Abstract
Dynamic parameters of preload have been widely recommended to guide fluid therapy based on the principle of fluid responsiveness and with regard to cardiac output. An equally important aspect is however to also avoid volume-overload. This accounts particularly when capillary leakage is present and volume-overload will promote impairment of microcirculatory blood flow. The aim of this study was to evaluate, whether an impairment of intestinal microcirculation caused by volume-load potentially can be predicted using pulse pressure variation in an experimental model of ischemia/reperfusion injury. The study was designed as a prospective explorative large animal pilot study. The study was performed in 8 anesthetized domestic pigs (German landrace). Ischemia/reperfusion was induced during aortic surgery. 6 h after ischemia/reperfusion-injury measurements were performed during 4 consecutive volume-loading-steps, each consisting of 6 ml kg−1 bodyweight−1. Mean microcirculatory blood flow (mean Flux) of the ileum was measured using direct laser-speckle-contrast-imaging. Receiver operating characteristic analysis was performed to determine the ability of pulse pressure variation to predict a decrease in microcirculation. A reduction of ≥ 10% mean Flux was considered a relevant decrease. After ischemia–reperfusion, volume-loading-steps led to a significant increase of cardiac output as well as mean arterial pressure, while pulse pressure variation and mean Flux were significantly reduced (Pairwise comparison ischemia/reperfusion-injury vs. volume loading step no. 4): cardiac output (l min−1) 1.68 (1.02–2.35) versus 2.84 (2.15–3.53), p = 0.002, mean arterial pressure (mmHg) 29.89 (21.65–38.12) versus 52.34 (43.55–61.14), p < 0.001, pulse pressure variation (%) 24.84 (17.45–32.22) versus 9.59 (1.68–17.49), p = 0.004, mean Flux (p.u.) 414.95 (295.18–534.72) versus 327.21 (206.95–447.48), p = 0.006. Receiver operating characteristic analysis revealed an area under the curve of 0.88 (CI 95% 0.73–1.00; p value < 0.001) for pulse pressure variation for predicting a decrease of microcirculatory blood flow. The results of our study show that pulse pressure variation does have the potential to predict decreases of intestinal microcirculatory blood flow due to volume-load after ischemia/reperfusion-injury. This should encourage further translational research and might help to prevent microcirculatory impairment due to excessive fluid resuscitation and to guide fluid therapy in the future.
Subject terms: Translational research, Aneurysm, Aortic diseases
Introduction
Dynamic parameters of preload like pulse pressure variation (PPV) were shown to be capable of predicting macrohemodynamic responses to fluid administration1,2. This led to the recommendation to use these parameters for guiding fluid therapy in critically-ill patients both perioperatively as well as in intensive care unit settings3–5. The rationale for optimizing macrohemodynamics using PPV aims to increase cardiac output, assuming that this would result in improved tissue perfusion. However, coherence between macro- and microcirculation is frequently lost emphasizing the need for direct microcirculatory evaluation6–8.
An important aspect in guiding fluid therapy is that both hypovolemia but also particularly volume-overload have to be avoided9,10. In an earlier study in an experimental model of systemic inflammation we were able to demonstrate that already full utilization of preload reserve will result in volume-overload accompanied by impairments of microcirculatory blood flow and endothelial function11. This is clinically important since the occurrence of microcirculatory disturbances is a major cause for the development of multiple-organ failure and accounts for increased mortality12–14. Tissue perfusion and thus oxygenation depend on the perfusion of the microvasculature6. The effects of fluid administration on microcirculatory blood flow so far have not been sufficiently addressed. While some investigators have revealed promising results, other studies have failed to show an improvement of microcirculation due to fluid therapy, even if therapy was guided in accordance with macrohemodynamic goals15–17.
With regard to PPV, certain values can discriminate fluid-responders from non-responders, while also a zone of intermediate values with limited discriminatory power has been identified18,19. Beneath this zone an increase of stroke volume is rather unlikely, which on the other hand makes it all the more likely that fluid administration will promote volume-overload and consequently also a decrease of microcirculatory blood flow. This might be of particular interest in conditions of disturbed endothelial function and increased capillary leakage.
Therefore, we hypothesized that the underlying physiological principles of PPV could also be used to identify whether fluid administration results in a decrease of microcirculatory blood flow. We used a porcine model of ischemia/reperfusion injury during experimental aortic surgery as a model for a condition with microcirculatory disturbances, endothelial dysfunction and increased capillary leakage20,21. The aim of this study was to evaluate whether PPV does have the potential to predict an impairment of intestinal microcirculatory blood flow due to volume-load in our experimental setting.
Results
Study population
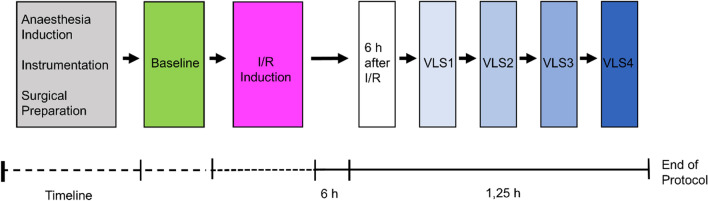
8 animals were studied. All animals survived until completion of protocol. No adverse events (e.g. severe bleeding, cardiac arrest) occurred. Mean body weight was 78.1 kg (95% CI 75.9–80.3). The study protocol is given in Fig. 1.
Figure 1.
Experimental protocol. After completion of anesthesia induction, instrumentation and surgical preparations, baseline measurements were performed. Thereafter, ischemia/reperfusion (I/R) was induced. 6 h after ischemia/reperfusion induction, measurements were repeated and 4 consecutive volume-loading-steps (VLS) performed.
Hemodynamic parameters
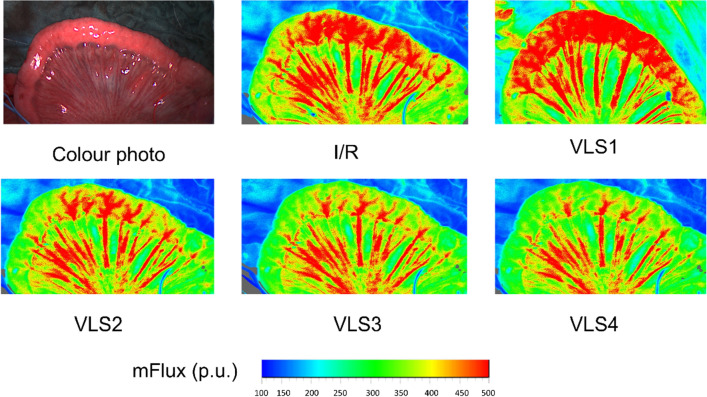
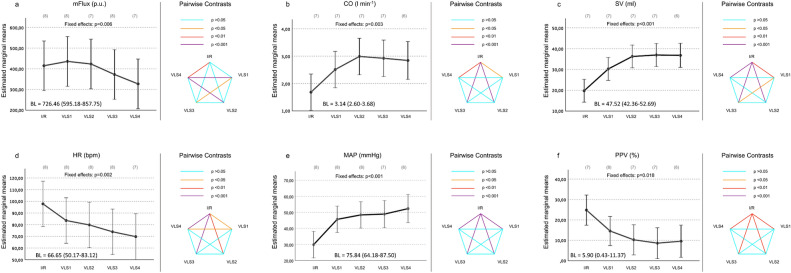
Sample pictures of intestinal mean Flux are given in Fig. 2. Baseline values prior ischemia/reperfusion as well as changes of micro- and macrohemodynamic parameters throughout the experimental protocol after ischemia/reperfusion are shown in Fig. 3.
Figure 2.
