Abstract
Autoimmune diseases are group of disorders where an immune response is mounted against the self. The prevalence and burden of this well established and recognised entity is on the rise. Irrespective of being a systemic or organ specific autoimmune disorder, the common underlying mechanism of action, is the imbalance in immune system resulting in loss of tolerance to self-antigens. The oral cavity is no alien to these disorders or to their influences. Pemphigus group of lesions, systemic lupus erythematosus, psoriasis and even Sjogren’s syndrome are some of the established autoimmune disorders with prominent oral manifestations. Though these diseases are well documented and enumerated, however addressing them is where the dilemma lies. Science, research and discoveries are a crucial part of medical discipline which help in looking beyond the horizon. With the introduction of selective targeted immunotherapies for autoimmune diseases as a treatment modality either in solitary or in combination with the conventional immunosuppressive treatments, are emerging as a promising elixir for patients enduring them. However, being unique, exploration of these biologics from its inception, to its mechanism of action, recognition of its application and evaluation of its safety norms are equally vital. Therefore, this review aims to provide a comprehensive particular on the novel biologics, the immunotherapies in autoimmune disorders targeting the different cells, their receptors or cytokines of the immune system.
Keywords: Autoimmune disorders, Anti-CD 20mAb immunotherapy, Anti-TNF targeted Therapy, Pemphigus, Rheumatoid arthritis
1. Introduction
Autoimmune diseases (AD), can be defined as a clinical syndrome caused by the activation of T cells or B cells or both, in the absence of an ongoing infection or other discernible cause. They can be classified clinically as either systemic (e.g systemic lupus erythematosus) or organ specific (e.g type I diabetes mellitus).1 Epidemiological studies have estimated a prevalence of 7.6%–9.4% among the world population. With varying degrees of severity and morbidity, they are considered as one of the top ten leading cause of mortality around the world, imposing a huge burden on public health service and economy.2 These unregulated self-reactive immune cells, damage the target tissue/organ through the production of a range of proinflammatory cytokines, like tumour necrosis factor (TNF-α), interleukins (IL), interferon gamma (IFN-γ) and so on, which play a key role in its pathogenesis.3 Further, the bidirectional relationship shared between autoimmune diseases and oral health is another factor that highlights the importance of recognition and awareness of the treatment regimes advocated. Direct or indirect impact of these disorders on oral cavity with their presentations ranging from either blisters and painful erosions as seen in pemphigus (P) group; erythema, striae and erosions in systemic lupus erythematosus (SLE), TMJ dysfunction and periodontal diseases in rheumatoid arthritis (RA); xerostomia in Sjogren’s syndrome (SjS) leading to dental caries and the relationship between periodontal diseases and type I Diabetes are some of the validated and documented literatures on AD.4 Irrespective of the direct influence or the indirect presentation in the oral cavity, it has been put forth by many researchers that oral signs may be one of the first manifestations of AD which accentuate their identification and importance of cognizance of the treatment protocol delivered among the dental fraternity.
Though the traditional and conventional treatment regimes, the immunostimulators and immunosuppressants such as the cyclosporine, tacrolimus that have been implemented in the treatment of these autoimmune diseases were successful, however with the advent of novel selective target immunotherapies have revolutionized the treatment strategy employed for autoimmune diseases. Hence, this review aims to provide a comprehensive particular on immunotherapies in autoimmune disorders.
Selecting the immune cells as a target, new host of biologic agents are developed as a therapeutic approach for autoimmune diseases. These immunotherapies can be broadly categorised into antigenic specific and non specific therapies. Inhibition of the immune cell activation and their pathways along with triggering factors including the cytokine and their receptors are considered as the non specific immunotherapies which are efficiently catalogued.5 On the contrary, directing the immunotherapeutic treatment towards elimination, tolerize and regulation of the effector lymphocytes which have evaded control check and contribute to the pathogenesis of auto immune diseases are antigen specific therapies.6 Though this therapeutic strategy is still in its early infancy, concerns related to the same are present. The possibility of hypersensitivity and exacerbation of the disease due to the related antigen introduction in priorly sensitized environment cannot be excluded.5
On introduction of any novel therapeutic agent, thorough observation from its origination to application and evaluation are crucial steps that needs to be assessed. Therefore, we try and make an attempt to accumulate and present a brief overview of the available literature on B cell, T cell, dendritic cell and some cytokines targeted therapies for autoimmune diseases.
