Abstract
Objective: To determine the changes of gene and protein expression through Rho/ROCK signaling pathway in EA treated spinal cord injury (SCI) rats and to unveil the possible underlying mechanism.
Design: Animal study.
Setting: Affiliated Hospital of Jiangxi University of Traditional Chinese Medicine.
Participants: Eighty Male Sprague Dawley rats.
Interventions: Electroacupuncture at Yaoyangguan (GV3), Dazhui (GV14), Zusanli (ST36) and Ciliao (BL32) and/or blocking agent Y27632 treatment.
Outcome Measures: Protein expression was detected by ELISA and Western blotting, mRNA expression was detected by quantitative PCR and in situ hybridization. Morphological changes in spinal cord were evaluated by HE-staining and Nissl staining. Hindlimb motor function in the rats was evaluated by Basso–Beattie–Bresnahan (BBB) assessment methods.
Results: Compared with injured rats in SCI group, EA, blocking agent Y27632 and EA + blocking agent Y27632 treatment had significantly reduced mRNA and protein expression levels of RhoA and ROCKII, decreased p-MLC protein expression and p-MLC/MLC ratio, suppressed cPLA2 activity and PGE2 level, improved spinal cord tissue morphology and BBB score of lower limb movement function at 7 days and at 14 days (P < 0.01 or <0.05).
Conclusion: Similar to the blocking agent Y27632, EA may have a notable inhibitory effect on the Rho/ROCK signaling pathway after SCI, therefore reducing the inhibition of axonal growth and inflammatory reaction may be a key mechanism of EA treatment for SCI.
Keywords: RhoA, ROCKII, SCI, MLC, cPLA2, PGE2, Y27632, Electroacupuncture
Introduction
Traumatic paraplegia caused by spinal cord injury (SCI) is a severe neurological injury that often leads to profound disability,1,2 and SCI results in high mortality and mutilation.3 Unfortunately, to date, there is no effective pharmacological or methodological treatment for SCI in modern medicine.4
Recently, clinical studies showed that electroacupuncture (EA) was used to treat SCI and EA could be beneficial to SCI patients, especially to those with incomplete paraplegia.5–7 However, the exact mechanisms remain to be fully elucidated.
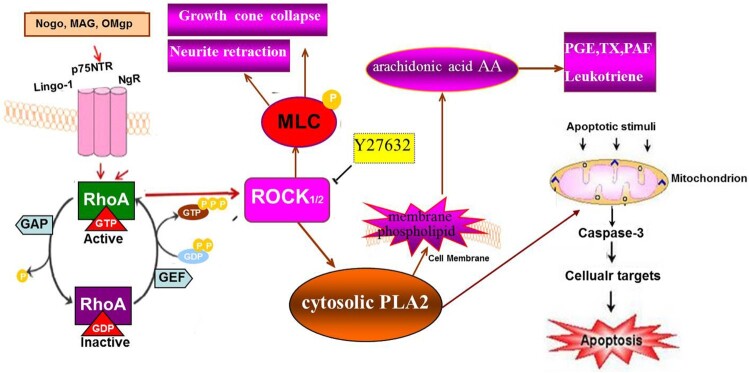
It is current theory that extracellular inhibitory signaling factors, such as myelin-associated glycoprotein (MAG), Nogo protein, oligodendrocyte myelin glycoprotein (OMgp),8–10 initiated intracellular signaling transduction and finally activates the same downstream Rho/ROCK signaling pathway. It is acceptable that inactive form of Rho (Rho-GDP) can be converted to active form of Rho (Rho-GTP), activating ROCK to influence its downstream effector molecule, such as myosin light chain (MLC), a substrate of ROCK, and cPLA2.11,12 Small molecule Y27632, a specific inhibitor of ROCKII, could inhibit the activities of ROCKII then promote neural axon growth, injured CNS axon growth and functional recovery.13,14 Phosphorylated MLC can stimulate the combination of myosin and actin, and trigger contraction of myosin, and then influence the structure of cytoskeleton, that cause the growth cone to collapse and retraction.15,16 cPLA2 is an important metabolic and regulatory enzyme catalyzing phospholipids to hydrolyze into free fatty acids and lysophospholipids mainly arachidonic acid (AA).17,18 AA and other substances with strong biological activitycan be further hydrolyzed into prostaglandin (PGE) and platelet activation factor, etc. by cyclic oxidase and lipooxidase.18 As shown in Figure 1.
