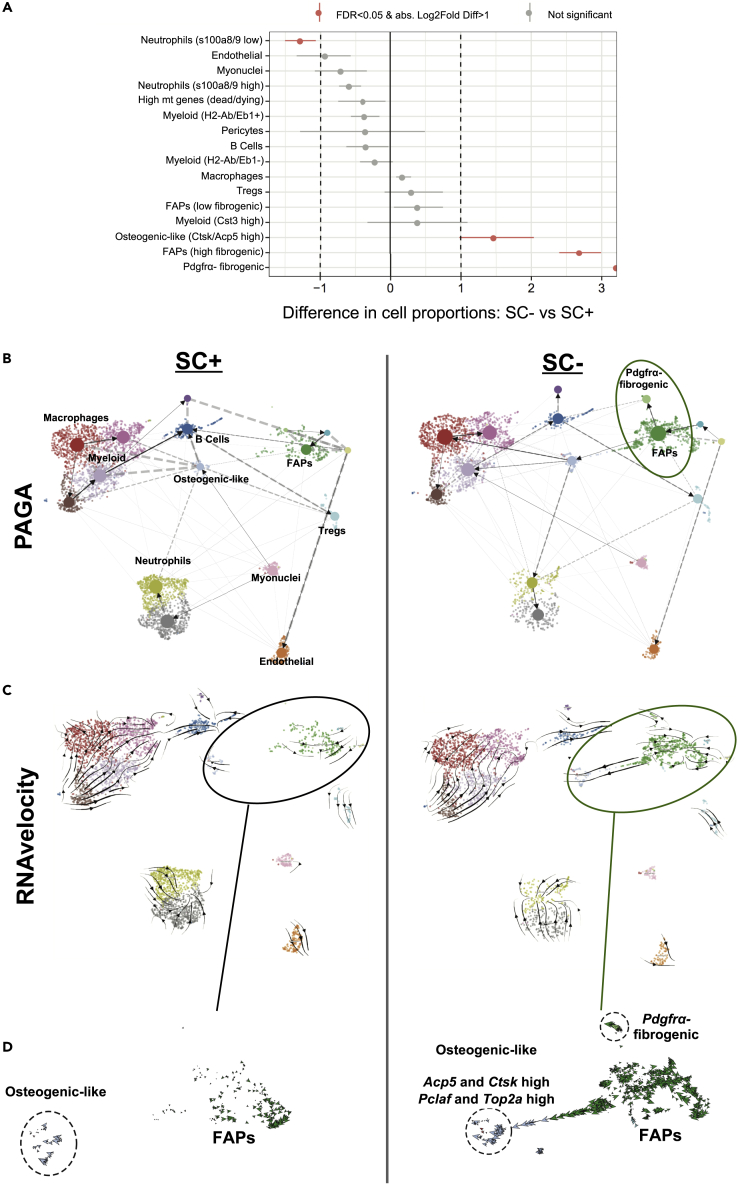
We sought to gather more detailed information on how satellite cells affect mononuclear cell fate progression during the early phase of MOV. We therefore conducted RNA velocity to model cell differentiation trajectories over pseudotime (Bergen et al., 2020; La Manno et al., 2018) and extended the results with the orthogonal method partition-based graph abstraction (which combines clustering with pseudotemporal ordering for trajectory analysis) (Wolf et al., 2019). These analyses involve the relative abundance of spliced and un-spliced transcripts to predict cell fate trajectories (La Manno et al., 2018). Following satellite cell depletion, three cell populations were statistically over-represented after 96 hr of MOV in the spliced read data set (Figure 6A). In addition to Pdgfrα-expressing FAPs, a cluster of Pdgfrα-negative cells enriched with ECM genes was over-represented in the absence of satellite cells (Figure 6A and see Figure 4B). Since Pdgfrα expression typically defines FAPs (Joe et al., 2010; Uezumi et al., 2010; Wosczyna et al., 2019), this unique cluster either represented fibroblasts (Chapman et al., 2016) or transitional-state FAPs (Oprescu et al., 2020) and demonstrated high expression of ECM-related genes such as collagens, Lox, and Sparc. We observed that some FAPs were indeed predicted to be transitioning toward Pdgfrα-negative fibrogenic cells with satellite cell depletion (Figures 6B and 6C).
Figure 6.
scRNA-seq trajectory inference analyses after 96 hr of mechanical overload (MOV) in the presence and absence of satellite cells (SCs)
(A) Statistical differences in Log2 cell proportions in scRNA-seq experiments between 96 hr of MOV in SC+ and SC- plantaris muscles.
(B) Partition-based graph abstraction (PAGA) analysis illustrates the appearance of a Pdgfrα-negative fibrogenic cell population in the absence of satellite cells.
(C) RNA velocity analysis shows FAPs predicted to be transitioning toward two distinct cell populations in the absence of satellite cells.
(D) Magnified view of the FAP cluster from RNA velocity showing a Pdgfrα-negative fibrogenic cell population in close proximity to FAPs, as well as an osteogenic-like cell population predicted to arise from FAPs preferentially in the absence of SCs during MOV.
Another cell population, seemingly with a continuous trajectory from the FAP cluster and enriched with cell cycle markers such as Pclaf and Top2a, was also abundant in the absence of satellite cells (Figures 6A and 6B). This cluster featured high expression of Acp5 and Ctsk, which are characteristic of osteogenic cells (Hayman et al., 1996; Inaoka et al., 1995; Minkin, 1982; Tezuka et al., 1994), but undetectable Pdgfrα (Figure 6D). In addition to being fibrogenic/adipogenic, FAPs also have osteogenic potential (Lees-Shepard et al., 2018; Wosczyna et al., 2012), especially in an altered muscle inflammatory environment (Eisner et al., 2020). We hypothesize that dysregulation of inflammatory-related signaling throughout the muscle in the absence of satellite cells may have augmented the likelihood of osteogenic FAP appearance during MOV, contributing to the transition we observe in our trajectory analyses. Furthermore, Wisp1 is well characterized as a crucial mediator of osteogenic cell fate progression (French et al., 2004; Inkson et al., 2008, 2009; Maeda et al., 2015; Ono et al., 2011) and is significantly elevated in Pdgfrα-expressing fibrogenic FAPs in the absence of satellite cells (see above). Also worth noting is that miR-206 (which inhibits Wisp1) is known to suppress osteogenic cell fate progression (Inose et al., 2009). Osteogenic FAPs are cleared by macrophages after injury which prevents muscle ossification (Eisner et al., 2020), but understanding how satellite cells affect this process during MOV warrants further investigation.
