Abstract
Background:
The ability of the pretreatment lymphocyte to monocyte ratio (LMR) to predict outcomes of patients with hepatocellular carcinoma (HCC) receiving sorafenib is not conclusively determined.
Methods:
We retrospectively studied patients treated with sorafenib for HCC in two tertiary referral centres in Asia and North America. Primary endpoints were overall survival (OS) and progression-free survival (PFS). Predictive factors for the outcomes were determined by Cox proportional hazards models. A risk-assessment tool was developed.
Results:
Compared to the North America cohort, the Asia cohort was more heavily pretreated (72.1% vs. 35.2%; P<0.001), had higher hepatitis B virus infection (87.6% vs. 5.6%; P<0.001), and more distant metastases (83.2% vs. 25.4%; P<0.001). Lower monocyte count in the Asia cohort (median, 462.7 vs. 600.0/μL; P=0.023) resulted in a higher LMR (median, 2.6 vs. 1.8; P<0.001). High LMR was associated with a significantly higher OS (hazard ratio [HR], 0.88; 95% confidence interval [CI], 0.81‒0.97; P=0.007). This was confirmed in a sensitivity analysis including patients treated in Asia only (HR, 0.89; 95% CI, 0.81‒0.97; P=0.010). An OS nomogram was constructed with following variables selected in the multivariate Cox model: LMR, treatment location, previous treatment, performance status, AFP, lymph node metastasis, and Child‒Pugh score. The concordance score was 0.71 (95% CI, 0.67‒0.75). LMR did not predict PFS.
Conclusion:
LMR measured before sorafenib administration predicts OS in advanced HCC patients. Our OS nomogram, incorporating LMR, can be offered to clinicians to improve their ability to assess prognosis, strengthen the prognosis-based decision making, and inform patients in the clinic.
Keywords: Lymphocyte, Monocyte, Liver Cancer, Chemotherapy, Overall Survival
1. Introduction
Lymphocyte to monocyte ratio (LMR) is a simple and straightforward parameter reflecting the state of immune homeostasis. In cancer, lymphocytes represent the ability of the host to fight against the tumour, whereas monocytes protect tumour cells by differentiating into tumour-associated macrophages (TAM) (1–3). TAMs secrete cytokines and growth factors that contribute to angiogenesis, local invasion, and distant metastasis (1–3).
Lymphocytes and monocytes are important prognostic factors in hepatocellular carcinoma (HCC). A recent meta-analysis reported that preoperative LMR was associated with overall survival (OS) and progression-free survival (PFS) in surgically-treated HCC patients (4). However, studies so far lack generalisability because all of the analyses were performed in patients who underwent surgery (4–6). In addition, some of the important factors that might have influenced LMR, such as information about previous cytotoxic therapy, were missed or not adequately controlled.
Sorafenib is a current standard of treatment for patients with advanced HCC.(7) A previous publication that analysed two randomised controlled trials reported that the neutrophil to lymphocyte ratio was associated with OS (8). In addition, evidence from preclinical studies indicates that sorafenib has immunomodulatory effects such as inactivation of lymphocytes and accumulation of regulatory T-cells (7, 9–11). These findings suggest that baseline immune status can be implicated in predicting outcomes of sorafenib-treated HCC patients; however, the significance of LMR as a measure of immune status has not been well studied in this patient population.
To determine the clinical significance of the pretreatment LMR in predicting OS and PFS, we analysed data from patients with advanced HCC who were treated with sorafenib at two tertiary referral centres in South Korea and United States. We also sought to develop a tool integrating LMR into a scoring system that can predict prognosis.
2. Methods
2.1. Study Design and Population
This study used data from two centres, the Asan Medical Center in South Korea and the Mayo Clinic in the United States. Patients who met the criteria for advanced HCC, either as an initial diagnosis or as a result of recurrence, were all included. However, patients who received other anti-cancer treatment(s) within three months before initiation of sorafenib were not considered for inclusion, as the treatments could potentially influence the blood cell counts.
Exclusion criteria included sorafenib treatment for less than four weeks, previous history of liver transplantation, concomitant malignancies within three months of HCC diagnosis, and the presence of human immunodeficiency virus infection. The Asia cohort comprised patients treated between 2007 and 2011 and North America cohort consisted of those who received sorafenib between 2008 and 2017.
The primary endpoints of this study were 1) overall survival (OS) and 2) progression-free survival (PFS). Time-to-primary endpoints were calculated by subtracting the first date of sorafenib administration from the date of primary endpoints. Data was collected until primary endpoints were reached or loss to follow-up, whichever came first. Follow-up status was assessed on December 31st, 2018.
Baseline characteristics were accessed by review of the electronic medical record. Lymphocyte and monocyte counts were calculated from complete blood cell measurement.
This study was approved by the institutional review board of the Asan Medical Center (2019–0685) and the Mayo Clinic (15–006298) and complied with the principles of the Declaration of Helsinki.
2.2. Statistical Analysis
Cox proportional hazards models were utilised to calculate the hazard ratio (HR) of each variable for OS and PFS in the entire population. In cases for which the primary endpoint of progression did not occur, death of any cause was considered as a censor. Subsequently, HRs were calculated in subgroups of patients treated in Asia and North America, separately, considering the inherent differences in baseline characteristics, including the blood cell counts. Finally, based on the results derived from the Cox proportional hazards models, scoring systems predicting clinical outcomes were constructed in the form of nomogram. The median survival was approximately 1 year and the 75th percentile of the follow-up was at 3 years, hence nomograms were constructed predicting survival at 1 year and 3 years. We assessed nomogram model performance by concordance score.
All statistical methods were implemented in SAS statistical software (SAS Institute, Cary, NC, USA), version 9.4.
3. Results
3.1. Patient Characteristics
Overall, 297 patients treated with sorafenib due to advanced stage HCC were enrolled, after the exclusion of 279 patients meeting the exclusion criteria (Fig. 1). Those excluded were 197 patients treated with sorafenib for less than four weeks, 47 patients who received liver transplantation before sorafenib initiation, 33 patients who were diagnosed with other concomitant malignancies within three months of HCC diagnosis, and 2 patients infected with human immunodeficiency virus.
Fig. 1.
