Abstract
Purpose: Up to 1.8% of youth identify as transgender; many will be treated with a gonadotropin-releasing hormone agonist (GnRHa). The impact of GnRHa on insulin sensitivity and body composition in transgender youth is understudied. We aimed to evaluate differences in insulin sensitivity and body composition in transgender youth on GnRHa therapy compared with cisgender youth.
Methods: Transgender participants were matched to cisgender participants on age, body mass index, and sex assigned at birth. Transgender males (n=9, ages 10.1–16.0 years) on GnRHa (mean±standard deviation duration of exposure: 20.9±19.8 months) were compared with cisgender females (n=14, ages 10.6–16.2). Transgender females (n=8, ages 12.6–16.1) on GnRHa (11.3±7 months) were compared with cisgender males (n=17, ages 12.5–15.5). Differences in insulin sensitivity (1/[fasting insulin], homeostatic model of insulin resistance [HOMA-IR]), glycemia (hemoglobin A1C [HbA1c], fasting glucose), and body composition (dual-energy X-ray absorptiometry) were evaluated using a mixed linear regression model.
Results: Transgender males had lower 1/fasting insulin and higher HOMA-IR (p=0.031, p=0.01, respectively), fasting glucose (89±4 vs. 79±13 mg/dL, p=0.012), HbA1c (5.4±0.2 vs. 5.2±0.2%, p=0.039), and percent body fat (36±7 vs. 32±5%, p=0.042) than matched cisgender females. Transgender females had lower 1/fasting insulin and higher HOMA-IR (p=0.028, p=0.035), HbA1c (5.4±0.1% vs. 5.1±0.2%, p=0.007), percent body fat (31±9 vs. 24±10%, p=0.002), and lower percent lean mass (66±8 vs. 74±10%, p<0.001) than matched cisgender males.
Conclusion: Transgender youth on a GnRHa have lower estimated insulin sensitivity and higher glycemic markers and body fat than cisgender controls with similar characteristics. Longitudinal studies are needed to understand the significance of these changes. Clinical Trial.gov ID: NCT02550431.
Keywords: assigned sex, body composition, gender dysphoria, gonadotropin-releasing hormone agonist, insulin resistance, transgender
Introduction
In the United States, 0.7–1.8% of youth identify as transgender (gender identity differs from sex at birth).1,2 The Endocrine Society recommends initiation of gonadotropin-releasing hormone agonist (GnRHa) therapy at Tanner stage 2 to halt pubertal changes that do not align with the individual's gender identity.3 There is a rise in the number of patients seeking this treatment at centers around the world.4
Transgender youth treated with GnRHa have improved psychosocial functioning5 and depressive symptoms.6 Furthermore, young transgender children who live openly in their affirmed gender identity and are supported by their families have rates of depression similar to age-matched controls or nontransgender siblings.7 The Endocrine Society and American Academy of Pediatrics recommend gender-affirmative care.3,8 However, despite puberty being a time of significant change in metabolism, the impact of pausing puberty on cardiometabolic health is largely unknown.
Prior studies have shown an increase in body fat and decrease in lean mass in transgender adolescents on GnRHa.9,10 However, participants in one study10 were older and had a more advanced pubertal stage9,10 than the Tanner 2 pubertal development recommended for GnRHa initiation in the Endocrine Society guidelines.3 Therefore, as there are known changes in body composition during puberty11 some of these changes may have already taken place before GnRHa initiation. Thus, data are lacking on the impact of GnRHa on body composition at younger ages or lower pubertal stages. It is also unknown whether GnRHa treatment affects insulin sensitivity or cardiometabolic health in transgender youth, a treatment known to be associated with increased risk of incident diabetes and coronary heart disease in adults.12 We hypothesized that GnRHa treatment during puberty would maintain or improve insulin sensitivity and body composition. This study addresses these gaps by evaluating markers of cardiometabolic health in transgender adolescents in the United States on GnRHa therapy, compared with cisgender adolescents.
The aims of this cross-sectional study were to evaluate insulin sensitivity and body composition among adolescent transgender females (assigned male at birth) and transgender males (assigned female at birth) on GnRHa treatment (before initiation of testosterone or estradiol), matched on sex assigned at birth, age, and body mass index (BMI) to cisgender adolescents. Second, we evaluated the association of insulin sensitivity with body fat and duration of GnRH treatment.