The presence of satellite cells for the first 96 hr of MOV is sufficient to support long-term hypertrophy, independent from myonuclear accretion
Long-term hypertrophy is blunted by the complete absence of satellite cells (Englund et al. 2020a, 2020b; Fry et al. 2014a, 2017). To test whether the presence of satellite cell communication early during MOV is sufficient to support long-term hypertrophy, we subjected Pax7-DTA mice to 8 weeks of MOV but administered vehicle (SC+) or tamoxifen (SC-) beginning at 96 hr after synergist ablation surgery (96hr, Figure 7A); sham-operated mice served as controls. Satellite cells were effectively reduced with tamoxifen treatment (Figure 7B, p < 0.05), and myonuclear accretion was prevented in SC 96 hr muscle after 8 weeks of MOV (Figure 7C, p < 0.05). The accumulation of muscle mass (Figure 7D), large muscle fibers (rightward shift, Figures 7E and 7F), fiber number on cross section (Figure 7G), and ECM content (Figures 7H and 7I) were comparable between SC+ and SC- muscles. While wet weight was not different between groups, the proportion of very large fibers (>2800 μm2) was numerically lower in the absence of satellite cells (Figure 7F), raising the possibility that an upper limit to the “myonuclear domain” was approached, affecting further myofiber growth. Collectively, these findings complement our previous long-term MOV data in which satellite cells were depleted after 7 days (Fry et al., 2017), but the complete prevention of myonuclear accretion with satellite cell depletion at 96 hr observed here suggests that satellite cell secretory function early during MOV is permissive for long-term muscle hypertrophy in adult fast-twitch plantaris muscles.
Figure 7.
The presence of satellite cells (SCs) for the first 96 hr of mechanical overload (MOV) is sufficient to control extracellular matrix deposition and support long-term hypertrophy of the plantaris muscle
(A) Experimental design schematic demonstrating the Veh and Tam treatment strategy after 96 hr of MOV (SC+96 hr and SC-96 hr, respectively) followed by 47 days of MOV (to 8 total weeks) in Pax7-DTA mice. Three mice were removed from this analysis: two in the tamoxifen-treated MOV group due to poor satellite cell depletion and one vehicle-treated MOV due to marked signs of regeneration and/or fiber splitting (numerous central nuclei and large heterogeneity in fiber size) (Murach et al., 2019), resulting in n = 4 for sham and n = 5 for MOV in each condition.
(B) Satellite cell quantity in Veh- and Tam-treated mice. †p < 0.05 effect of tamoxifen, #p < 0.05 effect of MOV.
(C) Myonuclei per fiber in SC+96 hr and SC-96 hr muscles, measured in isolated single muscle fibers. p < 0.05 interaction effect.
(D) Muscle weight in the presence and absence of satellite cells. #p < 0.05 effect of MOV.
(E) Total muscle fiber cross-sectional area (CSA) distribution in SC+96 hr and SC-96 hr muscles.
(F) Muscle fiber CSA presented as relative frequency. #p < 0.05 effect of MOV.
(G) Fiber number on cross section, #p < 0.05 effect of MOV.
(H) Representative image of extracellular matrix via picrosirius red (PSR) staining after MOV in SC+96 hr and SC-96 hr, scale bar, 100 μm.
(I) PSR area normalized to muscle area, p = 0.07 main effect for MOV. Data are presented as mean ± SEM.
Discussion
Using the Pax7-tdT reporter mouse and scRNA-seq, we show that myogenic cells (satellite cells and/or myofibers) are potentially communicating with endothelial cells in vivo via EVs following 14 days of MOV, which is related to an altered transcriptional profile in target cells. EV-mediated communication broadens the scope of myogenic cell-to-endothelial cell secretory signaling described in other models (Christov et al., 2007; Nie et al., 2019; Rhoads et al., 2009; Verma et al., 2018). After 96 hr of MOV, communication specifically from satellite cells is most evident in fibrogenic cells. The absence of satellite cells was associated with transcriptionally distinct fibrogenic populations, and this could be related to EV-mediated delivery of miR-206 that represses pro-fibrogenic Wisp1 in FAPs. Worth noting is that the removal of Vcam+ cells in our experiments, while done in both conditions, may have excluded a recently described Vcam+ FAP population that emerges during regeneration and disease (Malecova et al., 2018). Nevertheless, our satellite cell deplete data are consistent with the nearly two-fold induction of proliferating fibrogenic cells observed early after degenerative injury in the absence of satellite cells (Murphy et al., 2011), as well as the amplified accrual of ECM-generating cells that manifest after a longer period without satellite cells following MOV or injury (Fry et al., 2014a, 2017; Murphy et al., 2011). Our findings also comport with activated satellite cell EV miRNA cargo being principally targeted toward ECM deposition and remodeling processes (Fry et al., 2017; Murach et al., 2020c). The predicted appearance of osteogenic-like (seemingly FAP-derived) cells after 96 hr of MOV, which was accentuated in the absence of satellite cells, further points to robust satellite cell regulation of FAPs in vivo. When satellite cells were only present for the first 96 hr of MOV, successful hypertrophy after 8 weeks illustrates the powerful secretory function of these cells in supporting adult muscle hypertrophy, independent from myonuclear accretion (Murach et al., 2018a, 2018b).
