Abstract
Background
Rheumatoid arthritis (RA) is a systemic autoimmune disease. The combination therapy of methotrexate (MTX) and Janus kinase inhibitor (JAKi) is commonly used. Patients with RA are at increased risk of malignancy, however, it remains unclear whether the combination therapy is associated with a higher risk.
Objective
To assess the malignancy risk among patients with RA receiving combination therapy of JAKi and MTX compared to MTX alone.
Methods
PubMed, Cochrane and Embase were thoroughly searched for randomized controlled trials (RCTs) in patients with RA receiving JAKi and MTX, from inception to July 2020. Primary endpoints were malignancy events, Non melanomatous skin cancer (NMSC) and malignancy excluding NMSC and secondary endpoints were serious adverse events (SAE), deaths. Risk ratio (RR) and 95% CI were calculated using the Mantel–Haenszel random-effect method.
Results
659 publications were screened and 13 RCTs with a total of 6911 patients were included in the analysis. There was no statistically significant difference in malignancy [RR = 1.42; 95% CI (0.59, 3.41)], neither NMSC [RR = 1.44 (0.36, 5.76)] nor malignancies excluding NMSC [RR = 1.12 (0.40, 3.13)]. No statistically significant difference between the two groups for SAE [RR = 1.15 (0.90, 1.47)] and deaths [RR = 1.99 (0.75, 5.27)] was found.
Conclusion
The adjunction of JAKi to MTX is not associated with an increased risk of malignancy when compared to MTX alone. There is no increased risk of SAE and deaths when compared to MTX alone in patients with RA.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13317-021-00153-5.
Keywords: Rheumatoid arthritis, Tofacitinib, Baricitinib, Upadacitinib, Filgotinib, Peficitinib, Decernotinib, Jak inhibitors, Methotrexate
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease, estimated to affect approximately 0.5% to 1% of population [1]. Patients with RA are predisposed to an increased risk for malignancy, especially malignant lymphomas [2–7], lung cancers [5, 6] and non-melanoma skin cancer [7]. Higher mortality was associated with the presence of cancer, varied by stage of malignancy [8]. Persistent inflammation triggers the development and progression of cancer [9, 10]. It has been well-established that severity of inflammation in RA is positively correlated with the risk of lymphoma [11]. Taking the inflammation into control may reduce the risk of developing malignancy.
Janus kinase (JAK) can initiate lymphocyte activation, function and proliferation via tyrosine phosphorylation of the receptors and downstream signal transducer and activator of transcription (STAT) signaling [12]. JAK-STAT signaling pathways mediate a double-edged sword effect on both antitumor defense and tumor progression [13]. Inhibiting JAK-STAT pathways raises the concern of losing immune cell function to malignancy, as well as proposes the possibility of suppressing tumor formation.
JAK can be divided into 4 types: JAK1, JAK2, JAK3 and Tyrosine kinase 2 (Tyk2), responsive to myriad cytokines. Various Janus kinase inhibitors (JAKi) are being widely investigated in randomized controlled trials (RCTs) and proved efficacy in patients with RA [14]. JAKi consist of tofacitinib, selectively inhibiting JAK1 and JAK3, baricitinib, blocking JAK1 and JAK2, peficitinib, acting on all types of JAK, decernotinib, highly selective for JAK3, upadacitinib and filgotinib, selectively targeting JAK1. Limited evidence demonstrated no statistical difference in malignancy incidences in patients receiving tofacitinib compared to the general population [15]. Since most patients with RA are treated with combinations of traditional disease-modifying antirheumatic drugs (DMARDs), especially methotrexate, it is important to explore the safety profile of these therapies. The role of different JAKi in the risk of malignancy remains undetermined. Even scarce data exists, to see the malignancy outcomes of JAKi when used in combination with methotrexate.
In consideration of the current unclear malignancy risk of JAKi and MTX combination, we sought to explore a potential association between JAKi and MTX combination and malignancies in patients with RA.
Methods
Literature search
A systematic search was performed in PubMed, Embase and Cochrane Library without language limitations from inception to July 30, 2020. Search terms included “rheumatoid arthritis”, “tofacitinib”, “baricitinib”, “upadacitinib”, “filgotinib”, “peficitinib”, “decernotinib”, “jak inhibitors”, “methotrexate”. References of the retrieved articles were searched to identify further relevant studies suitable for this meta-analysis. Search strategy is listed in Table 1.
Table 1.
Search strategies
| PubMed search strategies | |||
|---|---|---|---|
| Search number | Query | Filters | Results |
| 1 | (Rheumatoid arthritis) OR (rheumatoid arthritis[MeSH Terms]) | 149,891 | |
| 2 | Janus kinase inhibitors | 5361 | |
| 3 | Tofacitinib | 1435 | |
| 4 | Baricitinib | 362 | |
| 5 | Upadacitinib | 115 | |
| 6 | Peficitinib | 59 | |
| 7 | Decernotinib | 22 | |
| 8 | Filgotinib | 101 | |
| 9 | #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 | 6367 | |
| 10 | #1 AND #9 | 972 | |
| 11 | #1 AND #9 | Randomized controlled trial | 101 |
| Embase search strategies | ||
|---|---|---|
| No. | Query | Results |
| #1 | Rheumatoid arthritis' OR 'rheumatoid arthritis':ti,ab,kw | 233,332 |
| #2 | Janus kinase inhibitor' OR 'janus kinase inhibitor':ti,ab,kw | 3663 |
| #3 | Tofacitinib' OR 'tofacitinib':ti,ab,kw | 4688 |
| #4 | Baricitinib' OR 'baricitinib':ti,ab,kw | 1216 |
| #5 | Upadacitinib' OR 'upadacitinib':ti,ab,kw | 419 |
| #6 | Peficitinib' OR 'peficitinib':ti,ab,kw | 155 |
| #7 | Decernotinib' OR 'decernotinib':ti,ab,kw | 122 |
| #8 | Filgotinib' OR 'filgotinib':ti,ab,kw | 403 |
| #9 | #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 | 8061 |
| #10 | #1 AND #9 | 3049 |
| #11 | #10 AND 'randomized controlled trial'/de | 400 |
| Cochrane search strategies | ||
|---|---|---|
| ID | Search hits | Search results |
| #1 | MeSH descriptor: [Arthritis, Rheumatoid] explode all trees | 6056 |
| #2 | MeSH descriptor: [Janus Kinase Inhibitors] explode all trees | 43 |
| #3 | (filgotinib): ti,ab,kw (word variations have been searched) | 132 |
| #4 | (tofacitinib):ti,ab,kw (word variations have been searched) | 699 |
| #5 | (baricitinib):ti,ab,kw (word variations have been searched) | 354 |
| #6 | (decernotinib):ti,ab,kw (word variations have been searched) | 8 |
| #7 | (upadacitinib):ti,ab,kw (word variations have been searched) | 196 |
| #8 | (peficitinib):ti,ab,kw (Word variations have been searched) | 23 |
| #9 | #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 | 1414 |
| #10 | #1 AND #9 | 161 |
Eligibility criteria
Two independent authors (VS and AM) screened all titles and abstracts for potential inclusion. Any discrepancy among the selected studies were resolved by a third author (RN). Double-blind RCTs that reported malignancy events in adult patients with RA receiving the combination of methotrexate with any JAKi, with methotrexate in the control arm. Studies that did not report malignancy outcomes were excluded. Exclusion criteria included reviews, editorials, letters, observational studies, non human studies. Long term extension studies with single arms and trials involving other biologic DMARDs were excluded as well.
