Abstract
Background: Alzheimer's disease (AD) is a chronic neurodegenerative disorder frequently accompanied by cerebral small vessel disease (CSVD). However, the influence of CSVD on the brain functional connectivity in subjects along the AD continuum is still largely unknown. The current study combined the static and dynamic functional network connectivity (FNC) to explore the underlying mechanism.
Materials and Methods: In this study, we included 182 healthy controls, 27 individuals with subjective cognitive decline (SCD), 27 with SCD+CSVD, 104 with mild cognitive impairment (MCI), 123 with MCI+CSVD, 16 with AD, and 62 with AD+CSVD. We examined the static and dynamic FNC within the default mode, salience, and cognitive control domains. We also assessed the association between atypical FNC patterns and cognitive impairments, as well as the pathologies.
Results: Static FNC results showed progressively increased within-domain connectivity and decreased between-domain connectivity along the AD continuum, especially in CSVD subjects. Dynamic FNC in CSVD subjects showed more occurrences in a highly modularized state and fewer occurrences in the diffusely connected state. Further analysis showed that neuropathology and CSVD burden divergently affect the FNC changes.
Conclusions: The overall results demonstrate divergent abnormalities of FNC in CSVD and non-CSVD individuals along the AD continuum, which were divergently affected by neuropathology and CSVD burden. Specifically, those with CSVD show more static and dynamic FNC impairments, associated with cognitive decline. These findings may advance our understanding of the effect of CSVD on AD onset and progression, and provide potential hints for clinical treatment.
Impact statement
By combining the static and dynamic brain functional network connectivity (FNC), our study explored the effect of vascular pathology on FNC in subjects along the Alzheimer's disease (AD) continuum. Results demonstrate divergent FNC abnormalities in individuals with/without cerebral small vessel disease (CSVD) along the AD continuum, which could be divergently affected by neuropathology (amyloid and tau) and CSVD burden. Notably, AD-continuum subjects with CSVD show more static and dynamic FNC impairments, associated with cognitive decline. Conclusively, these findings may advance our understanding of the effect of vascular pathology on AD onset and progression, and provide potential hints for clinical treatment.
Keywords: Alzheimer's disease, cerebral small vessel disease, functional network connectivity, resting-state functional connectivity, white matter hyperintensities
Introduction
Alzheimer's disease (AD) is a multifactorial neurodegenerative disease, clinically characterized by progressive memory problems and other cognitive impairments. In addition to the neuropathology (i.e., β-amyloid and neurofibrillary tangles), cerebral small vessel disease (CSVD) has been recognized as crucial pathogenesis in AD (Azarpazhooh et al., 2018). The coexistence of neuropathology and vascular pathology is related to higher dementia risk and greater cognitive decline (Vemuri et al., 2015). Modifying vascular risks may be helpful to reduce the risk of AD (Xu et al., 2015). Thus, understanding the effects of CSVD on subjects in the AD continuum may unveil the neural mechanism of vascular pathology on dementia and shed light on future clinical interventions.
Derived from functional magnetic resonance imaging data, functional network connectivity (FNC) between intrinsic connectivity networks has shown great promise in diverse applications to neurodegenerative disease (Fu, 2019). AD is regarded as a disease with a wide range of functional disconnection featuring large-scale brain network impairments (Delbeuck et al., 2003), especially involving the triple network model (Menon, 2011), including the default mode network (DMN), the salience network (SN), and the cognitive control (CC) network. Functionally, the DMN is associated with memory (Buckner et al., 2008), and the CC is associated with executive function (Seeley et al., 2007), with the SN serving as the “dynamic switch” between them (Menon and Uddin, 2010). The integration and segregation of these three networks are essential for maintaining cognition (Greicius, 2012) and work as the key mechanism underlying cognitive impairments in AD.
Previous neuroimage studies reported functional connectivity (FC) impairments of SN and CC in AD (He et al., 2014; Zhao et al., 2019). Moreover, the functional disconnection between the SN and CC/DMN was also found to be associated with AD progression (He et al., 2014). This may be related to the pathological changes in these networks. For example, DMN is vulnerable to AD-related amyloid accumulation years before AD onset (Kvavilashvili et al., 2020), which is accompanied by disruptions in FC and further leads to cognitive impairments (Greicius et al., 2009; Sebastian Palmqvist et al., 2017). On the contrary, the CSVD has also been associated with large-scale brain dysfunction, especially the disruption of FC involved CC and DMN (Chong et al., 2017; Kim et al., 2016; Vipin et al., 2018). Considering that both previous AD and CSVD studies reported overlapping FC abnormalities and CSVD is a common feature accompanied with AD, it is important to figure out the influence of CSVD on FC abnormalities in subjects along the AD continuum.
The abovementioned studies focused on static FC and did not study the wealth of information that can be derived from its temporal properties. Increasing evidence has shown that studying the dynamic fluctuations of brain connectivity might reveal more information that cannot be observed from its static counterpart (Allen et al., 2014; Calhoun et al., 2014; de Lacy et al., 2019; Espinoza et al., 2019; Fu et al., 2019). Several studies focusing on neurodegenerative disease reported the correlation between dynamic FC and clinical symptoms, as well as pathology. Hahn A et al. identified a significant association between Aβ deposition in DMN and dynamic FC in pre-clinical AD (Hahn et al., 2019). Fu et al. found that AD patients show distinct dynamic FC patterns with patients with subcortical ischemic vascular disease (Fu et al., 2019). Taken together, it is reasonable to believe that studying dynamic FC in addition to static FC would provide a more comprehensive picture of functional brain abnormalities that are related to AD and CSVD.