Sample picture of small intestine and color-coded examples of Laser-Speckle-Contrast-Imaging. Sample picture of exposed small intestine (ileum) and color-coded examples of Laser-Speckle-Contrast-Imaging derived mean intestinal microcirculatory blood flow (mFlux) at all points of measurement in an exemplary animal. I/R = ischemia/reperfusion; VLS = volume-loading-step.
Figure 3.
Changes of hemodynamic parameters throughout the experimental protocol. Changes throughout the experimental protocol of (a) mean microcirculatory blood flow (mFlux), (b) cardiac output (CO), (c) stroke volume (SV), (d) heart rate (HR), (e) mean arterial pressure (MAP) and (f) central pulse-pressure-variation (PPV). Data are presented as baseline adjusted estimated marginal means with 95% confidence intervals (left side). Results of pairwise comparisons are illustrated with color-coding of significance (right side). Points of measurements are 6 h after ischemia/reperfusion (I/R), volume loading steps 1–4 6 h after ischemia/reperfusion (VLS1-4). Number of valid values for each point of measurement are given in brackets. Baseline values (BL) prior ischemia/reperfusion are given in addition.
Prediction of intestinal microcirculatory blood flow decrease in ischemia/reperfusion
In each animal 4 volume-loading-steps were performed resulting in a total of 32 volume-loading-steps. 3 volume-loading-steps had to be excluded due to artefacts in microcirculatory measurements. Of the remaining 29 volume-loading-steps, 3 PPV values had to be excluded due to cardiac arrhythmias. Thus, in total 26 volume-loading-steps were analyzed. There were 11 positive and 15 negative states. In detail for predicting decrease of intestinal microcirculatory blood flow in ischemia/reperfusion central PPV presented with an AUC of 0.88 (95% CI 0.74–1.00; p < 0.001). The receiver operating characteristic curve is presented in Fig. 4.
Figure 4.
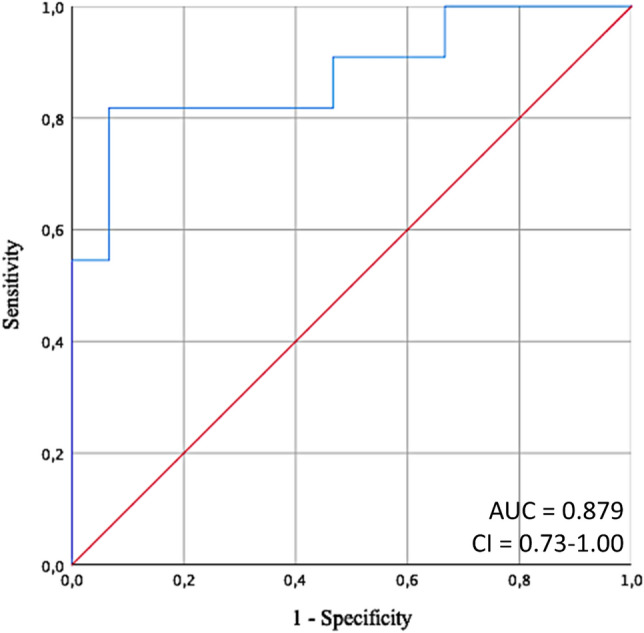
Receiver operating characteristic curve. Receiver operating characteristic curve to determine the ability of central pulse pressure variation to predict a ≥ 10% decrease of small intestinal microcirculation after ischemia/reperfusion. Direction of the test was set to decreasing values of pulse pressure variation. AUC = area under the curve, CI = 95% confidence interval.
According to the receiver operating characteristic analysis in this experimental model of ischemia/reperfusion in pigs, the following cutoff-values for the prediction of microcirculatory decreases can be proposed: PPV ≤ 7.7% (Youden Index 0.75; sensitivity 81.82%, specificity 93.33%, positive predictive value 0.90, negative predictive value 0.88).
In addition, receiver operating characteristics analysis was performed using peripheral PPV. Peripheral PPV presented with an AUC of 0.83 (95% CI 0.66 to 1.00; p = 0.005) for prediction of intestinal microcirculatory blood flow decline with a cutoff value ≤ 8.0 (Youden Index 0.59, sensitivity 72.73%, specificity 86.67%, positive predictive value 0.80, negative predictive value 0.81). Details on receiver operating characteristics analysis are given as additional table 2 h in the additional file.
Discussion
Our study demonstrates that PPV potentially could be used to predict decreases of intestinal microcirculatory blood flow due to excessive volume-load in an experimental setting of ischemia/reperfusion. Full utilization of preload reserve as reflected in low values for PPV aggravated impairment of intestinal microcirculatory flow. This suggests that dynamic indicators of preload may also be suitable to prevent microcirculatory deterioration when guiding fluid therapy in the future.
Over-infusion can bring about aggravated tissue edema leading to a deterioration of microcirculatory blood flow, while microcirculatory deterioration will result in oxygen deficits, multiple-organ failure and is associated with increased mortality12–14. Existing studies focusing on the effects of fluid administration on microcirculation often have shown a failure to improve microcirculation during fluid therapy and a saturation of the volume effect has been already described15,16,22. Therefore, identifying potential parameters that would enable prediction of microcirculatory behavior is of highest interest. Dynamic preload parameters such as pulse pressure variation can be used to assess fluid responsiveness3,23–26. Moreover, they may be useful to prevent volume-overload19,27,28. However, studies considering microcirculatory effects are scarce.
Loss of hemodynamic coherence has been described for different scenarios including perioperative medicine6,7,29–31. Microcirculatory alterations have a huge impact on prognosis and outcome not only in sepsis and heart failure, but also in surgical patients29,31,32. Moreover, anesthesia itself affects microcirculation31,33,34. Ischemia/reperfusion occurs in several medical conditions and leads to microcirculatory deterioration, accounts for the development of multiple-organ failure and is associated with increased mortality12,13,20,21,35,36. Alterations in microcirculatory blood flow have been shown to correlate with clinical outcome after liver, kidney and pancreas transplanation31,37–39. Ischemia/reperfusion induced by aortic cross clamping has been shown to influence microcirculation31. Loss of hemodynamic coherence with impairments of microcirculation as well as endothelial dysfunction has been described and microcirculation correlated with outcome following aortic cross clamping and vascular surgery8,40–42. In contrast, protection of splanchnic microcirculation with use of colloids has been shown to reduce complications in abdominal aortic surgery43. Intestinal microcirculatory alterations have also been shown to be associated with anastomotic leakage31. The small intestine is particularly susceptible to ischemia/reperfusion-injury resulting in intestinal-barrier dysfunction, bacterial translocation, systemic inflammation and multiple-organ failure21,44,45. Microcirculatory deteriorations are important determinants for manifestation of intestinal ischemia/reperfusion injury and have major prognostic impact46. The results of this study has revealed a promising new field of application for PPV as being capable of predicting impairments of intestinal microcirculatory blood flow in ischemia/reperfusion. Since effects of fluids on microvascular perfusion are associated with outcome32, use of PPV for prevention of small intestinal microcirculatory deterioration during fluid administration does have the potential to improve outcome.
In our experimental setting of ischemia/reperfusion PPV presented with a good AUC for predicting decline of microcirculatory blood flow due to volume-load. The cut-off value ≤ 7.7% in our series would be interpreted as being in the gray zone or to indicate and correspond to non-responders with regard to macrocirculatory responses to a fluid challenge. Cannesson and colleagues identified PPV-values between 9 and 13% as being inconclusive while Biais and others also showed that PPV-values between 4 and 17% failed to accurately predict fluid responsiveness18,19. Biais and others have reported, that this “grey zone” is already associated with the potential risk of over-infusion19.
