Abstract
Growing evidence suggests that docosahexaenoic acid (DHA) exerts neuroprotective effects, although the mechanism(s) underlying these beneficial effects are not fully understood. Here we demonstrate that DHA, but not arachidonic acid (ARA), suppressed oligomeric amyloid-β peptide (oAβ)–induced reactive oxygen species (ROS) production in primary mouse microglia and immortalized mouse microglia (BV2). Similarly, DHA but not ARA suppressed oAβ-induced increases in phosphorylated cytosolic phospholipase A2 (p-cPLA2), inducible nitric oxide synthase (iNOS), and tumor necrosis factor-α (TNF-α) in BV2 cells. LC-MS/MS assay indicated the ability for DHA to cause an increase in 4-hydroxyhexenal (4-HHE) and suppress oAβ-induced increase in 4-hydroxynonenal (4-HNE). Although oAβ did not alter the nuclear factor erythroid 2–related factor 2 (Nrf2) pathway, exogenous DHA, ARA as well as low concentrations of 4-HHE and 4-HNE upregulated this pathway and increased production of heme oxygenase-1 (HO-1) in microglial cells. These results suggest that DHA modulates ARA metabolism in oAβ-stimulated microglia through suppressing oxidative and inflammatory pathways and upregulating the antioxidative stress pathway involving Nrf2/HO-1. Understanding the mechanism(s) underlying the beneficial effects of DHA on microglia should shed light into nutraceutical therapy for the prevention and treatment of Alzheimer’s disease (AD).
Keywords: Alzheimer’s disease, Fish oil, Omega-3 fatty acids, Phospholipase A2, Lipid peroxidation
Introduction
Docosahexaenoic acid (DHA) is one of the most abundant polyunsaturated fatty acids (PUFAs) in the central nervous system [1]. Reduction of DHA in brains and low dietary DHA intake have been found associated with Alzheimer’s disease (AD) [1–5], whereas DHA-enriched diet is associated with a lower AD risk [2–4]. Thus, there is substantial interest to examine whether DHA exerts neuroprotective effects to alleviate the pathological events of AD. Studies with AD mouse models indicated a high-DHA diet to reduce amyloid-β plaque burden [5, 6], and cerebral amyloid angiopathy, including cerebrovascular amyloid-β (Aβ) deposition and microhemorrhages [7]. Given the evidence showing transport of plasma-derived DHA across the blood-brain barrier [8, 9], it is of interest to examine the beneficial and/or detrimental effects arising from the interactions of different neuronal cells with DHA and its peroxidation products. For example, oxidized DHA has been reported to increase amyloidogenic amyloid precursor protein (APP) processing in neurons [10]. DHA enhanced non-amyloidogenic APP processing in differentiated human neuroblastoma cells, possibly due to its ability to fluidize the plasma membranes [11, 12]. In addition, DHA was shown to enhance phagocytosis of Aβ and decrease inflammatory markers in microglia [13].
Besides DHA, arachidonic acid (ARA) is another abundant PUFA with similar concentrations to DHA in the brain. DHA and ARA in membrane phospholipids are released through hydrolytic reactions mediated by phospholipases A2 (PLA2). While the release of DHA has been attributed to the action of Ca2+-independent PLA2 (iPLA2) [14–17], the release of ARA is largely mediated by the Ca2+-dependent cytosolic phospholipase A2 (cPLA2) [18]. Unlike DHA, ARA is a lipid mediator for triggering a wide range of inflammatory responses through the synthesis of eicosanoids and prostanoids catalyzed by cyclooxygenases and lipoxygenases [18, 19]. In contrast, DHA is shown to impose pro-resolving and pro-homeostatic effects through synthesis of oxylipins such as resolvin D and neuroprotection D1 [20, 21].
PUFAs undergo lipid peroxidation due to a number of factors, and recent studies have focused on production of 4-hydroxyhexenal (4-HHE) from DHA and 4-hydroxynonenal (4-HNE) from ARA [22]. Consistent with oxidative stress–induced cPLA2 activation and release of ARA in AD patients [23], higher levels of protein-bound 4-HNE and free 4-HNE have been found in plasma, urine, and cerebrospinal fluid in AD and amnestic mild cognitive impairment patients [24–28]. Being reactive aldehydes and electrophiles, 4-HNE and 4-HHE can form adducts with macromolecules such as DNA, proteins, and enzymes to alter cell functions [29–31]. In fact, 4-HNE has been shown to covalently modify Aβ and accelerate the formation of more neurotoxic Aβ protofibrils while inhibiting the production of the mature fibrils [32]. Aside from the detrimental effects, these α,β-unsaturated aldehydes can also elicit hormetic functions, in part, through promoting the nuclear factor erythroid 2–related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) antioxidant pathway [33–40]. Our recent in vitro study demonstrated that low concentrations of 4-HNE and 4-HHE upregulated the Nrf2/HO-1 antioxidant pathway in microglial cells [41].
Microglia are resident macrophage cells in the brain and are responsible for scavenging cell debris, plaques, and damaged neurons and synapses [42]. A recent transcriptome study reported that microglial cell–enriched genes overlapped significantly with genes associated with neurodegenerative diseases and psychiatric disorders, and more than half of the genes associated with AD were preferentially expressed in microglia [43]. These findings suggest that microglia play crucial roles in neurodegenerative diseases, including AD. Therefore, investigating the mechanisms underlying microglial functions has been a major line of AD research. For example, the microglial cell–mediated clearance of Aβ has been shown to be governed by a range of receptors [44–57], and cPLA2 in microglia can facilitate Aβ uptake through its action to regulate membrane-cytoskeleton connectivity [58]. Due to the relatively high expression of nicotinamide adenine di-nucleotide phosphate (NADPH) oxidase in microglia, activation of microglial NADPH oxidase is the primary source of Aβ-induced ROS [59]. In addition, Aβ-induced activation of microglia contributes to neuroinflammation by upregulating reactive nitrogen intermediates and TNF-α [60].
To further understand the effects of DHA on AD pathology, this study examined whether DHA and its peroxidation product, 4-HHE, modulate oxidative stress, cPLA2 activation, the 4-HNE level, inflammatory responses, and the Nrf2/HO-1 pathway in oligomeric amyloid-β peptide (oAβ)–stimulated microglia. Results from this study should help unveil mechanisms underlying the beneficial effects of DHA and shed light into nutraceutical therapy for the prevention and treatment of AD.
Materials and Methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), Fluo-4-AM, A23187, and penicillin/streptomycin (P/S, 10,000 units/ml) were obtained from Life Technologies (Grand Island, NY). Fetal bovine albumin (FBS), bovine serum albumin (BSA), and Ham’s F-12 media were from GE Healthcare Life Sciences (Logan, UT). 4-HHE (1 mg in 0.1 ml of ethanol), 4-HNE (1 mg in 0.1 ml of ethanol), DHA (50 mg in 0.2 ml of ethanol), ARA (50 mg in 0.2 ml of ethanol), and lipopolysaccharide (LPS) were purchased from Cayman Chemical (Ann Arbor, MI). BSA (fatty acid free), cell proliferation reagent WST-1, 1,3-cyclohexanedione (CHD, 97%), ammonium acetate (HPLC grade), acetic acid (ACS grade) and formic acid (mass spectrometry grade), dimethyl sulfoxide (DMSO), 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), cOmplete™ protease inhibitor cocktail, and PhosSTOP™ phosphatase inhibitor cocktail were purchased from Sigma-Aldrich (St. Louis, MO). Human Aβ1–42 was purchased from California Peptide (Salt Lake City, UT), and scramble human Aβ1–42 was purchased from AnaSpec (Fremont, CA). Radioimmunoprecipitation assay (RIPA) buffer, the bicinchoninic acid (BCA) protein assay kit, SuperSignal™ West Pico plus chemiluminescent substrate, Restore™ PLUS Western blot stripping buffer, and TNF-α mouse uncoated ELISA kit were purchased from Thermo Scientific (Waltham, MA). CM-H2DCFDA (DCF) was purchased from Invitrogen, Inc. (Eugene, OR). Primary antibodies against HO-1, phosphorylated cytosolic phospholipase A2 (p-cPLA2), and cPLA2 were from Cell Signaling (Beverly, MA); monoclonal anti-β-actin peroxidase antibody was from Sigma-Aldrich (St. Louis, MO); anti-inducible nitric oxide synthase (iNOS) antibody was from Abcam (Cambridge, MA); and anti-Nrf2 antibody was from GeneTex (Irvine, CA). C18 Sep-Pak cartridges (1 ml, 100 mg) were obtained from Waters Corporation (Milford, MA). Phospholipid removal cartridges (Phree™, 1 ml) were purchased from Phenomenex, Inc. (Torrance, CA). All solvents (HPLC grade) used for LC and MS analysis were obtained from Thermo Fisher Scientific, Inc. (Fair Lawn, NJ). Neural Tissue Dissociation kit (P), gentleMACS Dissociator, MACS buffer, and CD11b microbeads were from Miltenyi Biotec (Bergisch Gladbach, Germany).
Primary Mouse Microglial Isolation
Timed pregnant C57BL/6 mice were purchased from Charles River (Wilmington, MA). All animal care and experimental protocols were carried out with permission from the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Chicago. Cerebral cortices and hippocampi were dissected from mouse pups (P1–P5). After removal of meninges, brain tissues were subjected to a magnetic cell sorting protocol [61]. Briefly, brain tissue was homogenized using the Neural Tissue Dissociation kit (P) and the gentleMACS Dissociator. Cells were pelleted at 300×g for 10 min at 4 °C, resuspended in ice-cold MACS buffer containing CD11b microbeads, and further incubated at 4 °C for 15 min. After incubation with microbeads, cells were washed, resuspended in ice-cold MACS buffer, and passed through the magnetized LS columns (Miltenyi Biotec) and microglia were collected according to the manufacturer’s protocol. Experiments with primary mouse microglia were carried out immediately after microglial isolation.
BV2 Cell Culture
BV2 cells were provided by Dr. Grace Sun (University of Missouri, MO) who originally obtained from Dr. Rosario Donato (University of Perugia, Italy). BV2 cells were cultured in DMEM supplied with 5% FBS and 1% P/S. Morphology of BV2 cells was routinely examined under a Nikon Eclipse Ti microscope before experiment. For measuring ROS and calcium flux, BV2 cells were seeded into 96-well plates. For Western blot analysis, BV2 cells were seeded into 6-well plates. For LC-MS/MS analysis, BV2 cells were seeded into 60-mm dishes. Experiments were conducted when cells reached 80–90% confluency. BV2 cells were serum starved for 3 h followed by oAβ treatment with different treatment times: 30 min for studying ROS production and calcium flux; 6 h for LC-MS/MS analysis of 4-HHE and 4-HNE; 6 h for Western blot analysis of p-cPLA2/cPLA2, iNOS, Nrf2, and HO-1; and 23 h for TNF-α ELISA assay. DHA and ARA were dissolved in 2% fatty acid–free BSA, and 4-HHE and 4-HNE were dissolved in DMSO.
Preparation of oAβ
oAβ was prepared according to the protocol described by Dahlgren et al. (2002) cited in Ref. [58]. Briefly, 10 mg Aβ was dissolved in 2.2 ml HFIP and incubated for 60 min at room temperature. The Aβ-HFIP mixture was then aliquoted into 0.5-ml microcentrifuge tubes. The tube was then opened, set in fume hood overnight, and placed in a speed vacuum system (Thermo Scientific) for HFIP evaporation. A clear film of Aβ appeared at the bottom of each tube, and the tube was stored in a − 80 °C freezer until use. For preparation of oAβ, the peptide was first resuspended in DMSO, di-luted in ice-cold phenol red–free Ham’s F12 medium to a final concentration of 100 μM, and aged at 4 °C for 24 h before use.
