Abstract
Objective: To assess the efficacy and safety of closed-loop control (CLC) insulin delivery system in adolescents and young adults with type 1 diabetes.
Research Design and Methods: Prespecified subanalysis of outcomes in adolescents and young adults aged 14–24 years old with type 1 diabetes in a previously published 6-month multicenter randomized trial. Participants were randomly assigned 2:1 to CLC (Tandem Control-IQ) or sensor augmented pump (SAP, various pumps+Dexcom G6 CGM) and followed for 6 months.
Results: Mean age of the 63 participants was 17 years, median type 1 diabetes duration was 7 years, and mean baseline HbA1c was 8.1%. All 63 completed the trial. Time in range (TIR) increased by 13% with CLC versus decreasing by 1% with SAP (adjusted treatment group difference = +13% [+3.1 h/day]; 95% confidence interval [CI] 9–16, P < 0.001), which largely reflected a reduction in time >180 mg/dL (adjusted difference −12% [−2.9 h/day], P < 0.001). Time <70 mg/dL decreased by 1.6% with CLC versus 0.3% with SAP (adjusted difference −0.7% [−10 min/day], 95% CI −1.0% to −0.2%, P = 0.002). CLC use averaged 89% of the time for 6 months. The mean adjusted difference in HbA1c after 6 months was 0.30% in CLC versus SAP (95% CI −0.67 to +0.08, P = 0.13). There was one diabetic ketoacidosis episode in the CLC group.
Conclusions: CLC use for 6 months was substantial and associated with improved TIR and reduced hypoglycemia in adolescents and young adults with type 1 diabetes. Thus, CLC has the potential to improve glycemic outcomes in this challenging age group.
The clinical trial was registered with ClinicalTrials.gov (NCT03563313).
Keywords: Closed-loop control insulin delivery, Adolescents, Young adult, Time in range
Introduction
Despite improvements in care, a majority of youth with type 1 diabetes still fail to meet glycemic control targets. In 2018, the prevailing American Diabetes Association goal HbA1c of <7.5% (<58 mmol/mol) was reached by only 17% of adolescents1; current guidelines have lowered the goal to <7% (<53 mmol/mol) for many in this age group.2 Based on registries in the United States and northern Europe, average HbA1c increases at adolescence and remains elevated into young adulthood.3 Youth with suboptimal glycemic control are at increased risk of diabetic ketoacidosis (DKA), microvascular and macrovascular complications, and premature mortality.4–6
Closed-loop insulin delivery is a promising approach for improving glycemic control, but outcomes data are scarce in pediatric and young adult populations. Based on short-term studies in monitored clinical settings and at diabetes camps, closed-loop insulin delivery systems can increase time in range (TIR) and reduce both hyper- and hypoglycemia.7–11 However, there are few randomized trials in free-living settings, and we are unaware of any published reports of trials longer than 3 months.
A retrospective study of a clinically available closed-loop control (CLC) system (Medtronic 670G) suggests that successful use of the automated system was associated with increased TIR and reduced HbA1c in youth.12 However, many youth discontinued use of the hybrid closed-loop system within the first 6 months of use. Difficulty with calibrations, alarms, expense, and the workload required to ensure correct function of the system were reported as the most challenging aspects of using the hybrid closed-loop system.13,14 Thus, there is an unmet need for a robust, safe, and user-friendly system that automates insulin delivery for youth with type 1 diabetes.
We recently reported on a new CLC insulin delivery system (Tandem Control-IQ) that was successful at improving continuous glucose monitor (CGM)-derived glycemic metrics and HbA1c in a 6-month randomized trial comparing CLC versus sensor-augmented pump in 168 individuals with type 1 diabetes aged 14–70 years old.15 This closed-loop system provides automated insulin correction boluses administered using CGM data, without the need for fingerstick glucose calibrations. The system also incorporates a hypoglycemia safety system that attenuates or discontinues insulin delivery using CGM and insulin-on-board information. Users are expected to enter carbohydrate intake for meal boluses.
This CLC algorithm has previously been shown to partially compensate for missed meal boluses in adolescents due, in part, to automated correction boluses.16 To assess the efficacy of this CLC system in adolescents and young adults, we conducted an a priori-defined subgroup analysis of outcomes in the 14–24-year-old participants in the study (n = 63).
Research Design and Methods
Details of the study protocol have been reported elsewhere15 and are summarized hereunder. The protocol was approved by a central institutional review board and written informed consent (or parental consent and assent for youth <18 years old) obtained from all participants. An investigational device exemption was approved by the Food and Drug Administration. An independent data and safety monitoring board provided trial oversight.