1.1. B cell targeting therapies in autoimmune diseases
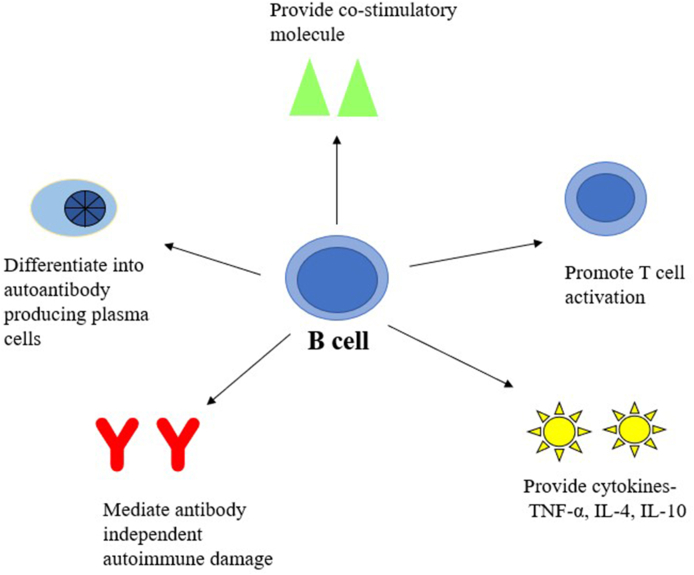
Priming the autoimmune reactions, an attribute off late recognised in antibody producing B cells twirls the management strategizes towards targeting autoreactive effector B cells depletion in these disorders.7 However, variance in the disease pathogenesis challenges the role of B cells (Fig. 1) and hence, though questions the performance of the treatment strategies advocated, the trail and lookout continued. Monoclonal antibodies (mAb) as an immunotherapeutic agent has witnessed burgeoning prosperity from its inception, a murine monoclonal antibody to chimeric, humanized and fully human monoclonal antibody.8 The very first mAb were muronomab and anti-idiotype mAbs which were murine mAb, their inevitable immunogenic nature limited their application. This was followed by infliximab, the foremost chimeric mAb that gained Food and Drug administration (FDA) approval for its inclusion in treatment of autoimmune disease. These therapies are directed either against the cell surface markers such as CD20 and CD22, cell activating factors such as BAFF and other cytokines.9
Fig. 1.
Schematic representation of B cell function in autoimmune diseases.
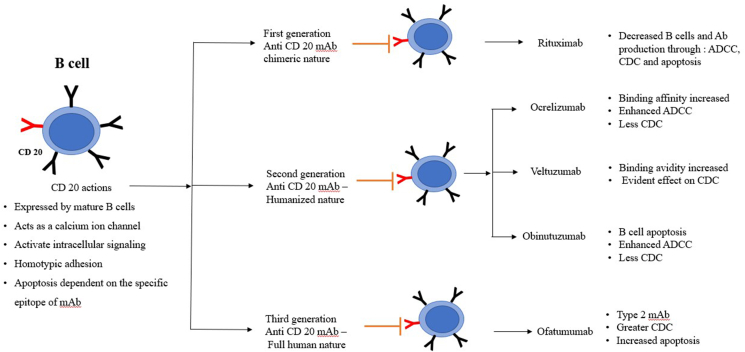
CD 20, a surface antigen expressed on the B cell, plays a key role in latters activation. Hence, it is hypothesised that, CD 20 as a target in question may be advantageous in the management of largely B cell mediated diseases.10 The effector action displayed by anti-CD 20mAb enables them to be grouped into Type 1mAb, strong activators of complement e.g rituximab, while type 2 are strong inducers of homotypic adhesion and apoptosis, e.g Ofatumumab.8 Though they successfully deplete the mature B cells, their action is ineffective on plasma cells and previously produced antibodies.9
Rituximab, a chimeric first generation anti-CD 20 mAb was approved for RA management. As literature emceed encouraging outcome through invitro studies and clinical trials, it further established the need to progress towards more humanized mAb development. Less immunogenic second generation anti-CD 20mAb development paved way for Ofatumumab, Ocrelizumab and veltuzumab mAb. Obinutuzumab, TRU-015, AME133V and Pro131921 are the third generation anti-CD 20mAb governed by enhanced effector functions.11 Better tolerance and potent nature of the newer generation though looks promising yet necessitates guidelines of its applications and dosage to be delineated through monitored systematic clinical trials for autoimmune diseases.12 (Fig. 2)
Fig. 2.
Schematic representation of anti CD 20 targeted immunotherapy in autoimmune diseases.