Numerous investigations demonstrate reporter transfer from one cell type to another in vivo (Cossetti et al., 2014; Murach et al., 2020c; Ortin-Martinez et al., 2017; Pearson et al., 2016; Wang et al., 2016) that is likely mediated by EVs, which are shown to package and transfer genetic reporters (Aswad et al., 2014; Cossetti et al., 2014; Flaherty et al., 2019; Forterre et al., 2014; Gao et al., 2020; Kanada et al., 2015; Murach et al., 2020c). The specificity of recombination in inducible Cre-driven reporters is well accepted, but when “leaky” recombination has been observed, it is generally restricted to the lineage being traced (Álvarez-Aznar et al., 2020; Chappell-Maor et al., 2019; Stifter and Greter, 2020; Van Hove et al., 2020). Recombination in the Pax7-tdT model is well accepted as specific to satellite cells (Cho and Doles, 2017; Keefe et al., 2015; Matthews et al., 2016; Pawlikowski et al., 2015; Summers et al., 2017), so our control experiments, taken together with the literature, collectively suggest that EV delivery of cytoplasmic-localized reporter protein and/or transcript may explain, at least in part, the appearance of tdT in non-satellite cell populations during MOV. We cannot rule out that muscle fibers, in addition to satellite cells, were communicating throughout muscle in our model due to satellite cell fusion by 14 days (evidenced by tdT expression), but this communication appeared preferential toward endothelial cells, which satellite cells are known to communicate with (Verma et al., 2018). More sensitive EV-tracking tools, as well as advances in scRNA-seq, may be required to better characterize satellite cell EV-specific communication throughout the muscle very early during hypertrophy. Future investigations will aim to address these limitations, but the current data collectively suggest that satellite cell EV communication is directed toward fibrogenic cells during the early stage of hypertrophy and may have greater influence on endothelial cells later during growth.
In a recent investigation, we incubated myotubes with MPC EVs and then conducted RNA-seq on the myotubes (Murach et al., 2020c). Apart from “ECM organization”, pathway analysis (Herwig et al., 2016) of differentially repressed myotube genes revealed “TNF signaling” and “cytokine-cytokine receptor interaction” as the processes most downregulated by MPC EVs; specifically, Ccl2 and Ccl7 were repressed in myotubes (Murach et al., 2020c). Ccl2 and Ccl7, along with Cxcl5, were also appreciably lower in satellite cells replete relative to deplete whole plantaris muscles after 7 days of MOV (Murach et al., 2020c). Pathway analysis similarly showed “immune system” and “cytokine-cytokine receptor interaction” among the topmost downregulated processes in the muscle in the presence versus absence of satellite cells with MOV (Reactome and KEGG). Accordingly, our scRNA-seq data indicate that satellite cell regulation of chemokine gene expression in individual mononuclear cells throughout the muscle is conserved, with the most noticeable effect in FAPs. FAPs play a role in muscle mass regulation (Wosczyna et al., 2019), and the immunomodulatory function of FAPs (De Micheli et al., 2020; Oprescu et al., 2020; Santini et al., 2020), as well as the complex interplay between FAPs and immune cells in muscle repair (Lemos et al., 2015), are being recognized. Through secreted factors, we propose that satellite cells serve as intermediaries ,for immune signaling, as well as fibrogenic cell function early during adaptation which ensures proper ECM remodeling and hypertrophy during long-term loading. Additionally, miR-206 delivery from satellite cell EVs may directly influence FAP behavior via regulation of Wisp1, which is associated with fibrogenic cell expansion and FAP fate progression (Ono et al., 2018; Oprescu et al., 2020; Venkatachalam et al., 2009). While typically low in fibrogenic cells (Fry et al., 2017), recent evidence suggests that miR-206 can be produced by FAPs under pathological conditions (Sandonà et al., 2020), and FAP-derived Wisp1 may be required to maintain myogenic cell function during aging (Lukjanenko et al., 2019). Satellite cell-FAP cross talk involving Wisp1 and miR-206 is likely condition dependent; however, Wisp1 is higher in whole muscle during MOV in the absence versus the presence of satellite cells (Murach et al., 2020c), which supports our observations that satellite cells signal to Wisp1-enriched FAPs during hypertrophy. Our data collectively agree with a proposed “cell circuit” framework where the early interplay between fibrogenic and inflammatory signaling, often influenced by a third cell type (in this case satellite cells), tunes fibrogenic cell behavior and dictates long-term ECM deposition and homeostasis (Adler et al., 2020).
Resident myonuclei have a robust transcriptional reserve capacity during short-term hypertrophy in adult muscle (Kawano et al., 2017; Kirby et al., 2016b; Murach et al., 2018a); however, it has been unclear whether attenuated long-term growth in the absence of satellite cells is due to a lack of myonuclear accretion or excess ECM deposition (Fry et al., 2014a, 2017). In mice depleted of satellite cells prior to experimentation, we recently reported that muscle fiber growth during running exercise ensues without excess ECM deposition but is impaired relative to satellite cell replete mice (Englund et al., 2020a, 2020b). In context with our current findings, we posit that there is likely an upper limit to the transcriptional capabilities of resident myonuclei for supporting adult muscle fiber growth (Petrella et al., 2008). This limit could be related to the stimulus (endurance and hypertrophic versus primarily hypertrophic) (Murach et al., 2020a), muscle or fiber type (Fry et al., 2014b), and/or the oxidative metabolic/protein synthetic demands placed on muscle fibers during loading (Murach et al., 2018b). While satellite cell fusion positively regulates myonuclear transcription during exercise (Englund et al., 2020a), fusion-independent satellite cell communication to fibrogenic cells (Fry et al., 2014a, 2017), muscle fibers (Murach et al., 2020c), and throughout muscle is also pro-hypertrophic. Evidence for the secretory functions of satellite cells provides an additional process that can be targeted to enhance stem cell-based therapeutic strategies for improving muscle mass or ameliorating fibrosis in disease and aging.