Measurement of ROS Production
For measuring ROS production in primary mouse microglia, freshly collected primary mouse microglia were suspended in 96-well plates and pretreated with 10 μM DHA or ARA for 2 h, followed by 2.5 μM oAβ treatment for 30 min. For ROS production measurement in BV2 cells, cells were starved for 3 h prior to pretreatment with 10 μM DHA or ARA for 24 h and incubated in fresh medium for 1 h before treating with 2.5 μM oAβ for 30 min; 5 μM DCF was then added to each well and incubated with cells for 1 h. The fluorescent intensity of DCF was measured using a Synergy H1 Plate Reader (BioTek Instruments, Inc., St. Louis, MO) with an excitation wavelength of 490 nm and an emission wavelength of 520 nm.
Western Blot Analysis
BV2 cells were seeded into 6-well plates and serum starved for 3 h before incubation with DHA, ARA, 4-HHE, or 4-HNE for 1 h. This was followed by treating cells with 2.5 μM oAβ and further incubation for 6 h. Cells were then lysed in RIPA buffer with protease and phosphatase inhibitors. Cell lysates were collected and centrifuged at 14,000×g for 15 min at 4 °C, and supernatants were collected. Protein concentration was determined by BCA assay. Equal amount of samples was loaded onto SDS-PAGE for electrophoresis. Then, proteins were transferred to 0.2-μm PVDF membranes at 100 V for 1 h at 4 °C. Membranes were then blocked with 5% non-fat milk in Tris-buffered saline with 0.1% Tween 20 (TBS-T) for 1 h at room temperature. The blots were incubated with antibodies against HO-1 (1:1000 dilution), p-cPLA2 (1:1000 dilution) or cPLA2 (1:1000 dilution), iNOS (1:200 dilution), Nrf2 (1:800 dilution), and β-actin (1:50,000 dilution) overnight at 4 °C. After washing with TBS-T, blots were incubated with HRP-conjugated anti-rabbit IgG antibody (1:1000 dilution) for 1 h at room temperature. Signals were developed using SuperSignal™ West Pico plus chemiluminescent substrate and captured with a myECL imager (Thermo Scientific). The optical density of bands was measured with the Image Studio Lite 5.2 (LI-COR Biotechnology, Lincoln, NE).
TNF-α ELISA Assay
The concentration of TNF-α in medium was determined by sandwich ELISA. Briefly, cells were treated as described above, and medium was collected and centrifuged at 4000×g for 5 min. The levels of TNF-α were assessed using a TNF-α ELISA kit following the manufacturer’s instruction. The re-maining cells were lysed with RIPA buffer and used for total protein determination with BCA assay.
LC-MS/MS Analysis of 4-HHE and 4-HNE in BV2 Cells
LC-MS/MS analysis was carried out as described earlier [41, 62, 63]. Briefly, cells were subcultured in 60-mm dishes, and after different treatment conditions, the culture medium was removed and 0.5 ml of phosphate-buffered saline-methanol (1:1, v/v) was added. An aliquot of cell suspension was added to an equal volume of internal standard (4-HHE-d3), and ace-tonitrile containing 1% formic acid was added to the mixture. Solid-phase extraction (SPE) was carried out using a Phree™ cartridge. 4-HHE, 4-HNE, and 4-HHE-d3 were derivatized by adding freshly prepared acidified 1,3-cyclohexanedione reagent at 60 °C for 1 h. After the tubes were cooled to room temperature, the derivatized 4-HHE and 4-HNE were desalted using a C18 SPE cartridge. The eluate from the C18 SPE cartridge was evaporated to dryness under a stream of nitrogen gas. An aliquot of the reconstituted solution was injected into a Waters Xevo TQ-S triple quadrupole mass spectrometer (Proteomics Center, University of Missouri, Columbia, MO). The multiple reaction monitoring transitions m/z 326.3 > 216.1 Da, 284.2 > 216.1 Da, and 287.2 > 216.1 Da were chosen for simultaneous monitoring of 4-HNE, 4-HHE, and 4-HHE-d3 derivatives, respectively. MassLynx software (v4.1, Waters) was used for all data acquisitions.
Calcium Measurement in BV2 Cells
BV2 cells were seeded into 96-well plates and starved for 3 h before the addition of 2 μM Fluo-4-AM for 30 min. Cells were then pretreated with 50 μM DHA or ARA for 1 h, followed by stimulation with 2.5 μM oAβ or treatment with 1 μM A23187 as positive control for 30 min. Cells were read with an excitation wavelength of 492 nm and an emission wavelength of 520 nm using a BioTek Synergy H1 Plate Reader.
Statistical Analysis
Data are expressed as mean ± standard deviation (SD) from at least three independent experiments with single lane. An unpaired two-tailed Student’s t test was used for statistical analysis between two groups. Statistical analysis between multiple groups was carried out using one-way ANOVA followed by Tukey’s post hoc HSD test in GraphPad Prism (version 8.10). A p value < 0.05 was considered statistically significant.
Results
Effects of DHA on oAβ-Induced ROS Production in Primary Mouse Microglia and BV2 Cells
To study mechanisms underlying the beneficial effects of DHA, we began to test if DHA can suppress ROS production induced by oAβ in freshly isolated primary mouse microglia (i.e., ex vivo condition) and in immortalized microglia (BV2). We found that pretreatment with 10 μM DHA for 2 h suppressed oAβ (2.5 μM for 30 min)–induced ROS production in primary mouse microglia (Fig. 1a). In contrary, pretreatment with 10 μM ARA did not alter ROS induced by oAβ (Fig. 1b). Similar results were obtained with BV2 cells upon pretreatment of cells with DHA or ARA for 24 h, followed by treatment with 2.5 μM oAβ for 30 min (Fig. 1a, b). In this study with BV2 cells, we tested the specificity of oAβ to induce ROS production by using scrambled Aβ. As shown in Fig. 1 a, scrambled Aβ did not induce ROS production under similar conditions. Our previous studies demonstrated ability for LPS to induce ROS in microglial cells [61]. Using LPS as a positive control, results from this study demonstrated ability for LPS to induce a large increase in ROS in BV2 cells (Fig. 1a). Since we obtained similar results on the effects of DHA on ROS production between BV2 cells and primary mouse microglia, we fur-thered our cell signaling studies using BV2 cells.
Fig. 1.
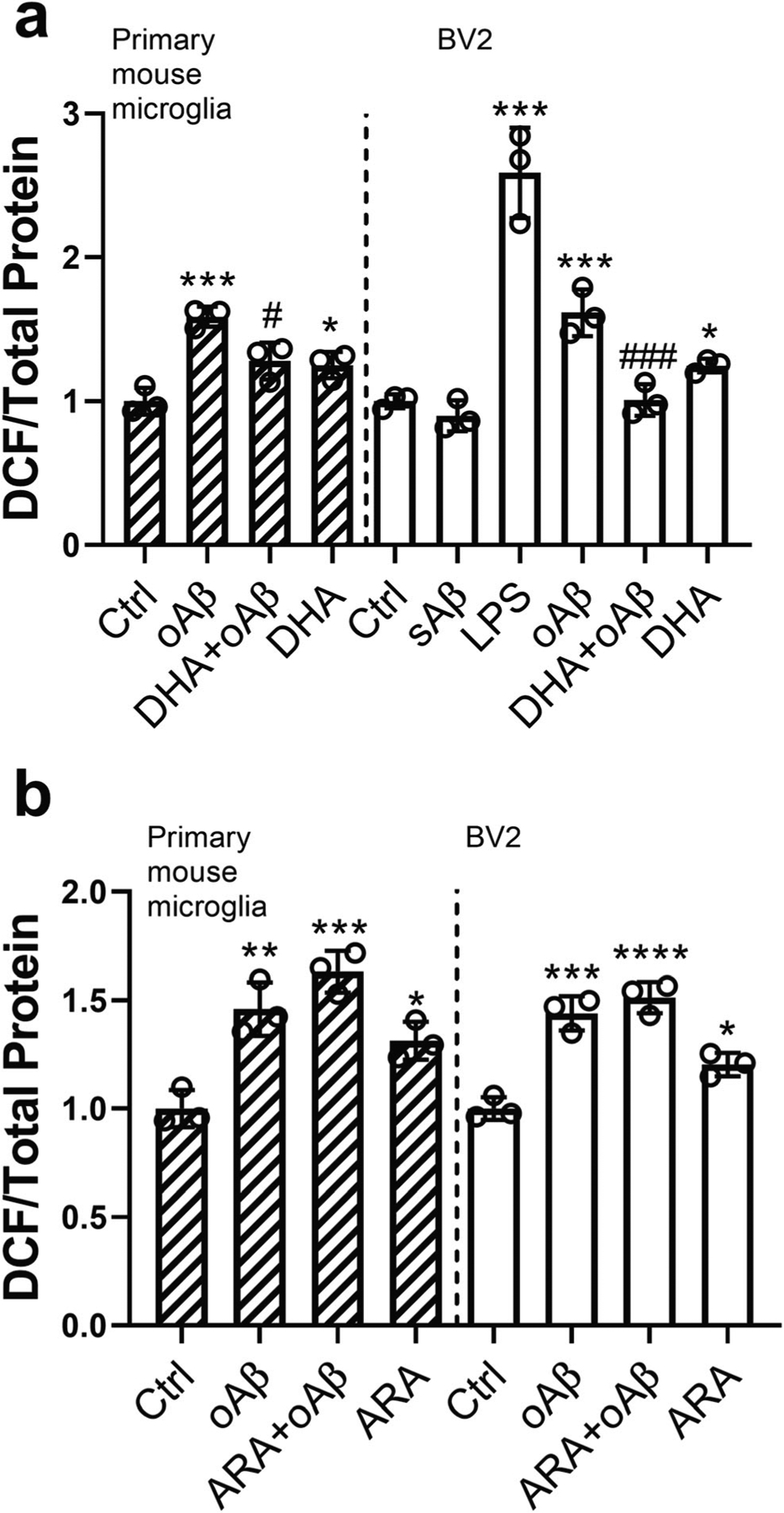
Effects of DHA and ARA on oAβ-induced reactive oxygen species (ROS) production in primary mouse microglia and BV2 cells. Primary microglia cells were pretreated with 10 μM DHA (a) or ARA (b) for 2 h, followed by treatment with 2.5 μM oAβ for 30 min. BV2 cells were incubated with 10 μM DHA (a) or ARA (b) for 24 h and with fresh medium for 1 h, followed by 2.5 μM oAβ stimulation for 30 min. BV2 cells were also treated with 2.5 μM scramble Aβ1–42 for 30 min as a negative control or 100 ng/ml LPS for 11 h as a positive control. Data are represented as mean ± SD from three independent experiments (n = 3). (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, compared with the control group; #p < 0.05, ###p < 0.001, compared with the oAβ treatment group.) From the left to the right bar (mean ± SD): a 1 ± 0.09, 1.59 ± 0.07, 1.28 ± 0.13, 1.25 ± 0.09, 1 ± 0.05, 0.9 ± 0.11, 2.59 ± 0.31, 1.62 ± 0.16, 1.01 ± 0.11, 1.25 ± 0.05; b 1 ± 0.09, 1.46 ± 0.12, 1.63 ± 0.1, 1.31 ± 0.09, 1 ± 0.05, 1.44 ± 0.08, 1.51 ± 0.07, 1.2 ± 0.05
Modulations of 4-HHE and 4-HNE by DHA and ARA in Microglia
In addition to the ability of DHA to suppress oAβ-induced ROS production in microglia, LC-MS/MS experiment was used to examine the effects of DHA and ARA on levels of their peroxidation products, 4-HHE and 4-HNE, respectively. Results showed that treatment with 2.5 μM oAβ for 6 h did not impose any effect on the 4-HHE level (Fig. 2a) but instead significantly increased the 4-HNE level (Fig. 2b, e). Treatment with 50 μM DHA for 7 h resulted in a significant increase in 4-HHE level, and this level was further increased when cells were treated with DHA for 1 h and followed by treatment with oAβ for 6 h (Fig. 2a). The addition of DHA alone appeared to lower (not significant) the 4-HNE level, and DHA suppressed the increase in 4-HNE induced by oAβ (Fig. 2b). Subsequently, oAβ increased the ratio of the 4-HNE to 4-HHE level (i.e., 4-HNE/4-HHE), which was dramatically decreased by the pretreatment of DHA (Fig. 2c). In contrast, oAβ, pretreatment with ARA prior to oAβ treatment, and ARA alone did not impose any effect on the 4-HHE level (Fig. 2d). While oAβ increased 4-HNE, pretreatment with ARA further increased 4-HNE (Fig. 2e). Subsequently, the increase in 4-HNE/4-HHE ratio induced by oAβ was enhanced by the pretreatment with ARA (Fig. 2f). It is also interesting to note that DHA modulated the peroxidation product of ARA (Fig. 2b), but ARA did not modulate the peroxidation product of DHA (Fig. 2d).
