Abstract
Purpose of Review:
Myelodysplastic syndrome (MDS) is a heterogeneous hematological disorder characterized by a spectrum of clinical presentation, cytogenetic, and somatic gene mutations and the risk of transformation to acute leukemia. Management options include observation, supportive care, blood transfusion, administration of growth factors and/or hypomethylating agents and hematopoietic cell transplant (HCT) either upfront or after disease progression.
Recent Findings:
Currently, HCT is the only curative therapy available for patients with MDS, with multiple factors such as donor availability, patient and disease characteristics being involved in making the decision to proceed with transplant.
Summary:
In this article, we summarize 1) overall prognosis and natural history of MDS, 2) currently available non-HCT therapy with a focus on hypomethylating agents (HMA), 3) outcomes after HCT in patients with MDS, 4) factors to be considered to proceed to HCT for treatment of MDS, and 5) more recent/ongoing studies relevant to HCT decision making processes.
Keywords: Myelodysplastic syndrome (MDS), International Prognostic Scoring System (IPSS), Revised International Prognostic Scoring System (IPSS-R), hypomethylating agents (HMA), allogeneic hematopoietic cell transplant (HCT)
INTRODUCTION
Myelodysplastic syndrome (MDS) is a heterogeneous group of hematologic disorders described as an acquired clonal hematopoietic cell disorder, clinically characterized by ineffective hematopoiesis leading to various degrees of cytopenia, dysplastic morphology in the bone marrow, and a tendency to transform into acute myelogenous leukemia (AML).(1) The median age at diagnosis is 70 years.(1) Clinical presentation of MDS can range from mild asymptomatic to severe symptomatic with transfusion-dependent cytopenia, recurrent infections, and rapid progression to AML. Despite better understanding of the molecular pathogenesis of the disease, advances in treatment options and supportive care in the past decades, availability of therapeutic agents that has led to prolongation of life,(2) allogeneic hematopoietic cell transplantation (HCT) remains as the only potentially curative therapy for patients with MDS. However, HCT is associated with a significant risk of transplant-related mortality (TRM) primarily due to infections, graft-versus-host disease (GVHD), regimen-related toxicities, and graft rejection. In addition, disease relapse can still occur in 15–30% of patients. Therefore, the decision-making process to proceed with HCT is complex and highly individualized, weighing the risks of “HCT-related complications” against risks of “low survival rate without HCT”. Heterogeneity in clinical presentations and the pace of disease progression makes this decision-making process even more challenging and complicated. Moreover, as the risks of “proceeding” versus “not proceeding HCT” can change overtime during the treatment course, a dynamic and adaptive discussions with patients and their families is required. In this article, we summarize 1) overall prognosis and natural history of MDS, 2) currently available non-HCT therapy with a focus on hypomethylating agents (HMA), 3) outcomes after HCT in patients with MDS, 4) factors to be considered to proceed to HCT for treatment of MDS, and 5) more recent/ongoing studies relevant to HCT decision making processes.
1). MDS Prognosis and natural history
Given the wide spectrum of disease severity and relatively slow, yet, progressive course of MDS, better understanding of the disease prognosis and clinical course is critically important to guide the therapy for patients. The International Prognostic Scoring System (IPSS) was originally developed by the International MDS Risk Analysis Workshop (IMRAW) based on the combination of cytogenetic, morphologic, and clinical data.(3) (Table 1) As described in Table 1b, median survival of 0.4, 1.1, 3.5, and 5.7 years are expected for high-, intermediate-2, intermediate-1, and low-risk patients, respectively. Later on in 2012, Revised International Prognostic Scoring System (IPSS-R)(4) was developed by the International Working Group for the Prognosis of MDS (IWG-PM) defining five risk groups for MDS, as presented in Table 2. Most of the clinical studies are currently using R-IPSS for the eligibility/analyses, however it should be noted that many studies in the past were dependent on the classic IPSS for describing HCT outcomes/HCT decision analyses. More recently, somatic mutations in several genes including TP53, EZH2, ETV6, RUNX1, and ASXL1 were found to hold independent prognostic value in MDS.(5, 6) Thus, a combined analysis of genetic mutations with IPSS-R/IPSS would likely further refine the risk stratification strategy for patients with MDS. In fact, a recent study by Nazha et al. incorporated molecular data into the IPSS-R using a cohort of patients treated at the Cleveland Clinic.(7)
Table 1a.
The risk score IPSS(3)
| Score value | |||||
|---|---|---|---|---|---|
| Prognostic variable | 0 | 0.5 | 1 | 1.5 | 2.0 |
| Bone marrow blast (%) | <5 | 5–10 | 11–20 | 21–30 | |
| Karyotype1 | Good | Intermediate | Poor | ||
| Cytopenia2 | 0/1 | 2/3 | |||
Definition of karyotype
Good Normal, Y-, 5q-, 20q-
Intermediate All other
Poor Chromosome 7 aberration and/or ≥ Chromosomal aberration
Cytopenia
Hemoglobin < 100 g/L (10 g/dL)
Neutrophil count < 1.8 G/L (1.800/μl)
Platelet count < 100 G/L (100.000/μl)
Table 1b.
The risk score IPSS(3)
| IPSS group | IPSS total score | Survival (Median; Years) | 25% AML evaluation (Years) | ||
|---|---|---|---|---|---|
| Age at dagnosis | Age at dagnosis | ||||
| ≤70 years | >70 years | ≤70 years | >70 years | ||
| Low | 0 | 9 | 3.9 | >9.4 (NR) | >5.8 (NR) |
| Intermediate 1 | 0.5–1.0 | 4.4 | 2.4 | 5.5 | 2.2 |
| Intermediate 2 | 1.5–2 | 1.3 | 1.2 | 1.0 | 1.4 |
| High | ≥2.5 | 0.4 | 0.4 | 0.2 | 0.4 |
Table 2a.
Cytogenetic scoring system. Data from patients in IWG-PM database(4)
| Prognostic subgroup | Cytogenetic abnormalities | Median survival, yrs | Median AML evolution, 25%, yrs |
|---|---|---|---|
| Very good | −Y, del(11q) | 5.4 | NR |
| Good | Normal, del(5q), del(12p), del(20q), double including del(5q) | 4.8 | 9.4 |
| Intermediate | del(7q), +8, +19, i(17q), any other single or double independent clones | 2.7 | 2.5 |
| Poor | −7, inv(3)/t(3q)/del(3q), double including −7/del(7q), complex: 3 abnormalities | 1.5 | 1.7 |
| Very poor | −7, inv(3)/t(3q)/del(3q), double including −7/del(7q), complex: 3 abnormalities | 0.7 |
Patient-related factors (i.e., age, performance status, and comorbidities) impact the overall prognosis for MDS patients, and should be considered in determining their treatment options. The MDS-CI was developed based on a large Italian cohort of MDS patients using five comorbidities (cardiac, moderate to severe hepatic, severe pulmonary, renal, solid tumor), which were found to be independently associated with the risk of non-leukemic death in multivariable analysis. A dynamic MDS-CI was also developed consisting of 3 risk groups (65% low, 29% intermediate, and 6% high) and predictive of survival and non-leukemic death independent of age, sex, WHO classification, cytogenetics, and transfusion dependence.(8) In an attempt to further define prognostic scores for MDS patients undergoing HCT, Shaffer at al. reported a HCT-specific prognostic scoring system derived from the CIBMTR data.(8)
These estimated risks based on the disease and patient factors would inform physicians and patients in establishing individualized goals of care. For example, for medically fit patients with high-risk MDS, the goal of care is generally to achieve long-term survival with the possibility of cure while for others who are considered to be too high-risk for HCT, the primary goals tend to be palliative to improve patients’ quality of life, and/or prolong life.