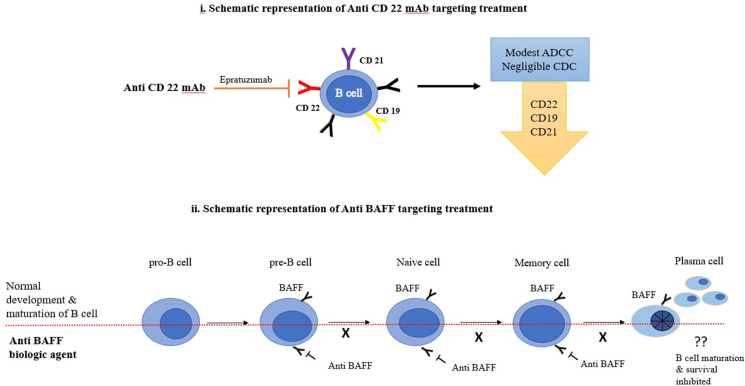
Rituximab (RTX), a genetically engineered chimeric monoclonal antibody with specific affinity to CD 20 surface antigen on B cells, intercepts two major functions, the cell cycle progression and differentiation of B cells.13 Being a B cell depleting agent, it is considered as beneficial or even curative by curbing the savage clique of self-perpetuating auto-reactive B cells in autoimmune disorders, though guarded by certain limitations.14 RTX as a treatment modality has gained importance in RA owing to the B cell synovial activity. From monotherapy to combination therapy, from minimal sample to major studies, application in conventional treatment resistant cases have shown promising results. However, it is to be noted that the drug mechanism of action may be ineffective as addressing pathogenesis of the said autoimmune disease is not governed by B cell depletion alone.15 RTX is considered relatively safe in both malignant and non-malignant conditions though infusion related adverse effects, infections, serum sickness, neutropenia and reactions to its chimeric nature have been recorded.16(Table 1) CD22, B-lymphocyte-restricted adhesion molecule is a membrane receptor which primarily activates B cells and aid in T cell interactions.17 This receptor is expressed from pro B stage to plasma cell differentiation stage.9 This membrane receptor had been a target for analysis in autoimmune diseases owing to its contribution in SLE pathogenesis.18 Epratuzumab, a humanized anti-CD 22mAb demonstrated a rapid, marked decline in CD22 and a marginal reduction of CD19, CD21 and CD79β, and also B cells with additional features of well tolerance and improvement of disease activity in moderate to severe SLE patients.19 However, the results were not uniform and hence is still controversial on the mechanism of action owing to the modest and negligible antibody dependent cellular cytotoxicity (ADCC) and complement dependent cytotoxicity (CDC) respectively.20 This necessitates further research to evaluate the mechanism of action and mode of response to further the use of anti-CD 22 mAb in autoimmune diseases.19(Fig. 3-i)
Table 1.
Targeted Immunotherapies and the autoimmune conditions.
| Targeted therapy | Biologic agent | Autoimmune conditions |
|---|---|---|
| Anti CD20 targeted therapy | Rituximab | RA, AHA, ITP, TTP, SLE, RD, WG, ABS, GHD allogeneic transplantation |
| Anti IL 6 targeted therapy | Tocilizumab | RA, SLE, SS, AHA, Systemic juvenile idiopathic arthritis, Acquired hemophilia A |
| Anti TNF targeted therapy | Etanercept | Juvenile RA, RA, AS, Plaque psoriasis, Psoriatic arthritis |
| Adalimumab | RA, AS, CD, Psoriatic arthritis | |
| Golimumab | RA, UC | |
| Certolizumab pegol | CD, Psoriatic arthritis | |
| Infliximab | RA, AS, CD, UC, Psoriatic arthritis, Chronic plaque psoriasis | |
| Anti Th 17 A targeted therapy | Secukinumab | RA, AS, Psoriasis, Psoriatic arthritis |
| Anti CD22 targeted therapy | Epratuzumab | SLE, Primary SjS |
RA- Rheumatoid arthritis, AHA- Autoimmune haemolytic anaemia, ITP-Immune thrombocytopenic purpura,TTP-Thrombotic thrombocytopenic purpura, SLE-Systemic lupus erythematosus, RD- Refractory dermatomyositis, WG-Wegener’s granulomatosis, ABS- Autoimmune bullous diseases of skin, GHD-Graft-versus-host disease, SS- Systemic sclerosis, CD- Crohn’s disease, AS- Ankylosing spondylitis, UC- Ulcerative colitis, SjS- Sjogren syndrome.
Fig. 3.
Schematic representation of B cell targeted immunotherapy in autoimmune diseases.