Since most of clinical studies use PPV derived by femoral or radial artery blood pressure measurements18,47, peripheral PPV was assessed in addition. Although the AUC for peripheral PPV was lower, which may be explained be our experimental model using an aortic graft potentially affecting PPV distal of the graft48, it still presented with an acceptable AUC. Therefore, our results suggest that either central or peripheral PPV can be used for prediction of microcirculatory decline, which would facilitate translation to a clinical scenario.
Our results suggest that utilization of preload reserve after ischemia/reperfusion should be performed with particular caution avoiding fluid administration if stroke volume increase is uncertain as indicated by PPV-values below or inside the grey zone. In this regard, a prospective randomized clinical trial demonstrated an improved outcome using a fluid sparing regimen and limiting fluid boluses to presence of hypotension, low cardiac output and high stroke volume variation27. Biais and colleagues already tried to take into account the patients’ clinical condition according to PaO2/FiO2 ratios when trying to define optimal threshold values for PPV aiming to minimize the risk of volume-overload19. Volume-overload has clearly been shown to be associated with worsened outcome10,49,50. However, in patients at increased risk for complications during major abdominal surgery, a restrictive fluid regimen was not associated with improved survival and was associated with a higher rate of acute kidney injury51. Nevertheless, a fluid regimen tailored to microcirculatory demands may improve outcome, even if it is guided by macrohemodynamic parameters. An association between elevated central venous pressure and microcirculatory deterioration has already been described for septic patients52. Moreover, goal-directed therapy based on macrohemodynamic parameters has been shown to improve microcirculation in experimental pancreatitis, major surgery as well as abdominal surgery with important implications for outcome, and even in aortic surgery, prevention of microcirculatory decline could recently been shown by use of a goal directed hemodynamic management53–56. While hemodynamic coherence is frequently lost in various conditions6,31, association between pulse pressure variation and intestinal microcirculation may still exist. A potential association between pulse pressure variation and microcirculation may also be suggested by previous studies. While a loss of hemodynamic coherence was found for septic patients reflected by a dissociation between sublingual microcirculation and cardiac index as well as mean arterial pressure, this was only found in the late phase of sepsis. In the early phase of sepsis there was no dissociation between macro- and microcirculatory parameters. Most interesting, this was accompanied by a significant reduction of pulse pressure variation, while in the late phase, no significant change of pulse pressure variation was seen16. Moreover, an improvement of microcirculatory parameters following passive leg raise as well as fluid administration has already been shown for preload dependent septic patients15. In our project, there was a dissociation between the systemic variables cardiac output, stroke volume and mean arterial pressure, which has previously been discussed57. Nevertheless, pulse pressure variation predicted microcirculatory decline. The results of our study are also in line with the results of a recent study conducted in patients undergoing major surgery by Bouattour et al.. In this clinical study, the authors could demonstrate that fluid administration in conditions of preload dependency as identified by a pulse pressure variation > 13% led to an improved microcirculation as assessed with sublingual sidestream darkfield imaging47. These results underline the usefulness of PPV to guide treatment of microcirculation. Based on the ability of PPV to predict microcirculatory decline due to fluid administration as shown in this study, we propose that in ischemia/reperfusion conditions, a PPV-based fluid management may help to protect intestinal microcirculation.
Our study has certain limitations. First, we limited the number of animals in this hypothesis generating pilot study. Sensible a priori input data to be used for sample size and power calculations were not available, hence, we chose a sample size that appeared feasible with respect to size of the study protocol and in accordance to ARRIVE and FELASA guidelines as well as 3R-principles reducing animal numbers to a reasonable sample size while being able to address the scientific topic properly58. Moreover, the number of animals as well as volume-loading-steps is comparable with existing studies59–64. In addition, the number of volume-loading-steps is also comparable to a number of clinical studies65–72. As this was an pilot study for generating hypotheses rather than confirmatory test pre-specified hypotheses, alpha error adjustment for multiple testing was not done. In addition, we did not include a control group. However, we assumed that the risk for microcirculatory deterioration due to fluid therapy would be low in healthy conditions and that there would be to few positive states to reliably assess the ability of PPV for prediction of microcirculatory decline in a control group. Another limitation would be that duration of vessel ischemia was not intended as standardized ischemia times but was dependent on the surgical techniques used. Although ischemic times were different between animals, the variation of vessel ischemia would reflect the clinical situation of open thoraco-abdominal aortic repair, especially since surgical procedures were performed by experienced vascular surgeons and the advantage of the study would therefore be the comparability to the clinical scenario73–75. Moreover, besides predicting decreases of intestinal microcirculatory blood flow caused by volume-overload, the clinical impact of this microcirculatory decline was not further assessed in our study. However, this has already been shown by previous publications31,32. Choice of anesthesia can also influence microcirculation31. Therefore, we have used a standardized approach with identical anesthesia doses and identical ventilation settings. Moreover, in a recent study on the effects of total intravenous vs. balanced anesthesia, use of a goal-directed therapy prevented deterioration of microcirculatory perfusion as well as oxygenation for both groups56. Therefore, our results may also be translated to patients receiving balanced anesthesia. Different effects of vasoactive drugs on microcirculation have been described31,76. Although all vasopressors used during surgical phases have been terminated for at least 30 min prior measurements, we cannot rule out residual effects of vasopressors even if they are very much unlikely after 30 min of cessation. While the choice of fluids does not influence the predictive value of PPV26, colloids may be different from crystalloids in regard to microcirculation. While some authors found no benefits or even detrimental effects for colloids, others have found beneficial effects in regard to microcirculation27,31,77–89. Choice of fluids was based on the experience from previous studies showing pronounced effects of hydroxyethyl-starch colloids on macro- as well as microcirculation in our porcine models11,53,90–94. Moreover, detrimental effects on microcirculation have been described for crystalloids as well95–98. Nevertheless, future studies are needed to test the ablity of pulse pressure varation to predict microcirculatory decline during fluid administration with crystalloids as well. In regard to laser speckle contrast imaging it should be noted that capillaries have a diameter of about 7 µm99,100, while laser speckle contrast imaging technique has a maximum resolution of about 10 µm, so it is barely possible to directly visualize capillaries with laser speckle contrast imaging. However, it is possible to detect perfusion in a capillary bed even when capillaries cannot be identified individually100. Nevertheless, the limitation of the used method for microcirculatory evaluation should be regarded. Future studies should also investigate the usefulness of dynamic preload parameters for maintenance and optimization of microcirculation in different clinical settings and other organs.
In conclusion, the results of our study show that PPV does have the potential to predict decreases of intestinal microcirculatory blood flow caused by volume-load in an experimental setting of ischemia/reperfusion. Full utilization of preload as reflected in low values of PPV resulted in impaired microcirculatory blood flow. This should encourage further translational research and might help to prevent microcirculatory impairment due to excessive fluid resuscitation and to guide fluid therapy in the future.
Methods
Study design
The study was conducted as a prospective explorative pilot study in 8 anesthetized domestic pigs (German landrace) both female and male using animals weighing approximately 75–80 kg. This study was performed in combination with a feasibility study for a new hybrid-graft implantation in accordance to ARRIVE and FELASA guidelines and 3Rs-principles reducing animal number. The animals received care in compliance with the ‘Guide for the Care and Use of Laboratory Animals’ (NIH publication No. 86–23, revised 2011) as well as FELASA guidelines and recommendations and experiments were carried out according to the ARRIVE guidelines58,101. Please see the ARRIVE guidelines checklist as well as additional comments on the ARRIVE guidelines given in the additional file. Results on microcirculatory behavior have been previously reported and methods have been previously described57,102.