2. Currently Available Non-Transplant Therapies
Treatments for MDS begins with best supportive care with transfusions, prophylactic/therapeutic antibiotics, and growth factor support as appropriate.(9) Additionally, in patients with lower-risk MDS, lenalidomide is used as an immunomodulating agent.(10–12) Benefits of lenalidomide have been particularly evident for patients with the del(5q) chromosomal abnormality. High-intensity induction chemotherapy may have the potential to change the natural history of the disease. However, there is a greater risk of regimen-related morbidity and mortality with limited response rates/durability. Comparative studies have not shown a benefit in any of the intensive chemotherapy regimens used for MDS treatment.(13, 14) However, for patients with a high tumor burden who have a potential hematopoietic cell donor, achievement of even a partial remission may be sufficient to permit for the HCT.
Hypomethylating Agents:
Azacitidine and decitabine are HMAs that are currently approved by the Food and Drug Administration (FDA) for treatment of MDS. The rationale for hypomethylation therapy was based on the observation that aberrant DNA methylation is a dominant process in patients with MDS.(15–18) HMAs indirectly deplete methylcytosine, resulting in hypomethylation of promoters of target genes that are involved in disease initiation or progression, making them appropriate targets for pharmacologic therapy. Administration of HMAs have been leading to a varying degree of hematological response with steady improvement in cell counts in 40% of treated patients and delay in progression to AML in patients with higher-risk MDS.(19) The duration of response is, however, usually limited to less than two years with a median duration of response of 11–15 months.(20, 21) Furthermore, the prognosis after azacytidine failure in patients with high-risk MDS was dismal with the median OS of 5.6 months, and 2-year survival probability of only 15%.(22) Overall survival data from large phase II/III trials using azacitidine for treatment of MDS are summarized in Table 3.
Table 3.
Survival data from Phase II/III trials using azacitidine for treatment of high-risk MDS
| Design/Therapy | Total N | Median Age (range) | Overall Survival |
|---|---|---|---|
| Cohort: Outcome after AZA-failure(22) | 435 | 69 (not available) | 15% (2 year) |
| Cohort: Prognostic Factors in Compassionate Use AZA(81) | 282 | 71(20–89) | ~20% (3 year by survival curve) |
| Cohort: Compassionate Use AZA(82) | 282 | 71(20–89) | 17.5% (3 year) |
| Ph III: Low-Dose Decitabine vs. BSC(83) | 233 | 70 (60–90) | 19% (2 year) |
| Ph III: European AZA-001: AZA vs. BSC(2) | 358 | 69 (38–88) | 50.8% (2 year), ~30% (3 year by survival curve) |
| Ph III: Decitabine vs. BSC(19) | 170 | 70 (62–76) | Not available |
| Ph III: CALGB AZA vs. BSC(84) | 191 | 69 (31–92) | ~45% (2 year), ~25% at (3 year by survival curves) |
| Retrospective: HCT in 60–70 yo vs. No Donor + AZA(45) | 178 | 66 (60–70) | 23% (2 year) |
| Decision Analysis (<60yo)(43) | 184 | 49.8 (18–60) | <5% for high, ~20% for Int 2 (3 year by survival curve) |
Combination therapy of HMA and lenalidomie(14) or vorinostat(23) have also been explored and demonstrated promising results in early phase trials. However, a three-arm randomized phase II study by the Southwest Oncology Group (SWOG) evaluating azacytidine alone or in combination with lenalidomide or with vorinostat for higher risk myelodysplastic syndromes failed to show clinical advantage of the combination therapy over the control group (azacitidine alone).(24) More recently, HMA in combination with venetoclax showed favorable result in AML,(25) and this promising combination is now being explored in high-risk MDS. PRIMA-1Met (APR-246) is a methylated derivative of PRIMA-1, which induces apoptosis in human tumor cells through restoration of the transcriptional transactivation function of mutant TP53. APR-246, has demonstrated reactivation of mutant TP53 in clinical trials, currently tested in a phase III trial in combination with HMAs (NCT03745716). These novel therapeutic options might change the expected clinical course of MDS, which in turn, can impact the decision process for HCT. Effective non-HCT therapies can increase the number of transplant candidates due to a better disease control, and can also possibly delay the need for HCT in some cases.
3. Hematopoietic Cell Transplant in MDS
Allogeneic HCT offers patients a potential cure for MDS through intensive conditioning chemoradiotherapy and potent graft-versus-leukemia (GVL) effects. Conventional high dose myeloablative conditioning (MAC) regimens include myeloablative doses of chemotherapy and/or radiation. These regimens are often poorly tolerated by older patients or those with significant comorbidities and are generally offered to medically fit patients under the age of 50–55 years. The introduction of reduced intensity conditioning (RIC) regimens over the last decade has allowed expansion of the upper age limit for allogeneic HCT, providing the possibility of transplantation to patients above 70 years old. Over the last several years, multiple groups have used allogeneic HCT with RIC to treat MDS with 2–3-year overall survival rates, ranging from 27% to 70% depending on the cohort and regimen characteristics.(26),(27–31) Lim et al, analyzed a group of 1,333 patients reported to the European Group for Blood and Marrow Transplantation (EBMT), ages 50 to 74 years (median age: 62); of whom 62% received RIC.(31) The four-year survival rate was 31%, the non-relapse mortality (NRM) rate was 39% and the relapse rate was 36%. No significant impact of age or transplantation regimen on outcomes was noted. Advanced disease stage at transplantation was the major independent predictor of poor outcomes. In another study, McClune et al, investigated the effect of age on outcome of RIC HCT for older patients (median age: 67 years); with MDS using the data from the Center for International Blood and Marrow Transplant Research (CIBMTR),(32) and reported a two-year survival rate of 45%, the NRM rate of 35% and the relapse rate of 29% in this population. As in the EBMT study, the CIBMTR data showed no significant impact of age on HCT outcomes. These two recent registry-based studies of older patients transplanted for MDS reflect results in the community-at-large and support the safety of allogeneic HCT for older patients with MDS.
Another large registry study from the CIBMTR evaluated outcomes of 701 adult MDS patients who underwent allogeneic HCT between 2002 and 2006.(33) This study focused on the impact of donor source on the outcomes of allogeneic HCT, comparing matched-related donor (MRD), 8/8 HLA allele matched unrelated donor (MUD), and 7/8 MUD. The median age was 53 years (range: 22–78) and 40% of patients received RIC regimens. The adjusted 3-year overall survival estimates were 47% for MRD, 38% for 8/8 MUD, and 31% for 7/8 MUD HCT recipients. In multivariate analysis, 8/8 MUD HCT recipients had similar DFS and survival rates compared to MRD HCT recipients, while 7/8 MUD HCT recipients had an inferior DFS and survival compared to MRD HCT recipients. Differences in outcome were largely related to excess TRM. Unrelated donor status or mismatch was not associated with less relapse (overall p value=0.33).
