Abstract
Background:
The objective was to compare ultrasonographic findings with clinical and radiographic findings in osteoarthritis (OA)-affected knee joints.
Methods:
This prospective study was conducted in Subharti Medical College, Meerut, after getting clearance from the ethical committee. Eighty-five symptomatic knees fulfilling American College of Rheumatology criteria for OA were included in the study. Patients with trauma, inflammatory, and infective conditions of the knee and with a history of intra-articular interventions and surgery were excluded. Demographic data, body mass index (BMI), visual analog scale (VAS), and Western Ontario and McMaster Universities Arthritis (WOMAC) questionnaire score were obtained. Kellgren–Lawrence (K-L) score was obtained on radiography. Ultrasonographic findings which were recorded include effusion, meniscal extrusion, femorotrochlear cartilage grading, maximum length of osteophytes at medial and lateral compartments, and presence or absence of Baker cyst.
Results:
A total of 85 consecutive symptomatic knees were examined. The male: female ratio was 22:63, with a mean age of 54.52 ± 9.44 years, mean duration of disease of 24.24 ± 19.14 months, mean BMI of 28.91 ± 3.69 kg/m2, and mean score of VAS and WOMAC pain scale of 6.27 ± 1.45 and 62.45 ± 10.96, respectively. K-L grading of 1, 2, 3, and 4 was reported in 12.9%, 21.2%, 25.9%, and 40% of the knees, respectively. The mean VAS score and WOMAC score showed statistically significant correlation with KL grading (P < 0.05). Knees with the presence of osteophytes, medial meniscal extrusion, effusion, and medial femoral trochlear cartilage grading showed statistically significant correlation with VAS and WOMAC scores (P < 0.05). However, the correlation was not significant for lateral meniscus extrusion and lateral femoral trochlear cartilage grading.
Conclusion:
Our study found that K-L grading and few ultrasonographic criteria showed a significant positive correlation with pain scores, while few other ultrasonographic criteria did not. Both imaging modalities are complementary to each other, rather than one being superior to the other.
Keywords: Cartilage, Kellgren–Lawrence grading, ultrasonography, visual analog scale score, Western Ontario and McMaster Universities Arthritis Scores
INTRODUCTION
Knee osteoarthritis (OA) is one of the most common degenerative joint diseases with a prevalence of 28.7% in India.[1] Its occurrence increases with advancing age and has a clear female predilection. It is the leading cause of pain and disability in the elderly, reflecting major implications for public health care. Knee OA leads to typical degradation of articular cartilage and soft-tissue structures encircling the knee joint. Though it can be diagnosed clinically, imaging techniques aid specifically in the identification of structures involved, adequacy of treatment planning, and preventing the factors responsible for progression.[2,3] In addition, availability of newer nonsurgical and surgical treatment options for knee OA-affected patients demands more precise imaging assessment.[4,5]
Conventional radiography is the initial imaging investigation in knee OA. It detects bony structural abnormality, but its findings did not show much correlation with the severity of pain in knee OA. This could possibly be because of the fact that soft-tissue structures cannot be viewed on radiographs, thus providing only an indirect evidence of cartilage and other soft-tissue damage. Joint space narrowing seen on radiography is considered an indirect indicator of femoro-tibial cartilage loss, however the assessment of actual joint space depends on both cartilage thickness and meniscal integrity, both of which cannot be directly evaluated with the help of radiography.[6,7] Till date, magnetic resonance imaging (MRI) is considered the most accurate imaging method for quantifying degenerative changes in knee OA. It can evaluate osseous abnormalities as well as soft-tissue changes with high sensitivity and specificity, but it cannot be used as a routine investigation because of its high cost and relatively low availability.[8] High-frequency ultrasound imaging is a noninvasive, widely available, and relatively inexpensive technique that can be used to reliably assess periarticular and superficial intra-articular abnormalities in knee OA. With time, ultrasonography has become the first-line imaging technique chosen by rheumatologists to obtain real-time imaging information in patients with painful joints.[9,10] Utility of ultrasound examination in shoulder imaging as well as interventions is well known.[11,12]
Many studies have been performed to establish the possible correlations between ultrasonographic findings and knee OA symptoms. Recent studies have indicated that ultrasonography is a useful and reliable method for identifying knee osteophytes, medial meniscal protrusion, and morphological changes in the cartilage in the medial femoral condyle, but no clear conclusions were drawn. All the authors underlined the need for more work in this field.[13,14,15,16,17,18,19,20,21,22,23] Due to paucity of literature in India, this study was undertaken to find out the possible correlation between knee pain scores and radiographic as well as ultrasonographic findings in knee OA and to further explore, if ultrasonographic findings presented better association with knee pain scores than conventional radiography.
