Abstract
Background: Gestational diabetes mellitus (GDM) is defined as glucose intolerance first identified during pregnancy. Delays in diagnosis and challenges in management can lead to serious adverse outcomes for the mother and child. As rates of GDM diagnosis increase worldwide, health systems and maternity services have become increasingly strained, especially with new restrictions around in-person care due to the current COVID-19 pandemic. Mobile health (mHealth) has increasingly shown promise for management of chronic disease, driven by smartphone adoption and increased internet connectivity. The aim of this work was to evaluate the adoption and multidisciplinary care coordination of an mHealth platform called M♡THer in a cohort of women with first-time diagnosis of GDM.
Methods: The mHealth platform for GDM management was developed incorporating a smartphone application, clinician portal, and secure cloud data storage. Forty participants with a first-time diagnosis of GDM were recruited to use the app during their pregnancy. User attitudes from clinicians and women were captured through post-hoc surveys, and app-usage metrics.
Results: Clinicians and women indicated satisfaction and ease of use of the mHealth platform, with some technological challenges around wireless connectivity. Blood glucose reviews and antenatal contact were higher with use of the M♡THer app compared with a matched historical sample.
Conclusion: The M♡THer mHealth platform is a new comprehensive tool for health care of women with GDM, and may provide an effective new avenue to enhance multidisciplinary care in the face of COVID-19 disruptions and challenges to traditional care pathways.
Keywords: mHealth, Smartphone app, Gestational diabetes, Remote monitoring, Blood glucose monitoring
Background
Gestational diabetes mellitus (GDM) is defined as glucose intolerance first identified in pregnancy and can have serious adverse effects on pregnancy outcomes and negative implications for the long-term well-being of the mother and child.1 An increasing number of women during pregnancy are being diagnosed with GDM, reflecting increases in maternal age, higher prevalence of obesity, and new diagnostic criteria widely adopted in Australia (2014) and internationally.2 In Australia, GDM now affects ∼15% of pregnancies,3 but can occur in up to 30% of pregnancies in high-risk populations.4
Increasing incidence of GDM has strained existing support systems and maternity services, with an associated rise in related health care expenditure. Growing costs are one of several systemic barriers to effective health care of women with GDM, as are challenges in communication between multidisciplinary care teams, and limited resources in rural and remote communities.5 Even in adequately resourced environments, challenges in management remain. Daily blood glucose levels (BGLs) are routinely recorded by using paper-based diaries and can be influenced by poor recall, inattention to detail, and delays in transmission of results to the treating clinician and members of the health care team.6 Suboptimal blood glucose control can increase the risk to the mother and unborn child with potentially serious medical consequences.7 This situation may arise in cases with delayed diagnosis where extended periods of high or low BGLs may occur before the first face:face review and intervention by health care staff.
Mobile health (mHealth) technologies have been increasingly adapted for management of chronic disease, driven by smartphone adoption and growing access to the internet. Smartphones are an attractive technology platform for health services deployment, as they can functionally integrate different monitoring and communication technologies for a comprehensive approach to health management.8 Owing to their near ubiquity, smartphone-based mHealth platforms are increasingly used by health care providers to deliver health advice and interventions at a personal level to patients.9 Recent studies have demonstrated the effective use of mHealth in self-management and medication adherence for patients with diabetes, supporting their potential to expand clinical influence beyond the traditional settings and into the home.10
At the Redland Hospital GDM clinic (Metro South Health [MSH], Redland City, Queensland, Australia), a survey of patient preferences was completed to determine preference for use of mobile technology as an adjunct or alternative to traditional face-to-face clinical care in women with GDM.11 Responses indicated that women with GDM would benefit from being offered a choice of individualized options, including mobile- and personally delivered clinical care. It was therefore anticipated that an mHealth platform would support not only the women who were pregnant, but also clinical care providers during management of GDM from diagnosis until birth. In response, an innovative mHealth platform was developed through a collaboration between MSH and the Commonwealth Scientific and Industrial Research Organisation (CSIRO), and was trialed in a cohort of women with a first-time diagnosis of GDM. The aim of the current study was to evaluate adoption (use and user satisfaction), multidisciplinary care coordination, and health care utilization through use of the mHealth platform, called M♡THer. We hypothesized that the smartphone- and internet-based interactive support system would be readily adopted and convenient for all users in a small observational study at Redland Hospital.
Methods
Study design
This feasibility study involved the research and development of an mHealth platform for GDM management, preliminary implementation at Redland Hospital, and feedback from clinicians and end-users.