4. Factors to be considered in decision making processes for HCT
Both patient- and disease-specific factors play an integral role in decision making whether to offer/recommend HCT to a patient with MDS and in predicting transplant outcomes.(34) Recently, the international expert panel, consisting of members of the EBMT, European LeukemiaNet (ELN), the Blood and Marrow Transplant Clinical Trial Group (BMT CTN), and the International Myelodysplastic Syndromes Foundation, developed the recommendations regarding indication/patient selection strategies for HCT.(35) Figures 1a, 1b depicts the therapeutic algorithm for making decisions for HCT candidacy and timing, showing that both patient and disease characteristics should be considered in computing the risk of proceeding or not proceeding with HCT.
Figure 1.
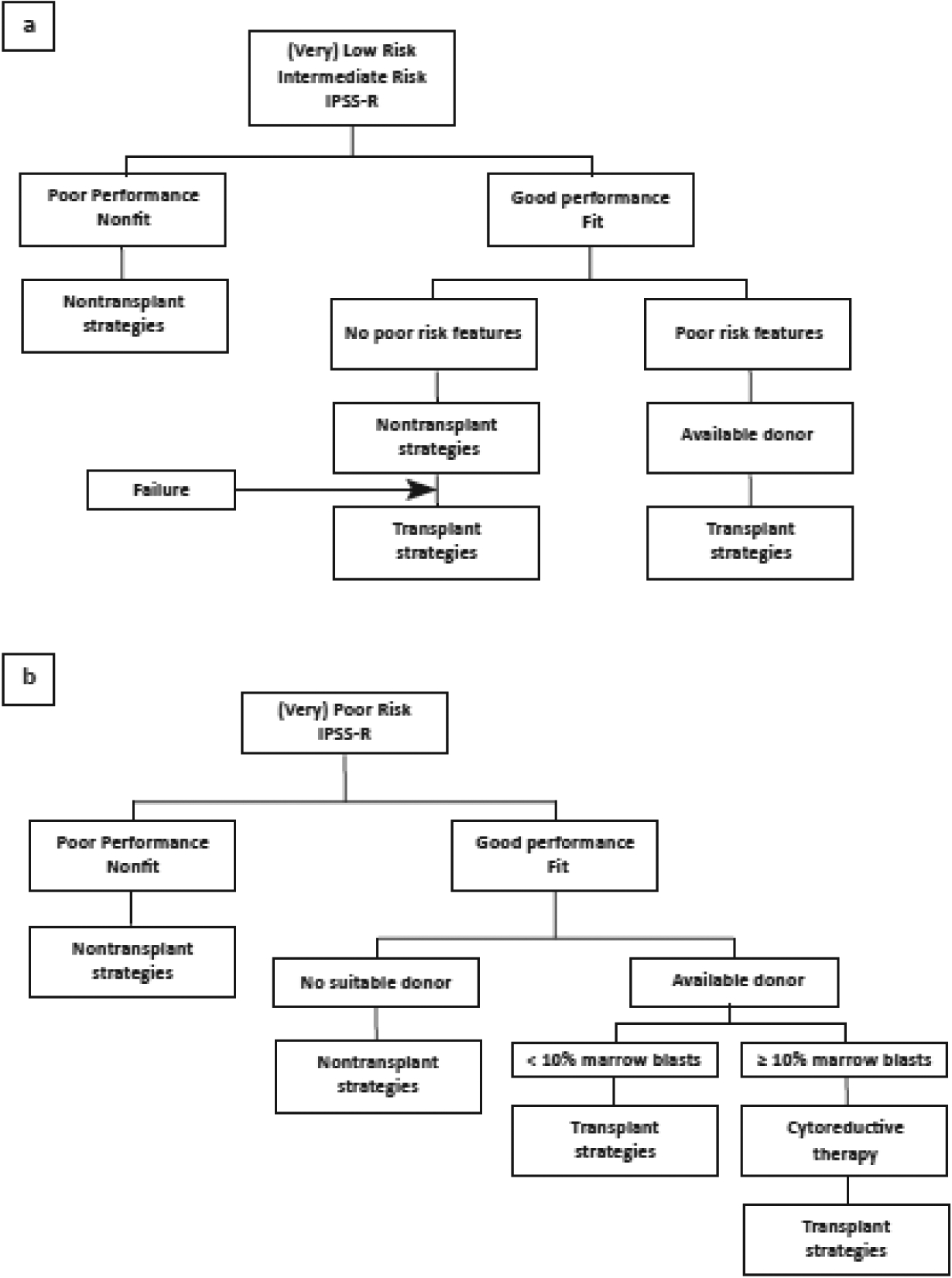
Therapeutic algorithm for adults with MDS (a) patients with (very) low-risk or intermediate IPSS-R risk score and (b)patients with (very) poor risk IRSS-R risk score.
