SARS-CoV-2 is the trigger of MIS-C, which suggests that other viruses may trigger different forms of Kawasaki disease. The discovery of inborn errors of immunity underlying MIS-C would facilitate that of inborn errors of immunity to viruses underlying Kawasaki disease.
Abstract
Multisystem inflammatory syndrome in children (MIS-C) emerged in April 2020 in communities with high COVID-19 rates. This new condition is heterogenous but resembles Kawasaki disease (KD), a well-known but poorly understood and clinically heterogenous pediatric inflammatory condition for which weak associations have been found with a myriad of viral illnesses. Epidemiological data clearly indicate that SARS-CoV-2 is the trigger for MIS-C, which typically occurs about 1 mo after infection. These findings support the hypothesis of viral triggers for the various forms of classic KD. We further suggest that rare inborn errors of immunity (IEIs) altering the immune response to SARS-CoV-2 may underlie the pathogenesis of MIS-C in some children. The discovery of monogenic IEIs underlying MIS-C would shed light on its pathogenesis, paving the way for a new genetic approach to classic KD, revisited as a heterogeneous collection of IEIs to viruses.
Introduction
As the COVID-19 pandemic continues, it is becoming increasingly clear that severe clinical manifestations of SARS-CoV-2 infection remain rare in children, accounting for only 1.5% of all COVID-19 hospital admissions (Docherty et al., 2020; Götzinger et al., 2020). However, in the spring of 2020, clusters of children admitted to the hospital with a multisystem hyperinflammatory syndrome, presenting with fever, abdominal pain and/or rash, myocarditis, and other clinical features reminiscent of Kawasaki disease (KD), were reported in communities with high rates of COVID-19, across Europe, and in North and South America (Fig. 1 and Table 1; de Farias et al., 2020; Farias et al., 2020; Jones et al., 2020; Riphagen et al., 2020; Toubiana et al., 2020b; Verdoni et al., 2020; Whittaker et al., 2020). Initial reports described a condition they referred to as an atypical form of KD with an incidence 30 times higher than in previous years (Verdoni et al., 2020). This newly identified syndrome was given the name pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 by the UK Royal College of Pediatrics and Child Health (Whittaker et al., 2020), multisystem inflammatory syndrome in children (MIS-C) by the US Centers for Disease Control and Prevention (CDC) and the World Health Organization, or COVID-19–associated KD (COVID-KD) by many investigators (Pouletty et al., 2020; Toubiana et al., 2021; Verdoni et al., 2020). MIS-C cases were typically reported 3–6 wk after the peak of SARS-CoV-2 infection in the local population, suggesting a temporal association with the ongoing pandemic (Belot and Levy-Bruhl, 2020; Belot et al., 2020; Dufort et al., 2020; Feldstein et al., 2020; Toubiana et al., 2020a). About 84% of MIS-C cases test positive for anti–SARS-CoV-2 antibodies and/or in viral PCR tests, and all reported cases have a history of exposure to SARS-CoV-2 (Ahmed et al., 2020). There is, therefore, strong viral and epidemiological evidence to suggest that SARS-CoV-2 is the trigger for MIS-C.
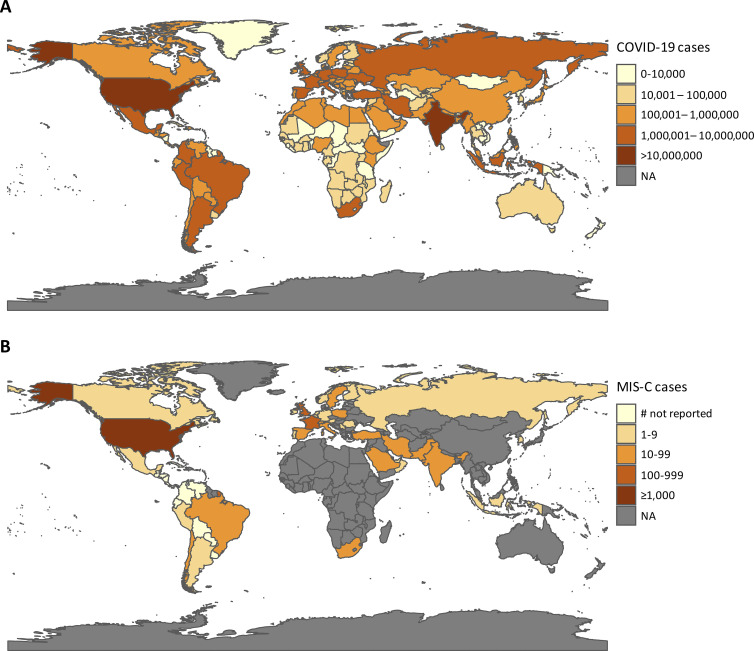
Figure 1.
Geographic distribution of COVID-19 and MIS-C cases. (A) Choropleth map of cumulative COVID-19 cases, by country, from World Health Organization data, as of February 11, 2021. (B) Choropleth map of MIS-C cases, by country, as reported in published studies. Countries that have reported cases but have not disclosed the number of cases are denoted as “# not reported.” Only MIS-C cases reported in English-language journals are included. A list of the articles included can be found in Table S1. NA, not applicable.
Table 1. A summary of the reported MIS-C cases, with information about ethnicitya.
| Location | Number of cases (M/F) | Ethnicity | Distribution of ethnic groups within the country concerned | References |
|---|---|---|---|---|
| England | 58 (38/20) | 38% Black | 84.9% White British/all other white | Whittaker et al., 2020; Office for National Statistics, 2021 |
| 31% Asian | 8% Asian/Asian British | |||
| 21% White | 3.5% Black/African/Caribbean/Black British | |||
| 10% Other | 1.9% Other ethnicity | |||
| 1.8% Mixed/multiple ethnic groups | ||||
| England | 29 (20/9) | 41% Caucasian | Felsenstein et al., 2020 | |
| 21% Southeast Asian | ||||
| 17% Unknown or Multi-ethnic background | ||||
| 14% African/Caribbean | ||||
| 7% East Asian | ||||
| England | 25 (15/10) | 40% White | Carter et al., 2020 | |
| 36% Black | ||||
| 20% Asian | ||||
| 4% Other | ||||
| England | 15 (11/4) | 40% African/Afro-Caribbean | Ramcharan et al., 2020 | |
| 40% South Asian | ||||
| 13% Mixed | ||||
| 7% Other | ||||
| France | 16 (8/8) | 62% Afro-Caribbean | Data not available | Pouletty et al., 2020 |
| 25% European | ||||
| 12% Middle Eastern | ||||
| France | 21 (9/12) | 57% Sub-Saharan African/Afro-Caribbean | Toubiana et al., 2020b | |
| 29% European | ||||
| 10% Asian | ||||
| 5% Middle Eastern | ||||
| US | 2,617b (1,544/1,073) | 35% Hispanic or Latino | 60.1% White | US Centers for Disease Control and Prevention, 2021; Kaiser Family Foundation, 2019 |
| 31% Black, non-Hispanic | 18.5% Hispanic | |||
| 25% White, non-Hispanic | 12.2% Black | |||
| 4% Other | 5.6% Asian | |||
| 3% Multiple | 0.7% American Indian/Alaska Native | |||
| 2% Asian | 0.2% Native Hawaiian/Other Pacific Islander | |||
| 0% American Indian/Alaska Native | 2.8% Multiple | |||
| 0% Native Hawaiian/Other Pacific Islander |
F, female; M, male.
Only papers in English, reporting 15 patients or more, are included in this table.
198 of the 2,617 cases did not report race/ethnicity data. CDC data reported as of March 1, 2021.
The exact incidence of MIS-C remains unknown, due to a lack of comprehensive SARS-CoV-2 testing data for children. A New York–based study reported an incidence of MIS-C of 2/100,000 in individuals age <21 yr in a population with an incidence of confirmed SARS-CoV-2 infection of 322/100,000 between March 1 and May 10, 2020 (Ahmed et al., 2020; Dufort et al., 2020). Another study reported a conservative estimate of the incidence of SARS-CoV-2 infection of no more than 5% in children under the age of 15 yr, with MIS-C detected in <2 in every 10,000 infected children (Belot et al., 2020). Unlike children with acute severe SARS-CoV-2 infection, most children presenting with MIS-C display no detectable active viral infection by PCR assays in the upper respiratory tract at the time of MIS-C diagnosis. Instead, they display signs of prior SARS-CoV-2 infection or contact with an infected individual ∼1 m before the onset of MIS-C symptoms (Toubiana et al., 2021). The clinical and immunological features of MIS-C and pediatric COVID-19 pneumonia are different (Gruber et al., 2020; Swann et al., 2020), whereas those of MIS-C and KD, both of which are heterogenous inflammatory conditions, overlap (Carter et al., 2020; Consiglio et al., 2020; Hoste et al., 2021; Toubiana et al., 2021; Whittaker et al., 2020). The emergence of MIS-C provided the first clear evidence to support the notion that KD-like inflammatory illness can be triggered by a virus (Toubiana et al., 2021). The case definition of MIS-C is evolving with improvements in our understanding of the spectrum of disease. MIS-C may correspond to a continuous distribution of different clinical entities. A key question that remains is whether there are common pathogenic mechanisms underlying MIS-C and classic KD. The starting point for addressing this question is a comparison of the epidemiological, clinical, and immunological features of MIS-C and KD.
Epidemiology of MIS-C and KD
MIS-C and KD display several notable epidemiological differences. The median age of children with MIS-C is 8–9 yr (Dufort et al., 2020; Feldstein et al., 2020; Godfred-Cato et al., 2020; Swann et al., 2020; Whittaker et al., 2020), whereas most children with KD are under the age of 5 yr with a median age of 3 yr (Abrams et al., 2020) and a peak of incidence in infants <1 yr old (Ae et al., 2020; Holman et al., 2010). Children who develop MIS-C are generally also older than those with severe pediatric COVID-19, although the lower mean age of patients with severe pediatric COVID-19 is largely due to the high proportion of infants among these patients (Diorio et al., 2020; Gale et al., 2021; Götzinger et al., 2020; Swann et al., 2020). In both MIS-C and severe pediatric COVID-19, most of the patients were previously healthy (Davies et al., 2020; Diorio et al., 2020; Feldstein et al., 2020), although a possible association with obesity in adolescents has been suggested (Dufort et al., 2020; Feldstein et al., 2020; Godfred-Cato et al., 2020). KD affects more boys than girls (sex ratio of ∼1.5:1), whereas no clear sex bias has been observed in MIS-C patients (Ahmed et al., 2020). The fatality rate of MIS-C has been estimated at 1–2%, much higher than that reported for KD (estimated KD case fatality rate of <0.1% in Japan; Ahmed et al., 2020; McCrindle et al., 2017). Interestingly, KD rates appear to be highest in East Asia, whereas individuals of South Asian and Latin American descent appear to be overrepresented in studies of MIS-C performed in the United States and/or Western European countries in which ancestry was reported (Table 1; Dufort et al., 2020; Feldstein et al., 2020; Godfred-Cato et al., 2020; Whittaker et al., 2020). There has been a striking paucity of MIS-C cases from East Asia, despite the high incidence of KD in this geographic area (Kim et al., 2020b; Li et al., 2019). Only two cases of MIS-C have been reported to date in South Korea, and no cases have been reported in Japan or China (Toyo Keizai Online COVID-19 Task Team, 2021), contrasting with the higher numbers of cases from the Americas, Europe, Africa, South Asia, and the Middle East (Almoosa et al., 2020; Dufort et al., 2020; Falah et al., 2020; Feldstein et al., 2020; Jain et al., 2020; Kim et al., 2020a; Kim et al., 2020b; Lee et al., 2021; Mamishi et al., 2020; Ulloa-Gutierrez et al., 2020; Verdoni et al., 2020; Webb et al., 2020). Whether this is merely due to a lower number of COVID-19 cases in East Asia remains to be seen, bearing in mind the possibility of ascertainment bias (Fig. 1, A and B). Some areas in Europe saw a marked increase in the incidence of KD-like illnesses during the first wave of the pandemic, whereas South Korea observed no change in KD incidence relative to previous years (Kim et al., 2020b; Verdoni et al., 2020). Population-related risk factors specific to either condition are therefore plausible.
Environmental factors may contribute to the geographic distribution of MIS-C incidence, but social determinants of health disparities also place particular ethnic minority populations at higher risk of SARS-CoV-2 exposure and associated disease (Abrams et al., 2020; Godfred-Cato et al., 2020). The contributions of viral, host genetic, and other biological factors to this higher risk remain unclear. It has been suggested that viral factors have contributed to geographic differences in the incidence of MIS-C. Following the spread of the COVID-19 pandemic from East Asia, new variants of SARS-CoV-2 carrying a D839Y/N/E substitution were identified in Europe and the United States. Computational modeling has suggested that the tyrosine substitution at position 839 in the SARS-CoV-2 spike protein strengthens the binding of the spike protein to the β variable (Vβ) region of the host T cell receptor, resulting in superantigen characteristics potentially capable of driving the uncontrolled immune activation seen in MIS-C (Cheng et al., 2020). However, human genetic factors may also underlie the differences in incidence between MIS-C and KD in Western versus Asian countries. Interestingly, KD studies predating COVID-19 suggested differences between ethnic groups for Kawasaki shock syndrome, a condition that affects ∼7% of KD patients (Alsaied et al., 2021). KD shock syndrome has a higher incidence in Western countries than in Asia (Li et al., 2019; Toubiana et al., 2020b), consistent with the observed geographic distribution of MIS-C. These striking epidemiological features highlight the need to define the genetic, immunological, and other factors, such as concurrent environmental exposures (SARS-CoV-2 or other factors), underlying MIS-C and KD.