Figure 1.
Extracellular inhibitory signaling factors activate the same downstream Rho/ROCK signaling pathway through transmembrane protein NgR. That inactive Rho-GDP gets converted to active Rho-GTP then activates ROCK to further influence the downstream effector molecules, such as myosin light chain (MLC), leads to the combination of myosin and actin, triggering contraction of myosin, and destruction of cytoskeleton structure, that cause the growth cone collapse and neurite retraction. ROCK also activate cPLA2, an important metabolic and regulatory enzyme, that could hydrolyze phospholipids into free fatty acids and lysophospholipids mainly arachidonic acid (AA). AA and other substances with strong biological activity, such as prostaglandin (PGE) and platelet activation factor (PAF), etc. to regulate the cellular inflammatory response. And cPLA2 could also induce cell apoptosis by stimulating mitochondrion produce caspase-3.
We hypothesized the Rho/ROCK signaling pathway, activated after SCI, contributes to inhibition of axons regeneration. EA could promote axon regrowth and induce recovery after SCI through inhibiting activation of Rho/ROCK signaling pathway. Previous studies indicate that acupuncture could regulate the genes and proteins expression levels of RhoA and ROCKII.19 Does EA could influence Rho/ROCK signaling pathway after SCI? How does EA treat SCI by affecting the Rho/ROCK signaling pathway?
Methods
Experimental animals
Eighty healthy, clean, female, Sprague–Dawley rats, aged 8 weeks (weighing 200 ± 20 g) were purchased from the Slack-Jingda Laboratory Animals Co., Ltd. of Hunan Province, China (certificate No. SCXK (Xiang) 2011-0003). Rats were fed with standard fodder and with food and water freely available, in a controlled environment with a constant temperature 20–25°C and a 12/12-hour light/dark cycle. Following a 3-day adaptation, experiments were initiated. We used female rats in our studies based on previous investigations.20
Modeling
Models of SCI were established in accordance with published methods.3 Prior to experiments, all rats were anesthetized with 3% pentobarbital sodium (1.5 mL/kg, Sigma, USA) intraperitoneally and an incision made. The T10 vertebral lamina and spinous process was removed by rongeur forceps to expose the spinal cord completely. The injury was then induced by striking the spinal cord with an electric cortical contusion impactor (n = 64). Strike parameters consisted of a strike tip with a diameter of 3 mm, velocity of 5 m/s, retention time of 0.5 s and compression of 1.5 mm.19
The spinous process at the L5-S1 joints was removed using the same method mentioned above. A small hole was made on the surface of the dura mater of the spinal cord with microscissors, avoiding the median vessel. A PE 10 hose was clamped and pressed under the dura mater and inserted into the arachnoid cavity slowly if clear cerebrospinal fluid was observed overflowing from the outside of the catheter, indicating that catheterization was successful.3 The wound was washed with saline and penicillin, and the injured rats were raised in separate cages.
Sham operation control animals received laminectomy only.
Grouping and treatment
After being evaluated for hindlimb motor function according to Basso–Beattie–Bresnahan (BBB) method, 64 SCI model rats were randomly subdivided into an SCI model group (SCI, n = 16), EA treatment group (EA, n = 16), blocking agent Y27632 treatment group (Y27632, n = 16), and EA plus blocking agent Y27632 treatment group (EA + Y, n = 16). All treatments were started at 1 d after SCI.