In this study, we aimed to explore large-scale brain network changes in subjects along the AD continuum with and without CSVD by combining the static and dynamic FNC analyses. We hypothesize that subjects along the AD continuum with and without CSVD might have common and also different changes in both static and dynamic FNC, which might also associate with cognitive dysfunction.
Materials and Methods
Study participants
Data used in this study are obtained from the Alzheimer's disease Neuroimaging Initiative (ADNI) database (Supplementary Material S1 provides detailed information about ADNI). The ADNI study was approved by all participating sites' institutional review boards, and all individuals provided written informed consent. Each subject underwent T1-weighted structural scan, T2 fluid attenuated inversion recovery image, resting-state functional magnetic resonance imaging (rsfMRI), and comprehensive neuropsychological assessments (Supplementary Material S2 provides detailed information about the MRI acquisition).
We included 299 healthy controls (HCs), 54 subjects with subjective cognitive decline (SCD), 227 subjects with mild cognitive impairments (MCIs), and 78 AD patients from the ADNI database (see flowchart in Supplementary Material S3). Notably, SCD serves as the typical presymptomatic stage along the AD developing continuum (Jessen et al., 2014) and is currently an important AD research target (detailed description of SCD is listed in Supplementary Material S4). Similar to previous work (Lin et al., 2017), we used the 50th percentile of the white matter hyperintensity (WMH) volume as the cutoff point to separate all subjects into the CSVD group (above the 50th percentile) and non-CSVD group (under the 50th percentile) (Supplementary Material S5, detailed information about WMH segmentation and assessment). Accordingly, we arranged all subjects into seven groups: 182 HC, 27 SCD, 27 SCD+CSVD, 104 MCI, 123 MCI+CSVD, 16 AD, and 62 AD+CSVD.
Neuropsychological assessment and cerebrospinal fluid assessment
Each subject finished comprehensive neuropsychological tests, including assessment of general mental status (Mini-Mental State Examination), memory (ADNI memory composite score), and executive function (ADNI executive function composite score). More detailed information about cognitive scores is provided in Supplementary Material S6.
Cerebrospinal fluid (CSF) biomarkers include Aβ1–42, total tau (T-tau), and P-tau181, measured by the multiplex xMAP Luminex platform as previously described (Shaw et al., 2009).
Image preprocessing
The rsfMRI data preprocessing was performed using the data processing assistant and resting-state fMRI toolbox (DPARSF) (Chao-Gan and Yu-Feng, 2010) based on the Statistical Parametric Mapping 12 (SPM12). The first 5 rsfMRI scans were discarded for the signal equilibrium and subject's adaptation to the scanning noise (Chao-Gan and Yu-Feng, 2010). The remaining 135 images were corrected for timing differences in slice acquisition (Friston et al., 1996). After that, a rigid body motion correction was performed to correct the head motion of the fMRI scans. Subjects with more than 3 mm maximum displacement in any of the x, y, or z directions or 3° of any angular motion were discarded. Then, the fMRI data were subsequently warped to a Montreal Neurological Institute space using the echo-planar images template (Calhoun et al., 2017) and then resampled into 3 × 3 × 3 mm3 cubic voxel. Finally, the fMRI data were smoothed using an 8 mm full-width at half-maximum kernel.
Group independent component analysis
The group independent component (IC) analysis of fMRI Toolbox (GIFT; trendscenter.org/software/gift) version 4.0b was used to extract meaningful functional networks and calculate FNC between networks (Calhoun et al., 2001; Du and Fan, 2013). The NeuroMark framework was applied for the back-reconstruction of individual spatial maps and time-courses (TCs) (Du et al., 2019). Based on the triple-network model, we targeted the independent components (ICs) that are within three functional domains: default mode domain (DM), salience domain (SA), and CC domain.
Static FNC analysis
Before calculating the static FNC and dynamic FNC, we performed the following postprocessing procedures on the TCs to remove physiological and scanner noise: (1) detrending linear, quadratic, and cubic trends; (2) despiking detected outliers using the AFNI 3D DESPIKE function; (3) filtering with a high-frequency cutoff of 0.15 Hz, and (4) conducting multiple regressions of the six realignment parameters and their temporal derivatives.
We calculated Pearson correlation coefficients between the TCs as the measure of static FNC, resulting in a static FNC matrix with a dimension of 17 × 17 for each subject. We then examined the differences between the control group and each noncontrol group using a general linear model (GLM) with age and gender as covariates (i.e., HC vs. SCD, HC vs. SCD+CSVD, HC vs. MCI, HC vs. MCI+CSVD, HC vs. AD, and HC vs. AD+CSVD). We also investigated the static FNC difference between each AD continuum with and without CSVD (i.e., SCD vs. SCD+CSVD, MCI vs. MCI+CSVD, AD vs. AD+CSVD). Results were corrected for multiple comparisons using the false discovery rate (FDR) (Benjamini and Hochberg, 1995) with a correction threshold of q = 0.05.