Limitations of the study
We provide multiple lines of evidence that indicate myogenic cells communicate with mononuclear cells throughout the muscle via EVs during hypertrophic adaptation; however, the current lack of a technology to prevent EV release in a cell-type-specific fashion means that other secretory mechanisms could in part explain some of our results. Furthermore, at earlier time points of MOV, more sensitive EV tracking tools may be required to define the extent of EV communication from satellite cells to mononuclear cells.
Resource availability
Lead contact
Further information and requests for resources should be directed to Charlotte Peterson, PhD (cpete4@uky.edu) and John McCarthy, PhD (jjmcca2@uky.edu).
Material availability
This work did not generate or use any new or unique reagents.
Data and code availability
The accession number for the scRNA-seq data reported in this paper is GEO: GSE168872.The code to find the proportional difference in cell populations between two samples is available as an R library on GitHub: https://github.com/rpolicastro/scProportionTest/releases/tag/v1.0.0.
Methods
All methods can be found in the accompanying transparent methods supplemental file.
Acknowledgments
The authors wish to thank Jennifer Strange of the University of Kentucky Flow Cytometry Core and Dr. Doug Harrison of the University of Kentucky Biology Department/Genetics and Genomics Imaging Center for their technical expertise with fluorescent activated cell sorting and single-cell RNA sequencing, respectively. Partial computational support was provided by the University of Kentucky High Performance Computing complex. The authors thank Dr. Ferdinand von Walden for providing mice for this study, Dr. Yuan Wen for his technical expertise, and Dr. Eric Wang for providing resources. This work was supported by funding from the NIH National Institutes of Arthritis and Musculoskeletal and Skin Diseases (AR060701 to C.A.P. and J.J.M. and AR071753 to K.A.M), National Institute on Aging (AG049086 to C.A.P. and J.J.M. and AG063994 to K.A.M), National Institute of Diabetes and Digestive and Kidney Diseases (DK119619 to C.A.P. and J.J.M), and National Institute of General Medical Sciences (GM130349 to J.J.S.)
Author contribution
K.A.M., C.S.F., C.A.P., and J.J.M. designed experiments. K.A.M., B.D.P., D.W.V.P., L.T.D., I.J.V., B.D.P., C.M.D., C.R.B., C.S.F., X.F., and C.I.R. performed experiments. K.A.M., B.D.P., R.A.P., D.W.V.P., I.J.V., B.D.P., C.R.B., X.F., C.I.R., and J.J.S. analyzed data. G.E.Z. and E.E.D.-V. provided resources. K.A.M wrote the manuscript and prepared the figures. C.A.P. and J.J.M. provided funding support and supervised the study. C.A.P., J.J.M., and R.A.P. assisted with data interpretation and manuscript writing. All authors edited and approved the final manuscript.
Declaration of interests
The authors have no conflicts to declare.
Published: April 23, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102372.
Contributor Information
John J. McCarthy, Email: jjmcca2@uky.edu.
Charlotte A. Peterson, Email: cpete4@uky.edu.
Supplemental information
References
- Adler M., Mayo A., Zhou X., Franklin R.A., Meizlish M.L., Medzhitov R., Kallenberger S.M., Alon U. Principles of cell circuits for tissue repair and fibrosis. iScience. 2020;23:100841. doi: 10.1016/j.isci.2020.100841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez-Aznar A., Martinez-Corral I., Daubel N., Betsholtz C., Mäkinen T., Gängel K. Tamoxifen-independent recombination of reporter genes limits lineage tracing and mosaic analysis using CreER T2 lines. Trans. Res. 2020;29:53–68. doi: 10.1007/s11248-019-00177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswad H., Forterre A., Wiklander O.P.B.,, Guillaume V., Danty-Berger E., Jalabert A., Lamaziere A., Meugnier E., Pesenti S., Ott C. Exosomes participate in the alteraion of muscle homeostasis during lipid-induced insulin resistance in mice. Diabetologia. 2014;57:2155–2164. doi: 10.1007/s00125-014-3337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman J.F., Klose A., Liu W., Paris N.D., Blanc R.S., Schmalz M., Knapp E., Chakkalakal J.V. Prepubertal skeletal muscle growth requires Pax7-expressing satellite cell-derived myonuclear contribution. Development. 2018;145:dev167197. doi: 10.1242/dev.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen V., Lange M., Peidli S., Wolf F.A., Theis F.J. Generalizing RNA velocity to transient cell states through dynamical modeling. Nat. Biotech. 2020;38:1408–1414. doi: 10.1038/s41587-020-0591-3. [DOI] [PubMed] [Google Scholar]
- Berschneider B., Ellwanger D.C., Baarsma H.A., Thiel C., Shimbori C., White E.S., Kolb M., Neth P., Königshoff M. miR-92a regulates TGF-β1-induced WISP1 expression in pulmonary fibrosis. Int. J. Biochem. Cell Biol. 2014;53:432–441. doi: 10.1016/j.biocel.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Berschneider B., Königshoff M. WNT1 inducible signaling pathway protein 1 (WISP1): a novel mediator linking development and disease. Int. J. Biochem. Cell Biol. 2011;43:306–309. doi: 10.1016/j.biocel.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Chapman M.A., Mukund K., Subramaniam S., Brenner D., Lieber R.L. Three distinct cell populations express extracellular matrix proteins and increase in number during skeletal muscle fibrosis. Am. J. Physiol. Cell Physiol. 2016;312:C131–C143. doi: 10.1152/ajpcell.00226.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell-Maor L., Kolesnikov M., Kim J.S., Shemer A., Haimon Z., Grozovski J., Boura-Halfon S., Masuda T., Prinz M., Jung S. Comparative analysis of CreER transgenic mice for the study of brain macrophages: a case study. Eur. J. Immunol. 2019;50:353–362. doi: 10.1002/eji.201948342. [DOI] [PubMed] [Google Scholar]