Participants
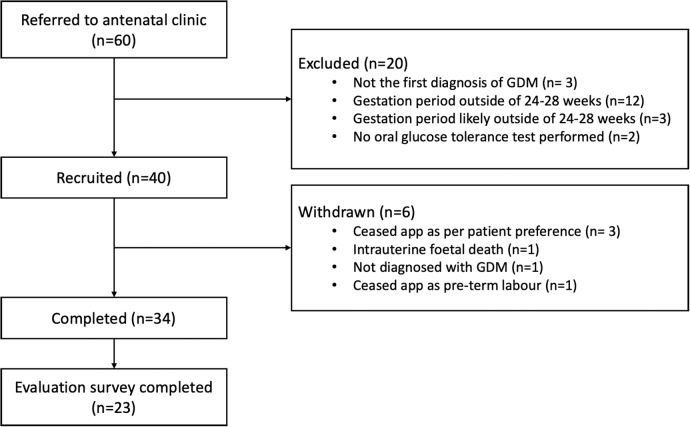
Recruitment of participants between August 2017 and April 2018 occurred at Redland Hospital with a goal of recruiting n = 40 women with a first-time diagnosis of GDM. Inclusion criteria included a confirmed oral glucose tolerance test diagnosis of GDM between 24 and 28 weeks of gestation, at least 16 years of age, owning and the ability to use a smart mobile phone, and the ability to speak and understand English. Exclusion criteria included women who had any other type of diabetes, severe comorbidities that would limit participation, or a known history of major psychiatric illness. Study recruitment is illustrated in Figure 1. Women with GDM who did not wish to be involved in the research were offered the usual antenatal care and excluded from data analysis. Ethical considerations specific to women who are pregnant were followed during study conception and design as outlined by the National Statement on Ethical Conduct in Human Research 2015.12 All study procedures were approved by the CSIRO Medical Research Ethics Committee (#2020_047_RR), and Metro South Hospital and Health Service (HREC/16/QPAH/785) before enrollment commenced.
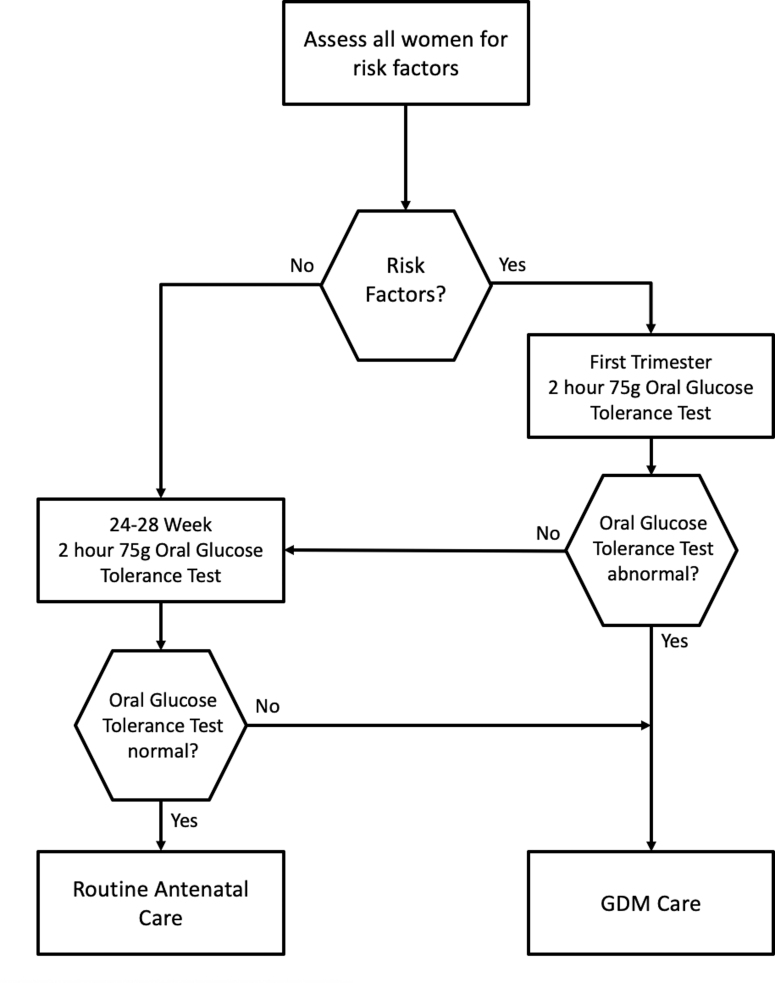
FIG. 1.
Schematic illustration of a GDM diagnosis (Queensland Clinical Guideline: Gestational Diabetes Mellitus13) and study recruitment.
