Abstract
Attention to urban agriculture (UA) has recently grown among practitioners, scientists, and the public, resulting in several initiatives worldwide. Despite the positive perception of modern UA and locally grown, fresh produce, the potential food safety risks connected to these practices may be underestimated, leading to regulatory gaps. Thus, there is a need for assessment tools to evaluate the food safety risks connected to specific UA initiatives, to assist practitioners in self-evaluation and control, and to provide policy makers and scholars a means to pursue and assess food safety in city regions, avoiding either a lack or an excess of regulation that could ultimately hinder the sector. To address this aim, this paper reviews the most recent and relevant literature on UA food safety assessments. Food safety indicators were identified first. Then, a food safety assessment framework for UA initiatives was developed. The framework uses business surveys and food analyses (if available) as a data source for calculating a food safety index for single UA businesses and the whole UA landscape of a given city region. The proposed framework was designed to allow its integration into the CRFS (City Region Food System) toolkit developed by FAO (Food and Agriculture Organization of the United Nations), RUAF foundation (Resource Centres on Urban Agriculture and Food Security) and Wilfrid Laurier University.
Keywords: Food safety indicators, CRFS framework Assessment, Risk assessment, Food policy
Highlights
-
•
Connection of several biological and chemical food safety risks to UA techniques.
-
•
Identifiable food safety risk factors for diverse UA practices.
-
•
Framework for the assessment of food safety levels of UA initiatives.
-
•
Development of a risk-based assessment that can be integrated into the FAO CRFS framework.
1. Urban agriculture: goals, benefits and perception
Urban agriculture (UA) is a strategic tool for food security in cities of the global south, as well as a powerful asset for urban sustainability and climate change adaptation in the global north (Orsini et al., 2020). In recent years, the importance of UA has largely increased, with the flourishing of many diverse initiatives and pilots, such as urban orchards, vertical farms, aquaponics or even more complex “green industry” plants (e.g., water treatment plants integrating hydroponics and heat generation). UA initiatives have great potential for positive social and economic impacts (Hallett et al., 2016; Horst et al., 2017; Kunpeuk et al., 2020) as well as resilient food provision processes or contributions that shorten the municipal food supply chain (European Commission, 2015) in both the global north and south (De Zeeuw et al., 2011; Hallett et al., 2016). Moreover, technologically advanced UA projects, provided that they are economically sustainable, can have a positive impact on employment, food carbon footprint reduction, efficient land management and food market diversification (Gómez et al., 2019; Rothwell et al., 2016; Sanyé-Mengual et al., 2019; Savvas & Gruda, 2018). Such initiatives integrate well with municipal networks of food producers, distributors, processors, and consumers. Therefore, UA may contribute to global food policy objectives (Local Governments for Sustainability [ICLEI], 2015; Food and Agriculture Organization of United Nations [FAO] & Resource Centres for Urban Agriculture and Food Security [RUAF], 2015), ultimately enabling sustainable and resilient urban or regional food supply chains, as recently defined within the framework for City Region Food System (CRFS) (FAO & RUAF, 2015; RUAF, 2015). The CRFS approach promotes sustainable and resilient food systems among urban centres, peri-urban areas and rural territories, forming a city region by strengthening their connections. Following this approach, the FAO and RUAF CRFS toolkit evaluates the status of a food system through specific indicators for different policy areas, helping decision-makers pursue desired goals (Blay-Palmer et al., 2018; FAO & RUAF, 2015). The strengthening of a CRFS through modern and intersectional UA production initiatives is recognized as an extremely valuable tool for policy challenges and the creation of social and economic value (Maye, 2019).
UA initiatives are diverse; more traditional initiatives, such as farmers' markets or community gardens, are usually well accepted by consumers and residents, especially with regard to locally produced food (Feldmann & Hamm, 2015). The same is not necessarily true for more technologically advanced systems, such as aquaponics, insect farming, algae and indoor vegetable production, for which there is a different degree of consumer acceptance (Specht et al., 2019). Undeniably, the literature reveals that consumers tend to overestimate the food safety level of locally grown food compared to large-scale retail trade produce, as they perceive locally grown food as more “genuine” (Khouryieh et al., 2019; Mohammad et al., 2019; Yu et al., 2017). However, shortening the distance from farm to fork may result in the avoidance of safety and/or quality controls and an oversimplification of safety-assuring procedures. Moreover, peri-urban agriculture and, especially, urban allotment gardens may suffer significantly from urban-related environmental pollution (Säumel et al., 2012). On the other hand, modern and innovative cultivation techniques (such as plant factories with artificial lighting [PFALs], recirculating systems, soilless systems, or vertical farms in general) are not necessarily considered appealing to consumers. This could be a consequence of a lack of awareness (Ercilla-Montserrat et al., 2019) or of the common narrative for which traditionally grown food is better and more “natural”. However, while advanced UA production systems are generally well accepted (Jürkenbeck et al., 2019; Miličić et al., 2017; Pollard et al., 2017; Sanyé-Mengual et al., 2018; Specht et al., 2016), studies on consumers' safety perception of their produce are scarce and locally based (Kaiser et al., 2015). Apart from their perception, short food supply chains, farmers’ markets, directly sold produce, and modern cultivation techniques do not intrinsically guarantee high levels of food safety and have potential biological and chemical risks (Antisari et al., 2015; Riggio et al., 2019; Säumel et al., 2012).
1.1. Food safety regulations and CRFS assessment approaches
Within the European Union (EU), food and feed sanitization rules for all supply-chain operators (“from farm to fork”) were adopted in 2004 through Regulations (EC) 852/2004, 853/2004, and 854/2004 that came into force on January 1, 2006. A certain degree of flexibility was also considered for microenterprises and some food producers. These exceptions allowed small local businesses to stay in the market without being overwhelmed by unaffordable costs required to comply with sanitization regulations, provided some conditions were satisfied. For example, EU member states can grant derogations to some animal-based “traditional foods” recognized within the EU. Additionally, farm shops or micro-producers selling their own products directly to consumers in small quantities can be subject to simplified regulations, although more stringent member state regulations can still be in force. Nevertheless, the risk for avoiding food control still exists due to intrinsic traceability difficulties justified by small lots and an extremely large number of micro-food businesses, accounting for over two hundred thousand food and drinking micro-enterprises (European Executive Agency for Small- and Medium-sized Enterprises [EASME], 2019).
In many Western countries, locally and traditionally grown food from small businesses has a better reputation to consumers, despite being, in many cases, less regulated and less systematically controlled than larger scale retail and imported food (Herman et al., 2012; Pussemier et al., 2012). Short supply-chain food (including produce from UA initiatives) should not meet a lower food safety standard than traditional large-scale retail trade produce (for which regulations and controls are broadly in place). Adequate quality controls should also be adopted for short supply-chain food whenever they do not already exist. A simplified and adaptable company structure, in addition to low distribution costs, should allow the competitiveness of short supply-chain businesses rather than safety control avoidance.
The contribution of UA to local food system performance and to the improvement of urban resilience and sustainability can be effectively assessed through the CRFS methodological approach (Blay-Palmer et al., 2018). Nevertheless, as the increase in the strength of UA initiatives is being considered, a fundamental investment in food policy becomes crucial (FAO, 2019; von der Leyen, 2019), especially regarding food safety risk assessments of UA initiatives and local food businesses. Specifically, there is a concurrent need to a) ensure that efficient control protocols are in place in small traditional businesses, b) prevent possible risks in UA practices of different natures/sources, and c) improve the public perception of food safety in technological systems.
Currently, with the CRFS assessment proposed by the FAO and RUAF (Carey & Dubbeling, 2017), the global food safety level of the CRFS can be generally assessed by surveys and secondary data, as some general food safety indicators are proposed (e.g., indicators 50 to 55). Carey and Dubbeling (2017) suggest, whenever possible, gathering disaggregated data. In this paper, a more detailed approach is proposed for UA, consisting of a) production of a UA initiatives inventory and b) assessment of their individual food safety risk level through specific risk-based scores. Public administrators and policy makers could rely on a more detailed assessment of local UA food businesses and producers to help them become empowered. Moreover, businesses themselves could benefit from these assessments. In fact, businesses would be able to improve their own food control programs, link analyses with proper risk management, and provide tools for good manufacturing or agricultural practices (GMP and GAP) and training.
This study detects the main food safety risks connected to UA vegetable production practices through an extensive scientific literature investigation, with the following aims:
-
•
Identify the main food safety risks from the available literature;
-
•
Develop an analytical framework to evaluate the food safety of single UA initiatives; and
-
•
Propose an index that enables UA businesses to improve their own food safety control.
The proposed framework, even if aimed at evaluating single UA initiatives, was developed to be compatible with the CRFS assessment and monitoring approaches contained in the FAO/RUAF CRFS toolkit (Carey & Dubbeling, 2017; FAO, RUAF, & Wilfrid Laurier University 2018).
2. Literature research methodology
2.1. Background setting and protocol study definition
An initial literature overview of food safety and health risk assessments related to UA food production was performed in May 2020 to provide a basis to set up the search keywords for the subsequent systematic review. The following string was adopted when searching the PubMed, Scopus, and Web of Science databases:
(“Urban Agriculture” OR “CRFS” OR “City Region Food Systems”) AND “Food Safety”
No chronological constraints were set. The search produced a total of 73 results. The entries were hence screened by discarding textbook chapters (8), non-English texts (3), false positives completely unrelated to food safety (5), a duplicate entry (1) and a paper with no full text available (1).
The 55 resulting papers were diverse in scope and perspective, documenting UA initiatives from very different parts of the world (Figure SM1). Only 31 of them referred to specific food safety risks of UA initiatives (e.g., foodborne pathogens and potentially toxic elements), while the remaining 24 either addressed food safety in general without addressing any specific risks or did not present any risk at all (only 2 papers). Thirty-five papers considered the social, economic and sustainability impacts of UA, while only 19 were actual food safety assessments.
The geographical distribution of the 55 papers (Figure SM1A) confirmed that the issue of food safety connected to UA is not predominant in the world's global south, where possibly other issues associated with UA (e.g., its contribution to food security) prevail (Orsini et al., 2013). Thus, the risk of a geographic bias of the search results was deemed negligible.
Overall, this preliminary research suggested the following possible risks: a) difficulty in distinctively differentiating studies related to UA rather than to rural agriculture, mostly due to the generic definition of UA and b) a possible risk of a high number of false positives, in particular studies addressing food security and sustainability.
2.2. Systematic literature search protocol
A qualitative systematic literature review (SLR) assessing the main food safety issues connected to UA was performed from March to June 2020. General principles for a SLR were adopted (Gough et al., 2017), as well as most appliable PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) recommendations (Moher et al., 2009). Web of Science and Scopus database were chosen. The search was set with a total of 79 keywords related to UA techniques (e.g., hydroponics and vertical farming) and hazards (e.g., Salmonella, nitrates, and heavy metals). The query used for the literature search included all “context” and “assessment” keywords (x and y, respectively), as listed in Table 1, by the formula:
| (x1 OR x2 OR x3 OR … xn) AND (y1 OR y2 OR y3 OR … yn) | (1) |
Table 1.
List of X (context) and Y (assessment) keywords used in the literature search with the query (x1 OR x2 OR x3 OR … xn) AND (y1 OR y2 OR y3 OR … yn).