a). Patient-Specific Factors:
Factors such as patients’ age at HCT, comorbidities, and performance status are considered while selecting patients for transplant.(34, 36)
Age:
In the past, the age cut-off of 60 years had been used in most transplant centers, and age older than 60 years was considered to be a risk factor for poor transplant outcomes. With advances in supportive care and the use of RIC, HCTs are increasingly feasible in even ‘older’ patients.(32, 36) In fact, HCTs for patients over the age of 70 years are increasingly used with promising results.(37) A CIBMTR study, restricted to patients older than age 40 undergoing HCT with RIC (n=535), showed that age up to 70 is not a prognostic factor for patients with MDS.(32) Similarly, more recent data from a CIBMTR study conducted for evidence development for Centers for Medicare and Medicaid Services (CMS) indicate similar survival in patients with MDS who are older than 65 years compared to those aged 55–64.(38)
Comorbidities:
Multiple indices have been developed to predict transplant outcomes based on patient comorbidities but the comorbidity index (HCT-CI) developed by Sorror et al. has shown prognostic significance in clinical studies and is being widely used to predict transplant outcomes and risk of mortality.(34, 39, 40) Patients with scores of 0–2 have comparable outcomes regardless of the conditioning regimen, and patients with scores of 3–4 and 5 have a higher risk of mortality with MAC.(34). Above mentioned MDS-CI can also inform patients and physicians about more refined prognostic predictions incorporating patient-related factors in addition to the disease-related prognostic factors.(8) In addition, geriatric assessments for functional reserve and resiliency are increasingly incorporated into HCT practice, and likely provide insight and guidance towards personalized decisions for HCT in elderly MDS patients.(41, 42)
b). Disease-Specific Factors
IPSS/IPSS-R:
As discussed above IPSS/IPSS-R have been used as the standard prognostic scoring system for MDS, and it is generally accepted that patients with Int-2/high risk by IPSS or high/very high risk by IPSS-R would benefit from an early HCT. A decision analysis study by Cutler et al, indicated that in MAC HCT from a matched sibling donor, patients in the Int-2 and high risk IPSS groups have longer life expectancy when transplanted early, and delay of HCT results in loss of life years.(43) In contrast, patients in the low-risk group have the best life expectancy if HCT is delayed until there is evidence of disease progression. A study by Koreth et al, extended a Markov decision model to elderly MDS patients and showed for patients with de novo MDS, aged 60–70 years with low/Int-1 disease risk, early transplantation is not the preferred strategy unless MDS-associated quality of life impairment is substantial.(44) For Int-2/high IPSS risk, early RIC HCT can offer a life expectancy benefit, with quality adjusted survival benefit detectable earlier. A retrospective study by Platzbecker et al, compared allogeneic HCT and azacitidine treatment for Int-2/high-risk MDS (and REAB-T/CMML with >=5% blasts) and showed a survival advantage for allogeneic HCT in medically fit patients with Int-2/high-risk MDS (and REAB-T/CMML with >=5% blasts) at age 60–70 years.(45)
It should be noted that the above-mentioned decision analysis studies used data based on the IPSS, and not the IPSS-R. Among lower risk patients (i.e., IPSS Int-1 or IPSS-R Intermediate risk) those who have prolonged transfusion-dependent cytopenia refractory growth factors or HMA can be considered to be candidates for an HCT.(35)
Somatic Mutations:
Mutations in genes ASXL1, SRSF2, RUNX1, U2AF1, and TP53 are associated with poor prognosis for MDS.(46) A combination of TP53 mutation with complex karyotype is associated with inferior prognosis especially in post-HCT setting.(47) A large CIBMTR cohort was also studied for somatic mutations and showed a negative impact of TP53 mutation on survival. This study also indicated that presence of RAS pathway mutations is associated with shorter survival due to relapse while the presence of JAK2 mutations is associated with shorter survival due to NRM.(48) On the contrary, Aldoss et al, reported that the survival outcome of therapy-related MDS was not different between TP53 mutated vs. unmutated cases.(49) Therefore, additional studies investigating the impact of specific mutations on treatment outcomes (HCT and non-HCT) are required to better inform clinicians and patients, especially in patients with lower risk category (i.e. IPSS-R, low/intermediate) who carry high-risk mutations.
c). Transplant-related factors
Donor Availability:
HLA matching serves as the basis of donor identification for HCT.(50, 51) Potential donors for allogeneic HCT include matched related/sibling donors (MSD), MUD from the donor registry, and alternative donors, including haploidentical donors, mismatched unrelated donors (MMUD) and cord blood units. Donor age, gender, degree of HLA match with the recipient,(52) ABO blood group, and cytomegalovirus (CMV) serostatus are important factors playing a role in donor selection process based on the algorithms developed to select the best donor.(53, 54) A matched young sibling donor is preferred as the first choice. However, as most patients with MDS are older, and a younger MSD may not be available, a MUD would be preferred.(55) Syngeneic donor HCT has been performed in MDS patients with good outcome.(56, 57) Studies, including the above mentioned CIBMTR study,(33) have shown comparable results with MSD and MUD transplants.(58) Additional summary of the outcome data form the CIBMTR is shown in Table 4. As the use of haploidentical donor HCT is increasing, but its relative efficacy compared with MSD/MUD remains to be evaluated.
Table 4.
Survival Probabilities by Donor Type
| MRD | WMUD | PMUD | Cord blood | Haploidentical | p-value | |
|---|---|---|---|---|---|---|
| # Eval | 1458 | 1091 | 273 | 153 | 95 | <0.001* |
| @ 1 year | 60 (57–62) | 56 (53–59) | 45 (39–51) | 45 (38–53) | 48 (38–58) | <0.001** |
| @ 3 years | 45(43–48) | 42 (39–45) | 29 (23–34) | 29 (22–37) | 33 (23–43) | <0.001** |
MRD: matched related donor; WMUD: well-matched unrelated donor; PMUD: partially matched unrelated donor
Log-rank
pointwise
Hematopoietic Stem Cell Source:
Either Bone marrow stem cells (BMSC) or peripheral blood granulocyte growth factor stimulated stem cells (PBSC) can be used for HCT. Several large, randomized trials compared PBSC and BMT in HLA-matched sibling donor HCTs, and showed that PBSC infusion result in better engraftment but higher risk of acute and chronic GVHD.(59–64) Some studies reported a decreased rate of relapse and better survival with PBSC, especially among patients with high-risk blood-cell cancers. In MUD HCTs, the use of PBSC is associated with a higher rate of chronic GVHD without impacting survival or relapse rates.(65) A multicenter retrospective study specifically evaluating MDS patients showed that PBSC grafts were associated with better transplant outcomes with improved overall survival and reduced risk of relapse.(66) The source of stem cells needs to be selected based on an individual basis, with consideration also for donor’s preference, and per institutional guidelines.
Timing of HCT:
Patients who have a higher-risk disease at the time of diagnosis are recommended to undergo HCT early after diagnosis if otherwise eligible for this intensive therapy.(67) In patients who have lower-risk disease on IPSS/IPSS-R at the time of diagnosis and hence lower risk of progression to acute leukemia are generally recommended to undergo HCT at the time of progression, as this approach has been shown to offer survival benefit secondary to NRM associated with earlier HCT.(68) The decision making process for HCT is dynamic involving the time required for donor search, disease response to HMA, and/or disease progression from low-risk to high-risk. Patient factors would also change over time; while all patients age over time, some may improve their functional status after treatment for MDS (i.e., with improved blood counts). For patients who are responding to HMA, it is currently unknown how many cycles (months) of HMA to be continued for the maximum benefit without compromising the organ function/performance status or without emergence of HMA resistance.
Pre-HCT Cytoreductive Therapy:
Several retrospective studies have shown that the percentage of myeloblasts in the bone marrow at the time of diagnosis in patients with MDS can predict post-HCT outcomes.(66, 69) While a few studies showed a beneficial effect in pre-HCT cytoreductive therapy,(35, 69) others suggest no apparent difference.(70) Gerds et al, reported that pre-HCT administration of HMA is associated with outcomes similar to more intensive induction chemotherapy.(71) A study by Damaj et al, made similar observations.(72) Experts recommend against pre-HCT cytoreductive treatment in high-risk MDS patients with less than 10% marrow blasts.(35)
Conditioning therapy for HCT in patients with MDS:
The role of the intensity of conditioning therapy on the transplant outcomes in patients with MDS has been analyzed in multiple retrospective studies. The decision to choose RIC versus MAC depends on numerous patient and disease factors. Studies have shown almost similar survival outcomes between RIC and MAC. The use of RIC may increase the risk of relapse or progressive disease with relatively lower TRM. On the other hand, the use of MAC would lead to a reduced risk of relapse but with increased TRM.