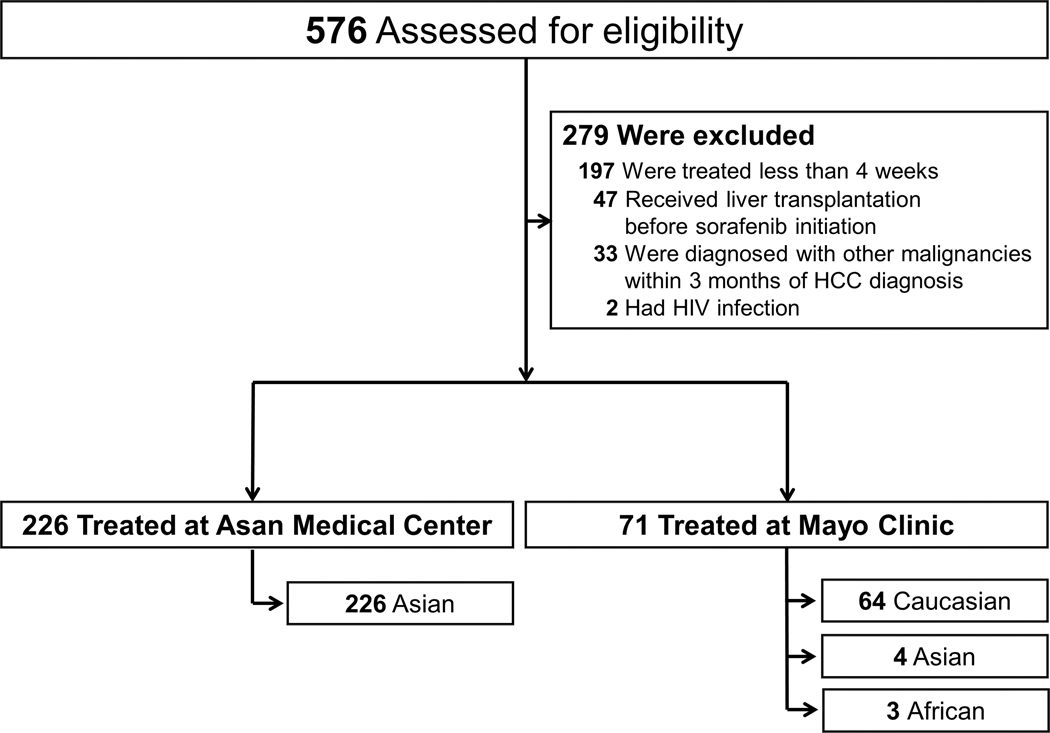
Study Cohort.
Both cohorts consisted of more than 85% male patients, and the mean age was significantly younger in the Asia cohort (54.2 vs. 61.5 years; P<0.001; Table 1). Patients in the Asia cohort were heavily treated previously. In addition, the majority of the Asia cohort had hepatitis B virus (HBV) infection as the underlying cause of liver disease (198 [88%]), whereas the most common aetiology in the North America cohort was chronic hepatitis C (27 [18%]). Patients with alcohol-associated HCC (7 [3.1%] in the Asia cohort and 13 [18.3%] in the North America cohort) did not have significant alcohol consumption within 3 months of sorafenib initiation.
Table 1.
Demographic and Clinical Characteristics of the Patients at Baseline by Treatment Site.
| Characteristic | Asan Medical Center (N = 226) | Mayo Clinic (N = 71) | P |
|---|---|---|---|
| Age – yr, mean ± SD | 54.2 ± 11.0 | 61.5 ± 12.5 | < 0.001 |
| Male sex – no. (%) | 196 (86.7) | 61 (85.9) | 0.86 |
| BMI – kg/m2, median (IQR) | 23.2 (21.8, 25.2) | 28.1 (24.7, 30.6) | < 0.001 |
| Race – no. (%) | < 0.001 | ||
| Asian | 226 (100.0) | 4 (5.6) | |
| Caucasian | 0 (0.0) | 64 (90.1) | |
| African | 0 (0.0) | 3 (4.2) | |
| Previous treatment, no. (%) | |||
| Surgery | 66 (29.2) | 4 (5.6) | < 0.001 |
| Ablation | 39 (17.3) | 5 (7.0) | 0.036 |
| Transarterial embolization | 137 (60.6) | 23 (32.4) | < 0.001 |
| Radiation* | 33 (14.6) | 4 (5.6) | 0.06 |
| Others | 19 (8.4) | 0 (0.0) | 0.009 |
| Etiology of liver disease, no. (%) | < 0.001 | ||
| Hepatitis B virus | 198 (87.6) | 4 (5.6) | |
| Hepatitis C virus | 6 (2.7) | 27 (38.0) | |
| Alcohol | 7 (3.1) | 13 (18.3) | |
| Non-alcoholic fatty liver | 8 (3.5) | 4 (5.6) | |
| Others or unknown | 7 (3.1) | 23 (32.4) | |
| Cirrhosis, no. (%) | 144 (63.7) | 53 (74.6) | 0.09 |
| ECOG Performance Status, no. (%) | 0.11 | ||
| 0 | 120 (53.1) | 30 (42.3) | |
| 1 or higher | 106 (46.9) | 41 (57.7) | |
| Lymphocyte – /μL, median (IQR) | 1184.9 (808.2, 1711.3) | 1020.0 (662.1, 1490.0) | 0.05 |
| Monocyte – /μL, median (IQR) | 462.7 (332.4, 647.7) | 600.0 (455.0, 770.0) | 0.023 |
| LM ratio, median (IQR) | 2.6 (0.3, 10.5) | 1.8 (0.7, 3.8) | < 0.001 |
| Platelet – ×103/μL, median (IQR) | 122.5 (82.0, 173.0) | 146.0 (107.5, 241.0) | 0.029 |
| Albumin – g/dL, median (IQR) | 3.5 (3.2, 3.9) | 3.5 (3.1, 3.9) | 0.74 |
| Alanine aminotransferase – IU/L, median (IQR) | 34.0 (24.0, 53.0) | 52.0 (28.0, 84.0) | 0.005 |
| Bilirubin – mg/dL, median (IQR) | 1.1 (0.9, 1.5) | 1.3 (0.6, 2.1) | 0.027 |
| PT – INR, median (IQR) | 1.1 (1.1, 1.2) | 1.2 (1.1, 1.4) | 0.004 |
| Creatinine – mg/dL, median (IQR) | 0.8 (0.7, 0.9) | 0.9 (0.8, 1.0) | 0.19 |
| Log10AFP – ng/mL, median (IQR) | 2.6 (1.2, 3.6) | 2.6 (1.4, 3.3) | 0.22 |
| Hepatitis B virus DNA – IU/mL, median (IQR) | 1085.5 (27.0, 72900.0) | 84.2 (2.3, 704.3) | 0.19 |
| Tumor characteristics, no. (%) | < 0.001 | ||
| Nodular intrahepatic lesion(s) | 87 (38.5) | 29 (40.8) | |
| No intrahepatic lesion | 65 (28.8) | 1 (1.4) | |
| Infiltrative intrahepatic lesion(s) | 74 (32.7) | 41 (57.7) | |
| Portal vein thrombosis, no. (%) | 99 (43.8) | 32 (45.1) | 0.85 |
| Regional lymph node metastasis, no. (%) | 41 (18.1) | 18 (25.4) | 0.18 |
| Distant metastasis, no. (%) | 188 (83.2) | 18 (25.4) | < 0.001 |
| Child-Pugh score, median (IQR) | 6.0 (5.0, 6.0) | 6.0 (5.0, 8.0) | 0.002 |
Radiotherapy to intrahepatic lesions (including portal vein thrombosis).