Comparison of the clinical features of MIS-C and KD
Patients with MIS-C were initially described as having conjunctivitis, cheilitis, rash, and shock (de Farias et al., 2020; Farias et al., 2020; Jones et al., 2020; Riphagen et al., 2020; Toubiana et al., 2020b; Verdoni et al., 2020; Whittaker et al., 2020), immediately inciting comparisons with KD, which has been described as a multisystem inflammatory vasculitis presenting with fever, acute mucocutaneous inflammation, and KD shock syndrome (McCrindle et al., 2017; Riphagen et al., 2020). Indeed, all MIS-C patients present with fever, and about half have a rash and bilateral bulbar conjunctival injection, as observed in KD. The American Heart Association criteria for KD (McCrindle et al., 2017) have not been systematically evaluated in all studies on MIS-C, but MIS-C cases seem to be more likely to fulfill the criteria for the incomplete form of KD (Belhadjer et al., 2020b; Belot et al., 2020; Capone et al., 2020; Cheung et al., 2020; Davies et al., 2020; Dufort et al., 2020; Feldstein et al., 2020; Moraleda et al., 2020; Pouletty et al., 2020; Ramcharan et al., 2020; Riphagen et al., 2020; Toubiana et al., 2020b; Verdoni et al., 2020). Some studies comparing cases of MIS-C with historical cases of KD have confirmed similarities with classic KD in terms of clinical features (Pouletty et al., 2020; Toubiana et al., 2021; Verdoni et al., 2020; Whittaker et al., 2020), but all these studies also highlighted specific features of COVID-KD cases (Abrams et al., 2020; Toubiana et al., 2021). More than 80% of MIS-C cases present with gastrointestinal (GI) symptoms (Abrams et al., 2020), some even suffering intense abdominal pain with acute pseudosurgical abdomen, peritoneal effusion, and gallbladder hydrops (Allali et al., 2021; Miller et al., 2020). Neurological symptoms, such as meningitis-like symptoms or encephalitis, are also more frequently observed in MIS-C than in classic KD (Toubiana et al., 2021). These symptoms are observed early in the disease and can be misinterpreted, sometimes leading to unnecessary treatments or surgery (Allali et al., 2021). One particular feature specific to MIS-C is the high prevalence of myocarditis associated with cardiogenic-vasoplegic shock. Depending on the definition of myocarditis and the inclusion criteria used (cardiovascular or KD-like illness), cardiac dysfunction has been reported to affect 20–100% of patients (Abrams et al., 2020; Dufort et al., 2020; Friedman et al., 2020; Matsubara et al., 2020; Theocharis et al., 2020). Recent cardiac magnetic resonance imaging data have shown these patients to have a myocardial edema, rather than the necrosis often observed in common viral myocarditis (Alsaied et al., 2021; Blondiaux et al., 2020). All these symptoms may reflect local vasculitis and inflammation of the affected organ, and may account for the rapid, syndrome-resolving response to corticosteroid treatment (Belhadjer et al., 2020a; Ouldali et al., 2021).
The proportion of myocardial functional defects, including the depression of ventricular function (Dufort et al., 2020; Friedman et al., 2020; Theocharis et al., 2020), is higher in MIS-C patients than in KD patients, but the rate of coronary artery dilations is similar to that generally reported for KD, although these abnormalities do not often progress to aneurysm (Davies et al., 2020; Dufort et al., 2020; Feldstein et al., 2020; Godfred-Cato et al., 2020; Matsubara et al., 2020; McCrindle et al., 2017; Nelson et al., 2020; Whittaker et al., 2020). Some MIS-C cohort studies have reported the occurrence of coronary artery dilation (Dufort et al., 2020; Feldstein et al., 2020; Godfred-Cato et al., 2020; Matsubara et al., 2020; Whittaker et al., 2020) and/or aneurysms (Davies et al., 2020; Dufort et al., 2020; Nelson et al., 2020) in 4–20% of patients, as previously reported for KD (McCrindle et al., 2017). However, unlike the coronary artery dilations and/or aneurysms observed in MIS-C, which are typically transient and have an onset that coincides with that of fever, the coronary artery aneurysms seen in KD peak ∼3 wk after fever onset (Jhaveri et al., 2021; Matsubara et al., 2020; Tsuda and Hashimoto, 2021). Importantly, coronary artery abnormalities have been observed not only in the youngest MIS-C patients meeting the full KD criteria, who would be more likely to be considered to have “classic” KD, but also in adolescent patients (Nelson et al., 2020). Physicians should, therefore, consider treating MIS-C patients with low-dose aspirin and intravenous Ig (IVIG) in addition to appropriate cardiovascular resuscitation and steroids, because IVIG (with or without steroids) is the only treatment that has been shown to reduce coronary complications in KD (Henderson et al., 2020; McCrindle et al., 2017; Oates-Whitehead et al., 2003). MIS-C is also associated with higher levels of inflammatory markers (including C-reactive protein [CRP], procalcitonin, and troponins) and brain natriuretic peptide (BNP), more profound lymphopenia, thrombocytopenia rather than the common thrombocytosis of prolonged KD, and higher ferritin levels than are observed in KD (Toubiana et al., 2021; Verdoni et al., 2020; Whittaker et al., 2020). However, all these characteristics are similar to those of the historic KD shock syndrome, the most severe form of KD (Kanegaye et al., 2009). Thus, MIS-C has several specific characteristics, but displays a significant clinical overlap with KD, and with KD shock syndrome in particular.
Clinical spectrum of MIS-C
With the progression of the pandemic, the definition of MIS-C has evolved, although definitions still vary between countries and research studies (Diorio et al., 2020; Dufort et al., 2020; Feldstein et al., 2020; Godfred-Cato et al., 2020; Jain et al., 2020; Mamishi et al., 2020; Riphagen et al., 2020; Verdoni et al., 2020; Whittaker et al., 2020). The CDC defines MIS-C as a persistent fever and inflammation (e.g., increases in the levels of CRP, erythrocyte sedimentation rate, D-dimers, procalcitonin, or the serum concentrations of cytokines, including IL-10 and TNF; Diorio et al., 2020) in patients <21 yr requiring hospitalization for severe illness with multiple-organ dysfunction (e.g., GI, cardiovascular, dermatological, neurological, nephrological, and/or respiratory; Godfred-Cato et al., 2020). Patients must also either have tested positive for SARS-CoV-2 (serologic test and/or PCR) or have clearly been exposed to SARS-CoV-2 (Godfred-Cato et al., 2020). Multiple-organ dysfunction is a typical clinical feature of MIS-C. The dysfunction of at least four organ systems has been reported in >70% of patients (Feldstein et al., 2020). As also discussed above, the most common presenting symptoms include multiple days of fever (>5 d in most cases; Feldstein et al., 2020), GI symptoms (e.g., diarrhea, vomiting, and abdominal pain) and, in some patients, conjunctivitis, neurological symptoms (e.g., headache, confusion, and lethargy) or respiratory symptoms (it is uncommon for these to be primary, and invasive or noninvasive ventilation is required in only a minority of patients; Davies et al., 2020). Patients often present with shock (Feldstein et al., 2020; Whittaker et al., 2020) and other aspects of impaired cardiovascular function, together with dysfunctions of multiple other systems. This multiple-organ dysfunction necessitates admission to intensive care units for 64–80% of MIS-C patients (Dufort et al., 2020; Feldstein et al., 2020; Godfred-Cato et al., 2020). At least half these patients require inotropes and/or fluid resuscitation (Belot et al., 2020; Whittaker et al., 2020).
These aspects are common to the general MIS-C patient population, but severity and the nature of individual clinical presentations vary widely, and patients can be grouped into different subsets on the basis of different strategies. A recently published consensus management pathway for MIS-C developed in the United Kingdom divides the syndrome into KD-like (based on American Heart Association criteria) and nonspecific inflammatory phenotypes (Harwood et al., 2021). A second strategy distinguishes between a febrile inflammatory phenotype (lacking shock and without KD criteria), KD-like illness (e.g., meeting complete or incomplete KD criteria but without shock or multisystem involvement), and shock with hyperinflammation (Whittaker et al., 2020; PIER Network, 2020). A third strategy for dividing MIS-C patients into three groups was developed on the basis of a latent class analysis (Godfred-Cato et al., 2020). Class 1 (∼35% of patients) has the highest frequency of multiple-organ dysfunction, generally including GI and cardiovascular abnormalities with shock and myocarditis, and laboratory testing abnormalities, including lymphopenia and high CRP, troponin, and BNP levels. Clinically, the features of the patients in this class do not overlap with COVID-19, but some overlap is observed with KD shock syndrome, and there is a resemblance to systemic-onset juvenile idiopathic arthritis (Binstadt et al., 2005). Class 2 (∼30%) has a larger overlap with typical COVID-19, with more pulmonary syndromes (e.g., cough and/or acute respiratory distress syndrome), higher rates of SARS-CoV-2 PCR positivity, and the highest mortality rate (5%). Finally, class 3 (∼35%) is more similar to KD, with a younger median age, a higher frequency of rash and mucocutaneous alterations, and lower levels of inflammatory markers. Given the clinical parallels, treatment attempts initially focused on approaches already shown to alleviate the symptoms of KD, such as IVIG, steroids, and aspirin. IVIG in addition to methylprednisolone has been shown to be associated with a more favorable course of fever and better cardiac recovery in MIS-C than treatment with IVIG alone (Belhadjer et al., 2020a; Ouldali et al., 2021). Some teams have also used biological therapies, including antibodies against IL-6 or TNF, and recombinant IL-1 receptor antagonist (IL-1RA; Diorio et al., 2020; Feldstein et al., 2020; Henderson et al., 2020; Radia et al., 2020; Whittaker et al., 2020). Despite the severe illness presented by patients, the mean duration of hospital stay is only 6–7 d (Dufort et al., 2020; Feldstein et al., 2020; Godfred-Cato et al., 2020). Most patients receive medium- to long-term clinical follow-up at the treating institution to monitor for late complications.
Comparison of the immunological features of MIS-C and KD
MIS-C and KD both involve hyperinflammatory responses, presenting clinically with persistent high fever, often accompanied by a visible rash and conjunctivitis. These two conditions remain elusive. They appear to be related but different conditions, with a clinical and immunological overlap (Fig. 2). The pathogenesis of KD remains unclear, but the postinfectious nature of MIS-C, and possibly also of KD, is consistent with an autoinflammatory and/or autoimmune hyperinflammatory process initially triggered by viral infection (Rowley et al., 1997; Rowley et al., 2020). The immune response in patients with KD is typically characterized by pro-inflammatory signatures, including increases in IL-1, IL-8, IL-6, and IL-17A levels (Bajolle et al., 2014; Nelson et al., 2020; Oates-Whitehead et al., 2003), marked leukocytosis, eosinophilia, and high levels of monocytes (Kanegaye et al., 2009; Radia et al., 2020), accompanied by high CRP, erythrocyte sedimentation rate, procalcitonin, alanine aminotransferase, and γ-glutamyl transferase levels (Henderson et al., 2020; Radia et al., 2020). Many of these features are common to MIS-C (including high levels of cytokines, such as IL-1, IL-8, IL-6, etc.), but tend to be stronger in MIS-C patients, and this is particularly true for markers of inflammation (such as CRP, procalcitonin, troponin, and ferritin) and BNP levels (Toubiana et al., 2021). KD-specific increases in the number of IgA plasma cells, and tissue-specific changes, mostly concerning the arterial wall, have been described (Marrani et al., 2018; Menikou et al., 2019; Rowley et al., 1997). Finally, it has been suggested that immune complexes and autoantibodies (Marrani et al., 2018; Menikou et al., 2019) are involved in the pathogenesis of KD (Jia et al., 2010), and probably also in MIS-C, but with markedly different specificities (Consiglio et al., 2020; Gruber et al., 2020).
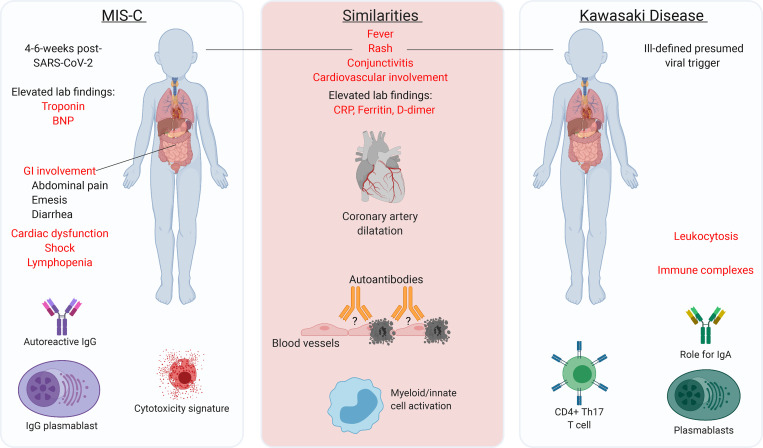
Figure 2.
A comparison of MIS-C and KD. The common and different clinical and immunological features of MIS-C and KD are shown. The major characteristic similarities or differences between the two conditions are highlighted in red. Th17, T helper type 17 cell.
In a study comparing MIS-C patients with KD patients that have been identified before the COVID-19 pandemic, these two groups of patients were shown to have different cytokine profiles, both during the hyperinflammatory phase and before treatment (Consiglio et al., 2020). The hyperinflammation observed in MIS-C also differs from that seen in acute, severe COVID-19, with lower IL-8 and IL-7 levels in MIS-C (Consiglio et al., 2020). KD patients have high frequencies of T helper type 17 cells in the bloodstream, consistent with their high plasma IL-17A concentrations (Jia et al., 2010). This T helper type 17 cell response may, therefore, be a key feature distinguishing between KD and MIS-C hyperinflammation in untreated patients (Consiglio et al., 2020), although other studies have reported high plasma IL-17A levels in MIS-C patients too (Esteve-Sole et al., 2021; Gruber et al., 2020; Lee et al., 2020). The autoantibody profiling of serum from untreated children with MIS-C and KD has revealed many candidate targets, some of which differ between MIS-C and KD. The presence of autoantibodies against DEL-1, a potent anti-inflammatory protein that antagonizes the integrin ICAM-1 and limits vascular inflammation, has been reported in patients with KD (Shin et al., 2015), but not in MIS-C patients (Consiglio et al., 2020). By contrast, one study showed that MIS-C patients have autoantibodies against proteins of the casein kinase family (Consiglio et al., 2020). These enzymes are activated in cells infected with SARS-CoV-2 and may play a role in viral replication (Bouhaddou et al., 2020), and other processes (Consiglio et al., 2020; Gruber et al., 2020). However, it remains unclear whether these autoantibodies played a role in the disease pathogenesis of KD or MIS-C. It therefore appears likely that both KD and MIS-C are postinfectious inflammatory diseases, with some overlapping clinical and immunological features, but different inflammatory signatures. They are, thus, probably mediated by related but different inflammatory pathways and, possibly, related but different pathogenic mechanisms.