Festuccia et al, studied HCT outcome in patients with MDS based on minimal residual/identifiable disease (MRD/MID) by flow cytometry and cytogenetics with respect to the intensity of conditioning therapy.(73) In this study, relapse was the main factor responsible for increased mortality after HCT in patients with MRD/MID positive disease who underwent RIC HCT. A recent multi-center randomized trial by the BMT CTN compared MAC and RIC in patients with AML or MDS, demonstrating a significant advantage with MAC in relapse-free survival, primarily due to reduced relapse rate despite reduced transplant-related mortality.(74) However, MDS represented only about 20% of the entire cohort and specific conclusion on MDS could not be made.
As a general rule, more fit and younger patients with no significant comorbidities are generally considered for MAC, while relatively frail patients with comorbidities would likely benefit from HCT with RIC. However, a recent analysis by Millymaki et al, showed that shorter blood cell telomere length in patients >40 years old is associated with a significantly elevated risk of early NRM with MAC HCT.(75) Rare germline TERT variants were also identified in this study. Thus, clinically unrecognized germline mutations in the telomerase genes TERT, TERC, and DKC1 may define a distinct subset of adult patients with sporadic MDS and short telomeres who have poor transplant outcomes.
5. Ongoing Studies/Recent Development
Prospective randomized trials comparing HCT and non-HCT therapy are always difficult and unfeasible. Retrospective comparative studies also suffer from inherent selection bias for better fit/psychosocially supported patients towards HCT groups. A biologic assignment design based on the availability of a matched donor can allow for better identification of patients who are HCT eligible and equally fit, yet, not proceeding with HCT, creating a clinically comparable cohort against HCT cohort. In such an effort, Robin et al, conducted a study on behalf of the SFGM-TC and GFM to compare overall survival in MDS patients who are considered as candidates for HCT according to donor availability.(76) The study accrued 162 patients (50: without an available donor, 112: with an available donor) and demonstrated that 4-year overall survival was significantly better in patients with an HLA matched donor (37%) who underwent HCT compared to patients who did not receive HCT due to the lack of an available donor (15%).
In the United States, a larger prospective multi-center study (BMT CTN #1102) comparing RIC HCT with an HMA/best supportive care in higher-risk MDS patients (IPSS Int-2/high by IPSS) aged 50–75 years is underway.(77) This trial is consistent with the National Comprehensive Cancer Center (NCCN) Treatment Guidelines MDS, and with suggestions from a recent review article by Giralt et al. regarding clinical trials to provide evidence for Medicare coverage of HCT for MDS.(78) The design consists of assigning a patients to HCT when a matched donor is available, or best supportive care/non-transplant therapy including HMA when a suitable matched related or unrelated donor is not available. Patients with an HLA-matched sibling or unrelated donor will proceed to HCT utilizing an institutionally-approved RIC regimen. The primary objective is to determine a benefit of HCT, assessed by 3-year overall survival (primary endpoint) from the time of study registration (at the time of HCT center referral, prior to donor search) among those who find a donor and undergo RIC HCT when compared to those who were similarly fit and HCT eligible, yet not undergoing HCT due to the lack of a suitable donor. The expected results from this study would support at least early referral and discussions about HCTs as an integrated part of management strategies in patients with MDS. Another prospective longitudinal observational study was recently conducted at the Dana-Farber/Harvard Cancer Center, designed to examine survival, quality of life, and other outcomes for RIC HCT versus non-HCT approaches for HCT-eligible patients with advanced MDS ages 60 to 75.(79) The data from the study suggested that a treatment strategy that included HCT was associated with better overall survival.
It should be noted that, as both HCT and non-HCT therapies are constantly evolving and improving, relative benefits and risks of HCT against non-HCT therapy would be changing overtime. Recently, HMA in combination with venetoclax showed favorable result in treatment of patients with AML,(25) and this promising combination is now being explored in high-risk MDS. As some of these patients’ responses have been durable, HCT may be less beneficial in some of these responders. At the same time there are advances in HCT including newer GVHD prophylaxis and supportive care with greater applicability in older patients and ethnic/racial minorities. A complex decision-making process incorporating multiple variables on an individual basis may, in the future, be supported by artificial intelligence (AI)-based algorithms. In fact, Nazha et al reported a robust and validated personalized prediction model developed with AI and machine learning algorithms from a training cohort of 1471 patients and validated this algorithm in a cohort of 831 patients composed of 23 clinical variables, including 11 somatic mutations, with significantly better predictability than existing prognostic scoring systems for overall and leukemia-free survivals.(80)
In summary, the decision-making process to proceed or not proceed to HCT for patients with MDS is complex, and requires considerations for both the disease-related and patient-related factors in a dynamic/longitudinal fashion. Early referral to HCT is highly encouraged, at least for detailed discussions about the risks and benefits of HCT in the context of overall MDS treatments.
Table2b.
The risk score IPSS–R (4)
| Prognostic variable | 0 | 0.5 | 1 | 1.5 | 2 | 3 | 4 |
|---|---|---|---|---|---|---|---|
| Cytogenetics | Very good | - | Good | - | Intermediate | Poor | Very poor |
| Bone marrow blasts % | ≤ 2 | - | >2%–< 5% | - | 5%–10% | > 10% | - |
| Hemoglobin | ≥ 10 | - | 8– < 10 | < 8 | - | - | - |
| Platelet | ≥ 100 | 50–< 100 | < 50 | - | - | - | |
| Neutrophil count | ≥ 0.8 | < 0.8 | - | - | - | - | - |
Table 2c.
