Abstract
Objective: This study analysis was designed to examine the 24-h effects of exercise on glycemic control as measured by continuous glucose monitoring (CGM).
Methods: Individuals with type 1 diabetes (ages: 15–68 years; hemoglobin A1c: 7.5% ± 1.5% [mean ± standard deviation (SD)]) were randomly assigned to complete twice-weekly aerobic, high-intensity interval, or resistance-based exercise sessions in addition to their personal exercise sessions for a period of 4 weeks. Exercise was tracked with wearables and glucose concentrations assessed using CGM. An exercise day was defined as a 24-h period after the end of exercise, while a sedentary day was defined as any 24-h period with no recorded exercise ≥10 min long. Sedentary days start at least 24 h after the end of exercise.
Results: Mean glucose was lower (150 ± 45 vs. 166 ± 49 mg/dL, P = 0.01), % time in range [70–180 mg/dL] higher (62% ± 23% vs. 56% ± 25%, P = 0.03), % time >180 mg/dL lower (28% ± 23% vs. 37% ± 26%, P = 0.01), and % time <70 mg/dL higher (9.3% ± 11.0% vs. 7.1% ± 9.1%, P = 0.04) on exercise days compared with sedentary days. Glucose variability and % time <54 mg/dL did not differ significantly between exercise and sedentary days. No significant differences in glucose control by exercise type were observed.
Conclusion: Participants had lower 24-h mean glucose levels and a greater time in range on exercise days compared with sedentary days, with mode of exercise affecting glycemia similarly. In summary, this study offers data supporting frequency of exercise as a method of facilitating glucose control but does not suggest an effect for mode of exercise.
Keywords: Type 1 diabetes, Exercise, Time in range, Continuous glucose monitoring, hypoglycemia, hyperglycemia, physical activity
Introduction
Regular exercise is recommended for a variety of reasons for people living with type 1 diabetes.1,2 It improves cardiorespiratory fitness, cardiovascular health, body composition, insulin sensitivity, well-being, and quality of life.3–5 The effect of regular exercise on hemoglobin A1c (HbA1c) levels in type 1 diabetes is equivocal, however, with some meta-analyses demonstrating modest improvement,5,6 and others showing little to no effect.4,7
While HbA1c is a meaningful metric of glycemic control that is associated with the development and progression of several diabetes-related complications,8–11 it only reflects average glucose over the last 2–3 months and does not provide information about the amount of exposure to hypo- or hyperglycemia or about glucose variability. Moreover, HbA1c fails to identify the potentially transient impact of a therapy, such as exercise, on glucose control in the hours surrounding the event.
As an alternative to HbA1c, continuous glucose monitoring (CGM) may be a more useful tool for determining if an exercise session has a time-restricted, but clinically meaningful impact on glycemic control as measured by time in range (TIR; 70–180 mg/dL) and the various components of time out of glycemic range, including time below range (<70 and <54 mg/dL) or time above range (>180 and >250 mg/dL).12,13 Examining the impact of exercise on short-term TIR might help inform existing physical activity guidelines1,2 on the frequency and mode of exercise that could help facilitate improved glucose control and other safe exercise training principles in type 1 diabetes.
A study was designed to examine the effect of various forms of exercise (i.e., continuous aerobic, high-intensity intervals, resistance) on acute measures of glycemic control as measured by CGM. The purpose of this analysis was to determine if days with structured exercise sessions have a different glycemic profile, as measured by TIR and other related metrics, compared to days with no structured exercise.
Methods
Study design
To examine the factors that influence the glucose responses to various forms of exercise in people living with type 1 diabetes under real-world living conditions, data were captured using wearable, connected devices and smart phone applications. The protocol and informed consent forms were approved by an institutional review board. Informed consent was obtained from each participants. Participants were either enrolled at clinic sites or remotely. Participants were randomly assigned to complete one of the following study exercise videos at home twice weekly for 4 weeks: (1) continuous moderate-intensity aerobic training; (2) high-intensity interval training (HIIT); or (3) resistance training.
Participants enrolled at clinic sites also completed two of these video-led exercise sessions in-clinic. Each exercise video was ∼30 min in duration, led by a certified exercise professional and designed with activities so that participants could achieve a target heart rate of 70%–80% of age-predicted maximal heart rate (aerobic video), or with intervals of heart rate up to 80%–90% of age-predicted maximal heart rate (HIIT video), or with resistance-band activities that elicited major muscle group fatigue after three sets of eight repetitions (resistance video), similar to what is recommended in current guidelines.1 In addition to the study exercise videos, participants were encouraged to continue their usual physical activity regimen of training, sports, and/or other structured physical activity events.
Participants used a study-developed, cloud-connected smartphone application (T1Dexi smart phone app) to enter all of their study-assigned structured exercise events (time, duration, and type of activity) and other personal exercise activities (i.e., exercise session other than the study-assigned exercise video) as well as other potential variables thought to influence the glycemic response to exercise (i.e., time since last meal, if a snack was eaten, if the activity was associated with competition stress, etc.). Meal content was also captured by the T1Dexi smart phone app through self-reported kilocalorie, carbohydrate, fat, and protein content accompanied by food photographs taken on the phone.