Dynamic FNC analyses
Similar to most previous studies (Allen et al., 2014; Fu et al., 2019), we used a sliding window approach to estimate the dynamic FNC for each subject. We applied a tapered window (window size = 20, time of repetitions [TRs] = 3 s) with a sliding step as 1 TR, which got T = 115 windows for each subject. The tapered window was obtained by convolving a rectangular window with a Gaussian (σ = 3TR). Notably, tapered windows provide better suppression of spurious correlations and may reduce sensitivity to outliers (Zalesky et al., 2014), and were thus recommended to estimate the sliding window connectivity (Mokhtari et al., 2019). Then, we applied the k-means clustering algorithm (Lloyd, 1982) to explore reoccurring dynamic FNC patterns across subjects. The L1 distance function (city distance) was used to cluster the windowed covariance matrices since the L1-norm works as a more effective similarity measure than the L2 (Euclidean) distance for high-dimensional data (Aggarwal and Keim, 2001). The optimal number of clusters was determined as k = 2 based on the elbow criterion, which is consistent with several previous studies on other types of brain disorders (Kim et al., 2017; Tu et al., 2019). After obtaining the state vector (a vector indicating which state each time point is assigned to), we calculated the fractional occupancy (number of total windows/number of windows assigned to each state) to evaluate the occurrence of dynamic states. Like the statistical analysis on static FNC, we examined the group differences (between controls and noncontrols and between AD continuum with and without CSVD) using a GLM with age and gender as covariates. Results were corrected for multiple comparisons using the FDR with a correction threshold of q = 0.05.
Correlation between FNC features and cognitive scores, pathological changes
We further examined the potential relationship between FNC features and cognitive performance (i.e., memory and executive function), as well as the pathological index (i.e., WMH volume, Aβ, P-tau, and T-tau). The correlation analysis focused on those atypical FNC features with significant group differences. To explore the possible pathological mechanism, we performed Pearson correlation analysis between the static and dynamic FNC alternations and AD neuropathology, as well as WMH volume. To explore the physiological signification, we computed the Pearson correlation between the static and dynamic FNC alternations and cognitive scores.
Results
Demographic and clinical characteristics
Detailed demographic and clinical information is provided in Table 1. We used a chi-squared test and analysis of variance (ANOVA) for categorical (gender, apolipoprotein E [APOE]) and continuous data (age, education), respectively (SPSS, version 19.0). Then, post hoc analysis using a two-sample t-test was performed to reveal the source of ANOVA difference (p < 0.05).
Table 1.
Demographic and Neuropsychological Data
| Demographic characteristics | HC (n = 182) | SCD (n = 27) | SCD+CSVD (n = 27) | MCI (n = 104) | MCI+CSVD (n = 123) | AD (n = 16) | AD+CSVD (n = 62) | F-value | Sig. |
|---|---|---|---|---|---|---|---|---|---|
| Age | 69.48 ± 6.33 | 71.97 ± 4.93 | 75.44 ± 6.27 | 69.09 ± 7.53 | 75.42 ± 6.39 | 70.63 ± 10.62 | 76.39 ± 7.85 | 17.72 | <0.001bdfgh |
| Gender (female) | 120/182 | 16/27 | 16/27 | 50/104 | 52/123 | 8/16 | 25/62 | 4.10 | <0.001cdf |
| Education | 16.71 ± 2.33 | 16.93 ± 2.67 | 16.56 ± 2.49 | 16.63 ± 2.33 | 16.16 ± 2.74 | 16.19 ± 3.04 | 15.77 ± 2.52 | 1.65 | 0.13 |
| APOE 4 | 48/127 | 8/26 | 7/27 | 37/80 | 29/79 | 7/12 | 33/43 | 5.12 | <0.001f |
| GDS | 0.67 ± 1.08 | 1.44 ± 1.34 | 1.33 ± 1.14 | 1.83 ± 1.46 | 1.53 ± 1.37 | 2.50 ± 1.55 | 1.79 ± 1.31 | 14.62 | <0.001abcdef |
| Cognitive scores | |||||||||
| MMSE | 29.23 ± 0.97 | 28.96 ± 1.32 | 29.15 ± 0.91 | 28.14 ± 1.72 | 27.98 ± 1.76 | 21.75 ± 2.67 | 21.56 ± 3.72 | 163.88 | <0.001cdef |
| CDR global | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.50 ± 0.00 | 0.50 ± 0.00 | 0.78 ± 0.26 | 0.91 ± 0.41 | 446.64 | <0.001cdef |
| CDR sum | 0.03 ± 0.11 | 0.02 ± 0.10 | 0.06 ± 0.16 | 1.40 ± 0.85 | 1.51 ± 1.00 | 4.53 ± 1.87 | 4.81 ± 1.75 | 255.79 | <0.001cdef |
| ADNI_MEM | 1.14 ± 0.52 | 1.33 ± 0.62 | 0.87 ± 0.62 | 0.41 ± 0.54 | 0.27 ± 0.64 | −0.72 ± 0.60 | −1.00 ± 0.62 | 107.69 | <0.001bcdefg |
| ADNI_EF | 1.28 ± 0.75 | 1.02 ± 0.72 | 0.79 ± 0.81 | 0.59 ± 0.97 | 0.33 ± 0.88 | −0.63 ± 1.02 | −1.06 ± 0.93 | 52.35 | <0.001bcdef |
| CSF | |||||||||
| Available CSF data (%) | 57.14% | 88.89% | 92.59% | 70.19% | 57.72% | 62.50% | 64.52% | ||
| Aβ1–42 (pg/mL) | 1366.93 ± 687.05 | 1697.16 ± 724.77 | 1192.3 ± 552.78 | 1141.97 ± 590.58 | 1179.58 ± 701.24 | 716.96 ± 409.84 | 640.64 ± 296.93 | 10.23 | <0.001acefg |
| T-Tau (pg/mL) | 234.03 ± 81.82 | 253.94 ± 75.01 | 233.26 ± 90.88 | 272.33 ± 132.84 | 268.72 ± 109.81 | 364.58 ± 127.49 | 354.81 ± 130.18 | 7.93 | <0.001cdef |
| P-Tau181 (pg/mL) | 21.13 ± 8.61 | 22.75 ± 6.62 | 21.21 ± 10.64 | 26.35 ± 15.40 | 25.18 ± 11.82 | 35.94 ± 14.97 | 34.54 ± 13.12 | 8.46 | <0.001cdef |
| WMH | |||||||||
| WMH volume | 0.67 ± 0.52 | 0.80 ± 0.57 | 7.14 ± 8.37 | 0.58 ± 0.51 | 8.32 ± 7.60 | 0.84 ± 0.61 | 8.14 ± 7.70 | 50.91 | <0.001bdfghi |
Data are presented as means ± standard deviations. Notably, not all subjects have CSF data since collecting them is invasive. Thus, 104 out of 182 HC, 24 out of 27 SCD, 25 out of 27 SCD+CSVD, 73 out of 104 MCI, 71 out of 123 MCI+CSVD, 10 out of 16 AD, 40 out of 62 AD+CSVD with CSF data were included in the neuropathological correlation analysis.