Materials and Methods
Participants
Transgender youth who had been on a GnRHa for ≥3 months were recruited between 2016 and 2019 from the TRUE Center for Gender Diversity at Children's Hospital Colorado (CHCO). All transgender females and five transgender males were recruited from the same cross-sectional study.13 An additional four transgender males were recruited from a separate longitudinal study, and data from their baseline visit are included here, when they were on a GnRHa alone. None of the participants were receiving testosterone or estradiol treatment at the time of this study. Youth were excluded if they had significant medical or psychiatric comorbidities (including diabetes or antipsychotic treatment), or were using hormones not prescribed by a physician. The study was approved by the Colorado Multiple Institutional Review Board; consent and assent were obtained from all participants and a guardian.
Data on healthy cisgender controls were obtained from two studies performed at our institution: the RESistance to InSulin in Type 1 ANd Type 2 diabetes (RESISTANT) study14 and the Health Influences in Puberty (HIP) study.15 Inclusion criteria for both RESISTANT14 and HIP15 have previously been described. We matched on sex assigned at birth to be able to examine how GnRHa treatment in birth-assigned males and females impacts insulin sensitivity and body composition, relative to birth-assigned male and female controls, respectively, not receiving GnRHa.
Research visit
All transgender participants had a research visit in the morning at the CHCO Clinical Translational Research Center (CTRC) after an overnight fast. Fasted blood samples were drawn. Pubertal staging was performed by a pediatric endocrinologist using the standards of Marshall and Tanner for breast development (using inspection and palpation) or testicular development, and pubic hair.16,17 Testicular volume was assessed using a Prader orchidometer and assigned a Tanner stage equivalent as follows: Tanner 1<4 mL; Tanner 2≥4 mL and <8 mL; Tanner 3≥8 mL and <12 mL; Tanner 4≥12 mL and ≤15 mL; Tanner 5>15 mL. Height was measured on a Harpenden stadiometer and weight on a digital electronic scale in light clothing. Height and weight were recorded to the ∼0.1 cm and kg, respectively. Blood pressure was measured after ∼5 min of seated rest, with an age- and size-appropriate manual cuff. Weight, height, and blood pressure were each measured twice and averaged. As all participants were <20 years, pediatric norms for BMI were used (percentile, where 5 to <85 percentile is normal weight, 85 to <95 percentile is overweight, and ≥95 percentile is obese). The 2000 Centers for Disease Control and Prevention Growth Charts were used to calculate percentiles using sex assigned at birth.18 Body composition (i.e., fat-free mass and fat mass) was measured by dual-energy X-ray absorptiometry (Discovery A or Hologic Horizon W, Hologic, Marlborough, MA). Participants filled out a demographic questionnaire, and all study data were managed using REDCap electronic data capture tools hosted at the University of Colorado Anschutz Medical Campus (CU-AMC).19
The methods and equipment used for the RESISTANT and HIP studies were the same as used in the transgender participants, including fasted laboratories, anthropomorphics, body composition, and puberty examination unless otherwise noted.
Laboratory assays
Serum/plasma fasted blood samples were assayed for glucose, insulin, lipid panel, aspartate aminotransferase (AST), alanine aminotransferase (ALT), hemoglobin A1C (HbA1c), leptin, sex hormone-binding globulin (SHBG), luteinizing hormone, follicle stimulating hormone, estradiol, and total testosterone. Laboratory assays were performed by the CU-AMC CTRC Core Laboratory and the UC Health Clinical Laboratory, and inter- and intra-assay coefficient of variations (CVs) have previously been described.13 Insulin sensitivity was estimated by the inverse of the fasting insulin concentration (1/[fasting insulin]) and by the homeostatic model of insulin resistance (HOMA-IR), which are both correlated with insulin sensitivity measured with a hyperinsulinemic euglycemic clamp20,21; lower values for 1/fasting insulin and higher values for HOMA-IR indicate worse insulin sensitivity.
Testosterone, estradiol, and SHBG were measured by chemiluminescence (Beckman Coulter, Brea, CA). Testosterone inter- and intra-assay CVs were 5.1% and 2.1%, respectively, and sensitivity 17 ng/dL; estradiol inter- and intra-assay CVs 8.2% and 4.3%, respectively, and sensitivity 10.0 pg/mL; SHBG inter- and intra-assay CVs 5.7% and 3.6%, respectively, and sensitivity 3 nmol/L.
Total cholesterol, triglycerides, and high-density lipoprotein cholesterol were directly measured, and low-density lipoprotein cholesterol was calculated using the Friedewald formula (for units in mg/dL).22
Because the AST and ALT for the cisgender participants were measured by a different assay, a correction factor was applied to the transgender participants: corrected AST=(measured AST + 14.374)/0.8334 and corrected ALT=(measured ALT + 10.058)/0.9319. Deming regression was used to build a regression model and determine the correction needed to make the results equivalent using the parameter estimates obtained from the regression.