Data extraction and quality assessment
Two independent investigators (VS and AM) performed the search and relevant studies were selected based on the inclusion and exclusion criteria. VS and AM extracted the data using a predefined data abstraction form: age, sex, duration of rheumatoid arthritis, mean number of tender and swollen joints, length of follow-up. Patient-years were calculated based on the extracted data. Any discordance between these two authors was resolved by the third author (RN). The Cochrane quality assessment tool for RCTs was used to assess risk of bias [16].
Outcomes of interest
The primary endpoints of interest were the incidence of malignancy, non melanomatous skin cancers (NMSC) and malignancies excluding NMSC. The secondary endpoints were incidence of serious adverse events (SAE) and deaths.
Statistical analysis
Extracted data were combined using Review Manager (RevMan) software (Cochrane collaboration) Version 5.4. Risk ratio (RR) of malignancies, NMSC, malignancies excluding NMSC, SAE, death was calculated with 95% confidence intervals (CIs) based on Mantel–Haenszel random-effect method. I2 was used to evaluate heterogeneity among the studies (< 25% considered low heterogeneity and > 50% considered significant heterogeneity). Publication bias was assessed via the funnel plot for the primary endpoint. Sensitivity analysis was performed by leave-one-out method.
Results were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocol (PRISMA-P) 2015 statement [17]. The protocol for this systematic review and meta-analysis was registered with PROSPERO (CRD42020201473).
Results
A total of thirteen RCTs comprising 6911 patients met the inclusion criteria [18–30], as summarised in Fig. 1, with 2377 patients in the MTX group and 4534 patients in the combination of MTX and JAKi group. Total patient-years in the MTX group and JAKi and MTX combination group were 988 and 3684, respectively. Among all the trials included, 3 trials used tofacitinib, 3 trials for baricitinib, 3 trials for upadacitinib, 2 trials for peficitinib and 1 trial for filgotinib in combination with MTX (Additional files 1, 2, 3, 4, 5, and 6).
Fig. 1.
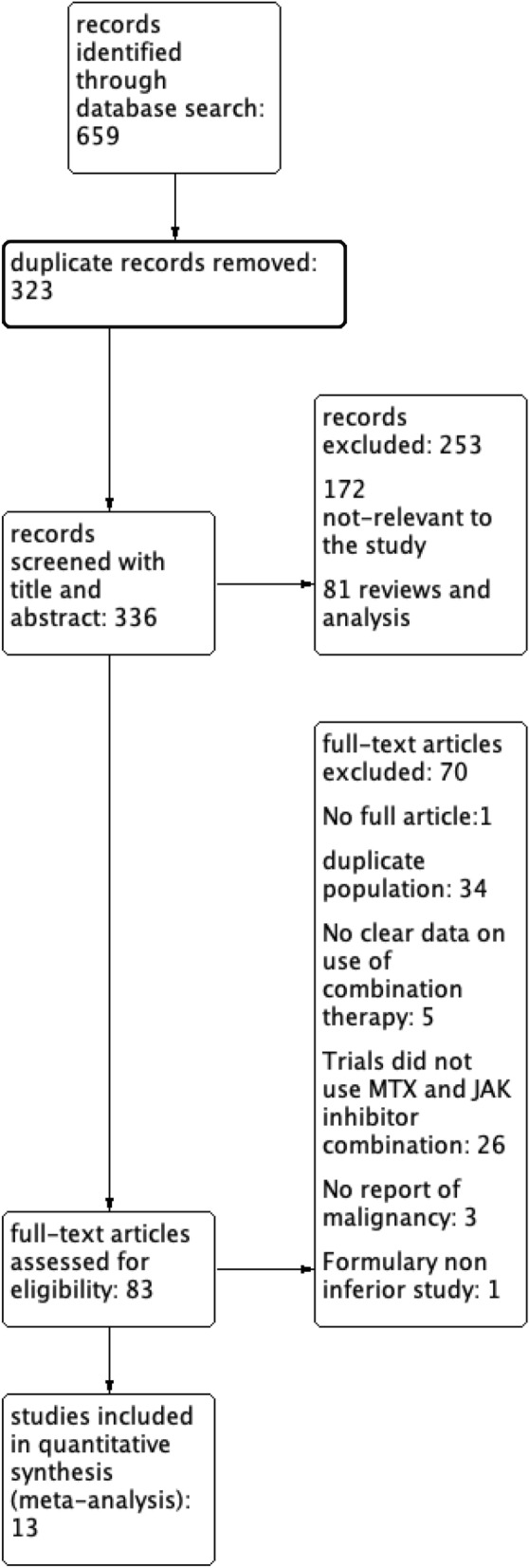
Flow chart of included randomized controlled trials
Study characteristics
The baseline characteristics of the patients with RA included in the studies were comparable among the two groups, as summarised in Table 2. The length of follow-up of the trials ranged from 12 weeks to 24 months. The mean age for the JAKi and MTX combination group was 53 ± 11.7 years and 53 ± 11.8 years in the MTX alone group. The average duration of RA was 7.6 years in the MTX alone group and 8.2 years in the JAKi and MTX combination group. The crude incidence rates of malignancies (NMSC/non NMSC) in JAKi and MTX combination group and MTX group were, respectively, 1.086 (0.497/0.651) and 0.709 (0.202/0.409) per 100 patient years.