a–i: post hoc analysis further revealed the source of ANOVA difference (a: HC vs. SCD; b: HC vs. SCD+CSVD; c: HC vs. MCI; d: HC vs. MCI+CSVD; e: HC vs. AD; f: HC vs. AD+CSVD; g: SCD vs. SCD+CSVD; h: MCI vs. MCI+CSVD; i: AD vs. AD+CSVD) (p < 0.05, significant difference between groups).
AD, Alzheimer's disease; ADNI, the AD Neuroimaging Initiative; ADNI-EF, the composite scores for executive function in ADNI; ADNI-MEM, the composite scores for memory in ADNI; ANOVA, analysis of variance; CDR, Clinical Dementia Rating; CSVD, cerebral small vessel disease; GDS, Geriatric Depression Scale; HC, healthy control; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; SCD, subjective cognitive control; WMH, white matter hyperintensities.
As for the neuropsychological scale, MCI and AD subjects (both CSVD and non-CSVD) showed worse general mental status, memory, and executive function than HC. There was no significant group difference between MCI and AD with and without CSVD, respectively. SCD+CSVD showed worse memory and executive function when compared with HC, and worse memory when compared with SCD without CSVD.
As for the neuropathological data in subgroups, MCI and AD subjects (both CSVD and non-CSVD) showed decreased Aβ1–42, increased P-tau181, and T-tau compared with HC. While there was no difference between MCI/AD with and without CSVD, SCD+CSVD showed decreased Aβ1–42 when compared with SCD without CSVD.
Intrinsic connectivity networks
Based on the triple-network model in AD, we included 17 ICs from the NeuroMark template, organized into three functional domains: DM (ICs 32, 40, 51, 63, 71, and 94), SA (ICs 17, 23, and 33), and CC (ICs 37, 38, 43, 55, 67, 70, 88, and 96). The spatial maps of ICs and the other component information are provided in Supplementary Material S7.
Static FNC results
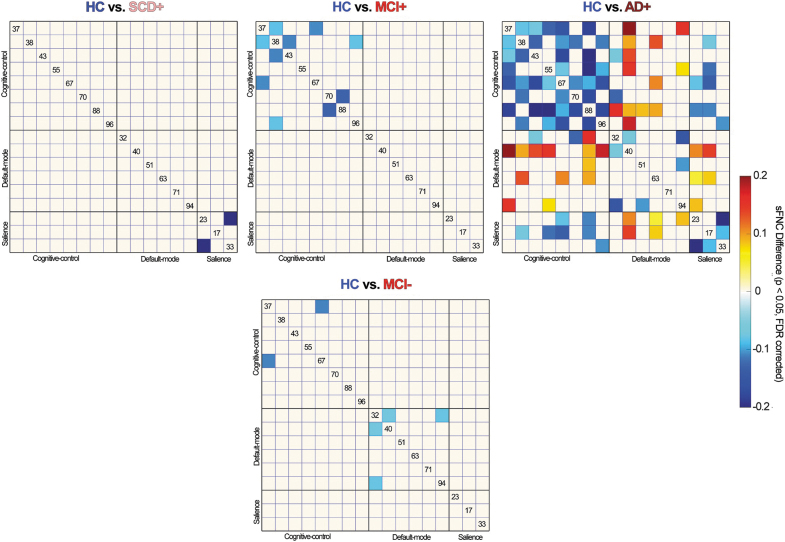
Static FNC analysis shows increased within-domain connectivity, while decreased between-domain connectivity along the AD continuum in subjects with CSVD (Fig. 1). To be specific, compared with HC, subjects with SCD+CSVD show increased within-SA connectivity (between anterior cingulate cortex [ACC] and insula). Subjects with MCI+CSVD show increased within-CC connectivity (between middle cingulate cortex [MCC] and inferior frontal gyrus [IFG], between middle frontal gyrus [MiFG] and MCC, between MiFG and superior medial frontal gyrus [SMFG], between MiFG and IFG, between MiFG and superior frontal gyrus [SFG]). Subjects with AD+CSVD show increased connectivity within domains, DM, SA, and CC, while decreased between-domain connectivity involving DM-CC and DM-SA. Moreover, MCI without CSVD shows increased connectivity within DM (between posterior cingulate cortex [PCC] and precuneus) and CC (between MCC and IFG).