In the EA treatment group, the rats were restrained on a board. Stainless steel 0.18-mm-diameter needles (Hwato Disposable Acupuncture Needle; Jiangsu Medical Supplies Factory, Jiangsu Province, China) were inserted to a depth of approximately 5 mm at the following acupoints: Yaoyangguan (GV3) and Dazhui (GV14), and Zusanli (ST36), Ciliao (BL32) on both sides.21 Then, the needles were connected to an Hwato SDZ-II EA apparatus (Suzhou, Jiangsu Province, China). Alternating strings of dense-sparse frequencies (100 Hz for 1.5 ms and 2 Hz for 1.5 ms alternately) were selected. The intensity was adjusted to induce a slight twitch of the hindlimb. EA treatment was performed for 20 min once per day, lasting for 14 days. Rats in the Y27632 treatment group received the blocking agent Y27632 (Qilu Pharmaceutical Co., Ltd., Jinan, Shandong Province, China) by subdural injection via a microinjector from a homemade PE10 tube (the 18 μg, 18 μg Y27632 lyophilized powders were dissolved in 30 μL phosphate buffered saline solution; each rat received 0.54 mL/kg), once per day, lasting for 14 days.8
Rats in the EA + Y treatment group received both EA and Y27632 subdural injection treatment.
Rats in the sham operation group, EA treatment group and SCI model group were received normal saline by subdural injection according to the methods mentioned above.
Target observation and detection
Behavioral assessments
The BBB Scale locomotor tests of all rats were blindly performed before treatment and on the 7th and 14th day when treatment began by 2 trained observers.19 Before the assessment, the bladder of each rat was emptied to minimize any influence of a full bladder on locomotor function. Rats’ bladder were voided every 8 h in normal times. Briefly, rats were placed in an open field and observed for 3 min. Each rat was evaluated three times, and the average integer value was recorded.
Sampling
At the 7th and 14th day after treatment, eight rats in each group were randomly selected to be deeply anesthetized, a10-mm spinal cord segment containing the injury epicenter was isolated from each animal and the segment was cut in half equally at the injury epicenter for analysis, with one half soaked in the 4% paraformaldehyde solution for storage and the other half frozen in liquid nitrogen and stored at −80°C.
Histological assessments
Four paraffin blocks were selected randomly and the tissue was cut into 4–7-μm-thick serial sections (250 μm apart and spanning the entire rostrocaudal extent of the lesion). The sections were deparaffinized with xylene and dehydrated in gradient alcohol. One set of sections was stained with hematoxylin and counterstained with eosin. The others were stained with cresyl violet–eosin for tigroid body with amethyst. The lesion and spared white matter area of the injured cord were visualized, outlined, and quantified using an Olympus BX60 microscope.
Reverse transcription real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the spinal cords of rats in each group using Trizol solution (Invitrogen, Carlsbad, CA, USA). The mRNA expression levels of RhoA and ROCKII were measured using a RT-qPCR system with SYBR Green (Thermo Fisher Scientific, Waltham, MA, USA). RT-qPCR conditions were as follows: 94°C for 5 min, followed by 40 cycles of 95°C for 15 s, 60°C for 45 s and 72°C for 30 s. Fluorescence signaling was detected at 60°C and samples were finally extended at 72°C for 7 min. The amplification efficiency was compared between the target and reference control GAPDH (glyceraldehyde 3-phosphate dehydrogenase) using the delta-delta Ct (ΔΔCt) method.22 Primers employed are listed in Table 1.
Table 1. Primer sequences for real-time quantitative polymerase chain reaction.
| Gene | Full name | Sequences (5-3′) | Product size (bp) |
|---|---|---|---|
| RhoA | Ras homolog family member A | Sense primer: GAT GGA GCT TGT GGT AAG antisense primer: ATC AGT GTC TGG GTA GGA G |
150 |
| ROCKII | Rho associated kinase II | Sense primer: ATC TCA TTT GTG CCT TCC antisense primer: CTG GTG CTA CAG TGT CTC G |
143 |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase | Sense primer: GCA AGT TCA ACG GCA CAG antisense primer: GCC AGT AGA CTC CAC GAC AT |
146 |
Western blot assay
Western blot analysis was performed as described previously with minor modification.14 All spinal cord tissues obtained in each group were homogenized in lysis buffer (JRDUN Biotechnology, Shanghai, China). Monoclonal antibodies used for Western blot included rat monoclonal anti-RhoA (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Antibodies used in this study were rat monoclonal antibody,1:1 000, and were from Santa Cruz Biotechnology (Santa Cruz, CA, USA), except for those specifically indicated. The other antibodies included anti-ROCKII, anti-MLC, anti- p-MLC and anti-GAPDH. The grayscale values of bands were quantified using Image J software (Fujifilm, Tokyo, Japan). The relative expression of protein was calculated based on the ratio of target grayscale values to loading control grayscale values.