Table 2.
Characteristics of included trials
| Name of study | Type of study | Study phase | Population | Duration of enrollment | Intervention | Number of patients | Follow up duration | Countries | Number of centers | Treatment arms | Primary outcome | Secondary outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Burmester [18] | Double-blind | Phase 3 | RA with IR to TNF inhibitors | October, 2009 to March 2011 | Tofacitinib and placebo | 399 | 6 months | 13 countries including North America, Latin America and Europe | 82 | Tofacitinib 5 mg, 10 mg BID vs. placebo along with MTX | ACR 20, HAQ-DI | DAS28-ESR |
| Fleischman [20] | Double-blind | Phase 3/long term | RA with IR to MTX | NA | Upadactinib, placebo, adalimumab | 1629 | 48 weeks | 41 | 286 | Upadactinib 15 mg, adalimumab 40 mg, placebo | DAS28-CRP, ACR20, inhibition of radiographic progression | DAS28-CRP mean change of DAS28-CRP, HAQ DI, SF-36, PCS, FACIT-F, CDAI < 10 |
| Fleischman [19], RA-BEGIN | Double-blind | Phase 3 | RA with no or minimal csDMARDs and naive to bDMARDs | 01/13–08/14 | Barictinib, MTX or combination of Barictinib and MTX | 588 | 52 weeks | 18 counries | 198 | Baricitnib 4 mg, Baricitinib 4 mg + MTX | Noninferioritiy of baricitnib monotherapy to MTX monotherapy by ACR20 at 24 weeks | Superiority comparision by ACR 20, HAQ-DI, SDAI, DAS28-CRP, vdH-mTSS |
| Genovese [21] | Double-blind | Phase 2b | RA with IR to MTX | 03/14–07/15 | Upadacitinib, placebo | 300 | 12 weeks | 16 | 63 | Upadactinib 3 mg bid, 6 mg bid, 12 mg bid, 18 mg bid, 24 mg q day, placebo bid | ACR20 | ACR50, ACR70, DAS28-CRP, CDAI |
|
Van der Hejide [25] ORAL SCAN |
Double-blind | Phase 3 | Active RA with IR to MTX | NA | Tofacitinib vs. placebo | 797 | 24 months | NA | NA | Tofacitinib 5 mg, 10 mg BID vs. placebo | ACR20, ACR50, ACR70, mean changes in DAS28-ESR, CDAI, SDAI, HAQ-DI | NA |
| Kivitz [22] | Double blind | Phase 2b | Modearte to severe RA with IR to MTX | NA | Peficitinib | 378 | 12 weeks | (8) Usa, poland, hungary, Czech republic, Mexico, Bulgaria, Belgium, Colombia | 43 | Peficitinib 25, 50, 100, 150 mg | ACR20 using CRP at 12 weeks | ACR50, ACR70, DAS28-CRP, CDAI |
| Kremer [23] | Double blind | Phase 2b | RA with IR to MTX | 10/13–07/15 | Upadactinib, placebo, adalimumab | 276 | 12 weeks | 10 (North america, Europe, Australia) | 123 | Upadacitnib 3 mg, 6 mg, 12 mg, 18 mg | ACR20 | ACR50, ACR70, Low disease points/remission by DAS28-CRP, CDAI, Change in DAS28-CRP, ACR core set changes, MCID of HAQ DI |
| Li [24] | Double blind | Phase 3 | RA with IR to MTX | NA | Baricitinib, placebo | 290 | 52 weeks | 3 (China, Brazil, Argentina) | 30 | Baricitinib 4 mg | ACR 20 at 12 weeks | HAQ-DI, DAS28-CRP, remission and LDA, SDAI, CDAI, ACR50, ACR70 |
| Takeuchi [26] RAJ4 | Double blind | Phase 3 | RA pt with IR to MTX | July 2014-Nov 2017 | Peficitinib | 519 | 52 weeks | Japan | 161 | Peficitinib 100 mg, 150 mg | ACR20 at 12 weeks/ET, baseline change in mTSS at 28 weeks/ET | ACR20/50/70 response, DAS28-CRP, DAS28-ESR, CRP, ESR, PGA, TJC68, SJC66, CDAI, SDAI |
| Tanaka [27] | Double-blind | Phase 2b | pt with moderate to severe RA on MTX | 11/11–12/13 | Baricitinib, placebo | 145 | 12 weeks | Japan | 24 | Baricitinib 1 mg, 2 mg, 4 mg, 8 mg | ACR20 at 12 weeks/ET, baseline change in mTSS at 28 weeks/ET | ACR50, ACR70, ACR core components, DAS28-ESR, DAS28-CRP, SDAI, EULAR28 |
| Taylor [28] | Double-blind | Phase 3 | pt with RA on MTX | 11/12–09/14 | Baricitinib 4 mg, adalimumab 40q2wk | 1307 | 52 weeks | 26 | 281 | Baricitinib 4 mg, adalimumab 40q2wk | ACR20 at 12 weeks, | mTSS score at 24 weeks, HAQDI, DAS28-CRP, SDAI, PRO at week 12 |
|
Vollenhoven [29] ORAL standard study |