M♡THer platform development (intervention)
The M♡THer platform followed an evolutionary development process involving clinicians, patient advocates, health services information technology representatives, and researchers at all stages of development. The platform was architected with consultation on ideal user experiences for the app and a requested feature set for the clinician portal. Regular working group meetings incorporated feedback and revisions throughout the process.
After initial development of the mHealth platform, clinicians and research staff led a 2-week trial implementation, testing mock user creation and registration, app installation, and data input and review through the clinician portal. At the conclusion of the trial, contributors identified areas for immediate and future improvement, with functional bug fixes and feasible feature upgrades proceeding before enrollment of study participants. The M♡THer platform integrates smartphone (iOS/Android) delivery of multimedia content, app-based data entry of BGL and other health data, and a clinician web portal to support women managing GDM from diagnosis to childbirth (Fig. 2).
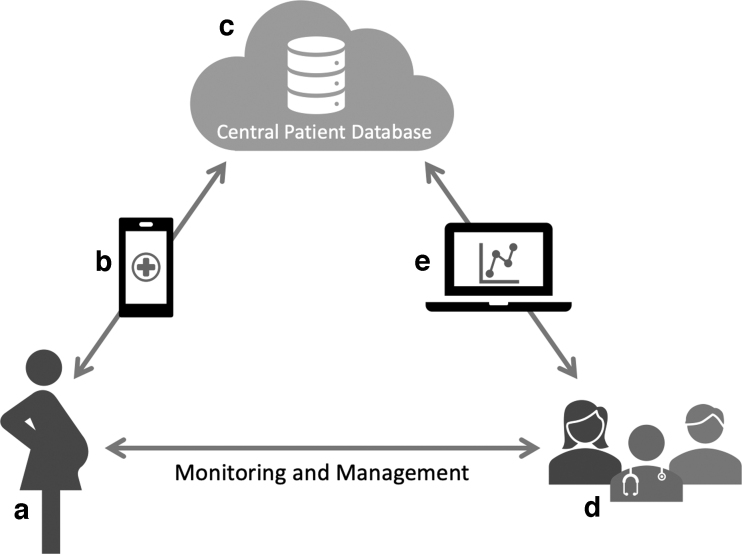
FIG. 2.
Schematic illustration of a GDM support platform using M♡THer. A woman with GDM (a) installs the M♡THer app on her smartphone (b). The M♡THer app is used for data input, such as BGL, weight, exercise, and symptoms, and delivery of motivational prompts and educational media. Patient data are uploaded to a secure centralized patient database (c), and can be accessed by the GDM care team (d) via a clinician web portal (e). Data can be reviewed via the web portal by health care practitioners during clinical appointments, aiding in discussions and management decisions.
Sampling procedures
Following recruitment, all participants were provided with a Bluetooth-enabled blood glucose meter (Roche Accu-Chek guide Meter, Rotkreuz, Switzerland). All participants received usual antenatal care, as well as access to the M♡THer app. The M♡THer app was installed on the participants' personal smartphone, and they were instructed in its use. The smartphone was carried by the women throughout the day to allow for health data logging (e.g., BGL, weight, exercise, diet, symptoms) and to provide access to adjunctive multimedia educational materials to assist them with their GDM management. Steps were automatically recorded through the built-in accelerometer, but other forms of exercise could be entered manually with indication of duration and intensity on a modified Borg scale. Health measures recorded through the app were individualized to each woman's needs through the clinician web portal. Data entered into the app were automatically updated to the clinician web portal for viewing by the health care practitioner at intervals determined by the Redland GDM service. Data could also be reviewed by health care practitioners during clinic appointments, aiding in discussions with women.
Historic data for comparison were obtained through retrospective analysis of available medical records for women with GDM. This review was performed by matched comparison of the recruited participants (matching criteria: age, prepregnancy weight, total number of participants). Data collected during the study and for historical comparison are outlined in Table 1.
Table 1.
Data Collected During Study and Through Retrospective Analysis of Historical Treatment Data
| Measure | Time of collection | Collected by | Collected from | Collected at |
|---|---|---|---|---|
| App usage | From enrollment in study to delivery | CSIRO engineer | Data server logs | CSIRO |
| User perceptions (women with GDM and health practitioners) | Postdelivery | GDM services | User satisfaction questionnaires | GDM services at Redland Hospital |
| BGL reviews | Weekly | GDM services | GDM service's logs | GDM services at Redland Hospital |
| Frequency of antenatal contact | At contact | Healthcare practitioners in GDM service | GDM service's logs | GDM services at Redland Hospital |
| Time to treatment (review with relevant health care professionals and commencement of medication if required) | From enrollment to delivery | GDM services | GDM service's logs | GDM services at Redland Hospital |
| Antenatal clinic visits | From enrollment to delivery | GDM services | GDM service's logs | GDM services at Redland Hospital |
BGL, blood glucose level; CSIRO, Commonwealth Scientific and Industrial Research Organisation; GDM, gestational diabetes mellitus.
