Currently, 1 out of 300 inhabitants of the Netherlands undergoes COVID-19 nasal swabs per day and this number is increasing now as commercial and at home testing kits become available. Frequently poking test-sticks into our noses is considered more and more to be an inconvenient but acceptable trade-off compared to the restrictions to our lives due to the possibility of unrevealed infections.
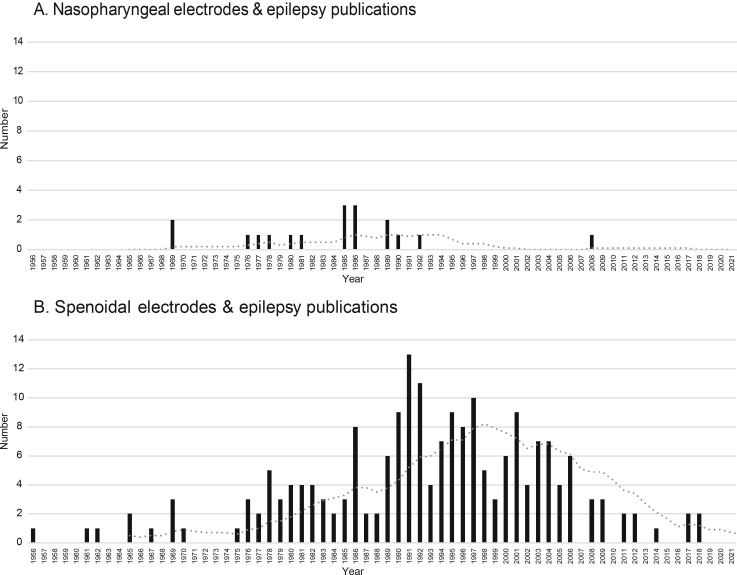
In the past, widespread clinical use of nasopharyngeal electrodes (NPE) to detect interictal discharges (IEDs) from the mesiotemporal structures has been hampered by discomfort of the patients during application of the electrodes. Up till 2008, some publications advocated the use of NPE due to their better detection of IEDs compared to with regular surface EEG (Goodin et al., 1990, Zijlmans et al., 2008); (Table 1 ). Since 2008 not even one paper on the specific use of NPE for epilepsy was published (Fig. 1 ). In most epilepsy centers its clinical use has diminished. Within 2021, five papers on a whole different type of NPE have already been published, namely miniaturized electrochemical immunosensors for SARS-CoV-2 detection in saliva (Fabiani et al., 2021).
Table 1.
Comparison of diagnostic yield of IEDs in mesiotemporal lobe epilepsy.
| Paper (# patients) | Diagnosis | Surface | NPE | SpE |
|---|---|---|---|---|
| Goodin et al. (50) | CP seizures | 58% | 97% | Not performed |
| Sperling et al. (44) | CP seizures | 54% | 57% | 99% |
| Yim et al. (229) | TLE | 56% | 76% | Not performed |
Three papers testing the added value of nasopharyngeal electrodes for detecting IEDs in mesiotemporal lobe epilepsy in comparison to surface EEG (including surface anterior temporal and surface ear electrodes). NPE = nasopharyngeal electrodes. SpE = Sphenoidal electrodes. CP = complex partial. TLE = temporal lobe epilepsy.
Fig. 1.
Overview on the number of publications per year over time on A. Nasopharyngeal electrodes and epilepsy (NNPE_tot = 18), and B. Sphenoidal electrodes and epilepsy (NSpE_tot = 178). The grey dotted line represents the 10 years average. Two peaks of popularity of the two types of electrodes separate in time can be observed.
Comparisons showed that sphenoidal electrodes were superior to NPE in detecting IEDs and seizure onsets from the mesiotemporal structures (Sperling et al. 1986). Follow-up papers mainly studied sphenoidal electrodes as adjuvant to the surface EEG and focused on improving the invasive placement and integrating sphenoidal electrodes into common presurgical evaluation and source localization (Cherian et al., 2012, Hamaneh et al., 2014). The number of papers on the use of sphenoidal electrodes has been gradually decreasing since 1990 (Fig. 1). A study comparing sphenoidal electrodes to identify IEDs with more invasive foramen ovale electrodes and surface EEG anterior temporal electrodes showed that the yield of detected IEDs in sphenoidal electrodes was much lower compared to foramen ovale electrodes but no significant increase in detection sensitivity by the sphenoidal electrodes was found compared with anterior temporal scalp electrodes (Torre, 1999). The use of sphenoidal electrodes for epilepsy surgery evaluation may have gone down with increased use of advanced EEG signal analysis, PET scanning, high field MRI, magnetoencephalography and post-processing of images like hippocampal volumetry.