Immunological spectrum of MIS-C
Some potential clues to the pathophysiological mechanisms underlying MIS-C have been identified. As discussed above, increases in the levels of inflammatory markers are variable in MIS-C (Diorio et al., 2020; Dufort et al., 2020; Vella et al., 2021). A broad spectrum of serum cytokine concentrations displays increases of various magnitudes (e.g., IL-1β, IL-6, IL-8, IL-10, IL-17, IL-18, IFN-γ, and TNF; Carter et al., 2020; Diorio et al., 2020; Gruber et al., 2020; Vella et al., 2021), with specific increases in IL-10 and TNF levels in MIS-C patients relative to patients with severe COVID-19. There is also evidence of a possible role for complement/coagulation (Ramaswamy et al., 2021) in MIS-C, and thrombotic microangiopathy in MIS-C and in some cases of severe pediatric COVID-19, based on increases in C5b-9 levels and on the increase in frequency of Burr cells and schistocytes on peripheral blood smears (Diorio et al., 2020). The immune profiling of MIS-C patients by a systems biology approach has also revealed other indicators of immune dysfunction (Gruber et al., 2020). The anti–SARS-CoV-2 antibodies in MIS-C patients effectively neutralize SARS-CoV-2 in vitro, like those from patients convalescing from COVID-19, although antibody levels differ (lower IgM, higher IgA, and anti-spike IgG; Anderson et al., 2020). Analyses of 92 secreted moieties have identified clear signs of inflammation in the plasma of MIS-C patients. CyTOF-based deep immunophenotyping identified a unique cytokine signature in MIS-C patients that was not present in healthy controls or COVID-19 patients and included strong signatures of inflammation (IL-18 and IL-6), lymphocytic and myeloid chemotaxis and activation (CCL3, CCL4, and CDCP1), and mucosal immune dysregulation (IL-17A, CCL20, and CCL28; Gruber et al., 2020). Interestingly, most recent immunological analyses of MIS-C have revealed a spectrum of immunological features, including changes in cytokine levels, with similarities and differences between subgroups of patients paralleling those between clinical subgroups (Esteve-Sole et al., 2021; Lee et al., 2020).
In addition, the mass cytometry immunophenotyping of peripheral mononuclear cells from MIS-C patients revealed decreases in the frequencies of nonclassical monocytes, and subsets of natural killer (NK) and T lymphocytes, suggesting extravasation to peripheral organs (Gruber et al., 2020). There is also evidence of significant T cell dysregulation in MIS-C, particularly for CD8 T cells, including increases in the populations of activated T cells (e.g., frequency of CD8 HLA-DR+CD38+ cells) similar to that seen in adults with severe COVID-19, together with an increase in the frequency of PD-1+CD39+CD8+ T cells in MIS-C (Vella et al., 2021). Moreover, HLA-DR levels on γδ and CD4+CCR7+ T cells, and CD64 (FcγRI) levels on neutrophils and monocytes, are high, indicating myeloid cell activation (Carter et al., 2020). Furthermore, an efflux of immature neutrophils with low CD10 and CD62L into the periphery has also been observed (Klocperk et al., 2020). Autoantibodies targeting the principal organs affected in MIS-C, including the GI tract, immune cells, cardiac and endothelial tissue, and some previously described autoantigens such as anti-La, have been described (Gruber et al., 2020; Ramaswamy et al., 2021), although it remains unclear whether these autoantibodies are specifically relevant to the pathogenesis of MIS-C. Finally, a profound expansion of some specific T cell receptor β variable (TRBV) genes, including TRBV11.2 (encoding Vβ 21.3), correlated with MIS-C severity and serum cytokine levels, has been observed in MIS-C patients and some adult COVID-19 patients with hyperinflammation as a major presenting phenotype (Cheng et al., 2020; Moreews et al., 2021 Preprint; Porritt et al., 2020; Ramaswamy et al., 2021). This observation is suggestive of superantigen-mediated activation as a key determinant of autoimmunity and hyperinflammation.
MIS-C may be a new presentation of KD triggered by a single virus
The pathogenesis of KD has remained enigmatic since its first description in 1967 (Kato et al., 1996; Kawasaki, 1967; Kawasaki et al., 1974; Nakamura, 2018; Newburger et al., 2016; Son and Newburger, 2018), resisting the assaults of both virologists, who tested the hypothesis of a virus-triggered inflammatory disease, and geneticists, who performed genome-wide association studies (GWASs) to test the hypothesis of a polygenic inflammatory disease. Many studies have provided anecdotal and suggestive, but not conclusive, evidence that KD can be triggered by viruses (Catalano-Pons et al., 2005; Jordan-Villegas et al., 2010; Oates-Whitehead et al., 2003). Interestingly, common coronaviruses have been suggested as a possible trigger for KD, but they accounted, at the time, for <10% of the viral infections associated with KD each year (Esper et al., 2005), and contradictory findings have been reported (Dominguez et al., 2006; Lehmann et al., 2009; Shimizu et al., 2005). Clearly, no virus “causes” KD with sufficient penetrance or in a sufficiently high proportion of cases to be identified as the principal trigger (Bajolle et al., 2014; Burns and Glodé, 2004; Catalano-Pons et al., 2005). There are two possible interpretations of these findings. Either known viruses do not trigger KD, or a great many known or unknown viruses trigger KD. One fact is indisputable: KD is not caused by a single known virus, or even a narrow set of known viruses, in the way that fulminant viral hepatitis is caused by the hepatitis A, B, and E viruses, for example. In parallel, many studies have also reported modest and suggestive, but not conclusive, genetic associations with KD (Kato et al., 1996; Kawasaki et al., 1974; Nakamura, 2018; Newburger et al., 2016; Son and Newburger, 2018). Again, no particular genotype “causes” KD with sufficient penetrance or in a sufficient proportion of cases to be identified as causal (Dietz et al., 2017; Onouchi, 2009). Again, there are two possible interpretations. Either KD is not determined by germline variants, or its genetic component follows a mode of inheritance that has not yet been tested.
The parallel failures of virologists and geneticists, tackling hypotheses in apparent opposition, are intriguing and possibly related. It could be speculated that KD is not itself a disease, but a spectrum of conditions caused by various viral and genetic factors. This would be consistent with its clinical and immunological heterogeneity. Moreover, the evidence that MIS-C can be caused by SARS-CoV-2 infection provides proof of principle for a viral etiology for at least one Kawasaki-like syndrome (Abdel-Mannan et al., 2020; Belot et al., 2020; Cheung et al., 2020; Dufort et al., 2020; Labé et al., 2020; Licciardi et al., 2020; McCrindle and Manlhiot, 2020; Ouldali et al., 2020; Riphagen et al., 2020; Toubiana et al., 2020b; Viner and Whittaker, 2020; Whittaker et al., 2020), suggesting that KD may also be caused by other viruses. MIS-C is itself clinically and immunologically heterogenous, suggesting that the greater heterogeneity of KD may be due, in part, to diverse viral etiologies. Does this discovery exclude the genetic hypothesis? On the contrary, it probably provides additional support for this hypothesis, as various life-threatening viral illnesses of childhood can be caused by “lacunar” inborn errors of immunity (IEIs; Casanova and Abel, 2020; Casanova and Abel, 2021). Moreover, it will probably facilitate the discovery of IEIs underlying KD, a task that might be rendered difficult by multiple viral etiologies, “multiplying” genetic heterogeneity, which is already high for each viral illness studied. By focusing on MIS-C, which may, in many ways, be considered as COVID-KD, we may be able to identify IEIs that result in vasculitis upon infection with SARS-CoV-2. Unlike inborn errors of type I IFNs, which result in disseminated COVID-19, with pulmonary and systemic inflammation ∼10 d after infection a consequence of viral spread with insufficient type I IFN immunity in the first few days of infection (Bastard et al., 2020; Trouillet-Assant et al., 2020; Zhang et al., 2020a; Zhang et al., 2020b), inborn errors underlying MIS-C may not affect normal viral control, but result in excessive inflammation of the vessels at about day 30. Both the spatial and temporal aspects of MIS-C are intriguing. The genome-wide search for single-gene or digenic rare IEIs should clarify this important question and shed light on the mechanism of MIS-C, thereby paving the way for new studies of KD in other patients.
Human genetic studies of KD: GWAS
Four GWASs on KD have identified several significant genome-wide hits (Table 2). An association of the missense variant (H131R) of FCGR2A with KD was first reported in European and East Asian cohorts (Khor et al., 2011), and later replicated in a GWAS on independent East Asian populations (Onouchi et al., 2012). The major A allele (encoding histidine) was found to be associated with a higher risk of KD, with an odds ratio of 1.32. FCGR2A encodes the FcγRIIA protein (CD32a), which belongs to a family of IgG receptors expressed on many immune cells, including NK cells, macrophages, and neutrophils. It is involved in cell activation and the uptake of immune complexes. The H131R substitution affects binding affinity, and can be used to stratify individuals as strong or weak responders to IgG subclasses (Bruhns et al., 2009). ITPKC, reported by Khor et al. (2011) as the second locus implicated in KD, was initially identified by linkage disequilibrium mapping in both Japanese and US children (Onouchi et al., 2008). The associated SNP, rs28493229, was shown to affect the splicing efficiency of the ITPKC mRNA, and the C allele reducing splicing was associated with an increase in the risk of KD, with an odds ratio of 1.52. ITPKC encodes inositol-trisphosphate 3-kinase, an enzyme responsible for down-regulating the calcium response in immune cells, thereby decreasing T cell activation (Kumrah et al., 2020; Lo, 2020).
Table 2. Genome-wide significant variants associated with KD susceptibility.
| Chr:position | SNP | Gene | Effect | Odds ratio | Risk allele frequency in 1000 Genomes, European/East Asian populations | References |
|---|---|---|---|---|---|---|
| 1:161479745 | rs1801274 | FCGR2A | Missense (H131R) | 1.32a | 0.49/0.72 | Khor et al., 2011 |
| 4:185568113 | rs2720378 | CASP3 | Intron | 1.20 | 0.73/0.30 | Johnson et al., 2020 |
| 6:27869631 | rs1873212 | TRX-CAT1-7 | Downstream | 1.2 | 0.07/0.17 | Johnson et al., 2020 |
| 6:28248594 | rs1778477 | PGBD1 | Upstream | 1.30 | 0.83/0.86 | Johnson et al., 2020 |
| 6:30411903 | rs1264516 | LOC105375012 | Downstream | 1.24 | 0.44/0.67 | Johnson et al., 2020 |
| 6:31533378 | rs2857602 | LTA | Intron | 1.28 | 0.40/0.34 | Johnson et al., 2020 |
| 6:32300809 | rs3129960 | C6orf10 | Intron | 1.37 | 0.15/0.15 | Johnson et al., 2020 |
| 6:32658079 | rs7775228 | HLA-DQB1 | Upstream | 1.26 | 0.16/0.23 | Johnson et al., 2020 |
| 6:32763514 | rs2857151 | HLA-DQB2–H-DOB | Intergenic | 1.47b | 0.67/0.69 | Onouchi et al., 2012 |
| 6:32782605 | rs2071473 | HLA-DOB | Intron | 1.26 | 0.39/0.43 | Johnson et al., 2020 |
| 8:11343973 | rs2736340c | BLK | Upstream | 1.55 | 0.24/0.72 | Johnson et al., 2020 |
| 14:107152027 | rs4774175 | IGHV1-69 | Downstream | 1.20 | 0.40/0.48 | Johnson et al., 2020 |
| 19:41224204 | rs28493229d | ITPKC | Intron | 1.52a/1.41 | 0.12/0.065 | Khor et al., 2011; Johnson et al., 2020 |
| 20:44746982 | rs1883832e | CD40 | 5′ UTR | 1.28 | 0.74/0.57 | Johnson et al., 2020 |
This table lists variants reaching genome-wide significance (P < 5 × 10−8) in the primary GWAS in which they were identified (Khor et al., 2011; Kim et al., 2017; Lee et al., 2012; Onouchi et al., 2012) or in a follow up meta-analysis (Johnson et al., 2020) of the three Asian GWASs (Kim et al., 2017; Lee et al., 2012; Onouchi et al., 2012). Chr, chromosome; SNP, single nucleotide polymorphism; UTR, untranslated region.
Odds ratio in the combined discovery GWAS and replication cohorts from the primary publication.
Odds ratio in the discovery GWAS from the primary publication.
In linkage disequilibrium (r2 > 0.5) in the 1000 Genomes Chinese and Japanese populations with rs2254546 (Onouchi et al., 2012), rs2618476 (Lee et al., 2012), and rs6993775 (Kim et al., 2017).
In linkage disequilibrium (r2 > 0.5) in the 1000 Genomes Chinese and Japanese populations with rs2233152 (Khor et al., 2011).
In linkage disequilibrium (r2 > 0.5) in the 1000 Genomes Chinese and Japanese populations with rs1569723 (Lee et al., 2012) and rs4813003 (Onouchi et al., 2012).