Measures
The primary endpoint of this study was user adoption through (1) usage as measured by number and frequency of BGL readings uploaded through the smartphone app and (2) user satisfaction measured using a mixed-methods approach incorporating the Likert scale and open-ended survey questions.
A secondary endpoint of the study was the comparison of measures of care using the M♡THer app to matched historical data. Comparison measures included (1) number of BGL clinical reviews by individual clinicians or antenatal care team, (2) frequency of antenatal contact, (3) occasions of service, related to GDM, attended by women, and (4) time from attendance at GDM group education to commencement of metformin and/or insulin treatment. The purpose of this comparison was to identify areas in which M♡THer app usage changed the participant's behavior or frequency of clinical contact, to refine the design for future implementation and translation.
Analyses were performed on data server logs, service delivery-related data, and deidentified patient data. App use was quantified by frequency and quantity of data uploads, specifically BGL recordings, from participant smartphones. User perceptions were assessed by evaluating responses to survey questionnaires and reported in descriptive form.
Results
A total of 40 women were recruited between August 2017 and April 2018 and 34 used the M♡THer app throughout the entirety of their pregnancy (Fig. 3). All women who met the inclusion criteria and were approached for participation accepted use of the mobile app for management of GDM. Twenty-three women completed the poststudy evaluation (65% response rate). Participants who completed the study and post-hoc user satisfaction survey were 23 to 42 years of age (n = 9: 23–27 years old, n = 6: 28–32 years old, n = 5: 33–37 years old, and n = 3: 38–42 years old). The average gestation at time of enrollment was 27 weeks (median: 27 weeks, range: 24–29 weeks). The average number of BGL readings uploaded to the portal was 229 (median: 230, range: 39–390) over an average of 65 days (median: 69 days, range: 19–98 days). Participants uploaded on average, three BGL readings per day.
FIG. 3.
M♡THer flow diagram of study participants.
User satisfaction surveys were completed by 23 women; 5 completed telephonically, 5 completed using paper surveys, and 13 using an electronic survey. The majority of survey respondents either strongly agreed or agreed that the M♡THer GDM app was helpful in recording their BGLs, facilitated better support from the health care team, and was overall helpful in managing their gestational diabetes (Table 2).
Table 2.
User Responses to Survey Questions
| Strongly agree | Agree | Neither agree nor disagree | Disagree | Strongly disagree | |
|---|---|---|---|---|---|
| The M♡THer GDM app was helpful in recording my BGLs | 11 | 12 | 0 | 0 | 0 |
| I feel confident that my health care team checked my BGLs | 11 | 9 | 3 | 0 | 0 |
| The M♡THer GDM app helped me to feel confident in managing my gestational diabetes | 9 | 12 | 2 | 0 | 0 |
| I felt supported by the health care team that was monitoring the M♡THer portal | 10 | 10 | 3 | 0 | 0 |
| Overall, I was satisfied with the M♡THer GDM app | 8 | 13 | 1 | 1 | 0 |
The user satisfaction survey sent to women who used the mobile application included two open-ended questions on what they liked most about the M♡THer app and what they would change about the app. They were also able to provide any additional comments about their experience during their participation in the study. Feedback indicated that many participants appreciated the easy access (not having to carry around a paper diary), ease of use, and the convenience of the app. They liked being able to monitor their own BGLs, the ability of the app to quickly connect them to, and have responses from their doctor. Negative responses primarily focused on technological problems experienced within the app or connecting the app through Bluetooth to the external blood glucose monitor. In addition, two participants indicated that despite using the M♡THer app to record their daily BGLs, the clinical care team still preferred to use paper-based records during in-person consultations.
When surveyed regarding their experience using the app for management of women with GDM, all clinicians either strongly agreed or agreed that the M♡THer app improved their efficiency in caring for their patients, as reported previously by Stoney et al.14 Diabetes educators indicated that increased burden at initiation was modest as the education of the app was incorporated into the general group education session and did not significantly increase time required. Women were instructed to download the app before the appointment and generally came with an idea of how it worked. Burden after that was reduced compared with the laborious phone and paper record system that was implemented prior.