Development, survival, differentiation and maturation of B cells are dependent on BAFF which bind to BAFF –R, TACI and BCMA receptors expressed by different stages of B cells through the progression of its development. Elevated levels of BAFF in the serum of SLE positive cases registered it as a therapeutic candidate to be reviewed. Belimumab, tabalumab and blisibimod are the targeted immunotherapeutic trials against BAFF in SLE (Fig. 3-ii). Of these only belimumab, fully humanized monoclonal antibody was successful in clearing the phase trials with good tolerance and efficacy. However, the cases selected for the trials were guarded with specific selection criteria and hence its efficacy is yet to be evaluated in cases with major organ involvement and combination therapy with other biologics or drugs which are still ongoing.21
1.2. TNF targeting therapies in autoimmune diseases
Tumor necrosis factor, a pleotropic cytokine, an important pro inflammatory mediator, is considered to perform a salient role in autoimmune diseases.22 On one hand TNF detection in the synovial samples of RA cases and the resultant diminished expression of pro inflammatory cytokines such as IL-1, IL-6, GM-CSF along with suppressed antigen-induced IFN-γ production on anti-TNF antibody introduction in the former, strengthens its immunoregulatory role in the former pathogenesis. This evidence based supportive data led to the development of anti-TNF biologics as a treatment strategy for autoimmune diseases. Infliximab, etanercept, adalimumab, golimumab, certolizumab are the anti TNF biologics currently in use.23
Infliximab is a recombinant chimeric anti TNF antibody that blocks TNF binding to its receptors and effectively mediates both CDC and ADCC. Etanercept, a human recombinant protein, inactivates TNF and lymphotoxin, thereby intercepting the latter interaction with its receptors. It helps in reliving the symptoms and inhibit the progression of the diseases. Adalimumab, fully humanized IgG1 mAb, is comparatively well tolerated owing to its minimal immunogenicity when compared to Infliximab and its longer half-life. Another fully humanized IgG1 mAb, with its greater affinity and human TNF specificity contributing to TNF effective neutralization is Golimumab. Certolizumab pegol, a humanized mAb, owing to its unique structure and distinct mechanism of action, contributes to its higher efficiency when compared with other TNF inhibitors.23(Table 1)
Anti-TNF biologics impediments irrespective of their effectiveness are governed primarily by lack of uniform response as nearly 40% patients fail to respond to the therapy.24 Further, the gene polymorphism of TNF and its receptor are associated with varied degree of response to the therapy. Increased risk of infections and malignancy as reported by few studies is another crucial factor observed in anti TNF biologics.25 Exacerbation of disease, de novo induction of other autoimmune disease, deleterious effect of the associated co morbidities also pose a limitation in its clinical application necessitating the need of through knowledge of TNF biological functions to develop more effective and safer targeted treatment methodologies.23,25
1.3. Interleukin 6 targeted therapy in autoimmune diseases
IL 6, another pleiotropic cytokine, has a crucial role in autoimmune diseases. From antibody and immunoglobulin secretion in B cells to differentiation of CD4+T heper cells and CD8+ Tcells into IL-17-producing T-helper cells (Th 17) and active cytotoxic cells respectively in T cells contributes to it substantiate role in the pathogenesis. Further, pathogenic role of IL 6 is demonstrated in numerous studies on animal models with suppression of the disease in preventive or therapeutic fashion by either blockage of the cytokine or administration of the antibody thereby compelling it as a target for therapeutic intervention.26,27
Tocilizumab, humanized anti-IL-6 receptor mAb, blocks IL 6 action by inhibiting its binding to transmembrane and its receptor. It is used widely for RA as a monotherapy or in combination with other drugs. Suppressed disease activity and halting the progression of joint deformity with improved functional activity favoured the clinical outcome in moderate to severe RA.27 The former accomplishments by the drug, have favoured numerous studies, case series to explore broader application in various systemic and organ specific autoimmune diseases which are under trials with further verification of its efficacy and safety (Table 1).
1.4. Th 17 lymphocytes targeted therapy in autoimmune diseases
T helper cells producing IL17 (Th 17), its identification, ability to stimulate inflammation, recognition of its role in the autoimmune pathogenesis supported by literature on animal and human models of both systemic and organ specific diseases have led to consider these cells and its pathways in the disorders. Th 17 cells produce pro-inflammatory cytokines IL17 A and IL17 F and are stimulated by IL6 and IL-23. Therefore, either targeting the produced cytokine or its receptor, IL17 and IL17 R or the cytokines that activate Th 17 cells are considered as a key for therapeutic strategy.28
Ustekinumab, fully human mAb, targeted IL-17 and IL-23 inhibition and hence the pathway of Th17. Most of the studies were in psoriasis, however other autoimmune diseases also achieved successful results in clinical trials. It was observed to be effective even in cases resistant to TNF inhibitors. However, on analysis of the trials, numerous adverse effects were identified emphasizing the monitoring, dosage and the safety norms.28,29 Briakinumab(targeting the IL-12/23p40 pathway),30 Brodalumab (human monoclonal anti-IL-17A receptor Ab),31 Ixekizumab(humanized mAb with selectivity for IL-17A)32 are the drugs under trial for psoriasis with promising results.