This secondary analysis included the data collected on participants aged 14 to <25 years. Study participants had a clinical diagnosis of type 1 diabetes treated with insulin for at least 1 year, with a minimum total daily insulin dose >10 U. Use of any noninsulin glucose-lowering agents other than metformin was an exclusion. There was no restriction on the HbA1c level at study entry. Outcomes included CGM-derived glycemic metrics, such as TIR 70–180 mg/dL (TIR), mean glucose, hyperglycemia (>180 mg/dL), hypoglycemia (<70 and <54 mg/dL), HbA1c, and adverse events.
Participants were randomly assigned in a 2:1 ratio to a closed-loop system (intervention group, CLC) or a sensor-augmented pump (control group, SAP) for 26 weeks. The closed-loop system consisted of a pump (t:slim X2 insulin pump with Control-IQ, Tandem Diabetes Care) and a CGM (Dexcom G6, Dexcom). Participants in the SAP group received the study CGM and either used their personal pump or, if not using a pump, were provided with a t:slim X2 insulin pump, without any automated insulin delivery features (including no use of low glucose suspend or predictive low glucose suspend features).
Both groups attended follow-up visits at 2, 6, 13, and 26 weeks and were contacted by telephone at 1, 4, 9, 17, and 21 weeks. In both groups, data-driven optimization of pump settings could be made at the 2-, 13-, and 26-week visits. HbA1c at randomization, 13, and 26 weeks was measured at a central laboratory at the University of Minnesota Advanced Research and Diagnostic Laboratory.
Reporting of adverse events was solicited throughout the trial. Reportable adverse events included serious adverse events, adverse events occurring in association with a trial device or procedure, severe hypoglycemia (defined as hypoglycemia leading to the need for active assistance because of altered consciousness), DKA, and hyperglycemia with ketonemia for which a health care provider was contacted. Participant satisfaction with the CLC system was assessed with the System Usability Scale, a 10-item technology agnostic questionnaire that measures the perceived usability of a system, with excellent usability associated with a mean score of ∼85.17
During the trial, use of the Control-IQ software was suspended temporarily for ∼4 weeks after the discovery of a software error that under certain circumstances could lead to erroneous insulin delivery; no adverse events occurred as a result of this error. Participants continued to use the study pump and CGM with the CLC feature turned off until a software update was deployed to participants. The analyses included all data recorded during the suspension period, even if the closed-loop mode was not in use, except for the reporting of the percentage of time CLC was used that excluded this time period.
Statistical analyses
All analyses were based on intent to treat, and all participants were included in the efficacy and safety analyses. Continuous outcome variables were compared between the two groups using a linear mixed-effects regression model that adjusted for the baseline level of the dependent variable, age, previous use of a CGM and pump, and clinical center (random effect). Binary outcomes were compared between the two groups using a similar logistic model. Descriptive statistics include means with standard deviations and medians with interquartile ranges (IQRs), depending on the distribution of data. All P values and confidence intervals were adjusted for multiplicity using the false discovery rate. All P values are two tailed. Analyses were performed with SAS software, version 9.4 (SAS Institute).
Results
Demographic and baseline clinical characteristics
The 63 adolescent and young adult participants included in this analysis ranged in age from 14 to 24 years with a mean age of 17 years (76% were <18 years old); 43% were female, 83% identified as white, and 11% reported Hispanic or Latino ethnicity. Mean type 1 diabetes duration was 7 years and screening HbA1c was 8.1% (65 mmol/mol); 19% were pump naive and 5% were CGM naive at entry (Table 1). Forty participants were randomly assigned to the CLC group and 23 to the SAP group. All 63 participants completed the 26-week study; there were no drop outs. Participant flow is shown in a Consort Diagram (Supplementary Fig. S1).
Table 1.