The primary focus of this feasibility study was adoption of both women with GDM and their treating clinicians. Secondary investigations of interest were the number of BGL reviews, utilization of antenatal services, and time to commencement of treatment when using the M♡THer app compared with matched historical data (n = 21). In this study, patient demographics (prepregnancy body mass index and time of diagnosis) were similar to past treatment data, while BGL clinical reviews and frequency of antenatal contact were higher with the introduction of M♡THer. Time to treatment and antenatal clinical visits were comparable whether or not M♡THer was used (Table 3).
Table 3.
Comparison Between Historical and M♡THer Participant Data
| Historical data (n = 21) |
M♡THer participants (n = 34) |
|||
|---|---|---|---|---|
| Mean | Median | Mean | Median | |
| Prepregnancy body mass index (kg/m2) | 25.4 | 25 | 24.8 | 24.6 |
| Weeks pregnant when diagnosed | 26.9 | 27 | 27 | 27 |
| Number of BGL clinical reviews | 12.6 | 11 | 27.3 | 25.5 |
| Frequency of antenatal contact | 9.7 | 9 | 15.5 | 14 |
| Time to treatment (weeks) | 2.6 | 2 | 2.8 | 3 |
| Antenatal clinic visits | 9.6 | 9 | 11 | 11 |
Discussion
The M♡THer platform is the first intervention in Australia to implement mobile technology to augment face-to-face clinical care for women with a first-time diagnosis of GDM. High treatment uptake and participant-reported satisfaction in a cohort of expectant mothers with GDM and their treating clinicians indicate that M♡THer is feasible and acceptable for use alongside traditional approaches. The 100% participation rate by women who were offered use of the app indicates the potential broad appeal of mobile technology for management of GDM, although trialled in a limited sample. Higher incidence of BGL reviews and greater frequency of antenatal contact with treating clinicians indicate the potential for greater treatment efficiency and earlier intervention through use of M♡THer compared with current management practice.
At the outset of this study, it was assumed that the frequency of low-utility clinic visits would reduce with the introduction of the M♡THer platform, however, an increase in these visits was observed compared with historical data. A possible explanation for this increase could be attendance to the clinic for intervention due to elevated BGLs identified through more frequent monitoring. A larger implementation of the M♡THer platform with more inclusive participant criteria would facilitate further investigations into integration issues, service utilization, and cost implications of offering M♡THer as a complement to standard clinical practice. Diabetes educators indicated that increased frequency of clinic visits did not significantly increase their clinical burden, a view that was shared by other members of the clinical care team. A significantly reduced burden was indicated by endocrinologists, although indirectly to the app. The app was introduced as part of a wider change process with streamlining of noncomplex GDM management resulting in reduced referrals to endocrinology clinics for patient review. The app provided the confidence that BGLs were being monitored and this was incorporated into the weekly diabetes educator/endocrinologist BGL review with no added burden. Advice on treatment or requests to refer to endocrinology could be more easily facilitated with greater BGL transparency. This process did happen before introduction of the app, but in a very labor-intensive way on the part of the diabetes educators.
Prior research suggests that one of the greatest challenges in the management of GDM is proper coordination between the multidisciplinary health care practitioners. mHealth technologies have shown potential but to date have had limited success in reducing data siloing and promoting coordination between members of the health care team. Our results in this study suggest that involving clinicians both in the initial design and ongoing development led to high levels of satisfaction and uptake among the health care team. Moreover, iterative consultation sessions with pregnant women with GDM during development allowed for issues in design and usability to be addressed early. Ease of use is one of the most significant predictors of intention-to-use smartphone health technologies and these revisions maximized acceptability of the M♡THer app with only a small number of participants (n = 3) choosing to discontinue use during the study.
Beyond patient adoption, challenges exist in translation of any mHealth platform to usual business. Funding models traditionally do not account for use of telehealth interventions, with lack of codes and reimbursement mechanisms acting as barriers to uptake in both public and private health settings.10 Clinical champions and supportive administrative processes can overcome some of these obstacles, as was demonstrated in this study. However, even with support mechanisms in place, interoperability with existing hospital practices and information technology systems (including data privacy regulations) must be prioritized from the very earliest stage of development. This need for planning is especially salient in the unequal speed at which technology advances, relative to slower moving regulatory reform.15–17
Although diabetes management has been the focus of many recent advancements in mHealth technology, few cover GDM, and many are developed with limited patient or clinical input and do not adhere to recognized medical guidelines and evidence-based practices.18 Importantly, no contemporary solutions for GDM management provide the ability for individualized patient customization by the clinician, but rather a static and standardized user experience.19–22 As health care pivots increasingly toward precision medicine, M♡THer is designed to easily implement the individual monitoring metrics and management strategies set forth by the treating clinician in collaboration with the women with GDM. This flexibility allows for easy integration into clinical practice through increased efficiency of care without increased demand on the patient or health care provider.