In situ hybridization
4-7-μm-thick section was incubated in 50 μL hybridization buffer containing 10 µM oligonucleotide probe at 95°C for 5 min and 37–40°C for 12 h, washed with saline sodium citrate, and treated with blocking buffer at 37°C for 15 min. Each section was then incubated in 30 µL biotinylated anti-digoxin antibody (1:50) at 37°C for 1 h, washed four times with 0.5 M PBS, incubated in streptavidin–biotin-peroxidase complex at 37°C for 30 min, and rinsed four times in 0.5 M PBS.23 The morphology of nerve cells in the spinal cord was observed under a microscope. Negative controls were incubated in 0.01 M PBS without primary antibody. The primers employed are shown in Table 2.
Table 2. Primer sequences for hybridization in situ.
| Gene | Sequences (5′−3′) |
|---|---|
| RhoA | AGGTAGAGTTGGCTTTATGGGACACAGCTGGACAGGAAGATTATGACCGTCTGAGGCCTCTCTCCTACCCAGACACTGATG |
| ROCKII | TCTTTATCACTTCCCAAC CAACTGTGAGGCCTGTATGAAGCCACTGT GGCACATGTTTAAACCTCCTCCTGCTCTAGAGTGCCGTAGATGCC |
Spinal cord tissue of prostaglandin E2 determination
Tissue levels of prostaglandin E2 (PGE2) in the spinal cord were assayed using enzyme linked immunosorbent assay (ELISA; Prostaglandin E2 ELISA kit; Shanghai Xinyu biology technology limited company, Shanghai, China). The supernatant extraction of spinal cord tissue was performed by conventional methods, and the supernatants were used for assay followed the manufacturer’s instructions.
Spinal cord tissue of cPLA2 activity assay
Spinal cord segments containing the injury epicenter were removed and were homogenized in ice-cold lysis buffer (0.1 M phosphate buffered solution, pH 7.4, 1 mM EDTA, 1 mM indomethacin; Cayman Chemical) using a tube pestle and centrifuged at 10,000 × g for 15 min at 4°C. The supernatants were then stored at −80°C, and assay followed the manufacturer’s instructions.
Statistical analyses
All data are presented as the mean ± SD. Data were analyzed by a two-way analysis of variance test followed by a post hoc Student–Newman–Keuls (SNK) test using the SPSS 17.0 sorfware (SPSS, Chicago, IL, USA), and a paired T test was used for intra-group comparison. A value of P < 0.05 was considered statistically significant.
Results
BBB scores
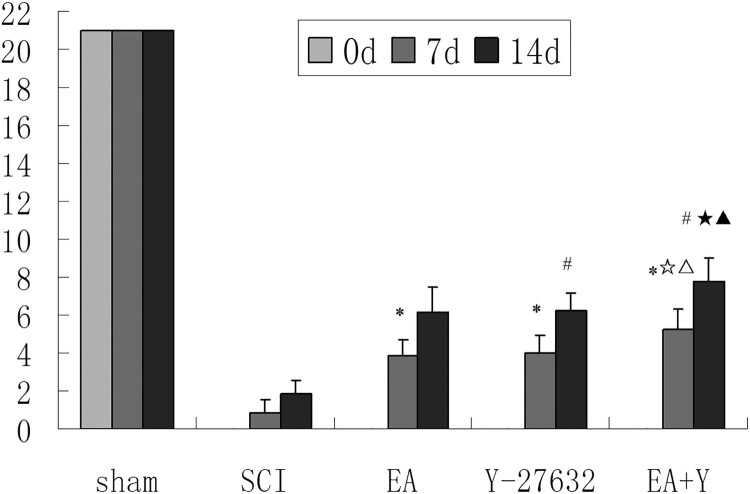
BBB scores in each group are shown in Figure 2. BBB scores of SCI rats were lower than that of the sham operation rats (P < 0.01) and each treating group rats at 7 or 14 days (P < 0.05). After treatment with EA or Y27632, or EA + Y27632, the hindlimb motor function of rats in any treating group has significant improvement compared with SCI rats (P < 0.05). BBB score was significantly lower in the SCI group (P < 0.05) than in any of the treatment groups (EA, Y27632, and EA + Y) at 7 days and at 14 days. BBB score in EA + Y27632 group was higher than that in EA group or Y27632 group (P < 0.01). However, there was no significant difference in BBB score between EA group and Y27632 group (P > 0.05). In each treatment group, the BBB score at 14 days was higher than that at 7 days (P < 0.05).