The study protocol is given in Fig. 1. This study was performed in coherence with current recommendations on the use of pulse pressure variation: All animals were mechanically ventilated with tidal volumes of 8 ml kg−1 total body weight, arrhythmic episodes were excluded and high respiratory rates avoided103,104. Ischemia/reperfusion was induced during aortic hybrid-graft implantation. Micro- and macrocirculation were measured at baseline prior ischemia/reperfusion and 6 h after ischemia/reperfusion induced by aortic hybrid-graft implantation. Thereafter 4 consecutive volume-loading-steps were performed followed by micro- and macrocirculatory measurements. Each volume-loading-step consisted of 6 ml kg−1 bodyweight−1 colloids (Voluven 6%, Fresenius Kabi, Bad Homburg, Germany). Each volume-loading-step was performed during a time period of 5 min using pressurized infusions. After completion of each volume-loading-step, 5 min were allowed for equilibration. At least 30 min prior measurements, all vasopressors used during the surgical phase were terminated to exclude effects of these substances.
Assessment of microcirculation
Microcirculatory blood flow was directly assessed using laser speckle contrast imaging as previously described57. Laser speckle contrast imaging has been frequently used for monitoring of the microcirculation including intestinal microcirculation as well models of ischemia/reperfusion105–115. For use of laser speckle contrast imaging, tissues are illuminated with coherent laser light. The backscattered light from the tissue then forms a random interference pattern at the detector. This interference pattern is called speckle pattern106. Laser speckle contrast imaging is based on the laser Doppler method, however, it has a much higher spatial and temporal resolution with use of full field laser measurements. It allows imaging of large surface area, is contact-free and can measure perfusion in real time111. It can detect mean microcirculatory blood flow (mFlux) up to a tissue depth of 3 mm. It is non-invasive and has good reproducibility115. The mean Flux (mFlux) is a dimensionless unit of microcirculatory blood flow, with the unit p.u.112. In detail, the speckle-laser (MoorFLPI-2, Moor Instruments, Axminster, UK) was positioned 25 cm above the intestinal segment using a target laser. For each measurement step microcirculatory blood flow was assessed for a 30 s period reducing variability due to respiration and organ movement57,111,116. A blinded investigator defined the region of interest off-line and calculated the mean Flux for the region of interest using a dedicated software (Moor FLPI-2 Review Software, v. 4.0, Moor Instruments, Axminster, UK)57.
Assessment of macrocirculation
Cardiac output and stroke volume were assessed invasively using a perivascular flow probe (Confidence PAU Flowprobe, chronic liner, 16 or 18 mm, Transonic Systems Inc., Ithaca, NY, USA) that was fit around the descending aorta and connected to the adapted hardware (Perivascular Flow Module, Transonic Systems Inc., Ithaca, NY, USA). Invasive pressure catheters (Millar Micro-Tip pressure catheters, Houston, Texas, USA) for assessment of arterial pressure were directly inserted into the ascending aorta via the carotid artery for arterial pressure measurement and assessment of central PPV as well as in the femoral artery for assessment of peripheral PPV using 8 Fr. introducer sheaths. Macrohemodynamic measurements were recorded simultaneously with microcirculatory assessment for a period of 2 min.
Data acquisition and processing
Invasive data were recorded by adapted hardware from ADInstruments (ADInstruments Bridge Amp and PowerLab, ADInstruments Ltd., Oxford, UK) and Transonic (Perivascular Flow Module, Transonic Systems Inc., Ithaca, NY, USA). Data analysis was performed off-line using LabChart software (LabChart Pro, version 8, ADInstruments Ltd., Oxford, UK). Central and peripheral PPV were calculated using data from 10 respiratory cycles. The following formula was applied:
Animal care, anesthesia and surgical procedures
Animal care, anesthesia and surgical procedures have been previously described57,102. Animals were brought to animal care facilities at least 7 days prior experiments and were housed in accordance to animal welfare recommendations. Animals were given food and water ad libitum and health status was regularly assessed by the responsible veterinarian. A fasting time of 12 h was maintained prior to the experiments102. Anesthesia was performed intravenously using a combination of an opiod together with different hypnotic agents to secure deep anesthesia during the entire protocol as previously described57. For anesthesia induction all animals received intramuscular injections of ketamine 10 mg kg−1 bodyweight−1, azaperone 4 mg kg−1 bodyweight−1, atropine 0.01 mg kg−1 bodyweight−1 and midazolam 10 mg for premedication. Thereafter, all animals were surgically tracheotomized and the trachea was intubated via the tracheotomy ostium. Maintenance of anesthesia was performed by continuous infusion of propofol (4 mg kg−1 bodyweight−1 h−1), fentanyl (10 µg kg−1 bodyweight−1 h−1), midazolam (0.3 mg kg−1 bodyweight−1 h−1) and ketamine (6 mg kg−1 bodyweight−1 h−1). This combination provides adequate anesthesia as well as hemodynamic stability and has been successfully used in previous studies57,117,118. Adequacy of anesthesia was assessed by careful observation of vital signs and ventilation parameters as well as by absence of any movements during the entire protocol with special attendance to phases of surgical stimulus. Additional bolus doses of fentanyl (50 µg) were given if there was any indication of pain or distress102. Pancuronium (0.1 mg kg−1 bodyweight−1) was only given for tracheotomy. A volume-controlled ventilation was established using tidal volumes of 8 ml kg−1 bodyweight−1 and a positive end-expiratory pressure of 8 cmH2O and ventilator frequency was adjusted to maintain an end-expiratory carbon dioxide tension (etCO2) of 35–40 mmHg (Julian, Dräger Medical, Lübeck, Germany). All animals were placed in supine position on a warming blanket to prevent heat loss57,102. Surgical procedures have been previously described57,118 and are given in detail in the additional file. Duration of vessel ischemia was not intended as standardized ischemia times but was dependent on the surgical techniques used. Times of vessel ischemia are given as additional table 1 in the additional file. The entire experimental procedure and handling of the animals was supervised by the responsible veterinarian.
Euthanasia
After completion of the study protocol all animals were sacrificed during deep anesthesia by fast injection of 40 mmol potassium chloride as previously described57,102.
Sample size calculation
As previously described, a priori input data to be used for sample size and power calculations were not available, hence, a sample size was chosen that appeared feasible with respect to size of the study protocol and in accordance to ARRIVE and FELASA guidelines and 3R-principles reducing animal numbers to a reasonable sample size while being able to address the scientific topic properly57.
Primary endpoint
Primary endpoint of this study was the ability of PPV to predict a mean microcirculatory flux decrease ≥ 10% following fluid administration.
Statistical analysis
The statistical plan was approved by all authors before start of the study. The dependent variables cardiac output, stroke volume, heart rate, mean arterial pressure, central PPV and mean Flux were subjected to general linear mixed model analyses, using the SPSS v. 24 routine GENLINMIXED for continuous data with an identity link function as previously described57. Models were specified with fixed effects for variable measurement point and random intercepts for animals. In addition, baseline values prior ischemia/reperfusion were included as fixed effects to the model as well to adjust for differences in baseline values between animals. Measurement points were considered as repeated measures within animals. Marginal means with 95% CI were computed for all dependent variables at all measurement points, followed by multiple pairwise comparisons of measurement point means via least significant difference tests. The assessment of the ability of PPV to predict microcirculatory decline was performed by calculating receiver operating characteristic curves. The response to fluid administration was considered significant, if mean microcirculatory flux decreased by at least 10%. Direction of the test was set to decreasing values of PPV, assuming that the likelihood of the state event increases with decreases in PPV. The value of the predictor variable resulting in a maximum Youden index119 was assumed to represent its ideal cutoff value. Statistical analyses were performed using the SPSS statistical software package 24 (IBM SPSS Statistics Inc., USA). Additional details on statistical analysis are given in the in the additional file as additional tables 2a–h. Variables are expressed as mean (95% confidence interval, CI). Two-tailed p values less than 0.05 were considered significant. In addition, results of general linear mixed model analyses that handled baseline values as part of the outcome and not as fixed effect covariate are given as additional table 3, along with p values of pairwise contrasts.