Participants used either their personal CGM or a blinded CGM (Dexcom G4 or G5 [San Diego, CA]) if they did not use a personal CGM, as well as a wearable activity and heart rate monitor (Garmin Vivosmart 3 [Olathe, KS] or a Verily Study Watch [San Francisco, CA]).
Identification of exercise and nonexercise days
Days were stratified as structured “exercise days” or “sedentary days.” To be included in these analyses, participants must have had at least one eligible exercise day and one sedentary day. A minimum of 8 h of CGM data were also required for each exercise or sedentary day. An exercise day was defined as a 24-h period following the end of a logged study-assigned or personal exercise session. For example, if an exercise event ended at 8 AM, then the exercise day would be the 24-h period starting at 8 AM. A sedentary day was defined as a 24-h period without an exercise session that began at least 24 h after the end of the last exercise session. For example, if an exercise ended at 8 AM, then the 24-h sedentary day would begin at 8 AM the next day, as long as no activity was documented for that 24-h sedentary period.
Both exercise and sedentary days were truncated if another exercise session occurred within the 24-h period. There is no overlap between sedentary and exercise days. An example of the categorization of exercise and sedentary days is provided in Supplementary Figure S1. While it is acknowledged that some habitual physical activity likely occurred on “sedentary” days (e.g., walking, shopping, etc.), we use the term sedentary to mean that there was no reported study-assigned or other personal exercise sessions with a duration of at least 10 min within that time frame. Because of study design, the time of day for exercise was not standardized and varied markedly.
Exercise days were further divided into 4-h time blocks to explore how long postexercise effects on glycemia lasted. Each exercise time block was paired with the closest time-matched sedentary block within 7 days. The 4-h time blocks required at least 2 h of CGM data to be included in the analyses.
Outcome measures and data analysis
Primary analyses assessed mean glucose, TIR, % time >180 mg/dL, and % time <70 mg/dL, according to the International Consensus on Time in Range.14 Secondary analyses included summarizing glucose coefficient of variation, % time <54 mg/dL and % time >250 mg/dL. These outcomes were compared on exercise versus sedentary days using a repeated measures linear regression model adjusting for assigned exercise type, study protocol (i.e., in-clinic or remote enrollment), baseline HbA1c, baseline age, baseline body mass index (BMI), and baseline pump use as fixed effects with a first-order autoregressive correlation structure. Power calculations were not performed for this observational study.
The effects of exercise duration and participant-reported competition status (yes/no) on glycemia were assessed on exercise days only using similar models with an additional adjustment for whether the exercise bout was performed in-clinic or at-home and baseline glucose before exercise.
Within each study-assigned exercise type, the following analyses were performed for the glycemic metrics: (1) testing for an interaction effect between day type (i.e., exercise, sedentary) and assigned exercise type (i.e., aerobic, HIIT, resistance) and (2) exercise versus sedentary day comparisons within each study-assigned exercise type. Exercise and sedentary glycemia were also averaged for each participant to assess the effect of exercise on an individual level.
Outcomes are summarized as means and standard deviations (SDs) or summary statistics appropriate to the distribution. For skewed outcomes, the 90% winsorized mean was reported by censoring at the lower 5th and upper 95th percentiles, and a rank-based Van der Waerden transformation was used for testing. Multiple comparisons were corrected for using the Benjamini–Hochberg adaptive false discovery rate correction procedure. All statistical tests were two-sided and assessed at the α = 0.05 significance level.
Results
Study participants
Forty-four participants, 15 to 68 years old ([mean ± SD]: 35 ± 15 years; BMI: 26.3 ± 3.1 kg/m2; HbA1c: 7.5% ± 1.5%) with type 1 diabetes (median diabetes duration was 16 [interquartile range (IQR): 9, 24] years) using either multiple daily injections (n = 9) or an insulin pump (n = 34) were randomly assigned to complete one of three study exercise videos (aerobic [n = 19], resistance [n = 14], or HIIT [n = 11]). Baseline characteristics for the 44 participants are described in Supplementary Table S1.
Overall glycemic control on exercise versus sedentary days
A total of 642 exercise days and 478 sedentary days were included in the analyses. Two hundred fifty-two (39%) of the exercise days included an assigned study exercise video. Table 1 summarizes the CGM-measured glucose metrics on exercise and sedentary days.
Table 1.