Figure 2.
BBB score of each group after 7 and 14 days of treatment. * P < 0.05 versus 7d SCI; # P < 0.05 versus 14d SCI; ☆ P < 0.05 versus 7d EA; ▵ P < 0.05 versus 7d Y-27632; ☆ P < 0.05 versus 14d EA; ▴ P < 0.05 versus 14d Y-27632. Data are expressed as the mean ± SD (two-way analysis of variance and Student–Newman–Keuls post hoc test, n = 16 or 8 rats/group). sham: sham operation; SCI: SCI; EA: electroacupuncture; Y: blocking agent Y27632.
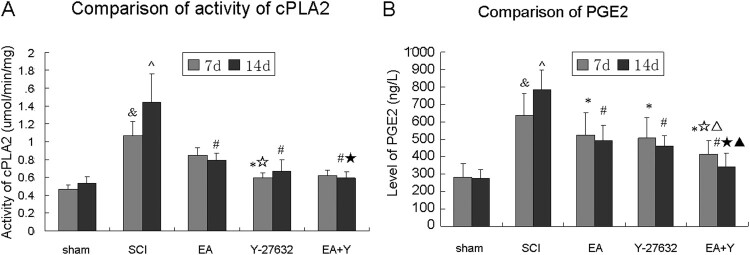
The activity of cPLA2 and the level of PGE2 in spinal cord tissue
The activity of cPLA2 and the level of PGE2 in each group are shown in Figure 3. cPLA2 activity and PGE2 level of SCI rats were higher than that of the sham operation rats at 7 days and at 14 days (P < 0.01). After treatment with EA or Y27632, or EA + Y27632, except for EA group at 7 days, cPLA2 activity and PGE2 level were significantly lower in the SCI group than in any of treating groups (EA, Y27632, and EA + Y) at 7 and 14 days (P < 0.01 or <0.05). As shown in Figure 3(A), cPLA2 activity in Y27632 and EA + Y27632 group was significantly lower than that in EA group at 7 days (P < 0.05) and cPLA2 activity in EA + Y27632 group was significantly lower than that in EA group at 14 days (P < 0.05). As shown in Figure 3(B), PGE2 level in EA + Y27632 group was significantly lower than those in EA group at 7 days (P < 0.01) and at 14 days (P < 0.05).
Figure 3.
Activity of cPLA2 and level of PGE2 following SCI. (A) Compiled results in a bar graph for the activity of cPLA2 at 7 and 14 days after treatment. (B) Compiled results in a bar graph for the level of PGE2 at 7 and 14 days after treatment. & P < 0.05 versus 7d sham; ^ P < 0.05 versus 14d sham; * P < 0.05 versus 7d SCI; # P < 0.05 versus 14d SCI; ☆ P < 0.05 versus 7d EA; △ P < 0.05 versus 7d Y-27632; ☆ P < 0.05 versus 14d EA; ▴ P < 0.05 versus 14d Y-27632. Data are shown as the mean ± standard error of the mean (two-way analysis of variance and Student–Newman–Keuls post hoc test, n = 8 rats/group). sham: sham operation; SCI: SCI; EA: electroacupuncture; Y: blocking agent Y27632.