Ethics approval and consent to participate
The study was approved by the Governmental Commission on the Care and Use of Animals of the City of Hamburg (Reference-No. 101/15). The animals received care in compliance with the ‘Guide for the Care and Use of Laboratory Animals’ (NIH publication No. 86–23, revised 2011) as well as FELASA guidelines and recommendations and experiments were carried out according to the ARRIVE guidelines. Please see the ARRIVE guidelines checklist as well as additional comments on the ARRIVE guidelines given in the additional file.
Supplementary Information
Acknowledgements
The authors would like to thank Dr. med. vet. Aline Reitmeier and Mrs. Jutta Dammann, Facility of Research Animal Care, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, for providing pre- and perioperative animal care and their technical assistance on animal handling. The authors further would like to thank Dr. Daniel Manzoni, Department of Vascular Surgery, Hôpital Kirchberg, Luxembourg for his technical assistance.
Abbreviation
- PPV
Pulse pressure variation
Author contributions
C.R.B.: study concept and design, acquisition and interpretation of data, draft of the manuscript, statistical analysis, final approval. M.F.G.: study concept and design, acquisition and interpretation of data, draft of the manuscript, critical revision of the manuscript for important intellectual content, final approval. T.F.: acquisition and interpretation of data, critical revision of the manuscript for important intellectual content, final approval. R.K.: acquisition and interpretation of data, critical revision of the manuscript for important intellectual content, final approval. H.O.P.: interpretation of data, statistical analysis, critical revision of the manuscript for important intellectual content, final approval. A.D.: acquisition and interpretation of data, critical revision of the manuscript for important intellectual content, final approval. E.S.D.: acquisition and interpretation of data, critical revision of the manuscript for important intellectual content, final approval. D.A.R.: study concept and design, interpretation of data, critical revision of the manuscript for important intellectual content, final approval. S.H.W.: study concept and design, acquisition and interpretation of data, critical revision of the manuscript for important intellectual content, final approval. C.J.C.T.: study concept and design, acquisition and interpretation of data, critical revision of the manuscript for important intellectual content, study supervision, final approval.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was supported by departmental funds of the Department of Anesthesiology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request. Additional details on statistical analysis are given in the additional file.
Competing interests
Constantin J. C. Trepte has received honorary for lectures by Maquet. All other authors declare no competing interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-88458-3.
References
- 1.Pinsky MR. Functional hemodynamic monitoring. Crit. Care Clin. 2015;31:89–111. doi: 10.1016/j.ccc.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monnet X, Teboul JL. Assessment of fluid responsiveness: recent advances. Curr. Opin. Crit. Care. 2018;24:190–195. doi: 10.1097/MCC.0000000000000501. [DOI] [PubMed] [Google Scholar]
- 3.Pinsky MR. Functional haemodynamic monitoring. Curr. Opin. Crit. Care. 2014;20:288–293. doi: 10.1097/MCC.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cecconi M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40:1795–1815. doi: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 6.Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit. care. 2015;19(Suppl 3):S8. doi: 10.1186/cc14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Backer D, Ortiz JA, Salgado D. Coupling microcirculation to systemic hemodynamics. Curr. Opin. Crit. Care. 2010;16:250–254. doi: 10.1097/MCC.0b013e3283383621. [DOI] [PubMed] [Google Scholar]
- 8.Siegemund M, et al. Aortic cross-clamping and reperfusion in pigs reduces microvascular oxygenation by altered systemic and regional blood flow distribution. Anesth. Analg. 2010;111:345–353. doi: 10.1213/ANE.0b013e3181e4255f. [DOI] [PubMed] [Google Scholar]
- 9.Bellamy MC. Wet, dry or something else? Br. J. Anaesth. 2006;97:755–757. doi: 10.1093/bja/ael290. [DOI] [PubMed] [Google Scholar]
- 10.Thacker JK, Mountford WK, Ernst FR, Krukas MR, Mythen MM. Perioperative fluid utilization variability and association with outcomes: considerations for enhanced recovery efforts in sample US surgical populations. Ann. Surg. 2016;263:502–510. doi: 10.1097/SLA.0000000000001402. [DOI] [PubMed] [Google Scholar]
- 11.Wodack KH, et al. Individualized early goal-directed therapy in systemic inflammation: is full utilization of preload reserve the optimal strategy? Crit. Care Med. 2014;42:e741–751. doi: 10.1097/CCM.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 12.Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit. Care Med. 2004;32:1825–1831. doi: 10.1097/01.CCM.0000138558.16257.3F. [DOI] [PubMed] [Google Scholar]
- 13.Trzeciak S, et al. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann. Emerg. Med. 2007;49:88–98. doi: 10.1016/j.annemergmed.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 14.De Backer D, et al. Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit. Care Med. 2013;41:791–799. doi: 10.1097/CCM.0b013e3182742e8b. [DOI] [PubMed] [Google Scholar]
- 15.Pottecher J, et al. Both passive leg raising and intravascular volume expansion improve sublingual microcirculatory perfusion in severe sepsis and septic shock patients. Intensive Care Med. 2010;36:1867–1874. doi: 10.1007/s00134-010-1966-6. [DOI] [PubMed] [Google Scholar]
- 16.Ospina-Tascon G, et al. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med. 2010;36:949–955. doi: 10.1007/s00134-010-1843-3. [DOI] [PubMed] [Google Scholar]
- 17.Edul VS, et al. Dissociation between sublingual and gut microcirculation in the response to a fluid challenge in postoperative patients with abdominal sepsis. Ann. Intensive Care. 2014;4:39. doi: 10.1186/s13613-014-0039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannesson M, et al. Assessing the diagnostic accuracy of pulse pressure variations for the prediction of fluid responsiveness: a "gray zone" approach. Anesthesiology. 2011;115:231–241. doi: 10.1097/ALN.0b013e318225b80a. [DOI] [PubMed] [Google Scholar]
- 19.Biais M, et al. Clinical relevance of pulse pressure variations for predicting fluid responsiveness in mechanically ventilated intensive care unit patients: the grey zone approach. Crit. Care. 2014;18:587. doi: 10.1186/s13054-014-0587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Granger DN. Ischemia-reperfusion: mechanisms of microvascular dysfunction and the influence of risk factors for cardiovascular disease. Microcirculation. 1999;6:167–178. doi: 10.1111/j.1549-8719.1999.tb00099.x. [DOI] [PubMed] [Google Scholar]
- 22.De Backer D, Orbegozo Cortes D, Donadello K, Vincent JL. Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence. 2014;5:73–79. doi: 10.4161/viru.26482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinsky MR. Heart lung interactions during mechanical ventilation. Curr. Opin. Crit. Care. 2012;18:256–260. doi: 10.1097/MCC.0b013e3283532b73. [DOI] [PubMed] [Google Scholar]