MATERIALS AND METHODS
This prospective study was conducted in the Department of Radio-diagnosis, Chattrapati Shivaji Subharti Hospital and Subharti Medical College, Meerut, India. Approval from the institutional ethical committee was obtained (IEC16547). Eighty-five adult patients fulfilling American College of Rheumatology criteria for OA, referred to radiology department for radiographic study, were recruited.[24] Patients with a prior history of trauma, surgery, and intra-articular interventions in relation to knee were excluded. Patients not giving informed consent and having inflammatory and infective conditions of the knee were also excluded. Informed consent of all the participants was obtained after explaining the procedure. Convenient sampling method was used, and data were collected on a preformed structured interviewer-administered questionnaire. More symptomatic knee of the patient was examined. A flowchart describing the selection of symptomatic patients is shown in Figure 1.
Figure 1.
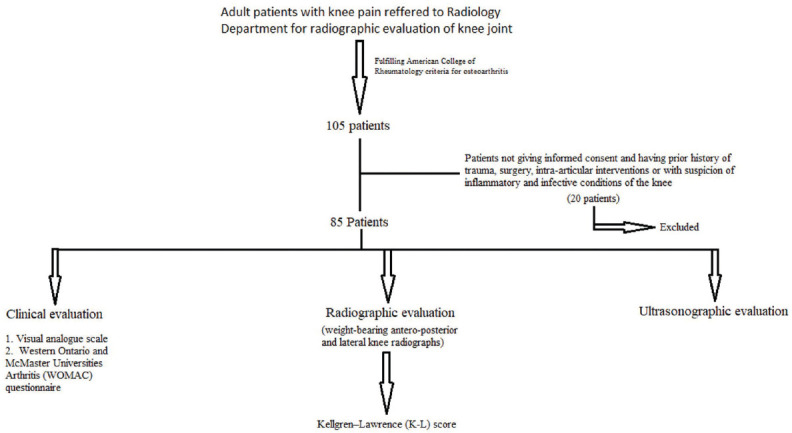
Flowchart showing selection of symptomatic patients and their clinical, radiographic, and ultrasonographic evaluation
Demographic data included age, sex, height, weight, and body mass index (BMI). Patients were then asked to score their average knee pain during the past month on a 100-mm visual analog scale (VAS) from 0 to 100 mm, where 0 mm represented no pain, while 100 mm represented extreme pain. Patients were then asked to self-complete Western Ontario and McMaster Universities Arthritis (WOMAC) questionnaire.[25] The questionnaire was translated in local language and patients were assisted by one of the investigators.
Weight-bearing anteroposterior and lateral knee radiographs of all the patients were taken. The radiographs were interpreted for severity of knee OA using Kellgren–Lawrence (K-L) score by a radiologist (blinded to the clinical and ultrasonographic findings).[26]
Ultrasonographic examination was performed by a radiologist having 5 years of experience in doing musculoskeletal ultrasonography (blinded to clinical and radiographic findings). All ultrasonographic examinations were performed on Samsung Madison HS70A machine (company-Samsung; Meerut, Uttar Pradesh, India) using a high-frequency linear probe. Ultrasound examination was performed with the patient lying in supine position and knee flexed to 20°–30° with a pillow under the knee for comfort. After applying abundant gel over the supra-patellar region, scanning was commenced in longitudinal and transverse planes applying minimal pressure over the area so as to avoid displacing the effusion sideward. Presence of effusion and synovial hypertrophy was noted. Quadriceps/patellar tendons and prepatellar/infrapatellar bursa were also evaluated.