FIG. 1.
Significant static functional network connectivity differences between groups. Top: comparisons between HCs and subjects along the AD continuum with CSVD; bottom: comparisons between HCs and subjects along the AD continuum without CSVD. sFNC with group differences (FDR corrected, q = 0.05) is highlighted in red and blue. AD, Alzheimer's disease; CSVD, cerebral small vessel disease; FDR, false discovery rate; HC, healthy control; MCI, mild cognitive impairment; SCD, subjective cognitive decline; sFNC, static functional network connectivity; −, subjects without CSVD; +, subjects with CSVD.
To visualize the trend clearly, we also provide the results under uncorrected p < 0.05 (Supplementary Material S8). Results show that the CSVD is more associated with within-CC functional alternations, while the non-CSVD is more associated with the within-DM functional alternations. Moreover, the CSVD shows the trend of decreased DM-CC FNC, which started from the SCD stage. In contrast, subjects along the AD continuum without CSVD tend to show the initially increased DM-CC FNC in SCD and MCI, while decreased DM-CC connectivity in AD.
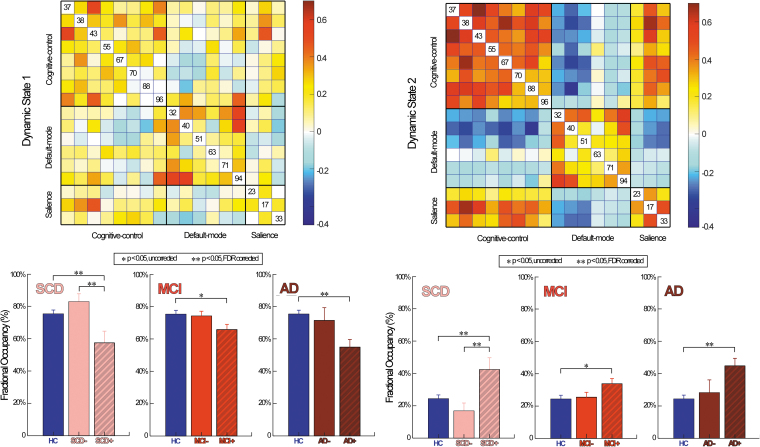
Dynamic FNC states and the temporal properties
Dynamic FNC analysis identified two highly structured FC states: a diffusely connected state 1 and a highly modularized state 2 (Fig. 2). Specifically, state 1 shows a moderate correlation within and between networks involving domains, DM, SA, and CC. By contrast, state 2 shows a high correlation within each functional domain, and between SA and CC, while low correlation between DM-CC, and DM-SA.
FIG. 2.
Dynamic functional network connectivity difference between groups. Top: FNC patterns for dynamic brain states. Bottom: statistical results on subjects along the AD continuum. Bar represents the mean of fractional occupancy of each group, while the error bar represents the standard error of mean. Significant group difference that passes the multiple comparisons is marked by two asterisks (FDR corrected, q = 0.05).
We found increased fractional occupancy in state 2, while decreased fractional occupancy in state 1 along AD continuum, especially in CSVD subjects (Fig. 2). To be specific, SCD+CSVD and AD+CSVD show significantly different occurrences of state changes when compared to HC (FDR, p < 0.05). Moreover, SCD+CSVD also shows significant dynamic FNC changes when compared to SCD. Notably, MCI+CSVD shows a similar trend of dynamic FNC changes when compared to HC but with relatively weaker significance (uncorrected, p < 0.05).
Associations between FNC features and cognitive scores, as well as pathologies
Detailed information about the identified correlations can be found in Supplementary Material S9. First, the atypical FNC features are significantly correlated with the pathological index. As for the dynamic FNC features, fractional occupancy of state 1 is negatively correlated with WMH volume, while fractional occupancy of state 2 is positively correlated with WMH volume. As for static FNC features, (1) increased within-CC (MCC-IFG, MCC-MiFG, MiFG-SMFG, MiFG-SFG, MiFG-IFG) and within-SA (ACC-insula) FNC are positively correlated with WMH volume; (2) increased within-DM (precuneus-PCC) FNC is positively correlated with neuropathology, especially the tau pathology; (3) increased FNC between DM and CC (precuneus-SMFG) is associated with neuropathology; and (4) decreased FNC between DM and CC (precuneus-MCC) is associated with WMH volume.
Second, atypical FNC features were significantly correlated with cognitive scores. For the dynamic FNC features, fractional occupancy of state 1 is positively correlated with memory and executive function, while fractional occupancy of state 2 is negatively correlated with memory and executive function (Fig. 3). As for the static FNC features, both the increased FNC within domains, DM, SA, and CC, and decreased FNC between DM and CC are negatively correlated with memory and executive function.
FIG. 3.
Correlation between dynamic functional network connectivity and cognition. The scatterplots illustrate the associations between cognitive scores and fractional occupancy in the whole samples.
To prove the stability and robustness of the results, we supplementarily repeat our analysis with the following changes: (1) based on whole-brain regions (Supplementary Material S10); (2) with different WMH volume cutoff points (Supplementary Material S11); (3) performing the dynamic FNC analysis using a sliding window approach with different window sizes (Supplementary Material S12); and (4) including APOE 4 genotype as a covariate (Supplementary Material S13).