- Chazaud B., Sonnet C., Lafuste P., Bassez G., Rimaniol A.-C., Poron F., Authier F.-J., Dreyfus P.A., Gherardi R.K. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J. Cell Biol. 2003;163:1133–1143. doi: 10.1083/jcb.200212046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho D.S., Doles J.D. Single cell transcriptome analysis of muscle satellite cells reveals widespread transcriptional heterogeneity. Gene. 2017;636:54–63. doi: 10.1016/j.gene.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christov C., Chrétien F., Abou-Khalil R., Bassez G., Vallet G., Authier F.-J., Bassaglia Y., Shinin V., Tajbakhsh S., Chazaud B. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol. Biol. Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colston J.T., de la Rosa S.D., Koehler M., Gonzales K., Mestril R., Freeman G.L., Bailey S.R., Chandrasekar B. Wnt-induced secreted protein-1 is a prohypertrophic and profibrotic growth factor. Am. J. Physiol. Heart Circ. Physiol. 2007;93:H1839–H1846. doi: 10.1152/ajpheart.00428.2007. [DOI] [PubMed] [Google Scholar]
- Contreras O., Rossi F.M., Brandan E. Adherent muscle connective tissue fibroblasts are phenotypically and biochemically equivalent to stromal fibro/adipogenic progenitors. Matrix Biol. Plus. 2019;2:100006. doi: 10.1016/j.mbplus.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossetti C., Lugini L., Astrologo L., Saggio I., Fais S., Spadafora C. Soma-to-germline transmission of RNA in mice xenografted with human tumour cells: possible transport by exosomes. PLoS One. 2014;9:e101629. doi: 10.1371/journal.pone.0101629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crewe C., Joffin N., Rutkowski J.M., Kim M., Zhang F., Towler D.A., Gordillo R., Scherer P.E. An endothelial-to-adipocyte extracellular vesicle axis governed by metabolic state. Cell. 2018;175:695–708. e613. doi: 10.1016/j.cell.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Micheli A.J., Laurilliard E.J., Heinke C.L., Ravichandran H., Fraczek P., Soueid-Baumgarten S., De Vlaminck I., Elemento O., Cosgrove B.D. Single-cell analysis of the muscle stem cell hierarchy identifies heterotypic communication signals involved in skeletal muscle regeneration. Cell Rep. 2020;30:3583–3595. e3585. doi: 10.1016/j.celrep.2020.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner C., Cummings M., Johnston G., Tung L.W., Groppa E., Chang C., Rossi F.M. Murine tissue-resident PDGFRα+ fibro-adipogenic progenitors spontaneously acquire osteogenic phenotype in an altered inflammatory environment. J. Bone Mineral. Res. 2020;35:1525–1534. doi: 10.1002/jbmr.4020. [DOI] [PubMed] [Google Scholar]
- Englund D., Figueiredo V., Dungan C., Murach K., Peck B., Petrosino J., Brightwell C., Dupont A., Neal A., Fry C. Satellite cell depletion disrupts transcriptional coordination and muscle adaptation to exercise. Function. 2020:zqaa033. doi: 10.1093/function/zqaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund D.A., Murach K.A., Dungan C.M., Figueiredo V.C., Vechetti I.J., Jr., Dupont-Versteegden E.E., McCarthy J.J., Peterson C.A. Depletion of resident muscle stem cells negatively impacts running volume, physical function and muscle hypertrophy in response to lifelong physical activity. Am. J. Physiol. Cell Physiol. 2020;318:C1178–C1188. doi: 10.1152/ajpcell.00090.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farup J., Madaro L., Puri P., Mikkelsen U.R. Interactions between muscle stem cells, mesenchymal-derived cells and immune cells in muscle homeostasis, regeneration and disease. Cell Death Dis. 2015;6:e1830. doi: 10.1038/cddis.2015.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty S.E., 3rd, Grijalva A., Xu X., Ables E., Nomani A., Ferrante A.W., Jr. lipase-independent pathway of lipid release and immune modulation by adipocytes. Science. 2019;363:989–993. doi: 10.1126/science.aaw2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forterre A., Jalabert A., Berger E., Baudet M., Chikh K., Errazuriz E., De Larichaudy J., Chanon S., Weiss-Gayet M., Hesse A.M. Proteomic analysis of C2C12 myoblast and myotube exosome-like vesicles: a new paradigm for myoblast-myotube cross talk? PLoS One. 2014;9:e84153. doi: 10.1371/journal.pone.0084153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French D.M., Kaul R.J., D'souza A.L., Crowley C.W., Bao M., Frantz G.D., Filvaroff E.H., Desnoyers L. WISP-1 is an osteoblastic regulator expressed during skeletal development and fracture repair. Am. J. Pathol. 2004;165:855–867. doi: 10.1016/S0002-9440(10)63348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry C.S., Kirby T.J., Kosmac K., McCarthy J.J., Peterson C.A. Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell. 2017;20:56–69. doi: 10.1016/j.stem.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry C.S., Lee J.D., Jackson J.R., Kirby T.J., Stasko S.A., Liu H., Dupont-Versteegden E.E., McCarthy J.J., Peterson C.A. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J. 2014;28:1654–1665. doi: 10.1096/fj.13-239426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry C.S., Noehren B., Mula J., Ubele M.F., Westgate P.M., Kern P.A., Peterson C.A. Fibre type-specific satellite cell response to aerobic training in sedentary adults. J. Physiol. 2014;592:2625–2635. doi: 10.1113/jphysiol.2014.271288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandham S., Su X., Wood J., Nocera A.L., Alli S.C., Milane L., Zimmerman A., Amiji M., Ivanov A.R. Technologies and standardization in research on extracellular vesicles. Trend Biotech. 2020;38:1066–1098. doi: 10.1016/j.tibtech.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Zhang Z., Mashimo T., Shen B., Nyagilo J., Wang H., Wang Y., Liu Z., Mulgaonkar A., Hu X.-L. Gliomas interact with non-glioma brain cells via extracellular vesicles. Cell Rep. 2020;30:2489–2500. e2485. doi: 10.1016/j.celrep.2020.01.089. [DOI] [PubMed] [Google Scholar]