Three subsequent GWASs conducted in Japanese (Onouchi et al., 2012), Taiwanese (Lee et al., 2012), and Korean (Kim et al., 2017) populations reported associations with a cluster of variants in the vicinity of the BLK gene. BLK is a src family B lymphoid tyrosine kinase present in B cells that transduces signals downstream from the B cell receptor. Significant associations were also reported for two noncoding variants in strong linkage disequilibrium close to CD40, a member of the TNF receptor (TNFR) superfamily, in the Taiwanese and Japanese GWASs (Lee et al., 2012; Onouchi et al., 2012). Significant genome-wide associations were also detected for SNPs in HLA class II genes in the Japanese population (Onouchi et al., 2012), whereas analyses performed with specific HLA class I alleles yielded nonreproducible results (Onouchi, 2009). Finally, a recent follow-up study of the three East Asian GWASs identified several additional significant loci (Johnson et al., 2020; Lee et al., 2012; Onouchi et al., 2012; Table 2). In particular, a higher risk of KD was found to be associated with the high-expression alleles of SNPs located in the Ig heavy variable gene (IGHV) cluster. Several of these associations with common variants suggest a role for B cells and/or endogenous Igs in KD pathogenesis. However, these epidemiological genetic studies have revealed only some of the factors involved, resulting in a 1.2- to 1.5-fold increase in disease risk. Thus, after many years of GWAS studies on KD, now might be the right time to consider alternative genetic hypotheses and approaches to tackling this question.
Monogenic disorders underlying virus-triggered hyperinflammatory disease: The example of hemophagocytic lymphohistiocytosis (HLH)
Virus-triggered hyperinflammation can be studied through a monogenic IEI approach, and HLH provides an example. HLH is a severe, often fatal condition characterized by extreme immune activation, as demonstrated by the infiltration of activated leukocytes into various organs; increases in serum concentrations of soluble CD25, IL-18, CXCL9, IFN-γ, ferritin, and triglycerides; hypofibrinogenemia; and low NK cell cytotoxicity (Janka, 2012). Viruses are associated with the onset of active HLH in most cases. Infections with herpes viruses, especially EBV and CMV, are most frequently associated with the pathogenesis of this disease (Brisse et al., 2017). The discovery of a series of well-defined monogenic IEIs has illustrated the pathogenic mechanism of HLH. On one hand, variants of PRF1 (encoding perforin) or UNC13D, STX11, STXBP2, LYST, RAB27A, or AP3B1, all of which encode molecules crucial for the trafficking/exocytosis of cytotoxic granules, cause isolated HLH with high penetrance, due to an impairment of granule-mediated cytotoxicity in CD8+ T cells and NK cells (Henderson et al., 2020; Janka, 2012). On the other hand, germline inactivating variants of SH2D1A (encoding SAP), CD27, or TNFRSF9 (4-1BB) cause HLH in 25–60% of affected individuals, together with additional clinical sequelae (hypogammaglobulinemia, B cell lymphoma; Tangye and Latour, 2020). These IEIs are unique in that affected individuals are often healthy and do not develop HLH until they become infected with specific viruses, typically EBV. These discoveries established that HLH results from germline variants disabling the appropriate activation of cytotoxic lymphocytes (CD8+ T cells, NK cells) to lyse pathogen-infected APCs, thereby preventing the rapid killing and efficient clearance of these APCs, leading to hyperimmune activation (Canna and Marsh, 2020; Janka, 2012; Tangye and Latour, 2020).
HLH can also result from hemizygous inactivating mutations of XIAP (due to EBV in ∼50% of cases) or monoallelic activating mutations of NLRC4. Mutations of XIAP or NLRC4 dysregulate inflammasome activation, leading to excessive and sustained production of IL-1β, IL-18, and CXCL9 (Canna and Marsh, 2020). Moreover, monoallelic mutations of CDC42 were recently reported to cause multisystemic inflammation, including HLH (Su and Orange, 2020). Levels of inflammatory mediators, IL-1β, IL-18, IFN-γ, and CXCL9, are very high in the serum of CDC42-deficient patients, and the constitutive production of these factors by bone marrow cells is also higher in patients than in healthy donors (Bucciol et al., 2020; Gernez et al., 2019; Lam et al., 2019). Consistent with these observations, treatment with inhibitors of IL-1 or IFN-γ was found to be effective in some cases of CDC42 deficiency (Gernez et al., 2019; Lam et al., 2019; Su and Orange, 2020). CDC42 deficiency may impair cytotoxic lymphocyte function and promote the production of inflammatory mediators, thereby fueling a cytokine storm. In XIAP deficiency, and perhaps in other deficiencies, colitis may be a separate form of severe inflammatory disease that can result from certain viral infections. Thus, an array of severe inflammatory conditions may emerge following viral infections in individuals with certain monogenic lesions.
Monogenic IEIs may underlie MIS-C in some children
MIS-C affects previously healthy children and adolescents, with most other subjects from the same age group infected with SARS-CoV-2 presenting only very mild illness or remaining asymptomatic. We recently showed that single-gene inborn errors of type I IFN immunity or neutralizing autoantibodies against type I IFNs can underlie life-threatening COVID-19 pneumonia in at least 10% of patients (Bastard et al., 2020, 2021; Zhang et al., 2020a, 2020b; van der Wijst et al., 2021 Preprint). We now suggest that some children may develop MIS-C because of a rare monogenic or digenic IEI that is clinically silent until infection with SARS-CoV-2, which triggers an inflammatory response. This hypothesis is consistent with previous findings of monogenic IEIs in other virus-triggered hyperinflammation diseases (i.e., HLH triggered by EBV). One key question is whether MIS-C has immunopathogenic mechanisms in common with KD, or even other childhood autoimmune and/or autoinflammatory conditions. The common and unique clinical, immunological, and laboratory features of these conditions (Fig. 2) suggest probable overlaps in their immunopathogenesis, at least to some extent. However, there are probably also different mechanisms at work, given the differences in the features of these conditions. As discussed above, the most intriguing and relevant comparison might be that between MIS-C and KD, the first being triggered by a single virus (SARS-CoV-2) whereas the second may perhaps be triggered by various infectious agents, such as other viruses (including other coronaviruses). Furthermore, despite being triggered by a single virus, MIS-C appears to be a clinically heterogeneous condition, making it possible to classify patients into subgroups on the basis of major clinical and immunological differences, as described above. The search for monogenic and digenic IEIs conferring a predisposition to MIS-C in previously healthy children and young adults should therefore focus primarily on the testing of genetic hypotheses in a genome-wide, agnostic manner, while bearing in mind the characteristic epidemiological, clinical, and immunological features of the condition, to facilitate the identification of candidate disease-causing genes (Casanova and Abel, 2020; Casanova and Abel, 2021; Casanova et al., 2020).
MIS-C may display both genetic homogeneity (the same gene mutated in two or more families) and genetic heterogeneity (one gene per family), and both physiological homogeneity (mutated genes immunologically related) and physiological heterogeneity (unrelated). Some disorders may be autosomal, whereas others are X-linked, and the traits involved may be recessive, dominant, or codominant. Depending on the gene, variants may be loss- or gain-of-function. Given the low incidence of this condition in the general population, the causal gene variants are probably equally rare. Clinical penetrance may be full or incomplete, depending on the nature of the gene defect. Incomplete penetrance may suggest that some cases are digenic, or even oligogenic. Some gene defects may have an age-dependent presentation, which may be particularly relevant here, given the age difference between MIS-C and KD patients. Finally, some defects may be present in specific ancestries. We anticipate that the discovery of monogenic IEI underlying MIS-C will unravel the molecular and cellular basis of the immunopathogenesis of the different classes of MIS-C in previously healthy children and young adults. Although generally considered to be a vasculitis, different types of disorders may be causal in the different clinical subgroups classified by the development of myocarditis, neurological symptoms, mild pneumonia symptoms, or Kawasaki-like symptoms only. The tragic pandemic of COVID-19 has provided us with a unique opportunity to discover the immunopathogenesis of a severe inflammatory disease triggered by a single virus via a monogenic approach.
Concluding remarks
Agnostic testing of genetic hypotheses can be used to search for rare monogenic or digenic IEIs conferring a predisposition to MIS-C, at both the cohort population and individual patient levels (https://www.covidhge.com). Not only should different genetic hypotheses be tested with genomic data from patients with MIS-C, but these data should also be analyzed in parallel with proper cohorts of controls consisting of healthy children and young adults who have encountered SARS-CoV-2 infection but remained asymptomatic or developed only a mild clinical syndrome. Moreover, COVID-19 pneumonia, neurological COVID-19, and MIS-C are probably caused by different types of human genetic disorders. However, it is tempting to think that the multisystem inflammatory syndrome in adults that has emerged very recently (Morris et al., 2020) may have some causative IEIs in common with MIS-C. It is also possible that “COVID toes,” a cutaneous inflammatory syndrome affecting patients of all ages, but mostly children and young adults (Hernandez and Bruckner, 2020), are caused by IEIs that are different but connected to those underlying MIS-C. Genetic analyses of these different SARS-CoV-2–related conditions can be performed in parallel, to identify the IEIs specific to each condition. Finally, genomic data from children with MIS-C may be analyzed in combination with or independently from data for children with KD. These two conditions may have some genetic causes in common if they share immunopathogenic mechanisms. Alternatively, monogenic and digenic IEIs may be detected more readily in genetic and/or physiological homogeneity approaches, for a condition triggered by a single virus, such as MIS-C. This will provide a basis for genetic diagnosis and counseling, while guiding the design of preventive and therapeutic interventions against MIS-C in children and young adults. It will also pave the way for studies of classic KD, which remains enigmatic, and other childhood autoimmune and/or autoinflammatory postinfectious conditions (e.g., acute disseminated encephalomyelitis, rheumatic fever, and Henoch-Schönlein purpura) through a rare IEI approach that will become as clinically important for children with KD or Kawasaki-like disease as the identification of the causal infectious triggers.
Online supplemental material
Table S1 lists reported MIS-C cases with geographic distribution.
Supplementary Material
lists reported MIS-C cases with geographic distribution.
Acknowledgments
We thank our colleagues from the COVID Human Genetic Effort consortium (https://www.covidhge.com) for discussions.
Our studies are funded by the Howard Hughes Medical Institute, the Rockefeller University, the St. Giles Foundation, the National Institutes of Health (R01AI088364 to J.-L. Casanova and S.-Y. Zhang; R01AI148963, R01AI151029, and R01AI150300 to D. Bogunovic; K08AI135091 to S.E. Henrickson; R21AI144315-02S1 to C.L. Lucas), the National Center for Advancing Translational Sciences, National Institutes of Health Clinical and Translational Science Award program (UL1 TR001866), the Yale Center for Mendelian Genomics and the GSP Coordinating Center funded by the National Human Genome Research Institute (UM1HG006504 and U24HG008956), Institut National de la Santé et de la Recherche Médicale and Université de Paris, the French National Research Agency (ANR) Résilience-Covid-19 grant GenMIS-C, the ANR “Investments for the Future” program (ANR-10-IAHU-01), the Integrative Biology of Emerging Infectious Diseases Laboratory of Excellence (ANR-10-LABX-62-IBEID), the ANR project AABIFNCOV (ANR-20-CO11-0001), the French Foundation for Medical Research (EQU201903007798), the French Foundation for Medical Research and ANR GENCOVID project, the ANRSCOV05 project, the Square Foundation, Grandir - Fonds de solidarité pour l’enfance, the Fondation du Souffle, the SCOR Corporate Foundation for Science, UK Research and Innovation Future Leader’s Fellowship (MR/S032304/1), the NIHR Imperial Biomedical Research Centre at Imperial College Healthcare NHS Trust (70931), the Burroughs Wellcome Fund Career Awards for Medical Scientists, the Clinical Immunology Society, the American Academy of Allergy Asthma and Immunology, the Michael Smith Foundation for Health Research, the National Health and Medical Research Council of Australia, and the University of New South Wales Sydney COVID Rapid Response Initiative (to S.G. Tangye).
Author contributions: V. Sancho-Shimizu, J.-L. Casanova, and S.-Y. Zhang conceptualized the study. V. Sancho-Shimizu, P. Brodin, A. Cobat, C.M. Biggs, J. Toubiana, C.L. Lucas, S.E. Henrickson, A. Belot, members of MIS-C@CHGE, S.G. Tangye, J.D. Milner, M. Levin, L. Abel, D. Bogunovic, J.-L. Casanova, and S.-Y. Zhang wrote and edited the manuscript.