Discussion
We combined the static and dynamic FNC analysis to investigate the effect of CSVD on functional brain connectivity in subjects along the AD continuum. Our results demonstrate two divergent functional impairment patterns in CSVD and non-CSVD subjects along the AD continuum. Further analysis showed that neuropathology and CSVD burden divergently affect the FNC changes. These static and dynamic FNC impairments in AD subjects with CSVD may provide insights into severer clinical symptoms and provide some hints for clinical treatment.
Divergent impairment patterns of static FNC in AD-continuum subjects with or without CSVD
Our study showed increased within-domain connectivity and decreased between-domain connectivity in all subjects along the AD continuum, which suggests a more segregated and less optimal network function. Thereinto, the subjects with CSVD showed more within-CC functional impairments, while the non-CSVD subjects showed more within-DM functional impairments. This pattern is similar to previous studies that found more DMN alternations in AD, while more CC changes in the CSVD (Chong et al., 2017; Kim et al., 2016; Vipin et al., 2018).
In the current study, the widespread increase of within-CC FNC was observed in all AD-continuum subjects, with the most prominent effect in subjects with CSVD. This may reflect greater influences on CC in the AD-continuum subjects with CSVD. Thereinto, MCC, MiFG, and SFG showed relatively early FC impairments. These results are similar to one previous study, which showed increased MiFG and SFG FC in AD and explained that as an indicator of damages (Zhao et al., 2018). We also found associations between increased within-CC FNC and higher WMH volume, which further proves that vascular pathology is the key mechanism. Moreover, the increased within-CC FNC correlated negatively with cognition. These findings suggested that functional impairment in CC is the representative of CSVD abnormalities, which is related to cognitive decline. Moreover, SCD+CSVD and AD+CSVD also showed increased within-SN FNC. This is similar to previous studies and suggested that SN is vulnerable to functional impairments at the early AD stage (He et al., 2014).
The DMN is essential in cognitive function and has been widely implicated in AD (Buckner et al., 2008; Greicius et al., 2004). Our study showed the trend of increased within-DM FNC in all groups. Thereinto, the non-CSVD tended to suffer relatively earlier and severer DMN FC impairments. Specifically, the precuneus showed the earliest and most significant FC changes. This is consistent with previous conclusions regarding precuneus as the initial target of AD (Buckner et al., 2005). Moreover, such increased within-DM FNC is associated with AD neuropathology, especially the tau, but not WMH volume. Similarly, the theory of cascading network failure in AD proposed that the precuneus predisposes to a tau-related degenerative process due to the high information processing load (Jones et al., 2017). Moreover, we observed the negative association between increased within-DM connectivity and cognition. Conclusively, our findings provide evidence for the influence of concurrent AD and CSVD on within FNC and cognition.
We also observed progressively decreased DM-CC FNC in the CSVD group. Such decreased between-domain FNC was associated with worse memory and executive function. This may reflect a worse communication between networks, suggesting that their whole-brain networks were fragmented into a few large isolated components. Similar results can be found in another study, which reported lower DMN-CC FNC in MCI+CSVD, AD, and AD+CSVD (Vipin et al., 2018). Interestingly, we also found increased FC between the precuneus and SMFG in AD+CSVD, which correlated with AD neuropathology. This may suggest the increased precuneus-SMFG FC as a compensatory alternation in response to AD neuropathology. By contrast, the non-CSVD group showed the trend of initially increased and then decreased FNC between CC and DMN, suggesting less internetwork changes under the separate effect of neuropathology. Moreover, we also observed increased SA-CC FNC, while decreased SA-DM FNC in subjects with CSVD, especially in AD+CSVD. This is partially in line with a previous study showing the functional disconnection between the SN and CC/DMN and proposed that as a marker of both normal aging and AD progression (He et al., 2014). Such alternated between-domain FNC suggested that the neural mechanism underlying executive function is relatively intact, while that of memory function was more impaired. This is consistent with previous ideas, suggesting that memory impairments are the primary manifestation of AD.
Severer dynamic FNC impairments in AD-continuum subjects with CSVD
Dynamic FNC can reflect the temporal properties of FC and provide extra information about the brain alternations underlying different pathologies. To be specific, state 1 features more diffuse FC within and between domains, including the positive couplings between DM and CC. This implied an efficient information transfer between functional networks and suggested an efficient global integration of the brain networks. State 2 was instead characterized by increased within-domain FNC in CC, DMN, and SN, with decreased connectivity between CC and DM. Such increased modularity can be interpreted as a reduced integration of neural networks, leaving each module relatively isolated.
We found increased fractional occupancy in the highly modularized state (state 2), and decreased fractional occupancy in the diffusely connected state (state 1) along the AD continuum, especially in CSVD subjects. Such dynamic FNC alternations were correlated with worsening of cognition, suggesting that an optimal global FC is important for cognition. Similarly, a previous study focused on Parkinson's disease with MCI also found abnormally increased modularity, which was associated with worse memory and visuospatial performance (Baggio et al., 2015). Notably, we also observed increased fractional occupancy in state 2, while decreased fractional occupancy in state 1 in SCD+CSVD when compared with SCD, which have not been shown in the static FNC analysis. This suggests that the effect of CSVD on AD occurs since the early stage. Further analysis showed the relationship between WMH volume and Aβ in SCD (CSVD and non-CSVD). Thus, we speculated that the existence of CSVD might aggravate amyloid deposition at the pre-clinical AD stage and further affect the FNC changes.