| X (context) keywords | Y (assessment) keywords | |
|---|---|---|
| City Region Food Systems | Food quality | Good agricultural practices |
| CRFS | Food safety | GAP |
| Hydroponics | Pesticides | Agricultural practices |
| Soilless system | Plant protection products | Polycyclic aromatic hydrocarbons |
| Roof garden | PPP | PAH |
| Aquaponics | Pesticide residues | Persistent organic pollutants |
| Food production | Heavy metals | POP |
| Rooftop greenhouse | Potentially toxic elements | Postharvest handling |
| Controlled environment | PTE | Processing practices |
| Indoor farming | Dioxins | Consumer handling |
| Urban farming | Dibenzofurans | Washing and sanitizing |
| Leafy Vegetables | Polychlorobiphenyls | Risk management |
| Fresh produce | PCB | Risk assessment |
| Hydroponic produce | Nitrates | Benefits in nutrition |
| Nutrient Film Technique | E. coli | Nutrient |
| NFT | E. coli O157:H7 | Additives |
| Recirculating nutrient solution | Salmonella | fortifica* OR biofortifica* |
| Recirculating aquaponic system | Listeria | Anti-nutrients |
| Irrigation water | Coliforms | Food chain |
| Urban soil | Foodborne illness | Food composition |
| Rural soil | Human health | Government food standards |
| zero km food | Community health | Bioactive non-nutrients |
| Urban horticulture | Health risk evaluation | Food contaminants |
| Urban agriculture | Quantitative microbial risk assessment | Shelf-life |
| Vertical Farming | QMRA | Nutraceuticals |
| Ultraviolet treatment | Microplastics | |
| Water disinfection treatment | Plastics | |
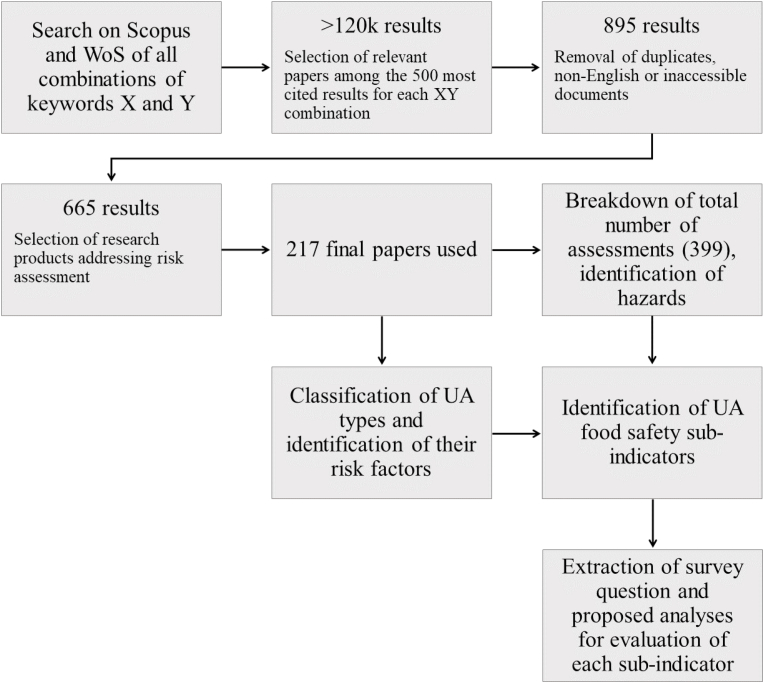
The search obtained more than a hundred thousand results, most of which were false positives. Hence, a further selection process was performed, as depicted in Fig. 1. Briefly, the first 500 results of each x:y combination were manually screened to keep the items relevant to UA and risk assessment only. Books and book chapters were discarded. The process resulted in 895 items selected. Furthermore, all duplicates were discarded, as well as unusable items (e.g., papers with unavailable full texts or non-English documents), resulting in 665 research products remaining. Ultimately, only actual UA risk assessment studies were considered suitable to the scope of the present SLR, and thus, the following were discarded: a) studies on non-food crops, b) field studies in contexts different from urban or peri-urban areas, and c) studies on animal husbandry, except for aquaculture and aquaponics. Papers addressed in the selected review papers were considered on a case-by-case basis. Eventually, 217 papers were selected and used for the qualitative analysis. The resulting entries spanned from 1962 to 2020, and approximately 60% of them were published from 2011 to 2020.
Fig. 1.
Scheme for the systematic literature search.
2.3. Data analysis for the development of a UA initiatives food safety assessment framework
Two key sets of information were extrapolated from the collected papers necessary for the development of the proposed framework. These were a) the identification of food safety hazards reported in UA practices and how they were assessed and b) the classification of UA types and identification of their inherent risk factors.
2.3.1. Identification of food safety hazards reported in UA practices
In this investigation, both regular and review articles were considered. The papers were singly considered to collect data about what hazards were identified and how the risk was assessed (specifically, what parameters were analysed). Eight hazard categories were identified based on the preliminary examination of title, abstract and keywords (: a) foodborne pathogens and microorganisms, b) potentially toxic elements (PTEs), c) pesticide residues, d) nitrate and nitrite contents, e) microfauna and pluricellular parasites, f) persistent organic pollutants (POPs), g) organic xenobiotics and pharmaceuticals, and h) hazardous materials. These hazard categories are described in detail in section 3.1.
Since most papers addressed more than one hazard, the 217 research papers were broken down into risk assessments, defined as the analytical determination (laboratory analysis) of one hazard on a specific matrix (e.g., growing medium, irrigation water, and food products). The papers contained a total of 399 risk assessments based on gathered data or direct analyses. As a brief example, Christou et al. (2016) investigated the quality and safety outputs of strawberries cultivated in ground-based greenhouses that received wastewater. Here, PTEs and pathogens were assessed on two matrices (irrigation water and fruit); thus, the paper was counted as four (4) assessments.
2.3.2. Classification of UA types and identification of their inherent risk factors
Six different UA classes were identified among the 217 selected papers: a) soil-based urban and peri-urban green, b) soilless UA systems, c) aquaponic plants, d) waste assimilating and experimental UA plants, e) processing and food industries, and f) local markets and retail. The first four (a to d) classes were UA production system types. Categories a, b and c were mutually exclusive. This simplified and generic classification was adopted to find similarities among different UA settings described in different papers. A more rigorous classification of UA production types is addressed in section 3.2.
The last two classes (e and f) were not UA production systems but destinations for UA production described or examined in the aforementioned papers. For example, Christou et al. (2016), previously described in section 2.3.1., accounted for the class categories “urban and peri-urban greens” and “waste assimilating and experimental UA plants”.
2.3.3. Identifying a framework for UA initiative food safety assessment: development of indicators, index of food safety for single initiatives, and global CRFS food safety index
Food safety risk indicators were developed to evaluate the food safety levels of single UA initiatives. The step-by-step procedure for their development was the following: a) indicators were defined as a measurement of risk, b) each indicator represented a defined risk factor connected to all possible UA production systems, c) each indicator had a different weight (or a different score range) reflecting the risk likelihood of the hazards represented, and d) each indicator fit a specific risk class (biological, chemical, and management).
The food safety index (FSI) of a specific UA initiative is calculated as the weighted average or algebraic sum of all its indicator scores. If an inventory of all UA initiatives within a CRFS is available, then a global FSI can also be calculated through a weighted average of the singular FSI (in this case, the weight is represented by the production volume of each initiative).
3. Results
3.1. Most common hazards connected to UA food production in scientific literature
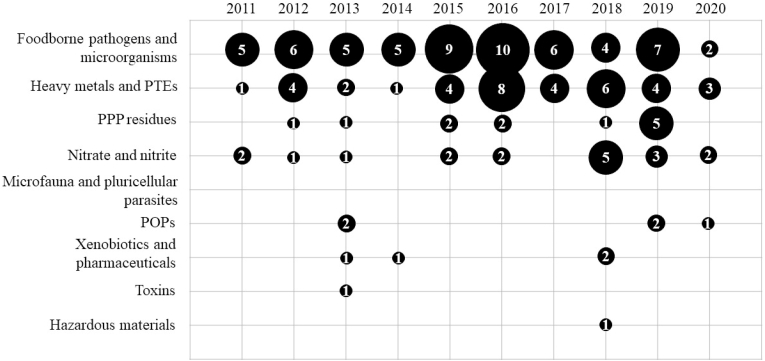
Overall, the risk assessments consisted of biological or chemical direct analyses of hazards within fresh produce from UA production systems, small local processing plants and/or local retailers. A synopsis of the number of assessments obtained per hazard category per period is shown in Table 2. For additional details, Fig. 2 displays the number of papers per hazard category per year (2011–2020).
Table 2.
Number of hazard assessments by category and period.
| Hazard category assessed | N° of assessments |
|||
|---|---|---|---|---|
| Period |
Total |
|||
| <2001 | 2001–2010 | 2011–2020 | ||
| Foodborne pathogens and microorganisms | 39 | 39 | 123 | 201 |
| PTEs and heavy metals | 13 | 20 | 51 | 84 |
| Pesticides residues | 5 | 9 | 13 | 27 |
| Nitrate and nitrite | 7 | 4 | 27 | 38 |
| Microfauna and pluricellular parasites | 4 | 13 | 0 | 17 |
| POPs | 5 | 3 | 9 | 17 |
| Xenobiotics (organic compounds) and pharmaceuticals | 1 | 1 | 10 | 12 |
| Toxins | 1 | 0 | 1 | 2 |
| Hazardous materials | 0 | 0 | 1 | 1 |
PTEs: potentially toxic elements; PPP: plant protection products; POPs: persistent organic pollutants.
Fig. 2.
Number of papers per hazard category per year (2011–2020).
3.1.1. Foodborne pathogens and microorganisms
As shown in Table 2, approximately 50% of the risk assessments contained in the selected papers addressed human pathogens. Such biological assessments were mostly from a) urban or peri-urban farms irrigated with greywater, b) hydroponic or aquaponic farms, and c) processing and sanitizing treatments of fresh produce. Human pathogens, such as Escherichia coli, Salmonella enterica, Listeria monocytogenes, Staphylococcus aureus, and Campylobacter serovars, are ubiquitous in the environment, commonly associated with faecal matter, and capable of causing foodborne diseases (Newell et al., 2010; Nyachuba, 2010). These microorganisms can be found in the growing media of plants (soil, solid substrates, nutrient solutions, etc.), especially when biological wastes are used as a nutrient source (Bernstein et al., 2008; Rababah & Ashbolt, 2000). Pathogens may come in contact with edible parts of crops and even be internalized in vegetable organisms (Golberg et al., 2011; Riggio et al., 2019). The most frequently assessed bacterial pathogens were the Shiga toxin-producing E. coli (STEC) (Deering et al., 2012; Koseki et al., 2011; Lopez-Galvez et al., 2014; Moriarty et al., 2019; Settanni et al., 2013), S. enterica (Castro-Ibáñez et al., 2015; Nousiainen et al., 2016; Phungamngoen & Rittisak, 2020), and L. monocytogenes (Sant’Ana et al., 2014; Y. J.; Wang et al., 2020). Irrigation water treatments (Dandie et al., 2019), postharvest sanitation, and GMP are considered crucial in reducing such biological risks (Olaimat & Holley, 2012; Trinetta et al., 2012).
Antimicrobial resistant strains of such pathogens were also of concern (Aarestrup et al., 2008), especially in environments where continuous exposure to antimicrobials could induce resistance in microbiota (Checcucci et al., 2020; García et al., 2020; Xi et al., 2015). Among protozoa, the addressed pathogens were mainly Cryptosporidium (Eregno et al., 2017) and Giardia (Srikanth & Naik, 2004).
Many research papers have addressed human viruses as well, mostly norovirus (Carducci et al., 2015; DiCaprio et al., 2012; Wang & Kniel, 2016). In fact, even though viruses are generally inactivated outside of a host, infecting doses can survive in the environment in multiple ways until reaching a new host (van Boxstael et al., 2013).
3.1.2. Potentially toxic elements
Approximately 49% of the reviewed papers and 21% of the total assessments addressed heavy metal and PTE traces in UA production (Table 2). Plants can accumulate hazardous doses of PTEs from contaminated growing media due to their capacity to uptake nutrients (e.g., cobalt, molybdenum, and copper) and translocate them into plant tissues. This scenario is also the case for elements relatively abundant in urban environments such as lead, cadmium, and zinc, as well as less abundant arsenic and mercury (Antisari et al., 2015; Joimel et al., 2020; Khan et al., 2008; Pennisi et al., 2016, 2017). In addition to the uptake from soil or growing substrates, plants grown in urban environments and exposed to open air may be contaminated by dry (particulate) and wet (smog) deposition of metal-containing particles that originate from traffic and heating pollution (Ercilla-Montserrat et al., 2018; Mu et al., 2017).
3.1.3. Pesticides residues
Less than 11% of the examined literature (7% of the assessments, Table 2) addressed residues of plant protection products (PPPs) or pesticides in UA. Most intensive horticulture areas or peri-urban farms have been studied (Polder et al., 2016; Zhang et al., 2013). Soilless cultures were also addressed (Savvas & Gruda, 2018) but were mainly used for modelling studies and did not reflect real case risks (Ni et al., 2018; Yang et al., 2019).
Active substances of PPP (mainly insecticides, herbicides, and fungicides) may persist on edible parts of plants for several days after treatment. In urban environments, pesticide treatments are also performed for non-agricultural purposes, such as mosquito or roadside herbicidal treatments (Md Meftaul et al., 2020). Normally, permitted treatments have precautionary limitations, such as a minimum latency period before harvest or a minimum distance from roadsides and residential buildings. Hence, the presence of pesticide residues in food would most commonly be caused by a) disregard for precautionary limitations, b) misuse of authorized active substances, and c) use of unauthorized substances. Unfortunately, detailed assessments on the use of pesticides in allotment gardens were missing, yet it can be assumed that risk is present (Atkinson et al., 1979; Voigt et al., 2015).
3.1.4. Nitrate and nitrite
Even if nitrate does not have high acute toxicity per se, it may be implicated in human methemoglobinemia disease (Hegesh & Shiloah, 1982; Langlois & Calabrese, 1992; Manassaram et al., 2010). Most importantly, nitrate metabolites and reaction products from human digestion (e.g., nitrite, N-nitroso compounds, and nitrosamines) have high toxicity levels and have been connected to gastrointestinal cancer, as stated by the European Food Safety Authority (EFSA) in 2017 (Mortensen et al., 2017). Nitrate and nitrite are naturally present in plants, where nitric nitrogen can be stored as a salt in cell vacuoles when it exceeds plant requirements. Nitrite is formed by the reduction of nitrate as a first step of nitrogen assimilation processes.