Secukinumab, humanized anti-IL-17A mAb is mainly studied in psoriasis. IL 17 A activity is inhibited, hence interferes with the disease pathway and promotes normalization of dermis and immune function without targeting IL17 F or Th17 function or Th1 pathway, thereby presenting with fewer adverse outcomes. These features have broadened its application in Psoriatic arthritis, Ankylosing spondylitis. Good efficacy, well tolerated and acceptable safety profile, minimal adverse effects as reported by studies and evaluated by meta-analysis suggest that with continued pharmacovigilance, this drug promises to be a potential therapeutic boon for psoriasis.33(Table 1)
1.5. T regs targeted therapy in autoimmune diseases
Regulatory T cells (T regs), either the nTregs derived from the thymus or the iTregs derived from the periphery on exposure to anti inflammatory cytokines are known to supress the autoimmune cells in an antigenic specific pattern. T cell recerptor (TCR) and its co stimulatory molecule CD28 are required for its activation. These cells express transcription factor, forkhead box P3 (Foxp3), with the ability to diminsh proinflammatory genes expression and upregulate anti inflammatory gene expression. Features such as specificity of the TCR, anti-inflammatory action, ability to traffic to the site of action and supression of autoimmune response, all favour it as a potential therapeutic target for autoimmune diseases.34,35
Reaseraches have hypothesised that the vulnerable spots to be targeted in T regs mechanism of suppression in autoimmune diseases when the T regs are in present in balanced number are 1. Target auto reactive CD4+ T effector memory cells by tolerogenic APC generation which allows antigen specific memory T cells to be targeted on reactivation by tolerogenic APC. 2. Driving exhausted CD8+ T cell to PD-1-PD-L1 mediated cell death by T regs when upsurge expression of increased affinity IL2 receptors (CD25) leads to local cytokine depletion. 3. Ability of T regs to inhibit B cell proliferation and its secretion of autoantibodies on activation by B cells. Further, the researchers addressed the key points in hypothesised situation when T regs are imbalanced in autoimmune diseases are 1. Promotion of T regs formation through IL2 due to their affinity of expression, 2. Adoptive Treg cell transfer by reconstituting the immune system prior to disease onset may inhibit the disease initiation.
However, given the small proportion of blood T regs, difficulty in its extraction and isolation, maintenance of its suppressive function is challenging. Various studies have approached to generate T regs both invitro and invivo. Either by expansion of polyclonal T regs by IL 2, rapamycin or autoantigenic specific T regs by antigen and dendritic cells, apoptosis antigen approach to achieve a pool of T regs with its suppressive action in function.34,35 Therefore, T regs as a target of immunotherapy can be addressed by either achieving a resaonable population either by its activation or promotion of its formation, which enables them to bring about their suppressive action. Literature suggests that studies are carried both in vitro and invivo to achieve the former.
1.6. Dendritic cell therapy
Dendritic cells (DC), they are the antigen presenting cells that present the antigen to the T cells. Directing the immune response, influencing the differentiated T regs, Th 17 cells, promoting tolerance, producing inflammatory or suppressive mediators, make them a potential target for immunotherapy. Tolerogenic DC are being used in early phase I trials for RA, known as Rheumavax. The principle applied in this form of therapy is similar to antigen specific therapy where the antigen loaded DC are introduced into the T cells during Ag presentation aiming to eliminate the T cells. This procedure needs to analysed for various factors such as the appropriate antigen, route, timing and frequency of administration along with monitoring the effects. Therefore, though in its initial stages, researchers are conducting clinical trials implementing the above principle in a quest to address the auto immune diseases.34,36
1.7. Immunotherapies in autoimmune diseases with oral manifestations
Autoimmune diseases, irrespective of being either organ or systemic in nature, may or may not include the oral cavity as its effector region, however, its influence in the oral cavity is undeniable. Being the mirror of the body’s health, some of the first signs are reported to appear in cavum oris. Pemphigus, systemic lupus erythematosus, rhematoid arthritis, psoriasis and even sjogrens syndrome are some of the autoimmune diseases with oral manifestations.37 Their immunotherapueuptic response are being evaluated, recorded and studied extensively.
Pemphigus are a group of life threating blistering epidermal and mucosal disorder, where the autoantibodies predominantly being Ig G4 and Ig G1 are directed towards the desmoglein (Dsg) 1 and 3 desmosomal components of the cell-cell adhesion complexes. RTX is found to be highly effective in pemphigus.38 It acts through incomplete B cells depletion, decrease in circulating anti Dsg autoantibodies, modulating both humoral and acquired immune function.39 Studies have found it not only effective in P vulgaris and P foliaceus, but also in refractory cases.40 Reports suggest its beneficial use even in B cell malagnancies associated with paraneoplastic pemphigus in few studies,41 while others present with much less consistent results.42 Though relapse is a common feature in RTX treatment,43 yet RTX as maintenance therapy has yeilded favourable results in pemphigus.42 Other B cell therapies that are studied with successful outcomes are ofatumumab44 and veltuzumab.45
SLE, an autoimmune disease in which B cells execute both antibody independent and dependent role in its pathogenesis have presented with promising results on treatment with RTX. Other than B cell depletion, achievement of complete response and better post treatment maintenance was the outcome of both monotherapy and combination therapy when evaluated by different studies. In addition, beneficiary action in lupus nephritis cases, down regulation of CD 40, CD 80, CD69, HLA-DR and NK cells were also observed in few studies.46 Ocrelizumab, an anti-CD 20mAb, has been used in SLE multicentric Phase III trials. Epratuzumab, an anti-CD 22mAb, anti-IL 10mAb, anti-TNF therapies are also considered and are under various stages of evaluation for SLE.47
Similarly, in rheumatoid arthritis which primarily manifests as chronic inflammatory arthropathy also has witnessed successful biological treatment. Currently, TNF inhibitors such as Adalimumab, certolizumab pegol, etanercept, golimumab and infliximab; Anakinra, an IL-1 inhibitor; Tocilizumab, IL-6 inhibitor; RTX, anti-CD20 inhibitor have revolutionized and presented options to the clinicians to choose from the drugs to be used either as first line treatment, monotherapy or in combination.48 As the mechanism of action and the pathogenesis of various biologics and autoimmune diseases are being systematically documented, has led to the possibilities of these drugs to be applied and evaluated for their beneficial mode of action through human and animal models in other autoimmune diseases too, their paving way to new era of treatment modalities.