Contributor Information
MIS-C@CHGE:
Elie Haddad, Kathie Beland, Aurora Pujol, Agatha Schlüter, Laura Planas-Serra, Sergio Aguilera-Albesa, Juan Valencia-Ramos, Agustí Rodríguez-Palmero, Marta Gut, Jacques G. Rivière, Roger Colobran, Pere Soler-Palacin, Carlos Rodriguez-Gallego, Rebeca Perez De Diego, Carlos Flores, Laia Alsina, Daniel Blazquez-Gamero, Iolanda Jordan, Sevgi Keles, Melike Emiroglu, Ozge Metin Akcan, Gulsum Alkan, Selma Erol Aytekin, Yahya Gul, Şadiye Kübra Tüter Öz, Sefika Elmas Bozdemir, Gulsum Iclal Bayhan, Saliha Kanık Yüksek, Aslınur Özkaya Parlakay, Belgin Gülhan, Aysun Yahşi, Ahmet Osman Kilic, Adem Karbuz, Emine Hafize Erdeniz, Esra Akyüz Özkan, Zerrin Orbak, Şehnaz Aydemir, Jale Bengi Celik, Bahar Kandemir, Gökhan Aytekin, Hasan Kapakli, Volkan Yarar, Alper Yosunkaya, Hulya Vatansev, Caner Aytekin, Selda Hancerli Torun, Serdar Nepesov, Taner Coskuner, Betül Sözeri, Yasemin Kendir Demirkol, Ozgur Kasapcopur, Mehmet Yıldız, Esra Sevketoglu, Nevin Hatipoğlu, Tayfun Özçelik, Osman Yesilbas, Zeynep Gökçe Gayretli Aydin, Anna Sediva, Adam Klocperk, Marketa Bloomfield, Isabelle Meyts, Selket Delafontaine, Filomeen Haerynck, Levi Hoste, Mohammad Shahrooei, Laura Marque, João Farela Neves, Giuseppe Novelli, Antonio Novelli, Alessandro Aiuti, Giorgio Casari, Amed Aziz Bousfiha, Saleh Zaid Almuhsen, Ali Sobh, Alenka Gagro, Fanny Bajolle, Damien Bonnet, Pierre Lebon, Weite Lei, Danyel Lee, Yoann Seeleuthner, Peng Zhang, Majistor Maglorius, Quentin Philippot, Simon Pelham, Paul Bastard, Qian Zhang, Emmanuelle Jouanguy, Anne Puel, Jethro Herberg, Taco W Kuijpers, Evangelos Bellos, Myrsini Kaforou, Stephanie Menikou, Qiang Pan-Hammarström, Lennart Hammarström, Hassan Abolhassani, Yenan Bryceson, Antonio Condino-Neto, Carolina Prando, Silvia Yumi Bando, Andre Cavalcanti, Jacques Fellay, Giradine Blanchard-Hohner, Davood Mansouri, Shima Mahmoudi, Oksana Boyarchuk, Alla Volokha, Anastasiia Bondarenko, Yuriy Stepanovskiy, Trine Mogensen, Diederik van de Beek, Evangelos Andreakos, Maria Papadaki, Ahmad Abou Tayoun, Rabih Halwani, Fahd Al-Mulla, José Luis Franco, Yu-Lung Lau, Mike Kwan, Kohsuke Imai, Satoshi Okada, Alexandre Bolze, Manish J. Butte, Elena Hsieh, Beth A Drolet, Lisa Arkin, Yuval Itan, Tom Maniatis, Moshe Arditi, Megan Cooper, Erica Schmitt, Samya Chakravorty, Mark S. Anderson, Helen C. Su, and Luigi D. Notarangelo
References
- Abdel-Mannan, O., Eyre M., Löbel U., Bamford A., Eltze C., Hameed B., Hemingway C., and Hacohen Y.. 2020. Neurologic and Radiographic Findings Associated With COVID-19 Infection in Children. JAMA Neurol. 77:1–6. 10.1001/jamaneurol.2020.2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams, J.Y., Godfred-Cato S.E., Oster M.E., Chow E.J., Koumans E.H., Bryant B., Leung J.W., and Belay E.D.. 2020. Multisystem Inflammatory Syndrome in Children Associated with Severe Acute Respiratory Syndrome Coronavirus 2: A Systematic Review. J. Pediatr. 226:45–54.e1. 10.1016/j.jpeds.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ae, R., Makino N., Kosami K., Kuwabara M., Matsubara Y., and Nakamura Y.. 2020. Epidemiology, Treatments, and Cardiac Complications in Patients with Kawasaki Disease: The Nationwide Survey in Japan, 2017-2018. J. Pediatr. 225:23–29.e2. 10.1016/j.jpeds.2020.05.034 [DOI] [PubMed] [Google Scholar]
- Ahmed, M., Advani S., Moreira A., Zoretic S., Martinez J., Chorath K., Acosta S., Naqvi R., Burmeister-Morton F., Burmeister F., et al. 2020. Multisystem inflammatory syndrome in children: A systematic review. EClinicalMedicine. 26:100527. 10.1016/j.eclinm.2020.100527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allali, S., Cohen J.F., Brice J., Khraiche D., and Toubiana J.. 2021. Gastrointestinal Symptoms Followed by Shock in a Febrile 7-Year-Old Child during the COVID-19 Pandemic. Clin. Chem. 67:54–58. 10.1093/clinchem/hvaa279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Lawati, Z., Al Rawahi H., and Al Yazidi L.S.F.. 2021. ACUTE APPENDICITIS MIMICKING MULTISYSTEM INFLAMMATORY SYNDROME IN CHILDREN: CASE REPORT AND REVIEW OF THE LITERATURE. J. Paediatr. Child Health. 57:461–462. 10.1111/jpc.15398 [DOI] [PubMed] [Google Scholar]
- Almoosa, Z.A., Al Ameer H.H., AlKadhem S.M., Busaleh F., AlMuhanna F.A., and Kattih O.. 2020. Multisystem Inflammatory Syndrome in Children, the Real Disease of COVID-19 in Pediatrics - A Multicenter Case Series From Al-Ahsa, Saudi Arabia. Cureus. 12:e11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaied, T., Tremoulet A.H., Burns J.C., Saidi A., Dionne A., Lang S.M., Newburger J.W., de Ferranti S., and Friedman K.G.. 2021. Review of Cardiac Involvement in Multisystem Inflammatory Syndrome in Children. Circulation. 143:78–88. 10.1161/CIRCULATIONAHA.120.049836 [DOI] [PubMed] [Google Scholar]
- Anderson, E.M., Diorio C., Goodwin E.C., McNerney K.O., Weirick M.E., Gouma S., Bolton M.J., Arevalo C.P., Chase J., Hicks P., et al. 2020. SARS-CoV-2 antibody responses in children with MIS-C and mild and severe COVID-19. J. Pediatric Infect. Dis. Soc.:piaa161. 10.1093/jpids/piaa161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydın, F., Çelikel E., Ekici Tekin Z., Coşkun S., Sezer M., Karagöl C., Kaplan M.M., Tekgöz N., Kurt T., Özcan S., et al. 2021. Comparison of baseline laboratory findings of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis and multisystem inflammatory syndrome in children. Int. J. Rheum. Dis. 24:542–547. 10.1111/1756-185X.14078 [DOI] [PubMed] [Google Scholar]
- Bahrami, A., Vafapour M., Moazzami B., and Rezaei N.. 2020. Hyperinflammatory shock related to COVID-19 in a patient presenting with multisystem inflammatory syndrome in children: First case from Iran. J. Paediatr. Child Health.:jpc.15048. 10.1111/jpc.15048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajolle, F., Meritet J.F., Rozenberg F., Chalumeau M., Bonnet D., Gendrel D., and Lebon P.. 2014. Markers of a recent bocavirus infection in children with Kawasaki disease: “a year prospective study”. Pathol. Biol. (Paris). 62:365–368. 10.1016/j.patbio.2014.06.002 [DOI] [PubMed] [Google Scholar]
- Bastard, P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V., et al. COVID Human Genetic Effort . 2020. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 370:eabd4585. 10.1126/science.abd4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard, P., Michailidis E., Hoffmann H.H., Chbihi M., Le Voyer T., Rosain J., Philippot Q., Seeleuthner Y., Gervais A., Materna M., et al. 2021. Auto-antibodies to type I IFNs can underlie adverse reactions to yellow fever live attenuated vaccine. J. Exp. Med. 218:e20202486. 10.1084/jem.20202486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista-Rodriguez, C., Sanchez-de-Toledo J., Clark B.C., Herberg J., Bajolle F., Randanne P.C., Salas-Mera D., Foldvari S., Chowdhury D., Munoz R., et al. 2021. Multisystem Inflammatory Syndrome in Children: An International Survey. Pediatrics. 147:e2020024554. 10.1542/peds.2020-024554 [DOI] [PubMed] [Google Scholar]
- Belhadjer, Z., Auriau J., Méot M., Oualha M., Renolleau S., Houyel L., and Bonnet D.. 2020a. Addition of Corticosteroids to Immunoglobulins Is Associated With Recovery of Cardiac Function in Multi-Inflammatory Syndrome in Children. Circulation. 142:2282–2284. 10.1161/CIRCULATIONAHA.120.050147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhadjer, Z., Méot M., Bajolle F., Khraiche D., Legendre A., Abakka S., Auriau J., Grimaud M., Oualha M., Beghetti M., et al. 2020b. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation. 142:429–436. 10.1161/CIRCULATIONAHA.120.048360 [DOI] [PubMed] [Google Scholar]
- Belot, A., Antona D., Renolleau S., Javouhey E., Hentgen V., Angoulvant F., Delacourt C., Iriart X., Ovaert C., Bader-Meunier B., et al. 2020. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 25:2001010. 10.2807/1560-7917.ES.2020.25.22.2001010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belot, A., and Levy-Bruhl D.. French Covid-19 Pediatric Inflammation Consortium . 2020. Multisystem Inflammatory Syndrome in Children in the United States. N. Engl. J. Med. 383:1793–1794. 10.1056/NEJMc2026136 [DOI] [PubMed] [Google Scholar]
- Binstadt, B.A., Levine J.C., Nigrovic P.A., Gauvreau K., Dedeoglu F., Fuhlbrigge R.C., Weindling S.N., Newburger J.W., and Sundel R.P.. 2005. Coronary artery dilation among patients presenting with systemic-onset juvenile idiopathic arthritis. Pediatrics. 116:e89–e93. 10.1542/peds.2004-2190 [DOI] [PubMed] [Google Scholar]
- Blondiaux, E., Parisot P., Redheuil A., Tzaroukian L., Levy Y., Sileo C., Schnuriger A., Lorrot M., Guedj R., and Ducou le Pointe H.. 2020. Cardiac MRI in Children with Multisystem Inflammatory Syndrome Associated with COVID-19. Radiology. 297:E283–E288. 10.1148/radiol.2020202288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhaddou, M., Memon D., Meyer B., White K.M., Rezelj V.V., Correa Marrero M., Polacco B.J., Melnyk J.E., Ulferts S., Kaake R.M., et al. 2020. The Global Phosphorylation Landscape of SARS-CoV-2 Infection. Cell. 182:685–712.e19. 10.1016/j.cell.2020.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisse, E., Wouters C.H., Andrei G., and Matthys P.. 2017. How Viruses Contribute to the Pathogenesis of Hemophagocytic Lymphohistiocytosis. Front. Immunol. 8:1102. 10.3389/fimmu.2017.01102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhns, P., Iannascoli B., England P., Mancardi D.A., Fernandez N., Jorieux S., and Daëron M.. 2009. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 113:3716–3725. 10.1182/blood-2008-09-179754 [DOI] [PubMed] [Google Scholar]
- Bucciol, G., Pillay B., Casas-Martin J., Delafontaine S., Proesmans M., Lorent N., Coolen J., Tousseyn T., Bossuyt X., Ma C.S., et al. 2020. Systemic Inflammation and Myelofibrosis in a Patient with Takenouchi-Kosaki Syndrome due to CDC42 Tyr64Cys Mutation. J. Clin. Immunol. 40:567–570. 10.1007/s10875-020-00742-5 [DOI] [PubMed] [Google Scholar]
- Buonsenso, D., Di Sante G., and Sali M.. CURE COVID-19 Study Group . 2020. Cytokine Profile in an Adolescent With Pediatric Multisystem Inflammatory Syndrome Temporally Related to COVID-19. Pediatr. Infect. Dis. J. 39:e213–e215. 10.1097/INF.0000000000002802 [DOI] [PubMed] [Google Scholar]
- Burns, J.C., and Glodé M.P.. 2004. Kawasaki syndrome. Lancet. 364:533–544. 10.1016/S0140-6736(04)16814-1 [DOI] [PubMed] [Google Scholar]
- Canna, S.W., and Marsh R.A.. 2020. Pediatric hemophagocytic lymphohistiocytosis. Blood. 135:1332–1343. 10.1182/blood.2019000936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone, C.A., Subramony A., Sweberg T., Schneider J., Shah S., Rubin L., Schleien C., Epstein S., Johnson J.C., Kessel A., et al. Northwell Health COVID-19 Research Consortium . 2020. Characteristics, Cardiac Involvement, and Outcomes of Multisystem Inflammatory Syndrome of Childhood Associated with severe acute respiratory syndrome coronavirus 2 Infection. J. Pediatr. 224:141–145. 10.1016/j.jpeds.2020.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, M.J., Fish M., Jennings A., Doores K.J., Wellman P., Seow J., Acors S., Graham C., Timms E., Kenny J., et al. 2020. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat. Med. 26:1701–1707. 10.1038/s41591-020-1054-6 [DOI] [PubMed] [Google Scholar]
- Casanova, J.L., and Abel L.. 2020. The human genetic determinism of life-threatening infectious diseases: genetic heterogeneity and physiological homogeneity? Hum. Genet. 139:681–694. 10.1007/s00439-020-02184-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova, J.L., and Abel L.. 2021. Lethal Infectious Diseases as Inborn Errors of Immunity: Toward a Synthesis of the Germ and Genetic Theories. Annu. Rev. Pathol. 16:23–50. 10.1146/annurev-pathol-031920-101429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova, J.L., and Su H.C.. COVID Human Genetic Effort . 2020. A Global Effort to Define the Human Genetics of Protective Immunity to SARS-CoV-2 Infection. Cell. 181:1194–1199. 10.1016/j.cell.2020.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano-Pons, C., Quartier P., Leruez-Ville M., Kaguelidou F., Gendrel D., Lenoir G., Casanova J.L., and Bonnet D.. 2005. Primary cytomegalovirus infection, atypical Kawasaki disease, and coronary aneurysms in 2 infants. Clin. Infect. Dis. 41:e53–e56. 10.1086/432578 [DOI] [PubMed] [Google Scholar]