Statistical analysis
Transgender participants were matched to cisgender controls on age (within a year or less), BMI (within category), and sex assigned at birth; transgender males (assigned female sex at birth, male gender identity) were matched to cisgender females and transgender females (assigned male sex at birth, female gender identity) were matched to cisgender males.
Transgender male participants (n=9, 10.1–16.0 years) were matched to cisgender females (n=14, 10.6–16.2 years) with a range of 1–2 cisgender female matches per transgender male case (1:1 n=4, 1:2 n=5). Transgender female participants (n=8, 12.6–16.1 years) were matched to cisgender males (n=17, 12.5–15.5 years) with a range of 1–3 matches per transgender female case (1:1 n=3, 1:2 n=1, 1:3 n=4).
Tests of differences between transgender and cisgender participants were performed based on mixed linear regression models with a random intercept for the matched set. Compound symmetry was used for the covariance structure, and the restricted maximum-likelihood method was used to estimate the covariance parameters. For group comparisons in which there was more than one cisgender match for the transgender participant, means of the matched set are presented (e.g., if three cisgender males were matched to one transgender female, the mean of outcome for the three cisgender males was obtained; the means and standard deviations (SDs) of the matched set means are reported).
Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC). p-Values <0.05 were considered significant. We did not correct for multiple comparisons because all findings in this pilot study are considered exploratory.
Results
Demographics of the overall group are presented in Table 1. Transgender males were treated with a GnRHa for an average (±SD) of 20.9±19.8 months (range 17.5–70.4 months) before study entry. Transgender females were treated with a GnRHa for an average of 11.3±7 months (range 4.7–24.2 months) before study entry. Five (56%) of the transgender males had menarche (average age 12±0.7 years) before starting a GnRHa and eight (57%) of cisgender females were postmenarchal (average age of menarche 12±1.3 years). None of the transgender male participants were having ongoing menses at the time of the study visit.
Table 1.
Demographics of Transgender and Cisgender Participants
| |
Assigned female at birth |
Assigned male at birth |
||
|---|---|---|---|---|
| Transgender males (n=9) | Cisgender females (n=14) | Transgender females (n=8) | Cisgender males (n=17) | |
| Age (years) | 13.8±1.7 | 13.9±1.7 | 13.7±1.2 | 13.9±0.9 |
| Age at initiation of GnRHa (years) | 12.1±1.9 | — | 12.8±1.3 | — |
| GnRHa duration (months) | 20.9±19.8 | — | 11.3±7 | — |
| Race | ||||
| White | 6 (67) | 10 (71) | 7 (88) | 13 (76) |
| Asian | 0 (0) | 1 (7) | 0 (0) | 1 (6) |
| African American | 1 (11) | 3 (21) | 0 (0) | 1 (6) |
| More than one race | 1 (11) | 0 (0) | 1 (12) | 0 (0) |
| Unknown/not reported | 1 (11) | 0 (0) | 0 (0) | 2 (12) |
| Ethnicity | ||||
| Hispanic/Latino | 4 (44) | 4 (29) | 4 (50) | 3 (18) |
| Not Hispanic/Latino | 5 (56) | 9 (64) | 4 (50) | 13 (76) |
| Unknown/not reported | 0 (0) | 1 (7) | 0 (0) | 1 (6) |
| Depression | 2 (22) | — | 3 (38) | — |
| Anxiety | 1 (11) | — | 1 (12) | — |
| Pubic hair Tanner stage | ||||
| 1 | 1 (11) | 1 (7) | 1 (12) | 1 (6) |
| 2 | 0 (0) | 0 (0) | 3 (38) | 5 (29) |
| 3 | 1 (11) | 2 (14) | 0 (0) | 3 (18) |
| 4 | 3 (33) | 4 (29) | 1 (12) | 2 (12) |
| 5 | 2 (22) | 1 (7) | 1 (12) | 1 (6) |
| Missing | 2 (22) | 6 (43) | 2 (25) | 5 (29) |
| Breast/testicular Tanner stage | ||||
| 2 | 1 (11) | 1 (7) | 5 (63) | 7 (41) |
| 3 | 3 (33) | 1 (7) | 0 (0) | 3 (18) |
| 4 | 2 (22) | 3 (21) | 1 (12) | 3 (18) |
| 5 | 2 (22) | 9 (64) | 2 (25) | 4 (24) |
| Missing | 1 (11) | 0 (0) | 0 (0) | 0 (0) |
| Family historya | ||||
| Hypertension | 5 (63) | 6 (75) | 5 (63) | 9 (75) |
| Hypercholesterolemia | 5 (63) | 7 (88) | 3 (38) | 8 (67) |
| Type 2 diabetes | 4 (50) | 7 (64) | 1 (13) | 4 (33) |
Values above represent the entire cohort used and are either presented as mean±SD or n (%).