Scanning in coronal planes over the medial and lateral aspects of the joint was done in longitudinal and transverse planes, and the status of menisci, collateral ligaments, Pes anserine bursitis, and iliotibial tract insertional tendinitis was noted. Maximum length of the osteophytes (bone step-ups) located at the edges of the medial and lateral joint surfaces was measured. Extrusion of menisci was also noted (defined as distance between the peripheral border of meniscus and outline of tibial plateau > 3 mm).[27]
Patients were then asked to flex the joint completely, and a probe was placed over trochlear notch for evaluation of the femorotrochlear articular cartilage degeneration. The degeneration was graded as: “Grade 0 – normal, Grade1 – loss of the normal sharpness of cartilage interfaces and/or increased echogenicity of the cartilage, Grade 2A – Grade 1 with clear local thinning (<50%) of the cartilage, Grade 2B – local thinning of the cartilage of more than 50% but < 100%, and Grade 3 – 100% focal loss of cartilage thickness.”[28] Patients were then asked to lie prone, and the posterior aspect of the knee joint was scanned in longitudinal and transverse planes. The distension of semimembranosus-medial head of gastrocnemius bursa was evaluated. Posterior joint recess was also evaluated for the presence of loose bodies. Tibial attachment of posterior cruciate ligament was also seen for any abnormality.
The data collected were tabulated in an Excel Sheet. Statistical analysis was performed using SPSS 22.00 for Windows; SPSS Inc., Chicago, IL, USA.
RESULTS
Eighty-five symptomatic knee OA cases were examined and analyzed. The study population included 63 (74.1%) females and 22 (25.9%) males, with a mean age of 54.52 ± 9.44 years. The mean duration of the symptoms was 24.24 ± 19.14 months. The mean BMI, VAS, and WOMAC scores were 28.91 ± 3.69 kg/m2, 6.27 ± 1.45, and 62.45 ± 10.96, respectively. K-L grading of 1, 2, 3, and 4 was reported in 12.9%, 21.2%, 25.9%, and 40% of the knees, respectively. The mean VAS score and WOMAC score increased with increase in K-L grading with statistically significant correlation (P < 0.05) [Table 1]. Weight-bearing frontal conventional radiographs of OA affected knee joints showing different K-L grading are shown in Figure 2.
Table 1.
Correlation of Kellgren-Lawrence grading with visual analog scale and Western Ontario and McMaster Universities Arthritis score
| KL Grading | Distribution, n (%) | VAS, mean±SD | WOMAC, mean±SD |
|---|---|---|---|
| Grade 1 | 11 (12.9) | 5.22±0.82 | 56.45±6.83 |
| Grade 2 | 18 (21.2) | 5.57±1.19 | 57.94±9.25 |
| Grade 3 | 22 (25.9) | 5.89±1.29 | 59.38±8.58 |
| Grade 4 | 34 (40) | 6.21±1.04 | 62.93±8.19 |
| ANOVA test** | 4.89 | 4.97 | |
| P | <0.04* | <0.04* |
KL Grading: Kellgren–Lawrence grading, VAS: Visual analog scale, WOMAC: Western Ontario and McMaster Universities Arthritis, n: Number of knees, SD: Standard deviation, ANOVA: Analysis of variance, *: Statistically significant
Figure 2.
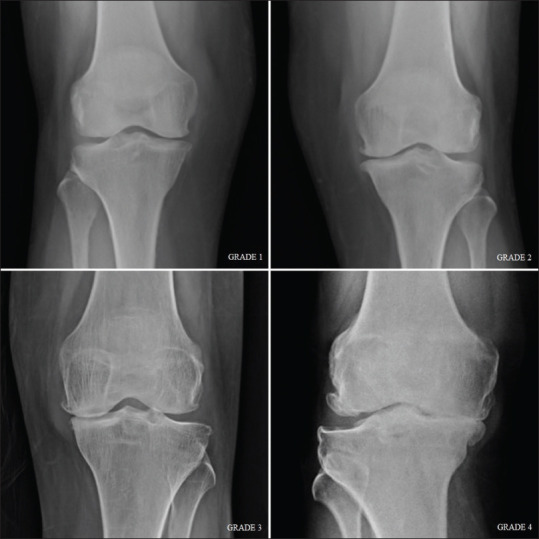
Radiographic grading of osteoarthritis in knee joint using Kellgren–Lawrence score
On ultrasonography, the most prevalent imaging finding was osteophytosis followed by medial meniscal extrusion, effusion, and lateral meniscal extrusion seen in 75 (88%), 57 (67.1%), 44 (51.8%), and 43 (50.6%) patients, respectively. Presence of medial meniscus extrusion, lateral meniscus extrusion, and joint effusion reported an increase in mean VAS and WOMAC scores, however statistically significant correlation was present with medial meniscus extrusion and effusion but not with lateral meniscus extrusion. Medial meniscus was not visualized in two cases. The mean length of osteophyte was greater at medial tibiofemoral joint space with a value of 3.91 ± 1.78 mm as compared to lateral tibiofemoral joint space with s value of 2.80 ± 1.74 mm. Pain scores increased with increasing length of osteophytes, and the findings were statistically significant.