- Hayman A.R., Jones S.J., Boyde A., Foster D., Colledge W.H., Carlton M.B., Evans M.J., Cox T.M. Mice lacking tartrate-resistant acid phosphatase (Acp 5) have disrupted endochondral ossification and mild osteopetrosis. Development. 1996;122:3151–3162. doi: 10.1242/dev.122.10.3151. [DOI] [PubMed] [Google Scholar]
- Herwig R., Hardt C., Lienhard M., Kamburov A. Analyzing and interpreting genome data at the network level with ConsensusPathDB. Nat. Prot. 2016;11:1889. doi: 10.1038/nprot.2016.117. [DOI] [PubMed] [Google Scholar]
- Inaoka T., Bilbe G., Ishibashi O., Tezuka K.-i., Kumegawa M., Kokubo T. Molecular cloning of human cDNA for cathepsin K: novel cysteine proteinase predominantly expressed in bone. Biochem. Biophys. Res. Commun. 1995;206:89–96. doi: 10.1006/bbrc.1995.1013. [DOI] [PubMed] [Google Scholar]
- Inkson C.A., Ono M., Bi Y., Kuznetsov S.A., Fisher L.W., Young M.F. The potential functional interaction of biglycan and WISP-1 in controlling differentiation and proliferation of osteogenic cells. Cells Tissues Organs. 2009;189:153–157. doi: 10.1159/000151377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inkson C.A., Ono M., Kuznetsov S.A., Fisher L.W., Robey P.G., Young M.F. TGF-β1 and WISP-1/CCN-4 can regulate each other’s activity to cooperatively control osteoblast function. J. Cell. Biochem. 2008;104:1865. doi: 10.1002/jcb.21754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inose H., Ochi H., Kimura A., Fujita K., Xu R., Sato S., Iwasaki M., Sunamura Sl, Takeuchi I., Fukumoto S. A microRNA regulatory network of osteoblast differentiation. Proc. Nat. Acad. Sci. 2009;106:20794–20799. doi: 10.1073/pnas.0909311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe A.W., Yi L., Natarajan A., Le Grand F., So L., Wang J., Rudnicki M.A., Rossi F.M. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010;12:153. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanada M., Bachmann M.H., Hardy J.W., Frimannson D.O., Bronsart L., Wang A., Sylvester M.D., Schmidt T.L., Kaspar R.L., Butte M.J. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc. Nat. Acad. Sci. 2015;112:E1433–E1442. doi: 10.1073/pnas.1418401112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano F., Ono Y., Fujita R., Watanabe A., Masuzawa R., Shibata K., Hasegawa S., Nakata K., Nakai N. Prenatal myonuclei play a crucial role in skeletal muscle hypertrophy in rodents. Am. J. Physiol. Cell Physiol. 2017;312:C233–C243. doi: 10.1152/ajpcell.00151.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe A.C., Lawson J.A., Flygare S.D., Fox Z.D., Colasanto M.P., Mathew S.J., Yandell M., Kardon G. Muscle stem cells contribute to myofibres in sedentary adult mice. Nat. Commun. 2015;6:7087. doi: 10.1038/ncomms8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby T.J., McCarthy J.J., Peterson C.A., Fry C.S. Synergist ablation as a rodent model to study satellite cell dynamics in adult skeletal muscle. Methods Mol. Biol. 2016;1460:43–52. doi: 10.1007/978-1-4939-3810-0_4. [DOI] [PubMed] [Google Scholar]
- Kirby T.J., Patel R.M., McClintock T.S., Dupont-Versteegden E.E., Peterson C.A., McCarthy J.J. Myonuclear transcription is responsive to mechanical load and DNA content but uncoupled from cell size during hypertrophy. Mol. Biol. Cell. 2016;27:788–798. doi: 10.1091/mbc.E15-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger J., Rehmsmeier M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nuc Acid Res. 2006;34:W451–W454. doi: 10.1093/nar/gkl243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Manno G., Soldatov R., Zeisel A., Braun E., Hochgerner H., Petukhov V., Lidschreiber K., Kastriti M.E., Lönnerberg P., Furlan A. RNA velocity of single cells. Nature. 2018;560:494–498. doi: 10.1038/s41586-018-0414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees-Shepard J.B., Yamamoto M., Biswas A.A., Stoessel S.J., Nicholas S.-A.E., Cogswell C.A., Devarakonda P.M., Schneider M.J., Cummins S.M., Legendre N.P. Activin-dependent signaling in fibro/adipogenic progenitors causes fibrodysplasia ossificans progressiva. Nat. Commun. 2018;9:1–14. doi: 10.1038/s41467-018-02872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos D.R., Babaeijandaghi F., Low M., Chang C.-K., Lee S.T., Fiore D., Zhang R.-H., Natarajan A., Nedospasov S.A., Rossi F.M. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat. Med. 2015;21:786–794. doi: 10.1038/nm.3869. [DOI] [PubMed] [Google Scholar]
- Liu W., Wei-LaPierre L., Klose A., Dirksen R.T., Chakkalakal J.V. Inducible depletion of adult skeletal muscle stem cells impairs the regeneration of neuromuscular junctions. Elife. 2015;4:e09221. doi: 10.7554/eLife.09221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukjanenko L., Karaz S., Stuelsatz P., Gurriaran-Rodriguez U., Michaud J., Dammone G., Sizzano F., Mashinchian O., Ancel S., Migliavacca E. Aging disrupts muscle stem cell function by impairing matricellular WISP1 secretion from fibro-adipogenic progenitors. Cell Stem Cell. 2019;24:433–446. e437. doi: 10.1016/j.stem.2018.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A., Ono M., Holmbeck K., Li L., Kilts T., Kram V., Noonan M., Yoshioka Y., McNerny E., Tantillo M. WNT1-induced secreted protein-1 (WISP1), a novel regulator of bone turnover and Wnt signaling. J. Biol. Chem. 2015;290:14004–14018. doi: 10.1074/jbc.M114.628818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecova B., Gatto S., Etxaniz U., Passafaro M., Cortez A., Nicoletti C., Giordani L., Torcinaro A., De Bardi M., Bicciato S. Dynamics of cellular states of fibro-adipogenic progenitors during myogenesis and muscular dystrophy. Nat. Commun. 