Family history is given out of total number of reported values (nonmissing). For transgender males, family history was missing for one participant. For cisgender females, family history was missing for hypertension (n=6), hypercholesterolemia (n=6), type 2 diabetes (n=3). Family history was not missing for any transgender female and was missing for 5 cisgender males (all conditions).
GnRHa, gonadotropin-releasing hormone agonist; SD, standard deviation.
Transgender males compared with cisgender females
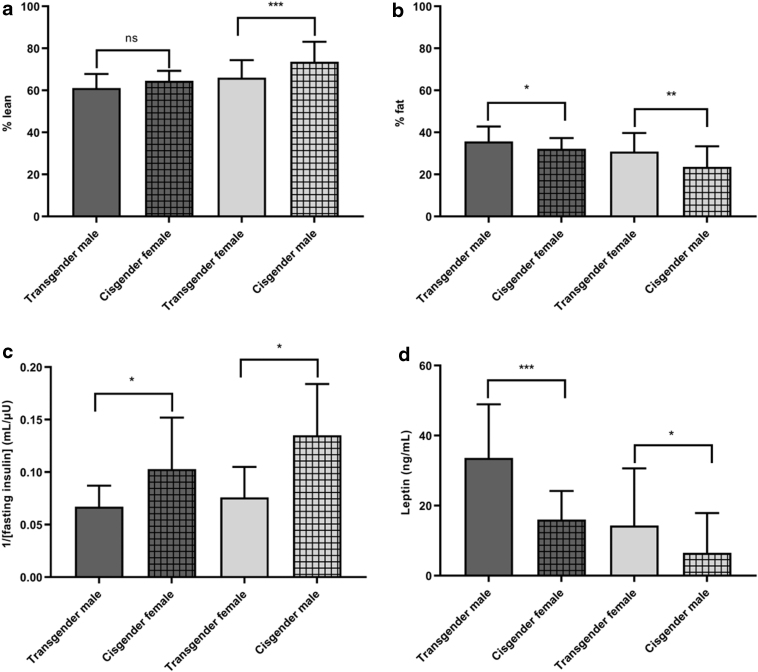
Markers of cardiometabolic health and hormone concentrations for transgender males are displayed in Table 2 and Figure 1. Transgender males had lower estimated insulin sensitivity than cisgender females, with lower inverse of fasted insulin (p=0.031) and higher HOMA-IR (p=0.010), higher fasted glucose (p=0.012) and higher HbA1c (p=0.039). Transgender males also had higher leptin (p<0.001) and AST (p=0.02) and lower total estradiol (p=0.014) than cisgender females. Transgender males had a higher percent body fat (36±7 vs. 32±5%, p=0.042) and lower total lean mass (32.3±5.2 vs. 36.4±7.8 kg, p=0.009), but not percent lean mass than cisgender females. The mean systolic blood pressure was lower among transgender males compared with cisgender females, although this did not reach statistical significance (p=0.08).
Table 2.
Markers of Cardiometabolic Health and Hormone Concentrations
| Assigned female at birth |
Assigned male at birth |
|||
|---|---|---|---|---|
| Transgender males (n=9) | Cisgender females (n=14a) | Transgender females (n=8) | Cisgender males (n=17a) | |
| BMI (percentile) | 62±32 | 67±29 | 44±39 | 45±38 |
| Systolic BP (mmHg) | 104±13 | 114±11 | 97±6 | 108±7** |
| Systolic BP (percentile) | 41±32 | 68±24 | 15±12 | 52±21*** |
| Diastolic BP (mmHg) | 67±5 | 65±9 | 65±11 | 65±3 |
| Diastolic BP (percentile) | 59±20 | 55±25 | 55±28 | 57±9 |
| Inverse of fasting insulin (mL/μU) | 0.067±0.020 | 0.103±0.049* | 0.076±0.029 | 0.135±0.049* |
| HOMA-IR | 3.7±1.7 | 2.3±1.1** | 3.5±1.4 | 2.2±1.3* |
| Fasting glucose (mg/dL) | 89±4 | 79±13* | 88±7 | 84±5 |
| Hemoglobin A1c (%) | 5.4±0.2 | 5.2±0.2* | 5.4±0.1 | 5.1±0.2** |
| AST (U/L) | 42±11 | 31±10* | 43±9 | 31±6** |
| ALT (U/L) | 28±13 | 24±9 | 34±17 | 24±6 |
| Total cholesterol (mg/dL) | 158±21 | 152±27 | 143±16 | 151±35 |
| Triglycerides (mg/dL) | 87±43 | 89±24 | 78±28 | 117±115 |
| HDL (mg/dL) | 53±16 | 48±13 | 51±6 | 54±13 |
| LDL (mg/dL) | 88±14 | 86±23 | 76±14 | 74±17 |
| Total estradiol (pg/mL) | 15±5 | 58±45* | 12±3 | 14±5 |
| Total testosterone (ng/dL) | 29±12 | 38±12 | 50±52 | 231±153*** |
| SHBG (nmol/L) | 53±34 | 47±19 | 50±24 | 54±26 |
| Luteinizing hormone (mIU/mL) | 1.6±1.8 | — | 0.8±0.6 | — |
| Follicle stimulating hormone (mIU/mL) | 2.7±2.3 | — | 0.8±0.3 | — |
Values are given as mean±SD.