Fig. 2.
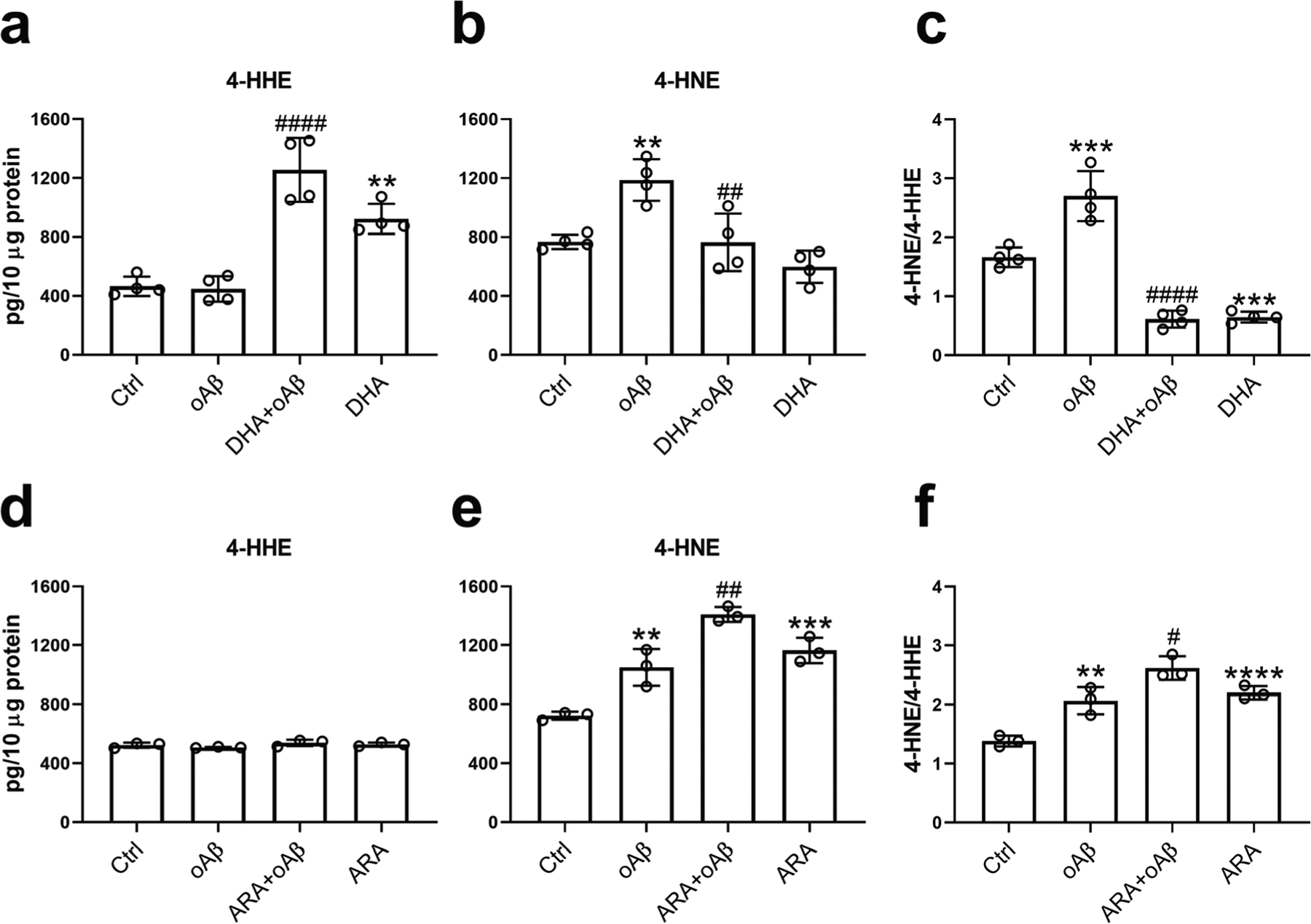
DHA and ARA modulated the levels of 4-HHE and 4-HNE in BV2 cells. BV2 cells were treated with 50 μM DHA (a–c) or 50 μM ARA (d–f) for 1 h, followed by stimulation with 2.5 μM oAβ for 6 h. The levels of 4-HHE and 4-HNE were measured by LC-MS/MS. Data are represented as mean ± SD from three or four independent experiments (n = 3 or 4). (**p < 0.01, ***p < 0.001, ****p < 0.0001, compared with the control group; #p < 0.05, ##p < 0.01, ####p < 0.0001, compared with the oAβ treatment group.) From the left to the right bar (mean ± SD): a 465.65 ± 66.1, 447.48 ± 86.16, 1254.83 ± 217.46, 922.35 ± 102.17; b 768.43 ± 48.87, 1187.2 ± 141.11, 764.08 ± 194.69, 599.38 ± 109.52; c 1.67 ± 0.17, 2.7 ± 0.42, 0.61 ± 0.14, 0.65 ± 0.09; d 523.49 ± 17.31, 507.73 ± 4.18, 539.14 ± 21.63, 528.88 ± 12.45; e 723.6 ± 27.41, 1049.67 ± 124.69, 1409.5 ± 50.82, 1164.7 ± 86.51; f 1.38 ± 0.09, 2.07 ± 0.23, 2.62 ± 0.2, 2.2 ± 0.11
Effects of DHA, ARA, 4-HHE, and 4-HNE on oAβ-Triggered cPLA2 Activation
It has been reported that aggregated Aβ activated cPLA2 in microglia [64]. Therefore, we examined if DHA, ARA, 4-HHE, and 4-HNE can alter oAβ-triggered cPLA2 activation, as indicated by phosphorylation of cPLA2 (i.e., p-cPLA2). Results showed that oAβ-triggered cPLA2 activation and this activation were suppressed by DHA (Fig. 3a), 4-HHE, and 4-HNE (Fig. 3c), but not by ARA (Fig. 3b).
Fig. 3.
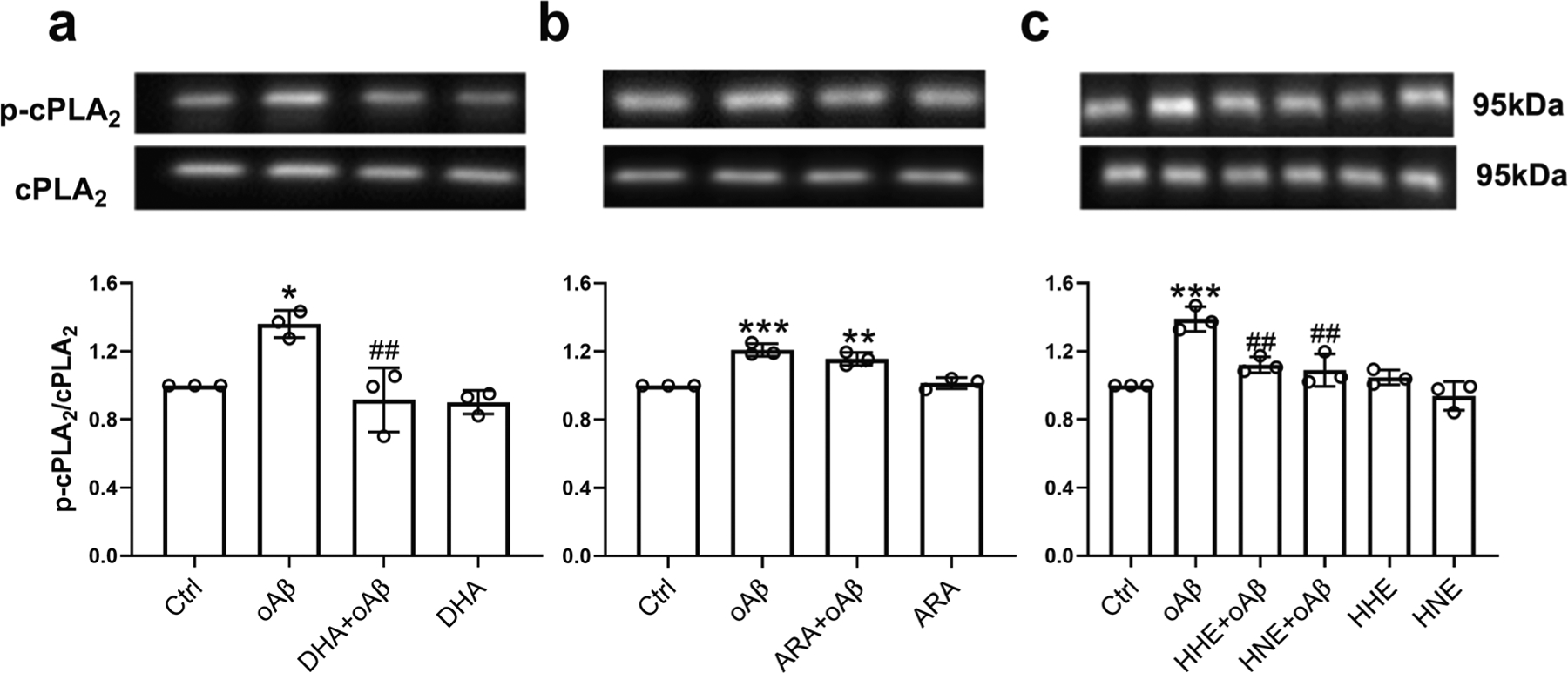
Effects of DHA, ARA, 4-HHE, and 4-HNE on oAβ-induced p-cPLA2 activation in BV2 cells. BV2 cells were pretreated with 50 μM DHA (a), 50 μM ARA (b), and 5 μM 4-HHE or 5 μM 4-HNE (c) for 1 h, followed by 2.5 μM oAβ treatment for 6 h. Data are represented as mean ± SD from three independent experiments (n = 3). (*p < 0.05, **p < 0.01, ***p < 0.001, compared with the control group; ##p < 0.01, compared with the oAβ treatment group.) From the left to the right bar (mean ± SD): a 1 ± 0, 1.36 ± 0.08, 0.92 ± 0.19, 0.9 ± 0.07; b 1 ± 0, 1.21 ± 0.04, 1.16 ± 0.04, 1.01 ± 0.03; c 1 ± 0, 1.39 ± 0.07, 1.12 ± 0.05, 1.09 ± 0.1, 1.05 ± 0.04, 0.94 ± 0.08
Effects of DHA, ARA, 4-HHE, and 4-HNE on oAβ-Induced iNOS and TNF-α
We also explored the effects of DHA, ARA, and their peroxidation products on oAβ-induced inflammatory responses in microglia. Results showed that oAβ-induced iNOS and TNF-α were suppressed by pretreatments with DHA (Fig. 4a, d), 4-HHE, and 4-HNE (Fig. 4c, f). However, pretreatment with ARA enhanced iNOS expression as compared with control but did not alter iNOS further with oAβ (Fig. 4b). Pretreatment with ARA did not alter the TNF-α level as compared with control, and oAβ together with ARA did not alter TNF-α as compared with oAβ alone (Fig. 4e). Interestingly, neither 4-HHE nor 4-HNE (at 5 μM) enhanced expression of iNOS or TNF-α as compared with control, but both 4-HHE and 4-HNE suppressed the increase in iNOS and TNF-α due to oAβ (Fig. 4c, f).