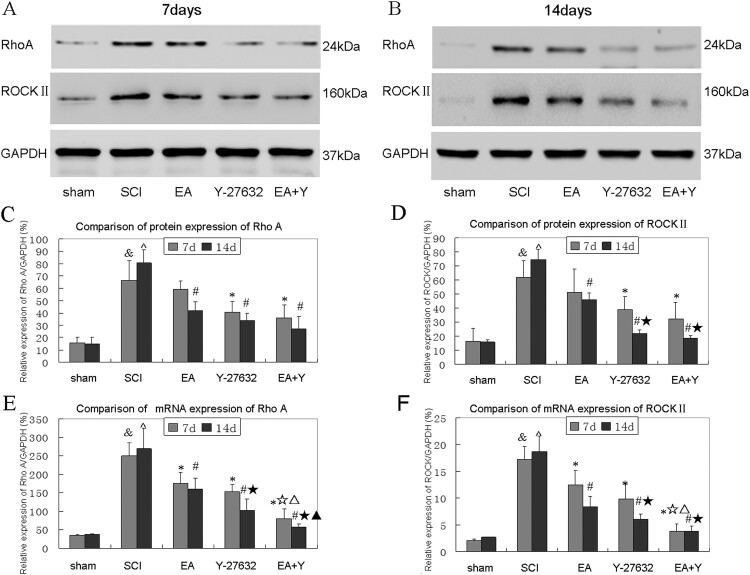
The mRNA and protein expression of RhoA and ROCKII in spinal cord tissue
As shown in Figure 4. The mRNA and protein expression levels of RhoA and ROCKII in SCI group rats were higher than that in the sham-operated group rats at 7 and 14 days (P < 0.05 or <0.01).
Figure 4.
Effect of EA on changes in mRNA and protein expression levels of RhoA and ROCKII of injured spinal cords. (A,B) Western blotting bands for RhoA, ROCKII and GAPDH in different groups at 7 and 14 days. (C) Compiled results in a bar graph for RhoA protein expression. (D) Compiled results in a bar graph for ROCKII protein expression. (E) Compiled results in a bar graph for RhoA mRNA expression. (F) Compiled results in a bar graph for ROCKII mRNA expression. Y-axis indicates the relative grayscale value of protein bands (the ratio of target grayscale values to loading control grayscale values). & P < 0.05 versus 7d sham; ^ P < 0.05 versus 14d sham; * P < 0.05 versus 7d SCI; # P < 0.05 versus 14d SCI; ☆ P < 0.05 versus 7d EA; △ P < 0.05 versus 7d Y-27632; ☆ P < 0.05 versus 14d EA; ▴ P < 0.05 versus 14d Y-27632. Data are expressed as the mean ± SD (two-way analysis of variance and Student–Newman–Keuls post hoc test, n = 4 rats/group). sham: sham operation; SCI:SCI; EA:electroacupuncture; Y:blocking agent Y27632. ROCKII: RhoAssociated kinase II. GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
As shown in Figure 4(A–D), after treatment with EA, Y27632, or EA + Y27632, the RhoA protein expression at 7 days suggested a decreasing trend in each treatment group(P > 0.05). But the RhoA protein expression in each treatment group was significantly lower than in the SCI group at 14 days (P < 0.01 or <0.05). For ROCKII protein levels, all treatment groups (except 7D) showed decreases. Except for EA group at 7 days, the ROCKII protein expression in each treatment group were significantly lower than in the SCI group at 7 and 14 days (P < 0.01 or <0.05). The protein expression of RhoA and ROCKII showed no significant difference between 7 and 14 days in each group (P > 0.05). And for protein expression of RhoA and ROCKII, there was no significant difference among three treatment groups (P > 0.05).
As shown in Figure 4(E,F), after treatment with EA or Y27632, or EA + Y27632, the mRNA expression of RhoA and ROCKII in each treatment group were significantly lower than that in SCI group at 14 and 7 days (P < 0.05 or <0.01); except for EA + Y27632 group, the mRNA expression of RhoA and ROCKII showed no significant difference between 7 and 14 days in each group (P > 0.05). Except for RhoA in Y27632 group and ROCKII in EA group at 14 days, the mRNA expression of RhoA and ROCKII showed no significant difference between 7 and 14 days in each group (P > 0.05).