Overall Glycemic Control on Exercise Versus Sedentary Days
| Exercise type | Metric | Exercise daya Summary statistics |
Sedentary day Summary statistics |
Pb |
|---|---|---|---|---|
| All study-assigned and nonstudy-assigned exercise | No. of participants | 44 | 44 | |
| No. of days | 642 | 478 | ||
| Hours of glucose readings, median (quartiles) | 21 (15, 24) | 22 (17, 24) | ||
| <12 h, n (%) | 92 (14%) | 50 (10%) | ||
| 12 to <16 h, n (%) | 81 (13%) | 59 (12%) | ||
| 16 to <20 h, n (%) | 87 (13%) | 76 (16%) | ||
| ≥20 h, n (%) | 382 (60%) | 293 (61%) | ||
| Mean glucose (mg/dL), mean (SD) | 150 (45) | 166 (49) | 0.01 | |
| Coefficient of variation (%), mean (SD) | 35% (12%) | 35% (12%) | ||
| % TIR 70–180 mg/dL, mean (SD) | 62% (23%) | 56% (25%) | 0.03 | |
| % Time below 70 mg/dLc, mean (SD) | 9.3% (11.0%) | 7.1% (9.1%) | 0.04 | |
| % Time below 54 mg/dLc, mean (SD) | 3.3% (5.6%) | 2.6% (4.5%) | 0.15 | |
| % Time above 180 mg/dLc, mean (SD) | 28% (23%) | 37% (26%) | 0.01 | |
| % Time above 250 mg/dLc, mean (SD) | 9% (13%) | 14% (17%) | 0.06 | |
| Study-assigned aerobic exercise | No. of participants | 19 | 19 | |
| No. of days | 116 | 224 | ||
| Hours of glucose readings, median (quartiles) | 22 (19, 24) | 22 (16, 24) | ||
| Mean glucose (mg/dL), mean (SD) | 150 (40) | 158 (45) | 0.43 | |
| Coefficient of variation (%), mean (SD) | 36% (12%) | 36% (13%) | ||
| % TIR 70–180 mg/dL, mean (SD) | 63% (19%) | 57% (24%) | 0.37 | |
| % Time below 70 mg/dLc, mean (SD) | 9.5% (11.0%) | 8.3% (9.5%) | 0.68 | |
| % Time below 54 mg/dLc, mean (SD) | 3.3% (5.8%) | 2.9% (4.6%) | 0.68 | |
| % Time above 180 mg/dLc, mean (SD) | 27% (20%) | 34% (25%) | 0.37 | |
| % Time above 250 mg/dLc, mean (SD) | 9% (11%) | 12% (15%) | 0.68 | |
| Study-assigned HIIT exercise | No. of participants | 14 | 14 | |
| No. of days | 72 | 140 | ||
| Hours of glucose readings, median (quartiles) | 21 (15, 23) | 22 (18, 24) | ||
| Mean glucose (mg/dL), mean (SD) | 154 (49) | 160 (45) | 0.37 | |
| Coefficient of variation (%), mean (SD) | 35% (12%) | 35% (12%) | ||
| % TIR 70–180 mg/dL, mean (SD) | 62% (23%) | 59% (23%) | 0.34 | |
| % Time below 70 mg/dLc, mean (SD) | 9.2% (10.9%) | 8.1% (10.3%) | 0.68 | |
| % Time below 54 mg/dLc, mean (SD) | 3.2% (4.9%) | 3.2% (5.1%) | 0.77 | |
| % Time above 180 mg/dLc, mean (SD) | 28% (24%) | 32% (23%) | 0.30 | |
| % Time above 250 mg/dLc, mean (SD) | 10% (16%) | 11% (15%) | 0.68 | |
| Study-assigned resistance exercise | No. of participants | 11 | 11 | |
| No. of days | 64 | 114 | ||
| Hours of glucose readings, median (quartiles) | 22 (17, 24) | 22 (16, 23) | ||
| Mean glucose (mg/dL), mean (SD) | 166 (54) | 188 (56) | 0.30 | |
| Coefficient of variation (%), mean (SD) | 35% (12%) | 32% (11%) | ||
| % TIR 70–180 mg/dL, mean (SD) | 55% (24%) | 49% (27%) | 0.43 | |
| % Time below 70 mg/dLc, mean (SD) | 7.0% (8.5%) | 3.4% (5.5%) | 0.31 | |
| % Time below 54 mg/dLc, mean (SD) | 2.3% (4.6%) | 1.1% (2.7%) | 0.57 | |
| % Time above 180 mg/dLc, mean (SD) | 37% (26%) | 47% (28%) | 0.30 | |
| % Time above 250 mg/dLc, mean (SD) | 13% (18%) | 20% (23%) | 0.57 |
Summary statistics are on an event-level. P = 0.49, >0.99, 0.68, 0.68, 0.75, and 0.68 for the day type by exercise group interaction effect for mean glucose, % time 70–180, <70, <54, >180, and >250 mg/dL, respectively.
Exercise days for all exercise types are inclusive of both study-assigned and nonstudy-assigned exercise. A minimum exercise duration of 10 min was required.
From a repeated measures linear regression model adjusting for baseline HbA1c, baseline age, baseline BMI, study protocol, and baseline pump use as fixed effects with a first-order autoregressive correlation structure. Assigned exercise type was also adjusted for when assessing the overall exercise effect. Due to skewed residuals, % time below 70 mg/dL, % time below 54 mg/dL, % time above 180 mg/dL, and % time above 250 mg/dL were rank-transformed. Multiple comparisons were adjusted for using the Benjamini–Hochberg adaptive false discovery rate procedure.
Due to skewed distribution, each metric was censored at the 5th and 95th percentile, and a winsorized mean and SD are reported.
HbA1c, hemoglobin A1c; HIIT, high-intensity interval training; SD, standard deviation; TIR, time in range.