- 24.Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann. Intensive Care. 2011;1:1. doi: 10.1186/2110-5820-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biais M, et al. Changes in stroke volume induced by lung recruitment maneuver predict fluid responsiveness in mechanically ventilated patients in the operating room. Anesthesiology. 2016 doi: 10.1097/ALN.0000000000001459. [DOI] [PubMed] [Google Scholar]
- 26.Yang X, Du B. Does pulse pressure variation predict fluid responsiveness in critically ill patients? A systematic review and meta-analysis. Crit. Care. 2014;18:650. doi: 10.1186/s13054-014-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo J, et al. Goal-directed fluid restriction during brain surgery: a prospective randomized controlled trial. Ann. Intensive Care. 2017;7:16. doi: 10.1186/s13613-017-0239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silversides JA, Perner A, Malbrain M. Liberal versus restrictive fluid therapy in critically ill patients. Intensive Care Med. 2019;45:1440–1442. doi: 10.1007/s00134-019-05713-y. [DOI] [PubMed] [Google Scholar]
- 29.Jhanji S, Lee C, Watson D, Hinds C, Pearse RM. Microvascular flow and tissue oxygenation after major abdominal surgery: association with post-operative complications. Intensive Care Med. 2009;35:671–677. doi: 10.1007/s00134-008-1325-z. [DOI] [PubMed] [Google Scholar]
- 30.De Backer D, et al. Microcirculatory alterations in cardiac surgery: effects of cardiopulmonary bypass and anesthesia. Ann. Thorac. Surg. 2009;88:1396–1403. doi: 10.1016/j.athoracsur.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Vellinga NA, Ince C, Boerma EC. Microvascular dysfunction in the surgical patient. Curr. Opin. Crit. Care. 2010;16:377–383. doi: 10.1097/mcc.0b013e32833a0633. [DOI] [PubMed] [Google Scholar]
- 32.De Backer D. Microcirculatory blood flow as a new tool for perioperative fluid management. In: Farag E, Kurz A, Troianos C, editors. Perioperative Fluid Management. Springer; 2020. pp. 169–180. [Google Scholar]
- 33.Koch M, et al. Effects of propofol on human microcirculation. Br. J. Anaesth. 2008;101:473–478. doi: 10.1093/bja/aen210. [DOI] [PubMed] [Google Scholar]
- 34.Turek Z, Sykora R, Matejovic M, Cerny V. Anesthesia and the microcirculation. Semin. Cardiothorac. Vasc. Anesth. 2009;13:249–258. doi: 10.1177/1089253209353134. [DOI] [PubMed] [Google Scholar]
- 35.Granger DN, et al. Splanchnic ischaemia-reperfusion injury: mechanistic insights provided by mutant mice. Acta Physiol. Scand. 2001;173:83–91. doi: 10.1046/j.1365-201X.2001.00888.x. [DOI] [PubMed] [Google Scholar]
- 36.De Backer D, Creteur J, Dubois MJ, Sakr Y, Vincent JL. Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am. Heart J. 2004;147:91–99. doi: 10.1016/j.ahj.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Puhl G, et al. Initial hepatic microcirculation correlates with early graft function in human orthotopic liver transplantation. Liver Transpl. 2005;11:555–563. doi: 10.1002/lt.20394. [DOI] [PubMed] [Google Scholar]
- 38.Schaser KD, et al. In vivo imaging of human pancreatic microcirculation and pancreatic tissue injury in clinical pancreas transplantation. Am. J. Transplant. 2005;5:341–350. doi: 10.1111/j.1600-6143.2004.00663.x. [DOI] [PubMed] [Google Scholar]
- 39.Schmitz V, Schaser KD, Olschewski P, Neuhaus P, Puhl G. In vivo visualization of early microcirculatory changes following ischemia/reperfusion injury in human kidney transplantation. Eur. Surg. Res. 2008;40:19–25. doi: 10.1159/000107683. [DOI] [PubMed] [Google Scholar]
- 40.Rehm M, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116:1896–1906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- 41.Donati A, et al. A comparison among portal lactate, intramucosal sigmoid Ph, and deltaCO2 (PaCO2—regional Pco2) as indices of complications in patients undergoing abdominal aortic aneurysm surgery. Anesth. Analg. 2004;99:1024–1031. doi: 10.1213/01.ANE.0000132543.65095.2C. [DOI] [PubMed] [Google Scholar]
- 42.Nakatsuka M. Assessment of gut mucosal perfusion and colonic tissue blood flow during abdominal aortic surgery with gastric tonometry and laser Doppler flowmetry. Vasc. Endovasc. Surg. 2002;36:193–198. doi: 10.1177/153857440203600306. [DOI] [PubMed] [Google Scholar]
- 43.Mahmood A, Gosling P, Barclay R, Kilvington F, Vohra R. Splanchnic microcirculation protection by hydroxyethyl starches during abdominal aortic aneurysm surgery. Eur. J. Vasc. Endovasc. Surg. 2009;37:319–325. doi: 10.1016/j.ejvs.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J. Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 45.Haglund U, Bergqvist D. Intestinal ischemia: the basics. Langenbecks Arch. Surg. 1999;384:233–238. doi: 10.1007/s004230050197. [DOI] [PubMed] [Google Scholar]
- 46.Vollmar B, Menger MD. Intestinal ischemia/reperfusion: microcirculatory pathology and functional consequences. Langenbecks Arch. Surg. 2011;396:13–29. doi: 10.1007/s00423-010-0727-x. [DOI] [PubMed] [Google Scholar]
- 47.Bouattour K, Teboul JL, Varin L, Vicaut E, Duranteau J. Preload dependence is associated with reduced sublingual microcirculation during major abdominal surgery. Anesthesiology. 2019;130:541–549. doi: 10.1097/ALN.0000000000002631. [DOI] [PubMed] [Google Scholar]
- 48.Whittlesey RH. Dynamic changes in arterial pressure pulses produced by a simulated saccular aneurysm. Am. J. Physiol. 1952;168:192–199. doi: 10.1152/ajplegacy.1951.168.1.192. [DOI] [PubMed] [Google Scholar]
- 49.Chapalain X, et al. Association between fluid overload and SOFA score kinetics in septic shock patients: a retrospective multicenter study. J. Intensive Care. 2019;7:42. doi: 10.1186/s40560-019-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malbrain M, et al. Intravenous fluid therapy in the perioperative and critical care setting: executive summary of the International Fluid Academy (IFA) Ann. Intensive Care. 2020;10:64. doi: 10.1186/s13613-020-00679-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Myles PS, et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N. Engl. J. Med. 2018;378:2263–2274. doi: 10.1056/NEJMoa1801601. [DOI] [PubMed] [Google Scholar]
- 52.Vellinga NA, Ince C, Boerma EC. Elevated central venous pressure is associated with impairment of microcirculatory blood flow in sepsis: a hypothesis generating post hoc analysis. BMC Anesthesiol. 2013;13:17. doi: 10.1186/1471-2253-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trepte CJ, et al. The impact of early goal-directed fluid management on survival in an experimental model of severe acute pancreatitis. Intensive Care Med. 2013;39:717–726. doi: 10.1007/s00134-012-2775-x. [DOI] [PubMed] [Google Scholar]
- 54.Jhanji S, et al. Haemodynamic optimisation improves tissue microvascular flow and oxygenation after major surgery: a randomised controlled trial. Crit. Care. 2010;14:R151. doi: 10.1186/cc9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hiltebrand LB, et al. Crystalloids versus colloids for goal-directed fluid therapy in major surgery. Crit. Care. 2009;13:R40. doi: 10.1186/cc7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loggi S, et al. Changes in the sublingual microcirculation following aortic surgery under balanced or total intravenous anaesthesia: a prospective observational study. BMC Anesthesiol. 2019;19:1. doi: 10.1186/s12871-018-0673-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Behem CR, et al. Volume based resuscitation and intestinal microcirculation after ischaemia/reperfusion injury: results of an exploratory aortic clamping study in pigs. Eur. J. Vasc. Endovasc. Surg. 2019;57:284–291. doi: 10.1016/j.ejvs.2018.08.055. [DOI] [PubMed] [Google Scholar]