Double blind | Phase 3 | RA pt with IR to MTX | 01/09–02/11 | Tofacitinib, adalimumab, placebo | 717 | 12 months | Worldwide | 115 | Tofacitinib 5 mg, 10 mg twice daily, 40 mg of adalimumab q2wks, placeebo | ACR20 reduction in tender and swollen joints at 6 months, 3/5ACR components, HAQ-DI at 3 months, DAS28-ESR | ACR20, ACR50, ACR70 with respect to tender and swollen joints and HAQ-DI |
| Westhovens [30] DARWIN 1 | Double blind | Phase 2b | Active RA with insufficient response to MTX | July 2013 to May 2015 | Filgotinib vs. placebo in combination with MTX | 594 | 24 weeks | 21 (North and South America, Europe, Asia, Australia) | 106 | Filgotinib 50 mg, 100 mg, 200 mg once or twice daily vs. placebo | ACR20 at 12 weeks | ACR20, ACR50, ACR70, ACR-N, DAS28-CRP, LDA/remission, EULAR response, EULAR remission, CADI, SDAI |
ACR American college of rheumatology, CRP C-reactive protein, CDAI clinical disease activity index, DAS28 disease activity score 28, ESR erythrocyte sedimentation rate, EULAR European League Against Rheumatism, FACIT-F functional assessment of chronic illness therapy fatigue scale, HAQ-DI health assessment questionnaire disability index, IR inadequate response, LDA low disease activity, MCID minimum clinically important difference, MTX methotrexate, NA not available, PCS physical component score, PGA physician’s global assessment of disease activity, PRO patient reported outcomes, RA rheumatoid Arthritis, SF-36 short-form 36, SDAI simplified disease activity index, SJC swollen joint count, TNF-alpha tumor necrosis factor-alpha, TJC total joint count, vdH-mTSS van der Heijde-modified total sharp score
Primary outcomes
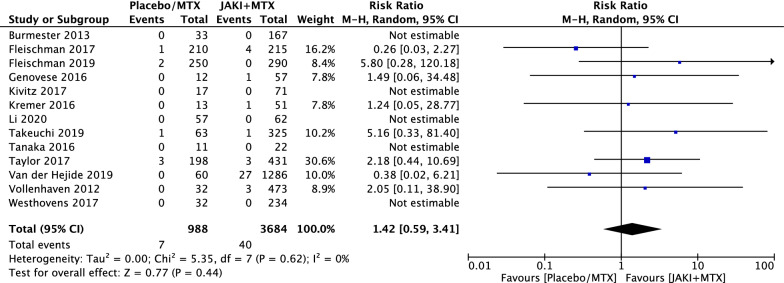
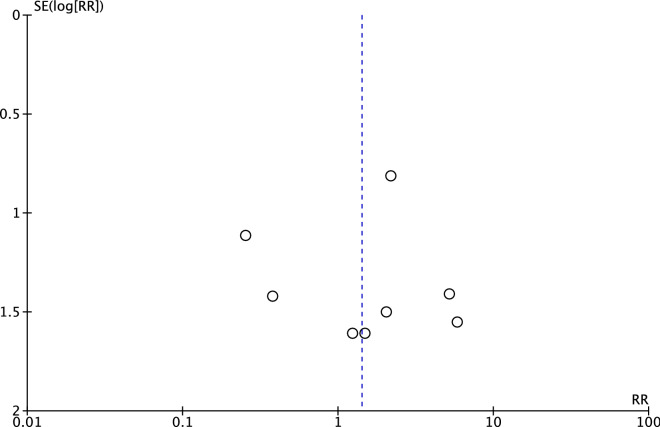
A total of forty patients suffering from malignancies were reported in the JAKi and MTX group among 3684 patient-years and seven malignancy events in the MTX group among 988 patient-years. There was no statistically significant difference in malignancy events (RR = 1.42; 95% CI 0.59 to 3.41, p = 0.44) between the combination group and the control group, seen in Fig. 2. We found a relatively low level of heterogeneity across all included RCTs (χ2 = 5.35, df = 7, p = 0.62, I2 = 0%). For the Mantel–Haenszel random methods, funnel plot showed no evidence of publication bias in all comparisons in Fig. 3.
Fig. 2.
Relative risks (RRs) of all malignancies in patients with rheumatoid arthritis (RA) treated with Janus kinase inhibitors (JAKi) and methotrexate (MTX) compared with MTX alone in randomised controlled trials (RCTs) using the Mantel–Haenszel (M–H) random-effect method
Fig. 3.
Funnel plots for the meta-analysis of occurrence of all malignancies among JAKi and MTX versus MTX
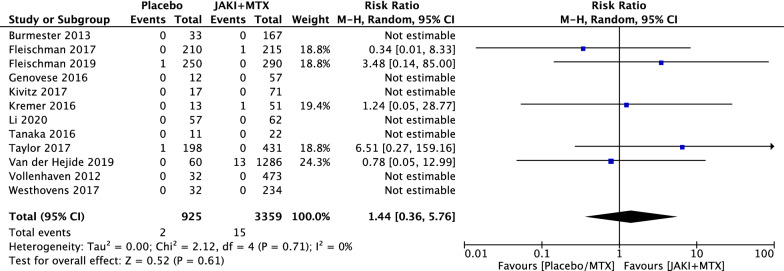
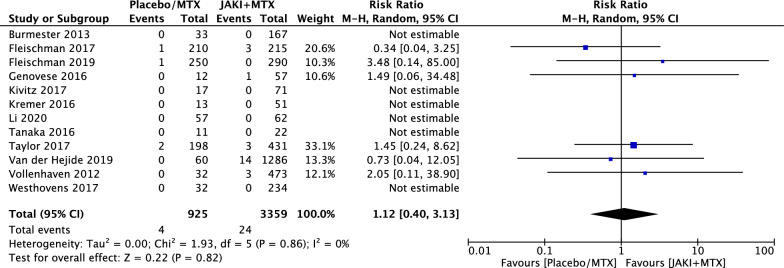
Considered separately, statistical differences remained undetectable for non melanomatous skin cancers (NMSC) (RR = 1.44; 95% CI 0.36 to 5.76, p = 0.61) (Fig. 4) and malignancies excluding NMSC (RR = 1.12; 95% CI 0.40 to 3.13, p = 0.82) (Fig. 5). Among 40 malignancy events in the JAKi and MTX combination group, 15 (37.5%) were NMSC and 24 (60%) were malignancies excluding NMSC. Among 7 malignancy events reported in MTX along group, 2 (28.5%) were NMSC and 4 (57%) were malignancies excluding NMSC. One malignancy in each group was not reported in detail. Among solid tumors for the MTX and JAKi combination group, the most common types were cervical cancer in 6 patients (25%), lung cancer in 5 patients (21%), breast cancer in 4 patients (17%) and ovarian cancer in 1 patient. Two cases of non-Hodgkin’s lymphoma and two cases of melanoma were reported.
Fig. 4.
RRs of non melanomatous skin cancer (NMSC) in patients with RA treated with JAKi and MTX compared to MTX alone in RCTs using the M–H random-effect method
Fig. 5.