These findings confirmed our static FNC results and further extended previous conclusions by findings that not only the brain activity strength but also the changes of brain activity fraction matter in AD. Specifically, the increased highly modularized state, while a decreased diffusely connected state, may work as the underlying mechanism of FNC changes in AD-continuum subjects with CSVD. Such temporal dynamics of FNC were closely associated with the clinical severity of AD, especially in subjects with CSVD.
Neuropathology and CSVD burden divergently affect the FNC changes
WMH volume is more associated with the static FNC changes in CC, while neuropathology is more associated with the static FNC changes in DMN. Moreover, the subjects with CSVD showed more between-domain impairments. These findings suggested that neuropathology and CSVD burden divergently influence FNC. Specifically, the AD neuropathology, namely, the amyloid and tau, starts to deposit from DMN and further leads to the FNC changes. By contrast, CSVD burden is histopathologically associated with myelin and oligodendrocyte changes, which lead to the white matter integrity changes and further affect the FNC (Khalsa et al., 2014).
Our subgroup analysis of the neuropathology showed the lower CSF Aβ in SCD+CSVD when compared with SCD. And further analysis showed the relationship between WMH volume and Aβ in SCD groups (both CSVD and non-CSVD). These findings suggest that CSVD may aggravate amyloid deposition at the early AD stage, while functioning as separate effects at the later stage. Conclusively, these results suggested the effect of CSVD on AD from two aspects: first, aggravating the neuropathology, and second, impairing the FNC.
Limitation
There exist several limitations to our study. First, the sample size of the AD group is relatively small, and thus, we only focused on characterizing those atypical static and dynamic features that show significant group differences. We would like to validate our present results in future studies with more subjects for each group. Second, some individuals did not have CSF data, which may reduce statistical power. Further studies with larger PET sample sizes are needed. Third, we mainly included the WMH volume as the CSVD index considering that different types of CSVD (such as lacunes and microbleeds) might have different etiologies and then affect FC differentially. Although this may help to reduce heterogeneity, it does not cover the effect of the other CSVD index. Further studies with a reasonable comprehensive CSVD index should be conducted.
Conclusion
Our study demonstrated differential patterns of FNC alternations in AD individuals with or without CSVD. The concurrent AD neuropathology and CSVD are associated with significant static FNC changes, namely, the increased within-domain while decreased between-domain FC. Moreover, AD subjects with CSVD tend to show more dynamic FNC changes, which were also closely associated with cognition. Results thus suggested that CSVD and AD have additive effects on FNC changes.
Authors' Contributions
KL and ZF contributed equally to this work. KL designed the study and wrote the first draft of the article. ZF analyzed the MRI data and wrote the protocol. XL, PH, and VDC assisted with study design and interpretation of findings. QZ collected clinical and MRI data. All authors have contributed to and approved the final article. All authors read and approved the final article.
Ethical Approval and Consent to Participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all participants and authorized representatives, and the study partners, before any protocol-specific procedures were carried out in the ADNI study.
Availability of Data and Material
The data sets generated and analyzed during the current study are available in the ADNI study. More details in www.adni-info.org.
Supplementary Material
Contributor Information
Collaborators: for the Alzheimer's Disease Neuroimaging Initiative
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was funded by the National Natural Science Foundation of China (grant no. 81901707) and the National Key Research and Development Program of China (grant no. 2016YFC1306600), as well as the National Institutes of Health grant RF1AG063153.
Supplementary Material
References
- Aggarwal C, Hinneburg A, Keim D. 2001. On the surprising behavior of distance metrics in high dimensional space. In Database Theory ICDT 420–434 [Google Scholar]
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. 2014. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 24:663–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarpazhooh MR, Avan A, Cipriano LE, Munoz DG, Sposato LA, Hachinski V. 2018. Concomitant vascular and neurodegenerative pathologies double the risk of dementia. Alzheimers Dement 14:148–156 [DOI] [PubMed] [Google Scholar]
- Baggio HC, Segura B, Sala-Llonch R, Marti MJ, Valldeoriola F, Compta Y, et al. 2015. Cognitive impairment and resting-state network connectivity in Parkinson's disease. Hum Brain Mapp 36:199–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Series B (Methodological) 57:289–300 [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. 2008. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, et al. 2005. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25:7709–7717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. 2001. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14:140–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Miller R, Pearlson G, Adalı T. 2014. The chronnectome: Time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron 84:262–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Wager TD, Krishnan A, Rosch KS, Seymour KE, Nebel MB, et al. 2017. The impact of T1 versus EPI spatial normalization templates for fMRI data analyses. Hum Brain Mapp 38:5331–5342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao-Gan Y, Yu-Feng Z. 2010. DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JSX, Liu S, Loke YM, Hilal S, Ikram MK, Xu X, et al. 2017. Influence of cerebrovascular disease on brain networks in prodromal and clinical Alzheimer's disease. Brain 140:3012–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lacy N ME, Kutz JN, Calhoun VD. 2019. Sex-related differences in intrinsic brain dynamism and their neurocognitive correlates. Neuroimage 202:116116. [DOI] [PubMed] [Google Scholar]