- 58.Guillen J. FELASA guidelines and recommendations. J. Am. Assoc. Lab. Anim. Sci. 2012;51:311–321. [PMC free article] [PubMed] [Google Scholar]
- 59.Endo Y, et al. Stroke volume variation (SVV) and pulse pressure variation (PPV) as indicators of fluid responsiveness in sevoflurane anesthetized mechanically ventilated euvolemic dogs. J. Vet. Med. Sci. 2017;79:1437–1445. doi: 10.1292/jvms.16-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacques D, et al. Pulse pressure variation and stroke volume variation during increased intra-abdominal pressure: an experimental study. Crit. Care. 2011;15:R33. doi: 10.1186/cc9980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.da Silva Ramos FJ, et al. Estimation of stroke volume and stroke volume changes by electrical impedance tomography. Anesth. Analg. 2018;126:102–110. doi: 10.1213/ANE.0000000000002271. [DOI] [PubMed] [Google Scholar]
- 62.Renner J, et al. Influence of increased intra-abdominal pressure on fluid responsiveness predicted by pulse pressure variation and stroke volume variation in a porcine model. Crit. Care Med. 2009;37:650–658. doi: 10.1097/CCM.0b013e3181959864. [DOI] [PubMed] [Google Scholar]
- 63.Jung YS, et al. Pulse oximeter plethysmograph variation during hemorrhage in beta-blocker-treated swine. J. Surg. Res. 2020;256:468–475. doi: 10.1016/j.jss.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 64.Broch O, et al. Dynamic and volumetric variables reliably predict fluid responsiveness in a porcine model with pleural effusion. PLoS ONE. 2013;8:e56267. doi: 10.1371/journal.pone.0056267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cannesson M, et al. Respiratory variations in pulse oximetry plethysmographic waveform amplitude to predict fluid responsiveness in the operating room. Anesthesiology. 2007;106:1105–1111. doi: 10.1097/01.anes.0000267593.72744.20. [DOI] [PubMed] [Google Scholar]
- 66.Cannesson M, et al. The ability of stroke volume variations obtained with Vigileo/FloTrac system to monitor fluid responsiveness in mechanically ventilated patients. Anesth. Analg. 2009;108:513–517. doi: 10.1213/ane.0b013e318192a36b. [DOI] [PubMed] [Google Scholar]
- 67.Haas S, et al. Prediction of volume responsiveness using pleth variability index in patients undergoing cardiac surgery after cardiopulmonary bypass. J. Anesth. 2012;26:696–701. doi: 10.1007/s00540-012-1410-x. [DOI] [PubMed] [Google Scholar]
- 68.Huang CC, et al. Prediction of fluid responsiveness in acute respiratory distress syndrome patients ventilated with low tidal volume and high positive end-expiratory pressure. Crit. Care Med. 2008;36:2810–2816. doi: 10.1097/CCM.0b013e318186b74e. [DOI] [PubMed] [Google Scholar]
- 69.Fellahi JL, et al. A comparison of endotracheal bioimpedance cardiography and transpulmonary thermodilution in cardiac surgery patients. J. Cardiothorac. Vasc. Anesth. 2012;26:217–222. doi: 10.1053/j.jvca.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 70.Fellahi JL, et al. Can endotracheal bioimpedance cardiography assess hemodynamic response to passive leg raising following cardiac surgery? Ann. Intensive Care. 2012;2:26. doi: 10.1186/2110-5820-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Biais M, Bernard O, Ha JC, Degryse C, Sztark F. Abilities of pulse pressure variations and stroke volume variations to predict fluid responsiveness in prone position during scoliosis surgery. Br. J. Anaesth. 2010;104:407–413. doi: 10.1093/bja/aeq031. [DOI] [PubMed] [Google Scholar]
- 72.Guinot PG, et al. Passive leg raising can predict fluid responsiveness in patients placed on venovenous extracorporeal membrane oxygenation. Crit. Care. 2011;15:R216. doi: 10.1186/cc10451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tshomba Y, et al. Clinical outcomes of hybrid repair for thoracoabdominal aortic aneurysms. Ann. Cardiothorac. Surg. 2012;1:293–303. doi: 10.3978/j.issn.2225-319X.2012.07.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chiesa R, et al. Use of a novel hybrid vascular graft for sutureless revascularization of the renal arteries during open thoracoabdominal aortic aneurysm repair. J. Vasc. Surg. 2014;60:622–630. doi: 10.1016/j.jvs.2014.03.256. [DOI] [PubMed] [Google Scholar]
- 75.Patel VI, Lancaster RT, Conrad MF, Cambria RP. Open surgical repair of thoracoabdominal aneurysms: the Massachusetts General Hospital experience. Ann. Cardiothorac. Surg. 2012;1:320–324. doi: 10.3978/j.issn.2225-319X.2012.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boerma EC, Ince C. The role of vasoactive agents in the resuscitation of microvascular perfusion and tissue oxygenation in critically ill patients. Intensive Care Med. 2010;36:2004–2018. doi: 10.1007/s00134-010-1970-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stens J, et al. Microcirculatory perfusion during different perioperative hemodynamic strategies. Microcirculation. 2015;22:267–275. doi: 10.1111/micc.12194. [DOI] [PubMed] [Google Scholar]
- 78.Bansch P, Statkevicius S, Bentzer P. Plasma volume expansion with 5% albumin compared to Ringer's acetate during normal and increased microvascular permeability in the rat. Anesthesiology. 2014;121:817–824. doi: 10.1097/ALN.0000000000000363. [DOI] [PubMed] [Google Scholar]
- 79.Kimberger O, et al. Goal-directed colloid administration improves the microcirculation of healthy and perianastomotic colon. Anesthesiology. 2009;110:496–504. doi: 10.1097/ALN.0b013e31819841f6. [DOI] [PubMed] [Google Scholar]
- 80.de Carvalho H, Dorigo D, Bouskela E. Effects of Ringer-acetate and Ringer-dextran solutions on the microcirculation after LPS challenge: observations in the hamster cheek pouch. Shock. 2001;15:157–162. doi: 10.1097/00024382-200115020-00013. [DOI] [PubMed] [Google Scholar]
- 81.Hoffmann JN, et al. Hydroxyethyl starch (130 kD), but not crystalloid volume support, improves microcirculation during normotensive endotoxemia. Anesthesiology. 2002;97:460–470. doi: 10.1097/00000542-200208000-00025. [DOI] [PubMed] [Google Scholar]
- 82.Steinbauer M, Harris AG, Messmer K. Effects of dextran on microvascular ischemia-reperfusion injury in striated muscle. Am. J. Physiol. 1997;272:H1710–1716. doi: 10.1152/ajpheart.1997.272.4.H1710. [DOI] [PubMed] [Google Scholar]
- 83.Schaper J, et al. Volume therapy with colloid solutions preserves intestinal microvascular perfusion in endotoxaemia. Resuscitation. 2008;76:120–128. doi: 10.1016/j.resuscitation.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 84.Obonyo NG, et al. Effects of volume resuscitation on the microcirculation in animal models of lipopolysaccharide sepsis: a systematic review. Intensive Care Med. Exp. 2016;4:38. doi: 10.1186/s40635-016-0112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kauvar DS, Baer DG, Dubick MA, Walters TJ. Effect of fluid resuscitation on acute skeletal muscle ischemia-reperfusion injury after hemorrhagic shock in rats. J. Am. Coll. Surg. 2006;202:888–896. doi: 10.1016/j.jamcollsurg.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 86.Schick MA, et al. Effects of crystalloids and colloids on liver and intestine microcirculation and function in cecal ligation and puncture induced septic rodents. BMC Gastroenterol. 2012;12:179. doi: 10.1186/1471-230X-12-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ergin B, et al. Fully balanced fluids do not improve microvascular oxygenation, acidosis and renal function in a rat model of endotoxemia. Shock. 2016;46:83–91. doi: 10.1097/SHK.0000000000000573. [DOI] [PubMed] [Google Scholar]