RRs of malignancies excluding NMSC in patients with RA treated with JAKi and MTX compared to MTX alone in RCTs using the M–H random-effect method
Secondary outcomes
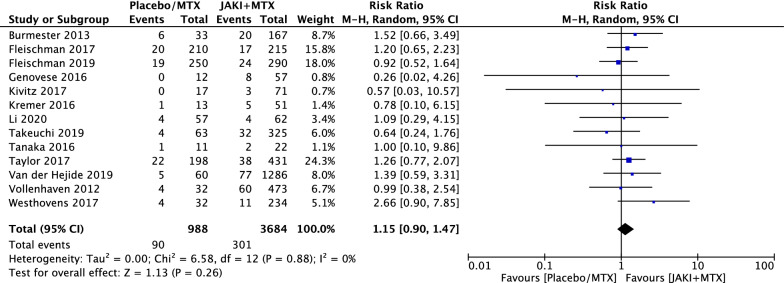
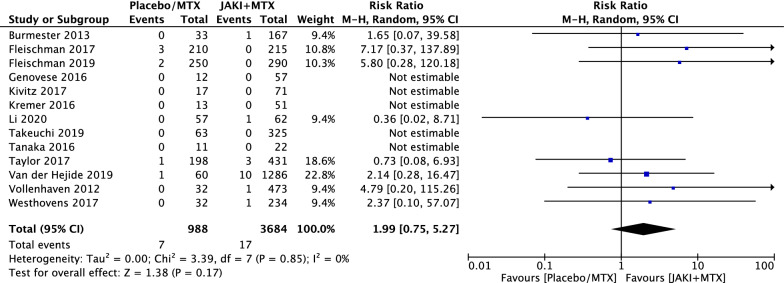
Although more serious adverse events and deaths were reported in the combination therapy group, there was no statistically significant difference between the two groups with RR = 1.15 (95% CI 0.90 to 1.47, p = 0.26) in SAE (Fig. 6) and RR = 1.99 (95% CI 0.75 to 5.27, p = 0.17) in deaths (Fig. 7).
Fig. 6.
RRs of safety adverse events (SAE) in patients with RA treated with JAKi and MTX compared to MTX alone in RCTs using the M–H random-effect method
Fig. 7.
RRs of deaths in patients with RA treated with JAKi and MTX compared to MTX alone in RCTs using the M–H random-effect method
Sensitivity analysis
The sensitivity analysis was performed for malignancy outcomes with leave one out method and did not show evidence of bias, as summarised in Table 3.
Table 3.
Sensitivity analysis by leave-one-out method
| Study excluded | Malignancy | NMSC | Malignancy excluding NMSC | SAE | Death |
|---|---|---|---|---|---|
| Burmester [18] | 1.42 (0.59, 3.41) | 1.44 (0.36, 5.76) | 1.12 (0.40, 3.13) | 1.12 (0.87, 1.45) | 2.03 (0.73, 5.64) |
| Fleischman [19] | 1.97 (0.75, 5.16) | 2.01 (0.43, 9.35) | 1.53 (0.48, 4.83) | 1.14 (0.87, 1.49) | 1.70 (0.61, 4.77) |
| Fleischman [20] | 1.24 (0.50, 3.12) | 1.18 (0.25, 5.47) | 0.99 (0.33, 2.91) | 1.21 (0.92, 1.59) | 1.76 (0.63, 4.92) |
| Genovese [21] | 1.41 (0.56, 3.53) | 1.44 (0.36, 5.76) | 1.09 (0.37, 3.21) | 1.16 (0.91, 1.49) | 1.99 (0.75, 5.27) |
| Van der Heijde [25] | 1.64 (0.65, 4.14) | 1.75 (0.36, 8.61) | 1.20 (0.40, 3.61) | 1.13 (0.88, 1.46) | 1.95 (0.64, 5.89) |
| Kivitz [22] | 1.42 (0.59, 3.41) | 1.44 (0.36, 5.76) | 1.12 (0.40, 3.13) | 1.16 (0.90, 1.48) | 1.99 (0.75, 5.27) |
| Kremer [23] | 1.43 (0.57, 3.58) | 1.49 (0.32, 6.99) | 1.12 (0.40, 3.13) | 1.16 (0.90, 1.48) | 1.99 (0.75, 5.27) |
| Li [24] | 1.42 (0.59, 3.41) | 1.44 (0.36, 5.76) | 1.12 (0.40, 3.13) | 1.15 (0.90, 1.48) | 2.37 (0.85, 6.59) |
| Takeuchi [26] | 1.22 (0.48, 3.09) | Not estimated | Not estimated | 1.19 (0.93, 1.54) | 1.99 (0.75, 5.27) |
| Tanaka [27] | 1.42 (0.59, 3.41) | 1.44 (0.36, 5.76) | 1.12 (0.40, 3.13) | 1.15 (0.90, 1.48) | 1.99 (0.75, 5.27) |
| Taylor [28] | 1.17 (0.41, 3.37) | 1.02 (0.22, 4.73) | 0.99 (0.28, 3.46) | 1.12 (0.84, 1.48) | 2.51 (0.85, 7.37) |
| Vollenhoven [29] | 1.37 (0.54, 3.43) | 1.44 (0.36, 5.76) | 1.03 (0.35, 3.08) | 1.16 (0.90, 1.50) | 1.82 (0.65, 5.05) |
| Westhovens [30] | 1.42 (0.59, 3.41) | 1.44 (0.36, 5.76) | 1.12 (0.40, 3.13) | 1.10 (0.86, 1.42) | 1.95 (0.70, 5.43) |
NMSC non melanomatous skin cancer, SAE serious adverse events
Risk of bias assessment
All RCTs (100%) adequately reported the generation of random sequence and 7 RCTs (53.8%) had adequate descriptions of concealed allocation. Blinding of participants, personnel, and outcome assessor was performed in all RCTs. Only 1 RCTs was unable to report the complete outcome. No selective reporting was discovered, seen in Fig. 8.
Fig. 8.

Risk of bias summary
Discussion
Patients with RA are predisposed to a higher risk for malignancy [2–7]. It remains not entirely clear if the increased risk of malignancy is primarily related to the pathogenesis of the disease or due to the immunosuppressive therapy. Currently, the combination of JAKi and MTX is widely used in patients with RA. However, whether biologic therapy increases the risk of malignancy has not been well addressed. According to our results, combination therapy of JAKi and MTX did not show any statistically significant increase in the number of malignancies as compared to MTX alone, neither in NMSC nor in malignancies excluding NMSC. Moreover, there are no statistical significant differences that were discovered in the incidence of SAE and mortality among these two groups. The majority of SAE are associated with infections including herpes zoster virus (HZV), urinary tract infection (UTI) and upper respiratory tract infections (URTI). To be noticed, the exposure time of patients with RA to JAKi and MTX is positively correlated to SAE as evidenced by the trials with longer follow up [19, 25, 26]. Among all the trials included, the mortality incidences were similar between patients under JAKi and MTX combination therapy and MTX monotherapy except ORAL SCAN trial [25], in which the combination group had a higher proportion of deaths compared to MTX alone. The deaths were predominantly contributed to multi-organ dysfunction, acute respiratory distress syndrome (ARDS) and major cardiovascular events (MACEs).