Some leafy vegetables have the tendency to accumulate high concentrations of nitrate in edible tissues (leaves especially). Approximately 13% of reviewed papers (9.5% of the assessments, Table 2) addressed nitrate excess in UA-produced vegetables as a food safety risk. Nevertheless, in most cases, the general purpose of the assessment was related to plant nutrition optimization rather than to proper safety risk determination. Vegetables such as spinach, kale, and chard (Bosnir et al., 2017) may contain up to hundreds or even thousands of mg of nitric nitrogen per kg of plant tissue. Rocket is one of the most nitrate-accumulating leafy vegetables, often containing several thousands of mg kg−1 tissue (Cavaiuolo & Ferrante, 2014). In traditional agriculture and non-protected environments, the seasonality of nitrate content in many vegetables is strong, as the content of nitrate in autumn harvests is significantly higher than that in other periods (Bosnir et al., 2017). In protected environments, the risk could be even higher, as nitrate levels frequently exceed plant requirements in nutrient solutions. This is especially the case for hydroponics (Guadagnin et al., 2005) and aquaponics (Pérez-Urrestarazu et al., 2019). As a result, leafy vegetables from any cultivation typology may contain nitrate concentrations higher than those recommended by the European Commission in 2011 (600–2000 mg kg−1 of product, EU Commission Regulation No 1258/2011). For the sake of clarity, it should be considered that nitrate intake includes multiple dietary and non-dietary sources other than vegetables, such as food additives, processed meat (Honikel, 2008) and drinking water (van den Brand et al., 2020).
3.1.5. Microfauna and pluricellular parasites
For the remaining sections, as the number of papers decreases, the difference between the % of total papers and the % of total assessments loses meaning, and only the second will be provided. As reported in Table 2, approximately 4% of the assessments addressed pluricellular parasites. Most of these studies focused on the effect of untreated wastewater utilization in extensive UA in the global south (Amoah et al., 2005) but not exclusively (Forslund et al., 2010). Ova of pluricellular worms such as Taenia, Fasciola and Ascaris are common in faeces, cattle sludge, and urban wastewater (Newell et al., 2010). Untreated water and compost can harbour active ova, and if used in UA, this water and compost may result in vegetable contamination, hence causing parasitosis to consumers, as is commonly reported (Al-Megrin, 2010; Matini et al., 2016).
3.1.6. Persistent organic pollutants
Approximately 4% of the assessments focused on POPs (Table 2). These are particularly hazardous and recalcitrant chemicals listed by the World Health Organization for special surveillance. Many POPs were originally used as pesticides prior to their prohibition, as was the case for aldrin, dieldrin, chlordane, DDT, etc. Other POPs are polychlorinated biphenyls (PCBs) and dioxin-like compounds such as polychlorinated dibenzodioxins or polychlorinated dibenzofurans (PCDDs and PCDFs, respectively), both by-products of waste combustion under certain conditions. While PCB use and production are currently banned, dioxin-like compounds are still generated from unauthorized waste combustion. The latter spread through contaminated particulate matter suspended in air and then are deposited on land. Heavily contaminated sites are generally under surveillance by local authorities. Few papers have addressed these contaminants in UA initiatives, mostly in contaminated urban and peri-urban sites or experimental set-ups (Tozzi et al., 2020; Urban et al., 2014; Wu et al., 2012).
3.1.7. Organic xenobiotics and pharmaceuticals
Approximately 3% of the assessments addressed other potentially hazardous organic xenobiotics and active principles (Table 2), which were not PPPs. The most notable examples were assessments of multiple xenobiotics in vegetables irrigated with reclaimed water (Dodgen et al., 2013; Margenat et al., 2018). Moreover, Mathews et al. (2014) assessed plant uptake of two antimicrobials under hydroponic conditions.
3.1.8. Toxins
The presence of toxins such as patulin, cyanotoxin, ochratoxin and aflatoxin is generally associated with their respective microbial contamination, as well as processing and/or storage mismanagement. Only 2 assessments (Table 2) addressed this risk in UA: Hao et al. (1999) assessed the presence of botulin in packaged vegetables, while Hariprasad et al. (2013) investigated the presence of aflatoxin in green leafy vegetables. Additionally, Lee and colleagues studied the internalization of cyanobacteria and the presence of cyanotoxin in lettuce through simulated scenarios of contaminated water irrigation (Lee et al., 2021).
3.1.9. Hazardous materials
Theoretically, certain materials may cause adverse health effects to consumers and operators when coming into contact with food or food production. This scenario occurs for hazardous materials such as asbestos, engineered nanoparticles and plastic micro or nanoparticles. No studies involving the presence or risk of asbestos in the context of UA were found. The only paper dealing with hazardous materials (a single assessment, as reported in Table 2) was from Ma et al. who reviewed the assimilation risks of nanoparticles, as well as the environmental effects on soil and plants in several studies (Ma et al., 2018). Asbestos and nanoparticle risks, however, are not inherently connected to UA practices, and their presence may be situational yet not negligible. Similarly, apart from the results of the present literature analysis, it may be necessary to mention another potential emerging risk to consider: the ubiquity of micro- and nanoplastics capable of contaminating the trophic chain in various ways, such as biomagnification, packaging contamination and internalization in crops (Rai et al., 2021; Senathirajah et al., 2021; Wang et al., 2019).
3.2. UA production systems and technologies as food safety risk factors
As reported by various authors (Eigenbrod & Gruda, 2015, Lovell, 2010, Mok et al., 2014), UA initiatives in developed countries are greatly varied and diverse in scope, structure, and production means. Neighbourhood and community gardens, allotment gardens, peri-urban farms, aquaponics, PFALs and experimental plants can all be included among UA initiatives (O'Sullivan et al., 2019). For this reason, a clear definition of UA typologies would be of great importance for UA initiative assessments. In the scope of this work, the use of different UA technologies involved different grades of food safety risk. According to the findings of our literature review, the most critical environmental aspects for the categorization of UA production systems were a) the use of natural soil rather than artificial media, b) exposure to pests, air pollution and/or atmospheric fallout, and c) the use of waste or by-products harbouring biological and/or chemical risks. The first two criteria were consistent with those proposed in Goldstein's taxonomy (Goldstein et al., 2016). Goldstein and co-authors proposed a UA classification based on four different types defined by two functional and technological criteria: a) integration with other buildings (ground-based vs building integrated) and 2) condition of the space (conditioned or nonconditioned). As stated before, soil-exposed UA farms may be seen as ground-based UA types, while open-air initiatives are the equivalent of non-conditioned types. Unfortunately, such specific cataloguing could not be systematically adopted in this study because of the heterogeneity and/or lack of information regarding the UA systems described in the selected papers. In addition, the use of wastes as growing media is not covered in Goldstein's taxonomy, according to which all four types have potential for liquid and solid waste assimilation. However, the use of risk-harbouring wastes is crucial for food safety assessments of UA. Since the authors consider Goldstein's taxonomy valuable, despite some limitations to the scope of the present work, in this paper the term “types” will refer to this taxonomy and used when possible; in addition, “productive system” or “system” will be used as a general term. In Table 3, the number of research papers per production system or context per hazard category and per time period are presented. In the following sections, the main food safety risks connected with specific aspects of UA production systems are presented.
Table 3.
Number of research papers per different hazard category and time period, categorized by five UA production contexts and local market assessments.
| Hazard category assessed | Period | Soil-based urban and peri-urban greens | Soilless UA systems | Aquaponic plants | Waste assimilating and experimental UA plants | Processing and food industry | Local market survey |
|---|---|---|---|---|---|---|---|
| Foodborne pathogens and microorganisms | <2001 | 4 | 1 | 0 | 5 | 18 | 3 |
| 2001–2010 | 5 | 7 | 0 | 5 | 3 | 0 | |
| 2011–2020 | 2 | 29 | 6 | 7 | 24 | 1 | |
| Heavy metals and PTEs | <2001 | 11 | 5 | 1 | 2 | 0 | 5 |
| 2001–2010 | 14 | 6 | 3 | 3 | 1 | 0 | |
| 2011–2020 | 23 | 9 | 2 | 7 | 2 | 0 | |
| PPP residues | <2001 | 3 | 2 | 0 | 0 | 0 | 0 |
| 2001–2010 | 2 | 3 | 0 | 0 | 1 | 0 | |
| 2011–2020 | 3 | 5 | 0 | 0 | 2 | 2 | |
| Nitrate and nitrite | <2001 | 1 | 3 | 0 | 0 | 1 | 1 |
| 2001–2010 | 1 | 3 | 0 | 1 | 0 | 0 | |
| 2011–2020 | 1 | 9 | 5 | 0 | 1 | 1 | |
| Microfauna and pluricellular parasites | <2001 | 1 | 0 | 0 | 1 | 1 | 0 |
| 2001–2010 | 3 | 1 | 0 | 4 | 0 | 0 | |
| 2011–2020 | 0 | 0 | 0 | 0 | 0 | 0 | |
| POPs | <2001 | 3 | 1 | 0 | 0 | 0 | 0 |
| 2001–2010 | 2 | 0 | 0 | 0 | 0 | 0 | |
| 2011–2020 | 2 | 2 | 0 | 1 | 0 | 0 | |
| Xenobiotics and pharmaceuticals | <2001 | 0 | 0 | 0 | 1 | 0 | 0 |
| 2001–2010 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2011–2020 | 2 | 1 | 0 | 1 | 0 | 0 | |
| Toxins | <2001 | 0 | 0 | 0 | 0 | 1 | 0 |
| 2001–2010 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2011–2020 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Hazardous materials | <2001 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2001–2010 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2011–2020 | 0 | 0 | 0 | 1 | 0 | 0 |
PTEs: Potentially toxic elements: PPP: plant protection products; PoPs: persistent organic pollutants.
3.2.1. Soil exposure: soil-based vs soilless systems
The most notable examples of soil-based UA systems or ground-based types (Goldstein et al., 2016) are allotment gardens, traditional peri-urban farms, community greens and urban orchards. They are generally found in city suburbs or peri-urban areas. Topsoil, in addition to possible irrigation techniques, is used to produce fresh vegetables and, less frequently, fruits. Small animal production (hens, rabbits, turkeys, honeybees, etc.) or fishing ponds may be present too. Soil-related pests are inevitable (such as moles, snails, nematodes and soil-borne plant pathogens), paving the way for possible utilization of PPPs (Zhang et al., 2013). Operators of these UA initiatives are gardening enthusiasts and amateurs (Teuber et al., 2019), workers enlisted through social programmes (Horst et al., 2017), and professional farmers. Many of these operators may not be specialized workers and have not received specific training, especially in allotments and community greens where the production is not meant for the market but for self-consumption.
Apart from their common traits, these UA initiative environments may be protected or unprotected (conditioned or nonconditioned ground-based types, according to Goldstein et al., 2016) and may receive solid and liquid waste (especially where freshwater is scarce). Their technological intensiveness may vary. For soil-based systems, the main risks are represented by the plant uptake of PTEs (Antisari et al., 2015; Bidar et al., 2020; Pennisi et al., 2016). As well documented in the literature, urban and peri-urban soil can be contaminated with several hazardous chemicals due to human activities (Yuan et al., 2021; Zheng et al., 2014). A full preliminary soil characterization would be advisable in soil-exposed UA, especially in areas where historical records of land use are not available from local authorities.
Soilless systems, on the other hand, are the norm for vertical farms and terrace gardens (which use inert substrates such as peat or perlite), hydroponics (which use a liquid nutrient solution), aeroponics (which use a vaporised nutrient solution) and PFALs in general. Most soilless UA systems are also enclosed in protected environments (conditioned types), but this is not always the case. As their technological level is generally higher with respect to soil-exposed systems, the control over production factors is higher, and their operators tend to be more specialized or better trained, resulting in reduced risks.
The chemical risks connected to the use of contaminated soil are negligible in soilless systems, provided that the artificial medium is controlled or comes from controlled sources (e.g., commercial peat). On the other hand, the risk for excessive nitrate uptake is higher in these systems with respect to uptake from soil-based ones, as reported in Table 3. In the papers reviewed, most nitrate assessments were conducted in soilless systems or aquaponics (Nozzi et al., 2018; Pérez-Urrestarazu et al., 2019).
3.2.2. Air exposure: unprotected vs protected environments
The addition of a physical shelter around crops allows for the modification of the growing environment, offering the advantage of enhancing crop productivity. Moreover, the presence of a physical barrier also has relevant implications for food safety. From the viewpoint of food risk assessment, there is a substantial difference between air-exposed, unprotected UA systems and protected environments (nonconditioned and conditioned types, respectively, according to Goldstein and colleagues).
Rooftop and terrace gardens, allotment gardens and open-air sky farms are a few examples of unprotected UA systems (Germer et al., 2011; Orsini et al., 2014; Orsini et al., 2020). These systems may be part of municipal beautification or revaluation projects, as they can also improve the appearance of a neighbourhood (similar to rooftop terraces and gardens). Vegetables exposed to urban air can be contaminated by hazardous compounds through precipitation, wet deposition (fog and smog) and dry deposition (particulate matter). PTEs such as lead, cadmium, copper, zinc, and mercury (Amato-Lourenço et al., 2016; von Hoffen & Säumel, 2014; Säumel et al., 2012) and even more hazardous POPs in particularly contaminated areas (Amodio et al., 2009; Polder et al., 2016) can end up in edible parts of vegetables. Moreover, pests such as insects, volatiles and rodents can more easily endanger crops, incentivizing the use of PPPs.