Baseline Characteristics
| CLC (N = 40) | SAP (N = 23) | Total (N = 63) | |
|---|---|---|---|
| Mean age, years | 17 (3) | 17 (3) | 17 (3) |
| No. of adolescents and young adults, n (%) | |||
| Age <18 years | 31 (78) | 17 (74) | 48 (76) |
| Age ≥18 years | 9 (22) | 6 (26) | 15 (24) |
| Gender, female | 17 (43) | 10 (43) | 27 (43) |
| Racea, n (%) | |||
| White | 31 (82) | 21 (91) | 52 (85) |
| Nonwhite | 7 (18) | 2 (9) | 9 (15) |
| Ethnicity, Hispanic, or Latino | 5 (13) | 2 (9) | 7 (11) |
| Household incomeb, n (%) | |||
| <$50,000 | 3 (10) | 1 (5) | 4 (8) |
| $50,000–$100,000 | 7 (23) | 6 (30) | 13 (25) |
| >$100,000 | 21 (68) | 13 (65) | 34 (67) |
| Private health insurancec, n (%) | 37 (93) | 19 (86) | 56 (90) |
| Mean HbA1c at screening (%) [mmol/mol] | 8.2 (1.1) [66 (12.0)] | 8.0 (1.2) [64 (13.1)] | 8.1 (1.1) [65 (12.0)] |
| Insulin modality, n (%) | |||
| Pumpd | 33 (82) | 18 (78) | 51 (81) |
| MDI | 7 (18) | 5 (22) | 12 (19) |
| CGM use, n (%) | |||
| Never | 1 (3) | 2 (9) | 3 (5) |
| In past, but not current | 10 (25) | 3 (13) | 13 (21) |
| Current | 29 (73) | 18 (78) | 47 (75) |
| Median type 1 diabetes duration (years) | 7 (4, 9) | 7 (5, 12) | 7 (4, 12) |
| C-peptide at randomization, n (%) | |||
| <0.02 nmol/L | 29 (72) | 15 (65) | 44 (70) |
| ≥0.02 nmol/L | 11 (28) | 8 (35) | 19 (30) |
| BMI percentiles, age <18 years | 78% (55%, 88%) | 68% (45%, 86%) | 77% (55%, 88%) |
| BMI (kg/m2), age ≥18 years | 29 (23, 35) | 26 (24, 26) | 26 (23, 31) |
Mean (SD), median (IQR), or n (%).
Two participants in the treatment group did not provide race information.
Nine participants in the treatment group and three in the control group did not provide income information.
One participant in the control group did not provide insurance information.
SAP group personal pumps included Animas (2), Insulet OmniPod (3), Medtronic 530G (3), Medtronic 670G (3), Medtronic Paradigm (1), and Tandem t:slim or t:slim X2 (6).
CLC, closed-loop control; IQR, interquartile range; SAP, sensor augmented pump; SD, standard deviation.
Glucose monitoring and system use
Median (IQR) percentage of CGM use for the 26 weeks of the trial was 96% (93%–97%) in the treatment group and 91% (83%–96%) in the control group. In the treatment group, the median percentage of time the system was in closed-loop mode was 89% (86%–92%) across all 26 weeks and consistent from month to month. On the System Usability Questionnaire, scores were high at 26 weeks (mean composite score 85.5 out of 100), indicating excellent usability. Participants performed fingerstick blood glucose checks a median of 0.15 times per day in the CLC group and 0.34 times per day in the SAP group.
Glycemic outcomes
TIR in the CLC group was 51% ± 16% at baseline and increased to 64% ± 8% over 26 weeks of follow-up, and TIR in the SAP group was 53% ± 13% at baseline and 52% ± 14% over 26 weeks of follow-up (Table 2). The risk-adjusted treatment group difference in TIR was +13% or 3.1 h/day, favoring the CLC group (95% confidence interval [CI] 9–16, P < 0.0001; Table 2).
Table 2.
Glycemic Outcomes
| CLC (n = 40) |
SAP (n = 23) |
|
|
|||
|---|---|---|---|---|---|---|