In our study, medial femoral trochlear cartilage of Grade 1, Grade 2A, Grade 2B, and Grade 3 was reported in 2.4%, 23.5%, 40%, and 34.1%, of the patients, respectively. Lateral femoral trochlear cartilage of Grade 1, Grade 2A, Grade 2B, and Grade 3 was reported in 7.1%, 40%, 50.6%, and 2.4% of the patients, respectively. The mean VAS score and WOMAC score increased with increase in femoral trochlear cartilage grading and was found maximum in Grade 3 trochlear cartilage both medially and laterally. When mean VAS and WOMAC scores were compared with the grading of femoral trochlear cartilage degeneration, it was found to be statistically significant (as P < 0.05) for both medial and lateral positions [Table 2].
Table 2.
Correlation of ultrasound findings with visual analog scale and Western Ontario and McMaster Universities Arthritis score
| Variables | Distribution, n (%) | VAS, mean±SD | WOMAC, mean±SD |
|---|---|---|---|
| Medial meniscus extrusion | |||
| Yes | 57 (67.1) | 6.53±1.44 | 64.58±10.49 |
| No | 26 (30.6) | 5.58±1.21 | 56.42±9.16 |
| Not visualized | 2 (2.4) | 8±1.41 | 80±2.83 |
| ANOVA test | 5.89 | 9.01 | |
| P | 0.004* | <0.01* | |
| Lateral meniscus extrusion | |||
| Yes | 43 (50.6) | 6.44±1.32 | 63.37±9.09 |
| No | 42 (49.4) | 6.10±1.57 | 61.50±12.64 |
| T-test | 1.22 | 0.62 | |
| P | 0.27 | 0.43 | |
| Effusion | |||
| Yes | 44 (51.8) | 6.75±1.16 | 65.86±9.13 |
| No | 41 (48.2) | 5.76±1.56 | 58.78±11.67 |
| T-test | 11.17 | 9.79 | |
| P | 0.001* | 0.002* | |
| Medial trochlear cartilage | |||
| Grade 1 | 2 (2.4) | 4±0.71 | 46.50±2.12 |
| Grade 2A | 20 (23.5) | 5.15±1.23 | 54.20±9.56 |
| Grade 2B | 34 (40) | 6.32±1.29 | 62.76±9.09 |
| Grade 3 | 29 (34.1) | 7.14±1.13 | 68.86±9.57 |
| ANOVA test | 12.96 | 11.78 | |
| P | <0.01* | <0.01* | |
| Lateral trochlear cartilage | |||
| Grade1 | 6 (7.1) | 4.67±1.63 | 50.17±8.91 |
| Grade 2A | 34 (40) | 5.97±1.34 | 59.71±10.06 |
| Grade 2B | 43 (50.6) | 6.63±1.29 | 65.74±10.19 |
| Grade 3 | 2 (2.4) | 8.50±0.71 | 75±7.07 |
| ANOVA test | 6.41 | 6.44 | |
| P | 0.001* | 0.001* |
*Statistically significant. VAS: Visual analog scale, WOMAC: Western Ontario and McMaster Universities Arthritis, n: Number of knees, SD: Standard deviation, ANOVA: Analysis of variance
Medial and lateral collateral ligaments were intact in all the patients, Thickening, hypoechogenicity and loss of fibrillar pattern are ultrasonographic features of collateral ligament degeneration and these findings were seen in MCL in 77.5% cases and in LCL in 41.8% cases. Both degenerated medial and lateral collateral ligaments were associated with increase in pain scores, but statistically significant correlation was found in relation with medial collateral ligament only. Baker's cyst was present in 9.4% and iliotibial tract insertional tendinopathy was seen in 11.6% of cases, and their presence was associated with higher VAS and WOMAC pain scores. The findings were not statistically correlated due to small sample size. Para-meniscal cyst and pes anserine bursitis were not seen in any case.
On applying Pearson's correlation, positive and statistically significant correlation was found between K-L grading and pain scores. Similar positive and statistically significant correlation was reported for medial sided osteophytes, medial meniscal extrusion, medial femorotrochlear cartilage grading, medial collateral ligament degeneration, and effusion in relation to VAS and WOMAC score. However, significant correlation was not found with lateral-sided osteophytes, lateral meniscus extrusion, and lateral femorotrochlear cartilage grading. High-frequency ultrasonographic images of the OA-affected knee joints in different patients are shown in Figures 3-5.