Fig. 4.
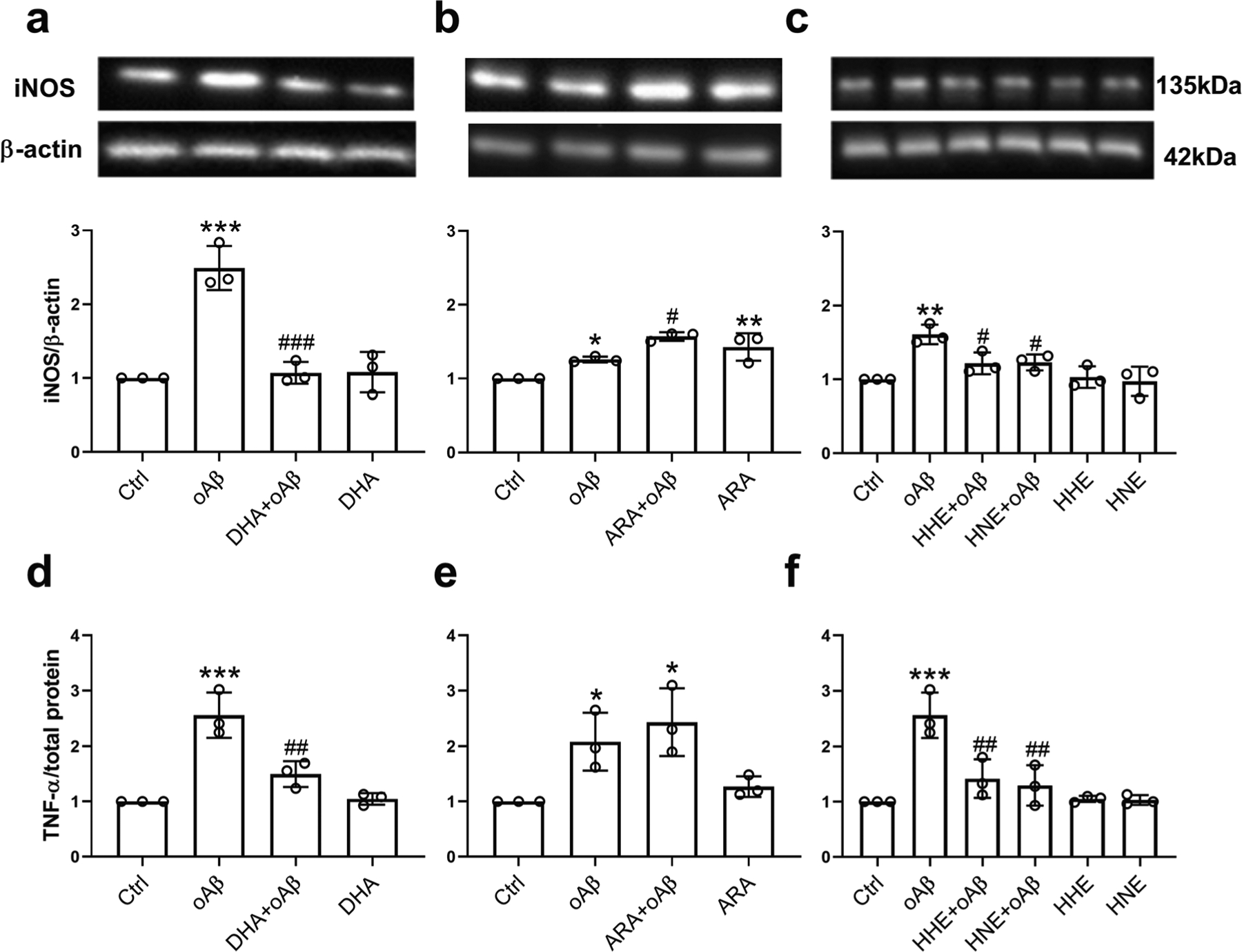
Effects of DHA, ARA, 4-HHE, and 4-HNE on oAβ-induced iNOS and TNF-α in BV2 cells. a–c For measuring iNOS expression, BV2 cells were pretreated with 50 μM DHA (a), 50 μM ARA (b), and 5 μM 4-HHE or 5 μM 4-HNE (c) for 1 h, followed by treatment with 2.5 μM oAβ for 6 h. d, e For measuring TNF-α expression, BV2 cells were pretreated with 10 μM DHA (d), 10 μM ARA (e), and 5 μM 4-HHE or 5 μM 4-HNE (f) for 1 h, followed by 1 μM oAβ treatment for 23 h. Data are represented as mean ± SD from three independent experiments (n = 3). (*p < 0.05, **p < 0.01, ***p < 0.001, compared with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001, compared with the oAβ treatment group.) From the left to the right bar (mean ± SD): a 1 ± 0, 2.49 ± 0.3, 1.07 ± 0.15, 1.08 ± 0.27; b 1 ± 0, 1.26 ± 0.04, 1.57 ± 0.06, 1.43 ± 0.19; c 1 ± 0, 1.61 ± 0.13, 1.22 ± 0.15, 1.23 ± 0.11, 1.03 ± 0.15, 0.97 ± 0.2; d 1 ± 0, 2.56 ± 0.41, 1.5 ± 0.23, 1.05 ± 0.11; e 1 ± 0, 2.08 ± 0.52, 2.44 ± 0.61, 1.27 ± 0.19; f 1 ± 0, 2.56 ± 0.41, 1.42 ± 0.35, 1.29 ± 0.37, 1.05 ± 0.06, 1.03 ± 0.09
DHA, ARA, 4-HHE, and 4-HNE Upregulated the Nrf2/HO-1 Antioxidant Pathway in oAβ-Stimulated Microglia
Our previous study demonstrated that exogenously added DHA, 4-HHE, and 4-HNE upregulated the antioxidant Nrf2/HO-1 pathway in microglia [41]. In this study, we examined whether DHA, ARA, 4-HHE, and 4-HNE also upregulated the Nrf2/HO-1 pathway in oAβ-stimulated microglia. Results showed that stimulation of microglia with oAβ alone did not impose any effect on Nrf2 and HO-1 expression levels (Fig. 5a–f), but both DHA and ARA (50 μM) upregulated Nrf2 and HO-1 regardless of the presence or absence of oAβ (Fig. 5a, b, d, e). Results also showed the ability for both 4-HHE and 4-HNE (5 μM) to upregulate Nrf2 and HO-1 regardless of the presence or absence of oAβ (Fig. 5c, f).
Fig. 5.
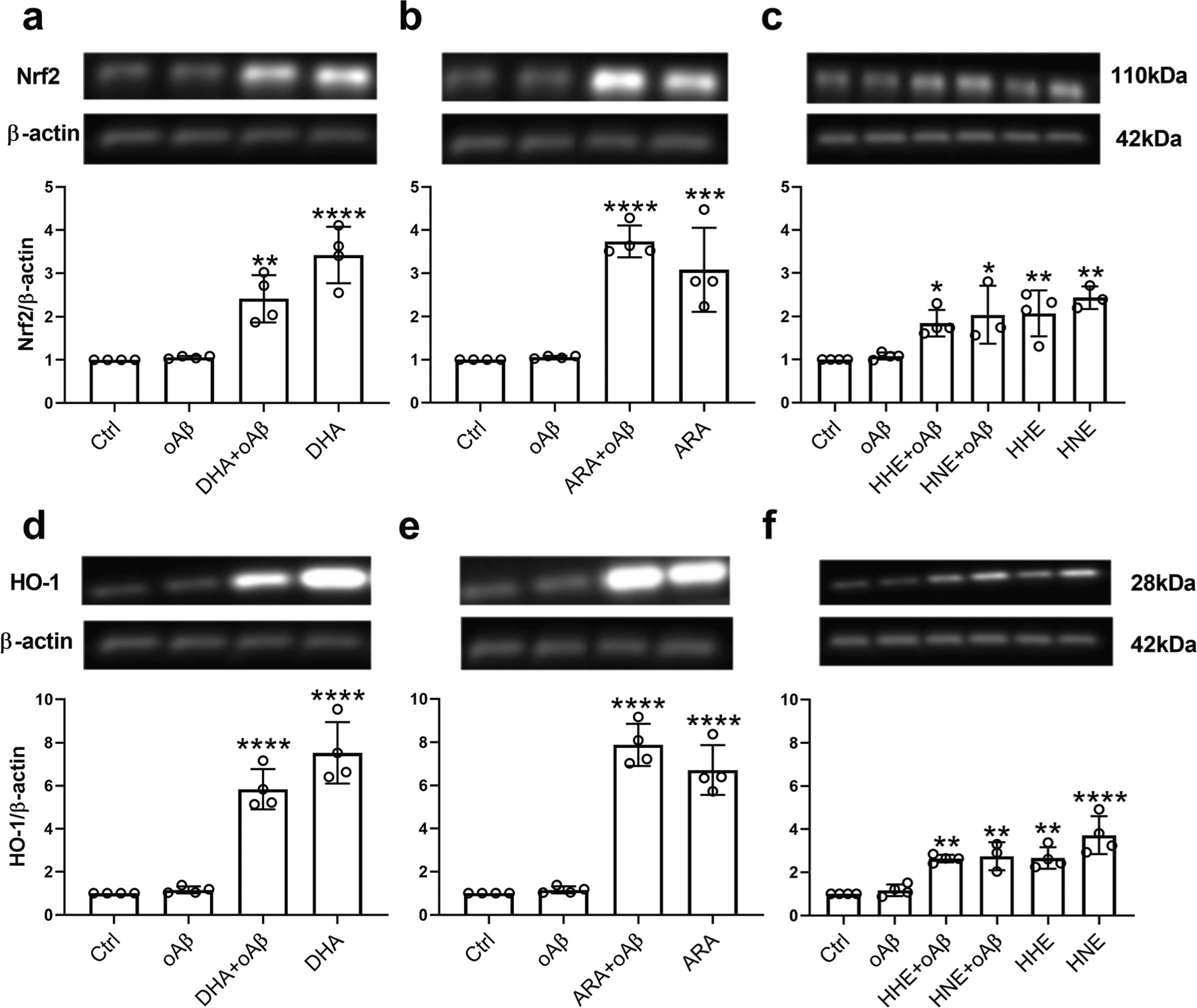
Effects of DHA, ARA, 4-HHE, and 4-HNE on oAβ-induced Nrf2 and HO-1 in BV2 cells. BV2 cells were pretreated with 50 μM DHA (a, d), 50 μM ARA (b, e), and 5 μM 4-HHE or 5 μM 4-HNE (c, f) for 1 h, followed by treatment with 2.5 μM oAβ for 6 h. Data are represented as mean ± SD from four independent experiments (n = 4). (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, compared with the control group.) From the left to the right bar (mean ± SD): a 1 ± 0, 1.06 ± 0.03, 2.41 ± 0.54, 3.42 ± 0.65; b 1 ± 0, 1.06 ± 0.03, 3.74 ± 0.37, 3.08 ± 0.97; c 1 ± 0, 1.08 ± 0.08, 1.84 ± 0.31, 2.04 ± 0.67, 2.07 ± 0.53, 2.43 ± 0.26; d 1 ± 0, 1.16 ± 0.16, 5.84 ± 0.94, 7.53 ± 1.43; e 1 ± 0, 1.16 ± 0.16, 7.88 ± 0.98, 6.72 ± 1.15; f 1 ± 0, 1.16 ± 0.27, 2.64 ± 0.17, 2.75 ± 0.65, 2.66 ± 0.5, 3.72 ± 0.88
DHA and ARA Imposed No Significant Effect on Calcium Influx in oAβ-Stimulated BV2 Cells
Since cPLA2 activity is calcium dependent, we examined the effects of DHA, ARA, and oAβ on calcium influx in BV2 cells; 2.5 μM oAβ increased calcium in cells by ~ 20%, but the increase was not statistically significant (Fig. 6). Pretreatment of cells with 50 μM DHA or ARA for 1 h did not impose any change in calcium in oAβ-stimulated cells (Fig. 6). Treating cells with 1 μM A23187, calcium ionophore, for 30 min increased calcium in cells by ~ 40%, as a positive control (Fig. 6). These results suggest that the changes in cPLA2 activation driven by DHA, ARA, and oAβ did not require changes in calcium influx.