In contrast, protected environment systems (conditioned types) are typically confined inside buildings, greenhouses, or opaque structures (Gould & Caplow, 2012). They can have different degrees of environmental control, starting from ground-based greenhouses to more advanced soilless PFALs with automated climate, irrigation, and nutrient controls (Gómez et al., 2019; Orsini et al., 2020). Thus, these systems are insulated from the urban environment to a certain degree and rarely experience the aforementioned risks. Other risks, as well as risks due to growing control failures or mismanagement, can still be present if not properly addressed with GAP (Ferentinos & Albright, 2003).
3.2.3. Waste assimilation: aquaponics and other experimental systems
The use of by-products and wastes (especially human or animal excreta) has always played a crucial role in agriculture, and this is also the case for UA. In fact, UA has great potential for the in situ assimilation of solid and liquid wastes, transforming them into nutrient-rich substrates through the action of microbiota and plant enzymes (Kawamura-Aoyama et al., 2014). This scenario is clearly the case for water-scarce countries in the global south, in which the use of greywater for crop fertigation in extensive peri-urban farms is crucial for water management and savings (Abubakari et al., 2011; Asafu-Adjaye, 2012; Keraita et al., 2007a; Menyuka et al., 2020). Modern UA techniques combine food production with waste transformation, such as aquaponics (Enduta et al., 2011; Magwaza et al., 2020; Rufí-Salís et al., 2020; Yep & Zheng, 2019).
Aquaponics and similar waste-assimilating UA systems exploit the joint ability of plants and microbiota to remove or degrade nutrients from growing media (Su et al., 2020). In the case of aquaponics, wastewater from an aquaculture system is used as a nutrient solution for plants. After nutrient abatement and other additional treatments, specifically, in the case of recirculating aquaponic systems (RAS), the water is generally recirculated into fish tanks multiple times, closing the cycle.
Waste material from faecal matter can harbour helminths and human pathogens (bacteria, viruses, and protozoa), potentially causing foodborne diseases and adverse health effects (Bartelme et al., 2019; Jeong et al., 2016). Moreover, human or animal excrement can contain several persistent pharmaceuticals if excreted unaltered from target organisms (Herklotz et al., 2010; Kinney et al., 2006). Several antibiotics commonly used in human therapy or animal husbandry have been shown to select antimicrobial resistant strains of bacteria in the environment (Finley et al., 2013; Manaia, 2017; Martinez, 2009), resulting in dangerous sanitary risks for UA operators and consumers, especially in recirculating systems (Li et al., 2019). Depending on the source of the waste administered in a UA system, many other contaminants could be present, such as PTEs or POPs (Chaney et al., 1996; Khan et al., 2008; Tremlová et al., 2013). Finally, excess nutrients should also be considered, as many leafy vegetables have the tendency to accumulate high concentrations of nitrate in edible parts (Hu et al., 2015; Nozzi et al., 2018).
Apart from compliance with pertinent regulatory systems (Joly et al., 2015), UA production systems using potentially hazardous inputs (e.g., aquaculture effluents, wastewater treatment plant sludge, contaminated soil, and by-products from the food industry) should continuously determine the potential health risks of their produce through food safety analyses and the adoption of advanced quality control systems. Water and nutrient solution quality parameters should continuously be monitored (e.g., implementing sensors for water quality parameters). The presence of pathogens or hazardous chemicals (contaminants and/or pharmaceuticals) should frequently be assessed and possibly reduced, implementing adequate techniques (e.g., stabilization of immature compost, water ozonation or UV treatment). Final products should be analysed as well.
3.2.4. Good practices and management of risk in UA initiatives
Compliance with GMP and GAP, considering the specifics of UA production systems through preliminary risk assessments, should guarantee that fresh produce in UA meets adequate quality and safety levels up to harvest. Presumably, respect for good practices and quality management systems would also ensure higher food quality levels in subsequent processes (harvest, sanitation, packaging, distribution, etc.) as is the case for traditional agricultural products. Food safety management practices, such as hazard analysis and critical control points (HACCP) (Wallace, 2014, pp. 226–239) in addition to related management standards such as the UNI EN ISO 22000 family (International Organization for Standardization [ISO], 2018)), remain valid for UA initiatives and businesses.
3.3. Focus on food safety: integration with CRFS assessment indicators
The official indicator framework proposed by the FAO, RUAF and Wilfrid Laurier University for the CRFS assessment and evaluation is a powerful tool for policymakers (Blay-Palmer et al., 2018; Carey & Dubbeling, 2017). Its holistic approach is useful for a first assessment of CRFS functionality and its adherence to given multilevel policy goals (e.g., environmental, social or economic sustainability). Here, the different food system components (namely, production, processing, distribution, retail, and consumption) were evaluated through six different sustainability macro-targets, consisting of different sets of indicators. These indicators were calculated using data collected through research (typically surveys) or existing databases. The 210 indicators proposed by the FAO, RUAF and Wilfrid Laurier University are clearly meant for evaluating multiple aspects of a local food system as a whole and generally address aggregated data. Food safety is not an objective per se, but it is an impact area among the macro-policy target “Social sustainability and equity (improve of health and well-being)”. Thus, FAO/RUAF food safety indicators are limited, do not take into account single initiatives or businesses, and tend to have general character, such as Presence of food safety legislation […] or Number of food safety incidents […] (FAO, RUAF, and Wilfrid Laurier University 2018b, p 26).
Nevertheless, the official CRFS assessment would not be incompatible per se with more detailed investigations, such as business inventories or catalogues. A CRFS assessment could include a detailed assessment (relevant to research, policy or management) of the food safety performances of selected UA initiatives and businesses. Such detailed analysis or monitoring could be implemented based on the most pertinent risk assessment and rely on food analyses. Thus, in this section, an operational framework was proposed for the development of a safety assessment of UA initiatives compatible with the official CRFS framework and based on relevant research on hazards connected to UA.
Through the subsequent proposed framework, it is possible to evaluate the food safety risk of a specific UA initiative. Additionally, if an inventory of all UA initiatives is available and evaluations for all initiatives are performed, then this framework allows an estimation of the overall food safety level of the whole CRFS and, in addition to the ordinary CRFS assessment, paves the way for further analyses based on non-aggregated data.
3.3.1. Food safety indicators
In Table 4, fifteen detailed indicators for a food safety assessment of UA initiatives are proposed. These were divided into three risk groups: biological, chemical, and management risk. Consistent with the results on hazard frequency described in section 3.1., each indicator has a score range representing its weight. Biological risk indicators accounted for approximately 33% of the risk, while chemical risk and management risk indicators accounted for approximately 55% and 12% of the risk, respectively. These three values were obtained as a sum of score ranges from indicators of the same group divided by the total score range. The higher weight of chemical risk was simply due to the presence of many different hazards in the chemical risk category. Each indicator is calculated based on one or more questions posed to UA initiative directors and should be validated through data, documentation, analyses, and other possible valid information, as suggested in Table 4. The scoring of each indicator was designed to intuitively suggest being negative for high-risk circumstances. A benchmark indicator score value for specific classes of UA initiatives could possibly be established after adequate testing and data collection in further works.
Table 4.
Synoptic table of possible indicators for food business safety assessment.
| Risk area | Indicator (minimum and maximum score value) | Data source | Survey question examples (indicator score variation) | |
|---|---|---|---|---|
|
Biological risk Chemical risk |
1 | Implementation of biological control (−15; +35) | Business survey, periodic safety controls and analyses (pathogen analyses on final produce) | Are pathogens and other biological analyses performed on final products? Which one? (multiple selection, +3 per selection, up to +15, −5 if no options are selected) If so, how frequently? (from 0 to +10) How many non-compliances are found (by you or by external entities) per volume of production? (from +10 to −10) |
| 2 | [Unlikelihood of] food exposed to uncontrolled fauna (e.g., rodents, pigeons) and/or zoonosis (−5; +5) | Business survey, periodic safety controls (traps) | Is the presence of pests and/or undesired animals controlled? (+5 if affirmative) | |
| How frequently pests and/or undesired animals are found in the growing environment? (from 0 to −5) | ||||
| 3 | [Unlikelihood of] food exposed, even occasionally, to growing media harbouring biological risk (−5; +5) | Business survey, periodic safety controls and analyses (raw material and supplies quality control) | Is any waste used in the production of biological origin, even occasionally? (−5 if affirmative) | |
| Is this material biologically controlled from an external provider or within your company? (from 0 to +5) | ||||
| 4 | [Unlikelihood of] food biologically altered during processing (spoilage or poor sanitization/preservation) (−5; +5) | Client/customer survey, periodic quality control (shelf life) | How are the products sanitized after harvest? (from 0 to +5) | |
| Is the shelf life satisfactory? (from −5 to 0) | ||||
| 5 | [Unlikelihood of] food exposed to waste harbouring chemical risk (−10; +10) | Business survey, periodic safety controls and analyses (PTEs and other hazardous substances) | Is any kind of non-biological waste used in production? (−5 if affirmative) | |
| Is natural soil used in production? (−5 if affirmative) | ||||
| Is the substrate material used for growing (natural or artificial) fully characterised? (from 0 to +10) | ||||
| 6 | [Unlikelihood of] food exposed to particulate, smog and/or atmospheric deposition (−10; 0) | Business survey, environmental service (air quality) | Is the growing environment isolated from the external atmosphere? (−5 if negative) If that is not the case (negative answer to previous question), is quality of air satisfactory in your area, according to environmental services? (+5 if affirmative, 0 if negative, −5 if answer is “no data available”) If the answer from the previous question is “no data available”, how clean would you judge the air to be in your area? (from 0 to +5) |
|
| 7 | [Unlikelihood of] food exposed to PPP and other pharmaceuticals (−25; +10) | Business survey, periodic safety controls and analyses (PPP) | Are any PPP used during the production cycles? (−5 if affirmative) | |
| If so, which ones are regularly used? (−2 for each active principle indicated, up to −10) | ||||
| How frequently are PPP residues on final products controlled? (from 0 to +10) | ||||
| How many non-compliances are found (by you or by external entities) per volume of production? (from 0 to −10) | ||||
| 8 | [Unlikelihood of] food exposed to regulated chemicals such as additives, and disinfectants, fertilizers (−10; +5) | Business survey, periodic safety controls and analyses (GMP and GAP) | Which other chemicals are used in the production environment and how likely are they to be found in final products? (from −10 to +5) | |
| 9 | [Unlikelihood of] food accumulating (e.g., by phytoextraction or biomagnification) potentially hazardous elements or nutrients (such as PTEs or nitrate) (−10; +20) | Business survey, periodic safety controls and analyses (PTEs, nitrate) | Which chemical analyses on final products are periodically performed? (+2 per PTE, maximum +10; +5 for nitrate in addition) | |
| How frequently? (from +1 to +5) | ||||
| How many non-compliant actions are found (by you or by external entities) per volume of production? (from 0 to −10) | ||||
| 10 | [Unlikelihood of] food exposed to undesired or hazardous materials and substances such as micro-plastics and asbestos. (−10; +5) | Business survey, periodic safety controls and analyses (hazardous materials) | Are these compounds likely to be found in final products? (multiple selection, from −10 to +5) | |
| 11 | [Unlikelihood of] food contaminated by toxins (−5; +5) | Business survey, periodic safety controls and analyses (toxins) | Are toxins controlled in final products? How frequent are non-compliant actions per volume of production? (from +5 to −5, 0 if not controlled) | |
| Management risk | 12 | [Prevention of] food adulteration, tampering and accidents (−5; 0) | Business survey | To your knowledge, have any adulteration or tampering episodes occurred? How frequently? (from 0 to −3) |
| How frequently does equipment malfunction? (from 0 to −2) | ||||
| 13 | High food safety education level of business operators (0; +5) | Business survey (operators and conductor) | How would you judge the training of your collaborators and employees? (from 0 to +3) | |
| How much do you invest in training? (from 0 to +2) | ||||
| 14 | Implementation of food quality system, traceability, scheduled controls, and management of non-compliance (0; +15) | Business survey | Have you adopted any quality management program other than those prescribed by law? Which one? (from 0 to +5) | |
| Have you obtained any quality certifications? Which ones? (from 0 to +5) | ||||
| Have you adopted any quality policy program other than those prescribed by law? Can you describe it? (from 0 to +5) | ||||
| 15 | [Rareness of] safety control and non-compliance (0; +5) | Periodic safety control (if present) | Could you describe your course of action in this particular scenario? (a scenario is illustrated, in which non-compliance is found) (discursive, from 0 to +5) | |
3.3.2. Food safety index
A numerical score for each indicator is calculated following established guidelines such as those proposed in Table 4. Then, a safety index can be calculated as a sum of each indicator score by the following formula:
| (2) |
where:
-
-
i is a given UA initiative.
-
-
j is the indicator.
-
-
FSIi is the safety index of initiative i.
-
-
M is the number of indicators (15).
-
-
Ii,j is the score of indicator j for UA initiative i.
The FSI score, using the proposed indicators, spans from +130 to −115. The maximum value represents the highest food safety level or a very good risk management.