Means of the matched set, *p<0.05, **p≤0.01, ***p≤0.001 (p-values represent significance from log-transformed variables when relevant).
BMI, body mass index; BP, blood pressure; HOMA-IR, homeostatic model of insulin resistance; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SHBG, sex hormone-binding globulin.
FIG. 1.
Body composition (a,b), insulin sensitivity (c), and leptin (d) among transgender and cisgender participants. Means and standard deviations are presented; lower 1/[fasting insulin] indicates worse insulin sensitivity, *p<0.05, **p<0.01, ***p<0.001. ns, not statistically significant.
Transgender females compared with cisgender males
Markers of cardiometabolic health and hormone concentrations for transgender females are displayed in Table 2 and Figure 1. Transgender females had lower estimated insulin sensitivity than cisgender males, with lower inverse of fasted insulin (p=0.028) and higher HOMA-IR (p=0.035), as well as higher HbA1c (p=0.007), AST (p=0.007), and leptin (p<0.001) and lower total testosterone (p<0.001) than cisgender males. Transgender females had higher percent body fat (31±9 vs. 24±10%, p=0.002) and lower percent lean mass (66±8 vs. 74±10%, p<0.001) than cisgender males, and lower systolic blood pressure and systolic blood pressure percentile (p=0.006).
Associations
In the pooled population of transgender and cisgender participants, insulin sensitivity assessed by 1/fasting insulin was inversely correlated with percent fat (r=−0.68 [95% confidence interval, CI: −0.81 to −0.48], p<0.0001) and inversely correlated with BMI percentile (r=−0.64 [95% CI: −0.79 to −0.41], p<0.0001). Duration of GnRHa treatment did not correlate with insulin sensitivity.
Discussion
In this study, we show that both transgender males and females on GnRHa have lower estimated insulin sensitivity, more evidence of dysglycemia, and higher body fat than age-, assigned sex-, and BMI-matched cisgender controls.
In adult cisgender individuals, there are known effects of having low endogenous sex steroids, either primarily or secondary to exogenous suppression, on body composition and insulin sensitivity. Testosterone deficiency in adult cisgender men is associated with greater visceral fat, insulin resistance, and metabolic syndrome.23 Blockade of endogenous testosterone with a GnRH agonist or antagonist results in increased body fat, decreased lean mass,24 and increased risk of incident diabetes.12,25 It is known that in cisgender adult women, ovariectomy or rapid, severe estradiol deficiency is associated with increased diabetes risk,23 although less is known about the impact of acute suppression of estradiol on insulin sensitivity.
However, these findings may not be directly applicable to adolescents. Puberty is closely associated with changes in metabolism and body composition, and may be a critical window for development of future cardiometabolic risk. For example, earlier pubertal timing is associated with metabolic syndrome-related derangements,26 and healthy adolescents develop transient insulin resistance that resolves after puberty is completed.27 In addition, there are known sex differences in body composition and insulin sensitivity that emerge during puberty; cisgender adolescent girls are known to be more insulin resistant, develop a higher percent body fat, have a greater decrease in habitual levels of physical activity, and have a higher incidence of type 2 diabetes than cisgender boys.28–30
Given the negative associations of puberty and early puberty with insulin sensitivity and cardiometabolic risk, we had hypothesized that those on a GnRHa would have better insulin sensitivity than controls. However, we showed that transgender youth on GnRHa had worse estimated insulin sensitivity than controls. Moreover, since puberty in cisgender individuals leads to greater increases in fat-free mass in males vs. great increases in fat mass in females, we hypothesized that GnRHa would maintain prepubertal body composition. Yet, body composition differences between transgender youth on a GnRHa and cisgender youth were largely in the same direction, regardless of sex assigned at birth. This may suggest that these differences are related to nonsex-specific impacts of GnRHa or to another factor common to transgender individuals. Therefore, our data suggest that suppression of sex steroids during puberty may negatively impact body composition, a finding consistent with prior publications.9,10
GnRHa is also used in younger children for the treatment of central precocious puberty (CPP). Youth with CPP treated with a GnRHa show increased fat mass and percent body fat standard deviation score (SDS) and decreased lean tissue mass SDS,31 although this is not a universal finding.32 After initiation of GnRHa for CPP, girls who are normal weight have an increase in BMI/BMI Z-score but no change in insulin sensitivity33,34; whereas girls who are overweight/obese have no significant change in BMI33,34 but may have worsening insulin sensitivity.34 Similar to this study, both of the cited CPP studies used HOMA-IR to estimate insulin sensitivity, as well as other validated estimates.