IPSS–R scoring risk groups
| Risk category | Risk score |
|---|---|
| Very low | ≤ 1.5 |
| Low | >1.5–3 |
| Intermediate | >3–4.5 |
| High | >4.5–6 |
| Very high | >6 |
Acknowledgments:
The authors would like to thank Dr. Sally Mokhtari for critical review and assistance with editing this manuscript.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
The authors declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed
References:
- 1.Steensma DP. Myelodysplastic syndromes current treatment algorithm 2018. Blood Cancer J. 2018;8(5):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. The Lancet Oncology. 2009;10(3):223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–88. [PubMed] [Google Scholar]
- 4.Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papaemmanuil E, Gerstung M, Malcovati L, Tauro S, Gundem G, Van Loo P, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122(22):3616–27; quiz 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haferlach T, Nagata Y, Grossmann V, Okuno Y, Bacher U, Nagae G, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 2014;28(2):241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nazha A, Narkhede M, Radivoyevitch T, Seastone DJ, Patel BJ, Gerds AT, et al. Incorporation of molecular data into the Revised International Prognostic Scoring System in treated patients with myelodysplastic syndromes. Leukemia. 2016;30(11):2214–20 [DOI] [PubMed] [Google Scholar]
- 8.Della Porta MG, Malcovati L, Strupp C, Ambaglio I, Kuendgen A, Zipperer E, et al. Risk stratification based on both disease status and extra-hematologic comorbidities in patients with myelodysplastic syndrome. Haematologica. 2011;96(3):441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberg PL, Stone RM, Al-Kali A, Barta SK, Bejar R, Bennett JM, et al. Myelodysplastic Syndromes, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. 2017;15(1):60. [DOI] [PubMed] [Google Scholar]
- 10.List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. The New England journal of medicine. 2006;355(14):1456–65. [DOI] [PubMed] [Google Scholar]
- 11.List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, et al. Efficacy of lenalidomide in myelodysplastic syndromes. The New England journal of medicine. 2005;352(6):549–57. [DOI] [PubMed] [Google Scholar]
- 12.Nimer SD. Clinical management of myelodysplastic syndromes with interstitial deletion of chromosome 5q. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(16):2576–82. [DOI] [PubMed] [Google Scholar]
- 13.Estey EH, Thall PF, Cortes JE, Giles FJ, O’Brien S, Pierce SA, et al. Comparison of idarubicin + ara-C-, fludarabine + ara-C-, and topotecan + ara-C-based regimens in treatment of newly diagnosed acute myeloid leukemia, refractory anemia with excess blasts in transformation, or refractory anemia with excess blasts. Blood. 2001;98(13):3575–83. [DOI] [PubMed] [Google Scholar]
- 14.Beran M, Shen Y, Kantarjian H, O’Brien S, Koller CA, Giles FJ, et al. High-dose chemotherapy in high-risk myelodysplastic syndrome: covariate-adjusted comparison of five regimens. Cancer. 2001;92(8):1999–2015. [DOI] [PubMed] [Google Scholar]
- 15.McCabe DC, Caudill MA. DNA methylation, genomic silencing, and links to nutrition and cancer. Nutr Rev. 2005;63(6 Pt 1):183–95. [DOI] [PubMed] [Google Scholar]
- 16.McCabe MT, Brandes JC, Vertino PM. Cancer DNA methylation: molecular mechanisms and clinical implications. Clin Cancer Res. 2009;15(12):3927–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blaise D, Furst S, Crocchiolo R, El-Cheikh J, Granata A, Harbi S, et al. Haploidentical T Cell-Replete Transplantation with Post-Transplantation Cyclophosphamide for Patients in or above the Sixth Decade of Age Compared with Allogeneic Hematopoietic Stem Cell Transplantation from an Human Leukocyte Antigen-Matched Related or Unrelated Donor. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2016;22(1):119–24. [DOI] [PubMed] [Google Scholar]
- 18.Blaise D, Nguyen S, Bay JO, Chevallier P, Contentin N, Dhedin N, et al. [Allogeneic stem cell transplantation from an HLA-haploidentical related donor: SFGM-TC recommendations (Part 1)]. Pathologie-biologie. 2014;62(4):180–4. [DOI] [PubMed] [Google Scholar]
- 19.Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106(8):1794–803. [DOI] [PubMed] [Google Scholar]
- 20.Roberts DA, Steensma DP. Outlook and Management of Patients with Myelodysplastic Syndromes Failed by Hypomethylating Agents. Curr Hematol Malig Rep. 2015;10(3):318–28. [DOI] [PubMed] [Google Scholar]
- 21.Komrokji RS. Treatment of Higher-Risk Myelodysplastic Syndromes After Failure of Hypomethylating Agents. Clin Lymphoma Myeloma Leuk. 2015;15 Suppl:S56–9. [DOI] [PubMed] [Google Scholar]
- 22.Prebet T, Gore SD, Esterni B, Gardin C, Itzykson R, Thepot S, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(24):3322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakai Y, Tanaka Y, Yanagihara T, Watanabe M, Duan X, Terasawa M, et al. The Rac activator DOCK2 regulates natural killer cell-mediated cytotoxicity in mice through the lytic synapse formation. Blood. 2013;122(3):386–93. [DOI] [PubMed] [Google Scholar]
- 24.Sekeres MA, Othus M, List AF, Odenike O, Stone RM, Gore SD, et al. Randomized Phase II Study of Azacitidine Alone or in Combination With Lenalidomide or With Vorinostat in Higher-Risk Myelodysplastic Syndromes and Chronic Myelomonocytic Leukemia: North American Intergroup Study SWOG S1117. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017;35(24):2745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura R, Palmer J, Parker P, Stein A, Stiller T, Pullarkat V, et al. Improved Outcome After Reduced Intensity Allogeneic Hematopoietic Stem Cell Transplantation (RI-HCT) for Myelodysplastic Syndrome (MDS) Using Tacrolimus/Sirolimus-Based Gvhd Prophylaxis. Blood. 2009;114:2771-. [Google Scholar]
- 27.Martino R, Perez-Simon JA, Moreno E, Queralto JM, Caballero D, Mateos M, et al. Reduced-intensity conditioning allogeneic blood stem cell transplantation with fludarabine and oral busulfan with or without pharmacokinetically targeted busulfan dosing in patients with myeloid leukemia ineligible for conventional conditioning. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2005;11(6):437–47. [DOI] [PubMed] [Google Scholar]
- 28.Oran B, Giralt S, Saliba R, Hosing C, Popat U, Khouri I, et al. Allogeneic hematopoietic stem cell transplantation for the treatment of high-risk acute myelogenous leukemia and myelodysplastic syndrome using reduced-intensity conditioning with fludarabine and melphalan. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13(4):454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laport GG, Sandmaier BM, Storer BE, Scott BL, Stuart MJ, Lange T, et al. Reduced-intensity conditioning followed by allogeneic hematopoietic cell transplantation for adult patients with myelodysplastic syndrome and myeloproliferative disorders. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14(2):246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura R, Rodriguez R, Palmer J, Stein A, Naing A, Tsai N, et al. Reduced-intensity conditioning for allogeneic hematopoietic stem cell transplantation with fludarabine and melphalan is associated with durable disease control in myelodysplastic syndrome. Bone marrow transplantation. 2007;40(9):843–50. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura R, Palmer JM, O’Donnell MR, Stiller T, Thomas SH, Chao J, et al. Reduced intensity allogeneic hematopoietic stem cell transplantation for MDS using tacrolimus/sirolimus-based GVHD prophylaxis. Leukemia research. 2012;36(9):1152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClune BL, Weisdorf DJ, Pedersen TL, Tunes da Silva G, Tallman MS, Sierra J, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(11):1878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saber W, Cutler CS, Nakamura R, Zhang MJ, Atallah E, Rizzo JD, et al. Impact of donor source on hematopoietic cell transplantation outcomes for patients with myelodysplastic syndromes (MDS). Blood. 2013;122(11):1974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorror ML, Sandmaier BM, Storer BE, Maris MB, Baron F, Maloney DG, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25(27):4246–54. [DOI] [PubMed] [Google Scholar]