Fig. 6.
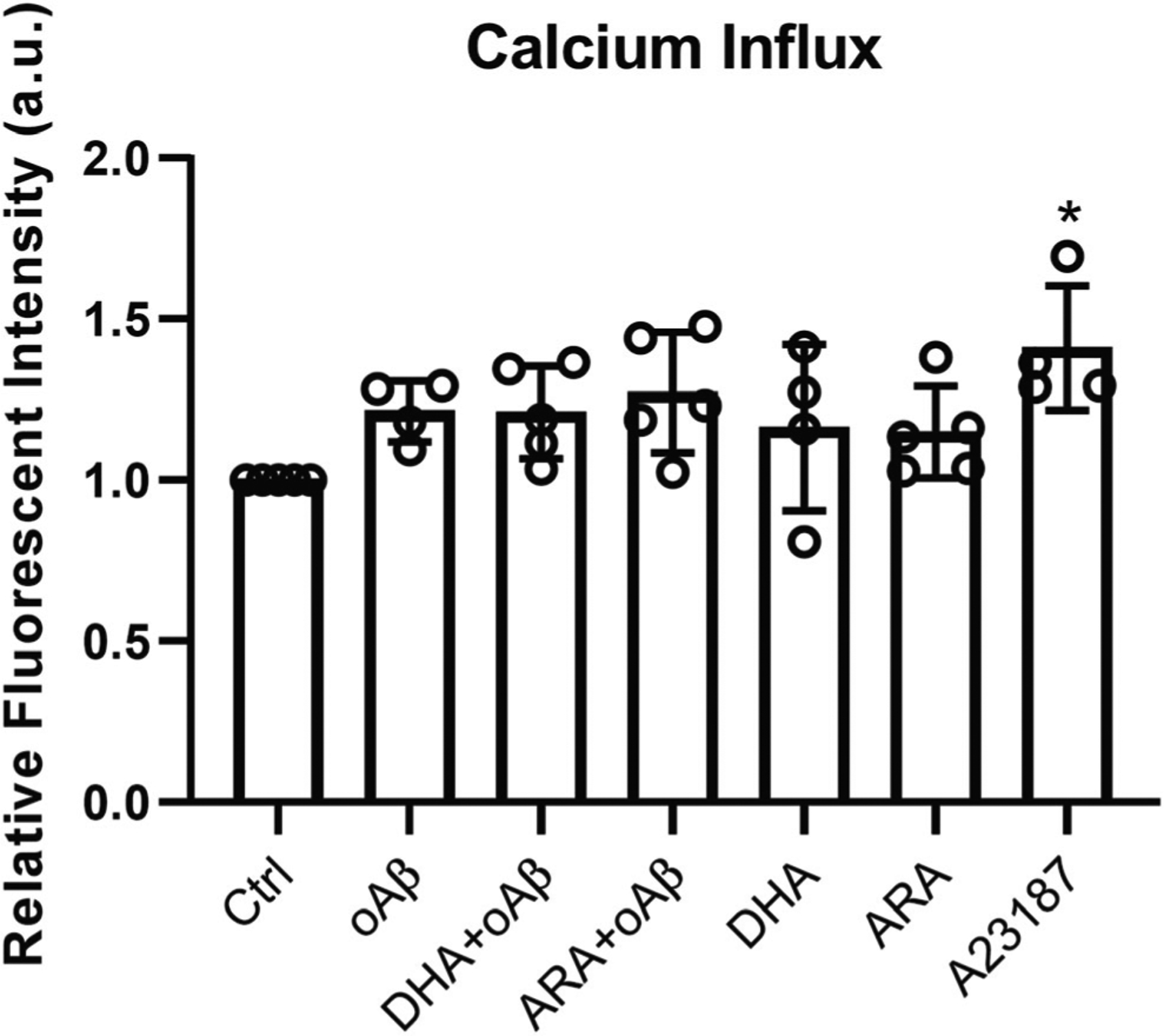
No significant effect of DHA and ARA on calcium influx in oAβ-stimulated BV2 cells. BV2 cells were incubated with 2 μM Fluo-4-AM for 30 min, followed by 50 μM DHA or ARA for 1 h, and were stimulated with 2.5 μM oAβ or 1 μM A23187 (positive control) for 30 min. Data are represented as mean ± SD from at least four independent experiments (n ≥ 4). (*p < 0.05, compared with the control group.) From the left to the right bar (mean ± SD): 1 ± 0, 1.21 ± 0.09, 1.21 ± 0.14, 1.27 ± 0.19, 1.16 ± 0.26, 1.15 ± 0.14, 1.41 ± 0.19
Discussion
There is growing evidence that DHA exerts neuroprotective effects on AD brains through multiple mechanisms, such as anti-inflammatory, anti-amyloid, anti-tau, enhanced non-amyloidogenesis activity, and preventing Aβ fibrillogenesis [5, 11, 12, 65–69]. In addition, dietary supplementation of DHA can directly impact microglial lipid content [70]. However, only few studies have addressed mechanism(s) whereby DHA exerts neuroprotective effects in AD, in particular, through its ability to modulate microglial activity. DHA has been found to enhance phagocytosis of Aβ and decrease inflammatory markers in human microglia [13]. DHA modulated microglial cell number and morphology in response to intracerebroventricular injection of Aβ40 in mice [71]. As a major source of oxidative stress and neuroinflammation, microglia can be activated by aggregated Aβ to produce superoxides through NADPH oxidase and trigger cPLA2 activity [72].
Although the mechanisms have yet to be fully elucidated, DHA has been reported to reduce oxidative stress through suppressing NADPH oxidase activity in various cell types, including endothelial cells, pancreatic islets, hepatocytes, keratinocytes, and monocytes/macrophages [73–78]. Consistent with our previous report on the ability of DHA to suppress LPS-induced ROS production in microglial BV2 cells [41], our study here showed that pretreatment with DHA suppressed oAβ-induced ROS production in both primary mouse microglia and microglial BV2 cells (Fig. 1a). It is important to note that dramatic downregulation of genes related to immune cell function and signaling as well as immune, blood vessel, and brain development have been observed at 6 h after plating primary microglia in cell culture [43]. Therefore, to minimize these dramatic alterations of gene expression levels and to maintain the expression levels of cells in the ex vivo condition, our experiments used primary mouse microglia immediately after cell isolation and purification. The freshly plated cells were pretreated with DHA or ARA for 2 h and then treatment with oAβ for 30 min prior to the addition of DCF for 1 h so that the total experimental time of cells in culture was between 3.5 and 6 h.
In this study, the effects of DHA on microglia were compared with ARA, another PUFA abundant in brain tissue. We found that pretreatment with ARA moderately enhanced oAβ-induced ROS production in both primary mouse microglia and BV2 cells (Fig. 1b). In fact, activation of cPLA2 is required for NADPH oxidase activity to produce ROS [64, 79], and NADPH oxidase activity can be restored by exogenous ARA in cPLA2-deficient human myeloid cells [79]. Our observations of ARA-enhanced ROS production induced by oAβ in microglia are consistent with previous findings regarding the role of ARA in NADPH oxidase activity.
In addition to the effects of DHA on NADPH oxidase activity, there is evidence that ω−3 fatty acids exert anti-inflammatory effects in various types of cells through stimulation of G protein–coupled receptor 120 (GPR120) [80–82]. ω−3 fatty acid–enriched diets have also been reported to activate GRP120-Nrf2 cross-talk to maintain balanced energy metabolism in mice overexpressing catalase [83]. Most recently, GPR120 was found to play a role in DHA-mediated inhibition of oxygen-glucose deprivation (OGD)–induced inflammation in primary microglia and BV2 cells [84]. In addition, inflammation is intimately related to peroxisome proliferator–activated receptors (PPARs) (see review from [85]). DHA has been reported as a ligand for PPARα, PPARβ/δ, and PPARγ [86–88] and inhibits advanced glycation end product (AGE)–induced inflammation in retinal microglia via suppression of the PPARγ/NF-κB pathway [89]. In turn, PPARγ regulates Nrf2 pathway and acts synergistically to suppress oxidative stress [90–93] and exert anti-inflammatory effects by inhibition of the NF-κB pathway [94, 95].
PUFAs are susceptible to free oxygen radical attacks and generate peroxidation products [1]. Our previous study demonstrated an increase in 4-HNE levels in LPS-stimulated microglial cells, an event known to link to cPLA2 activation and ARA production [41]. In the present study, an oAβ-induced increase in 4-HNE was similarly due to an increase in p-cPLA2 and ARA. Therefore, treatment of cells with DHA resulted in an increase in 4-HHE (Fig. 2a), whereas treating cells with ARA caused the increase in 4-HNE instead (Fig. 2e). These results suggest the endogenous and exogenous pools of free DHA and ARA are subject to peroxidation [23]. An interesting finding here is that DHA suppressed 4-HNE generation induced by oAβ (Fig. 2b), whereas ARA imposed no effect on the 4-HHE level (Fig. 2d). These findings are consistent with data in Fig. 1 demonstrating that DHA suppressed oAβ-induced ROS production and, in turn, suppressed downstream cellular processes, including cPLA2 activation (Fig. 3a) and ARA metabolism, thereby lowering the oAβ-elevated 4-HNE level (Fig. 2b). Since cPLA2 hydrolyses membrane phospholipids to produce lysophospholipids and ARA, the results showing that DHA suppressed oAβ-activated cPLA2 in Fig. 3a also help interpret recent in vivo studies that dietary DHA increased the level of DHA but decreased that of ARA in mouse brains [34, 62]. Interestingly, in the study with maternal DHA supplement, an increase in 4-HHE level was observed in the cerebral cortex and hippocampus but not in the cerebellum [62]. Despite of the increase in 4-HHE, animals supplemented with the DHA diet did not show changes in the 4-HNE levels in different brain regions [62]. These results are consistent with our in vitro study with BV2 cells that treatment with DHA increased 4-HHE levels but not the 4-HNE level (Fig. 2b).
Many neurologic dysfunctions including AD have demonstrated the increase in 4-HNE, in agreement with the increase in inflammatory cPLA2 and production of ARA [22]. Interestingly, a study by Bradley et al. [96] reported elevated levels of extractable and protein-bound HHE in multiple regions of AD brain. Recently, we have adopted a LC-MS/MS protocol to simultaneous determine levels of soluble 4-HHE and 4-HNE in cell and animal models [41, 62]. These studies indicated differences in metabolic pathways for production of 4-HHE and 4-HNE. In our previous study with BV2 microglial cells, exogenous 4-HHE and 4-HNE at 1–10 μM dose dependently suppressed LPS-induced inflammation and upregulated the antioxidant Nrf2/HO-1 [41]. While 5 μM of exogenous 4-HHE is reported to suppress Aβ-induced inflammation and upregulate the antioxidant Nrf2/HO-1 pathway in BV2 cells in this study, a dose greater than 25 μM significantly lowered the survival of rat cortical neurons and glucose uptake in primary cortical cultures [96], and 2.5 μM of 4-HHE impaired glutamate uptake in primary rat astrocytes [97].