Figure 3.
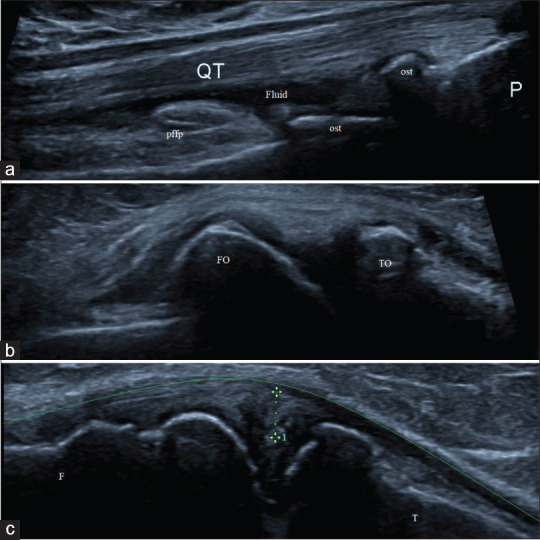
High-frequency ultrasonographic evaluation of osteoarthritis-affected knee joint showing; (a) presence of minimal effusion (fluid) in the supra-patellar recess and multiple osteophytes (ost) at patello-femoral joint; (b) presence of femoral and tibial osteophytes at joint margins (FO and TO); and (c) evidence of medial meniscal extrusion (calipers) causing displacement of medial collateral ligament (green line). F: Femur, T: Tibia, QT: Quadriceps tendon, pffp: Prefemoral fat pad and P: Patella
Figure 5.
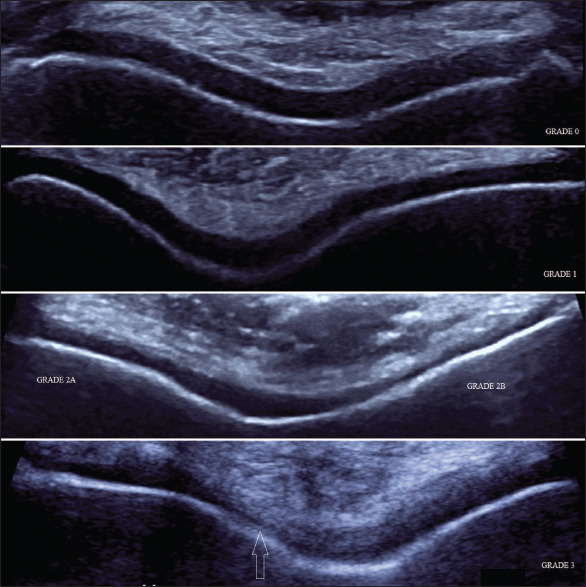
High-frequency ultrasonographic evaluation of osteoar thritis-affected knee joints showing different grading of femorotrochlear articular cartilage degeneration
Figure 4.
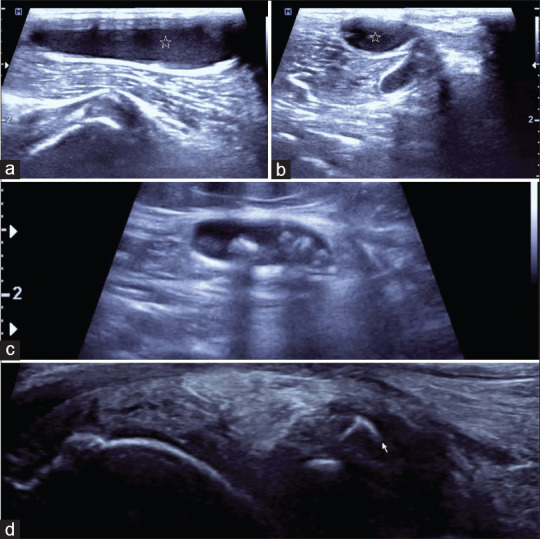
High frequency ultrasonographic evaluation of different patients with osteoarthritis affected knee joints showing (a,b) semimembranosus/medial gastrocnemius bursal distension also known as Baker cyst (star); (c) presence of multiple loose bodies present within the Baker cyst; and (d) presence of loose bodies (arrow) in the posterior joint recess
DISCUSSION
Our study comprised 85 patients with OA-affected knee joints, examined clinically, radiographically, and ultrasonographically. Comparison of the demographic and radiographic findings in our study with the published literature is shown in Table 3. The average age, sex ratio, and BMI of the patients in our study were comparable to those of the previous studies as shown in Table 3. The mean VAS and WOMAC scores in our study were 6.27 ± 1.45 and 62.45 ± 10.96, respectively. Serban et al.[16] reported similar mean score of VAS (6.58 ± 2.08), but low mean score of WOMAC (54.9 ± 16.7) in their study.