1.8. Limitations of biologics
The biological agents designed to target a specific molecular component of immune system which did demonstrate good safety and tolerabilty have been recorded with cetain adverse effects. The development of new autoimmune diseases irrespective of the present, during the treatment with biologics have been recorded. Either systemic and organ specific autoimmune diseases induced during the treatment with biologics predominantly with anti TNF agents along with therpies directed towards B cells or T cells or even the cytokines are been recognised and recorded lately.49,50
The main targets of the these therapies either target a cytokine, its receptor or the cells which constitute a part of the normal immune hemostasis and hence the physiological environment. Therefore, eliminating or blocking the former leads to adverse effcets such as cytokine storm, infections, hypersensitivity, immune imbalance with immune suppression, cross reactivity etc Therefore, this necessiates a thorough knowledge and understanding of the immunologic mechanisms of the physiologic environment and the biologics used with judicial monitoring of the procedure.49,50
2. Conclusion
Immunotherapies in autoimmune diseases have seen prominent amplification in its recognition, trials and in clinical applications. The armonary of immunotherapies seeing the light are expanding swiftly. Either monotherapy or their compatibility when used as a combination therapy with other biologics or drugs have been recorded. However, thorough comprehension of the molecular mechansim of action, their possible limitations, evaluation of parameters such as efficacy, tolerance, safety are crucial to present them as a promising novel therapuetic treatment for autoimmune diseases. Reasearchers are conducting trials which are in different phases both on animal and human model to acheieve and acquire a successful immunotherapeutic agent for autoimmune diseases.
Funding source
The author received no specific funding for this work.
Declaration of competing interest
All the author associated with present manuscript declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Davidson A., Diamond B. Autoimmune diseases. N Engl J Med. 2001;345(5):340–350. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 2.Bragazzi N.L., Watad A., Brigo F., Adawi M., Amital H., Shoenfeld Y. Public health awareness of autoimmune diseases after the death of a celebrity. Clin Rheumatol. 2017;36(8):1911–1917. doi: 10.1007/s10067-016-3513-5. [DOI] [PubMed] [Google Scholar]
- 3.Ellis J.S., Braley-Mullen H. Mechanisms by which B cells and regulatory T cells influence development of murine organ-specific autoimmune diseases. J Clin Med. 2017;6(2):13. doi: 10.3390/jcm6020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reilly R.J., Johnston W., Culshaw S. Autoimmunity and the oral cavity. Current Oral Health Reports. 2019;6(1):1–8. [Google Scholar]
- 5.Caspi R.R. Immunotherapy of autoimmunity and cancer: the penalty for success. Nat Rev Immunol. 2008;8(12):970–976. doi: 10.1038/nri2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peakman M., Dayan C.M. Antigen-specific immunotherapy for autoimmune disease: fighting fire with fire? Immunology. 2001;104(4):361. doi: 10.1046/j.1365-2567.2001.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiorina P., Vergani A., Dada S. Targeting CD22 reprograms B-cells and reverses autoimmune diabetes. Diabetes. 2008;57(11):3013–3024. doi: 10.2337/db08-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perosa F., Prete M., Racanelli V., Dammacco F. CD20-depleting therapy in autoimmune diseases: from basic research to the clinic. J Intern Med. 2010;267(3):260–277. doi: 10.1111/j.1365-2796.2009.02207.x. [DOI] [PubMed] [Google Scholar]
- 9.Musette P., Bouaziz J.D. B cell modulation strategies in autoimmune diseases: new concepts. Front Immunol. 2018;9:622. doi: 10.3389/fimmu.2018.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt E., Goebeler M. CD20-directed therapy in autoimmune diseases involving the skin: role of rituximab. Expet Rev Dermatol. 2008;3(3):259–278. [Google Scholar]
- 11.Boross P., Leusen J.H. Mechanisms of action of CD20 antibodies. Am. J. Cancer Res. 2012;2(6):676. [PMC free article] [PubMed] [Google Scholar]