Since DHA modulated the 4-HHE and 4-HNE levels in oAβ-stimulated microglia (Fig. 2), both 4-HHE and 4-HNE may also be involved in the effects of DHA on cell signaling. We found that not only DHA (Fig. 3a) but also both 4-HHE and 4-HNE suppressed oAβ-induced cPLA2 activation in BV2 cells (Fig. 3c). In addition, DHA, 4-HHE, and 4-HNE imposed anti-inflammatory activity to suppress oAβ-induced iNOS (Fig. 4a, c) and TNF-α (Fig. 4d, f) and enhanced the Nrf2/HO-1 pathway in both unstimulated and oAβ-stimulated microglial cells (Fig. 5a, c, d, f). However, pretreatment of DHA in a rat spinal cord injury (SCI) model has been found to activate pro-survival/anti-apoptotic pathways at least partly through AKt and cyclic AMP–responsive element binding protein (CREB) to protect NG2+, APC+, and NeuN+ cells, which may be independent of its anti-inflammatory effects on glial cells [98].
While exogenous ARA upregulated the Nrf2/HO-1 pathway (Fig. 5b, e), ARA did not suppress oAβ-induced ROS production (Fig. 1), cPLA2 activation (Fig. 3b), and iNOS (Fig. 4b) and TNF-α (Fig. 4e). In fact, the abilities of exogenous 4-HHE and 4-HNE to impose anti-oxidative and anti-inflammatory responses have been demonstrated in other cell types, including smooth muscle and endothelial cells [36, 39, 99]. In addition, the electrophilic properties of 4-hydroxyalkenals to upregulate Nrf2, resulting in increases in synthesis of HO-1 and other phase II enzymes, have contributed to the neuroprotective effects observed in DHA metabolism [30, 34, 100–103].
In this study, we demonstrate that exogenous 4-HNE upregulates the Nrf2/HO-1 antioxidant pathway. Study by Pizzimenti et al. [104] showed that 4-HNE forms adduct with HO-1 which results in the structural and functional impairment of HO-1. In turn, such modification of HO-1 by 4-HNE may impair HO-1/biliverdin reductase-A system, leading to increased oxidative stress and Tau hyper-phosphorylation in the brain [105–109]. In fact, the interactions of 4-HNE with various proteins to form 4-HNE-protein adducts have been found harmful in diseased brains and in body fluids of subjects affected by AD, Parkinson’s disease, Huntington disease, and amyotrophic lateral sclerosis and of animal models of these diseases (see review from [110]). Particularly in the case of AD, HNE-modified Aβ inhibits degradation of oxidized proteins by 20S proteasome [111]. HNE covalently modifies and induces cross-linking of neuronal cytoskeletal proteins [112] and upregulates BACE-1 expression and Aβ production in neurons [113].
In summary, this study demonstrated the effects of DHA on ROS production, cPLA2 activation, inflammatory responses, and the neuroprotective Nrf2/HO-1 pathway in oAβ-stimulated microglial cells, and the involvements of 4-HHE and 4-HNE in these effects, which should provide insights into the beneficial effects of DHA on AD.
Acknowledgments
We thank Dr. Brian Mooney, associate director of the Charles W. Gehrke Proteomics Center at the University of Missouri, for providing assistance with the LC-MS/MS.
Funding Information
This study is supported by a National Institutes of Health grant R01-AG044404 (to J. C. L.).
Footnotes
Conflict of Interest The authors declare that they have no competing interests.
References
- 1.Sun GY, Simonyi A, Fritsche KL, Chuang DY, Hannink M, Gu Z, Greenlief CM, Yao JK et al. (2018) Docosahexaenoic acid (DHA): an essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot Essent Fat Acids 136:3–13. 10.1016/j.plefa.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barberger-Gateau P, Raffaitin C, Letenneur L, Berr C, Tzourio C, Dartigues JF, Alperovitch A (2007) Dietary patterns and risk of dementia: the Three-City cohort study. Neurology 69(20):1921–1930. 10.1212/01.wnl.0000278116.37320.52 [DOI] [PubMed] [Google Scholar]
- 3.Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM (1997) Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol 42(5):776–782. 10.1002/ana.410420514 [DOI] [PubMed] [Google Scholar]
- 4.Laitinen MH, Ngandu T, Rovio S, Helkala EL, Uusitalo U, Viitanen M, Nissinen A, Tuomilehto J et al. (2006) Fat intake at midlife and risk of dementia and Alzheimer’s disease: a population-based study. Dement Geriatr Cogn Disord 22(1):99–107. 10.1159/000093478 [DOI] [PubMed] [Google Scholar]
- 5.Green KN, Martinez-Coria H, Khashwji H, Hall EB, Yurko-Mauro KA, Ellis L, LaFerla FM (2007) Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-beta and tau pathology via a mechanism involving presenilin 1 levels. J Neurosci Off J Soc Neurosci 27(16):4385–4395. 10.1523/JNEUROSCI.0055-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N Jr, Frautschy SA et al. (2005) A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci Off J Soc Neurosci 25(12):3032–3040. 10.1523/jneurosci.4225-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hur J, Mateo V, Amalric N, Babiak M, Bereziat G, Kanony-Truc C, Clerc T, Blaise R et al. (2018) Cerebrovascular beta-amyloid deposition and associated microhemorrhages in a Tg2576 Alzheimer mouse model are reduced with a DHA-enriched diet. FASEB J: official publication of the Federation of American Societies for Experimental Biology 32(9):4972–4983. fj201800200R. 10.1096/fj.201800200R [DOI] [PubMed] [Google Scholar]
- 8.Pan Y, Choy KHC, Marriott PJ, Chai SY, Scanlon MJ, Porter CJH, Short JL, Nicolazzo JA (2018) Reduced blood-brain barrier expression of fatty acid-binding protein 5 is associated with increased vulnerability of APP/PS1 mice to cognitive deficits from low omega-3 fatty acid diets. J Neurochem 144(1):81–92. 10.1111/jnc.14249 [DOI] [PubMed] [Google Scholar]
- 9.Pan Y, Scanlon MJ, Owada Y, Yamamoto Y, Porter CJ, Nicolazzo JA (2015) Fatty acid-binding protein 5 facilitates the blood-brain barrier transport of docosahexaenoic acid. Mol Pharm 12(12): 4375–4385. 10.1021/acs.molpharmaceut.5b00580 [DOI] [PubMed] [Google Scholar]
- 10.Grimm MO, Haupenthal VJ, Mett J, Stahlmann CP, Blumel T, Mylonas NT, Endres K, Grimm HS et al. (2016) Oxidized docosahexaenoic acid species and lipid peroxidation products increase amyloidogenic amyloid precursor protein processing. Neurodegener Dis 16(1–2):44–54. 10.1159/000440839 [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Sheng W, Sun GY, Lee JCM (2011) Effects of fatty acid unsaturation numbers on membrane fluidity and α-secretase-dependent amyloid precursor protein processing. Neurochem Int 58(3):321–329. 10.1016/j.neuint.2010.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Sun GY, Eckert GP, Lee JC (2014) Cellular membrane fluidity in amyloid precursor protein processing. Mol Neurobiol 50(1):119–129. 10.1007/s12035-014-8652-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hjorth E, Zhu M, Toro VC, Vedin I, Palmblad J, Cederholm T, Freund-Levi Y, Faxen-Irving G et al. (2013) Omega-3 fatty acids enhance phagocytosis of Alzheimer’s disease-related amyloid-beta42 by human microglia and decrease inflammatory markers. J Alzheimer’s Disease: JAD 35(4):697–713. 10.3233/jad-130131 [DOI] [PubMed] [Google Scholar]
- 14.Strokin M, Sergeeva M, Reiser G (2007) Prostaglandin synthesis in rat brain astrocytes is under the control of the n-3 docosahexaenoic acid, released by group VIB calcium-independent phospholipase A2. J Neurochem 102(6):1771–1782. 10.1111/j.1471-4159.2007.04663.x [DOI] [PubMed] [Google Scholar]
- 15.Ong WY, Yeo JF, Ling SF, Farooqui AA (2005) Distribution of calcium-independent phospholipase A2 (iPLA2) in monkey brain. J Neurocytol 34(6):447–458. 10.1007/s11068-006-8730-4 [DOI] [PubMed] [Google Scholar]
- 16.Green JT, Orr SK, Bazinet RP (2008) The emerging role of group VI calcium-independent phospholipase A2 in releasing docosahexaenoic acid from brain phospholipids. J Lipid Res 49(5):939–944. 10.1194/jlr.R700017-JLR200 [DOI] [PubMed] [Google Scholar]
- 17.Ramanadham S, Ali T, Ashley JW, Bone RN, Hancock WD, Lei X (2015) Calcium-independent phospholipases A2 and their roles in biological processes and diseases. J Lipid Res 56(9):1643–1668. 10.1194/jlr.R058701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun GY, Chuang DY, Zong Y, Jiang J, Lee JC, Gu Z, Simonyi A (2014) Role of cytosolic phospholipase A2 in oxidative and inflammatory signaling pathways in different cell types in the central nervous system. Mol Neurobiol 50(1):6–14. 10.1007/s12035-014-8662-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calder PC (2008) The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fat Acids 79(3–5):101–108. 10.1016/j.plefa.2008.09.016 [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG (2004) Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A 101(22):8491–8496. 10.1073/pnas.0402531101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serhan CN (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510(7503):92–101. 10.1038/nature13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang B, Fritsche KL, Beversdorf DQ, Gu Z, Lee JC, Folk WR, Greenlief CM, Sun GY (2019) Yin-yang mechanisms regulating lipid peroxidation of docosahexaenoic acid and arachidonic acid in the central nervous system. Front Neurol 10:642. 10.3389/fneur.2019.00642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephenson DT, Lemere CA, Selkoe DJ, Clemens JA (1996) Cytosolic phospholipase A2 (cPLA2) immunoreactivity is elevated in Alzheimer’s disease brain. Neurobiol Dis 3(1):51–63. 10.1006/nbdi.1996.0005 [DOI] [PubMed] [Google Scholar]
- 24.Lovell MA, Ehmann WD, Mattson MP, Markesbery WR (1997) Elevated 4-hydroxynonenal in ventricular fluid in Alzheimer’s disease. Neurobiol Aging 18(5):457–461 [DOI] [PubMed] [Google Scholar]
- 25.Markesbery WR, Lovell MA (1998) Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiol Aging 19(1):33–36 [DOI] [PubMed] [Google Scholar]
- 26.McGrath LT, McGleenon BM, Brennan S, McColl D, Mc IS, Passmore AP (2001) Increased oxidative stress in Alzheimer’s disease as assessed with 4-hydroxynonenal but not malondialdehyde. QJM 94(9):485–490 [DOI] [PubMed] [Google Scholar]
- 27.Montine KS, Olson SJ, Amarnath V, Whetsell WO Jr, Graham DG, Montine TJ (1997) Immunohistochemical detection of 4-hydroxy-2-nonenal adducts in Alzheimer’s disease is associated with inheritance of APOE4. Am J Pathol 150(2):437–443 [PMC free article] [PubMed] [Google Scholar]
- 28.Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA (1997) 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J Neurochem 68(5):2092–2097 [DOI] [PubMed] [Google Scholar]
- 29.Ayala A, Munoz MF, Arguelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med Cell Longev 2014:360438. 10.1155/2014/360438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long EK, Picklo MJ Sr (2010) Trans-4-hydroxy-2-hexenal, a product of n-3 fatty acid peroxidation: make some room HNE. Free Radic Biol Med 49(1):1–8. 10.1016/j.freeradbiomed.2010.03.015 [DOI] [PubMed] [Google Scholar]