| Baseline | 26 Weeks | Baseline | 26 Weeks | CLC-SAP difference (95% CI)a | Pa | |
| CGM metrics | ||||||
| % TIR 70–180 mg/dL | 51 (16) | 64 (8) | 53 (13) | 52 (14) | 13 (9 to 16) | <0.0001 |
| Mean glucose, mg/dL | 183 (31) | 167 (15) | 179 (25) | 183 (28) | −18 (−25 to −10) | <0.0001 |
| % Time >180 mg/dL | ||||||
| Median (IQR) | 44 (32, 54) | 33 (28, 40) | 43 (33, 56) | 45 (33, 54) | ||
| Mean (SD) | 46 (17) | 34 (8) | 44 (14) | 46 (15) | −12 (−16 to −8) | <0.0001 |
| % Time >250 mg/dL | ||||||
| Median (IQR) | 15.1 (9.1, 26.0) | 9.5 (6.3, 14.1) | 14.7 (6.9, 22.7) | 14.2 (10.6, 25.9) | ||
| Mean (SD) | 18.3 (13.3) | 10.9 (6.5) | 16.0 (10.7) | 18.1 (12.2) | −8.1 (−11.7 to −4.5) | <0.0001 |
| % Time >300 mg/dL | ||||||
| Median (IQR) | 4.8 (2.7, 12.8) | 3.1 (1.4, 5.7) | 5.1 (1.2, 10.4) | 5.1 (3.1, 11.8) | ||
| Mean (SD) | 8.3 (8.2) | 4 (3.8) | 6.5 (6.6) | 7.9 (7.9) | −4.4 (−6.7 to −2.1) | 0.0005 |
| CGM-measured hyperglycemic eventb rate per week | ||||||
| Median (IQR) | 4.3 (2.6, 7.0) | 2.6 (1.6, 4.5) | 4.5 (1.1, 7.0) | 4.4 (3.3, 7.4) | ||
| Mean (SD) | 4.8 (3.) | 3.3 (2.2) | 4.5 (3.3) | 5.0 (3.0) | −1.9 (−2.9 to −1.0) | <0.0001 |
| % Time <70 mg/dL | ||||||
| Median (IQR) | 3.1 (0.7, 4.6) | 1.4 (0.8, 2.3) | 2.1 (1.0, 4.6) | 1.8 (0.7, 3.0) | ||
| Mean (SD) | 3.2 (2.7) | 1.6 (1.0) | 2.9 (2.5) | 2.1 (1.5) | −0.7 (−1 to −0.2) | 0.002 |
| % Time <54 mg/dL | ||||||
| Median (IQR) | 0.4 (0.1, 1.3) | 0.2 (0.1, 0.4) | 0.2 (0.1, 0.7) | 0.3 (0.1, 0.5) | ||
| Mean (SD) | 0.9 (1.1) | 0.3 (0.3) | 0.7 (1.0) | 0.4 (0.3) | −0.09 (−0.2 to 0.05) | 0.21 |
| CGM-measured hypoglycemic eventc rate per week | ||||||
| Median (IQR) | 0.6 (0.0, 2.2) | 0.5 (0.2, 1.0) | 0.5 (0.0, 1.5) | 0.5 (0.2, 0.8) | ||
| Mean (SD) | 1.3 (1.6) | 0.7 (0.6) | 1.1 (1.6) | 0.7 (0.8) | −0.2 (−0.5 to 0.2) | 0.31 |
| Coefficient of variation, % | 39 (7) | 37 (4) | 38 (6) | 38 (5) | −2 (−3 to 0) | 0.02 |
| CGM binary outcomes | ||||||
| Participants with TIR 70–180 mg/dL >70% | 2 (5%) | 12 (30%) | 2 (9%) | 3 (13%) | 22 (−6 to 44) | 0.06 |
| Participants with time <54 mg/dL <1% | 26 (65%) | 38 (95%) | 19 (83%) | 21 (91%) | 9 (−32 to 44) | 0.32 |
| Participants with TIR 70–180 mg/dL >70% and time <54 mg/dL <1% | 1 (3%) | 12 (30%) | 2 (9%) | 3 (13%) | 22 (−6 to 44) | 0.06 |
| HbA1c outcomes | ||||||
| Mean HbA1c, % [mmol/mol] | 7.97 (0.93) [64 (10.20)] | 7.51 (0.74) [59 (8.1)] | 7.66 (0.86) [60 (9.4)] | 7.66 (1.14)d [60 (12.5)] | −0.30 (−0.67 to 0.08) [−3.3 (−7.3 to 0.9)] | 0.13 |
| Participants with HbA1c ≤ 7.0% | 4 (10%) | 8 (20%) | 5 (22%) | 5 (23%)d | 6 (−24 to 27) | 0.45 |
| Participants with HbA1c improvement from baseline ≥0.5% | NA | 15 (38%) | NA | 5 (23%)d | 13 (−18 to 39) | 0.34 |
Mean (SD), n (%), or median (IQR).
Adjusted for baseline value of dependent variable, age, prior CGM use, prior pump use, and clinical center (random effects). P values and confidence intervals adjusted for multiplicity using the false discovery rate.
At least 15 consecutive minutes >300 mg/dL (>16.6 mmol/L).
At least 15 consecutive minutes <54 mg/dL (<3.0 mmol/L).
For one participant in control group, the 26-week visit was outside the prespecified window, thus their HbA1c value was excluded from analyses.
TIR, time in range.