- 12.Du F.H., Mills E.A., Mao-Draayer Y. Next-generation anti-CD20 monoclonal antibodies in autoimmune disease treatment. Autoimmunity Highlights. 2017 Dec 1;8(1):12. doi: 10.1007/s13317-017-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rastetter W., Molina A., White C.A. Rituximab: expanding role in therapy for lymphomas and autoimmune diseases. Annu Rev Med. 2004;55:477–503. doi: 10.1146/annurev.med.55.091902.104249. [DOI] [PubMed] [Google Scholar]
- 14.Salvi M., Vannucchi G., Campi I. Efficacy of rituxim∗ab treatment for thyroid-associated ophthalmopathy as a result of intraorbital B-cell depletion in one patient unresponsive to steroid immunosuppression. Eur J Endocrinol. 2006;154(4):511–517. doi: 10.1530/eje.1.02119. [DOI] [PubMed] [Google Scholar]
- 15.Salvi M., Vannucchi G., Campi I. Treatment of Graves’ disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol. 2007 Jan 1;156(1):33–40. doi: 10.1530/eje.1.02325. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka Y.B. Cell targeting therapy using the anti-CD20 antibody rituximab in inflammatory autoimmune disease. Intern Med. 2007;46:1313–1315. doi: 10.2169/internalmedicine.46.1914. [DOI] [PubMed] [Google Scholar]
- 17.Leonard J.P., Link B.K. Immunotherapy of non-Hodgkin’s lymphoma with hLL2 (epratuzumab, an anti-CD22 monoclonal antibody) and Hu1D10 (apolizumab) Semin Oncol. 2002;29(1):81–86. [PubMed] [Google Scholar]
- 18.Dörner T., Shock A., Smith K.G. CD22 and autoimmune disease. Int Rev Immunol. 2012;31(5):363–378. doi: 10.3109/08830185.2012.709890. [DOI] [PubMed] [Google Scholar]
- 19.Li J., Wei M.M., Song Q., Guo X.H., Shao L., Liu Y. Anti-CD22 epratuzumab for systemic lupus erythematosus: a systematic review and meta-analysis of randomized controlled trials. Exp.Therapeut.Med. 2019;18(2):1500–1506. doi: 10.3892/etm.2019.7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossi E.A., Goldenberg D.M., Michel R., Rossi D.L., Wallace D.J., Chang C.H. Trogocytosis of multiple B-cell surface markers by CD22 targeting with epratuzumab. Blood. J. Am. Soc.Hematol. 2013;122(17):3020–3029. doi: 10.1182/blood-2012-12-473744. [DOI] [PubMed] [Google Scholar]
- 21.Lee W.S., Amengual O. B cells targeting therapy in the management of systemic lupus erythematosus. Immunological Medicine. 2020;43(1):16–35. doi: 10.1080/25785826.2019.1698929. [DOI] [PubMed] [Google Scholar]
- 22.Monaco C., Nanchahal J., Taylor P., Feldmann M. Anti-TNF therapy: past, present and future. Int Immunol. 2015;27(1):55–62. doi: 10.1093/intimm/dxu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li P., Zheng Y., Chen X. Drugs for autoimmune inflammatory diseases: from small molecule compounds to anti-TNF biologics. Front Pharmacol. 2017;8:460. doi: 10.3389/fphar.2017.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roda G., Jharap B., Neeraj N., Colombel J.F. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol. 2016;7(1):e135. doi: 10.1038/ctg.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerra I., Algaba A., Pérez-Calle J.L. Induction of psoriasis with anti-TNF agents in patients with inflammatory bowel disease: a report of 21 cases. Journal of Crohn’s and Colitis. 2012;6(5):518–523. doi: 10.1016/j.crohns.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Nishimoto N., Kishimoto T., Yoshizaki K. Anticytokine therapy in autoimmune diseases. Intern Med. 1999;38(2):178–182. doi: 10.2169/internalmedicine.38.178. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka T., Kishimoto T. Targeting interleukin-6: all the way to treat autoimmune and inflammatory diseases. Int J Biol Sci. 2012;8(9):1227. doi: 10.7150/ijbs.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabarkiewicz J., Pogoda K., Karczmarczyk A., Pozarowski P., Giannopoulos K. The role of IL-17 and Th17 lymphocytes in autoimmune diseases. Arch Immunol Ther Exp. 2015;63(6):435–449. doi: 10.1007/s00005-015-0344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffiths C.E., Strober B.E., van de Kerkhof P. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362(2):118–128. doi: 10.1056/NEJMoa0810652. [DOI] [PubMed] [Google Scholar]
- 30.Reich K., Langley R.G., Papp K.A. A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis. N Engl J Med. 2011;365(17):1586–1596. doi: 10.1056/NEJMoa1010858. [DOI] [PubMed] [Google Scholar]
- 31.Mease P.J., Genovese M.C., Greenwald M.W. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370(24):2295–2306. doi: 10.1056/NEJMoa1315231. [DOI] [PubMed] [Google Scholar]