2018;9:1–12. doi: 10.1038/s41467-018-06068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masschelein E., D'Hulst G., Zvick J., Hinte L., Soro-Arnaiz I., Gorski T., von Meyenn F., Bar-Nur O., De Bock K. Exercise promotes satellite cell contribution to myofibers in a load-dependent manner. Skelet. Muscle. 2020;10:21. doi: 10.1186/s13395-020-00237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B.G., Torreggiani E., Roeder E., Matic I., Grcevic D., Kalajzic I. Osteogenic potential of alpha smooth muscle actin expressing muscle resident progenitor cells. Bone. 2016;84:69–77. doi: 10.1016/j.bone.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J.J., Mula J., Miyazaki M., Erfani R., Garrison K., Farooqui A.B., Srikuea R., Lawson B.A., Grimes B., Keller C. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138:3657–3666. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkin C. Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif. Tissue Int. 1982;34:285–290. doi: 10.1007/BF02411252. [DOI] [PubMed] [Google Scholar]
- Murach K.A., Dungan C.M., Peterson C.A., McCarthy J.J. Muscle fiber splitting is a physiological response to extreme loading in animals. Exerc. Sport Sci. Rev. 2019;47:108–115. doi: 10.1249/JES.0000000000000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murach K.A., Englund D.A., Dupont-Versteegden E.E., McCarthy J.J., Peterson C.A. Myonuclear domain flexibility challenges rigid assumptions on satellite cell contribution to skeletal muscle fiber hypertrophy. Front. Physiol. 2018;9:635. doi: 10.3389/fphys.2018.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murach K.A., Fry C.S., Kirby T.J., Jackson J.R., Lee J.D., White S.H., Dupont-Versteegden E.E., McCarthy J.J., Peterson C.A. Starring or supporting role? Satellite cells and skeletal muscle fiber size regulation. Physiology. 2018;33:26–38. doi: 10.1152/physiol.00019.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murach K.A., McCarthy J.J., Peterson C.A., Dungan C.M. Making mice mighty: recent advances in translational models of load-induced muscle hypertrophy. J. Appl. Physiol. 2020;129:516–521. doi: 10.1152/japplphysiol.00319.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murach K.A., Mobley C.B., Zdunek C.J., Frick K.K., Jones S.R., McCarthy J.J., Peterson C.A., Dungan C.M. Muscle memory: myonuclear accretion, maintenance, morphology, and miRNA levels with training and detraining in adult mice. J. Cachexia Sarcopenia Muscle. 2020;11:1705–1722. doi: 10.1002/jcsm.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murach K.A., Vechetti I.J., Jr., Van Pelt D.W., Crow S.E., Dungan C.M., Figueiredo V.C., Kosmac K., Fu X., Richards C.I., Fry C.S. Fusion-independent satellite cell communication to muscle fibers during load-induced hypertrophy. Function. 2020;1:zqaa009. doi: 10.1093/function/zqaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murach K.A., White S.H., Wen Y., Ho A., Dupont-Versteegden E.E., McCarthy J.J., Peterson C.A. Differential requirement for satellite cells during overload-induced muscle hypertrophy in growing versus mature mice. Skelet. Muscle. 2017;7:14. doi: 10.1186/s13395-017-0132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.M., Lawson J.A., Mathew S.J., Hutcheson D.A., Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y., Sato Y., Garner R.T., Kargl C., Wang C., Kuang S., Gilpin C.J., Gavin T.P. Skeletal muscle-derived exosomes regulate endothelial cell functions via reactive oxygen species-activated nuclear factor-κB signalling. Exp. Physiol. 2019;104:1262–1273. doi: 10.1113/EP087396. [DOI] [PubMed] [Google Scholar]
- Ono M., Inkson C.A., Kilts T.M., Young M.F. WISP-1/CCN4 regulates osteogenesis by enhancing BMP-2 activity. J. Bone Mineral. Res. 2011;26:193–208. doi: 10.1002/jbmr.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Masaki A., Maeda A., Kilts T.M., Hara E.S., Komori T., Pham H., Kuboki T., Young M.F. CCN4/WISP1 controls cutaneous wound healing by modulating proliferation, migration and ECM expression in dermal fibroblasts via α5β1 and TNFα. Matrix Biol. 2018;68:533–546. doi: 10.1016/j.matbio.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oprescu S.N., Yue F., Qiu J., Brito L.F., Kuang S. Temporal dynamics and heterogeneity of cell populations during skeletal muscle regeneration. iScience. 2020;23:100993. doi: 10.1016/j.isci.2020.100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortin-Martinez A., Tsai E.L.S., Nickerson P.E., Bergeret M., Lu Y., Smiley S., Comanita L., Wallace V.A. A reinterpretation of cell transplantation: GFP transfer from donor to host photoreceptors. Stem Cells. 2017;35:932–939. doi: 10.1002/stem.2552. [DOI] [PubMed] [Google Scholar]