- 35.de Witte T, Bowen D, Robin M, Malcovati L, Niederwieser D, Yakoub-Agha I, et al. Allogeneic hematopoietic stem cell transplantation for MDS and CMML: recommendations from an international expert panel. Blood. 2017;129(13):1753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim Z, Brand R, Martino R, van Biezen A, Finke J, Bacigalupo A, et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol. 2010;28(3):405–11. [DOI] [PubMed] [Google Scholar]
- 37.Muffly L, Pasquini MC, Martens M, Brazauskas R, Zhu X, Adekola K, et al. Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States. Blood. 2017;130(9):1156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atallah E, Logan B, Chen M, Cutler C, Deeg J, Jacoby M, et al. Comparison of Patient Age Groups in Transplantation for Myelodysplastic Syndrome: The Medicare Coverage With Evidence Development Study. JAMA oncology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenberg PL, Attar E, Bennett JM, Bloomfield CD, Borate U, De Castro CM, et al. Myelodysplastic syndromes: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2013;11(7):838–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malcovati L, Hellstrom-Lindberg E, Bowen D, Ades L, Cermak J, Del Canizo C, et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood. 2013;122(17):2943–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jayani R, Rosko A, Olin R, Artz A. Use of geriatric assessment in hematopoietic cell transplant. Journal of geriatric oncology. 2019. [DOI] [PubMed] [Google Scholar]
- 42.Derman BA, Kordas K, Ridgeway J, Chow S, Dale W, Lee SM, et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood advances. 2019;3(22):3488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cutler CS, Lee SJ, Greenberg P, Deeg HJ, Perez WS, Anasetti C, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104(2):579–85. [DOI] [PubMed] [Google Scholar]
- 44.Koreth J, Pidala J, Perez WS, Deeg HJ, Garcia-Manero G, Malcovati L, et al. Role of reduced-intensity conditioning allogeneic hematopoietic stem-cell transplantation in older patients with de novo myelodysplastic syndromes: an international collaborative decision analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(21):2662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Platzbecker U, Schetelig J, Finke J, Trenschel R, Scott BL, Kobbe G, et al. Allogeneic hematopoietic cell transplantation in patients age 60–70 years with de novo high-risk myelodysplastic syndrome or secondary acute myelogenous leukemia: comparison with patients lacking donors who received azacitidine. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18(9):1415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malcovati L, Papaemmanuil E, Ambaglio I, Elena C, Galli A, Della Porta MG, et al. Driver somatic mutations identify distinct disease entities within myeloid neoplasms with myelodysplasia. Blood. 2014;124(9):1513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bejar R, Stevenson KE, Caughey B, Lindsley RC, Mar BG, Stojanov P, et al. Somatic mutations predict poor outcome in patients with myelodysplastic syndrome after hematopoietic stem-cell transplantation. J Clin Oncol. 2014;32(25):2691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindsley RC, Saber W, Mar BG, Redd R, Wang T, Haagenson MD, et al. Prognostic Mutations in Myelodysplastic Syndrome after Stem-Cell Transplantation. The New England journal of medicine. 2017;376(6):536–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aldoss I, Stiller T, Tsai NC, Song JY, Cao T, Bandara NA, et al. Therapy-related acute lymphoblastic leukemia has distinct clinical and cytogenetic features compared to de novo acute lymphoblastic leukemia, but outcomes are comparable in transplanted patients. Haematologica. 2018;103(10):1662–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Armitage JO. Bone marrow transplantation. N Engl J Med. 1994;330(12):827–38. [DOI] [PubMed] [Google Scholar]
- 51.Anasetti C, Amos D, Beatty PG, Appelbaum FR, Bensinger W, Buckner CD, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989;320(4):197–204. [DOI] [PubMed] [Google Scholar]
- 52.Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576–83. [DOI] [PubMed] [Google Scholar]
- 53.Worel N, Buser A, Greinix HT, Hagglund H, Navarro W, Pulsipher MA, et al. Suitability Criteria for Adult Related Donors: A Consensus Statement from the Worldwide Network for Blood and Marrow Transplantation Standing Committee on Donor Issues. Biol Blood Marrow Transplant. 2015;21(12):2052–60. [DOI] [PubMed] [Google Scholar]
- 54.Dehn J, Spellman S, Hurley CK, Shaw BE, Barker JN, Burns LJ, et al. Selection of unrelated donors and cord blood units for hematopoietic cell transplantation: guidelines from the NMDP/CIBMTR. Blood. 2019;134(12):924–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kroger N From nuclear to a global family: more donors for MDS. Blood. 2013;122(11):1848–50. [DOI] [PubMed] [Google Scholar]
- 56.Koreth J, Biernacki M, Aldridge J, Kim HT, Alyea EP 3rd, Armand P et al. Syngeneic donor hematopoietic stem cell transplantation is associated with high rates of engraftment syndrome. Biol Blood Marrow Transplant. 2011;17(3):421–8. [DOI] [PubMed] [Google Scholar]
- 57.Kroger N, Brand R, van Biezen A, Bron D, Blaise D, Hellstrom-Lindberg E, et al. Stem cell transplantation from identical twins in patients with myelodysplastic syndromes. Bone Marrow Transplant. 2005;35(1):37–43. [DOI] [PubMed] [Google Scholar]
- 58.Hows JM, Passweg JR, Tichelli A, Locasciulli A, Szydlo R, Bacigalupo A, et al. Comparison of long-term outcomes after allogeneic hematopoietic stem cell transplantation from matched sibling and unrelated donors. Bone Marrow Transplant. 2006;38(12):799–805. [DOI] [PubMed] [Google Scholar]
- 59.Blaise D, Kuentz M, Fortanier C, Bourhis JH, Milpied N, Sutton L, et al. Randomized trial of bone marrow versus lenograstim-primed blood cell allogeneic transplantation in patients with early-stage leukemia: a report from the Societe Francaise de Greffe de Moelle. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18(3):537–46. [DOI] [PubMed] [Google Scholar]
- 60.Powles R, Mehta J, Kulkarni S, Treleaven J, Millar B, Marsden J, et al. Allogeneic blood and bone-marrow stem-cell transplantation in haematological malignant diseases: a randomised trial. Lancet (London, England). 2000;355(9211):1231–7. [DOI] [PubMed] [Google Scholar]
- 61.Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. The New England journal of medicine. 2001;344(3):175–81. [DOI] [PubMed] [Google Scholar]
- 62.Morton J, Hutchins C, Durrant S. Granulocyte-colony-stimulating factor (G-CSF)-primed allogeneic bone marrow: significantly less graft-versus-host disease and comparable engraftment to GCSF-mobilized peripheral blood stem cells. Blood. 2001;98(12):3186–91. [DOI] [PubMed] [Google Scholar]
- 63.Couban S, Simpson DR, Barnett MJ, Bredeson C, Hubesch L, Howson-Jan K, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood. 2002;100(5):1525–31. [DOI] [PubMed] [Google Scholar]
- 64.Schmitz N, Beksac M, Hasenclever D, Bacigalupo A, Ruutu T, Nagler A, et al. Transplantation of mobilized peripheral blood cells to HLA-identical siblings with standard-risk leukemia. Blood. 2002;100(3):761–7. [DOI] [PubMed] [Google Scholar]