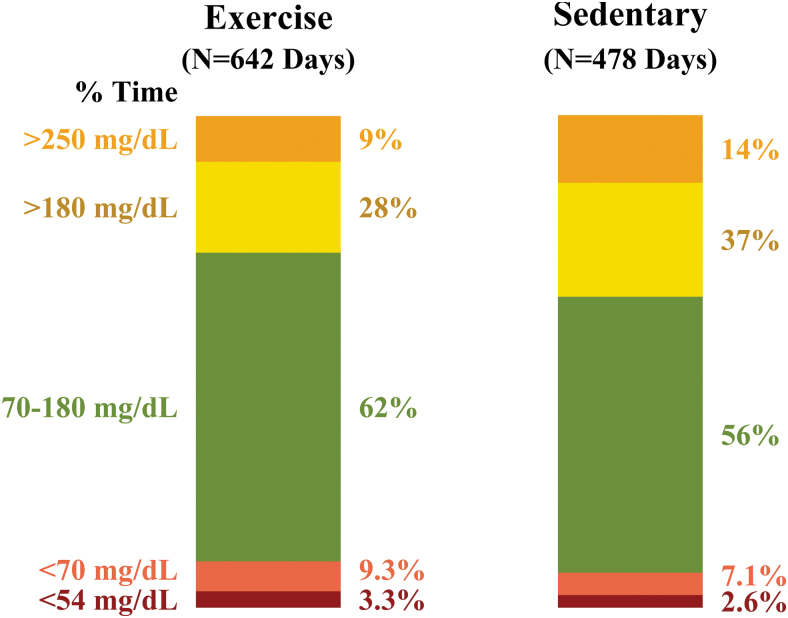
A stacked bar plot of mean TIR, time above range, and time below range on exercise and sedentary days is shown in Figure 1. Compared with sedentary days, exercise days had a lower mean glucose (150 ± 45 mg/dL exercise vs. 166 ± 49 mg/dL sedentary, P = 0.01), higher TIR (62% ± 23% vs. 56% ± 25%, P = 0.03), and lower % time >180 mg/dL (28% ± 23% vs. 37% ± 26%, P = 0.01). Exercise days also had higher % time <70 mg/dL compared with sedentary days (9.3% ± 11.0% vs. 7.1% ± 9.1%, P = 0.04), but no significant difference in % time below 54 mg/dL (3.3% ± 5.6% exercise vs. 2.6% ± 4.5% sedentary, P = 0.15). Mean glucose coefficient of variation on exercise and sedentary days were both 35% ± 12%.
FIG. 1.
Stacked bar plot of average % time <54, <70, 70 to 180, >180, and >250 mg/dL on exercise versus sedentary days. Percent time <54 mg/dL is a subset of % time <70 mg/dL, while % time >250 mg/dL is a subset of % time >180 mg/dL. Due to skewed distribution, % time <70, <54, >180, and >250 mg/dL values were each censored at the 5th and 95th percentile. Color graphics are available online.
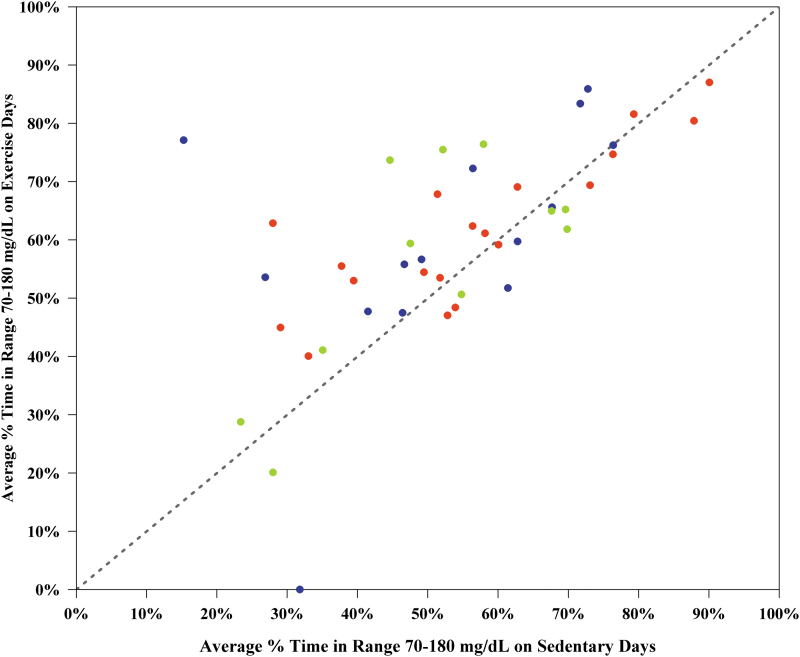
Trends were similar within each of the study-assigned exercise types, although there may be insufficient power to detect differences within each study-assigned exercise type due to the smaller sample size. A sensitivity analysis restricting to a minimum of 16 h of CGM data per exercise or sedentary day showed similar results (Supplementary Table S2). Most participants had better TIR postexercise, with 14/44 (32%) participants increasing their average TIR by >10% on exercise days and only 1/44 (2%) participant decreasing their average TIR by >10% on exercise days (Fig. 2). A cumulative distribution function plot of participant-level mean % TIR 70–180 mg/dL on exercise versus sedentary days is displayed in Supplementary Figure S2, showing higher participant-level mean % TIR 70–180 mg/dL on exercise days when compared with sedentary days.