Still, clinical neurophysiologists often encounter patients with typical clinical histories of (mesial) temporal lobe epilepsy but no abnormalities on surface EEG and negative MRI or other negative diagnostics, in whom we aim to quickly confirm the diagnosis of epilepsy but are reluctant to use invasive diagnostics. This is especially the case in patients with a differential diagnosis of psychiatric disease (Gupta et al., 1989). In this issue of Clinical Neurophysiology, Yim and colleagues studied the use of NPE in a large cohort of 229 persons with suspected temporal lobe epilepsy (Yim et al., 2021). They reviewed the EEGs by experienced and less experienced EEG reviewers and calculated the diagnostic yield of EEGs with and without NPE to identify IEDs. IEDs were detected in 76.4% of patients with NPE recordings compared to 55.5% with non-NPE recordings (p < 0.001). Bilateral independent IEDs were found in 26.2% and 11.4% of EEGs with NPE and non-NPE recordings. These results are intermediate to the yield presented by Goodin and by Sperling previously, being 56% for surface EEG and 76% for surface EEG with nasopharyngeal electrodes, though in smaller cohorts.
Reasons to use NPE are the potential increase of diagnostic yield and their minimal invasiveness to approach the basis of the brain. NPE are cheap and relatively easy to use once EEG technicians are acquainted to the insertion technique.
Reasons not to use NPE is that they do not easily stay in a stable position for long-term recordings, like sphenoidal electrodes can. Their yield seems less than the one of sphenoidal electrodes. Recording of mesiotemporal spikes is also possible by sleep-deprivation and long-term recordings. It has been shown that spikes that are recorded by foramen ovale electrodes in sleep stage NREM 2 will show up on surface EEG electrodes in sleep stage NREM3/4 (Clemens et al., 2003).
Sleep deprivation (SD) EEG is currently the diagnostic gold standard test after a normal routine EEG in suspected epilepsy and may enable capturing spreading of IEDs from mesiotemporal structures. The diagnostic yield of SD-EEG varies widely between studies and comparison of studies are hampered by small sample sizes and different SD protocols. Most early studies on SD-EEG were done after total sleep deprivation (TSD, defined as no sleep for 24 hours or more). For example, Rowan and colleagues found a significantly greater IED yield in EEGs after TSD compared with routine wake and drug-induced sleep EEGs (Rowan et al., 1982). However, IEDs were recorded only in 28% of their subjects after TSD. Degen and colleagues found that IEDs or seizures were more likely to be activated by sleep or sleep deprivation in patients who had idiopathic generalized epilepsy rather than focal epilepsy (Degen and Degen, 1991). Therefore, even after TSD the diagnostic yield of TSD in focal temporal epilepsy appears low (Roupakiotis et al., 2000, Leach et al., 2006). Recording an EEG with NPE may be an alternative to sleep deprivation EEG if temporal lobe seizures are suspected.
In this era of shared decision-making between neurologist and patient, the patients should be offered an informed choice on an EEG after sleep deprivation or an EEG with NPE. We may require a prospective study in which patients with suspected temporal lobe epilepsy are offered either short term EEG with NPE or 24-hours EEG recording or sleep deprivation EEG after an initial negative short-term surface EEG, to evaluate the diagnostic yield of NPE within regular clinical practice.
This should include a rough cost-validation to accurately weight the pros and cons of different methods. The global habituation to nasopharyngeal swaps due to the COVID pandemic may have resulted in a recent switch in preference by patients towards solving the diagnostic problem with a nasopharyngeal electrode, even if this requires poking in their noses.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
MZ and MvtK were funded by an ERC starting grant 803880 and TKI grant LSHM19080.