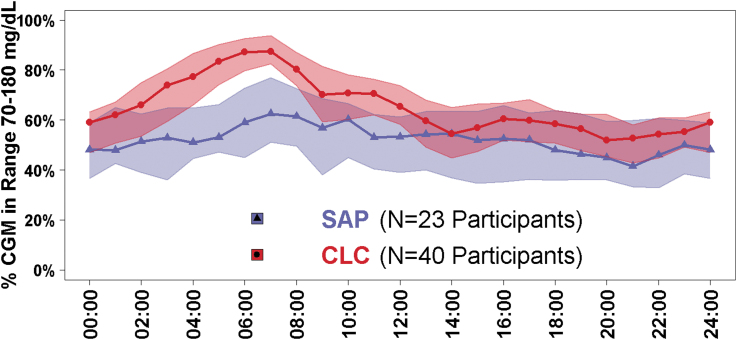
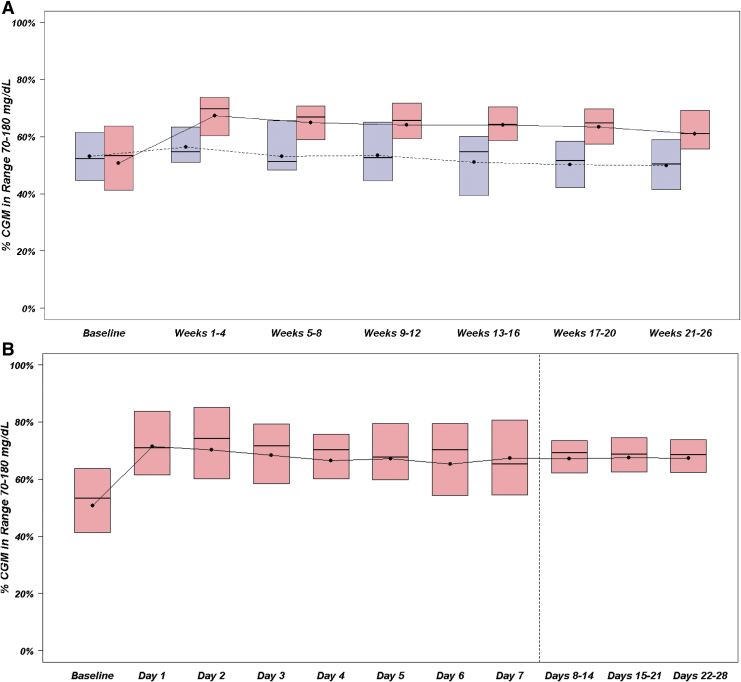
Improvements in TIR in the CLC group compared with the SAP group were especially substantial between 1 AM and 8 AM (Fig. 1). The risk-adjusted treatment group difference in TIR was +19% (95% CI 13–24, P < 0.0001) at night, and +11% (95% CI 7–14, P < 0.0001; Supplementary Table S1) during the day. From a longitudinal perspective, improvement in TIR in the CLC group was apparent after the first day of system use and was largely sustained over the remainder of the 26 weeks of follow-up (Fig. 2).
FIG. 1.
TIR by time of day and by treatment group. Blue triangles represent the SAP group, N = 23 participants. Red circles represent the CLC group, N = 40 participants. Lines with symbols denote hourly median values and the shaded regions are defined by the 25th and 75th percentiles. CLC, closed-loop control; SAP, sensor augmented pump; TIR, time in range.
FIG. 2.
(A) TIR over 26 weeks. Blue represents SAP group, N = 23 participants. Red represents CLC group, N = 40 participants. Black dots denote mean values, horizontal lines in the boxes denote medians, and the bottom and top of the boxes represent the 25th and 75th percentiles. (B) TIR during first 28 days in the CLC group. Red represents CLC group, N = 40 participants. Black dots denote mean values, horizontal lines in the boxes are medians, and bottom and top of the boxes represent the 25th and 75th percentiles. The range of the data are narrower after day 8 because it was averaged at a participant level over 7 days instead of 1 day, and consequently there would tend to be less variability in the point estimate for each participant.
Analysis of other CGM metrics for hyperglycemia, mean glucose, hypoglycemia, and glycemic variability all favored the CLC group compared with the SAP group, with the treatment group differences being greater during the night (12 AM–6 AM) than during the day (6 AM–12 PM) (Table 2; Supplementary Table S1 and Supplementary Figs. S2–S5). HbA1c was 7.97% (0.93) (63 [10.2 mmol/mol]) at baseline and 7.51% (0.74) (58 [8.1 mmol/mol]) at 26 weeks in the CLC group and was 7.66% (0.86) (60 [9.4 mmol/mol]) at baseline and 7.66% (0.74) (60 [8.1 mmol/mol]) at 26 weeks, in the SAP group (risk-adjusted treatment group difference = −0.30, 95% CI −0.67 to 0.08, P = 0.13) (Table 2).
A treatment effect on TIR was apparent in age groups 14 to <18 years old and 18 to <25 years old (Supplementary Table S2). In both groups, there did not appear to be meaningful differences comparing glycemic metrics on the weekdays versus the weekend (Supplementary Table S3). In jointly assessing TIR and hypoglycemia, 25 (63%) of the CLC group compared with 5 (22%) of the SAP group had an increase in TIR combined with a decrease in time <70 mg/dL (Supplementary Fig. S6).
Safety outcomes
No severe hypoglycemia episodes were reported in either group. One episode of DKA occurred in the CLC group due to infusion set failure (Supplementary Table S4). There were six additional episodes of hyperglycemia with ketosis reported in the CLC group versus one in the SAP group.