Despite an increasing number of studies on the impact of testosterone or estradiol on cardiometabolic health in transgender individuals, as reviewed in a recent meta-analysis,35 the impact of GnRHa treatment on cardiometabolic health remains a vastly understudied area. Similar to longitudinal studies showing transgender adolescents on a GnRHa have an increase in body fat and decrease in lean mass,9,10 we showed that transgender males and females have a higher body fat percentage than BMI-matched cisgender youth. One study from the Netherlands showed that in the first year of treatment with GnRHa, transgender individuals had increased percent body fat and absolute fat mass and decreased lean mass.9 However, they also had an increase in BMI, and the changes in body fat were neither adjusted for the changes in BMI, nor compared with a nontransgender population. Another study from the Netherlands evaluated body composition at the start of GnRHa, the start of testosterone or estradiol, and at 22 years of age.10 Results were adjusted for Tanner stage and BMI at the start of GnRHa treatment. The average age at the start of GnRHa was 14.5±1.8 years for transgender women and 15.3±2 for transgender men (84% had menarche), representing an older and more pubertally advanced population than our population presented here. Percent body fat increased and percent lean mass decreased between the start of GnRHa and the start of testosterone or estradiol.10 Data are lacking in transgender youth starting GnRHa at younger ages and pubertal stages, and very few comparing results with a matched cisgender population.
The clinical significance of higher HbA1c and/or fasting glucose values that are still within the normal range on cardiometabolic risk among healthy youth is not well understood. It has been suggested that youth should have a fasting plasma glucose <90 mg/dL, arguing that a mean fasting glucose ∼90 is of clinical significance.36 Our group has also shown that obese youth with normal HbA1c (<5.7%) have glucose excursions above the normal range.37 Others have shown that the relationship between fasting glucose and cardiovascular disease is J shaped.38 Since adult transgender individuals on hormone therapy have a higher risk of acute cardiovascular events than cisgender adults,39 future studies should assess the impact of hormonal interventions as well as physical activity, weight, diet, and other factors, with the goal of reducing future cardiovascular events for this population.
The current cross-sectional study has many strengths. There have been very few carefully performed metabolic studies in transgender youth. Although other studies have evaluated body composition,9,10 we are not aware of any that have employed a matched group of cisgender controls or that evaluated insulin sensitivity. There are also several limitations to our study. First, our study was cross-sectional, so we do not yet know about changes in insulin sensitivity before versus after GnRHa treatment. Second, the sample size was small. Third, testosterone and estradiol were not measured by mass spectrometry, although these were not our primary outcomes. Fourth, it is known that there are changes in insulin sensitivity during puberty. We were not powered to adjust for pubertal stage in the analyses, but participants were matched on age, a proxy for pubertal stage. In comparisons between transgender youth on a GnRHa and cisgender youth, future studies should evaluate whether it is best to match on Tanner stage or age (would be challenging or impossible to match on both since puberty is being halted in these transgender youth). Fifth, there was a wide range of length of time on a GnRHa. As our study was cross-sectional, it is also possible that other factors might explain the insulin sensitivity differences between transgender and cisgender youth, such as physical activity. We were not able to directly compare or control for physical activity, something that should be accounted for in future studies.
In conclusion, our results show that transgender adolescents on a GnRHa have lower estimated insulin sensitivity and have a metabolically less favorable body composition than cisgender youth with otherwise similar characteristics. Future studies are needed to determine if insulin sensitivity changes or is worse after initiation of GnRHa therapy in transgender youth (and whether timing of initiation impacts outcomes), as well as the subsequent effects of testosterone or estradiol treatment. Longitudinal studies are underway to evaluate these outcomes.