- Cheng, M.H., Zhang S., Porritt R.A., Noval Rivas M., Paschold L., Willscher E., Binder M., Arditi M., and Bahar I.. 2020. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation. Proc. Natl. Acad. Sci. USA. 117:25254–25262. 10.1073/pnas.2010722117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, E.W., Zachariah P., Gorelik M., Boneparth A., Kernie S.G., Orange J.S., and Milner J.D.. 2020. Multisystem Inflammatory Syndrome Related to COVID-19 in Previously Healthy Children and Adolescents in New York City. JAMA. 324:294–296. 10.1001/jama.2020.10374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, B.C., Sanchez-de-Toledo J., Bautista-Rodriguez C., Choueiter N., Lara D., Kang H., Mohsin S., Fraisse A., Cesar S., Sattar Shaikh A., et al. 2020. Cardiac Abnormalities Seen in Pediatric Patients During the SARS-CoV2 Pandemic: An International Experience. J. Am. Heart Assoc. 9:e018007. 10.1161/JAHA.120.018007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan, E., Foulon P., Cappeliez O., Dolle N., Vanfraechem G., and De Backer D.. 2020. Multisystem Inflammatory Syndrome With Complete Kawasaki Disease Features Associated With SARS-CoV-2 Infection in a Young Adult. A Case Report. Front. Med. (Lausanne). 7:428. 10.3389/fmed.2020.00428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consiglio, C.R., Cotugno N., Sardh F., Pou C., Amodio D., Rodriguez L., Tan Z., Zicari S., Ruggiero A., Pascucci G.R., et al. CACTUS Study Team . 2020. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell. 183:968–981.e7. 10.1016/j.cell.2020.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, P., Evans C., Kanthimathinathan H.K., Lillie J., Brierley J., Waters G., Johnson M., Griffiths B., du Pré P., Mohammad Z., et al. 2020. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc. Health. 4:669–677. 10.1016/S2352-4642(20)30215-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Farias, E.C.F., Pedro Piva J., de Mello M.L.F.M.F., do Nascimento L.M.P.P., Costa C.C., Machado M.M.M., Rodrigues T.D.S., Carvalho R.D.F.P., Alves M.C.B., Aires L.F.Q., et al. 2020. Multisystem Inflammatory Syndrome Associated With Coronavirus Disease in Children: A Multi-centered Study in Belem, Para, Brazil. Pediatr. Infect. Dis. J. 39:e374–e376. 10.1097/INF.0000000000002865 [DOI] [PubMed] [Google Scholar]
- Dhanalakshmi, K., Venkataraman A., Balasubramanian S., Madhusudan M., Amperayani S., Putilibai S., Sadasivam K., Ramachandran B., and Ramanan A.V.. 2020. Epidemiological and Clinical Profile of Pediatric Inflammatory Multisystem Syndrome - Temporally Associated with SARS-CoV-2 (PIMS-TS) in Indian Children. Indian Pediatr. 57:1010–1014. 10.1007/s13312-020-2025-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz, S.M., van Stijn D., Burgner D., Levin M., Kuipers I.M., Hutten B.A., and Kuijpers T.W.. 2017. Dissecting Kawasaki disease: a state-of-the-art review. Eur. J. Pediatr. 176:995–1009. 10.1007/s00431-017-2937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diorio, C., Henrickson S.E., Vella L.A., McNerney K.O., Chase J., Burudpakdee C., Lee J.H., Jasen C., Balamuth F., Barrett D.M., et al. 2020. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J. Clin. Invest. 130:5967–5975. 10.1172/JCI140970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty, A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., Holden K.A., Read J.M., Dondelinger F., Carson G., et al. ISARIC4C investigators . 2020. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 369:m1985. 10.1136/bmj.m1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolhnikoff, M., Ferreira Ferranti J., de Almeida Monteiro R.A., Duarte-Neto A.N., Soares Gomes-Gouvêa M., Viu Degaspare N., Figueiredo Delgado A., Montanari Fiorita C., Nunes Leal G., Rodrigues R.M., et al. 2020. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc. Health. 4:790–794. 10.1016/S2352-4642(20)30257-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez, S.R., Anderson M.S., Glodé M.P., Robinson C.C., and Holmes K.V.. 2006. Blinded case-control study of the relationship between human coronavirus NL63 and Kawasaki syndrome. J. Infect. Dis. 194:1697–1701. 10.1086/509509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufort, E.M., Koumans E.H., Chow E.J., Rosenthal E.M., Muse A., Rowlands J., Barranco M.A., Maxted A.M., Rosenberg E.S., Easton D., et al. New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team . 2020. Multisystem Inflammatory Syndrome in Children in New York State. N. Engl. J. Med. 383:347–358. 10.1056/NEJMoa2021756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esper, F., Shapiro E.D., Weibel C., Ferguson D., Landry M.L., and Kahn J.S.. 2005. Association between a novel human coronavirus and Kawasaki disease. J. Infect. Dis. 191:499–502. 10.1086/428291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve-Sole, A., Anton J., Pino-Ramirez R.M., Sanchez-Manubens J., Fumado V., Fortuny C., Rios-Barnes M., Sanchez-de-Toledo J., Girona-Alarcon M., Mosquera J.M., et al. 2021. Similarities and differences between the immunopathogenesis of COVID-19-related pediatric inflammatory multisystem syndrome and Kawasaki disease. J. Clin. Invest. 131:e144554. 10.1172/JCI144554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falah, N.U., Hashmi S., Ahmed Z., Jaan A., Akhtar A., Khalid F., Farooque U., Shera M.T., Ali S., and Javed A.. 2020. Kawasaki Disease-Like Features in 10 Pediatric COVID-19 Cases: A Retrospective Study. Cureus. 12:e11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias, E.C.F., Justino M.C.A., and Mello M.L.F.M.F.. 2020. Multisystem Inflammatory Syndrome in a Child Associated with Coronavirus Disease 19 in the Brazilian Amazon: Fatal Outcome in an Infant. Rev. Paul. Pediatr. 38:e2020165. 10.1590/1984-0462/2020/38/2020165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein, L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F., Newburger J.W., Kleinman L.C., Heidemann S.M., Martin A.A., et al. CDC COVID-19 Response Team . 2020. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 383:334–346. 10.1056/NEJMoa2021680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein, S., Willis E., Lythgoe H., McCann L., Cleary A., Mahmood K., Porter D., Jones J., McDonagh J., Chieng A., et al. 2020. Presentation, Treatment Response and Short-Term Outcomes in Paediatric Multisystem Inflammatory Syndrome Temporally Associated with SARS-CoV-2 (PIMS-TS). J. Clin. Med. 9:3293. 10.3390/jcm9103293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouriki, A., Fougère Y., De Camaret C., Blanchard Rohner G., Grazioli S., Wagner N., Relly C., Pachlopnik Schmid J., Trück J., Kottanatu L., et al. 2021. Case Report: Case Series of Children With Multisystem Inflammatory Syndrome Following SARS-CoV-2 Infection in Switzerland. Front Pediatr. 8:594127. 10.3389/fped.2020.594127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, K.G., Harrild D.M., and Newburger J.W.. 2020. Cardiac Dysfunction in Multisystem Inflammatory Syndrome in Children: A Call to Action. J. Am. Coll. Cardiol. 76:1962–1964. 10.1016/j.jacc.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale, C., Quigley M.A., Placzek A., Knight M., Ladhani S., Draper E.S., Sharkey D., Doherty C., Mactier H., and Kurinczuk J.J.. 2021. Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: a prospective national cohort study using active surveillance. Lancet Child Adolesc. Health. 5:113–121. 10.1016/S2352-4642(20)30342-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernez, Y., de Jesus A.A., Alsaleem H., Macaubas C., Roy A., Lovell D., Jagadeesh K.A., Alehashemi S., Erdman L., Grimley M., et al. 2019. Severe autoinflammation in 4 patients with C-terminal variants in cell division control protein 42 homolog (CDC42) successfully treated with IL-1β inhibition. J. Allergy Clin. Immunol. 144:1122–1125.e6. 10.1016/j.jaci.2019.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatasheh, G., Al Dhanhani H., Goyal A., Noureddin M.B., Al Awaad D., and Peerwani Z.. 2021. COVID-19-Related Giant Coronary Aneurysms in an Infant with Multisystem Inflammatory Disorder in Children: The First Case Report from the United Arab Emirates and the Arab Region. Case Rep. Infect. Dis. 2021:8872412. 10.1155/2021/8872412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfred-Cato, S., Bryant B., Leung J., Oster M.E., Conklin L., Abrams J., Roguski K., Wallace B., Prezzato E., Koumans E.H., et al. California MIS-C Response Team . 2020. COVID-19-Associated Multisystem Inflammatory Syndrome in Children - United States, March-July 2020. MMWR Morb. Mortal. Wkly. Rep. 69:1074–1080. 10.15585/mmwr.mm6932e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götzinger, F., Santiago-García B., Noguera-Julián A., Lanaspa M., Lancella L., Calò Carducci F.I., Gabrovska N., Velizarova S., Prunk P., Osterman V., et al. ptbnet COVID-19 Study Group . 2020. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc. Health. 4:653–661. 10.1016/S2352-4642(20)30177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazioli, S., Tavaglione F., Torriani G., Wagner N., Rohr M., L’Huillier A.G., Leclercq C., Perrin A., Bordessoule A., Beghetti M., et al. 2020. Immunological assessment of pediatric multisystem inflammatory syndrome related to COVID-19. J. Pediatric Infect. Dis. Soc.:piaa142. 10.1093/jpids/piaa142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber, C.N., Patel R.S., Trachtman R., Lepow L., Amanat F., Krammer F., Wilson K.M., Onel K., Geanon D., Tuballes K., et al. 2020. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell. 183:982–995.e14. 10.1016/j.cell.2020.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood, R., Allin B., Jones C.E., Whittaker E., Ramnarayan P., Ramanan A.V., Kaleem M., Tulloh R., Peters M.J., Almond S., et al. PIMS-TS National Consensus Management Study Group . 2021. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. Lancet Child Adolesc. Health. 5:133–141. 10.1016/S2352-4642(20)30304-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, L.A., Canna S.W., Friedman K.G., Gorelik M., Lapidus S.K., Bassiri H., Behrens E.M., Ferris A., Kernan K.F., Schulert G.S., et al. 2020. American College of Rheumatology Clinical Guidance for Pediatric Patients with Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with SARS-CoV-2 and Hyperinflammation in COVID-19. Version 2. Arthritis Rheumatol. 10.1002/art.41454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez, C., and Bruckner A.L.. 2020. Focus on “COVID Toes”. JAMA Dermatol. 156:1003. 10.1001/jamadermatol.2020.2062 [DOI] [PubMed] [Google Scholar]
- Holman, R.C., Belay E.D., Christensen K.Y., Folkema A.M., Steiner C.A., and Schonberger L.B.. 2010. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr. Infect. Dis. J. 29:483–488. 10.1097/INF.0b013e3181cf8705 [DOI] [PubMed] [Google Scholar]
- Hoste, L., Van Paemel R., and Haerynck F.. 2021. Multisystem inflammatory syndrome in children related to COVID-19: a systematic review. Eur. J. Pediatr. 18:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain, S., Sen S., Lakshmivenkateshiah S., Bobhate P., Venkatesh S., Udani S., Shobhavat L., Andankar P., Karande T., and Kulkarni S.. 2020. Multisystem Inflammatory Syndrome in Children With COVID-19 in Mumbai, India. Indian Pediatr. 57:1015–1019. 10.1007/s13312-020-2026-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain, M.K., Sahu S.K., Behera J.R., and Patnaik S.. 2021. Multisystem Inflammatory Syndrome in Children Associated with COVID 19 Treated with Oral Steroid. Indian J. Pediatr. 88:106. 10.1007/s12098-020-03497-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janka, G.E. 2012. Familial and acquired hemophagocytic lymphohistiocytosis. Annu. Rev. Med. 63:233–246. 10.1146/annurev-med-041610-134208 [DOI] [PubMed] [Google Scholar]
- Jhaveri, S., Ahluwalia N., Kaushik S., Trachtman R., Kowalsky S., Aydin S., and Stern K.. 2021. Longitudinal Echocardiographic Assessment of Coronary Arteries and Left Ventricular Function following Multisystem Inflammatory Syndrome in Children. J. Pediatr. 228:290–293.e1. 10.1016/j.jpeds.2020.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, S., Li C., Wang G., Yang J., and Zu Y.. 2010. The T helper type 17/regulatory T cell imbalance in patients with acute Kawasaki disease. Clin. Exp. Immunol. 162:131–137. 10.1111/j.1365-2249.2010.04236.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, T.A., Mashimo Y., Wu J.Y., Yoon D., Hata A., Kubo M., Takahashi A., Tsunoda T., Ozaki K., Tanaka T., et al. Korean Kawasaki Disease Genetics Consortium, Taiwan Kawasaki Disease Genetics Consortium, Taiwan Pediatric ID Alliance, Japan Kawasaki Disease Genome Consortium . 2020. Association of an IGHV3-66 gene variant with Kawasaki disease. J. Hum. Genet. 26:1–15. 10.1038/s10038-020-00864-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, V.G., Mills M., Suarez D., Hogan C.A., Yeh D., Segal J.B., Nguyen E.L., Barsh G.R., Maskatia S., and Mathew R.. 2020. COVID-19 and Kawasaki Disease: Novel Virus and Novel Case. Hosp. Pediatr. 10:537–540. 10.1542/hpeds.2020-0123 [DOI] [PubMed] [Google Scholar]
- Jordan-Villegas, A., Chang M.L., Ramilo O., and Mejías A.. 2010. Concomitant respiratory viral infections in children with Kawasaki disease. Pediatr. Infect. Dis. J. 29:770–772. 10.1097/INF.0b013e3181dba70b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser Family Foundation . 2019. Population Distribution by Race/Ethnicity. https://www.kff.org/other/state-indicator/distribution-by-raceethnicity (accessed December 27, 2020)