- 31.Cherkas A, Zarkovic N (2018) 4-Hydroxynonenal in redox ho-meostasis of gastrointestinal mucosa: implications for the stomach in health and diseases. Antioxidants (Basel) 7(9):E118. 10.3390/antiox7090118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siegel SJ, Bieschke J, Powers ET, Kelly JW (2007) The oxidative stress metabolite 4-hydroxynonenal promotes Alzheimer protofibril formation. Biochemistry 46(6):1503–1510. 10.1021/bi061853s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng R, Heck DE, Mishin V, Black AT, Shakarjian MP, Kong AN, Laskin DL, Laskin JD (2014) Modulation of keratinocyte expression of antioxidants by 4-hydroxynonenal, a lipid peroxidation end product. Toxicol Appl Pharmacol 275(2):113–121. 10.1016/j.taap.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakagawa F, Morino K, Ugi S, Ishikado A, Kondo K, Sato D, Konno S, Nemoto K et al. (2014) 4-Hydroxy hexenal derived from dietary n-3 polyunsaturated fatty acids induces anti-oxidative enzyme heme oxygenase-1 in multiple organs. Biochem Biophys Res Commun 443(3):991–996. 10.1016/j.bbrc.2013.12.085 [DOI] [PubMed] [Google Scholar]
- 35.Lin MH, Yen JH, Weng CY, Wang L, Ha CL, Wu MJ (2014) Lipid peroxidation end product 4-hydroxy-trans-2-nonenal triggers un-folded protein response and heme oxygenase-1 expression in PC12 cells: roles of ROS and MAPK pathways. Toxicology 315:24–37. 10.1016/j.tox.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 36.Ishikado A, Nishio Y, Morino K, Ugi S, Kondo H, Makino T, Kashiwagi A, Maegawa H (2010) Low concentration of 4-hydroxy hexenal increases heme oxygenase-1 expression through activation of Nrf2 and antioxidative activity in vascular endothelial cells. Biochem Biophys Res Commun 402(1):99–104. 10.1016/j.bbrc.2010.09.124 [DOI] [PubMed] [Google Scholar]
- 37.Siow RC, Ishii T, Mann GE (2007) Modulation of antioxidant gene expression by 4-hydroxynonenal: atheroprotective role of the Nrf2/ARE transcription pathway. Redox Rep: Communications in Free Radical Research 12(1):11–15. [DOI] [PubMed] [Google Scholar]
- 38.Zhang M, Wang S, Mao L, Leak RK, Shi Y, Zhang W, Hu X, Sun B et al. (2014) Omega-3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1. J Neurosci Off J Soc Neurosci 34(5):1903–1915. 10.1523/jneurosci.4043-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishikado A, Morino K, Nishio Y, Nakagawa F, Mukose A, Sono Y, Yoshioka N, Kondo K et al. (2013) 4-Hydroxy hexenal derived from docosahexaenoic acid protects endothelial cells via Nrf2 activation. PLoS One 8(7):e69415. 10.1371/journal.pone.0069415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen G, Riahi Y, Sunda V, Deplano S, Chatgilialoglu C, Ferreri C, Kaiser N, Sasson S (2013) Signaling properties of 4-hydroxyalkenals formed by lipid peroxidation in diabetes. Free Radic Biol Med 65:978–987. 10.1016/j.freeradbiomed.2013.08.163 [DOI] [PubMed] [Google Scholar]
- 41.Yang B, Li R, Michael Greenlief C, Fritsche KL, Gu Z, Cui J, Lee JC, Beversdorf DQ et al. (2018) Unveiling anti-oxidative and anti-inflammatory effects of docosahexaenoic acid and its lipid peroxidation product on lipopolysaccharide-stimulated BV-2 microglial cells. J Neuroinflammation 15(1):202. 10.1186/s12974-018-1232-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gehrmann J, Matsumoto Y, Kreutzberg GW (1995) Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev 20(3):269–287 [DOI] [PubMed] [Google Scholar]
- 43.Gosselin D, Skola D, Coufal NG, Holtman IR, Schlachetzki JCM, Sajti E, Jaeger BN, O’Connor C et al. (2017) An environment-dependent transcriptional network specifies human microglia identity. Science (New York, NY) 356(6344):eaal3222. 10.1126/science.aal3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zuroff L, Daley D, Black KL, Koronyo-Hamaoui M (2017) Clearance of cerebral Abeta in Alzheimer’s disease: reassessing the role of microglia and monocytes. Cell Mol Life Sci. 10.1007/s00018-017-2463-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frenkel D, Wilkinson K, Zhao L, Hickman SE, Means TK, Puckett L, Farfara D, Kingery ND et al. (2013) Scara1 deficiency impairs clearance of soluble amyloid-beta by mononuclear phago-cytes and accelerates Alzheimer’s-like disease progression. Nat Commun 4:2030. 10.1038/ncomms3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C et al. (2013) TREM2 variants in Alzheimer’s disease. N Engl J Med 368(2):117–127. 10.1056/NEJMoa1211851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, Abraham R, Hamshere ML et al. (2011) Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet 43(5):429–435. 10.1038/ng.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J et al. (2013) Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med 368(2):107–116. 10.1056/NEJMoa1211103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koenigsknecht J, Landreth G (2004) Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism. J Neurosci Off J Soc Neurosci 24(44):9838–9846. 10.1523/JNEUROSCI.2557-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, Gallins PJ, Buxbaum JD et al. (2011) Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet 43(5):436–441. 10.1038/ng.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE (2009) CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci Off J Soc Neurosci 29(38):11982–11992. 10.1523/JNEUROSCI.3158-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Udan ML, Ajit D, Crouse NR, Nichols MR (2008) Toll-like receptors 2 and 4 mediate Abeta(1–42) activation of the innate immune response in a human monocytic cell line. J Neurochem 104(2):524–533. 10.1111/j.1471-4159.2007.05001.x [DOI] [PubMed] [Google Scholar]
- 53.Ulland TK, Song WM, Huang SC, Ulrich JD, Sergushichev A, Beatty WL, Loboda AA, Zhou Y et al. (2017) TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell 170(4): 649–663.e613. 10.1016/j.cell.2017.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilkinson K, El Khoury J (2012) Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer’s disease. Int J Alzheimers Dis 2012:489456. 10.1155/2012/489456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang CN, Shiao YJ, Shie FS, Guo BS, Chen PH, Cho CY, Chen YJ, Huang FL et al. (2011) Mechanism mediating oligomeric Abeta clearance by naive primary microglia. Neurobiol Dis 42(3):221–230. 10.1016/j.nbd.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 56.Yeh FL, Wang Y, Tom I, Gonzalez LC, Sheng M (2016) TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron 91(2):328–340. 10.1016/j.neuron.2016.06.015 [DOI] [PubMed] [Google Scholar]
- 57.Yu Y, Ye RD (2015) Microglial Abeta receptors in Alzheimer’s disease. Cell Mol Neurobiol 35(1):71–83. 10.1007/s10571-014-0101-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teng T, Dong L, Ridgley DM, Ghura S, Tobin MK, Sun GY, LaDu MJ, Lee JC (2018) Cytosolic phospholipase A2 facilitates oligomeric amyloid-β peptide association with microglia via regulation of membrane-cytoskeleton connectivity. Mol Neurobiol 56:3222–3234. 10.1007/s12035-018-1304-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilkinson BL, Landreth GE (2006) The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer’s disease. J Neuroinflammation 3:30. 10.1186/1742-2094-3-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meda L, Cassatella MA, Szendrei GI, Otvos L Jr, Baron P, Villalba M, Ferrari D, Rossi F (1995) Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature 374(6523): 647–650. 10.1038/374647a0 [DOI] [PubMed] [Google Scholar]
- 61.Chuang DY, Simonyi A, Kotzbauer PT, Gu Z, Sun GY (2015) Cytosolic phospholipase A2 plays a crucial role in ROS/NO signaling during microglial activation through the lipoxygenase pathway. J Neuroinflammation 12:199. 10.1186/s12974-015-0419-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang B, Li R, Woo T, Browning JD Jr, Song H, Gu Z, Cui J, Lee JC et al. (2019) Maternal dietary docosahexaenoic acid alters lipid peroxidation products and (n-3)/(n-6) fatty acid balance in offspring mice. Metabolites 9(3):40. 10.3390/metabo9030040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun GY, Li R, Yang B, Fritsche KL, Beversdorf DQ, Lubahn DB, Geng X, Lee JC et al. (2019) Quercetin potentiates docosahexaenoic acid to suppress lipopolysaccharide-induced oxidative/inflammatory responses, alter lipid peroxidation products, and enhance the adaptive stress pathways in BV-2 microglial cells. Int J Mol Sci 20(4):E932. 10.3390/ijms20040932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szaingurten-Solodkin I, Hadad N, Levy R (2009) Regulatory role of cytosolic phospholipase A2alpha in NADPH oxidase activity and in inducible nitric oxide synthase induction by aggregated Abeta1–42 in microglia. Glia 57(16):1727–1740. 10.1002/glia.20886 [DOI] [PubMed] [Google Scholar]
- 65.Amen DG, Harris WS, Kidd PM, Meysami S, Raji CA (2017) Quantitative erythrocyte omega-3 EPA plus DHA levels are related to higher regional cerebral blood flow on brain SPECT. J Alzheimer’s Disease: JAD 58(4):1189–1199. 10.3233/JAD-170281 [DOI] [PubMed] [Google Scholar]
- 66.El Shatshat A, Pham AT, Rao PPN (2019) Interactions of polyunsaturated fatty acids with amyloid peptides Abeta40 and Abeta42. Arch Biochem Biophys 663:34–43. 10.1016/j.abb.2018.12.027 [DOI] [PubMed] [Google Scholar]
- 67.Heras-Sandoval D, Pedraza-Chaverri J, Perez-Rojas JM (2016) Role of docosahexaenoic acid in the modulation of glial cells in Alzheimer’s disease. J Neuroinflammation 13(1):61. 10.1186/s12974-016-0525-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang TL (2010) Omega-3 fatty acids, cognitive decline, and Alzheimer’s disease: a critical review and evaluation of the literature. J Alzheimer’s Disease: JAD 21(3):673–690. 10.3233/JAD-2010-090934 [DOI] [PubMed] [Google Scholar]
- 69.Pan Y, Khalil H, Nicolazzo JA (2015) The impact of docosahexaenoic acid on Alzheimer’s disease: is there a role of the blood-brain barrier? Curr Clin Pharmacol 10(3):222–241 [DOI] [PubMed] [Google Scholar]
- 70.Rey C, Nadjar A, Joffre F, Amadieu C, Aubert A, Vaysse C, Pallet V, Layé S et al. (2018) Maternal n-3 polyunsaturated fatty acid dietary supply modulates microglia lipid content in the offspring. Prostaglandins Leukot Essent Fat Acids 133:1–7. 10.1016/j.plefa.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 71.Hopperton KE, Trepanier MO, Giuliano V, Bazinet RP (2016) Brain omega-3 polyunsaturated fatty acids modulate microglia cell number and morphology in response to intracerebroventricular amyloid-beta 1–40 in mice. J Neuroinflammation 13(1):257. 10.1186/s12974-016-0721-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abdullah L, Evans JE, Emmerich T, Crynen G, Shackleton B, Keegan AP, Luis C, Tai L et al. (2017) APOE epsilon4 specific imbalance of arachidonic acid and docosahexaenoic acid in serum phospholipids identifies individuals with preclinical mild cognitive impairment/Alzheimer’s disease. Aging 9(3):964–985. 10.18632/aging.101203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Niazi ZR, Silva GC, Ribeiro TP, Leon-Gonzalez AJ, Kassem M, Mirajkar A, Alvi A, Abbas M et al. (2017) EPA:DHA 6:1 prevents angiotensin II-induced hypertension and endothelial dysfunction in rats: role of NADPH oxidase- and COX-derived oxidative stress. Hyper Res: official journal of the Japanese Society of Hypertension 40(12):966–975. 10.1038/hr.2017.72 [DOI] [PubMed] [Google Scholar]