SD, standard deviation; IQR, interquartile ranges; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; LM ratio, lymphocyte/monocyte ratio; PT, prothrombin time; INR, international normalized ratio; AFP, alpha-fetoprotein
Comparing blood indices in the Asia vs. the North America cohort, lymphocyte counts were higher (median, 1184.9/μL vs. 1020.0/μL; P=0.05) and monocyte counts were significantly lower (median, 462.7/μL vs. 600.0/μL; P=0.023), leading to a significantly higher LMR in the Asia cohort (median, 2.6 vs. 1.8; P<0.001). In terms of tumour characteristics, the North America cohort had significantly more patients with infiltrative HCC (41 [58%]) than the Asia cohort (74 [33%]; P<0.001). In contrast, a significantly higher proportion of patients in the Asia cohort did not have any intrahepatic tumours (65 [28.8%] vs. 1 [1.4%] in the North America cohort; P<0.001), with most having metastases to distant organs (188 [83.2%]) vs. 18 [25.4%]; P<0.001).
When patients were classified according to the high vs. low LMR based on the median value of 2.43, the low LMR group included more males (92% vs. 82%; P=0.028) and those treated in North America (26% vs. 8.2%; P<0.001; Table 2). In contrast, the high LMR group had a higher proportion of patients who did not have intrahepatic tumours (31% vs. 16%; P=0.009) and who have fewer metastases to regional lymph nodes (14% vs. 24%; P=0.044), but who had more distant metastases (80% vs. 68%; P=0.026).
Table 2.
Demographic and Clinical Characteristics of the Patients at Baseline by Lymphocyte to Monocyte Ratio
| Characteristic | Lymphocyte to Monocyte Ratio ≥ 2.43 (N = 134) | Lymphocyte to Monocyte Ratio < 2.43 (N = 133) | P |
|---|---|---|---|
| Age – yr, mean ± SD | 55.4 ± 11.4 | 55.9 ± 11.8 | 0.71 |
| Male sex – no. (%) | 110 (82.1) | 122 (91.7) | 0.028 |
| Treatment location – no. (%) | <0.001 | ||
| Asia | 123 (91.8) | 99 (74.4) | |
| North America | 11 (8.2) | 34 (25.6) | |
| BMI – kg/m2, median (IQR) | 23.8 (22.3, 26.0) | 23.2 (21.7, 25.4) | 0.14 |
| Previous treatment, no. (%) | |||
| Surgery | 45 (33.6) | 22 (16.5) | 0.002 |
| Ablation | 23 (17.2) | 19 (14.3) | 0.62 |
| Transarterial embolization | 75 (56.0) | 76 (57.1) | 0.90 |
| Radiation* | 8 (6.0) | 27 (20.3) | 0.001 |
| Others | 10 (7.5) | 8 (6.0) | 0.81 |
| Etiology of liver disease, no. (%) | 0.29 | ||
| Hepatitis B virus | 106 (79.1) | 91 (68.4) | |
| Hepatitis C virus | 9 (6.7) | 13 (9.8) | |
| Alcohol | 7 (5.2) | 8 (6.0) | |
| Non-alcoholic fatty liver | 5 (3.7) | 6 (4.5) | |
| Others or unknown | 7 (5.2) | 15 (11.3) | |
| Cirrhosis, no. (%) | 86 (64.2) | 89 (66.9) | 0.70 |
| ECOG Performance Status, no. (%) | 0.54 | ||
| 0 | 72 (53.7) | 66 (49.6) | |
| 1 or higher | 62 (46.3) | 67 (50.4) | |
| Lymphocyte – /μL, median (IQR) | 1465.4 (1099.5, 1994.2) | 917.6 (600.0, 1206.0) | < 0.001 |
| Monocyte – /μL, median (IQR) | 404.5 (290.0, 517.1) | 584.6 (442.5, 788.0) | < 0.001 |
| Platelet – ×103/μL, median (IQR) | 122.5 (87.8, 174.3) | 127.0 (78.0, 193.5) | 0.80 |
| Albumin – g/dL, median (IQR) | 3.7 (3.4, 4.0) | 3.4 (3.0, 3.7) | < 0.001 |
| Alanine aminotransferase – IU/L, median (IQR) | 32.0 (22.0, 50.5) | 39.5 (27.0, 69.8) | 0.002 |
| Bilirubin – mg/dL, median (IQR) | 1.0 (0.9, 1.4) | 1.2 (0.9, 1.8) | 0.08 |
| PT – INR, median (IQR) | 1.1 (0.9, 1.8) | 1.1 (1.1, 1.2) | 0.001 |
| Creatinine – mg/dL, median (IQR) | 0.9 (0.7, 1.0) | 0.8 (0.7, 1.0) | 0.27 |
| Log10AFP – ng/mL, median (IQR) | 2.4 (1.2, 3.6) | 2.7 (1.4, 3.7) | 0.46 |
| Hepatitis B virus DNA – IU/mL, median (IQR) | 1100.0 (0.0, 36607.0) | 960.0 (83.5, 220000.0) | 0.22 |
| Tumor characteristics, no. (%) | 0.009 | ||
| Nodular intrahepatic lesion(s) | 50 (37.3) | 55 (41.4) | |
| No intrahepatic lesion | 42 (31.3) | 21 (15.8) | |
| Infiltrative intrahepatic lesion(s) | 42 (31.3) | 57 (42.9) | |
| Portal vein thrombosis, no. (%) | 54 (40.3) | 65 (48.9) | 0.18 |
| Regional lymph node metastasis, no. (%) | 19 (14.2) | 32 (24.1) | 0.044 |
| Distant metastasis, no. (%) | 107 (79.9) | 90 (67.7) | 0.026 |
| Child-Pugh score, median (IQR) | 5.0 (5.0, 6.0) | 6.0 (5.0, 7.0) | < 0.001 |
Radiotherapy to intrahepatic lesions (including portal vein thrombosis).
SD, standard deviation; IQR, interquartile ranges; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; LM ratio, lymphocyte/monocyte ratio; PT, prothrombin time; INR, international normalized ratio; AFP, alpha-fetoprotein
3.2. Clinical Outcomes
3.2.1. Entire Cohort
The median follow-up time was 18 months (IQR, 10.0‒36.4 months). During follow-up, 272 patients (Kaplan-Meier estimate, 91.6%; Fig., Supplemental Digital Content 1) had the primary endpoint of death. By univariate analysis, age, treatment location, previous treatment, cirrhosis, Eastern Cooperative Oncology Group (ECOG) performance status, laboratory components of Child-Pugh score, i.e. albumin, bilirubin, and prothrombin time (PT), alpha-fetoprotein (AFP), tumour characteristics, portal vein thrombosis, and LMR were associated with OS (Table 3). Subsequent multivariate analysis identified that LMR was significantly associated with OS (HR, 0.88; 95% CI, 0.81‒0.97; P=0.007). Treatment in Asia (HR, 3.80, 95% CI, 2.03‒7.10; P<0.001), previous treatment (HR, 0.58; 95% CI, 0.42‒0.81; P=0.001), ECOG performance status (HR, 1.33; 95% CI, 1.00‒1.76; P=0.049), log10AFP (HR, 1.25; 95% CI, 1.13‒1.38; P<0.001), lymph node metastasis (HR, 1.50; 95% CI, 1.05‒2.13; P=0.025), and Child‒Pugh score (HR, 1.19; 95% CI, 1.03‒1.38; P=0.017) were also significantly predicted OS.