Conclusions
Adolescents and young adults with type 1 diabetes represent a challenging population who have generally been less successful with the use of diabetes devices and, as a result, have not gained the same benefits as either young children (8–14 years) or adults (>25 years) using such devices.18 As a result, we specifically targeted the age group of 14–24 years old for a preplanned secondary analysis of a 6-month randomized control trial to assess the efficacy and safety of CLC compared with SAP. Notably, we found that this sample of adolescents and young adults successfully used the CGM >90% of the time during the 6-month trial and the closed-loop system was active 89% of the time.
Glycemic outcomes in the adolescents and young adults were as positive as those observed in the adults in the study, with the younger participants increasing their glucose TIR over 3 h/day. Moreover, use of the CLC insulin delivery system was associated with lower mean glucose, and reduced time spent in both hyperglycemia and hypoglycemia. This effect was seen within 24 h of starting closed loop and was sustained for 6 months. This immediate improvement was the result of automated insulin delivery overnight, automated correction doses given during the day, and automated modulation of insulin delivery to decrease hypoglycemia, as it occurred before a change in human behavior, such as bolusing habits, seems possible. Of note, although trending in the same direction, changes in HbA1c were not significantly different between treatment groups, in contrast to the CGM metrics' results.
Use of CLC was especially effective at increasing TIR overnight, consistent with the design of the CLC algorithm, which intensifies control overnight by sliding the target range down to ∼110–120 mg/dL. This finding is especially salient in the adolescent population, where nocturnal and early morning glycemic control can be challenging due to changes in growth hormone, which can lead to hyperglycemia, as well as variation in exercise, which can trigger delayed hypoglycemia particularly overnight when exercise has been in the afternoon.19
Effects of CLC in this adolescent and young adult subgroup are consistent with the magnitude of improvements in glycemic control reported among older participants 25–71 years old (N = 105) in the overall randomized trial, where TIR improved from 66% to 76% with CLC compared with 63% to 64% with SAP.15 Thus, our results suggest that this CLC system has comparable efficacy for 6 months among adolescents and young adults as in older adults.
We are aware of only one other closed-loop randomized trial of at least 3 months that has reported results in this age group for comparison with our findings. In a 12-week randomized trial, Tauschmann et al.20 reported among 13–21 years old an improvement from baseline in TIR of 14% using CLC (N = 11) versus 4% using SAP (N = 8). In the 3-month Medtronic 670G single-arm study, TIR improved from 60% at baseline to 67% during follow-up among the 30 adolescents aged 14–21 years.21
Maximizing the proportion of time in automated insulin delivery mode is critical if closed-loop systems are to deliver on their promise of improving glycemic outcomes and lessening patient burden. Indeed, higher time spent in CLC mode is correlated with improvements in TIR, lower HbA1c, and decreased glycemic variability.12,22 Our observed 89% time in CLC mode, which was maintained over the 26 weeks of the study, stands in contrast to lower auto mode use described with the 670G system. In the 670G single-arm trial, median time in auto mode over 3 months was only 76%.21 Messer et al. reported an initial auto mode use of 87% declining to 72% by the end of 3 months of 670G use in patients of ages 14–26 years.23 A real-world single-center retrospective study reported median auto mode time of 38% in pediatric clinic patients of ages 10–21 years.12
From a safety perspective, there were no instances of severe hypoglycemia in either the CLC or the SAP group. However, we did note a trend for increased frequency of hyperglycemia with ketosis in the CLC group due to infusion set problems. This trend might reflect differences in adverse-event reporting between the groups, since the insulin pump used in the CLC group was an investigational device, whereas many participants in the SAP group were using an approved personal insulin pump.
An alternative explanation is that the participants in the CLC group may have become less attentive to hyperglycemia symptoms that could have led to a delay in identifying an infusion set failure. It is important to note that use of a closed-loop system has the same potential for infusion set failure as with using an insulin pump without automated insulin delivery, and CLC system users like all pump users should be mindful of the potential for infusion set failure producing hyperglycemia and ketosis.
Strengths of our study include the 6 months of follow-up (which is to our knowledge longer than any previous randomized clinical trial (RCT) of closed-loop insulin delivery in this age group) and the 100% retention of participants. The major limitation in interpretation of the results is that the study cohort was not fully representative of the general population of adolescents and young adults with type 1 diabetes. Despite broad inclusion criteria, most of our study population had high socioeconomic status, most were pump and CGM users before the study, and baseline HbA1c level on average was better than that in the general population for this age group. In addition, study participants may have had more frequent visits and phone contacts than might be expected outside of a study.
In conclusion, use of CLC for 6 months was substantial and associated with improved TIR and reduced hypoglycemia in adolescents and young adults with type 1 diabetes. Thus, CLC has the potential to improve glycemic outcomes in this challenging age group.