To our knowledge, this is the first systematic review exclusively to explore the safety profile, especially from the malignancy perspective, of JAKi and MTX combination therapy.
According to observational studies, patients with RA have an increased risk of malignancy [3, 4, 31]. This is well studied in non-Hodgkin’s lymphoma (NHL), with diffuse large B-cell lymphoma (DLBCL) as the most common type of NHL among patients with RA. These patients are also at increased risk of non-hematologic malignancy such as lungs, kidney, nasopharyngeal carcinoma [32]. The pathophysiology is associated with organ damage from chronic inflammation, genetic mutations, and autoimmune B lymphocyte activation [3, 32]. Viral infection, such as Epstein–Barr virus (EBV) infection may also play a role in the development of B cell lymphoma [33]. It was estimated that patients with RA have approximately 12-fold risk of developing lymphoma and twofold for lung cancer [34]. The risk of developing malignancy is closely related to disease activity [11]. Overtime, the stimulated immune cells from chronic inflammation may undergo malignant transformation, eventually leading to lymphoma. Interestingly, the chronic use of non-steroid anti-inflammatory drugs (NSAIDs) in these patients was associated with a decreased risk of developing colorectal cancer [34].
In terms of treatment, safety profiles, especially the effects on malignancy, should be carefully addressed. Previous studies have shown association between specific therapies and their corresponding risk of malignancy. The role of MTX on the incidence of malignancies remains controversial. Several population based studies did not show any increased risk of malignancies with MTX use compared to baseline risk among patients with RA [35, 36]. MTX may even decrease the risk of lymphoma by suppressing immunologic activation in RA activity but at the same time can increase the risk of other lymphomas [37]. The effects of JAKi on the risk of malignancies still remain unclear. Although genetic variation of JAK-STAT pathway is a known risk factor for malignancy [38], it is unclear whether the use of JAKi would lead to a decreased incidence of malignancies. US Corrona RA registry, a 5-year prospective observational study, did not detect any statistically significant difference in developing malignancy between tofacitinib and other biologic DMARDs [39]. There was no increased risk of malignancy for tofacitinib in patients with RA, when compared to conventional synthetic DMARDs or tumor necrosis factor-α inhibitors per a meta-analysis of observational studies [40]. With the widespread use of JAKi, not only tofacitinib, and MTX among patients with RA, there is accumulating data from multiple RCTs and a definite necessity to explore the safety profile of the combination therapy.
It is important that the effect of dose and exposure time of JAKi and MTX combination on malignancy should be interpreted with caution. Among all the trials included in our study, ORAL SCAN [25] study using tofacitinib, reported the largest number of malignancies. This trial is also distinguished for the longest follow up period of 24 months. However, a definitive conclusion should not be drawn from this single study and more data from long term extension studies is warranted to establish the association between exposure length and incidence of malignancy. Moreover, there is no significant difference in risk of malignancy between high dose and low dose JAKi and MTX.
The results of our secondary analysis are consistent with previous studies, while still with few differences [41]. A previously published meta-analysis also showed no significant increase in the number of malignancies with JAKi treatment but it did not specifically account for the effect of MTX [41]. Safety profile of JAKi and MTX combination is comparable to MTX alone as reported in previous studies. Incidence of SAE and deaths in our analysis were similar to prior published studies. It was reported that higher doses of JAKi were associated with increased SAE, but studies included in our analysis had comparable SAE across different dose ranges of JAKi without large differences [41]. Although not included in our analysis, cardiovascular mortality was also not significantly high in patients receiving JAKi when compared to placebo [42]. Overall JAKi has an acceptable safety profile and combination with MTX did not change it.
There are several limitations in our study and the results should be carefully interpreted. First, the patient-year exposure in the control group was much less compared to the combination group. The increased number of malignancies in the combination group, may be partly related to longer inflammation exposure in the setting of RA. Secondly, long term extension studies were excluded as they were mostly single arm studies without a control group, which could result in potential bias. Last, the majority of malignancies have a latency period and develop over the course of months to years and some trials included in our analysis had relatively limited follow up duration which may underestimate the actual malignancy rate. The strengths of our study is that it included large patient-years to detect any differences among the groups. There was no heterogeneity among the studies included in our analysis and the results of sensitivity analyses are consistent with overall results, proving the robustness of the study.
Conclusion
Our meta-analysis showed combination therapy of JAKi and MTX did not increase the malignancies in rheumatoid arthritis patients when compared to MTX alone. SAE and deaths are also not significantly different among the two groups. These results have been consistent among all the studies included in the analysis suggesting overall acceptable safety profile of JAKi and MTX combination.
Supplementary Information
Additional file 1: Figure S1. RRs of all malignancy in patients with RA treated with JAKi and MTX compared to MTX alone in RCTs using the M–H random-effect method, subgroup by JAKi and dose.
Additional file 2: Figure S2. RRs of all malignancy in patients with RA treated with Tofacitinib and MTX compared to MTX alone in RCTs using the M–H random-effect method.
Additional file 3: Figure S3. RRs of all malignancy in patients with RA treated with Baricitinib and MTX compared to MTX alone in RCTs using the M–H random-effect method.
Additional file 4: Figure S4. RRs of all malignancy in patients with RA treated with Updacitinib and MTX compared to MTX alone in RCTs using the M–H random-effect method.
Additional file 5: Figure S5. RRs of all malignancy in patients with RA treated with Filgotinib and MTX compared to MTX alone in RCTs using the M–H random-effect method.
Additional file 6: Figure S6. RRs of all malignancy in patients with RA treated with Peficitinib and MTX compared to MTX alone in RCTs using the M–H random-effect method.