- Patsalos A., Pap A., Varga T., Trencsenyi G., Contreras G.A., Garai I., Papp Z., Dezso B., Pintye E., Nagy L. In situ macrophage phenotypic transition is affected by altered cellular composition prior to acute sterile muscle injury. J. Physiol. 2017;595:5815–5842. doi: 10.1113/JP274361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlikowski B., Pulliam C., Betta N.D., Kardon G., Olwin B.B. Pervasive satellite cell contribution to uninjured adult muscle fibers. Skelet. Muscle. 2015;5:42. doi: 10.1186/s13395-015-0067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R., Gonzalez-Cordero A., West E., Ribeiro J., Aghaizu N., Goh D., Sampson R., Georgiadis A., Waldron P., Duran Y. Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nat. Commun. 2016;7:13029. doi: 10.1038/ncomms13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrella J.K., Kim J.S., Mayhew D.L., Cross J.M., Bamman M.M. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J. Appl. Physiol. 2008;104:1736–1742. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- Rhoads R.P., Johnson R.M., Rathbone C.R., Liu X., Temm-Grove C., Sheehan S.M., Hoying J.B., Allen R.E. Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducible factor pathway. Am. J. Physiol. Cell Physiol. 2009;296:C1321–C1328. doi: 10.1152/ajpcell.00391.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandonà M., Consalvi S., Tucciarone L., De Bardi M., Scimeca M., Angelini D.F., Buffa V., D'Amico A., Bertini E.S., Cazzaniga S. HDAC inhibitors tune miRNAs in extracellular vesicles of dystrophic muscle-resident mesenchymal cells. EMBO Rep. 2020;21:e50863. doi: 10.15252/embr.202050863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini M.P., Malide D., Hoffman G., Pandey G., D’Escamard V., Nomura-Kitabayashi A., Rovira I., Kataoka H., Ochando J., Harvey R.P. Tissue-fesident PDGFRα+ progenitor cells contribute to fibrosis versus healing in a context-and spatiotemporally dependent manner. Cell Rep. 2020;30:555–570. e557. doi: 10.1016/j.celrep.2019.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifi-Alan M., Shamsi R., Behmanesh A., Mirfakhraie R., Omrani M.D., Ghafouri-Fard S. MIR-206 target prediction in breast cancer subtypes by bioinformatics tools. Int. J. Cancer Manage. 2018;11:e69554. [Google Scholar]
- Stifter S.A., Greter M. STOP floxing around: specificity and leakiness of inducible Cre/loxP systems. Eur. J. Immunol. 2020;50:338–341. doi: 10.1002/eji.202048546. [DOI] [PubMed] [Google Scholar]
- Summers M.A., Mikulec K., Peacock L., Little D.G., Schindeler A. Limitations of the Pax7-creER(T2) transgene for driving deletion of Nf1 in adult mouse muscle. Int. J. Dev. Biol. 2017;61:531–536. doi: 10.1387/ijdb.170182as. [DOI] [PubMed] [Google Scholar]
- Tatsumi R., Sankoda Y., Anderson J.E., Sato Y., Mizunoya W., Shimizu N., Suzuki T., Yamada M., Rhoads R.P., Jr., Ikeuchi Y. Possible implication of satellite cells in regenerative motoneuritogenesis: HGF upregulates neural chemorepellent Sema3A during myogenic differentiation. Am. J. Physiol. Cell Physiol. 2009;297:C238–C252. doi: 10.1152/ajpcell.00161.2009. [DOI] [PubMed] [Google Scholar]
- Tezuka K.-I., Tezuka Y., Maejima A., Sato T., Nemoto K., Kamioka H., Hakeda Y., Kumegawa M. Molecular cloning of a possible cysteine proteinase predominantly expressed in osteoclasts. J. Biol. Chem. 1994;269:1106–1109. [PubMed] [Google Scholar]
- Uezumi A., Fukada S.-i., Yamamoto N., Takeda S.i., Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010;12:143. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- Van Hove H., Antunes A.R.P., De Vlaminck K., Scheyltjens I., Van Ginderachter J.A., Movahedi K. Identifying the variables that drive tamoxifen-independent CreERT2 recombination: implications for microglial fate mapping and gene deletions. Eur. J. Immunol. 2020;50:459–463. doi: 10.1002/eji.201948162. [DOI] [PubMed] [Google Scholar]
- Vechetti I.J., Jr., Valentino T., Mobley C.B., McCarthy J.J. The role of extracellular vesicles in skeletal muscle and systematic adaption to exercise. J. Physiol. 2020;599:845–861. doi: 10.1113/JP278929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K., Venkatesan B., Valente A.J., Melby P.C., Nandish S., Reusch J.E., Clark R.A., Chandrasekar B. WISP1, a pro-mitogenic, pro-survival factor, mediates tumor necrosis factor-α (TNF-α)-stimulated cardiac fibroblast proliferation but inhibits TNF-α-induced cardiomyocyte death. J. Biol. Chem. 2009;284:14414–14427. doi: 10.1074/jbc.M809757200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma M., Asakura Y., Murakonda B.S.R., Pengo T., Latroche C., Chazaud B., McLoon L.K., Asakura A. Muscle satellite cell cross-talk with a vascular niche maintains quiescence via VEGF and notch signaling. Cell Stem Cell. 2018;23:530–543. e539. doi: 10.1016/j.stem.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.-X., Qiu Y.-R., Zhong X.-P. Intercellular protein transfer from thymocytes to thymic epithelial cells. PLoS One. 2016;11:e0152641. doi: 10.1371/journal.pone.0152641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf F.A., Hamey F.K., Plass M., Solana J., Dahlin J.S., Gottgens B., Rajewsky N., Simon L., Theis F.J. PAGA: graph abstraction reconciles clustering with trajectory inference through a topology preserving map of single cells. Genome Biol. 2019;20:59. doi: 10.1186/s13059-019-1663-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosczyna M.N., Biswas A.A., Cogswell C.A., Goldhamer D.J. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J. Bone Miner. Res. 2012;27:1004–1017. doi: 10.1002/jbmr.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosczyna M.N., Konishi C.T., Carbajal E.E.P., Wang T.T., Walsh R.A., Gan Q., Wagner M.W., Rando T.A. Mesenchymal stromal cells are required for regeneration and homeostatic maintenance of skeletal muscle. Cell Rep. 2019;27:2029–2035. e2025. doi: 10.1016/j.celrep.2019.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosczyna M.N., Rando T.A. A muscle stem cell support group: coordinated cellular responses in muscle regeneration. Dev. Cell. 2018;46:135–143. doi: 10.1016/j.devcel.2018.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession number for the scRNA-seq data reported in this paper is GEO: GSE168872.The code to find the proportional difference in cell populations between two samples is available as an R library on GitHub: https://github.com/rpolicastro/scProportionTest/releases/tag/v1.0.0.