Limitations
This study had some limitations, including a relatively small sample size recruited from a single hospital, limiting the generalizability of the findings. In addition, recruitment and use of the M♡THer app and clinician portal were driven by a group of engaged clinical champions, somewhat mitigating integration and interoperability challenges inherent to the broader health care system. In this study, we evaluated the usage of the M♡THer app for BGL and health tracking, but were unable to measure the uptake and access frequency of the adjunctive educational materials as they were hosted by third-party health services and organizations. The use and utility of educational content will be a primary focus in the next phase of research, an implementation study. Another limitation of this study was the retrospective comparison with a historical sample of women with GDM, rather than a concurrent cohort. This was done to limit the additional burden on clinicians during the study recruitment period. Historical care data were retrieved from the same hospital and clinical care team from a time period immediately before the study commencement, and so, care pattern personnel were substantially similar between comparison cohorts. In addition, tight criteria for study inclusion limited the pool of eligible women and the overall diversity of participants, and so, broad acceptability in different health care settings and populations will be a primary objective of the implementation study.
Strengths/future directions
The M♡THer platform is the first evidence-based, individually customizable mHealth intervention for management of GDM in Australia. The key strength of this platform is the ability of the clinician to carefully adapt the data collection and reporting metrics for each woman with GDM, allowing focused management of GDM while minimizing additional assessment burdens on expectant mothers. The value of this bespoke approach was validated in this study by improvement in multidisciplinary care coordination between health care practitioners and subsequent earlier intervention for BGL excursions in the study cohort.
The COVID-19 pandemic has significantly disrupted the traditional pathways for care and management of both acute and chronic health conditions. mHealth platforms have been increasingly utilized to virtualize care due to restrictions to in-person consultations, with both successes and challenges.23 Proactive implementation of telehealth models of care requires increased coordination between researchers and clinicians, but ultimately is more likely to generate long-term benefits that are robust to disruptions such as a global pandemic.24 The rationale for this approach has been demonstrated with ongoing demand and uptake of the M♡THer app in hospitals in Queensland, and broad interest across Australia. The ultimate vision for the M♡THer platform is to facilitate greater efficiency of care for both expectant mothers and health care providers. The platform will provide women with the support they need from their own mobile device, thereby expanding clinical care beyond hospital walls to wherever they are without unduly increasing the burden on the clinical care team. By reducing their need to attend the clinic, the mobile app provides expectant mothers with the ability to have tailored support, while simultaneously providing real-time actionable health data to the clinical care team. This has the potential to facilitate greater adherence to BGL and lifestyle monitoring and to facilitate earlier intervention where required. Ultimately, the goal of this mobile platform is to increase the quality of care for all women with GDM, regardless of circumstance, location, or other barriers to access.
From the outcomes of this feasibility study, several design and implementation elements have been identified to improve the M♡THer app and experience for women with GDM. Broader recruitment criteria and early availability will allow immediate commencement with the M♡THer app after diagnosis for all women with GDM and improve the continuity of care and management throughout pregnancy. To increase the reach and access, the M♡THer app is currently available in several different selected languages. A multisite implementation study that includes all three MSH antenatal clinics (Redland Hospital, Logan Hospital, and Beaudesert Hospital), as well as the Mater Mothers' Hospital in Brisbane, has commenced in June 2020. This 12-month study (HREC/2019/QMS/56607 [MSH HREC] and ANZCTR submission: 379026) aims to recruit >1000 women with GDM and in addition to the endpoints similar to the feasibility study, clinical data for future risk modeling and provider costs savings that might be associated with changes in outpatient clinic attendance will be evaluated.