Table 3.
Comparison of the demographic and radiographic findings of the patients in our study with that of the published literature
| Study | Age (years) | Female:male ratio | BMI (kg/m2) | Duration (months) | KL Grading (%) | |||
|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | |||||
| Present study | 54.52±9.44 | 63:22 (2.86) | 28.91±3.69 | 24.24±19.14 | 12.9 | 21.2 | 25.9 | 40 |
| Naredo et al.[19] | 64±7.6 | Not assessed | 31.8±5.1 | Not assessed | 25.5 | 37.7 | 33.3 | 3.3 |
| Chan et al.[15] | 59.0±13.9 | 143:50 (2.86) | 24.9±4.1 | Not assessed | 17.1 | 22.3 | 22.3 | 16.6 |
| Bevers et al.[22] | 57±9.2 | 120:60 (2.00) | 28.8±7.4 | 103±114 | 23.9 | 38.9 | 17.8 | 8.3 |
| Podlipská et al.[20] | 59.9±7.8 | 49:30 (1.63) | 29.1±4.3 | Not assessed | 26.6 | 24.1 | 25.3 | 21.5 |
| Serban et al.[16] | 63.44±9.49 | 33:19 (1.73) | 31.73±6.42 | Not assessed | 41.5 | 31.7 | 24.4 | 2.4 |
BMI: Body mass index, KL grading: Kellgren–Lawrence grading
As far as K-L grading is considered, 40% of the knees were in Grade IV, which differed from that of previous published literature. This difference in K-L grading might be due to the fact that demography of the patients in surrounding area is rural and they involved in agricultural and strenuous work who seeks medical attention late in the disease course. The mean pain scores increased with increase in K-L grading. A positive and significant correlation was found between K-L grading and VAS as well as WOMAC scores. Therefore, it can be said that with increase in K-L grading, pain also increases, which is in line with a recent systematic review that higher Grade of OA (K-L 3 or above) is a stronger predictor of pain than lower Grades (K-L 2 or less). Serban et al.[16] also revealed significantly higher VAS and WOMAC scores as the K-L score increased.
A positive and significant correlation was reported for effusion in relation to VAS as well as WOMAC scale. Similarly, Hill et al.[29] reported a strong association between effusion and pain in knee OA. Naredo et al.[19] found a strong association between effusion and pain in knee on motion as well as rest, and this finding was independent of radiographic OA severity, age, disease duration, and BMI. However, Serban et al.[16] did not find any correlation between effusion and pain scores.
A positive and significant correlation was reported for medial meniscus extrusion in relation to VAS as well as WOMAC score. Kijima et al.[30] found correlation between pain with medial meniscus extrusion (the higher the extrusion degree, the higher the level of pain). However, Serban et al.[16] did not find any correlation between the medial meniscal extrusion and pain scores. A positive correlation was reported for lateral meniscus extrusion in relation to VAS as well as WOMAC scale, however the results were statistically insignificant. Similar findings were noted by Serban et al.,[16] who did not find lateral meniscus extrusion to be an independent predictor of VAS score, however they found it to be an independent predictor of WOMAC score.
The mean length of osteophytes was greater at medial tibiofemoral joint space (3.91 ± 1.78 cm) as compared to lateral tibiofemoral joint space (2.80 ± 1.74 cm), which may be due to the fact that medial compartment of knee is a weight-bearing part and exposed to greater stress as compared to the lateral compartment. Significant Pearson's correlation was found with pain scores and medial osteophytes but not with lateral osteophytes. Chan et al.[15] and Serban et al.[16] also found similar correlation.