Table 3.
Univariate and multivariate analyses of the factors for overall survival in the entire cohort
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| Age – per 1 yr | 0.99 (0.98‒1.00) | 0.046 | 0.99 (0.98‒1.00) | 0.15 |
| Male sex | 1.33 (0.93‒1.89) | 0.12 | ||
| Treatment location | 0.049 | < 0.001 | ||
| North America | Reference | Reference | ||
| Asia | 1.35 (1.00‒1.84) | 3.80 (2.03‒7.10) | ||
| BMI – per 1 kg/m2 | 0.98 (0.95‒1.02) | 0.28 | ||
| Previous treatment | 0.61 (0.47‒0.78) | < 0.001 | 0.58 (0.42‒0.81) | 0.001 |
| Etiology of liver disease | 0.07 | 0.35 | ||
| HBV | Reference | Reference | ||
| Hepatitis C virus | 1.22 (0.82‒1.82) | 2.02 (1.02‒3.99) | ||
| Alcohol | 0.93 (0.56‒1.53) | 1.34 (0.67‒2.69) | ||
| NAFLD | 0.65 (0.34‒1.22) | 1.19 (0.55‒2.60) | ||
| Others or unknown | 0.59 (0.37‒0.92) | 1.13 (0.56‒2.25) | ||
| Cirrhosis | 1.34 (1.04‒1.72) | 0.024 | 1.09 (0.82‒1.47) | 0.55 |
| ECOG PS, 1 or higher | 1.52 (1.20‒1.94) | < 0.001 | 1.33 (1.00‒1.76) | 0.049 |
| Lymphocyte, per 100/μL | 0.98 (0.96‒1.00) | 0.014 | ||
| Monocyte, per 100/μL | 1.08 (1.03‒1.15) | 0.005 | ||
| LM ratio, per 1 unit | 0.88 (0.81‒0.95) | 0.001 | 0.88 (0.81‒0.97) | 0.007 |
| Platelet, per 10000/μL | 1.01 (1.00‒1.03) | 0.10 | ||
| Albumin, per 1 g/dL | 0.46 (0.36‒0.59) | < 0.001 | ||
| Bilirubin, per 1 mg/dL | 1.30 (1.18‒1.43) | < 0.001 | ||
| ALT, per 1 IU/L | 1.00 (1.00‒1.01) | 0.10 | ||
| PT INR, per 1 unit | 4.77 (2.83‒8.04) | < 0.001 | ||
| Creatinine, per 1 mg/dL | 0.91 (0.66‒1.25) | 0.54 | ||
| Log10AFP, per 1 ng/mL | 1.12 (1.08‒1.16) | < 0.001 | 1.25 (1.13‒1.38) | < 0.001 |
| HBV DNA, 20,000 IU/mL or higher | 1.36 (0.92‒2.00) | 0.13 | ||
| Tumor characteristics | < 0.001 | 0.72 | ||
| Nodular lesion(s) | Reference | Reference | ||
| No liver lesion | 0.62 (0.45‒0.85) | 0.87 (0.61‒1.25) | ||
| Infiltrative lesion(s) | 1.39 (1.06‒1.82) | 1.01 (0.69‒1.49) | ||
| Portal vein thrombosis | 1.78 (1.40‒2.27) | < 0.001 | 1.27 (0.91‒1.78) | 0.17 |
| Lymph node metastasis | 1.33 (0.98‒1.79) | 0.07 | 1.50 (1.05‒2.13) | 0.025 |
| Distant metastasis | 0.99 (0.76‒1.29) | 0.96 | ||
| CP score, per 1 point | 1.34 (1.20‒1.49) | < 0.001 | 1.19 (1.03‒1.38) | 0.017 |
CI, confidence interval; BMI, body mass index; HBV, hepatitis B virus; NAFLD, non-alcoholic fatty liver disease; ECOG PS, Eastern Cooperative Oncology Group Performance Status; LM ratio, lymphocyte/monocyte ratio; ALT, alanine aminotransferase; PT, prothrombin time; INR, international normalized ratio; AFP, alpha-fetoprotein; CP score, Child-Pugh score.
In terms of PFS, a total of 238 (80%) patients progressed during sorafenib treatment (Fig., Supplemental Digital Content 2). When analysed by Cox proportional hazards models, age, treatment location, body mass index, aetiology of liver disease, albumin, AFP levels, portal vein thrombosis, and distant metastasis were significantly associated with progression by univariate analysis (Table, Supplemental Digital Content 3). Neither LMR nor lymphocyte or monocyte counts predicted PFS (HR for LMR, 0.96; 95% CI, 0.88‒1.03; P=0.25). After multivariate analysis, age (HR, 0.98; 95% CI, 0.96‒0.99; P=0.003), treatment in Asia (HR, 3.31; 95% CI, 1.69‒6.49; P<0.001), albumin (HR, 0.65; 95% CI, 0.48‒0.88; P=0.006), and log10AFP (HR, 1.13; 95% CI, 1.02‒1.25; P=0.024) remained statistically significant.
3.2.2. Asia Cohort
Due to the impact of treatment location on clinical outcomes and the significant differences in blood cell counts observed between the two institutions, we performed a subgroup analysis including patients treated in South Korea only. Similar to the results derived from the entire cohort, previous treatment, cirrhosis, ECOG performance status, platelet count, AFP level, tumour characteristics, portal vein thrombosis, lymph node metastasis, Child‒Pugh score and the laboratory components of the Child‒Pugh score were shown to be significant predictors for OS by the univariate analysis (Table 4). Lymphocytes (HR, 0.97, 95% CI, 0.95‒0.99; P=0.004), monocytes (HR, 1.11; 95% CI, 1.04‒1.17; P<0.001), and the LMR (HR, 0.84, 95% CI, 0.77‒0.92; P<0.001) were all associated with OS. After multivariate adjustment, low LMR predicted OS (HR, 0.89; 95% CI, 0.81‒0.97; P=0.010), together with previous treatment (HR, 0.61; 95% CI, 0.43‒0.87; P=0.006), ECOG performance status (HR, 1.37; 95% CI, 1.01‒1.84; P=0.042), AFP levels (HR for log10 transformed values, 1.27; 95% CI, 1.14‒1.41; P<0.001), lymph node metastasis (HR, 1.77; 95% CI, 1.23‒2.54; P=0.002), and Child‒Pugh score (HR, 1.24; 95% CI, 1.05‒1.47; P=0.010).
Table 4.