3.3.3. UA initiatives inventory and evaluation of CRFS global FSI for UA
In contrast to the CRFS framework approach, the proposed methodology aims to calculate (at least for all UA initiatives) a general food safety macro-indicator starting from a food safety evaluation and assessment of single initiatives (bottom-up approach). To implement this approach, it is necessary to build an inventory of UA food-producing initiatives in the CRFS, gathering data available in the territory. Some criteria needed for the inventory are suggested below.
The inventory should include any active initiative from allotment gardens to experimental vertical farms in a yearly timeframe. Thus, contiguous allotment gardens should be considered as a single UA initiative. For each initiative, data need to be gathered concerning a) identification, b) production types based on identifiable and well-recognized taxonomy systems for UA initiatives (Goldstein et al., 2016), c) production volumes (e.g., estimated gross production and value), d) destination of products (e.g., market segments or other destinations), and e) conductor, manager, or other representative of the initiative.
Since the food safety assessment for UA initiatives suggested in this review is meant for plant-producing initiatives, only these initiatives should be included. Animal urban farms, food processors, food distributors and hospitality/catering initiatives (HORECA) should be treated separately (optimally, with different dedicated indicators for their assessment). Ideally, each initiative in the inventory should be examined, but in case this is not feasible, a representative sample may be defined. In this study, a global FSI is calculated as a weighted mean of all the safety indexes (in this context, the weight factor would be represented by the estimated gross annual production of each initiative).
| (3) |
where:
-
-
GFSIua is the CRFS global food safety index for UA initiatives and businesses.
-
-
PT is the total production (or market) volume of all the considered UA initiatives within the CRFS.
-
-
i is a given UA initiative.
-
-
N is the number of all UA initiatives considered.
-
-
Pi is the production (or market) share of initiative i.
-
-
FSIi is calculated by formula (2).
Many different data analyses can be performed with this approach (e.g., the relationship between UA types and their FSI in a given CRFS, the identification of specific risks through the global study of a single indicator in a CRFS, and the relationship between economic performances of UA initiatives and their FSI in a given CRFS).
For example, data analysis might be performed through a scatter plot. Each initiative i could be plotted as:
| (4) |
where:
-
-
FSIi is calculated by formula (2).
-
-
i is a given UA initiative.
-
-
is the sample mean of the food safety indexes for the N initiatives.
-
-
Pi is the production (or market) share of initiative i.
-
-
is the sample mean of production or market shares for the N initiatives.
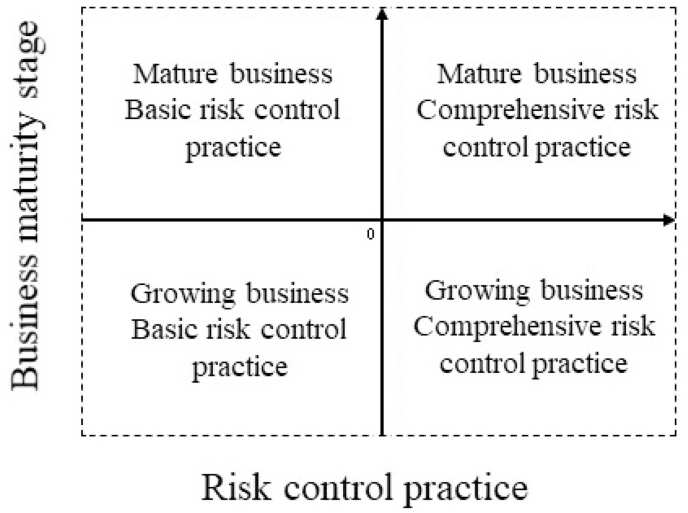
In this context, points (initiatives) in the upper-right quadrant would represent mature businesses with better-than-average risk control practices (as shown in Fig. 3). Bottom-right quadrant points would be initiatives with better-than-average risk with potential for market expansion. Upper-left quadrant points would be larger-than-average businesses with lower-than-average risk management. Finally, bottom-left points would represent smaller-than average initiatives with lower-than-average food safety indexes.
Fig. 3.
Reference scatter plot for analysis between FSI and business maturity.
4. Conclusions
In this work, the most relevant scientific papers addressing food safety assessments for UA techniques were reviewed to establish a framework for the evaluation of food safety in UA initiatives.
Among the 217 selected research papers, 120 addressed human pathogens either exclusively or not exclusively. These results indicated the use of excreta-derived nutrient sources (implicit, as in the case of aquaponics, or facultative, as in the case of greywater fertigation) or fresh produce sanitation failures as a source of risk. Approximately 94 papers addressed PTEs, indicating air pollution exposure or contaminated substrates as risk sources. Less frequent, but not of lesser importance, other hazards considered in UA food safety assessments were nitrate excess (28 papers, mostly in recirculating systems) and pesticide residues (23 papers). The hazards connected with the use of solid or liquid wastes were POPs (11 papers), organic xenobiotics and pharmaceuticals (5 papers) and hazardous materials (1 paper). Toxins were rarely assessed (2 papers).
Three main criteria determining the risk level of UA initiatives were identified: a) use of soil rather than inert substrate/media, b) exposition of crops to atmospheric deposition, and 3) use of solid or liquid wastes in the growing cycle.
Therefore, an operational framework for a qualitative-quantitative assessment of food safety levels in UA initiatives was developed. The proposed approach is based on the CRFS assessment indicator framework and is meant to contribute to or benefit from it. The methodology integrates the use of business surveys and food analyses (where available) to evaluate food safety risks through specific indicators. A food safety index can then be calculated for each initiative by averaging the indicator scores. Moreover, a global UA food safety index may be derived within the CRFS by weighing each UA initiative index according to their production share. Apart from data handling and simplicity of implementation, another advantage lies in the possibility of comparing risks among very different kinds of UA initiatives. Instead, some limits are due to the falsifiability of survey answers (which could be corrected by validating the answers with biological or chemical analyses); in addition, some limits occur because the definition of UA is not strict (sometimes even arbitrary), and thus. what is considered UA may vary in different CRFSs. This proposed framework supports decision-making and enables stakeholders from an initiative to the policy-making level to improve food safety control in a UA. This framework thereby contributes to protecting and improving the health and well-being of future consumers in a CRFS. A rigorous and homogeneous scoring system for each food safety indicator must still be implemented and validated in further and applied studies to fully utilize the potential of this framework, enabling it to benefit the public sector, policy makers and academia.
Acknowledgments
The research leading to this publication has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 862663. The publication reflects the authors' views. The Research Executive Agency (REA) is not liable for any use that may be made of the information contained therein. Giuseppe Calore, Giampaolo Nitti and Youssef Rouphael are gratefully acknowledged for their contribution to literature search.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.foodcont.2021.108085.
Author contribution statement
E. Buscaroli: conceptualization, investigation, data curation, writing – original draft, writing – review & editing, supervision.
I. Braschi: conceptualization, writing – review & editing, supervision, project administration.
C. Cirillo: investigation, writing – review & editing.
A. Fargue-Lelièvre: investigation, writing – review & editing.
G. C. Modarelli: investigation, data curation, writing – review & editing.
G. Pennisi: writing – review & editing, visualization.
I. Righini: investigation, writing – review & editing.
K. Specht: investigation, writing – review & editing.
F. Orsini: conceptualization, writing – review & editing, supervision, project administration, funding acquisition.
Declaration of competing interest statement
Authors declare no conflicts of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aarestrup F.M., Wegener H.C., Collignon P. Resistance in bacteria of the food chain: Epidemiology and control strategies. Expert Review of Anti-infective Therapy. 2008;6(5):733–750. doi: 10.1586/14787210.6.5.733. [DOI] [PubMed] [Google Scholar]
- Abubakari A.-H., Husseini R., Addi P.E. Strategies for minimising health risks of wastewater for poor farmers in the urban environment. Acta Horticulturae. 2011;911:123–132. doi: 10.17660/ActaHortic.2011.911.13. [DOI] [Google Scholar]
- Al-Megrin W.A.I. Prevalence of intestinal parasites in leafy vegetables in riyadh, Saudi Arabia. International Journal of Zoological Research. 2010;6(3):190–195. doi: 10.3923/ijzr.2010.190.195. [DOI] [Google Scholar]
- Amato-Lourenço L.F., Moreira T.C.L., De Oliveira Souza V.C., Barbosa F., Jr., Saiki M., Saldiva P.H.N., Mauad T. The influence of atmospheric particles on the elemental content of vegetables in urban gardens of Sao Paulo, Brazil. Environmental Pollution. 2016;216:125–134. doi: 10.1016/j.envpol.2016.05.036. [DOI] [PubMed] [Google Scholar]
- Amoah P., Drechsel P., Abaidoo R.C. Irrigated urban vegetable production in Ghana: Sources of pathogen contamination and health risk elimination. Irrigation and Drainage. 2005;54(S1):S49–S61. doi: 10.1002/ird.185. [DOI] [Google Scholar]
- Amodio M., Caselli M., de Gennaro G., Tutino M. Particulate PAHs in two urban areas of Southern Italy: Impact of the sources, meteorological and background conditions on air quality. Environmental Research. 2009;109(7):812–820. doi: 10.1016/j.envres.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Antisari L.V., Orsini F., Marchetti L., Vianello G., Gianquinto G.P. Heavy metal accumulation in vegetables grown in urban gardens. Agronomy for Sustainable Development. 2015;35(3):1139–1147. doi: 10.1007/s13593-015-0308-z. [DOI] [Google Scholar]
- Asafu-Adjaye P. The tendency to urban-farm in Accra: A cultural lag-labor surplus nexus. Journal of Third World Studies. 2012;29(2):159–182. https://www.scopus.com/inward/record.uri?eid=2-s2.0-84873589209&partnerID=40&md5=9ff5e4dd1d5d88f910f5890d2820c419 [Google Scholar]
- Atkinson H.J., Gibson N.H.E., Evans H. A study of common crop pests in allotment gardens around Leeds. Plant Pathology. 1979;28(4):169–177. doi: 10.1111/j.1365-3059.1979.tb01122.x. [DOI] [Google Scholar]
- Bartelme R.P., Smith M.C., Sepulveda-Villet O.J., Newton R.J. Component microenvironments and system biogeography structure microorganism distributions in recirculating aquaculture and aquaponic systems. mSphere. 2019;4(4) doi: 10.1128/mSphere.00143-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein N., Guetsky R., Friedman H., Bar-Tal A., Rot I. Monitoring bacterial populations in an agricultural greenhouse production system irrigated with reclaimed wastewater. The Journal of Horticultural Science & Biotechnology. 2008;83(6):821–827. doi: 10.1080/14620316.2008.11512467. [DOI] [Google Scholar]
- Bidar G., Pelfrêne A., Schwartz C., Waterlot C., Sahmer K., Marot F., Douay F. Urban kitchen gardens: Effect of the soil contamination and parameters on the trace element accumulation in vegetables – a review. The Science of the Total Environment. 2020;738:139569. doi: 10.1016/j.scitotenv.2020.139569. [DOI] [PubMed] [Google Scholar]
- Blay-Palmer A., Santini G., Dubbeling M., Renting H., Taguchi M., Giordano T. Validating the city region food system Approach: Enacting inclusive, transformational city region food systems. Sustainability. 2018;10(5):1680. doi: 10.3390/su10051680. [DOI] [Google Scholar]
- Bosnir D.B.J., Bevardi M., Boskovic A.G., Lasic S.M.D., Krivohlavek A., Racs A., Mojosovic-Cuic A., Trstenjak N.U. Nitrate in leafy green vegetables and estimated intake. African Journal of Traditional, Complementary and Alternative Medicines. 2017;14(3):31–41. doi: 10.21010/ajtcam.v14i3.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxstael S., Habib I., Jacxsens L., De Vocht M., Baert L., Van De Perre E., Rajkovic A., Lopez-Galvez F., Sampers I., Spanoghe P., De Meulenaer B., Uyttendaele M. Food safety issues in fresh produce: Bacterial pathogens, viruses and pesticide residues indicated as major concerns by stakeholders in the fresh produce chain. Food Control. 2013;32(1):190–197. doi: 10.1016/j.foodcont.2012.11.038. [DOI] [Google Scholar]