Acknowledgments
The authors thank the participants and families who participated and the Clinical Translational Research Center nursing staff at Children's Hospital Colorado. An earlier version of this article was posted on a preprint server. These data were presented at the Building Interdisciplinary Research Careers in Women's Health (BIRCWH) Annual Conference at the National Institutes of Health on December 11, 2019 and abstract in the Journal of Women's Health Vol. 28, No. 11, 2019.
Abbreviations Used
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- BP
blood pressure
- CHCO
Children's Hospital Colorado
- CPP
central precocious puberty
- CTRC
Clinical Translational Research Center
- CU-AMC
University of Colorado Anschutz Medical Campus
- CV
coefficient of variations
- GnRHa
gonadotropin-releasing hormone agonist
- HbA1c
hemoglobin A1C
- HDL
high-density lipoprotein
- HIP
Health Influences in Puberty
- HOMA-IR
homeostatic model of insulin resistance
- LDL
low-density lipoprotein
- RESISTANT
RESistance to InSulin in Type 1 ANd Type 2 diabetes
- SD
standard deviation
- SDS
standard deviation score
- SHBG
sex hormone-binding globulin
Author Disclosure Statement
N.J.N. previously consulted for Antares Pharma, Inc.
Funding Information
Institutional funding: National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (NCATS) Colorado CTSA UL1 TR001082. Dr. Nokoff is supported by a T32 grant (T32 DK 63687), University of Colorado Anschutz Medical Campus Center for Women's Health Research, Endocrine Fellow Foundation, and the Doris Duke University of Colorado School of Medicine Fund to Retain Clinical Scientists. Drs. Nokoff and Kelsey are supported by the Building Interdisciplinary Research Careers in Women's Health NIH/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) BIRCWH K12 (HD057022).
The HIP study was supported by Dr. Kelsey's American Diabetes Association Junior Faculty Award (1-11-JF-23) and the Children's Hospital Colorado Research Institute Research Scholar Award. The RESISTANT study was supported by NCRR K23 RR020038, NIH BIRCWH K12 HD057022, ADA 7-11-CD-08, JDRF Award 11-2010-343, NIDDK R56 DK088971, ADA 1-11-JF-23, and NIH/NCATS Colorado.
Cite this article as: Nokoff NJ, Scarbro SL, Moreau KL, Zeitler P, Nadeau KJ, Reirden D, Juarez-Colunga E, Kelsey MM (2021) Body composition and markers of cardiometabolic health in transgender youth on gonadotropin-releasing hormone agonists, Transgender Health 6:2, 111–119, DOI: 10.1089/trgh.2020.0029.
References
- 1. Herman JL, Flores AR, Brown TNT, et al. Age of Individuals Who Identify As Transgender in the United States. Los Angeles, CA: The Williams Institute, 2017 [Google Scholar]
- 2. Johns MM, Lowry R, Andrzejewski J, et al. Transgender identity and experiences of violence victimization, substance use, suicide risk, and sexual risk behaviors among high school students—19 states and large urban school districts, 2017. MMWR Morb Mortal Wkly Rep. 2019;68:67–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869–3903 [DOI] [PubMed] [Google Scholar]
- 4. Skordis N, Butler G, de Vries MC, et al. ESPE and PES international survey of centers and clinicians delivering specialist care for children and adolescents with gender dysphoria. Horm Res Paediatr. 2018;90:326–331 [DOI] [PubMed] [Google Scholar]
- 5. de Vries ALC, McGuire JK, Steensma TD, et al. Young adult psychological outcome after puberty suppression and gender reassignment. Pediatrics. 2014;134:696–704 [DOI] [PubMed] [Google Scholar]
- 6. de Vries ALC, Steensma TD, Doreleijers TAH, Cohen-Kettenis PT. Puberty suppression in adolescents with gender identity disorder: a prospective follow-up study. J Sex Med. 2011;8:2276–2283 [DOI] [PubMed] [Google Scholar]
- 7. Olson KR, Durwood L, DeMeules M, McLaughlin KA. Mental health of transgender children who are supported in their identities. Pediatrics. 2016;137:e20153223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rafferty J. Ensuring comprehensive care and support for transgender and gender-diverse children and adolescents. Pediatrics. 2018;142:e20182162. [DOI] [PubMed] [Google Scholar]
- 9. Schagen SE, Cohen-Kettenis PT, Delemarre-van de Waal HA, Hannema SE. Efficacy and safety of gonadotropin-releasing hormone agonist treatment to suppress puberty in gender dysphoric adolescents. J Sex Med. 2016;13:1125–1132 [DOI] [PubMed] [Google Scholar]
- 10. Klaver M, de Mutsert R, Wiepjes CM, et al. Early hormonal treatment affects body composition and body shape in young transgender adolescents. J Sex Med. 2018;15:251–260 [DOI] [PubMed] [Google Scholar]
- 11. Loomba-Albrecht LA, Styne DM. Effect of puberty on body composition. Curr Opin Endocrinol Diabetes Obes. 2009;16:10–15 [DOI] [PubMed] [Google Scholar]