- 32.Tham L.S., Tang C.C., Choi S.L., Satterwhite J.H., Cameron G.S., Banerjee S. Population exposure–response model to support dosing evaluation of ixekizumab in patients with chronic plaque psoriasis. J Clin Pharmacol. 2014;54(10):1117–1124. doi: 10.1002/jcph.312. [DOI] [PubMed] [Google Scholar]
- 33.Frieder J., Kivelevitch D., Menter A. Secukinumab: a review of the anti-IL-17A biologic for the treatment of psoriasis. Ther. Adv.Chronic Dis. 2018;9(1):5–21. doi: 10.1177/2040622317738910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang D., Tu E., Kasagi S., Zanvit P., Chen Q., Chen W. Manipulating regulatory T cells: a promising strategy to treat autoimmunity. Immunotherapy. 2015;7(11):1201–1211. doi: 10.2217/imt.15.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulivieri C., Baldari C.T. T-cell-based immunotherapy of autoimmune diseases. Expet Rev Vaccine. 2013;12(3):297–310. doi: 10.1586/erv.12.146. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch D.L., Ponda P. Antigen-based immunotherapy for autoimmune disease: current status. ImmunoTargets Ther. 2015;4:1. doi: 10.2147/ITT.S49656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saccucci M., Di Carlo G., Bossù M., Giovarruscio F., Salucci A., Polimeni A. Autoimmune diseases and their manifestations on oral cavity: diagnosis and clinical management. J. Immunol.Res. 2018;2018 doi: 10.1155/2018/6061825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollmann R., Schmidt T., Eming R., Hertl M. Pemphigus: a comprehensive review on pathogenesis, clinical presentation and novel therapeutic approaches. Clin Rev Allergy Immunol. 2018;54(1):1–25. doi: 10.1007/s12016-017-8662-z. [DOI] [PubMed] [Google Scholar]
- 39.Amber K.T., Maglie R., Solimani F., Eming R., Hertl M. Targeted therapies for autoimmune bullous diseases: current status. Drugs. 2018;78(15):1527–1548. doi: 10.1007/s40265-018-0976-5. [DOI] [PubMed] [Google Scholar]
- 40.Cianchini G., Lupi F., Masini C., Corona R., Puddu P., De Pità O. Therapy with rituximab for autoimmune pemphigus: results from a single-center observational study on 42 cases with long-term follow-up. J Am Acad Dermatol. 2012;67(4):617–622. doi: 10.1016/j.jaad.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Maglie R., Didona D., Eming R., Hertl M. Pemphigus: current and future therapeutic strategies. Front Immunol. 2019;10:1418. doi: 10.3389/fimmu.2019.01418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwatra S.G., Boozalis E., Pasieka H., Anhalt G.J. Decreased recognition of paraneoplastic pemphigus in patients previously treated with anti-CD 20 monoclonal antibodies. Br J Dermatol. 2019;180(5):1238–1239. doi: 10.1111/bjd.17577. [DOI] [PubMed] [Google Scholar]
- 43.Wang H.H., Liu C.W., Li Y.C., Huang Y.C. Efficacy of rituximab for pemphigus: a systematic review and meta-analysis of different regimens. Acta Derm Venereol. 2015;95(8):928–932. doi: 10.2340/00015555-2116. [DOI] [PubMed] [Google Scholar]
- 44.Rapp M., Pentland A., Richardson C. Successful treatment of pemphigus vulgaris with ofatumumab. J Drugs Dermatol JDD: J Drugs Dermatol JDD. 2018;17(12):1338–1339. [PubMed] [Google Scholar]
- 45.Ellebrecht C.T., Choi E.J., Allman D.M. Subcutaneous veltuzumab, a humanized anti-CD20 antibody, in the treatment of refractory pemphigus vulgaris. JAMA dermatology. 2014;150(12):1331–1335. doi: 10.1001/jamadermatol.2014.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karim M.Y., Pisoni C.N., Khamashta M.A. Update on immunotherapy for systemic lupus erythematosus—what’s hot and what’s not! Rheumatology. 2009;48(4):332–341. doi: 10.1093/rheumatology/ken476. [DOI] [PubMed] [Google Scholar]
- 47.Dörner T., Kaufmann J., Wegener W.A., Teoh N., Goldenberg D.M., Burmester G.R. Initial clinical trial of epratuzumab (humanized anti-CD22 antibody) for immunotherapy of systemic lupus erythematosus. Arthritis Res Ther. 2006;8(3):R74. doi: 10.1186/ar1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meier F.M., Frerix M., Hermann W., Müller-Ladner U. Current immunotherapy in rheumatoid arthritis. Immunotherapy. 2013;5(9):955–974. doi: 10.2217/imt.13.94. [DOI] [PubMed] [Google Scholar]
- 49.Her M., Kavanaugh A. Alterations in immune function with biologic therapies for autoimmune disease. J Allergy Clin Immunol. 2016;137(1):19–27. doi: 10.1016/j.jaci.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 50.Perez-Alvarez R., Pérez-de-Lis M., Ramos-Casals M., BIOGEAS Study Group Biologics-induced autoimmune diseases. Curr Opin Rheumatol. 2013;25(1):56–64. doi: 10.1097/BOR.0b013e32835b1366. [DOI] [PubMed] [Google Scholar]