- 88.Guven G, Hilty MP, Ince C. Microcirculation: physiology, pathophysiology, and clinical application. Blood Purif. 2020;49:143–150. doi: 10.1159/000503775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Komori M, et al. Effects of crystalloids and colloids on microcirculation, central venous oxygen saturation, and central venous-to-arterial carbon dioxide gap in a rabbit model of hemorrhagic shock. J. Anesth. 2019;33:108–117. doi: 10.1007/s00540-018-2594-5. [DOI] [PubMed] [Google Scholar]
- 90.Trepte CJ, et al. Prediction of volume-responsiveness during one-lung ventilation: a comparison of static, volumetric, and dynamic parameters of cardiac preload. J. Cardiothorac. Vasc. Anesth. 2013;27:1094–1100. doi: 10.1053/j.jvca.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 91.Wodack KH, et al. Assessment of central hemodynamic effects of phenylephrine: an animal experiment. J. Clin. Monit. Comput. 2019;33:377–384. doi: 10.1007/s10877-018-0204-6. [DOI] [PubMed] [Google Scholar]
- 92.Haas SA, et al. An assessment of global end-diastolic volume and extravascular lung water index during one-lung ventilation: is transpulmonary thermodilution usable? Anesth. Analg. 2013;117:83–90. doi: 10.1213/ANE.0b013e31828f2c39. [DOI] [PubMed] [Google Scholar]
- 93.Bachmann KA, et al. Effects of thoracic epidural anesthesia on survival and microcirculation in severe acute pancreatitis: a randomized experimental trial. Crit. Care. 2013;17:R281. doi: 10.1186/cc13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kluttig R, et al. Invasive hemodynamic monitoring of aortic and pulmonary artery hemodynamics in a large animal model of ARDS. J. Vis. Exp. 2018 doi: 10.3791/57405. [DOI] [PubMed] [Google Scholar]
- 95.Byrne L, et al. Unintended consequences: fluid resuscitation worsens shock in an ovine model of endotoxemia. Am. J. Respir. Crit. Care Med. 2018;198:1043–1054. doi: 10.1164/rccm.201801-0064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Orbegozo D, et al. Effects of different crystalloid solutions on hemodynamics, peripheral perfusion, and the microcirculation in experimental abdominal sepsis. Anesthesiology. 2016;125:744–754. doi: 10.1097/ALN.0000000000001273. [DOI] [PubMed] [Google Scholar]
- 97.Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte(R) 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann. Surg. 2012;256:18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- 98.Funk W, Baldinger V. Microcirculatory perfusion during volume therapy. A comparative study using crystalloid or colloid in awake animals. Anesthesiology. 1995;82:975–982. doi: 10.1097/00000542-199504000-00022. [DOI] [PubMed] [Google Scholar]
- 99.Zweifach BW, Lipowsky HH. Quantitative studies of microcirculatory structure and function. III. Microvascular hemodynamics of cat mesentery and rabbit omentum. Circ. Res. 1977;41:380–390. doi: 10.1161/01.res.41.3.380. [DOI] [PubMed] [Google Scholar]
- 100.De Backer D, Donadello K, Cortes DO. Monitoring the microcirculation. J. Clin. Monit. Comput. 2012;26:361–366. doi: 10.1007/s10877-012-9383-8. [DOI] [PubMed] [Google Scholar]
- 101.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthr. Cartil. 2012;20:256–260. doi: 10.1016/j.joca.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 102.Behem CR, et al. Real-time assessment of spinal cord microperfusion in a porcine model of ischemia/reperfusion. J. Vis. Exp. 2020 doi: 10.3791/62047. [DOI] [PubMed] [Google Scholar]
- 103.Mahjoub Y, et al. Evaluation of pulse pressure variation validity criteria in critically ill patients: a prospective observational multicentre point-prevalence study. Br. J. Anaesth. 2014;112:681–685. doi: 10.1093/bja/aet442. [DOI] [PubMed] [Google Scholar]
- 104.Teboul JL, Monnet X, Chemla D, Michard F. Arterial pulse pressure variation with mechanical ventilation. Am. J. Respir. Crit. Care Med. 2019;199:22–31. doi: 10.1164/rccm.201801-0088CI. [DOI] [PubMed] [Google Scholar]
- 105.Briers D, et al. Laser speckle contrast imaging: theoretical and practical limitations. J. Biomed. Opt. 2013;18:066018. doi: 10.1117/1.JBO.18.6.066018. [DOI] [PubMed] [Google Scholar]
- 106.Heeman W, Steenbergen W, van Dam G, Boerma EC. Clinical applications of laser speckle contrast imaging: a review. J. Biomed. Opt. 2019;24:1–11. doi: 10.1117/1.JBO.24.8.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McGuire PG, Howdieshell TR. The importance of engraftment in flap revascularization: confirmation by laser speckle perfusion imaging. J. Surg. Res. 2010;164:e201–212. doi: 10.1016/j.jss.2010.07.059. [DOI] [PubMed] [Google Scholar]
- 108.Wu CY, et al. Laser speckle contrast imaging for assessing microcirculatory changes in multiple splanchnic organs and the gracilis muscle during hemorrhagic shock and fluid resuscitation. Microvasc. Res. 2015;101:55–61. doi: 10.1016/j.mvr.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 109.Bezemer R, et al. Validation of near-infrared laser speckle imaging for assessing microvascular (re)perfusion. Microvasc. Res. 2010;79:139–143. doi: 10.1016/j.mvr.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 110.Ding C, et al. Laser speckle contrast imaging for assessment of abdominal visceral microcirculation in acute peritonitis: does sequential impairments exist? Microvasc. Res. 2014;95:26–30. doi: 10.1016/j.mvr.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 111.Li CH, et al. The monitoring of microvascular liver blood flow changes during ischemia and reperfusion using laser speckle contrast imaging. Microvasc. Res. 2014;94:28–35. doi: 10.1016/j.mvr.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 112.Sturesson C, Milstein DM, Post IC, Maas AM, van Gulik TM. Laser speckle contrast imaging for assessment of liver microcirculation. Microvasc. Res. 2013;87:34–40. doi: 10.1016/j.mvr.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 113.Senarathna J, Rege A, Li N, Thakor NV. Laser speckle contrast imaging: theory, instrumentation and applications. IEEE Rev. Biomed. Eng. 2013;6:99–110. doi: 10.1109/RBME.2013.2243140. [DOI] [PubMed] [Google Scholar]
- 114.Klijn E, et al. Laser speckle imaging identification of increases in cortical microcirculatory blood flow induced by motor activity during awake craniotomy. J. Neurosurg. 2013;118:280–286. doi: 10.3171/2012.10.JNS1219. [DOI] [PubMed] [Google Scholar]
- 115.Eriksson S, Nilsson J, Lindell G, Sturesson C. Laser speckle contrast imaging for intraoperative assessment of liver microcirculation: a clinical pilot study. Med. Devices (Auckl.) 2014;7:257–261. doi: 10.2147/MDER.S63393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rousseau P, et al. Increasing the "region of interest" and "time of interest", both reduce the variability of blood flow measurements using laser speckle contrast imaging. Microvasc. Res. 2011;82:88–91. doi: 10.1016/j.mvr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 117.Debus ES, et al. Feasibility study of a novel thoraco-abdominal aortic hybrid device (SPIDER-graft) in a translational pig model. Eur. J. Vasc. Endovasc. Surg. 2018;55:196–205. doi: 10.1016/j.ejvs.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 118.Wipper S, et al. Impact of hybrid thoracoabdominal aortic repair on visceral and spinal cord perfusion: the new and improved SPIDER-graft. J. Thorac. Cardiovasc. Surg. 2019;158:692–701. doi: 10.1016/j.jtcvs.2018.11.133. [DOI] [PubMed] [Google Scholar]
- 119.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request. Additional details on statistical analysis are given in the additional file.