- van den Brand A.D., Beukers M., Niekerk M., van Donkersgoed G., van der Aa M., van de Ven B., Bulder A., van der Voet H., Sprong C.R. Assessment of the combined nitrate and nitrite exposure from food and drinking water: Application of uncertainty around the nitrate to nitrite conversion factor. Food Additives & Contaminants: Part A. 2020;37(4):568–582. doi: 10.1080/19440049.2019.1707294. [DOI] [PubMed] [Google Scholar]
- Carducci A., Caponi E., Ciurli A., Verani M. Possible internalization of an enterovirus in hydroponically grown lettuce. International Journal of Environmental Research and Public Health. 2015;12(7):8214–8227. doi: 10.3390/ijerph120708214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey J., Dubbeling M. City region food system indicator framework. RUAF. 2017 [Google Scholar]
- Castro-Ibáñez I., Gil M.I., Tudela J.A., Allende A. Microbial safety considerations of flooding in primary production of leafy greens: A case study. Food Research International. 2015;68:62–69. doi: 10.1016/j.foodres.2014.05.065. [DOI] [Google Scholar]
- Cavaiuolo M., Ferrante A. Nitrates and glucosinolates as strong determinants of the nutritional quality in rocket leafy salads. Nutrients. 2014;6(4):1519–1538. doi: 10.3390/nu6041519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney R.L., Ryan J.A., O'Connor G.A. Organic contaminants in municipal biosolids: Risk assessment, quantitative pathways analysis, and current research priorities. The Science of the Total Environment. 1996;185(1–3):187–216. doi: 10.1016/0048-9697(96)05051-6. [DOI] [Google Scholar]
- Checcucci A., Trevisi P., Luise D., Modesto M., Blasioli S., Braschi I., Mattarelli P. Exploring the animal waste resistome: The spread of antimicrobial resistance genes through the use of livestock manure. Frontiers in Microbiology. 2020;11 doi: 10.3389/fmicb.2020.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou A., Maratheftis G., Elia M., Hapeshi E., Michael C., Fatta-Kassinos D. Effects of wastewater applied with discrete irrigation techniques on strawberry plants' productivity and the safety, quality characteristics and antioxidant capacity of fruits. Agricultural Water Management. 2016;441(2):48–54. doi: 10.1016/j.agwat.2016.04.027. [DOI] [Google Scholar]
- Dandie C.E., Ogunniyi A.D., Ferro S., Hall B., Drigo B., Chow C.W.K., Venter H., Myers B., Deo P., Donner E., Lombi E. Disinfection options for irrigation water: Reducing the risk of fresh produce contamination with human pathogens. Critical Reviews in Environmental Science and Technology. 2019:1–31. doi: 10.1080/10643389.2019.1704172. 0(0) [DOI] [Google Scholar]
- De Zeeuw H., Van Veenhuizen R., Dubbeling M. The role of urban agriculture in building resilient cities in developing countries. Journal of Agricultural Science. 2011;149(S1):153–163. doi: 10.1017/S0021859610001279. [DOI] [Google Scholar]
- Deering A.J., Mauer L.J., Pruitt R.E. Internalization of E. coli O157:H7 and Salmonella spp. in plants: A review. Food Research International. 2012;45(2):567–575. doi: 10.1016/j.foodres.2011.06.058. [DOI] [Google Scholar]
- DiCaprio E., Ma Y., Purgianto A., Hughes J., Li J. Internalization and dissemination of human norovirus and animal caliciviruses in hydroponically grown romaine lettuce. Applied and Environmental Microbiology. 2012;78(17):6143–6152. doi: 10.1128/AEM.01081-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodgen L.K., Li J., Parker D., Gan J.J. Uptake and accumulation of four PPCP/EDCs in two leafy vegetables. Environmental Pollution. 2013;182:150–156. doi: 10.1016/j.envpol.2013.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EASME – Executive Agency for SMEs, European Commission . 2019. Annual report on European SMEs 2018/2019. [Google Scholar]
- Eigenbrod C., Gruda N. Urban vegetable for food security in cities. A review. Agronomy for Sustainable Development. 2015;(35):483–498. doi: 10.1007/s13593-014-0273-y. [DOI] [Google Scholar]
- Enduta A., Jusoh A., Ali N., Wan Nik W.B. Nutrient removal from aquaculture wastewater by vegetable production in aquaponics recirculation system. Desalination and Water Treatment. 2011;32(1–3):422–430. doi: 10.5004/dwt.2011.2761. [DOI] [Google Scholar]
- Ercilla-Montserrat M., Muñoz P., Montero J.I., Gabarrell X., Rieradevall J. A study on air quality and heavy metals content of urban food produced in a Mediterranean city (Barcelona) Journal of Cleaner Production. 2018;195:385–395. doi: 10.1016/j.jclepro.2018.05.183. [DOI] [Google Scholar]
- Ercilla-Montserrat M., Sanjuan-Delmás D., Sanyé-Mengual E., Calvet-Mir L., Banderas K., Rieradevall J., Gabarrell X. Analysis of the consumer's perception of urban food products from a soilless system in rooftop greenhouses: A case study from the mediterranean area of barcelona (Spain) Agriculture and Human Values. 2019;36(3):375–393. doi: 10.1007/s10460-019-09920-7. [DOI] [Google Scholar]
- Eregno F.E., Moges M.E., Heistad A. Treated greywater reuse for hydroponic lettuce production in a green wall system: Quantitative health risk assessment. Water. 2017;9(7) doi: 10.3390/w9070454. [DOI] [Google Scholar]
- European Commission, EIP-AGRI Focus Group . 2015. Innovative short food supply chain management final report 2015. November; p. 80. [Google Scholar]
- Feldmann C., Hamm U. Consumers' perceptions and preferences for local food: A review. Food Quality and Preference. 2015;40:152–164. doi: 10.1016/j.foodqual.2014.09.014. [DOI] [Google Scholar]
- Ferentinos K.P., Albright L.D. Fault detection and diagnosis in deep-trough hydroponics using intelligent computational tools. Biosystems Engineering. 2003;84(1):13–30. doi: 10.1016/S1537-5110(02)00232-5. [DOI] [Google Scholar]
- Finley R.L., Collignon P., Larsson D.G.J., McEwen S.A., Li X.-Z., Gaze W.H., Reid-Smith R., Timinouni M., Graham D.W., Topp E. The scourge of antibiotic resistance: The important role of the environment. Clinical Infectious Diseases. 2013;57(5):704–710. doi: 10.1093/cid/cit355. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations . FAO; 2019. FAO framework for the urban food agenda. [DOI] [Google Scholar]
- Food and Agriculture Organization of the United Nations, & Resource Centres for Urban Agriculture and Food Security A vision for City Region Food Systems: Building sustainable and resilient city regions. Urban Agriculture Magazine. 2015;29:3–4. [Google Scholar]
- Food and Agriculture Organization of the United Nations, Resource Centres for Urban Agriculture and Food Security, & Wilfrid Laurier University City region food system toolkit tool/example. 2018. http://www.fao.org/3/i9255en/I9255EN.pdf
- Forslund A., Ensink J.H.J., Battilani A., Kljujev I., Gola S., Raicevic V., Jovanovic Z., Stikic R., Sandei L., Fletcher T., Dalsgaard A. Faecal contamination and hygiene aspect associated with the use of treated wastewater and canal water for irrigation of potatoes (Solanum tuberosum) Agricultural Water Management. 2010;98(3):440–450. doi: 10.1016/j.agwat.2010.10.007. [DOI] [Google Scholar]
- García J., García-Galán M.J., Day J.W., Boopathy R., White J.R., Wallace S., Hunter R.G. A review of emerging organic contaminants (EOCs), antibiotic resistant bacteria (ARB), and antibiotic resistance genes (ARGs) in the environment: Increasing removal with wetlands and reducing environmental impacts. Bioresource Technology. 2020:123228. doi: 10.1016/j.biortech.2020.123228. [DOI] [PubMed] [Google Scholar]
- Germer J., Sauerborn J., Asch F., de Boer J., Schreiber J., Weber G., Müller J. Skyfarming an ecological innovation to enhance global food security. Journal Für Verbraucherschutz Und Lebensmittelsicherheit. 2011;6(2):237–251. doi: 10.1007/s00003-011-0691-6. [DOI] [Google Scholar]
- Golberg D., Kroupitski Y., Belausov E., Pinto R., Sela S. Salmonella Typhimurium internalization is variable in leafy vegetables and fresh herbs. International Journal of Food Microbiology. 2011;145(1):250–257. doi: 10.1016/j.ijfoodmicro.2010.12.031. [DOI] [PubMed] [Google Scholar]
- Goldstein B., Hauschild M., Fernández J., Birkved M. Urban versus conventional agriculture, taxonomy of resource profiles: A review. Agronomy for Sustainable Development. 2016;36(1):9. doi: 10.1007/s13593-015-0348-4. [DOI] [Google Scholar]
- Gómez C., Currey C.J., Dickson R.W., Kim H.-J., Hernández R., Sabeh N.C., Raudales R.E., Brumfield R.G., Laury-Shaw A., Wilke A.K., Lopez R.G., Burnett S.E. Controlled environment food production for urban agriculture. HortScience. 2019;54(9):1448–1458. doi: 10.21273/HORTSCI14073-19. [DOI] [Google Scholar]
- Gough D., Oliver S., Thomas J. SAGE Publications Ltd; 2017. An introduction to systematic reviews. [Google Scholar]
- Gould D., Caplow T. Metropolitan sustainability: Understanding and improving the urban environment. 2012. Building-integrated agriculture: A new approach to food production; pp. 147–170. [DOI] [Google Scholar]
- Guadagnin S.G., Rath S., Reyes F.G.R. Evaluation of the nitrate content in leaf vegetables produced through different agricultural systems. Food Additives & Contaminants. 2005;22(12):1203–1208. doi: 10.1080/02652030500239649. [DOI] [PubMed] [Google Scholar]
- Hallett S., Hoagland L., Toner E. Vol. 44. 2016. Urban agriculture: Environmental, economic, and social perspectives; pp. 65–120. (Horticultural reviews). [DOI] [Google Scholar]
- Hao Y.-Y., Brackett R.E., Beuchat L.R., Doyle M.P. Microbiological quality and production of botulinal toxin in film-packaged broccoli, carrots, and green beans. Journal of Food Protection. 1999;62(5):499–508. doi: 10.4315/0362-028X-62.5.499. [DOI] [PubMed] [Google Scholar]
- Hariprasad P., Durivadivel P., Snigdha M., Venkateswaran G. Natural occurrence of aflatoxin in green leafy vegetables. Food Chemistry. 2013;138(2–3):1908–1913. doi: 10.1016/j.foodchem.2012.11.093. [DOI] [PubMed] [Google Scholar]
- Hegesh E., Shiloah J. Blood nitrates and infantile methemoglobinemia. Clinica Chimica Acta. 1982;125(2):107–115. doi: 10.1016/0009-8981(82)90187-5. [DOI] [PubMed] [Google Scholar]
- Herklotz P.A., Gurung P., Vanden Heuvel B., Kinney C.A. Uptake of human pharmaceuticals by plants grown under hydroponic conditions. Chemosphere. 2010;78(11):1416–1421. doi: 10.1016/j.chemosphere.2009.12.048. [DOI] [PubMed] [Google Scholar]
- Herman L., Heyndrickx M., De Reu K., Van Coillie E., Uyttendaele M. Microbiological safety and quality aspects in relation to the short food supply chain. In: Huyghebaert A., Van Huffel X., Houins G., editors. Food safety of the short supply chain. Federal Agency for the Safety of the Food Chain (FASFC); Brussels: 2012. pp. 33–44. [Google Scholar]
- von Hoffen L.P., Säumel I. Orchards for edible cities: Cadmium and lead content in nuts, berries, pome and stone fruits harvested within the inner city neighbourhoods in Berlin, Germany. Ecotoxicology and Environmental Safety. 2014;101:233–239. doi: 10.1016/j.ecoenv.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Honikel K.-O. The use and control of nitrate and nitrite for the processing of meat products. Meat Science. 2008;78(1):68–76. doi: 10.1016/j.meatsci.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Horst M., McClintock N., Hoey L. The intersection of planning, urban agriculture, and food justice: A review of the literature. Journal of the American Planning Association. 2017;83(3):277–295. doi: 10.1080/01944363.2017.1322914. [DOI] [Google Scholar]
- Hu Z., Lee J.W., Chandran K., Kim S., Brotto A.C., Khanal S.K. Effect of plant species on nitrogen recovery in aquaponics. Bioresource Technology. 2015;188:92–98. doi: 10.1016/j.biortech.2015.01.013. [DOI] [PubMed] [Google Scholar]
- International Organization for Standardization Food safety management systems - requirements for any organization in the food chain (ISO Standard No. 22000) 2018. https://www.iso.org/standard/65464.html Retrieved from.