When mean VAS and WOMAC scores were compared statistically with grades of trochlear cartilage damage medially and laterally, it was found to be statistically significant as P < 0.05. These results were in accordance with the study done by Serban et al.[16] Similar findings of association of the hyaline cartilage damage or loss, with pain scores, have also been demonstrated in ultrasound study by Chan et al.[15] and MRI study by Yusuf et al.[31]
Limitations
Some of the limitations of our study should be taken into account. First, we did not compare the symptomatic patients with the control group. Second, ultrasound findings were not compared with MRI and scoring system was not used for different ultrasound features. Third, data of patient's treatment were not taken into account. A lack of standardization and validation over the definition and scoring system for different ultrasonographic signs in OA-affected knee joint definitely exists. These constraints can be sorted by conducting a multicentered study involving larger sample size and including controls as well. Newer ultrasonographic techniques such as elastography and three-dimensional/four-dimensional ultrasound and use of ultrasound contrast agents will also help in understanding the pathophysiology of OA in a better way.
CONCLUSION
This was the first study in the Indian scenario comparing high-frequency ultrasonographic evaluation of OA-affected knee joints with K-L grading and pain scores. The present study revealed that findings of conventional radiography (K-L grading) and few ultrasonographic criteria such as medial tibio-femoral osteophyte, medial meniscal extrusion, medial femorotrochlear cartilage grading, medial collateral ligament degeneration, and effusion showed a significant positive correlation with pain scores of the knees. Hence, it can be said that although conventional radiography and musculoskeletal ultrasound measure different structural tissues of the osteoarthritic knee, both imaging modalities are complementary to each other, rather one being superior to other.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Pal CP, Singh P, Chaturvedi S, Pruthi KK, Vij A. Epidemiology of knee osteoarthritis in India and related factors. Indian J Orthop. 2016;50:518–22. doi: 10.4103/0019-5413.189608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guermazi A, Hayashi D, Eckstein F, Hunter DJ, Duryea J, Roemer FW. Imaging of osteoarthritis. Rheum Dis Clin North Am. 2013;39:67–105. doi: 10.1016/j.rdc.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Roemer FW, Eckstein F, Hayashi D, Guermazi A. The role of imaging in osteoarthritis. Best Pract Res Clin Rheumatol. 2014;28:31–60. doi: 10.1016/j.berh.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Sato M, Yamato M, Mitani G, Takagaki T, Hamahashi K, Nakamura Y, et al. Combined surgery and chondrocyte cell-sheet transplantation improves clinical and structural outcomes in knee osteoarthritis. NPJ Regen Med. 2019;4:4. doi: 10.1038/s41536-019-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Power CK, Galvin D, Foto T, Mahmoud AA, Kostopoulos NG. Thermal Microcautery, a form of peripheral nerve field stimulation for treatment of painful knee osteoarthritis: Non-randomized controlled trial. Pain Studies Treatment. 2019;7:33–54. [Google Scholar]
- 6.Hunter DJ, Zhang YQ, Tu X, Lavalley M, Niu JB, Amin S, et al. Change in joint space width: Hyaline articular cartilage loss or alteration in meniscus? Arthritis Rheum. 2006;54:2488–95. doi: 10.1002/art.22016. [DOI] [PubMed] [Google Scholar]
- 7.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: A systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score) Osteoarthritis Cartilage. 2011;19:990–1002. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grassi W, Filippucci E, Busilacchi P. Musculoskeletal ultrasound. Best Pract Res Clin Rheumatol. 2004;18:813–26. doi: 10.1016/j.berh.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Gibbon WW. Seminars in Musculoskeletal Radiology. Vol. 8. New York, USA: Thieme Medical Publishers, Inc; 2004. Applications of ultrasound in arthritis; pp. 313–28. [DOI] [PubMed] [Google Scholar]