As determined by in situ hybridization, the RhoA and ROCKII mRNA had a weak expression in sham-operated rats at 7 and 14 days. By contrast, injury induced an increased RhoA and ROCKII mRNA expression at 7 and 14 days. In contrast, treatment of EA, Y27632 or EA + Y27632 could decrease mRNA expression of RhoA and ROCKII at 7 and 14 days. As shown in Figure 5(A–D).
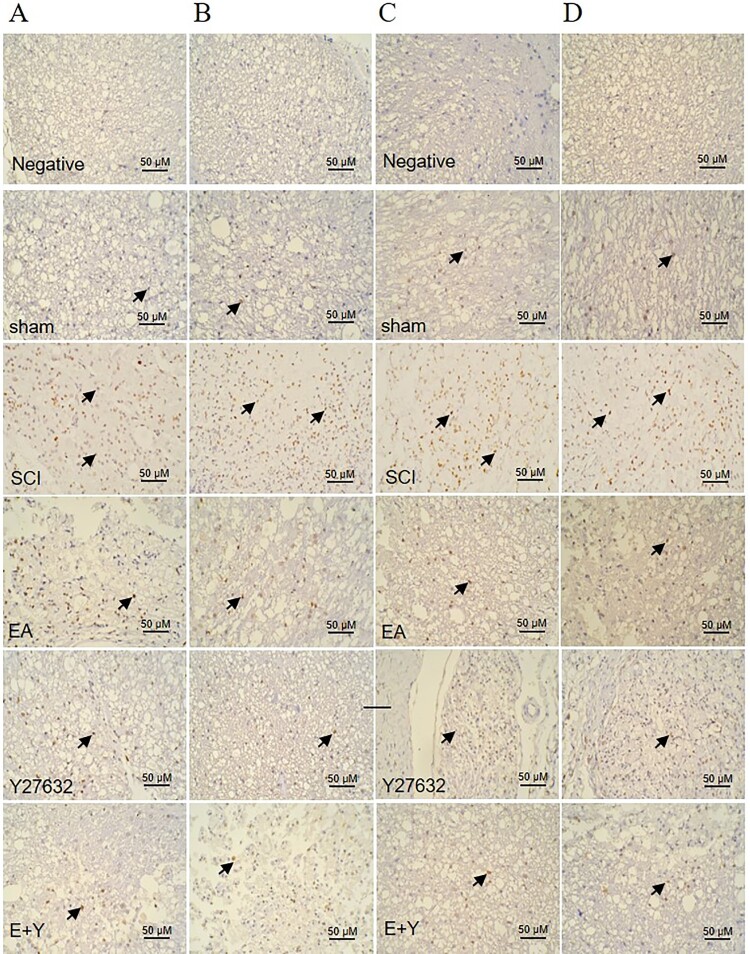
Figure 5.
mRNA expression of RhoA and ROCKII in injured spinal cords at 7 and 14 days. In situ hybridization positive cells contain brown spot or particles as indicated by the arrows. sham: sham operation; SCI: SCI; EA: electroacupuncture; Y: blocking agent Y27632; ROCK II: Rho-associated kinase II. (A) mRNA expression of Rho-A at 7 days; (B) mRNA expression of RhoA at 14 days; (C) mRNA expression of ROCKII at 7 days; (D) mRNA expression of ROCKII at 14 days. (n = 4 rats/group).