FIG. 2.
Scatterplot of average % time 70–180 mg/dL on exercise versus sedentary days (n = 44 participants). Dots above the line of identity represent participants with greater time in range on exercise days compared with sedentary days. Red, blue, and green dots represent participants assigned to aerobic, HIIT, and resistance study exercise videos, respectively. HIIT, high-intensity interval training. Color graphics are available online.
Exercise events with shorter durations had lower % time <70 mg/dL (P = 0.03) and lower % time <54 mg/dL (P = 0.03) in comparison to exercise events with longer durations. Shorter exercise durations had higher TIR when compared to longer exercise sessions; however, this effect did not reach statistical significance (P = 0.06). There was no significant association between mean glucose, % time >180 mg/dL, or % time >250 mg/dL and exercise duration (Table 2). Postexercise glycemia was similar for competitive and noncompetitive exercises, although competitive exercise trended toward a higher mean glucose than noncompetitive exercise (P = 0.14) (Supplementary Table S3).
Table 2.
Relationship Between Postexercise Glycemia and Exercise Duration on Exercise Days
| Metric | Exercise duration (min) |
Pa | ||
|---|---|---|---|---|
| 10 to 30 | 31 to <60 | ≥60 | ||
| No. of participants/no. of days | 31/160 | 41/348 | 28/111 | |
| Hours of glucose readings, median (quartiles) | 20 (13, 23) | 22 (18, 24) | 21 (14, 23) | |
| Baseline glucose before exercise (mg/dL)b, mean (SD) | 138 (58) | 156 (69) | 143 (60) | |
| Mean glucose (mg/dL), mean (SD) | 144 (43) | 153 (44) | 147 (44) | 0.40 |
| Glucose coefficient of variation (%), mean (SD) | 34% (12%) | 36% (11%) | 36% (12%) | |
| % TIR 70 to 180 mg/dL, mean (SD) | 67% (23%) | 61% (22%) | 60% (24%) | 0.06 |
| % Time below 70 mg/dLc, mean (SD) | 9.1% (10.8%) | 8.9% (10.3%) | 11.4% (14.5%) | 0.03 |
| % Time below 54 mg/dLc, mean (SD) | 2.8% (5.0%) | 3.0% (5.2%) | 5.5% (9.1%) | 0.03 |
| % Time above 180 mg/dLc, mean (SD) | 23% (23%) | 29% (23%) | 27% (25%) | 0.37 |
| % Time above 250 mg/dLc, mean (SD) | 7% (13%) | 10% (14%) | 8% (12%) | 0.36 |
Summary statistics are on a day-level.
From a repeated measures linear regression model adjusting for baseline age, BMI, HbA1c, pump use, baseline glucose before exercise, whether or not the exercise session was done in clinic, protocol, and exercise type with a first-order autoregressive correlation structure. Due to a skewed outcome, % time >180, >250, <70, and <54 mg/dL were rank-transformed.
Average glucose in the hour before exercise.
Due to skewed distribution, each metric was censored at the 5th and 95th percentile, and a winsorized mean and SD are reported.
BMI, body mass index.
Glycemic control within 24 h of exercise
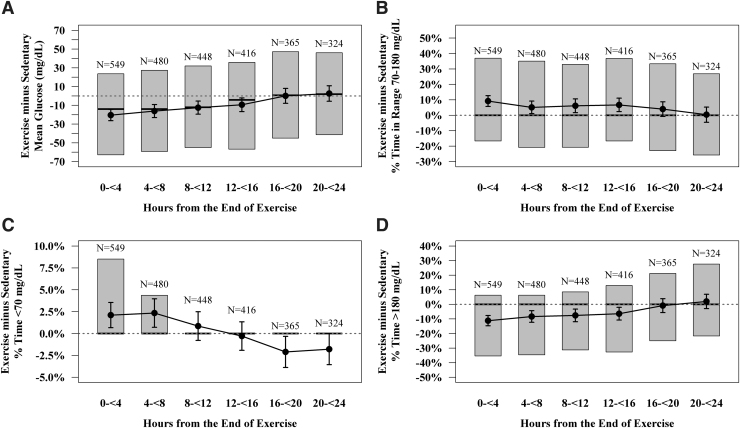
A total of 640 exercise events had at least one 4-h postexercise time block that could be matched with an equivalent sedentary time block. Figure 3 and Supplementary Table S4 summarize the differences in CGM-measured glucose metrics on exercise and sedentary days in 4-h postexercise time blocks.
FIG. 3.
Boxplots of glycemic control in the 24 h following exercise days in comparison to equivalent time periods on sedentary days, analyzed on an exercise event-level. Shaded boxes represent the interquartile range. Vertical bars represent the 95% confidence interval around the mean (black dots). Solid black lines in the middle of the shaded boxes represent medians. Number above boxes indicate number of exercise events. Due to skewed distribution, % time below 70 mg/dL and % time above 180 mg/dL values were each censored at the 5th and 95th percentile.