Univariate and multivariate analyses of the factors for overall survival in Asia cohort
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| Age – per 1 yr | 0.99 (0.98‒1.01) | 0.27 | ||
| Male sex | 1.20 (0.81‒1.78) | 0.35 | ||
| BMI – per 1 kg/m2 | 0.98 (0.93‒1.03) | 0.40 | ||
| Previous treatment | 0.43 (0.32‒0.58) | < 0.001 | 0.61 (0.43‒0.87) | 0.006 |
| Etiology of liver disease | 0.69 | |||
| HBV | Reference | |||
| Hepatitis C virus | 1.36 (0.60‒3.06) | |||
| Alcohol | 1.51 (0.71‒3.21) | |||
| NAFLD | 0.94 (0.46‒1.90) | |||
| Others or unknown | 0.74 (0.33‒1.67) | |||
| Cirrhosis | 1.32 (1.00‒1.74) | 0.049 | 1.13 (0.83‒1.54) | 0.44 |
| ECOG PS, 1 or higher | 1.57 (1.20‒2.06) | < 0.001 | 1.37 (1.01‒1.84) | 0.042 |
| Lymphocyte, per 100/μL | 0.97 (0.95‒0.99) | 0.004 | ||
| Monocyte, per 100/μL | 1.11 (1.04‒1.17) | < 0.001 | ||
| LM ratio, per 1 unit | 0.84 (0.77‒0.92) | < 0.001 | 0.89 (0.81‒0.97) | 0.010 |
| Platelet, per 10000/μL | 1.03 (1.01‒1.04) | 0.001 | 1.01 (0.99‒1.03) | 0.29 |
| Albumin, per 1 g/dL | 0.42 (0.31‒0.56) | < 0.001 | ||
| Bilirubin, per 1 mg/dL | 1.75 (1.41‒2.17) | < 0.001 | ||
| ALT, per 1 IU/L | 1.00 (1.00‒1.00) | 0.88 | ||
| PT INR, per 1 unit | 9.61 (3.91‒23.61) | < 0.001 | ||
| Creatinine, per 1 mg/dL | 0.95 (0.71‒1.26) | 0.72 | ||
| Log10AFP, per 1 ng/mL | 1.34 (1.21‒1.47) | < 0.001 | 1.27 (1.14‒1.41) | < 0.001 |
| HBV DNA, 20,000 IU/mL or higher | 1.35 (0.91‒2.00) | 0.13 | ||
| Tumor characteristics | < 0.001 | 0.89 | ||
| Nodular lesion(s) | Reference | Reference | ||
| No liver lesion | 0.56 (0.40‒0.78) | 0.92 (0.64‒1.33) | ||
| Infiltrative lesion(s) | 1.52 (1.12‒2.08) | 1.02 (0.68‒1.54) | ||
| Portal vein thrombosis | 1.86 (1.42‒2.44) | < 0.001 | 1.24 (0.86‒1.78) | 0.25 |
| Lymph node metastasis | 1.69 (1.20‒2.38) | 0.003 | 1.77 (1.23‒2.54) | 0.002 |
| Distant metastasis | 0.80 (0.56‒1.15) | 0.23 | ||
| CP score, per 1 point | 1.55 (1.36‒1.78) | < 0.001 | 1.24 (1.05‒1.47) | 0.010 |
CI, confidence interval; BMI, body mass index; HBV, hepatitis B virus; NAFLD, non-alcoholic fatty liver disease; ECOG PS, Eastern Cooperative Oncology Group Performance Status; LM ratio, lymphocyte/monocyte ratio; ALT, alanine aminotransferase; PT, prothrombin time; INR, international normalized ratio; CP score, Child-Pugh score.
LMR was not associated with PFS by the univariate analysis (HR, 0.90; 95% CI, 0.83‒0.98; P=0.017; Table, Supplementary Digital Content 4). However, age (HR, 0.98; 95% CI, 0.97‒1.00; P=0.012) and infiltrative tumours (HR, 1.43; 95% CI, 0.91‒2.25; P=0.046) remained significant after multivariate adjustment.
3.2.3. North America Cohort
Next we performed a subgroup analysis of the North America cohort. Fifty-two (73%) and 51 (72%) patients died and progressed, respectively, during the study period.
In this cohort, aetiology of liver disease (P=0.09), alanine aminotransferase (ALT) levels (P<0.001), ECOG performance status (P=0.06), portal vein thrombosis (P=0.06), and Child‒Pugh score (P=0.046) were associated with OS by the univariate analysis and ALT levels (HR, 1.02; 95% CI, 1.01‒1.03; P<0.001) remained significant after multivariate analysis (Table, Supplemental Digital Content 5). LMR did not show statistical significance by univariate analysis (HR, 0.93; 95% CI, 0.60‒1.44; P=0.75).
PFS was associated with albumin, ALT, and PT (Table, Supplemental Digital Content 6), and ALT levels (HR, 1.01; 95% CI, 1.00‒1.02; P=0.003) remained statistically significant in the multivariate analysis. LMR was not a significant predictor of PFS by the univariate analysis (HR, 0.95; 95% CI, 0.61‒1.49; P=0.83).
3.3. Survival Prediction Tool
We constructed a survival prediction tool incorporating clinical variables that were shown to be significant in the Cox proportional hazards models. For the entire cohort, OS was 31.7% (95% CI, 26.4–37.1) at 1 year and 11.4% (7.6–15.2) at 3 years. Fig. 2 shows the nomogram constructed to predict the probability of OS at 1 year and 3 years after sorafenib initiation. The concordance score was 0.71 (95% CI, 0.67–0.75). Supplemental Digital Content 7 (Fig.) shows the OS nomogram constructed in the Asia cohort (concordance score, 0.74; 95% CI, 0.70–0.78).
Fig. 2.
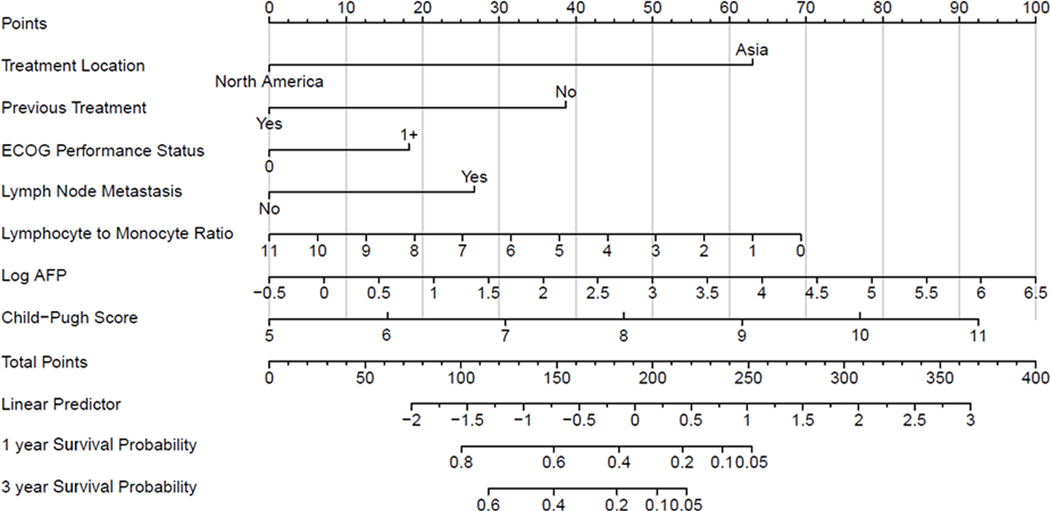
Overall Survival Nomogram.