- 65.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guardiola P, Runde V, Bacigalupo A, Ruutu T, Locatelli F, Boogaerts MA, et al. Retrospective comparison of bone marrow and granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells for allogeneic stem cell transplantation using HLA identical sibling donors in myelodysplastic syndromes. Blood. 2002;99(12):4370–8. [DOI] [PubMed] [Google Scholar]
- 67.Robin M, Porcher R, Ades L, Raffoux E, Michallet M, Francois S, et al. HLA-matched allogeneic stem cell transplantation improves outcome of higher risk myelodysplastic syndrome A prospective study on behalf of SFGM-TC and GFM. Leukemia. 2015;29(7):1496–501. [DOI] [PubMed] [Google Scholar]
- 68.Alessandrino EP, Porta MG, Malcovati L, Jackson CH, Pascutto C, Bacigalupo A, et al. Optimal timing of allogeneic hematopoietic stem cell transplantation in patients with myelodysplastic syndrome. Am J Hematol. 2013;88(7):581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sierra J, Perez WS, Rozman C, Carreras E, Klein JP, Rizzo JD, et al. Bone marrow transplantation from HLA-identical siblings as treatment for myelodysplasia. Blood. 2002;100(6):1997–2004. [PubMed] [Google Scholar]
- 70.Appelbaum FR, Anderson J. Allogeneic bone marrow transplantation for myelodysplastic syndrome: outcomes analysis according to IPSS score. Leukemia. 1998;12 Suppl 1:S25–9. [PubMed] [Google Scholar]
- 71.Gerds AT, Gooley TA, Estey EH, Appelbaum FR, Deeg HJ, Scott BL. Pretransplantation therapy with azacitidine vs induction chemotherapy and posttransplantation outcome in patients with MDS. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18(8):1211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Damaj G, Duhamel A, Robin M, Beguin Y, Michallet M, Mohty M, et al. Impact of azacitidine before allogeneic stem-cell transplantation for myelodysplastic syndromes: a study by the Societe Francaise de Greffe de Moelle et de Therapie-Cellulaire and the Groupe-Francophone des Myelodysplasies. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(36):4533–40. [DOI] [PubMed] [Google Scholar]
- 73.Festuccia M, Deeg HJ, Gooley TA, Baker K, Wood BL, Fang M, et al. Minimal Identifiable Disease and the Role of Conditioning Intensity in Hematopoietic Cell Transplantation for Myelodysplastic Syndrome and Acute Myelogenous Leukemia Evolving from Myelodysplastic Syndrome. Biol Blood Marrow Transplant. 2016;22(7):1227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017;35(11):1154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Myllymaki M, Redd RA, Cutler CS, Saber W, Hu Z-H, Wang T, et al. Telomere Length and Telomerase Complex Mutations Predict Fatal Treatment Toxicity after Stem Cell Transplantation in Patients with Myelodysplastic Syndrome. Blood. 2018;132(Supplement 1):796-. [Google Scholar]
- 76.Robin M, Porcher R, Adès L, Raffoux E, Michallet M, François S, et al. HLA-matched allogeneic stem cell transplantation improves outcome of higher risk myelodysplastic syndrome A prospective study on behalf of SFGM-TC and GFM. Leukemia. 2015;29(7):1496–501. [DOI] [PubMed] [Google Scholar]
- 77.Saber W, Le Rademacher J, Sekeres M, Logan B, Lewis M, Mendizabal A, et al. Multicenter biologic assignment trial comparing reduced-intensity allogeneic hematopoietic cell transplant to hypomethylating therapy or best supportive care in patients aged 50 to 75 with intermediate-2 and high-risk myelodysplastic syndrome: Blood and Marrow Transplant Clinical Trials Network #1102 study rationale, design, and methods. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014;20(10):1566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15(3):367–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.El-Jawahri A, Kim HT, Steensma DP, Cronin AM, Stone RM, Watts CD, et al. Does quality of life impact the decision to pursue stem cell transplantation for elderly patients with advanced MDS? Bone marrow transplantation. 2016;51(8):1121–6. [DOI] [PubMed] [Google Scholar]
- 80.Nazha A, Komrokji RS, Meggendorfer M, Mukherjee S, Al Ali N, Walter W, et al. A Personalized Prediction Model to Risk Stratify Patients with Myelodysplastic Syndromes. Blood. 2018;132(Supplement 1):793-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lubbert M, Bertz H, Ruter B, Marks R, Claus R, Wasch R, et al. Non-intensive treatment with low-dose 5-aza-2’-deoxycytidine (DAC) prior to allogeneic blood SCT of older MDS/AML patients. Bone marrow transplantation. 2009;44(9):585–8. [DOI] [PubMed] [Google Scholar]
- 82.Itzykson R, Thépot S, Quesnel B, Dreyfus F, Recher C, Wattel E, et al. Long-term outcome of higher-risk MDS patients treated with azacitidine: an update of the GFM compassionate program cohort. Blood. 2012;119(25):6172–3. [DOI] [PubMed] [Google Scholar]
- 83.Lubbert M, Suciu S, Baila L, Ruter BH, Platzbecker U, Giagounidis A, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(15):1987–96. [DOI] [PubMed] [Google Scholar]
- 84.Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(10):2429–40. [DOI] [PubMed] [Google Scholar]
Most Important References
- 33.Saber W, Cutler CS, Nakamura R, Zhang MJ, Atallah E, Rizzo JD, et al. Impact of donor source on hematopoietic cell transplantation outcomes for patients with myelodysplastic syndromes (MDS). Blood. 2013;122(11):1974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Authors of study reported that for patients with myelodysplastic syndromes, donor source remains an important determinant of post-transplantation outcomes.
- 38.Atallah E, Logan B, Chen M, Cutler C, Deeg J, Jacoby M, et al. Comparison of Patient Age Groups in Transplantation for Myelodysplastic Syndrome: The Medicare Coverage With Evidence Development Study. JAMA oncology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Data from this CIBMTR study conducted for evidence development for Centers for Medicare and Medicaid Services (CMS) indicate similar survival in patients with MDS who are older than 65 years compared to younger patients aged 55–64.
- 43.Cutler CS, Lee SJ, Greenberg P, Deeg HJ, Perez WS, Anasetti C, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104(2):579–85. [DOI] [PubMed] [Google Scholar]; * This decision analysis study by Cutler et al, indicated that in MAC HCT from a matched sibling donor, patients in the Int-2 and high risk IPSS groups have longer life expectancy when transplanted early, and delay of HCT results in loss of life years.
- 44.Koreth J, Pidala J, Perez WS, Deeg HJ, Garcia-Manero G, Malcovati L, et al. Role of reduced-intensity conditioning allogeneic hematopoietic stem-cell transplantation in older patients with de novo myelodysplastic syndromes: an international collaborative decision analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(21):2662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study by Koreth et al, extended a Markov decision model to elderly MDS patients and showed for patients with de novo MDS, aged 60–70 years with low/Int-1 disease risk, early transplantation is not the preferred strategy unless MDS-associated quality of life impairment is substantial. For Int-2/high IPSS risk, early RIC HCT can offer a life expectancy benefit, with quality adjusted survival benefit detectable earlier.
- 48.Lindsley RC, Saber W, Mar BG, Redd R, Wang T, Haagenson MD, et al. Prognostic Mutations in Myelodysplastic Syndrome after Stem-Cell Transplantation. The New England journal of medicine. 2017;376(6):536–47. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This large CIBMTR study for somatic mutations in MDS showed a negative impact of TP53 mutation on survival. The study also showed that the presence of RAS pathway mutations was associated with shorter survival due to relapse while the presence of JAK2 mutations was associated with shorter survival due to NRM.