- Kanegaye, J.T., Wilder M.S., Molkara D., Frazer J.R., Pancheri J., Tremoulet A.H., Watson V.E., Best B.M., and Burns J.C.. 2009. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 123:e783–e789. 10.1542/peds.2008-1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, H., Sugimura T., Akagi T., Sato N., Hashino K., Maeno Y., Kazue T., Eto G., and Yamakawa R.. 1996. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 94:1379–1385. 10.1161/01.CIR.94.6.1379 [DOI] [PubMed] [Google Scholar]
- Kawasaki, T. 1967. [Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children]. Arerugi. 16:178–222. [PubMed] [Google Scholar]
- Kawasaki, T., Kosaki F., Okawa S., Shigematsu I., and Yanagawa H.. 1974. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 54:271–276. [PubMed] [Google Scholar]
- Khor, C.C., Davila S., Breunis W.B., Lee Y.C., Shimizu C., Wright V.J., Yeung R.S., Tan D.E., Sim K.S., Wang J.J., et al. Blue Mountains Eye Study . 2011. Genome-wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nat. Genet. 43:1241–1246. 10.1038/ng.981 [DOI] [PubMed] [Google Scholar]
- Kim, J.J., Yun S.W., Yu J.J., Yoon K.L., Lee K.Y., Kil H.R., Kim G.B., Han M.K., Song M.S., Lee H.D., et al. Korean Kawasaki Disease Genetics Consortium . 2017. A genome-wide association analysis identifies NMNAT2 and HCP5 as susceptibility loci for Kawasaki disease. J. Hum. Genet. 62:1023–1029. 10.1038/jhg.2017.87 [DOI] [PubMed] [Google Scholar]
- Kim, H., Shim J.Y., Ko J.H., Yang A., Shim J.W., Kim D.S., Jung H.L., Kwak J.H., and Sol I.S.. 2020a. Multisystem Inflammatory Syndrome in Children Related to COVID-19: the First Case in Korea. J. Korean Med. Sci. 35:e391. 10.3346/jkms.2020.35.e391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.J., Park H., Choi Y.Y., Kim Y.K., Yoon Y., Kim K.R., and Choi E.H.. 2020b. Defining Association between COVID-19 and the Multisystem Inflammatory Syndrome in Children through the Pandemic. J. Korean Med. Sci. 35:e204. 10.3346/jkms.2020.35.e204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klocperk, A., Parackova Z., Dissou J., Malcova H., Pavlicek P., Vymazal T., Dolezalova P., and Sediva A.. 2020. Case Report: Systemic Inflammatory Response and Fast Recovery in a Pediatric Patient With COVID-19. Front. Immunol. 11:1665. 10.3389/fimmu.2020.01665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumrah, R., Vignesh P., Rawat A., and Singh S.. 2020. Immunogenetics of Kawasaki disease. Clin. Rev. Allergy Immunol. 59:122–139. 10.1007/s12016-020-08783-9 [DOI] [PubMed] [Google Scholar]
- Labé, P., Ly A., Sin C., Nasser M., Chapelon-Fromont E., Ben Saïd P., and Mahé E.. 2020. Erythema multiforme and Kawasaki disease associated with COVID-19 infection in children. J. Eur. Acad. Dermatol. Venereol. 34:e539–e541. 10.1111/jdv.16666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad, S.S., Kait S.P., Suryawanshi P.B., Mujawar J., Lad P., Khetre R., Jadhav L.M., Bhor A., Balte P., Kataria P., et al. 2021. Neurological Manifestations in Pediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2 (PIMS-TS). Indian J. Pediatr. 88:294–295. 10.1007/s12098-020-03530-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, M.T., Coppola S., Krumbach O.H.F., Prencipe G., Insalaco A., Cifaldi C., Brigida I., Zara E., Scala S., Di Cesare S., et al. 2019. A novel disorder involving dyshematopoiesis, inflammation, and HLH due to aberrant CDC42 function. J. Exp. Med. 216:2778–2799. 10.1084/jem.20190147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y.C., Kuo H.C., Chang J.S., Chang L.Y., Huang L.M., Chen M.R., Liang C.D., Chi H., Huang F.Y., Lee M.L., et al. Taiwan Pediatric ID Alliance . 2012. Two new susceptibility loci for Kawasaki disease identified through genome-wide association analysis. Nat. Genet. 44:522–525. 10.1038/ng.2227 [DOI] [PubMed] [Google Scholar]
- Lee, P.Y., Day-Lewis M., Henderson L.A., Friedman K.G., Lo J., Roberts J.E., Lo M.S., Platt C.D., Chou J., Hoyt K.J., et al. 2020. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J. Clin. Invest. 130:5942–5950. 10.1172/JCI141113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.H., Han H.S., and Lee J.K.. 2021. The Importance of Early Recognition, Timely Management, and the Role of Healthcare Providers in Multisystem Inflammatory Syndrome in Children. J. Korean Med. Sci. 36:e17. 10.3346/jkms.2021.36.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, C., Klar R., Lindner J., Lindner P., Wolf H., and Gerling S.. 2009. Kawasaki disease lacks association with human coronavirus NL63 and human bocavirus. Pediatr. Infect. Dis. J. 28:553–554. 10.1097/INF.0b013e31819f41b6 [DOI] [PubMed] [Google Scholar]
- Li, Y., Zheng Q., Zou L., Wu J., Guo L., Teng L., Zheng R., Jung L.K.L., and Lu M.. 2019. Kawasaki disease shock syndrome: clinical characteristics and possible use of IL-6, IL-10 and IFN-γ as biomarkers for early recognition. Pediatr. Rheumatol. Online J. 17:1. 10.1186/s12969-018-0303-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licciardi, F., Pruccoli G., Denina M., Parodi E., Taglietto M., Rosati S., and Montin D.. 2020. SARS-CoV-2-Induced Kawasaki-Like Hyperinflammatory Syndrome: A Novel COVID Phenotype in Children. Pediatrics. 146:e20201711. 10.1542/peds.2020-1711 [DOI] [PubMed] [Google Scholar]
- Lima-Setta, F., Magalhães-Barbosa M.C., Rodrigues-Santos G., Figueiredo E.A.D.N., Jacques M.L., Zeitel R.S., Sapolnik R., Borges C.T.D.S., Lanziotti V.S., Castro R.E.V., et al. Brazilian Research Network in Pediatric Intensive Care (BRnet-PIC) . 2020. Multisystem inflammatory syndrome in children (MIS-C) during SARS-CoV-2 pandemic in Brazil: a multicenter, prospective cohort study. J. Pediatr. (Rio J.).:S0021-7557(20)30225-4. 10.1016/j.jped.2020.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishman, J., Kohler C., de Vos C., van der Zalm M.M., Itana J., Redfern A., Smit L., and Rabie H.. 2020. Acute Appendicitis in Multisystem Inflammatory Syndrome in Children With COVID-19. Pediatr. Infect. Dis. J. 39:e472–e473. 10.1097/INF.0000000000002900 [DOI] [PubMed] [Google Scholar]
- Lo, M.S. 2020. A framework for understanding Kawasaki disease pathogenesis. Clin. Immunol. 214:108385. 10.1016/j.clim.2020.108385 [DOI] [PubMed] [Google Scholar]
- Mamishi, S., Movahedi Z., Mohammadi M., Ziaee V., Khodabandeh M., Abdolsalehi M.R., Navaeian A., Heydari H., Mahmoudi S., and Pourakbari B.. 2020. Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in 45 children: a first report from Iran. Epidemiol. Infect. 148:e196. 10.1017/S095026882000196X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrani, E., Burns J.C., and Cimaz R.. 2018. How Should We Classify Kawasaki Disease? Front. Immunol. 9:2974. 10.3389/fimmu.2018.02974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara, D., Kauffman H.L., Wang Y., Calderon-Anyosa R., Nadaraj S., Elias M.D., White T.J., Torowicz D.L., Yubbu P., Giglia T.M., et al. 2020. Echocardiographic Findings in Pediatric Multisystem Inflammatory Syndrome Associated With COVID-19 in the United States. J. Am. Coll. Cardiol. 76:1947–1961. 10.1016/j.jacc.2020.08.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrindle, B.W., and Manlhiot C.. 2020. SARS-CoV-2-Related Inflammatory Multisystem Syndrome in Children: Different or Shared Etiology and Pathophysiology as Kawasaki Disease? JAMA. 324:246–248. 10.1001/jama.2020.10370 [DOI] [PubMed] [Google Scholar]
- McCrindle, B.W., Rowley A.H., Newburger J.W., Burns J.C., Bolger A.F., Gewitz M., Baker A.L., Jackson M.A., Takahashi M., Shah P.B., et al. American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention . 2017. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation. 135:e927–e999. 10.1161/CIR.0000000000000484 [DOI] [PubMed] [Google Scholar]
- Meena, P., Pallavi D., Mishra D., Jhamb U., and Aggarwal M.. 2021. Low-Dose Dexamethasone Following IVIG in Pediatric Inflammatory Multisystem Syndrome in Temporal Association with COVID-19 (PIMS-TC). Indian J. Pediatr. 88:301–302. 10.1007/s12098-020-03509-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menikou, S., Langford P.R., and Levin M.. 2019. Kawasaki Disease: The Role of Immune Complexes Revisited. Front. Immunol. 10:1156. 10.3389/fimmu.2019.01156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, J., Cantor A., Zachariah P., Ahn D., Martinez M., and Margolis K.G.. 2020. Gastrointestinal Symptoms as a Major Presentation Component of a Novel Multisystem Inflammatory Syndrome in Children That Is Related to Coronavirus Disease 2019: A Single Center Experience of 44 Cases. Gastroenterology. 159:1571–1574.e2. 10.1053/j.gastro.2020.05.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraleda, C., Serna-Pascual M., Soriano-Arandes A., Simó S., Epalza C., Santos M., Grasa C., Rodríguez M., Soto B., Gallego N., et al. 2020. Multi-Inflammatory Syndrome in Children related to SARS-CoV-2 in Spain. Clin. Infect. Dis.:ciaa1042. 10.1093/cid/ciaa1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreews, M., Le Gouge K., Bellomo A., Malcus C., Pescarmona R., Khaldi-Plassart S., Djebali S., Mathieu A.L., Perret M., Villard M., et al. 2021. Superantigenic TCR Vbeta 21.3 signature in Multisystem Inflammatory Syndrome in Children. medRxiv. (Preprint posted February 12, 2021) 10.1101/2021.02.11.21251166 [DOI]
- Morris, S.B., Schwartz N.G., Patel P., Abbo L., Beauchamps L., Balan S., Lee E.H., Paneth-Pollak R., Geevarughese A., Lash M.K., et al. 2020. Case Series of Multisystem Inflammatory Syndrome in Adults Associated with SARS-CoV-2 Infection - United Kingdom and United States, March-August 2020. MMWR Morb. Mortal. Wkly. Rep. 69:1450–1456. 10.15585/mmwr.mm6940e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, Y. 2018. Kawasaki disease: epidemiology and the lessons from it. Int. J. Rheum. Dis. 21:16–19. 10.1111/1756-185X.13211 [DOI] [PubMed] [Google Scholar]
- Nelson, C., Ishimine P., Hayden S.R., Correia M., and Wardi G.. 2020. Multisystem Inflammatory Syndrome in Children (MIS-C) in an Adolescent that Developed Coronary Aneurysms: A Case Report and Review of the Literature. J. Emerg. Med. 59:699–704. 10.1016/j.jemermed.2020.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburger, J.W., Takahashi M., and Burns J.C.. 2016. Kawasaki Disease. J. Am. Coll. Cardiol. 67:1738–1749. 10.1016/j.jacc.2015.12.073 [DOI] [PubMed] [Google Scholar]
- Ng, K.F., Kothari T., Bandi S., Bird P.W., Goyal K., Zoha M., Rai V., and Tang J.W.. 2020. COVID-19 multisystem inflammatory syndrome in three teenagers with confirmed SARS-CoV-2 infection. J. Med. Virol. 92:2880–2886. 10.1002/jmv.26206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates-Whitehead, R.M., Baumer J.H., Haines L., Love S., Maconochie I.K., Gupta A., Roman K., Dua J.S., and Flynn I.. 2003. Intravenous immunoglobulin for the treatment of Kawasaki disease in children. Cochrane Database Syst. Rev. 4:CD004000. 10.1002/14651858.CD004000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office for National Statistics . 2021. Office for National Statistics. https://www.ons.gov.uk (accessed December 27, 2020)
- Toyo Keizai Online COVID-19 Task Team . 2021. https://toyokeizai.net/sp/visual/tko/covid19/en.html (accessed April 2, 2021)
- Onouchi, Y. 2009. Molecular genetics of Kawasaki disease. Pediatr. Res. 65:46R–54R. 10.1203/PDR.0b013e31819dba60 [DOI] [PubMed] [Google Scholar]
- Onouchi, Y., Gunji T., Burns J.C., Shimizu C., Newburger J.W., Yashiro M., Nakamura Y., Yanagawa H., Wakui K., Fukushima Y., et al. 2008. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat. Genet. 40:35–42. 10.1038/ng.2007.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onouchi, Y., Ozaki K., Burns J.C., Shimizu C., Terai M., Hamada H., Honda T., Suzuki H., Suenaga T., Takeuchi T., et al. US Kawasaki Disease Genetics Consortium . 2012. A genome-wide association study identifies three new risk loci for Kawasaki disease. Nat. Genet. 44:517–521. 10.1038/ng.2220 [DOI] [PubMed] [Google Scholar]
- Ouldali, N., Pouletty M., Mariani P., Beyler C., Blachier A., Bonacorsi S., Danis K., Chomton M., Maurice L., Le Bourgeois F., et al. 2020. Emergence of Kawasaki disease related to SARS-CoV-2 infection in an epicentre of the French COVID-19 epidemic: a time-series analysis. Lancet Child Adolesc. Health. 4:662–668. 10.1016/S2352-4642(20)30175-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouldali, N., Toubiana J., Antona D., Javouhey E., Madhi F., Lorrot M., Léger P.L., Galeotti C., Claude C., Wiedemann A., et al. French Covid-19 Paediatric Inflammation Consortium . 2021. Association of Intravenous Immunoglobulins Plus Methylprednisolone vs Immunoglobulins Alone With Course of Fever in Multisystem Inflammatory Syndrome in Children. JAMA. 325:855–864. 10.1001/jama.2021.0694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsurekci, Y., Gürlevik S., Kesici S., Akca U.K., Oygar P.D., Aykac K., Karacanoglu D., Sarıtas Nakip O., Ilbay S., Katlan B., et al. 2021. Multisystem inflammatory syndrome in children during the COVID-19 pandemic in Turkey: first report from the Eastern Mediterranean. Clin. Rheumatol. 10.1007/s10067-021-05631-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIER Network . 2020. Paediatric Inflammatory Multisystem Syndrome - Temporarily Associated with SARS-CoV2 (PIMS-TS). https://www.piernetwork.org/pims-ts.html (accessed February 9, 2021)