- 12. Keating NL, O'Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nokoff NJ, Scarbro SL, Moreau KL, et al. Body composition and markers of cardiometabolic health in transgender youth compared to cisgender youth. J Clin Endocrinol Metab. 2019;105:e704–e714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bjornstad P, Truong U, Pyle L, et al. Youth with type 1 diabetes have worse strain and less pronounced sex differences in early echocardiographic markers of diabetic cardiomyopathy compared to their normoglycemic peers: a RESistance to InSulin in Type 1 ANd Type 2 diabetes (RESISTANT) study. J Diabetes Complications. 2016;30:1103–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nokoff N, Thurston J, Hilkin A, et al. Sex differences in effects of obesity on reproductive hormones and glucose metabolism in early puberty. J Clin Endocrinol Metab. 2019;104:4390–4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000. CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002:1–190 [PubMed] [Google Scholar]
- 19. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. George L, Bacha F, Lee S, et al. Surrogate estimates of insulin sensitivity in obese youth along the spectrum of glucose tolerance from normal to prediabetes to diabetes. J Clin Endocrinol Metab. 2011;96:2136–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 22. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502 [PubMed] [Google Scholar]
- 23. Mauvais-Jarvis F. Gender differences in glucose homeostasis and diabetes. Physiol Behav. 2018;187:20–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chao J, Rubinow KB, Kratz M, et al. Short-term estrogen withdrawal increases adiposity in healthy men. J Clin Endocrinol Metab. 2016;101:3724–3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456 [DOI] [PubMed] [Google Scholar]
- 26. Widen E, Silventoinen K, Sovio U, et al. Pubertal timing and growth influences cardiometabolic risk factors in adult males and females. Diabetes Care. 2012;35:850–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moran A, Jacobs DR, Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48:2039–2044 [DOI] [PubMed] [Google Scholar]
- 28. Copeland KC, Zeitler P, Geffner M, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. 2011;96:159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention. A Report of the Surgeon General Physical Activity and Health Adolescents and Young Adults. Atlanta, GA: Department of Health and Human Services, 1996 [Google Scholar]
- 31. Boot AM, De Muinck Keizer-Schrama S, Pols HA, et al. Bone mineral density and body composition before and during treatment with gonadotropin-releasing hormone agonist in children with central precocious and early puberty. J Clin Endocrinol Metab. 1998;83:370–373 [DOI] [PubMed] [Google Scholar]
- 32. Alessandri SB, Pereira Fde A, Villela RA, et al. Bone mineral density and body composition in girls with idiopathic central precocious puberty before and after treatment with a gonadotropin-releasing hormone agonist. Clinics (Sao Paulo). 2012;67:591–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arcari AJ, Freire AV, Escobar ME, et al. One-year treatment with gonadotropin-releasing hormone analogues does not affect body mass index, insulin sensitivity or lipid profile in girls with central precocious puberty. J Pediatr Endocrinol Metab. 2019;32:181–186 [DOI] [PubMed] [Google Scholar]
- 34. Park J, Kim JH. Change in body mass index and insulin resistance after 1-year treatment with gonadotropin-releasing hormone agonists in girls with central precocious puberty. Ann Pediatr Endocrinol Metab. 2017;22:27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maraka S, Singh Ospina N, Rodriguez-Gutierrez R, et al. Sex steroids and cardiovascular outcomes in transgender individuals: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2017;102:3914–3923 [DOI] [PubMed] [Google Scholar]
- 36. RISE Consortium. Restoring Insulin Secretion (RISE): design of studies of β-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes Care. 2014;37:780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chan CL, Pyle L, Newnes L, et al. Continuous glucose monitoring and its relationship to hemoglobin A1c and oral glucose tolerance testing in obese and prediabetic youth. J Clin Endocrinol Metab. 2015;100:902–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park C, Guallar E, Linton JA, et al. Fasting glucose level and the risk of incident atherosclerotic cardiovascular diseases. Diabetes Care. 2013;36:1988–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nota NM, Wiepjes CM, de Blok CJM, et al. Occurrence of acute cardiovascular events in transgender individuals receiving hormone therapy. Circulation. 2019;139:1461–1462 [DOI] [PubMed] [Google Scholar]