Within 4 h after the end of exercise, average mean glucose was 20 ± 72 mg/dL lower than similar time periods on sedentary days. This difference in mean glucose level between exercise and sedentary days dropped to 0 ± 77 mg/dL by 16–20 h after exercise. Hyperglycemia followed a similar pattern: mean % time >180 mg/dL was 11% ± 42% lower in the 4 h postexercise compared with a comparable period on a sedentary day, but returned to levels experienced during equivalent sedentary time periods by 16–20 h after exercise.
Within the time frames examined, time below range was elevated with a higher frequency over the first 12 h after exercise compared to sedentary days. This elevation diminished with time in recovery, perhaps even with less time below range in the 12–24 h after exercise compared with nonexercise days.
When looking at data from study-assigned exercise sessions only, the postexercise glycemic control patterns were found to be largely similar within the aerobic, HIIT, and resistance exercise types. Participants showed better average TIR and less % of time >180 mg/dL control in the 12–20 h after resistance exercise than in the initial 12 h after resistance exercise (Supplementary Table S4).
Discussion
In this study, we found that mean glucose concentration is lower and TIR higher, with less % time >180 mg/dL, on days with structured exercise when compared to sedentary days in people living with type 1 diabetes. The improvement in mean glucose levels (156 ± 45 mg/dL vs. 166 ± 49 mg/dL) and TIR on exercise days when compared to sedentary days appear clinically relevant, with more than half of the participants having higher TIR by about 5% on days with structured exercise. We also found that exercise did not impact glucose variability but modestly increased the % time <70 mg/dL on exercise days compared with sedentary days, particularly in the first 12 h of exercise recovery.
Importantly, there was no overall statistically significant difference between exercise and sedentary days in time spent with serious, clinically significant hypoglycemia (i.e., % time <54 mg/dL was 3.3% ± 5.6% on exercise days compared with 2.6% ± 4.5% on sedentary days); however, the lack of significance may be attributed to low power, and longer exercise durations did significantly increase time below range. Finally, our study showed similar postexercise improvements in glycemia among all study-assigned exercise types (i.e., aerobic, resistance, HIIT), although there may have been insufficient power to conclude that these improvements were statistically significant. It also did not find a significant association between postexercise glycemia with competition.
The improvements in glycemia caused by structured exercise, as measured by mean glucose level and TIR, tended to peak within about 4 h after the end of exercise, with these metrics then reverting back toward the preexercise values within 16–20 h. This finding is in line with the temporary increase in insulin sensitivity and glucose disposal as measured by glucose clamp technique following aerobic exercise.15,16 The reduction in glucose levels typically caused by exercise may come at a cost, however, as we also observed that time below range tended to increase during at least the first 8 h after the end of exercise, as has previously been observed clinically17 and in more controlled laboratory-based studies.15,18,19
Interestingly, exercise days for the resistance group tended to have not only slightly lower TIR with more % time >180 mg/dL but also had the greatest improvement compared with sedentary days. However, the resistance exercise group also had less optimal glycemic control on sedentary days when compared with the sedentary days of the aerobic and interval groups. In our study, it is unclear if participants managed these modes of exercise differently with respect to insulin dose administration or carbohydrate or other macronutrient ingestion to help limit their anticipated changes in glucose level associated with the exercise event. Nonetheless, this study demonstrates clinically significant improvements in glycemic control in the 24 h following the end of exercise.
In some laboratory-based studies, both HIIT20,21 and resistance training22 have been associated with a rise in glucose and often hyperglycemia that can last for hours in recovery, particularly if performed in a fasted state. On the contrary, improved glucose control with home-based HIIT, compared to moderate-intensity continuous (aerobic) training has been demonstrated.23,24
In this study, we did not observe a rise in glucose levels caused by HIIT or resistance training. A recent study by Reddy et al. demonstrated that home-based resistance exercise training improves TIR over sedentary behavior or aerobic exercise training.25 The failure to observe the expected rise in glucose levels with HIIT in this study may be related to several uncontrolled factors such as the timing of exercise, insulin levels during and after HIIT, feeding behaviors, and meal content and/or the relative intensity of the HIIT-based sessions.
One advantage of measuring TIR in people living with type 1 diabetes, in addition to HbA1c, is that the former may allow for the observation of time-restricted impact on glycemia over the short-term, such as potentially the impact of active days compared with inactive days on overall glucose control. Measuring TIR, hypoglycemia, and hyperglycemia also allows for the potential to determine if a structured activity, like exercise, compromises control by increasing the percent of time out of range.
To date, only a limited number of studies have used TIR to contrast an exercise day with a nonexercise day or to compare days with two or more different exercise modes. In 12 adults with type 1 diabetes (9 on multiple daily injections; 3 on continuous subcutaneous insulin infusion (CSII)), TIR was similar among days with either ∼30 min of continuous moderate-intensity cycling (41.2% ± 25.0%) or ∼20 min of HIIT cycling (41.6% ± 26.8%), compared to nonexercise days (41.2% ± 25.4%).26
In another study of 12 adults using closed-loop insulin delivery, TIR was also similar between days with HIIT (median 79.5% [IQR: 73.2%, 87.6%]) and days with continuous moderate-intensity exercise (76.1% [IQR: 70.3%, 83.9%], P = 0.37) while no formal comparison was made to nonexercise days.27 Days under closed-loop control had higher TIR (71.3% ± 17.6%) than under open-loop control (64.7% ± 13.3%) in a prolonged outdoor ski study in adolescents with type 1 diabetes, but again no comparison was made to sedentary days.28
In a recent study, Scott et al. failed to observe any improvements in CGM-based glycemic metrics with ∼30 min of fasted in-clinic HIIT or continuous moderate-intensity aerobic exercise, compared to a sedentary day, in 14 previously sedentary people with type 1 diabetes.23 The failure to observe differences may have been related to the small sample size and the large glucose variability among the study participants.