- Jeong H., Kim H., Jang T. Irrigation water quality standards for indirect wastewater reuse in agriculture: A contribution toward sustainable wastewater reuse in South Korea. Water. 2016;8(4):169. doi: 10.3390/w8040169. [DOI] [Google Scholar]
- Joimel S., Cortet J., Consalès J.N., Branchu P., Haudin C.-S., Morel J.L., Schwartz C. Contribution of chemical inputs on the trace elements concentrations of surface soils in urban allotment gardens. Journal of Soils and Sediments. 2020 doi: 10.1007/s11368-020-02784-z. [DOI] [Google Scholar]
- Joly A., Junge R., Bardocz T. Aquaponics business in Europe: Some legal obstacles and solutions. Ecocycles. 2015;1(2):3–5. doi: 10.19040/ecocycles.v1i2.30. [DOI] [Google Scholar]
- Jürkenbeck K., Heumann A., Spiller A. Sustainability matters: Consumer acceptance of different vertical farming systems. Sustainability. 2019;11(15):4052. doi: 10.3390/su11154052. [DOI] [Google Scholar]
- Kaiser M.L., Williams M.L., Basta N., Hand M., Huber S. When vacant lots become urban gardens: Characterizing the perceived and actual food safety concerns of urban agriculture in Ohio. Journal of Food Protection. 2015;78(11):2070–2080. doi: 10.4315/0362-028X.JFP-15-181. [DOI] [PubMed] [Google Scholar]
- Kawamura-Aoyama C., Fujiwara K., Shinohara M., Takano M. Study on the hydroponic culture of lettuce with microbially degraded solid food waste as a nitrate source. Japan Agricultural Research Quarterly: Japan Agricultural Research Quarterly. 2014;48(1):71–76. doi: 10.6090/jarq.48.71. [DOI] [Google Scholar]
- Keraita B., Konradsen F., Drechsel P., Abaidoo R.C. Effect of low-cost irrigation methods on microbial contamination of lettuce irrigated with untreated wastewater. Tropical Medicine and International Health. 2007;12(SUPPL. 2):15–22. doi: 10.1111/j.1365-3156.2007.01937.x. [DOI] [PubMed] [Google Scholar]
- Khan S., Aijun L., Zhang S., Hu Q., Zhu Y.-G. Accumulation of polycyclic aromatic hydrocarbons and heavy metals in lettuce grown in the soils contaminated with long-term wastewater irrigation. Journal of Hazardous Materials. 2008;152(2):506–515. doi: 10.1016/j.jhazmat.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Khouryieh M., Khouryieh H., Daday J.K., Shen C. Consumers' perceptions of the safety of fresh produce sold at farmers' markets. Food Control. 2019;105:242–247. doi: 10.1016/j.foodcont.2019.06.003. [DOI] [Google Scholar]
- Kinney C.A., Furlong E.T., Werner S.L., Cahill J.D. Presence and distribution of wastewater-derived pharmaceuticals in soil irrigated with reclaimed water. Environmental Toxicology & Chemistry. 2006;25(2):317. doi: 10.1897/05-187R.1. [DOI] [PubMed] [Google Scholar]
- Koseki S., Mizuno Y., Yamamoto K. Comparison of two possible routes of pathogen contamination of spinach leaves in a hydroponic cultivation system. Journal of Food Protection. 2011;74(9):1536–1542. doi: 10.4315/0362-028X.JFP-11-031. [DOI] [PubMed] [Google Scholar]
- Kunpeuk W., Spence W., Phulkerd S., Suphanchaimat R., Pitayarangsarit S. The impact of gardening on nutrition and physical health outcomes: A systematic review and meta-analysis. Health Promotion International. 2020;35(2):397–408. doi: 10.1093/heapro/daz027. [DOI] [PubMed] [Google Scholar]
- Langlois C.J., Calabrese E.J. The interactive effect of chlorine, copper and nitrite on methaemoglobin formation in red blood cells of dorset sheep. Human & Experimental Toxicology. 1992;11(3):223–228. doi: 10.1177/096032719201100311. [DOI] [PubMed] [Google Scholar]
- Lee S., Kim J., Lee J. Colonization of toxic cyanobacteria on the surface and inside of leafy green: A hidden source of cyanotoxin production and exposure. Food Microbiology. 2021;94:103655. doi: 10.1016/j.fm.2020.103655. [DOI] [PubMed] [Google Scholar]
- von der Leyen U. A union that strives for more, my agenda for Europe - political guidelines for the next European commission 2019 - 2024. 2019. https://ec.europa.eu/commission/sites/beta-political/files/political-guidelines-next-commission_en.pdf
- Li D., Wong C.H., Seet M.F., Kuan N. Isolation, characterization, and inactivation of stenotrophomonas maltophilia from leafy green vegetables and urban agriculture systems. Frontiers in Microbiology. 2019;10 doi: 10.3389/fmicb.2019.02718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Local Governments for Sustainability . Vol. 3. 2015. (Seoul declaration “building A world of local action” for a sustainable urban future). [Google Scholar]
- Lopez-Galvez F., Allende A., Pedrero-Salcedo F., Alarcon J.J., Gil M.I. Safety assessment of greenhouse hydroponic tomatoes irrigated with reclaimed and surface water. International Journal of Food Microbiology. 2014;191:97–102. doi: 10.1016/j.ijfoodmicro.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Lovell S.T. Multifunctional urban agriculture for sustainable land use planning in the United States. Sustainability. 2010;2(8):2499–2522. doi: 10.3390/su2082499. [DOI] [Google Scholar]
- Magwaza S.T., Magwaza L.S., Odindo A.O., Mditshwa A. Hydroponic technology as decentralised system for domestic wastewater treatment and vegetable production in urban agriculture: A review. The Science of the Total Environment. 2020;698:134154. doi: 10.1016/j.scitotenv.2019.134154. [DOI] [PubMed] [Google Scholar]
- Manaia C.M. Assessing the risk of antibiotic resistance transmission from the environment to humans: Non-direct proportionality between abundance and risk. Trends in Microbiology. 2017;25(3):173–181. doi: 10.1016/j.tim.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Manassaram D.M., Backer L.C., Messing R., Fleming L.E., Luke B., Monteilh C.P. Nitrates in drinking water and methemoglobin levels in pregnancy: A longitudinal study. Environmental Health. 2010;9(1):60. doi: 10.1186/1476-069X-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margenat A., Matamoros V., Díez S., Cañameras N., Comas J., Bayona J.M. Occurrence and bioaccumulation of chemical contaminants in lettuce grown in peri-urban horticulture. The Science of the Total Environment. 2018;637–638:1166–1174. doi: 10.1016/j.scitotenv.2018.05.035. [DOI] [PubMed] [Google Scholar]
- Martinez J.L. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environmental Pollution. 2009;157(11):2893–2902. doi: 10.1016/j.envpol.2009.05.051. [DOI] [PubMed] [Google Scholar]
- Mathews S., Henderson S., Reinhold D. Uptake and accumulation of antimicrobials, triclocarban and triclosan, by food crops in a hydroponic system. Environmental Science and Pollution Research. 2014;21(9):6025–6033. doi: 10.1007/s11356-013-2474-3. [DOI] [PubMed] [Google Scholar]
- Matini M., Shamsi-Ehsan T., Maghsood A.H. The parasitic contamination of farm vegetables in Asadabad city, west of Iran, in 2014. Avicenna Journal of Clinical Microbiology and Infection. 2016;4(1) doi: 10.17795/ajcmi-32474. [DOI] [Google Scholar]
- Ma C., White J.C., Zhao J., Zhao Q., Xing B. Uptake of engineered nanoparticles by food crops: Characterization, mechanisms, and implications. Annual Review of Food Science and Technology. 2018;9(1):129–153. doi: 10.1146/annurev-food-030117-012657. [DOI] [PubMed] [Google Scholar]
- Maye D. ‘Smart food city’: Conceptual relations between smart city planning, urban food systems and innovation theory. City, Culture and Society. 2019;16:18–24. doi: 10.1016/j.ccs.2017.12.001. [DOI] [Google Scholar]
- Md Meftaul I., Venkateswarlu K., Dharmarajan R., Annamalai P., Megharaj M. Pesticides in the urban environment: A potential threat that knocks at the door. The Science of the Total Environment. 2020;711 doi: 10.1016/j.scitotenv.2019.134612. [DOI] [PubMed] [Google Scholar]
- Menyuka N.N., Sibanda M., Bob U. Perceptions of the challenges and opportunities of utilising organic waste through urban agriculture in the durban south basin. International Journal of Environmental Research and Public Health. 2020;17(4) doi: 10.3390/ijerph17041158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miličić V., Thorarinsdottir R., Santos M., Hančič M. Commercial aquaponics approaching the European market: To consumers' perceptions of aquaponics products in Europe. Water. 2017;9(2):80. doi: 10.3390/w9020080. [DOI] [Google Scholar]
- Mohammad Z.H., Yu H., Neal J.A., Gibson K.E., Sirsat S.A. Food safety challenges and barriers in southern United States farmers markets. Foods. 2019;9(1):12. doi: 10.3390/foods9010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty M.J., Semmens K., Bissonnette G.K., Jaczynski J. Internalization assessment of E. coli O157:H7 in hydroponically grown lettuce. Lebensmittel-Wissenschaft & Technologie. 2019;100:183–188. doi: 10.1016/j.lwt.2018.10.060. [DOI] [Google Scholar]
- Mok H.-F., Williamson V.G., Grove J.R., Burry K., Barker S.F., Hamilton A.J. Strawberry fields forever? Urban agriculture in developed countries: A review. Agronomy for Sustainable Development. 2014;34(1):21–43. doi: 10.1007/s13593-013-0156-7. [DOI] [Google Scholar]
- Mortensen A., Aguilar F., Crebelli R., Di Domenico A., Dusemund B., Frutos M.J., Galtier P., Gott D., Gundert‐Remy U., Lambré C., Leblanc J., Lindtner O., Moldeus P., Mosesso P., Oskarsson A., Parent‐Massin D., Stankovic I., Waalkens‐Berendsen I., Woutersen R.A., Younes M. Re‐evaluation of potassium nitrite (E 249) and sodium nitrite (E 250) as food additives. EFSA Journal. 2017;15(6) doi: 10.2903/j.efsa.2017.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D., Zhang R., Li C., Li Y., Tian L., Wang L. Influence of urban rainwater of city on yield, quality and heavy metal content of hydroponic lettuce. Nongye Gongcheng Xuebao/Transactions of the Chinese Society of Agricultural Engineering. 2017;33:348–354. doi: 10.11975/j.issn.1002-6819.2017.z1.052. [DOI] [Google Scholar]
- Newell D.G., Koopmans M., Verhoef L., Duizer E., Aidara-Kane A., Sprong H., Opsteegh M., Langelaar M., Threfall J., Scheutz F., van der Giessen J., Kruse H. Food-borne diseases - the challenges of 20 years ago still persist while new ones continue to emerge. International Journal of Food Microbiology. 2010;139:S3–S15. doi: 10.1016/j.ijfoodmicro.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J., Sun S.X., Zheng Y., Datta R., Sarkar D., Li Y.M. Removal of prometryn from hydroponic media using marsh pennywort ( Hydrocotyle vulgaris L.) International Journal of Phytoremediation. 2018;20(9):909–913. doi: 10.1080/15226514.2018.1448359. [DOI] [PubMed] [Google Scholar]
- Nousiainen L.L., Joutsen S., Lunden J., Hänninen M.L., Fredriksson-Ahomaa M. Bacterial quality and safety of packaged fresh leafy vegetables at the retail level in Finland. International Journal of Food Microbiology. 2016;232:73–79. doi: 10.1016/j.ijfoodmicro.2016.05.020. [DOI] [PubMed] [Google Scholar]
- Nozzi V., Graber A., Schmautz Z., Mathis A., Junge R. Nutrient management in aquaponics: Comparison of three approaches for cultivating lettuce, mint and mushroom herb. Agronomy. 2018;8(3):27. doi: 10.3390/agronomy8030027. [DOI] [Google Scholar]
- Nyachuba D.G. Foodborne illness: Is it on the rise? Nutrition Reviews. 2010;68(5):257–269. doi: 10.1111/j.1753-4887.2010.00286.x. [DOI] [PubMed] [Google Scholar]
- Olaimat A.N., Holley R.A. Factors influencing the microbial safety of fresh produce: A review. Food Microbiology. 2012;32(1):1–19. doi: 10.1016/j.fm.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Orsini F., Gasperi D., Marchetti L., Piovene C., Draghetti S., Ramazzotti S., Bazzocchi G., Gianquinto G.P. Exploring the production capacity of rooftop gardens (RTGs) in urban agriculture: The potential impact on food and nutrition security, biodiversity and other ecosystem services in the city of bologna. Food Security. 2014;6(6):781–792. doi: 10.1007/s12571-014-0389-6. [DOI] [Google Scholar]
- Orsini F., Pennisi G., Michelon N., Minelli A., Bazzocchi G., Sanyé-Mengual E., Gianquinto G.P. Features and functions of multifunctional urban agriculture in the global north: A review. Frontiers in Sustainable Food Systems. 2020;4 doi: 10.3389/fsufs.2020.562513. [DOI] [Google Scholar]
- Orsini F., Pennisi G., Zulfiqar F., Gianquinto G.P. Sustainable use of resources in plant factories with artificial lighting (PFALs) European Journal of Horticultural Science. 2020;85(5):297–309. doi: 10.17660/eJHS.2020/85.5.1. [DOI] [Google Scholar]
- Orsini F., Kahane R., Nono-Womdim R., Gianquinto G. Urban agriculture in the developing world: a review. Agronomy for Sustainable Development. 2013;(33):695–720. doi: 10.1007/s13593-013-0143-z. [DOI] [Google Scholar]