Supplementary Material
Contributor Information
Collaborators: for the iDCL Trial Research Group, Boris Kovatchev, Stacey Anderson, Emma Emory, Mary Voelmle, Katie Conshafter, Kim Morris, Mary Oliveri, Linda Gondor-Fredrick, Harry Mitchell, Kayla Calvo, Christian Wakeman, Marc Breton, Emily Flint, Kenny Kim, Lindsay Roethke, Mei Mei Church, Camille Andre, Molly Piper, David Lam, Grenye O'Malley, Camilla Levister, Selassie Ogyaadu, Jessica Lovett, Vinaya Simha, Vikash Dadlani, Shelly McCrady-Spitzer, Corey Reid, Kanchan Kumari, Greg Forlenza, G. Todd Alonso, Robert Slover, Emily Jost, Laurel Messer, Cari Berget, Lindsey Towers, Alex Rossick-Solis, Tali Jacobson, Marissa Town, Ideen Tabatabai, Jordan Keller, Evalina Salas, Francis Doyle, III, Eyal Dassau, Samantha Passman, Tiffany Campos, Carlos Murphy, Nandan Patibandla, Sarah Borgman, Guillermo Arreaza-Rubín, Neal Green, Boris Kovatchev, Sue Brown, Stacey Anderson, Marc Breton, Lori Laffel, Jordan Pinsker, Carol Levy, R. Paul Wadwa, Bruce Buckingham, Francis Doyle, III, Eric Renard, Claudio Cobelli, Yves Reznik, Guillermo Arreaza-Rubín, John Lum, Roy Beck, Robert Janicek, Deanna Gabrielson, Steven H. Belle, Jessica Castle, Jennifer Green, Laurent Legault, Steven M. Willi, Carol Wysham, and Thomas Eggerman
Authors' Contributions
E.I., R.W.B., and L.M.L. were involved in study design, researched data, contributed to discussion, and wrote/edited the article. D.R. performed statistical analyses and wrote/edited the article. L.A.-O., J.E.P., B.A.B., R.P.W., L.E., Y.C.K., C.J.L., G.P.F., C.K., J.W.L., and S.A.B. were involved in study design, researched data, contributed to discussion, and reviewed/edited the article.
Author Disclosure Statement
D.R. has no disclosures to report. L.A.-O. has received consulting fees from Dexcom. J.E.P. reports receiving grant support, provided to his institution, consulting fees, and speaker fees from Tandem Diabetes Care, grant support, provided to his institution, and advisory board fees from Medtronic, grant support, provided to his institution, and consulting fees from Eli Lilly, grant support and supplies, provided to his institution, from Insulet, and supplies, provided to his institution, from Dexcom. B.A.B. has received research funding from Tandem, Insulet Corp., Medtronic, Beta Bionics, Lilly, Convatec, and Dexcom. He has served on advisory boards for Medtronic, Novo Nordisk, and Convatec. R.P.W. reports receiving grant support and supplies, provided to his institution, from Tandem Diabetes Care, Dexcom, Beta Bionics, Eli Lilly, and MannKind and has served as a consultant/speaker for Eli Lilly and Tandem Diabetes Care. L.E. serves as a consultant for Tandem Diabetes Care. Y.C.K. received product support from Dexcom and Roche Diabetes and has consulted for Novo Nordisk. C.J.L. reports receiving advisory board fees from Sanofi, and grant support, paid to her institution, from Dexcom, Tandem Diabetes Care, Insulet, Abbott Diabetes, Senseonics, and Lexicon Pharmaceuticals. G.P.F. conducts research supported by Medtronic, Dexcom, Abbott, Tandem, Insulet, Beta Bionics, and Lilly and has been a consultant/speaker for Medtronic, Dexcom, Abbott, Tandem, Insulet, Beta Bionics, and Lilly. R.W.B. reports receiving consulting fees, paid to his institution, from Insulet, Bigfoot Biomedical, vTv Therapeutics, and Eli Lilly, grant support and supplies, provided to his institution, from Tandem and Dexcom, and supplies from Ascenia and Roche. C.K. has received consulting fees, paid to his institution, from Bigfoot Biomedical and grant support and supplies, provided to his institution, from Tandem and Dexcom. J.W.L. reports receiving consulting fees, paid to his institution, from Animas Corporation, Bigfoot Biomedical, Tandem Diabetes Care, and Eli Lilly. S.A.B. reports receiving grant support and supplies, provided to her institution, from Tandem Diabetes Care, Insulet, and Tolerion, and supplies, provided to her institution, from Dexcom and Roche Diagnostics. L.M.L. has received consulting fees from Johnson & Johnson, Sanofi, NovoNordisk, Roche, Dexcom, Insulet, Boehringer Ingelheim, ConvaTec, Medtronic, Lifescan, Laxmi, and Insulogic.