Acknowledgements
Not applicable.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- CI
Confidence interval
- DLBCL
Diffuse large B-cell lymphoma
- DMARDs
Disease-modifying antirheumatic drugs
- EBV
Epstein–Barr virus
- HZV
Herpes zoster virus
- JAK
Janus kinase
- JAKi
Janus kinase inhibitors
- MACEs
Major cardiovascular events
- MTX
Methotrexate
- NHL
Non-Hodgkin’s lymphoma
- NMSC
Non melanomatous skin cancer
- NSAIDs
Non-steroid anti-inflammatory drugs
- PRISMA-P
Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocol
- RA
Rheumatoid arthritis
- RCTs
Randomized controlled trials
- RR
Risk ratio
- SAE
Serious adverse events
- STAT
Signal transducer and activator of transcription
- Tyk2
Tyrosine kinase 2
- URTI
Upper respiratory tract infection
- UTI
Urinary tract infection
Authors’ contributions
VS participated in the selection of title, key words, selection papers, data extraction, analysis, and writing. AM participated in the selection papers, data extraction, and writing. RP participated in the writing. RN participated in the searching of papers, and writing. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
All relevant data are within the paper.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004–2014. Rheumatol Int. 2017;37(9):1551–1557. doi: 10.1007/s00296-017-3726-1. [DOI] [PubMed] [Google Scholar]
- 2.Ekström K, Hjalgrim H, Brandt L, Baecklund E, Klareskog L, Ekbom A, et al. Risk of malignant lymphomas in patients with rheumatoid arthritis and in their first-degree relatives. Arthritis Rheum. 2003;48(4):963–970. doi: 10.1002/art.10939. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y-J, Chang Y-T, Wang C-B, Wu C-Y. The risk of cancer in patients with rheumatoid arthritis: a nationwide cohort study in Taiwan. Arthritis Rheum. 2011;63(2):352–358. doi: 10.1002/art.30134. [DOI] [PubMed] [Google Scholar]
- 4.Hemminki K, Li X, Sundquist K, Sundquist J. Cancer risk in hospitalized rheumatoid arthritis patients. Rheumatol Oxf Engl. 2008;47(5):698–701. doi: 10.1093/rheumatology/ken130. [DOI] [PubMed] [Google Scholar]
- 5.Parikh-Patel A, Allen M, Cress R, White RH. Risk of cancer among rheumatoid arthritis patients in California. Cancer Causes Control. 2009;20(6):1001–1010. doi: 10.1007/s10552-009-9298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim XR, Xiang W, Tan JWL, Koh LW, Lian TY, Leong KP, et al. Incidence and patterns of malignancies in a multi-ethnic cohort of rheumatoid arthritis patients. Int J Rheum Dis. 2019;22(9):1679–1685. doi: 10.1111/1756-185X.13655. [DOI] [PubMed] [Google Scholar]
- 7.Askling J, Fored C, Baecklund E, Brandt L, Backlin C, Ekbom A, et al. Haematopoietic malignancies in rheumatoid arthritis: lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann Rheum Dis. 2005;64(10):1414–1420. doi: 10.1136/ard.2004.033241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simard JF, Ekberg S, Johansson ALV, Askling J. What is the impact of chronic systemic inflammation such as rheumatoid arthritis on mortality following cancer? Ann Rheum Dis. 2016;75(5):862–866. doi: 10.1136/annrheumdis-2014-207155. [DOI] [PubMed] [Google Scholar]
- 9.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5(10):749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 10.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res MCR. 2006;4(4):221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 11.Baecklund E, Iliadou A, Askling J, Ekbom A, Backlin C, Granath F, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 2006;54(3):692–701. doi: 10.1002/art.21675. [DOI] [PubMed] [Google Scholar]
- 12.Darnell JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 13.Owen KL, Brockwell NK, Parker BS. JAK-STAT signaling: a double-edged sword of immune regulation and cancer progression. Cancers. 2019;11(12):2002. doi: 10.3390/cancers11122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F, Sun L, Wang S, Davis JM, Matteson EL, Murad MH, et al. Efficacy and safety of tofacitinib, baricitinib, and upadacitinib for rheumatoid arthritis: a systematic review and meta-analysis. Mayo Clin Proc. 2020;95(7):1404–1419. doi: 10.1016/j.mayocp.2020.01.039. [DOI] [PubMed] [Google Scholar]
- 15.Curtis JR, Lee EB, Kaplan IV, Kwok K, Geier J, Benda B, et al. Tofacitinib, an oral Janus kinase inhibitor: analysis of malignancies across the rheumatoid arthritis clinical development programme. Ann Rheum Dis. 2016;75(5):831–841. doi: 10.1136/annrheumdis-2014-205847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 18.Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet Lond Engl. 2013;381(9865):451–460. doi: 10.1016/S0140-6736(12)61424-X. [DOI] [PubMed] [Google Scholar]
- 19.Fleischmann R, Schiff M, van der Heijde D, Ramos-Remus C, Spindler A, Stanislav M, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol. 2017;69(3):506–517. doi: 10.1002/art.39953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleischmann R, Pangan AL, Song I-H, Mysler E, Bessette L, Peterfy C, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol. 2019;71(11):1788–1800. doi: 10.1002/art.41032. [DOI] [PubMed] [Google Scholar]
- 21.Genovese MC, Smolen JS, Weinblatt ME, Burmester GR, Meerwein S, Camp HS, et al. Efficacy and safety of ABT-494, a selective JAK-1 inhibitor, in a phase IIb study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol. 2016;68(12):2857–2866. doi: 10.1002/art.39808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kivitz AJ, Gutierrez-Ureña SR, Poiley J, Genovese MC, Kristy R, Shay K, et al. Peficitinib, a JAK inhibitor, in the treatment of moderate-to-severe rheumatoid arthritis in patients with an inadequate response to methotrexate. Arthritis Rheumatol. 2017;69(4):709–719. doi: 10.1002/art.39955. [DOI] [PubMed] [Google Scholar]
- 23.Kremer JM, Emery P, Camp HS, Friedman A, Wang L, Othman AA, et al. A phase IIb study of ABT-494, a selective JAK-1 inhibitor, in patients with rheumatoid arthritis and an inadequate response to anti-tumor necrosis factor therapy. Arthritis Rheumatol. 2016;68(12):2867–2877. doi: 10.1002/art.39801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, Hu J, Bao C, Li X, Li X, Xu J, et al. Baricitinib in patients with rheumatoid arthritis with inadequate response to methotrexate: results from a phase 3 study. Clin Exp Rheumatol. 2020;38(4):732–741. [PubMed] [Google Scholar]
- 25.van der Heijde D, Strand V, Tanaka Y, Keystone E, Kremer J, Zerbini CAF, et al. Tofacitinib in combination with methotrexate in patients with rheumatoid arthritis: clinical efficacy, radiographic, and safety outcomes from a twenty-four-month, phase III study. Arthritis Rheumatol. 2019;71(6):878–891. doi: 10.1002/art.40803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeuchi T, Tanaka Y, Tanaka S, Kawakami A, Iwasaki M, Katayama K, et al. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III randomised, double-blind, placebo-controlled trial (RAJ4) in Japan. Ann Rheum Dis. 2019;78(10):1305–1319. doi: 10.1002/central/CN-01981196/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka Y, Emoto K, Cai Z, Aoki T, Schlichting D, Rooney T, et al. Efficacy and safety of baricitinib in Japanese patients with active rheumatoid arthritis receiving background methotrexate therapy: a 12-week, double-blind, randomized placebo-controlled study. J Rheumatol. 2016;43(3):504–511. doi: 10.3899/jrheum.150613. [DOI] [PubMed] [Google Scholar]
- 28.Taylor PC, Keystone EC, van der Heijde D, Weinblatt ME, del Carmen ML, Reyes Gonzaga J, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376(7):652–662. doi: 10.1056/NEJMoa1608345. [DOI] [PubMed] [Google Scholar]
- 29.van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367(6):508–519. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 30.Westhovens R, Kavanaugh A, Jamoul C, Tasset C, Harrison P, Van Der Aa A. Effect of baseline serum CRP levels on clinical efficacy in rheumatoid arthritis patients treated with filgotinib: post-hoc analysis from two phase 2b studies. Ann Rheum Dis. 2017;76((Westhovens R.) Rheumatology, KU Leuven, University Hospitals, Leuven, Belgium):148. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L621422116.