4. Discussion
The critical role of the immune system in various malignancies, in terms of carcinogenesis, progression, and response to therapy, has been previously demonstrated. LMR reflect the status of immune homeostasis. Lymphocytes in the peripheral blood of cancer patients play a role in the anti-cancer macroenvironment which fights against the tumour cells in general (12). Lymphocytes can also migrate to the tumour tissue, form tumour-infiltrating lymphocytes (TILs) within the tumour mass, and induce apoptosis of tumour cells (12). In contrast, monocytes move toward the tumour and differentiate into TAMs which function as a pro-cancer microenvironment by facilitating tumour cell migration, invasion, metastasis, and angiogenesis and by protecting the tumour from the anti-tumour immune response (1–3, 13–17). A pathologic evaluation of tumour tissue from patients who received liver transplantation due to HCC revealed that low LMR was associated with low CD3-positive to CD68-positive cell ratio (18).
Earlier studies that revealed the prognostic significance of LMR were mostly performed in patients with haematologic malignancies (19, 20). Later, LMR was shown to be prognostic in patients with solid organ cancers (21, 22); however, with regard to HCC, most studies were performed in patients who underwent surgical resection (23). For instance, preoperative LMR was significantly associated with OS and PFS in one study; however, all of the patients had HBV infection (5, 6). A similar study of surgically treated HBV-associated HCCs also reported that LMR was a significant predictor of OS and PFS; however, the study population comprised patients with both early and intermediate stage HCC (24).
With regard to patients treated with sorafenib, only one study has demonstrated an association of LMR with OS and PFS; however, the patient number was relatively small (n=142) and intermediate-stage patients (46 out of 142; 32.4%) were also included (25). In addition, key clinical information of potential impact on the clinical outcomes or blood cell counts, such as tumour characteristics, cirrhosis, and recent anti-cancer treatment, were not available or adequately controlled for in the analysis.
Several preclinical studies have reported that treatment with tyrosine kinase inhibitors is negatively associated with the function of antigen-presenting cells and T-lymphocytes (26, 27). These findings suggest that immune function is potentially important in sorafenib treatment, leading us to investigate the role of LMR in sorafenib-treated patients.
Consistent with the previous studies, lymphocytes and monocytes significantly predicted OS in our unadjusted analysis including HCC patients treated with sroafenib. The impact (HRs) and statistical significance (P values) were greater when lymphocytes and monocytes were combined into a single marker, LMR. However, a specific cut-off point which determined longer vs. shorter OS was not identified (data not shown). Therefore, we incorporated LMR as a continuous variable into the nomogram.
Our nomogram included variables related to demographics (treatment location), clinical history (previous treatment), performance status, tumour characteristics (AFP and lymph node metastasis), and liver function (Child–Pugh score), in addition to LMR. These covariates were selected based on a backward procedure. The highest HR was observed for the variable ‘treatment location’, which largely represent the difference in the patients’ race. Previous randomised controlled trials of sorafenib in HCC demonstrated that survival time of Asian patients were shorter than that of non-Asian patients (28, 29), even for those who received placebo. In a study which analysed these trials, Asian race was a poor prognostic factor for OS by the univariate analysis in the sorafenib arm (HR, 1.73; P=0.028), but was not statistically significant after multivariate adjustment (8). In our predictive model, Asia cohort, which consists of 100% Asian, was associated with a higher risk of death than North America cohort comprised of 90% Caucasian. It needs to be verified in future studies whether race is a significant predictor for OS in sorafenib-treated patients, after adjusting for immunologic biomarkers such as LMR.
AFP level and Child–Pugh score were significant in our multivariate model. This is consistent with the results of several prior studies (8, 30). Presence of lymph node metastasis was also reported to be a poor prognostic factor for OS in sorafenib-treated patients in a previous publication (31).
Of note, previous treatment was associated with a 42% reduction in risk of death. Findings from an observational registry of sorafenib-treated patients revealed that concomitant or prior treatment with TACE was associated with markedly longer OS time (32). A secondary analysis of a study which compared sorafenib vs. sunitinib also identified that prior surgical or locoregional therapy was associated with better OS in patients who received sorafenib (33). Considering more than half of patients receiving curative therapy for HCC experience recurrence and a considerable number of sorafenib candidates were treatment-experienced (28, 29), identifying ‘previous treatment’ as a predictive factor and including it in the risk prediction model could have utility in the clinical setting.
Meanwhile, in North America cohort where LMR was not associated with outcomes, higher ALT levels predicted poorer OS and PFS. In contrast, in Asia cohort where LMR was an independent predictor of the outcomes, ALT levels did not show statistical significance. As shown in our analyses, median HBV DNA levels were not markedly high and only 30% of patients showed HBV DNA titre of >20,000 IU/mL (data not shown), probably due to the use of nucleos(t)ide analogues. Also, the median ALT levels are significant lower in Asia cohort when compared to that of North America cohort, where chronic hepatitis C virus (HCV) is more prevalent and effective treatment options were limited before 2015. From these results, it can be speculated that LMR performs better in predicting outcomes of HCC patients when chronic inflammation, which definitely influences blood cell counts, is under good control. Thanks to direct-acting antivirals, HCV can be efficiently eradicated in >90% of patients who receive treatments (34). If these patients develop HCC, LMR might serve as a useful prognostic tool in North American patients as well.
LMR measured before sorafenib administration did not predict PFS. Studies of patients with ovarian cancer (35), gastric cancer (36), upper urinary tract cancer (37) did not observe an association between LMR and event-free or PFS. These findings suggest that response to anti-cancer therapy is determined by multiple factors including tumour characteristics and the mechanism of the drugs; however, the eventual outcome such as survival might be modulated by the patient’s overall immunologic status.
Of note, patients with low LMR showed more infiltrative tumours and invasion to regional lymph nodes whereas those with high LMR were more likely to have distant metastases at the time of sorafenib initiation. It is plausible that tumour cells, which invade blood vessels and enter into the systemic circulation, sensitise the immune system at the time of metastatic colonisation, resulting in increased lymphocyte counts and high LMR. Our findings suggest that the predictive ability of high LMR is primarily the result of eliminating tumour cells that are already in the systemic circulation, rather than suppression of regional proliferation in the localised disease, during sorafenib treatment.
Before utilising LMR and our nomogram in clinical practice, it would be helpful to demonstrate the direct correlation between LMR and tumour pathology. In this study, we did not determine whether patients with high LMR actually showed high TILs and low TAMs. Considering that the diagnosis of HCC does not mandate biopsy and physicians’ tendency to avoid invasive procedures in advanced-stage HCC patients due to their perceived bleeding risk, it may be neither realistic nor ethical to obtain the tumour tissue in every patient. However, additional analyses using readily available data from clinical trials to correlate with immunologic phenotypes derived from the tumour tissue and peripheral blood would be possible. Furthermore, our nomogram could be used as a surrogate for immunologic criteria during analyses of the results from clinical trials or the design of future studies, particularly those examining the efficacy of immunotherapy.