- Delbeuck X, Van der Linden M, Collette F. 2003. Alzheimer's disease as a disconnection syndrome? Neuropsychol Rev 13:79–92 [DOI] [PubMed] [Google Scholar]
- Du Y, Fan Y. 2013. Group information guided ICA for fMRI data analysis. NeuroImage 69:157–197 [DOI] [PubMed] [Google Scholar]
- Du Y, Fu Z, Sui J, Gao S, Xing Y, Lin D, et al. 2019. NeuroMark: a fully automated ICA method to identify effective fMRI markers of brain disorders. medRxiv:19008631 [Google Scholar]
- Espinoza FA, Liu J, Ciarochi J, Turner JA, Vergara VM, Caprihan A, et al. 2019. Dynamic functional network connectivity in Huntington's disease and its associations with motor and cognitive measures. Hum Brain Mapp 40:1955–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. 1996. Movement-related effects in fMRI time-series. Magn Reson Med 35:346–355 [DOI] [PubMed] [Google Scholar]
- Fu Z, Caprihan A, Chen J, Du Y, Adair JC, Sui J, et al. 2019. Altered static and dynamic functional network connectivity in Alzheimer's disease and subcortical ischemic vascular disease: shared and specific brain connectivity abnormalities. Hum Brain Mapp 40:3203–3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Du YCalhoun VD. 2019. The dynamic functional network connectivity analysis framework. Engineering 5:190–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Kimmel DL. 2012. Neuroimaging insights into network-based neurodegeneration. Curr Opin Neurol 25:727–734 [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. 2004. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A 101:4637–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. 2009. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19:72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Strandberg T, Stomrud E, Nilsson M, van Westen D, Palmqvist S, Ossenkoppele R, Hansson O. 2019. Association between earliest amyloid uptake and functional connectivity in cognitively unimpaired elderly. Cereb Cortex 29:2173–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Qin W, Liu Y, Zhang X, Duan Y, Song J, et al. 2014. Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer's disease. Hum Brain Mapp 35:3446–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Amariglio RE, van Boxtel M, et al. 2014. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement 10:844–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Graff-Radford J, Lowe VJ, Wiste HJ, Gunter JL, Senjem ML, et al. 2017. Tau, amyloid, and cascading network failure across the Alzheimer's disease spectrum. Cortex 97:143–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa S, Mayhew SD, Chechlacz M, Bagary M, Bagshaw AP. 2014. The structural and functional connectivity of the posterior cingulate cortex: comparison between deterministic and probabilistic tractography for the investigation of structure-function relationships. Neuroimage 102:118–127 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Cha J, Lee JM, Shin JS, Jung NY, Kim YJ, et al. 2016. Distinctive resting state network disruptions among Alzheimer's disease, subcortical vascular dementia, and mixed dementia patients. J Alzheimers Dis 50:709–718 [DOI] [PubMed] [Google Scholar]
- Kim J, Criaud M, Cho SS, Diez-Cirarda M, Mihaescu A, Coakeley S, et al. 2017. Abnormal intrinsic brain functional network dynamics in Parkinson's disease. Brain 140:2955–2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvavilashvili L, Niedźwieńska A, Gilbert SJ, Markostamou I. 2020. Deficits in spontaneous cognition as an early marker of Alzheimer's disease. Trends Cogn Sci 24:285–301 [DOI] [PubMed] [Google Scholar]
- Lin PC, Chang FC, Huang HC, Tsai JY, Lin YY, Chung CP. 2017. Greater periventricular white matter hyperintensity severity in basilar artery branch atheromatous disease. BMC Neurol 17:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd SP. 1982. Least squares quantization in PCM. IEEE Trans Inf Theory 28:129–137 [Google Scholar]
- Menon V. 2011. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15:483–506 [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214:655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhtari F, Akhlaghi MI, Simpson SL, Wu G, Laurienti PJ. 2019. Sliding window correlation analysis: modulating window shape for dynamic brain connectivity in resting state. Neuroimage 189:655–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist S, Schöll M, Strandberg O, Mattsson N, Stomrud E, Zetterberg H, Blennow K, et al. 2017. Earliest accumulation of β-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat Commun 8:1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. 2009. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol 65:403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y, Zeng F, Zeng F, Maleki N, Lan L, Li Z, et al. 2019. Abnormal thalamocortical network dynamics in migraine. Neurology 92:e2706–e2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Preboske GM, Kantarci K, et al. 2015. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain 138(Pt 3):761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vipin A, Loke YM, Liu S, Hilal S, Shim HY, Xu X, et al. 2018. Cerebrovascular disease influences functional and structural network connectivity in patients with amnestic mild cognitive impairment and Alzheimer's disease. Alzheimers Res Ther 10:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Tan L, Wang HF, Jiang T, Tan MS, Tan L, et al. 2015. Meta-analysis of modifiable risk factors for Alzheimer's disease. J Neurol Neurosurg Psychiatry 86:1299–1306 [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Cocchi L, Gollo LL, Breakspear M. 2014. Time-resolved resting-state brain networks. Proc Natl Acad Sci U S A 111:10341–10346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Lu H, Metmer H, Li WXY, Lu J. 2018. Evaluating functional connectivity of executive control network and frontoparietal network in Alzheimer's disease. Brain Res 1678:262–272 [DOI] [PubMed] [Google Scholar]
- Zhao Q, Sang X, Metmer H, Swati Z, Lu J; Alzheimer's Disease NeuroImaging I. 2019. Functional segregation of executive control network and frontoparietal network in Alzheimer's disease. Cortex 120:36–48 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated and analyzed during the current study are available in the ADNI study. More details in www.adni-info.org.