- Porritt, R.A., Paschold L., Rivas M.N., Cheng M.H., Yonker L.M., Chandnani H., Lopez M., Simnica D., Schultheiss C., Santiskulvong C., et al. 2020. Identification of a unique TCR repertoire, consistent with a superantigen selection process in Children with Multi-system Inflammatory Syndrome. bioRxiv 2020.11.09.372169. 10.1101/2020.11.09.372169 [DOI]
- Pouletty, M., Borocco C., Ouldali N., Caseris M., Basmaci R., Lachaume N., Bensaid P., Pichard S., Kouider H., Morelle G., et al. 2020. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann. Rheum. Dis. 79:999–1006. 10.1136/annrheumdis-2020-217960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radia, T., Williams N., Agrawal P., Harman K., Weale J., Cook J., and Gupta A.. 2020. Multi-system inflammatory syndrome in children & adolescents (MIS-C): A systematic review of clinical features and presentation. Paediatr. Respir. Rev.:S1526-0542(20)30117-2. 10.1016/j.prrv.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy, A., Brodsky N.N., Sumida T.S., Comi M., Asashima H., Hoehn K.B., Li N., Liu Y., Shah A., Ravindra N.G., et al. 2021. Immune dysregulation and autoreactivity correlate with disease severity in SARS-CoV-2-associated multisystem inflammatory syndrome in children. Immunity. 10.1016/j.immuni.2021.04.0031074-76131074-7613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramcharan, T., Nolan O., Lai C.Y., Prabhu N., Krishnamurthy R., Richter A.G., Jyothish D., Kanthimathinathan H.K., Welch S.B., Hackett S., et al. 2020. Paediatric Inflammatory Multisystem Syndrome: Temporally Associated with SARS-CoV-2 (PIMS-TS): Cardiac Features, Management and Short-Term Outcomes at a UK Tertiary Paediatric Hospital. Pediatr. Cardiol. 41:1391–1401. 10.1007/s00246-020-02391-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev, T., Antebi M., Eytan D., Shachor-Meyouhas Y., Ilivitzki A., Aviel Y.B., and Ben-Ari J.. 2020. Pediatric Inflammatory Multisystem Syndrome With Central Nervous System Involvement and Hypocomplementemia Following SARS-COV-2 Infection. Pediatr. Infect. Dis. J. 39:e206–e207. 10.1097/INF.0000000000002804 [DOI] [PubMed] [Google Scholar]
- Remppis, J., Ganzenmueller T., Kohns Vasconcelos M., Heinzel O., Handgretinger R., and Renk H.. 2021. A case series of children and young people admitted to a tertiary care hospital in Germany with COVID-19. BMC Infect. Dis. 21:133. 10.1186/s12879-021-05791-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riphagen, S., Gomez X., Gonzalez-Martinez C., Wilkinson N., and Theocharis P.. 2020. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 395:1607–1608. 10.1016/S0140-6736(20)31094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley, A.H., Eckerley C.A., Jäck H.M., Shulman S.T., and Baker S.C.. 1997. IgA plasma cells in vascular tissue of patients with Kawasaki syndrome. J. Immunol. 159:5946–5955. [PubMed] [Google Scholar]
- Rowley, A.H., Baker S.C., Arrollo D., Gruen L.J., Bodnar T., Innocentini N., Hackbart M., Cruz-Pulido Y.E., Wylie K.M., Kim K.A., and Shulman S.T.. 2020. A Protein Epitope Targeted by the Antibody Response to Kawasaki Disease. J. Infect. Dis. 222:158–168. 10.1093/infdis/jiaa066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadiq, M., Aziz O.A., Kazmi U., Hyder N., Sarwar M., Sultana N., Bari A., and Rashid J.. 2020. Multisystem inflammatory syndrome associated with COVID-19 in children in Pakistan. Lancet Child Adolesc. Health. 4:e36–e37. 10.1016/S2352-4642(20)30256-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbaznejad, L., Navaeifar M.R., Abbaskhanian A., Hosseinzadeh F., Rahimzadeh G., and Rezai M.S.. 2020. Clinical characteristics of 10 children with a pediatric inflammatory multisystem syndrome associated with COVID-19 in Iran. BMC Pediatr. 20:513. 10.1186/s12887-020-02415-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, C., Shike H., Baker S.C., Garcia F., van der Hoek L., Kuijpers T.W., Reed S.L., Rowley A.H., Shulman S.T., Talbot H.K., et al. 2005. Human coronavirus NL63 is not detected in the respiratory tracts of children with acute Kawasaki disease. J. Infect. Dis. 192:1767–1771. 10.1086/497170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, J., Maekawa T., Abe T., Hajishengallis E., Hosur K., Pyaram K., Mitroulis I., Chavakis T., and Hajishengallis G.. 2015. DEL-1 restrains osteoclastogenesis and inhibits inflammatory bone loss in nonhuman primates. Sci. Transl. Med. 7:307ra155. 10.1126/scitranslmed.aac5380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasetia, D.H., Malahayati T.T., Andriyani F.M., Setiabudi D., and Nataprawira H.M.. 2020. A fatal course of multiple inflammatory syndrome in children coinfection with dengue. A case report from Indonesia. IDCases. 22:e01002. 10.1016/j.idcr.2020.e01002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son, M.B.F., and Newburger J.W.. 2018. Kawasaki Disease. Pediatr. Rev. 39:78–90. 10.1542/pir.2016-0182 [DOI] [PubMed] [Google Scholar]
- Su, H.C., and Orange J.S.. 2020. The Growing Spectrum of Human Diseases Caused by Inherited CDC42 Mutations. J. Clin. Immunol. 40:551–553. 10.1007/s10875-020-00785-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann, O.V., Holden K.A., Turtle L., Pollock L., Fairfield C.J., Drake T.M., Seth S., Egan C., Hardwick H.E., Halpin S., et al. ISARIC4C Investigators . 2020. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 370:m3249. 10.1136/bmj.m3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffarel, P., Jorro Barón F., Rodríguez A.P., Widmer J., and Meregallia C.. 2021. Multisystem inflammatory syndrome in children related to COVID-19: An update regarding the presentation of two critically ill patients. Arch. Argent. Pediatr. 119:e26–e35. 10.5546/aap.2021.eng.e26 [DOI] [PubMed] [Google Scholar]
- Tam, H., El Tal T., Go E., and Yeung R.S.M.. 2020. Pediatric inflammatory multisystem syndrome temporally associated with COVID-19: a spectrum of diseases with many names. CMAJ. 192:E1093–E1096. 10.1503/cmaj.201600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangye, S.G., and Latour S.. 2020. Primary immunodeficiencies reveal the molecular requirements for effective host defense against EBV infection. Blood. 135:644–655. 10.1182/blood.2019000928 [DOI] [PubMed] [Google Scholar]
- Theocharis, P., Wong J., Pushparajah K., Mathur S.K., Simpson J.M., Pascall E., Cleary A., Stewart K., Adhvaryu K., Savis A., et al. 2020. Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur. Heart J. Cardiovasc. Imaging.:jeaa212. 10.1093/ehjci/jeaa212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, J.P., Izquierdo G., Acuña M., Pavez D., Reyes F., Fritis A., González R., Rivacoba C., Contardo V., and Tapia L.I.. 2020. Multisystem inflammatory syndrome in children (MIS-C): Report of the clinical and epidemiological characteristics of cases in Santiago de Chile during the SARS-CoV-2 pandemic. Int. J. Infect. Dis. 100:75–81. 10.1016/j.ijid.2020.08.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toubiana, J., Levy C., Allali S., Jung C., Leruez-Ville M., Varon E., Bajolle F., Ouldali N., Chareyre J., Béchet S., et al. 2020a. Association between SARS-CoV-2 infection and Kawasaki-like multisystem inflammatory syndrome: a retrospective matched case-control study, Paris, France, April to May 2020. Euro Surveill. 25:2001813. 10.2807/1560-7917.es.2020.25.48.2001813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toubiana, J., Poirault C., Corsia A., Bajolle F., Fourgeaud J., Angoulvant F., Debray A., Basmaci R., Salvador E., Biscardi S., et al. 2020b. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 369:m2094. 10.1136/bmj.m2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toubiana, J., Cohen J.F., Brice J., Poirault C., Bajolle F., Curtis W., Moulin F., Matczak S., Leruez M., Casanova J.L., et al. 2021. Distinctive Features of Kawasaki Disease Following SARS-CoV-2 Infection: a Controlled Study in Paris, France. J. Clin. Immunol. 41:526–535. 10.1007/s10875-020-00941-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracewski, P., Ludwikowska K.M., Szenborn L., and Kusa J.. 2020. The first case of pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 infection (PIMS-TS) in Poland, complicated by giant coronary artery aneurysms. Kardiol. Pol. 78:1064–1065. [DOI] [PubMed] [Google Scholar]
- Trouillet-Assant, S., Viel S., Gaymard A., Pons S., Richard J.C., Perret M., Villard M., Brengel-Pesce K., Lina B., Mezidi M., et al. COVID HCL Study group . 2020. Type I IFN immunoprofiling in COVID-19 patients. J. Allergy Clin. Immunol. 146:206–208.e2. 10.1016/j.jaci.2020.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda, E., and Hashimoto S.. 2021. Time Course of Coronary Artery Aneurysms in Kawasaki Disease. J. Pediatr. 230:133–139.e2. 10.1016/j.jpeds.2020.12.004 [DOI] [PubMed] [Google Scholar]
- Ulloa-Gutierrez, R., Ivankovich-Escoto G., Yock-Corrales A., and Tremoulet A.H.. 2020. Multisystem Inflammatory Syndrome Surveillance and COVID-19 in Latin America. Pediatr. Infect. Dis. J. 39:e473–e474. 10.1097/INF.0000000000002901 [DOI] [PubMed] [Google Scholar]
- US Centers for Disease Control and Prevention . 2021. Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States. https://www.cdc.gov/mis-c/cases/index.html (accessed March 1, 2021)
- Valverde, I., Singh Y., Sanchez-de-Toledo J., Theocharis P., Chikermane A., Di Filippo S., Kuciñska B., Mannarino S., Tamariz-Martel A., Gutierrez-Larraya F., et al. AEPC COVID-19 Rapid Response Team* . 2021. Acute Cardiovascular Manifestations in 286 Children With Multisystem Inflammatory Syndrome Associated With COVID-19 Infection in Europe. Circulation. 143:21–32. 10.1161/CIRCULATIONAHA.120.050065 [DOI] [PubMed] [Google Scholar]
- van der Wijst, M.G.P., Vazquez S.E., Hartoularos G.C., Bastard P., Grant T., Bueno R., Lee D.S., Greenland J.R., Sun Y., Perez R., et al. 2021. Longitudinal single-cell epitope and RNA-sequencing reveals the immunological impact of type 1 interferon autoantibodies in critical COVID-19. bioRxiv (Preprint posted March 10, 2021) 10.1101/2021.03.09.434529 [DOI]
- Vella, L.A., Giles J.R., Baxter A.E., Oldridge D.A., Diorio C., Kuri-Cervantes L., Alanio C., Pampena M.B., Wu J.E., Chen Z., et al. UPenn COVID Processing Unit . 2021. Deep immune profiling of MIS-C demonstrates marked but transient immune activation compared to adult and pediatric COVID-19. Sci. Immunol. 6:eabf7570. 10.1126/sciimmunol.abf7570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoni, L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., Bonanomi E., and D’Antiga L.. 2020. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 395:1771–1778. 10.1016/S0140-6736(20)31103-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viner, R.M., and Whittaker E.. 2020. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 395:1741–1743. 10.1016/S0140-6736(20)31129-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, K., Abraham D.R., Faleye A., McCulloch M., Rabie H., and Scott C.. Cape Town MISC-Team . 2020. Multisystem inflammatory syndrome in children in South Africa. Lancet Child Adolesc. Health. 4:e38. 10.1016/S2352-4642(20)30272-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker, E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P., Ramnarayan P., Fraisse A., Miller O., Davies P., et al. PIMS-TS Study Group and EUCLIDS and PERFORM Consortia . 2020. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA. 324:259–269. 10.1001/jama.2020.10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez, J.A., Alvarez-Risco A., and Delgado-Zegarra J.. 2020. Covid-19 in Peru: from supervised walks for children to the first case of Kawasaki-like syndrome. BMJ. 369:m2418. 10.1136/bmj.m2418 [DOI] [PubMed] [Google Scholar]
- Yozgat, C.Y., Uzuner S., Bursal Duramaz B., Yozgat Y., Erenberk U., Iscan A., and Turel O.. 2020. Dermatological manifestation of pediatrics multisystem inflammatory syndrome associated with COVID-19 in a 3-year-old girl. Dermatol. Ther. (Heidelb.). 33:e13770. 10.1111/dth.13770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q., Bastard P., Bolze A., Jouanguy E., Zhang S.Y., Cobat A., Notarangelo L.D., Su H.C., Abel L., and Casanova J.L.. COVID Human Genetic Effort . 2020a. Life-Threatening COVID-19: Defective Interferons Unleash Excessive Inflammation. Med (N Y). 1:14–20. 10.1016/j.medj.2020.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., Ogishi M., Sabli I.K.D., Hodeib S., Korol C., et al. NIAID-USUHS/TAGC COVID Immunity Group . 2020b. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 370:eabd4570. 10.1126/science.abd4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
lists reported MIS-C cases with geographic distribution.