- 74.Lucena CF, Roma LP, Graciano MF, Veras K, Simoes D, Curi R, Carpinelli AR (2015) Omega-3 supplementation improves pancreatic islet redox status: in vivo and in vitro studies. Pancreas 44(2):287–295. 10.1097/mpa.0000000000000249 [DOI] [PubMed] [Google Scholar]
- 75.Wales KM, Kavazos K, Nataatmadja M, Brooks PR, Williams C, Russell FD (2014) N-3 PUFAs protect against aortic inflammation and oxidative stress in angiotensin II-infused apolipoprotein E−/−mice. PLoS One 9(11):e112816. 10.1371/journal.pone.0112816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamagata K, Tusruta C, Ohtuski A, Tagami M (2014) Docosahexaenoic acid decreases TNF-alpha-induced lectin-like oxidized low-density lipoprotein receptor-1 expression in THP-1 cells. Prostaglandins Leukot Essent Fat Acids 90(4):125–132. 10.1016/j.plefa.2013.12.011 [DOI] [PubMed] [Google Scholar]
- 77.Depner CM, Philbrick KA, Jump DB (2013) Docosahexaenoic acid attenuates hepatic inflammation, oxidative stress, and fibrosis without decreasing hepatosteatosis in a Ldlr(−/−) mouse model of Western diet-induced nonalcoholic steatohepatitis. J Nutr 143(3): 315–323. 10.3945/jn.112.171322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rahman M, Kundu JK, Shin JW, Na HK, Surh YJ (2011) Docosahexaenoic acid inhibits UVB-induced activation of NF-kappaB and expression of COX-2 and NOX-4 in HR-1 hairless mouse skin by blocking MSK1 signaling. PLoS One 6(11): e28065. 10.1371/journal.pone.0028065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Levy R, Lowenthal A, Dana R (2000) Cytosolic phospholipase A2 is required for the activation of the NADPH oxidase associated H+ channel in phagocyte-like cells. Adv Exp Med Biol 479:125–135. 10.1007/0-306-46831-x_11 [DOI] [PubMed] [Google Scholar]
- 80.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ et al. (2010) GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142(5):687–698. 10.1016/j.cell.2010.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raptis DA, Limani P, Jang JH, Ungethum U, Tschuor C, Graf R, Humar B, Clavien PA (2014) GPR120 on Kupffer cells mediates hepatoprotective effects of omega3-fatty acids. J Hepatol 60(3): 625–632. 10.1016/j.jhep.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 82.Wellhauser L, Belsham DD (2014) Activation of the omega-3 fatty acid receptor GPR120 mediates anti-inflammatory actions in immortalized hypothalamic neurons. J Neuroinflammation 11:60. 10.1186/1742-2094-11-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amos D, Cook C, Santanam N (2019) Omega 3 rich diet modulates energy metabolism via GPR120-Nrf2 crosstalk in a novel antioxidant mouse model. Biochim Biophys Acta Mol Cell Biol Lipids 1864(4):466–488. 10.1016/j.bbalip.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ren Z, Chen L, Wang Y, Wei X, Zeng S, Zheng Y, Gao C, Liu H (2019) Activation of the omega-3 fatty acid receptor GPR120 protects against focal cerebral ischemic injury by preventing inflammation and apoptosis in mice. J Immunol 202(3):747–759. 10.4049/jimmunol.1800637 [DOI] [PubMed] [Google Scholar]
- 85.Korbecki J, Bobinski R, Dutka M (2019) Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflamm Res 68(6):443–458. 10.1007/s00011-019-01231-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Forman BM, Chen J, Evans RM (1997) Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A 94(9):4312–4317. 10.1073/pnas.94.9.4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krey G, Braissant O, L’Horset F, Kalkhoven E, Perroud M, Parker MG, Wahli W (1997) Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol 11(6):779–791. 10.1210/mend.11.6.0007 [DOI] [PubMed] [Google Scholar]
- 88.Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM et al. (1999) Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell 3(3):397–403 [DOI] [PubMed] [Google Scholar]
- 89.Wang L, Chen K, Liu K, Zhou Y, Zhang T, Wang B, Mi M (2015) DHA inhibited AGEs-induced retinal microglia activation via suppression of the PPARgamma/NFkappaB pathway and reduction of signal transducers in the AGEs/RAGE axis recruitment into lipid rafts. Neurochem Res 40(4):713–722. 10.1007/s11064-015-1517-1 [DOI] [PubMed] [Google Scholar]
- 90.Cho HY, Gladwell W, Wang X, Chorley B, Bell D, Reddy SP, Kleeberger SR (2010) Nrf2-regulated PPAR{gamma} expression is critical to protection against acute lung injury in mice. Am J Respir Crit Care Med 182(2):170–182. 10.1164/rccm.200907-1047OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ikeda Y, Sugawara A, Taniyama Y, Uruno A, Igarashi K, Arima S, Ito S, Takeuchi K (2000) Suppression of rat thromboxane synthase gene transcription by peroxisome proliferator-activated receptor gamma in macrophages via an interaction with NRF2. J Biol Chem 275(42):33142–33150. 10.1074/jbc.M002319200 [DOI] [PubMed] [Google Scholar]
- 92.Polvani S, Tarocchi M, Galli A (2012) PPARgamma and oxidative stress: con(beta) catenating NRF2 and FOXO. PPAR Res 2012: 641087. 10.1155/2012/641087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shih AY, Imbeault S, Barakauskas V, Erb H, Jiang L, Li P, Murphy TH (2005) Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. J Biol Chem 280(24):22925–22936. 10.1074/jbc.M414635200 [DOI] [PubMed] [Google Scholar]
- 94.Wardyn JD, Ponsford AH, Sanderson CM (2015) Dissecting molecular cross-talk between Nrf2 and NF-kappaB response pathways. Biochem Soc Trans 43(4):621–626. 10.1042/BST20150014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao XR, Gonzales N, Aronowski J (2015) Pleiotropic role of PPARgamma in intracerebral hemorrhage: an intricate system involving Nrf2, RXR, and NF-kappaB. CNS Neurosci Ther 21(4): 357–366. 10.1111/cns.12350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bradley MA, Xiong-Fister S, Markesbery WR, Lovell MA (2012) Elevated 4-hydroxyhexenal in Alzheimer’s disease (AD) progression. Neurobiol Aging 33(6):1034–1044. 10.1016/j.neurobiolaging.2010.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lovell MA, Bradley MA, Fister SX (2012) 4-Hydroxyhexenal (HHE) impairs glutamate transport in astrocyte cultures. J Alzheimer’s Disease: JAD 32(1):139–146. 10.3233/JAD-2012-120409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Figueroa JD, Cordero K, Baldeosingh K, Torrado AI, Walker RL, Miranda JD, Leon MD (2012) Docosahexaenoic acid pretreatment confers protection and functional improvements after acute spinal cord injury in adult rats. J Neurotrauma 29(3):551–566. 10.1089/neu.2011.2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang YC, Lii CK, Wei YL, Li CC, Lu CY, Liu KL, Chen HW (2013) Docosahexaenoic acid inhibition of inflammation is partially via cross-talk between Nrf2/heme oxygenase 1 and IKK/NF-kappaB pathways. J Nutr Biochem 24(1):204–212. 10.1016/j.jnutbio.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 100.Huang Y, Li W, Kong AN (2012) Anti-oxidative stress regulator NF-E2-related factor 2 mediates the adaptive induction of antioxidant and detoxifying enzymes by lipid peroxidation metabolite 4-hydroxynonenal. Cell Biosci 2(1):40. 10.1186/2045-3701-2-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen ZH, Saito Y, Yoshida Y, Sekine A, Noguchi N, Niki E (2005) 4-Hydroxynonenal induces adaptive response and enhances PC12 cell tolerance primarily through induction of thioredoxin reductase 1 via activation of Nrf2. J Biol Chem 280(51):41921–41927. 10.1074/jbc.M508556200 [DOI] [PubMed] [Google Scholar]
- 102.Maulucci G, Daniel B, Cohen O, Avrahami Y, Sasson S (2016) Hormetic and regulatory effects of lipid peroxidation mediators in pancreatic beta cells. Mol Asp Med 49:49–77. 10.1016/j.mam.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 103.Jazwa A, Cuadrado A (2010) Targeting heme oxygenase-1 for neuroprotection and neuroinflammation in neurodegenerative diseases. Curr Drug Targets 11(12):1517–1531 [DOI] [PubMed] [Google Scholar]
- 104.Pizzimenti S, Ciamporcero E, Daga M, Pettazzoni P, Arcaro A, Cetrangolo G, Minelli R, Dianzani C et al. (2013) Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front Physiol 4:242. 10.3389/fphys.2013.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barone E, Di Domenico F, Cassano T, Arena A, Tramutola A, Lavecchia MA, Coccia R, Butterfield DA et al. (2016) Impairment of biliverdin reductase-A promotes brain insulin resistance in Alzheimer disease: a new paradigm. Free Radic Biol Med 91:127–142. 10.1016/j.freeradbiomed.2015.12.012 [DOI] [PubMed] [Google Scholar]
- 106.Barone E, Di Domenico F, Cenini G, Sultana R, Cini C, Preziosi P, Perluigi M, Mancuso C et al. (2011) Biliverdin reductase—a protein levels and activity in the brains of subjects with Alzheimer disease and mild cognitive impairment. Biochim Biophys Acta 1812(4):480–487. 10.1016/j.bbadis.2011.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barone E, Di Domenico F, Cenini G, Sultana R, Coccia R, Preziosi P, Perluigi M, Mancuso C et al. (2011) Oxidative and nitrosative modifications of biliverdin reductase-A in the brain of subjects with Alzheimer’s disease and amnestic mild cognitive impairment. J Alzheimer’s Disease: JAD 25(4):623–633. 10.3233/JAD-2011-110092 [DOI] [PubMed] [Google Scholar]
- 108.Barone E, Di Domenico F, Sultana R, Coccia R, Mancuso C, Perluigi M, Butterfield DA (2012) Heme oxygenase-1 posttranslational modifications in the brain of subjects with Alzheimer disease and mild cognitive impairment. Free Radic Biol Med 52(11–12):2292–2301. 10.1016/j.freeradbiomed.2012.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sharma N, Tramutola A, Lanzillotta C, Arena A, Blarzino C, Cassano T, Butterfield DA, Di Domenico F et al. (2019) Loss of biliverdin reductase-A favors Tau hyper-phosphorylation in Alzheimer’s disease. Neurobiol Dis 125:176–189. 10.1016/j.nbd.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 110.Di Domenico F, Tramutola A, Butterfield DA (2017) Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of Alzheimer disease and other selected age-related neurodegenerative disorders. Free Radic Biol Med 111:253–261. 10.1016/j.freeradbiomed.2016.10.490 [DOI] [PubMed] [Google Scholar]
- 111.Shringarpure R, Grune T, Sitte N, Davies KJ (2000) 4-Hydroxynonenal-modified amyloid-beta peptide inhibits the proteasome: possible importance in Alzheimer’s disease. Cell Mol Life Sci 57(12):1802–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Montine KS, Kim PJ, Olson SJ, Markesbery WR, Montine TJ (1997) 4-hydroxy-2-nonenal pyrrole adducts in human neurodegenerative disease. J Neuropathol Exp Neurol 56(8):866–871. 10.1097/00005072-199708000-00004 [DOI] [PubMed] [Google Scholar]
- 113.Tamagno E, Parola M, Bardini P, Piccini A, Borghi R, Guglielmotto M, Santoro G, Davit A et al. (2005) Beta-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J Neurochem 92(3):628–636. 10.1111/j.1471-4159.2004.02895.x [DOI] [PubMed] [Google Scholar]