- 11.Wu WT, Chang KV, Mezian K, Naňka O, Lin CP, Özçakar L. Basis of shoulder nerve entrapment syndrome: An ultrasonographic study exploring factors influencing cross-sectional area of the suprascapular nerve. Front Neurol. 2018;9:902. doi: 10.3389/fneur.2018.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang JC, Chang KV, Wu WT, Han DS, Özçakar L. Ultrasound-guided standard vs.dual-target subacromial corticosteroid injections for shoulder impingement syndrome: A randomized controlled trial. Arch Phys Med Rehabil. 2019;100:2119–28. doi: 10.1016/j.apmr.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Tarhan S, Unlu Z. Magnetic resonance imaging and ultrasonographic evaluation of the patients with knee osteoarthritis: A comparative study. Clin Rheumatol. 2003;22:181–8. doi: 10.1007/s10067-002-0694-x. [DOI] [PubMed] [Google Scholar]
- 14.de Miguel Mendieta E, Cobo Ibáñez T, Usón Jaeger J, Bonilla Hernán G, Martín Mola E. Clinical and ultrasonographic findings related to knee pain in osteoarthritis. Osteoarthritis Cartilage. 2006;14:540–4. doi: 10.1016/j.joca.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Chan KK, Sit RW, Wu RW, Ngai AH. Clinical, radiological and ultrasonographic findings related to knee pain in osteoarthritis. PLoS One. 2014;9:e92901. doi: 10.1371/journal.pone.0092901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serban O, Porojan M, Deac M, Cozma F, Solomon C, Lenghel M, et al. Pain in bilateral knee osteoarthritis-correlations between clinical examination, radiological, and ultrasonographical findings. Med Ultrason. 2016;18:318–25. doi: 10.11152/mu.2013.2066.183.pin. [DOI] [PubMed] [Google Scholar]
- 17.Wu PT, Shao CJ, Wu KC, Wu TT, Chern TC, Kuo LC, et al. Pain in patients with equal radiographic grades of osteoarthritis in both knees: The value of gray scale ultrasound. Osteoarthritis Cartilage. 2012;20:1507–13. doi: 10.1016/j.joca.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Eşen S, Akarırmak U, Aydın FY, Unalan H. Clinical evaluation during the acute exacerbation of knee osteoarthritis: The impact of diagnostic ultrasonography. Rheumatol Int. 2013;33:711–7. doi: 10.1007/s00296-012-2441-1. [DOI] [PubMed] [Google Scholar]
- 19.Naredo E, Cabero F, Palop MJ, Collado P, Cruz A, Crespo M. Ultrasonographic findings in knee osteoarthritis: A comparative study with clinical and radiographic assessment. Osteoarthritis Cartilage. 2005;13:568–74. doi: 10.1016/j.joca.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Podlipská J, Guermazi A, Lehenkari P, Niinimäki J, Roemer FW, Arokoski JP, et al. Comparison of diagnostic performance of semi-quantitative knee ultrasound and knee radiography with MRI: Oulu Knee Osteoarthritis Study. Sci Rep. 2016;6:1–2. doi: 10.1038/srep22365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Razek AA, El-Basyouni SR. Ultrasound of knee osteoarthritis: Interobserver agreement and correlation with Western Ontario and McMaster Universities Osteoarthritis. Clin Rheumatol. 2016;35:997–1001. doi: 10.1007/s10067-015-2990-2. [DOI] [PubMed] [Google Scholar]
- 22.Bevers K, Zweers MC, van den Ende CH, Martens HA, Mahler E, Bijlsma JW, et al. Ultrasonographic analysis in knee osteoarthritis: Evaluation of inter-observer reliability. Clin Exp Rheumatol. 2012;30:673–8. [PubMed] [Google Scholar]
- 23.Malas FÜ, Kara M, Kaymak B, Akıncı A, Özçakar L. Ultrasonographic evaluation in symptomatic knee osteoarthritis: Clinical and radiological correlation. Int J Rheum Dis. 2014;17:536–40. doi: 10.1111/1756-185X.12190. [DOI] [PubMed] [Google Scholar]
- 24.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 25.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 26.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swamy N, Wadhwa V, Bajaj G, Chhabra A, Pandey T. Medial meniscal extrusion: Detection, evaluation and clinical implications. Eur J Radiol. 2018;102:115–24. doi: 10.1016/j.ejrad.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Saarakkala S, Waris P, Waris V, Tarkiainen I, Karvanen E, Aarnio J, et al. Diagnostic performance of knee ultrasonography for detecting degenerative changes of articular cartilage. Osteoarthritis Cartilage. 2012;20:376–81. doi: 10.1016/j.joca.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Hill CL, Gale DG, Chaisson CE, Skinner K, Kazis L, Gale ME, et al. Knee effusions, popliteal cysts, and synovial thickening: Association with knee pain in osteoarthritis. J Rheumatol. 2001;28:1330–7. [PubMed] [Google Scholar]
- 30.Kijima H, Yamada S, Nozaka K, Saito H, Shimada Y. Relationship between pain and medial meniscal extrusion in knee osteoarthritis. Adv Orthop. 2015;2015:210972. doi: 10.1155/2015/210972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yusuf E, Kortekaas MC, Watt I, Huizinga TW, Kloppenburg M. Do knee abnormalities visualised on MRI explain knee pain in knee osteoarthritis? A systematic review. Ann Rheum Dis. 2011;70:60–7. doi: 10.1136/ard.2010.131904. [DOI] [PubMed] [Google Scholar]