This study has not only several strengths but also some limitations that need to be acknowledged. This study was a large observational assessment of almost 700 exercise sessions captured in a free-living environment. We used instructional videos to standardize, at least to some degree, the at-home prescribed exercise modes and a newly developed smartphone app that allowed for the contextualization of all exercise events, including those activities outside of the study videos that may be associated with competition stress. However, this study relied on participants to sufficiently self-report exercises in the study-specific mobile app.
While some exercises may have been missed or the timing not properly recorded, this would have likely made exercise and sedentary days more similar; thus, the effect of exercise on glycemic control may be even greater than what is reported. In addition, while study-assigned exercise videos were randomized by participant, exercise and sedentary days themselves were not, which could have introduced confounding as participants were able to choose their own exercise schedule. This data set may also be insufficient to determine if the various exercise modes differ in their glycemic responses and to explain the variable effect that glucose has on glycemia in persons living with type 1 diabetes. Moreover, we did not determine if the time of nutritional intake, or insulin usage by the individuals who impacted their glycemic responses to exercise. A larger cohort is currently being recruited to address these limitations.
In summary, in postpubertal adolescents and adults living with type 1 diabetes, days with structured exercise were associated with lower mean glucose and improved TIR when compared with sedentary days, but with modestly increased nonserious hypoglycemia. These preliminary results suggest that this effect exists exclusive of the mode of exercise, thereby suggesting a take-home message that exercise, no matter the mode, can potentially have immediate clinically significant benefits for persons with type 1 diabetes.
Supplementary Material
Author Disclosure Statement
The intellectual property surrounding the Remote Food Photography Method© (RFPM©) is owned by Pennington Biomedical Research Center/Louisiana State University and CM is an inventor of the technology. M.C.R. has received speaker's honoraria from Medtronic Diabetes, Insulet, Ascensia Diabetes Program), Xeris Pharmaceuticals, Lilly Diabetes and Lilly Innovation. Z.L. has no disclosures. R.W.B. reports receiving consulting fees, paid to his institution, from Insulet, Bigfoot Biomedical, vTv Therapeutics, and Eli Lilly, grant support and supplies, provided to his institution, from Tandem and Dexcom, and supplies from Ascensia and Roche. R.L.G. has no disclosures. P.G.J. has no disclosures. J.R.C. reports receiving personal fees from Novo Nordisk, personal fees from AstraZeneca, personal fees from Zealand Pharma, personal fees from Dexcom, personal fees from Adocia, other from Pacific Diabetes Technologies, outside the submitted work. M.B.G. has no disclosures. M.C. has no disclosures. S.R.P. has no disclosures. E.D. is currently an employee of Eli Lilly and Company. The work presented in this poster/article was performed as part of E.D.'s academic appointment and is independent of his employment with Eli Lilly and Company. F.J.D. reports equity, licensed IP and is a member of the Scientific Advisory Board of Mode AGC. C.K.M. has no disclosures. P.C. has no disclosures. M.R.R. has received consulting honoraria from Semma Therapeutics and Sernova Corp. and research support from Xeris Pharmaceuticals.
Funding Information
This work was supported by a grant from the Leona M. and Harry B. Helmsley Charitable Trust and by U.S. Public Health Service research grants UL1 TR001878 (University of Pennsylvania Center for Human Phenomic Science), P30 DK19525 (University of Pennsylvania Diabetes Research Center), P30 DK072476 (Pennington Biomedical Research Center), and U54 GM104940 (Pennington Biomedical Research Center). This research was supported by a grant of no charge materials from Verily Life Sciences LLC. This research was supported by a grant of no charge materials from DIABNEXT LLC.