Future improvements to the app and interface will provide the ability to record dietary intake with greater precision, and allow for quantification of carbohydrate intake. It will also better monitor medications prescribed for women with GDM. One significant limitation yet to be overcome is the discontinuity in care for mothers with GDM after giving birth. Future efforts therefore should investigate the feasibility in expanding mobile app management and resources to new mothers and their children who are at higher risk of secondary complications. Building from recent advances in user retention of mHealth platforms, gamification and community-building elements may be incorporated to increase support and patient engagement.25 Finally, efforts are underway to integrate M♡THer into rural maternal health clinics to increase the quality of care in very remote areas, particularly in underserviced communities.26,27
Conclusion
Increasingly, the focus of chronic disease management is shifting to in-home and community settings, with mHealth delivery rapidly becoming the solution of choice. This trend is manifest in diabetes management through the proliferation of mHealth solutions, although the quality and evidence basis for offerings remain highly variable. Despite deficits in current management practices and the obvious benefit of introducing methods for precision health care, mHealth solutions for GDM have lagged behind. The M♡THer platform was developed to address this shortfall through close collaboration between engineers, clinicians, and research specialists, the first solution of its kind in Australia. This study has demonstrated that such a solution is acceptable to women with GDM and can be feasibly integrated into existing clinical practice and health care settings. The need for such a solution has been emphasized by increased demand for effective and safe telehealth technologies during disruptions to traditional face:face care models due to the COVID-19 pandemic. The M♡THer system has the potential to significantly benefit pregnant women by enabling faster interventions while simultaneously reducing unnecessary visits to GDM antenatal clinics and lends to the ability of clinicians to offer “virtual” telehealth clinics to women in their homes or workplaces. Future steps include implementation studies across multiple hospitals with a larger and more diverse population and expanding access to M♡THer for women in regional and rural areas.
Acknowledgments
Ms. Barbara Kleine—Senior Clinical Dietitian, Redland Hospital, Bayside Health Service, Metro South Health, Brisbane, Australia; Mr. Denis Morton—Chronic Disease Team Leader, Chronic Disease Service, Redland Hospital, Bayside Health Service, Metro South Health, Brisbane, Australia; Ms. Melissa Johnsson—Clinical Midwife, Women and Birthing Services, Redland Hospital, Bayside Health Service, Metro South Health, Brisbane, Australia; and Janet Knowles—Nurse Unit Manager, Women and Birthing Services, Redland Hospital, Bayside Health Service, Metro South Health, Brisbane, Australia.
Disclaimer
This article has been submitted solely to this journal and is not published, in press, or submitted elsewhere.
Authorship Confirmation Statement
All coauthors have reviewed and approved of this article. Author contributions are as follows: M.V.—research protocol development, app and portal development coordination, data analysis and interpretation, article preparation; C.R.—data analysis and interpretation, article preparation; R.M.S.—content design of the app, research protocol input, development of survey materials, ethics submission involvement, data interpretation and assistance with article preparation; L.H.—project management (planning, implementation, and evaluation), coordination of internal and external partners, and ethics coordination and submission; N.S.—end-user manual development, staff training, coordination of pilot implementation, dietetic clinical care, data collection and interpretation; R.W.—app content design, diabetes education clinical care, research protocol input, GDM app user instruction, diabetes education clinical care and portal registration and discharge; J.I.—content design of the app, research protocol input, endocrinologist clinical care input, data analysis and interpretation; J.R.—primary midwife patient contact for recruitment, portal registration and midwife clinical care during project implementation; and W.D.—conception and design of the app, research protocol input, obstetric clinical care, oversight of project implementation as the primary investigator, and data interpretation.
Author Disclosure Statement
No competing financial interests exist.
Funding Statement
This research was funded by the Metro South Hospital and Health Service Executive Planning and Innovation Committee and The Australian eHealth Research Centre.
References
- 1. McIntyre HD, Catalano P, Zhang C, et al. : Gestational diabetes mellitus. Nat Rev Dis Primers 2019;5:47. [DOI] [PubMed] [Google Scholar]
- 2. Nankervis A, McIntyre H, Moses R, et al. : ADIPS consensus guidelines for the testing and diagnosis of hyperglycaemia in pregnancy in Australia and New Zealand. Aust Diabet Pregnancy Soc 2014:1–8 [Google Scholar]