- O’Sullivan C.A., Bonnett G.D., McIntyre C.L., Hochman Z., Wasson A.P. Strategies to improve the productivity, product diversity and profitability of urban agriculture. Agricultural Systems. 2019;174:133–144. doi: 10.1016/j.agsy.2019.05.007. [DOI] [Google Scholar]
- Pennisi G., Gasperi D., Mancarella S., Vittori Antisari L., Vianello G., Orsini F., Gianquinto G.P. Soilless cultivation in urban gardens for reduced potentially toxic elements (PTEs) contamination risk. Acta Horticulturae. 2017;1189:377–381. doi: 10.17660/ActaHortic.2017.1189.72. [DOI] [Google Scholar]
- Pennisi G., Orsini F., Gasperi D., Mancarella S., Sanoubar R., Vittori Antisari L., Vianello G., Gianquinto G.P. Soilless system on peat reduce trace metals in urban-grown food: Unexpected evidence for a soil origin of plant contamination. Agronomy for Sustainable Development. 2016;36(4) doi: 10.1007/s13593-016-0391-9. [DOI] [Google Scholar]
- Pérez-Urrestarazu L., Lobillo-Eguíba J., Fernández-Cañero R., Fernández-Cabanás V.M. Food safety concerns in urban aquaponic production: Nitrate contents in leafy vegetables. Urban Forestry and Urban Greening. 2019;44(May):126431. doi: 10.1016/j.ufug.2019.126431. [DOI] [Google Scholar]
- Phungamngoen C., Rittisak S. Surface characteristics of leafy vegetables and their effects on Salmonella Attachment. E3S Web of Conferences. 2020;141 doi: 10.1051/e3sconf/202014103002. [DOI] [Google Scholar]
- Polder A., Müller M.B., Brynildsrud O.B., de Boer J., Hamers T., Kamstra J.H., Lie E., Mdegela R.H., Moberg H., Nonga H.E., Sandvik M., Skaare J.U., Lyche J.L. Dioxins, PCBs, chlorinated pesticides and brominated flame retardants in free-range chicken eggs from peri-urban areas in Arusha, Tanzania: Levels and implications for human health. The Science of the Total Environment. 2016;551–552:656–667. doi: 10.1016/j.scitotenv.2016.02.021. [DOI] [PubMed] [Google Scholar]
- Pollard G., Ward J.D., Koth B. Aquaponics in urban agriculture: Social acceptance and urban food planning. Horticulturae. 2017;3(2):39. doi: 10.3390/horticulturae3020039. [DOI] [Google Scholar]
- Pussemier L., Herman L., Van Huffel X., Huyghebaert A. Chemical aspects of food safety and quality in the short food supply chain. In: Huyghebaert A., Van Huffel X., Houins G., editors. Food safety of the short supply chain. Federal Agency for the Safety of the Food Chain (FASFC); Brussels: 2012. pp. 45–51. [Google Scholar]
- Rababah A.A., Ashbolt N.J. Innovative production treatment hydroponic farm for primary municipal sewage utilisation. Water Research. 2000;34(3):825–834. doi: 10.1016/s0043-1354(99)00231-6. [DOI] [Google Scholar]
- Rai P.K., Lee J., Brown R.J.C., Kim K.-H. Environmental fate, ecotoxicity biomarkers, and potential health effects of micro- and nano-scale plastic contamination. Journal of Hazardous Materials. 2021;403:123910. doi: 10.1016/j.jhazmat.2020.123910. [DOI] [PubMed] [Google Scholar]
- Resource Centres for Urban Agriculture and Food Security City region food systems. Urban Agriculture Magazine. 2015;29 [Google Scholar]
- Riggio G.M., Jones S.L., Gibson K.E. Risk of human pathogen internalization in leafy vegetables during lab-scale hydroponic cultivation. Horticulturae. 2019;5(1) doi: 10.3390/horticulturae5010025. [DOI] [Google Scholar]
- Rothwell A., Ridoutt B., Page G., Bellotti W. Environmental performance of local food: Trade-offs and implications for climate resilience in a developed city. Journal of Cleaner Production. 2016;114:420–430. doi: 10.1016/j.jclepro.2015.04.096. [DOI] [Google Scholar]
- Rufí-Salís M., Petit-Boix A., Villalba G., Sanjuan-Delmás D., Parada F., Ercilla-Montserrat M., Arcas-Pilz V., Muñoz-Liesa J., Rieradevall J., Gabarrell X. Recirculating water and nutrients in urban agriculture: An opportunity towards environmental sustainability and water use efficiency? Journal of Cleaner Production. 2020;261:121213. doi: 10.1016/j.jclepro.2020.121213. [DOI] [Google Scholar]
- Sant'Ana A.S., Franco B.D.G.M., Schaffner D.W. Risk of infection with Salmonella and Listeria monocytogenes due to consumption of ready-to-eat leafy vegetables in Brazil. Food Control. 2014;42:1–8. doi: 10.1016/j.foodcont.2014.01.028. [DOI] [Google Scholar]
- Sanyé-Mengual E., Specht K., Grapsa E., Orsini F., Gianquinto G.P. How can innovation in urban agriculture contribute to sustainability? A characterization and evaluation study from five western European cities. Sustainability. 2019;11(15) doi: 10.3390/su11154221. [DOI] [Google Scholar]
- Sanyé-Mengual E., Specht K., Krikser T., Vanni C., Pennisi G., Orsini F., Gianquinto G.P. Social acceptance and perceived ecosystem services of urban agriculture in Southern Europe: The case of Bologna, Italy. PloS One. 2018;13(9) doi: 10.1371/journal.pone.0200993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Säumel I., Kotsyuk I., Hölscher M., Lenkereit C., Weber F., Kowarik I. How healthy is urban horticulture in high traffic areas? Trace metal concentrations in vegetable crops from plantings within inner city neighbourhoods in Berlin, Germany. Environmental Pollution. 2012;165:124–132. doi: 10.1016/j.envpol.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Savvas D., Gruda N. Application of soilless culture technologies in the modern greenhouse industry – a review. European Journal of Horticultural Science. 2018 doi: 10.17660/eJHS.2018/83.5.2. November. [DOI] [Google Scholar]
- Senathirajah K., Attwood S., Bhagwat G., Carbery M., Wilson S., Palanisami T. Estimation of the mass of microplastics ingested – a pivotal first step towards human health risk assessment. Journal of Hazardous Materials. 2021;404:124004. doi: 10.1016/j.jhazmat.2020.124004. [DOI] [PubMed] [Google Scholar]
- Settanni L., Miceli A., Francesca N., Cruciata M., Moschetti G. Microbiological investigation of Raphanus sativus L. grown hydroponically in nutrient solutions contaminated with spoilage and pathogenic bacteria. International Journal of Food Microbiology. 2013;160(3):344–352. doi: 10.1016/j.ijfoodmicro.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Specht K., Weith T., Swoboda K., Siebert R. Socially acceptable urban agriculture businesses. Agronomy for Sustainable Development. 2016;36(1):17. doi: 10.1007/s13593-016-0355-0. [DOI] [Google Scholar]
- Specht K., Zoll F., Schümann H., Bela J., Kachel J., Robischon M. How will we eat and produce in the cities of the future? From edible insects to vertical farming-A study on the perception and acceptability of new approaches. Sustainability. 2019;11(16):4315. doi: 10.3390/su11164315. [DOI] [Google Scholar]
- Srikanth R., Naik D. Prevalence of Giardiasis due to wastewater reuse for agriculture in the suburbs of Asmara City, Eritrea. International Journal of Environmental Health Research. 2004;14(1):43–52. doi: 10.1080/09603120310001633912. [DOI] [PubMed] [Google Scholar]
- Su M.H., Azwar E., Yang Y., Sonne C., Yek P.N.Y., Liew R.K., Cheng C.K., Show P.L., Lam S.S. Simultaneous removal of toxic ammonia and lettuce cultivation in aquaponic system using microwave pyrolysis biochar. Journal of Hazardous Materials. 2020;396:122610. doi: 10.1016/j.jhazmat.2020.122610. [DOI] [PubMed] [Google Scholar]
- Teuber S., Schmidt K., Kühn P., Scholten T. Engaging with urban green spaces – a comparison of urban and rural allotment gardens in Southwestern Germany. Urban Forestry and Urban Greening. 2019;43:126381. doi: 10.1016/j.ufug.2019.126381. [DOI] [Google Scholar]
- Tozzi F., Del Bubba M., Petrucci W.A., Pecchioli S., Macci C., Hernández García F., Martínez Nicolás J.J., Giordani E. Use of a remediated dredged marine sediment as a substrate for food crop cultivation: Sediment characterization and assessment of fruit safety and quality using strawberry (Fragaria x ananassa Duch.) as model species of contamination transfer. Chemosphere. 2020;238:124651. doi: 10.1016/j.chemosphere.2019.124651. [DOI] [PubMed] [Google Scholar]
- Tremlová J., Száková J., Sysalová J., Tlustoš P. Bioavailability of arsenic, cadmium, iron and zinc in leafy vegetables amended with urban particulate matter suspension. Journal of the Science of Food and Agriculture. 2013;93(6):1378–1384. doi: 10.1002/jsfa.5903. [DOI] [PubMed] [Google Scholar]
- Trinetta V., Morgan M., Linton R. Microbial decontamination in the food industry: Novel methods and applications. Elsevier; 2012. Chlorine dioxide for microbial decontamination of food; pp. 533–562. [DOI] [Google Scholar]
- Urban J.D., Wikoff D.S., Bunch A.T.G., Harris M.A., Haws L.C. A review of background dioxin concentrations in urban/suburban and rural soils across the United States: Implications for site assessments and the establishment of soil cleanup levels. The Science of the Total Environment. 2014;466–467:586–597. doi: 10.1016/j.scitotenv.2013.07.065. [DOI] [PubMed] [Google Scholar]
- Voigt A., Latkowska M., Rutecka A., Poniży L., Mizgajski A., Breuste J., Haas K., Artmann M., Hursthouse A., Agboola J., Külvik M., Olonen A., Leitão T.E., Costa H. Environmental behaviour of urban allotment gardeners in Europe. ECLAS Landscape in flux. 2015. https://www.researchgate.net/publication/301231747_Environmental_Behaviour_of_Urban_Allotment_Gardeners_in_Europe
- Wallace C.A. Encyclopedia of food safety. Elsevier; 2014. Food safety assurance systems: Hazard analysis and critical control point system (HACCP): Principles and practice. [DOI] [Google Scholar]
- Wang Y.J., Deering A.J., Kim H.J. The occurrence of Shiga toxin-producing E. Coli in aquaponic and hydroponic systems. Horticulturae. 2020;6(1):1. doi: 10.3390/horticulturae6010001. [DOI] [Google Scholar]
- Wang Q., Kniel K.E. Survival and transfer of murine norovirus within a hydroponic system during kale and mustard microgreen harvesting. Applied and Environmental Microbiology. 2016;82(2):705–713. doi: 10.1128/AEM.02990-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Liu X., Li Y., Powell T., Wang X., Wang G., Zhang P. Microplastics as contaminants in the soil environment: A mini-review. The Science of the Total Environment. 2019;691:848–857. doi: 10.1016/j.scitotenv.2019.07.209. [DOI] [PubMed] [Google Scholar]
- Wu S., Xia X.H., Zhang S.W., Liu Q., Xu L. Distribution of polychlorinated biphenyls (PCBs) and toxic equivalency of dioxin-like PCB congeners in rural soils of Beijing, China. Journal of Environmental Informatics. 2012;20(1):12–19. doi: 10.3808/jei.201200216. [DOI] [Google Scholar]
- Xi X., Wang M., Chen Y., Yu S., Hong Y., Ma J., Wu Q., Lin Q., Xu X. Adaption of the microbial community to continuous exposures of multiple residual antibiotics in sediments from a salt-water aquacultural farm. Journal of Hazardous Materials. 2015;290:96–105. doi: 10.1016/j.jhazmat.2015.02.059. [DOI] [PubMed] [Google Scholar]
- Yang T., Doherty J., Guo H., Zhao B., Clark J.M., Xing B., Hou R., He L. Real-time monitoring of pesticide translocation in tomato plants by surface-Enhanced Raman spectroscopy. Analytical Chemistry. 2019;91(3):2093–2099. doi: 10.1021/acs.analchem.8b04522. [DOI] [PubMed] [Google Scholar]
- Yep B., Zheng Y. Aquaponic trends and challenges – a review. Journal of Cleaner Production. 2019;228:1586–1599. doi: 10.1016/j.jclepro.2019.04.290. [DOI] [Google Scholar]
- Yuan X., Xue N., Han Z. A meta-analysis of heavy metals pollution in farmland and urban soils in China over the past 20 years. Journal of Environmental Sciences. 2021;101:217–226. doi: 10.1016/j.jes.2020.08.013. [DOI] [PubMed] [Google Scholar]
- Yu H., Gibson K.E., Wright K.G., Neal J.A., Sirsat S.A. Food safety and food quality perceptions of farmers' market consumers in the United States. Food Control. 2017;79:266–271. doi: 10.1016/j.foodcont.2017.04.010. [DOI] [Google Scholar]
- Zhang F., He J., Yao Y., Hou D., Jiang C., Zhang X., Di C., Otgonbayar K. Spatial and seasonal variations of pesticide contamination in agricultural soils and crops sample from an intensive horticulture area of Hohhot, North-West China. Environmental Monitoring and Assessment. 2013;185(8):6893–6908. doi: 10.1007/s10661-013-3073-y. [DOI] [PubMed] [Google Scholar]
- Zheng T., Ran Y., Chen L. Polycyclic aromatic hydrocarbons (PAHs) in rural soils of dongjiang river basin: Occurrence, source apportionment, and potential human health risk. Journal of Soils and Sediments. 2014;14(1):110–120. doi: 10.1007/s11368-013-0753-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.