This study included a relatively large number of patients who were uniformly treated with sorafenib due to advanced stage HCC at two international tertiary referral centres; however, the majority of patients were of Asian or Caucasian race. Considering the high prevalence of HBV infection and the typical diagnosis of HCC at advanced stages in African patients (38), it is definitely important to validate whether LMR and our nomogram are prognostic in African HCC patients, as well as in a larger Caucasian cohort, particularly in whom HCV is well controlled. Finally, as the LMR was identified as a predictor of OS in advanced HCC patients treated with sorafenib, it would be of interest to assess the dynamic change in LMR during treatment on a regular basis and determine whether changes in LMR are associated with clinical outcomes in future studies.
In conclusion, LMR measured prior to initiation of sorafenib was significantly associated with OS in patients with advanced-stage HCC. When considering sorafenib treatment in advanced HCC patients, a new OS nomogram incorporating LMR can aid in educating patients, prognosticating and making prognosis-based decisions for physicians. In addition, it could potentially be utilised in selecting eligible patients for future clinical trials.
Supplementary Material
Supplemental Digital Content 1 Kaplan-Meier Estimate of Overall Survival in the Entire Cohort. CI, Confidence Interval.
Supplemental Digital Content 2 Kaplan-Meier Estimate of Progression-Free Survival in the Entire Cohort. CI, Confidence Interval.
Supplemental Digital Content 4 Univariate and Multivariate Analyse of the Factors for Progression-Free Survival in Asia Cohort.
Supplemental Digital Content 5 Univariate and Multivariate Analyses of the Factors for Overall Survival in North American Cohort.
Supplemental Digital Content 6 Univariate and Multivariate Analyses of the Factors for Progression-Free Survival in North America Cohort.
Supplemental Digital Content 3 Univariate and Multivariate Analyses of the Factors for Progression-Free Survival in the Entire Cohort.
Supplemental Digital Content 7 Overall Survival Nomogram in Asia Cohort.
Acknowledgments
Compliance with Ethical Requirements
Financial Disclosure: National Institutes of Health to LRR (R01 CA 186566), the Mayo Clinic Hepatobiliary SPORE (P50 CA 210964), the Mayo Clinic Cancer Center (P30 CA 15083), the Mayo Clinic Center for Clinical and Translational Science (UL1 TR 002377)
Dr. Lewis Roberts has received grant support from ARIAD Pharmaceuticals, Bayer, BTG International, Exact Sciences, Gilead Sciences, Glycotest Inc., RedHill Inc., Target PharmaSolutions, and Wako Diagnostics; he has provided advisory services to Bayer, Exact Sciences, Gilead Sciences, GRAIL Inc., QED Therapeutics and TAVEC.
Informed consent in Human Subjects: The institutional review board of the Asan Medical Center (approval number: 2019-0685) and the Mayo Clinic (approval number: 15-006298) granted a waiver of informed consent and approval of this observational study with deidentified data.
Abbreviations
- LMR
lymphocyte to monocyte ratio
- TAM
tumour-associated macrophage
- HCC
hepatocellular carcinoma
- OS
overall survival
- PFS
progression-free survival
- SD
standard deviation
- IQR
interquartile ranges
- HR
hazard ratio
- HBV
hepatitis B virus
- ECOG
Eastern Cooperative Oncology Group
- PT
prothrombin time
- AFP
alpha-fetoprotein
- TIL
tumour-infiltrating lymphocytes
- ALT
alanine aminotransferase
- HCV
hepatitis C virus
Footnotes
Conflicts of Interest statements: The other authors have no conflicts of interests to declare.
References
- 1.Dirkx AE, Oude Egbrink MG, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol. 2006;80(6):1183–96. Epub 2006/09/26. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- 2.Ribatti D, Nico B, Crivellato E, Vacca A. Macrophages and tumor angiogenesis. Leukemia. 2007;21(10):2085–9. Epub 2007/09/20. doi: 10.1038/sj.leu.2404900. [DOI] [PubMed] [Google Scholar]
- 3.Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284(49):34342–54. Epub 2009/10/17. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song W, Tian C, Wang K, Zhang R-j, Zou S-b. The pretreatment lymphocyte to monocyte ratio predicts clinical outcome for patients with hepatocellular carcinoma: A meta-analysis. Sci Rep. 2017;7:46601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin ZX, Ruan DY, Li Y, Wu DH, Ma XK, Chen J, et al. Lymphocyte-to-monocyte ratio predicts survival of patients with hepatocellular carcinoma after curative resection. World J Gastroenterol. 2015;21(38):10898–906. Epub 2015/10/20. doi: 10.3748/wjg.v21.i38.10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu SJ, Lin YX, Ye H, Li FY, Xiong XZ, Cheng NS. Lymphocyte to monocyte ratio and prognostic nutritional index predict survival outcomes of hepatitis B virus-associated hepatocellular carcinoma patients after curative hepatectomy. J Surg Oncol. 2016;114(2):202–10. Epub 2016/05/21. doi: 10.1002/jso.24297. [DOI] [PubMed] [Google Scholar]
- 7.Cabrera R, Ararat M, Xu Y, Brusko T, Wasserfall C, Atkinson MA, et al. Immune modulation of effector CD4+ and regulatory T cell function by sorafenib in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2013;62(4):737–46. Epub 2012/12/12. doi: 10.1007/s00262-012-1380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J Hepatol. 2017;67(5):999–1008. Epub 2017/07/09. doi: 10.1016/j.jhep.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 9.Hipp MM, Hilf N, Walter S, Werth D, Brauer KM, Radsak MP, et al. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood. 2008;111(12):5610–20. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Huang Y, Reiberger T, Duyverman AM, Huang P, Samuel R, et al. Differential effects of sorafenib on liver versus tumor fibrosis mediated by stromal-derived factor 1 alpha/C-X-C receptor type 4 axis and myeloid differentiation antigen-positive myeloid cell infiltration in mice. Hepatology. 2014;59(4):1435–47. Epub 2013/11/19. doi: 10.1002/hep.26790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R, et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology. 2014;59(4):1415–26. Epub 2013/09/05. doi: 10.1002/hep.26731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411(6835):380–4. Epub 2001/05/18. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 13.Malek E, de Lima M, Letterio JJ, Kim BG, Finke JH, Driscoll JJ, et al. Myeloid-derived suppressor cells: The green light for myeloma immune escape. Blood Rev. 2016;30(5):341–8. Epub 2016/05/02. doi: 10.1016/j.blre.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206(6):1327–37. Epub 2009/05/20. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–8. Epub 2004/01/07. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 16.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–6. Epub 2006/01/28. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362(10):875–85. Epub 2010/03/12. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mano Y, Yoshizumi T, Yugawa K, Ohira M, Motomura T, Toshima T, et al. Lymphocyte-to-Monocyte Ratio Is a Predictor of Survival After Liver Transplantation for Hepatocellular Carcinoma. Liver Transpl. 2018;24(11):1603–11. Epub 2018/06/13. doi: 10.1002/lt.25204. [DOI] [PubMed] [Google Scholar]