- 31.Buchbinder R, Barber M, Heuzenroeder L, Wluka AE, Giles G, Hall S, et al. Incidence of melanoma and other malignancies among rheumatoid arthritis patients treated with methotrexate. Arthritis Rheum. 2008;59(6):794–799. doi: 10.1002/art.23716. [DOI] [PubMed] [Google Scholar]
- 32.Andersen CL, Lindegaard H, Vestergaard H, Siersma VD, Hasselbalch HC, de Fine ON, et al. Risk of lymphoma and solid cancer among patients with rheumatoid arthritis in a primary care setting. PLoS ONE. 2014;9(6):e99388. doi: 10.1371/journal.pone.0099388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56(9):2886–2895. doi: 10.1002/art.22864. [DOI] [PubMed] [Google Scholar]
- 34.Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015;17(1):212. doi: 10.1186/s13075-015-0728-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams CA, Bloch DA, Sibley J, Haga M, Wolfe F, Raynauld JP, et al. Lymphoma and luekemia in rheumatoid arthritis: are they associated with azathioprine, cyclophosphamide, or methotrexate? J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis. 1996;2(2):64–72. [PubMed] [Google Scholar]
- 36.Wolfe F, Michaud K. Lymphoma in rheumatoid arthritis: the effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum. 2004;50(6):1740–1751. doi: 10.1002/art.20311. [DOI] [PubMed] [Google Scholar]
- 37.Wilton KM, Matteson EL. Malignancy incidence, management, and prevention in patients with rheumatoid arthritis. Rheumatol Ther. 2017;4(2):333–347. doi: 10.1007/s40744-017-0064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hosseini A, Gharibi T, Marofi F, Javadian M, Babaloo Z, Baradaran B. Janus kinase inhibitors: a therapeutic strategy for cancer and autoimmune diseases. J Cell Physiol. 2020;235(9):5903–5924. doi: 10.1002/jcp.29593. [DOI] [PubMed] [Google Scholar]
- 39.Comparison of malignancy and mortality rates between tofacitinib and biologic DMARDs in clinical practice: five-year results from a US-based rheumatoid arthritis registry. ACR Meeting Abstracts. https://acrabstracts.org/abstract/comparison-of-malignancy-and-mortality-rates-between-tofacitinib-and-biologic-dmards-in-clinical-practice-five-year-results-from-a-us-based-rheumatoid-arthritis-registry/. Accessed 19 Mar 2021.
- 40.Xie W, Yang X, Huang H, Gao D, Ji L, Zhang Z. Risk of malignancy with non-TNFi biologic or tofacitinib therapy in rheumatoid arthritis: a meta-analysis of observational studies. Semin Arthritis Rheum. 2020;50(5):930–937. doi: 10.1016/j.semarthrit.2020.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Malignancies and serious infections in randomized controlled trials of janus kinase inhibitors in patients with rheumatoid arthritis: a systematic review and meta-analysis. ACR Meeting Abstracts. https://acrabstracts.org/abstract/malignancies-and-serious-infections-in-randomized-controlled-trials-of-janus-kinase-inhibitors-in-patients-with-rheumatoid-arthritis-a-systematic-review-and-meta-analysis/. Accessed 8 Jan 2021.
- 42.Xie W, Huang Y, Xiao S, Sun X, Fan Y, Zhang Z. Impact of janus kinase inhibitors on risk of cardiovascular events in patients with rheumatoid arthritis: systematic review and meta-analysis of randomised controlled trials. Ann Rheum Dis. 2019;78(8):1048–1054. doi: 10.1136/annrheumdis-2018-214846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. RRs of all malignancy in patients with RA treated with JAKi and MTX compared to MTX alone in RCTs using the M–H random-effect method, subgroup by JAKi and dose.
Additional file 2: Figure S2. RRs of all malignancy in patients with RA treated with Tofacitinib and MTX compared to MTX alone in RCTs using the M–H random-effect method.
Additional file 3: Figure S3. RRs of all malignancy in patients with RA treated with Baricitinib and MTX compared to MTX alone in RCTs using the M–H random-effect method.
Additional file 4: Figure S4. RRs of all malignancy in patients with RA treated with Updacitinib and MTX compared to MTX alone in RCTs using the M–H random-effect method.
Additional file 5: Figure S5. RRs of all malignancy in patients with RA treated with Filgotinib and MTX compared to MTX alone in RCTs using the M–H random-effect method.
Additional file 6: Figure S6. RRs of all malignancy in patients with RA treated with Peficitinib and MTX compared to MTX alone in RCTs using the M–H random-effect method.
Data Availability Statement
All relevant data are within the paper.