See Article, pages 1741–1751
References
- Cherian A., Radhakrishnan A., Parameswaran S., Varma R., Radhakrishnan K. Do sphenoidal electrodes aid in surgical decision making in drug resistant temporal lobe epilepsy? Clin Neurophysiol. 2012;123:463–470. doi: 10.1016/j.clinph.2011.07.041. [DOI] [PubMed] [Google Scholar]
- Clemens Z., Janszky J., Szucs A., Békésy M., Clemens B., Halász P. Interictal epileptic spiking during sleep and wakefulness in mesial temporal lobe epilepsy: a comparative study of scalp and foramen ovale electrodes. Epilepsia. 2003;44:186–192. doi: 10.1046/j.1528-1157.2003.27302.x. [DOI] [PubMed] [Google Scholar]
- Degen R., Degen H.E. Sleep and sleep deprivation in epileptology. Epilepsy Res Suppl. 1991;2:235–260. [PubMed] [Google Scholar]
- Fabiani L., Saroglia M., Galatà G., De Santis R., Fillo S., Luca V. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens Bioelectron. 2021;171 doi: 10.1016/j.bios.2020.112686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández Torre J.L., Alarcón G., Binnie C.D., Polkey C.E. Comparison of sphenoidal, foramen ovale and anterior temporal placements for detecting interictal epileptiform discharges in presurgical assessment for temporal lobe epilepsy. Clin Neurophysiol. 1999;110:895–904. doi: 10.1016/s1388-2457(99)00039-5. [DOI] [PubMed] [Google Scholar]
- Goodin D.S., Aminoff M.J., Laxer K.D. Detection of epileptiform activity by different noninvasive EEG methods in complex partial epilepsy. Ann Neurol. 1990;27:330–334. doi: 10.1002/ana.410270317. [DOI] [PubMed] [Google Scholar]
- Gupta B.K., Yerevanian B., Charlton M. Nasopharyngeal EEG recording in psychiatric patients. J Clin Psychiatry. 1989;50:262–264. [PubMed] [Google Scholar]
- Hamaneh M.B., Kaiboriboon K., Dimitriu D., Turnbull J.P., Lüders H.O., Loparo K.A. A method for the inclusion of sphenoidal electrodes in realistic EEG source imaging. J Clin Neurophysiol. 2014;31:429–436. doi: 10.1097/WNP.0000000000000052. [DOI] [PubMed] [Google Scholar]
- Leach J.P., Stephen L.J., Salveta C., Brodie M.J. Which electroencephalography (EEG) for epilepsy? The relative usefulness of different EEG protocols in patients with possible epilepsy. J Neurol Neurosurg Psychiatry. 2006;77:1040–1042. doi: 10.1136/jnnp.2005.084871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roupakiotis S.C., Gatzonis S.D., Triantafyllou N., Mantouvalos V., Chioni A., Zournas C. The usefulness of sleep and sleep deprivation as activating methods in electroencephalographic recording: contribution to a long-standing discussion. Seizure. 2000;9:580–584. doi: 10.1053/seiz.2000.0462. [DOI] [PubMed] [Google Scholar]
- Rowan A.J., Veldhuisen R.J., Nagelkerke N.J. Comparative evaluation of sleep deprivation and sedated sleep EEGs as diagnostic aids in epilepsy. Electroencephalogr Clin Neurophysiol. 1982;54:357–364. doi: 10.1016/0013-4694(82)90199-7. [DOI] [PubMed] [Google Scholar]
- Sperling M.R., Mendius J.R., Engel J., Jr. Mesial temporal spikes: a simultaneous comparison of sphenoidal, nasopharyngeal, and ear electrodes. Epilepsia. 1986;27:81–86. doi: 10.1111/j.1528-1157.1986.tb03505.x. [DOI] [PubMed] [Google Scholar]
- Yim SH, Cho KH, Choi YH, Kim HI, Cho Y-J, Heo K. Nasopharyngeal electrodes in temporal lobe epilepsy: A reappraisal of their diagnostic utility. Clin Neurophysiol 2021;132:1741–1751. [DOI] [PubMed]
- Zijlmans M., Huiskamp G.M., van Huffelen A.C., Spetgens W.P.J., Leijten F.S.S. Detection of temporal lobe spikes: comparing nasopharyngeal, cheek and anterior temporal electrodes to simultaneous subdural recordings. Clin Neurophysiol. 2008;119:1771–1777. doi: 10.1016/j.clinph.2008.04.011. [DOI] [PubMed] [Google Scholar]