- 19.Lin B, Chen C, Qian Y, Feng J. Prognostic role of peripheral blood lymphocyte/monocyte ratio at diagnosis in diffuse large B-cell lymphoma: a meta-analysis. Leuk Lymphoma. 2015;56(9):2563–8. Epub 2015/02/18. doi: 10.3109/10428194.2015.1014367. [DOI] [PubMed] [Google Scholar]
- 20.Porrata LF, Ristow K, Colgan JP, Habermann TM, Witzig TE, Inwards DJ, et al. Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in classical Hodgkin’s lymphoma. Haematologica. 2012;97(2):262–9. Epub 2011/10/14. doi: 10.3324/haematol.2011.050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishijima TF, Muss HB, Shachar SS, Tamura K, Takamatsu Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev. 2015;41(10):971–8. Epub 2015/10/21. doi: 10.1016/j.ctrv.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Mao Y, Chen D, Duan S, Zhao Y, Wu C, Zhu F, et al. Prognostic impact of pretreatment lymphocyte-to-monocyte ratio in advanced epithelial cancers: a meta-analysis. Cancer Cell Int. 2018;18:201. Epub 2018/12/12. doi: 10.1186/s12935-018-0698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang YT, Jiang JH, Yang HJ, Wu ZJ, Xiao ZM, Xiang BD. The lymphocyte-to-monocyte ratio is a superior predictor of overall survival compared to established biomarkers in HCC patients undergoing liver resection. Sci Rep. 2018;8(1):2535. Epub 2018/02/09. doi: 10.1038/s41598-018-20199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li GJ, Ji JJ, Yang F, Xu HW, Bai Y. Preoperative lymphocyte-to-monocyte ratio predicts survival in primary hepatitis B virus-positive hepatocellular carcinoma after curative resection. Onco Targets Ther. 2017;10:1181–9. Epub 2017/03/07. doi: 10.2147/ott.s110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Z, Xu L, Zhuang L, Ning Z, Zhang C, Yan X, et al. Role of monocyte-to-lymphocyte ratio in predicting sorafenib response in patients with advanced hepatocellular carcinoma. Onco Targets Ther. 2018;11:6731–40. Epub 2018/10/24. doi: 10.2147/ott.s173275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appel S, Rupf A, Weck MM, Schoor O, Brummendorf TH, Weinschenk T, et al. Effects of imatinib on monocyte-derived dendritic cells are mediated by inhibition of nuclear factor-kappaB and Akt signaling pathways. Clin Cancer Res. 2005;11(5):1928–40. Epub 2005/03/10. doi: 10.1158/1078-0432.Ccr-04-1713. [DOI] [PubMed] [Google Scholar]
- 27.Borg C, Terme M, Taieb J, Menard C, Flament C, Robert C, et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest. 2004;114(3):379–88. Epub 2004/08/03. doi: 10.1172/jci21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. Epub 2008/12/20. doi: 10.1016/s1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 29.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. Epub 2008/07/25. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 30.Sohn W, Paik YH, Cho JY, Lim HY, Ahn JM, Sinn DH, et al. Sorafenib therapy for hepatocellular carcinoma with extrahepatic spread: treatment outcome and prognostic factors. J Hepatol. 2015;62(5):1112–21. Epub 2014/12/17. doi: 10.1016/j.jhep.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Cho JY, Paik YH, Lim HY, Kim YG, Lim HK, Min YW, et al. Clinical parameters predictive of outcomes in sorafenib-treated patients with advanced hepatocellular carcinoma. Liver international : official journal of the International Association for the Study of the Liver. 2013;33(6):950–7. Epub 2013/04/23. doi: 10.1111/liv.12168. [DOI] [PubMed] [Google Scholar]
- 32.Geschwind JF, Kudo M, Marrero JA, Venook AP, Chen XP, Bronowicki JP, et al. TACE Treatment in Patients with Sorafenib-treated Unresectable Hepatocellular Carcinoma in Clinical Practice: Final Analysis of GIDEON. Radiology. 2016;279(2):630–40. Epub 2016/01/09. doi: 10.1148/radiol.2015150667. [DOI] [PubMed] [Google Scholar]
- 33.Abdel-Rahman O. Impact of baseline characteristics on outcomes of advanced HCC patients treated with sorafenib: a secondary analysis of a phase III study. J Cancer Res Clin Oncol. 2018;144(5):901–8. Epub 2018/02/20. doi: 10.1007/s00432-018-2610-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghany MG, Morgan TR. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2020;71(2):686–721. Epub 2019/12/10. doi: 10.1002/hep.31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwon BS, Jeong DH, Byun JM, Lee TH, Choi KU, Song YJ, et al. Prognostic value of preoperative lymphocyte-monocyte ratio in patients with ovarian clear cell carcinoma. J Cancer. 2018;9(7):1127–34. Epub 2018/04/21. doi: 10.7150/jca.24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma JY, Liu Q. Clinicopathological and prognostic significance of lymphocyte to monocyte ratio in patients with gastric cancer: A meta-analysis. Int J Surg. 2018;50:67–71. Epub 2018/01/14. doi: 10.1016/j.ijsu.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Zhu X, Wu SQ, Xu R, Wang YH, Zhong ZH, Zhang L, et al. The evaluation of monocyte lymphocyte ratio as a preoperative predictor in urothelial malignancies: a pooled analysis based on comparative studies. Sci Rep. 2019;9(1):6280. Epub 2019/04/20. doi: 10.1038/s41598-019-42781-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang JD, Mohamed EA, Aziz AO, Shousha HI, Hashem MB, Nabeel MM, et al. Characteristics, management, and outcomes of patients with hepatocellular carcinoma in Africa: a multicountry observational study from the Africa Liver Cancer Consortium. The lancet Gastroenterology & hepatology. 2017;2(2):103–11. Epub 2017/04/14. doi: 10.1016/s2468-1253(16)30161-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1 Kaplan-Meier Estimate of Overall Survival in the Entire Cohort. CI, Confidence Interval.
Supplemental Digital Content 2 Kaplan-Meier Estimate of Progression-Free Survival in the Entire Cohort. CI, Confidence Interval.
Supplemental Digital Content 4 Univariate and Multivariate Analyse of the Factors for Progression-Free Survival in Asia Cohort.
Supplemental Digital Content 5 Univariate and Multivariate Analyses of the Factors for Overall Survival in North American Cohort.
Supplemental Digital Content 6 Univariate and Multivariate Analyses of the Factors for Progression-Free Survival in North America Cohort.
Supplemental Digital Content 3 Univariate and Multivariate Analyses of the Factors for Progression-Free Survival in the Entire Cohort.
Supplemental Digital Content 7 Overall Survival Nomogram in Asia Cohort.