Supplementary Material
References
- 1. Colberg SR, Sigal RJ, Yardley JE, et al. : Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016;39:2065–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riddell MC, Gallen IW, Smart CE, et al. : Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol 2017;5:377–390 [DOI] [PubMed] [Google Scholar]
- 3. Chimen M, Kennedy A, Nirantharakumar K, et al. : What are the health benefits of physical activity in type 1 diabetes mellitus? A literature review. Diabetologia 2012;55:542–551 [DOI] [PubMed] [Google Scholar]
- 4. Ostman C, Jewiss D, King N, Smart NA: Clinical outcomes to exercise training in type 1 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract 2018;139:380–391 [DOI] [PubMed] [Google Scholar]
- 5. Wu N, Bredin SSD, Guan Y, et al. : Cardiovascular health benefits of exercise training in persons living with type 1 diabetes: a systematic review and meta-analysis. J Clin Med 2019;8:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yardley JE, Hay J, Abou-Setta AM, et al. : A systematic review and meta-analysis of exercise interventions in adults with type 1 diabetes. Diabetes Res Clin Pract 2014;106:393–400 [DOI] [PubMed] [Google Scholar]
- 7. Kennedy A, Nirantharakumar K, Chimen M, et al. : Does exercise improve glycaemic control in type 1 diabetes? A systematic review and meta-analysis. PLoS One 2013;8:e58861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group: Effect of intensive diabetes treatment on carotid artery wall thickness in the epidemiology of diabetes interventions and complications. Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group. Diabetes 1999;48:383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group, Lachin JM, White NH, et al. : Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes 2015;64:631–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group: Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014;37:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miller RG, Anderson SJ, Costacou T, et al. : Hemoglobin A1c level and cardiovascular disease incidence in persons with type 1 diabetes: an application of joint modeling of longitudinal and time-to-event data in the Pittsburgh Epidemiology of Diabetes Complications Study. Am J Epidemiol 2018;187:1520–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Houlder SK, Yardley JE: Continuous glucose monitoring and exercise in type 1 diabetes: past, present and future. Biosensors (Basel) 2018;8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tagougui S, Taleb N, Rabasa-Lhoret R: The benefits and limits of technological advances in glucose management around physical activity in patients type 1 diabetes. Front Endocrinol (Lausanne) 2018;9:818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Battelino T, Danne T, Bergenstal RM, et al. : Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davey RJ, Howe W, Paramalingam N, et al. : The effect of midday moderate-intensity exercise on postexercise hypoglycemia risk in individuals with type 1 diabetes. J Clin Endocrinol Metab 2013;98:2908–2914 [DOI] [PubMed] [Google Scholar]
- 16. McMahon SK, Ferreira LD, Ratnam N, et al. : Glucose requirements to maintain euglycemia after moderate-intensity afternoon exercise in adolescents with type 1 diabetes are increased in a biphasic manner. J Clin Endocrinol Metab 2007;92:963–968 [DOI] [PubMed] [Google Scholar]
- 17. MacDonald MJ: Postexercise late-onset hypoglycemia in insulin-dependent diabetic patients. Diabetes Care 1987;10:584–588 [DOI] [PubMed] [Google Scholar]
- 18. Maran A, Pavan P, Bonsembiante B, et al. : Continuous glucose monitoring reveals delayed nocturnal hypoglycemia after intermittent high-intensity exercise in nontrained patients with type 1 diabetes. Diabetes Technol Ther 2010;12:763–768 [DOI] [PubMed] [Google Scholar]
- 19. Tsalikian E, Mauras N, Beck RW, et al. : Impact of exercise on overnight glycemic control in children with type 1 diabetes mellitus. J Pediatr 2005;147:528–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aronson R, Brown RE, Li A, Riddell MC: Optimal insulin correction factor in post-high-intensity exercise hyperglycemia in adults with type 1 diabetes: the FIT study. Diabetes Care 2019;42:10–16 [DOI] [PubMed] [Google Scholar]
- 21. Harmer AR, Chisholm DJ, McKenna MJ, et al. : High-intensity training improves plasma glucose and acid-base regulation during intermittent maximal exercise in type 1 diabetes. Diabetes Care 2007;30:1269–1271 [DOI] [PubMed] [Google Scholar]
- 22. Turner D, Gray BJ, Luzio S, et al. : Similar magnitude of post-exercise hyperglycemia despite manipulating resistance exercise intensity in type 1 diabetes individuals. Scand J Med Sci Sports 2016;26:404–412 [DOI] [PubMed] [Google Scholar]
- 23. Scott SN, Cocks M, Andrews RC, et al. : Fasted high-intensity interval and moderate-intensity exercise do not lead to detrimental 24-hour blood glucose profiles. J Clin Endocrinol Metab 2019;104:111–117 [DOI] [PubMed] [Google Scholar]
- 24. Scott SN, Shepherd SO, Andrews RC, et al. : A multidisciplinary evaluation of a virtually supervised home-based high-intensity interval training intervention in people with type 1 diabetes. Diabetes Care 2019;42:2330–2333 [DOI] [PubMed] [Google Scholar]
- 25. Reddy R, Wittenberg A, Castle JR, et al. : Effect of aerobic and resistance exercise on glycemic control in adults with type 1 diabetes. Can J Diabetes 2019;43:406–414.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee AS, Way KL, Johnson NA, Twigg SM: High-intensity interval exercise and hypoglycaemia minimisation in adults with type 1 diabetes: a randomised cross-over trial. J Diabetes Complications 2020;34:107514. [DOI] [PubMed] [Google Scholar]
- 27. Lee MH, Vogrin S, Paldus B, et al. : Glucose and counterregulatory responses to exercise in adults with type 1 diabetes and impaired awareness of hypoglycemia using closed-loop insulin delivery: a randomized crossover study. Diabetes Care 2020;43:480–483 [DOI] [PubMed] [Google Scholar]
- 28. Breton MD, Chernavvsky DR, Forlenza GP, et al. : Closed-loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the Artificial Pancreas Ski Study. Diabetes Care 2017;40:1644–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.