Funding Information
E.I. reports grant support from NICHD R21HD091974, NIDDK (P30DK057521 and UC4DK108483), the Harold Hamm Foundation, and the Peabody Foundation. NIH/NIDDK Grant UC4 108483. The University of Virginia Strategic Investment Fund Project #88 provided institutional and regulatory support. Tandem Diabetes Care provided the experimental closed-loop systems used in the trial, system-related supplies including the Dexcom CGM and Roche glucometer, and technical expertise. Tandem Diabetes Care was not involved in data analysis and was provided a copy of the article for review before publication.
Supplementary Material
References
- 1. Foster NC, Beck RW, Miller KM, et al. : State of type 1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Diabetes Association: 13. Children and adolescents: standards of medical care in diabetes-2020. Diabetes Care 2020;43:S163–S182 [DOI] [PubMed] [Google Scholar]
- 3. Hermann JM, Miller KM, Hofer SE, et al. : The transatlantic HbA1c gap; differences in glycaemic control across the lifespan between people included in the US T1D Exchange Registry and those included in the German/Austrian DPV registry. Diabet Med 2020;37:848–855 [DOI] [PubMed] [Google Scholar]
- 4. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group: intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC Study 30-Year Follow-up. Diabetes Care 2016;39:686–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group: Mortality in type 1 diabetes in the DCCT/EDIC versus the general population. Diabetes Care 2016;39:1378–138327411699 [Google Scholar]
- 6. Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, et al. : the effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 7. Ekhlaspour L, Forlenza GP, Chernavvsky D, et al. : Closed loop control in adolescents and children during winter sports: use of the Tandem Control-IQ AP system. Pediatr Diabetes 2019;20:759–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ly TT, Buckingham BA, DeSalvo DJ, et al. : Day-and-night closed-loop control using the unified safety system in adolescents with type 1 diabetes at camp. Diabetes Care 2016;39:e106–e107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ly TT, Keenan DB, Roy A, Han J, et al. : Automated overnight closed-loop control using a proportional-integral-derivative algorithm with insulin feedback in children and adolescents with type 1 diabetes at diabetes camp. Diabetes Technol Ther 2016;18:377–384 [DOI] [PubMed] [Google Scholar]
- 10. Phillip M, Battelino T, Atlas E, et al. : nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med 2013;368:824–833 [DOI] [PubMed] [Google Scholar]
- 11. Russell SJ, Hillard MA, Balliro C, et al. : Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol 2016;4:233–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duffus SH, Ta'ani ZA, Slaughter JC, et al. : Increased proportion of time in hybrid closed-loop “Auto Mode” is associated with improved glycaemic control for adolescent and young patients with adult type 1 diabetes using the MiniMed 670G insulin pump. Diabetes Obes Metab 2020;22:688–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berget C, Messer LH, Vigers T, et al. : Six months of hybrid closed loop in the real-world: an evaluation of children and young adults using the 670G system. Pediatr Diabetes 2020;21:310–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Messer LH, Berget C, Vigers T, et al. : Real world hybrid closed-loop discontinuation: predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes 2020;21:319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown SA, Kovatchev BP, Raghinaru D, et al. : Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chernavvsky DR, DeBoer MD, Keith-Hynes P, et al. : Use of an artificial pancreas among adolescents for a missed snack bolus and an underestimated meal bolus. Pediatr Diabetes 2016;17:28–35 [DOI] [PubMed] [Google Scholar]
- 17. Bangor A, Kortum P, Miller J: Determining what individual SUS scores mean: adding an adjective rating scale. J Usab Stud 2009;4:114–123 [Google Scholar]
- 18. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group; Tamborlane WV, Beck RW, Bode BW, et al. : Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 19. Sherr JL: Closing the loop on managing youth with type 1 diabetes: children are not just small adults. Diabetes Care 2018;41:1572–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tauschmann M, Thabit H, Bally L, et al. : Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet 2018;392:1321–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garg SK, Weinzimer SA, Tamborlane WV, et al. : Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017;19:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Akturk HK, Giordano D, Champakanath A, et al. : Long-term real-life glycaemic outcomes with a hybrid closed-loop system compared with sensor-augmented pump therapy in patients with type 1 diabetes. Diabetes Obes Metab 2020;22:583–589 [DOI] [PubMed] [Google Scholar]
- 23. Messer LH, Forlenza GP, Sherr JL, et al. : Optimizing hybrid closed-loop therapy in adolescents and emerging adults using the MiniMed 670G System. Diabetes Care 2018;41:789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.