- 3. Australian Institute of Health and Welfare: Incidence of gestational diabetes in Australia. 2019. https://www.aihw.gov.au/reports/diabetes/incidence-of-gestational-diabetes-in-australia (accessed June24, 2020)
- 4. Fujimoto W, Samoa R, Wotring A: Gestational diabetes in high-risk populations. Clin Diabetes 2013;31:90–94 [Google Scholar]
- 5. Colagiuri S, Falavigna M, Agarwal MM, et al. : Strategies for implementing the WHO diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. Diabetes Res Clin Pract 2014;103:364–372 [DOI] [PubMed] [Google Scholar]
- 6. Buchanan TA, Xiang AH, Page KA: Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol 2012;8:639–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cosson E, Baz B, Gary F, et al. : Poor reliability and poor adherence to self-monitoring of blood glucose are common in women with gestational diabetes mellitus and may be associated with poor pregnancy outcomes. Diabetes care 2017;40:1181–1186 [DOI] [PubMed] [Google Scholar]
- 8. Klasnja P, Pratt W: Healthcare in the pocket: mapping the space of mobile-phone health interventions. J Biomed Inform 2012;45:184–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gagnon MP, Ngangue P, Payne-Gagnon J, Desmartis M. m-Health adoption by healthcare professionals: a systematic review. Journal of the American Medical Informatics Association. 2016 Jan 1;23(1):212–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garabedian LF, Ross-Degnan D, Wharam JF: Mobile phone and smartphone technologies for diabetes care and self-management. Curr Diab Rep 2015;15:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stoney R, Hardie A, Kleine B: Is there a role for mobile technology in the management of gestational diabetes mellitus? Nutr Diet 2017;74:9–49 [Google Scholar]
- 12. National Health and Medical Research Council: National statement on ethical conduct in human research 2007 (updated May 2015). 2015. https://www.nhmrc.gov.au/about-us/publications/national-statement-ethical-conduct-human-research (accessed June26, 2020)
- 13. Health QDo: Gestational diabetes mellitus. In: Guidelines MaNC, ed. Online, 2020. https://www.health.qld.gov.au/qcg/documents/g-gdm.pdf (accessed June26, 2020)
- 14. Stoney R, Dutton W, Varnfield M, et al. : MoTHer Digital Solution: a smartphone app and web-based portal for enhanced service delivery and care of women with gestational diabetes mellitus. World Hosp Health Serv 2019;55:28–32 [Google Scholar]
- 15. Shan R, Sarkar S, Martin SS: Digital health technology and mobile devices for the management of diabetes mellitus: state of the art. Diabetologia 2019;62:877–887 [DOI] [PubMed] [Google Scholar]
- 16. Schoenfeld AJ, Sehgal NJ, Auerbach A: The challenges of mobile health regulation. JAMA Intern Med 2016;176:704–705 [DOI] [PubMed] [Google Scholar]
- 17. Sim I: Mobile devices and health. N Engl J Med 2019;381:956–968 [DOI] [PubMed] [Google Scholar]
- 18. Fatehi F, Menon A, Bird D: Diabetes care in the digital era: a synoptic overview. Curr Diab Rep 2018;18:38. [DOI] [PubMed] [Google Scholar]
- 19. Hirst JE, Mackillop L, Loerup L, et al. : Acceptability and user satisfaction of a smartphone-based, interactive blood glucose management system in women with gestational diabetes mellitus. J Diabetes Sci Technol 2015;9:111–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mackillop L, Hirst JE, Bartlett KJ, et al. : Comparing the efficacy of a mobile phone-based blood glucose management system with standard clinic care in women with gestational diabetes: randomized controlled trial. JMIR Mhealth Uhealth 2018;6:e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Skar J: Women's experiences of using a smartphone-application, the Pregnant+ app, to manage Gestational Diabetes Mellitus-a qualitative study, Høgskolen i Oslo og Akershus. Institutt for sykepleie og helsefremmende arbeid 2017. https://oda-hioa.archive.knowledgearc.net/handle/10642/5222 (accessed June26, 2020)
- 22. Skar JB, Garnweidner-Holme LM, Lukasse M, Terragni L: Women's experiences with using a smartphone app (the Pregnant+ app) to manage gestational diabetes mellitus in a randomised controlled trial. Midwifery 2018;58:102–108 [DOI] [PubMed] [Google Scholar]
- 23. Mehrotra A, Ray K, Brockmeyer DM, et al. : Rapidly converting to “virtual practices”: outpatient care in the era of Covid-19. N Engl J Med 2020;1 [Google Scholar]
- 24. Smith AC, Thomas E, Snoswell CL, et al. : Telehealth for global emergencies: Implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare 2020;26:309–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saric K, Redd C, Varnfield M, O'Dwyer J, Karunanithi M. Increasing Health Care Adherence Through Gamification, Video Feedback, and Real-World Rewards. In 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 2018 Jul 18 (pp. 1584–1587). IEEE; [DOI] [PubMed] [Google Scholar]
- 26. Ishak M, Petocz P, Ishak M, Petocz P: Gestational diabetes among Aboriginal Australians: prevalence, time trend, and comparisons with non-Aboriginal Australians. Ethn Dis 2003;13:55–60 [PubMed] [Google Scholar]
- 27. Gardner K, Sibthorpe B, Chan M, et al. : Implementation of continuous quality improvement in Aboriginal and Torres Strait Islander primary health care in Australia: a scoping systematic review. BMC Health Serv Res 2018;18:541. [DOI] [PMC free article] [PubMed] [Google Scholar]