Histological changes
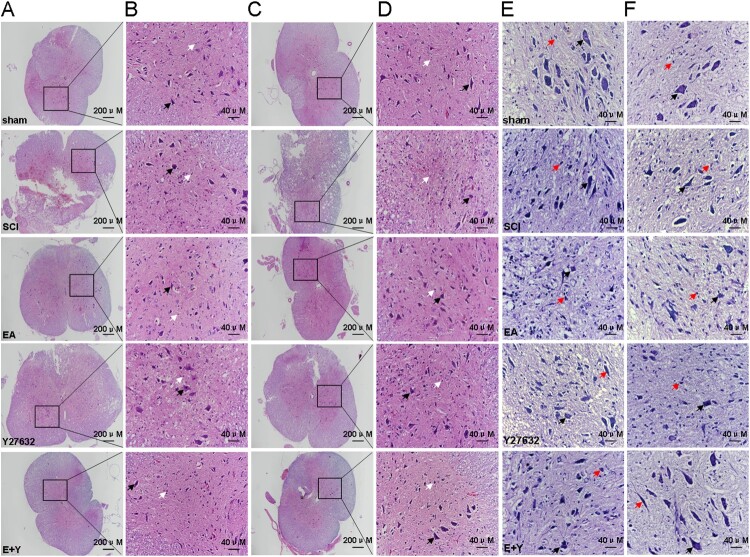
As shown in Figure 6(A–F), the spinal cord tissues in the sham operation group were dense in structure, and the boundary between the gray matter and white matter was clear. The gray matter resembled a butterfly, with the white matter neatly arranged, and the motor neurons were regular in shape. In the SCI model group, the spinal cord tissue structure was damaged, with an unclear boundary between the gray matter and white matter. The amount of neurons in the gray matter area was lost, and a large number of microglial cells were proliferated. As shown in Figure 6(E,F), determined by Nissl staining, there were bluish-purple plaques in the motoneuronal cytoplasm in the gray matter of the spinal tissue in the sham operation group. And the Nissl-stained cells were reduced or even absent, the remaining Nissl cells were cord-like, with deep blue staining, which was most obvious in the central nucleus in SCI group. After treatment with EA or Y27632, or EA + Y27632, the pathological morphology of the spinal cord was improved compared with that in the SCI model group, but there was still edema with microglial cell infiltration. In addition, the Nissl stain was visible, and the neurons in the cytoplasm began to display blue–purple patches. Compared with that in the SCI model group, the pathological morphology was significantly improved, and the Nissl staining was relatively weak.
Figure 6.
HE and Nissl staining in each group. Neuron cell was indicated by the black arrows, and neuroglia cell was indicated by the white arrows in HE staining and by the red arrows in Nissl staining. (A,B) HE staining at 7 days; (C,D): HE staining at 14 days; (E) Nissl staining at 7 days; (F) Nissl staining at 14 days. (n = 4 rats/group) sham: sham operation; SCI: SCI; EA: electroacupuncture; Y: blocking agent Y27632.
The protein expression of p-MLC and MLC by western blotting
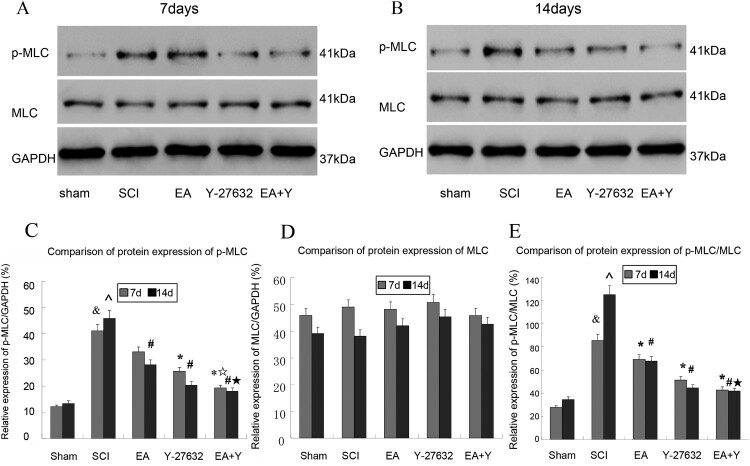
The protein expressions of p-MLC and MLC in each group are shown in Figure 7. The protein expression of p-MLC and the ratio of p-MLC/MLC in SCI group were higher than that in the sham-operated rats (P < 0.01). After treatment with EA or Y27632, or EA + Y27632, except for EA group at 7 days, the protein expression of p-MLC and the ratio of p-MLC/MLC decreased in each treatment group and were significantly lower in the SCI group than in any of the three treatment groups at 7 and 14 days (P < 0.05 or < 0.01). However, there was no significant difference among three treatment groups (P > 0.05). As shown in Figure 7(A–E).
Figure 7.
Phosphorylated myosin light chain(p-MLC) and MLC activation following SCI. (A,B) Western blotting bands for p-MLC, MLC and GAPDH in different groups at 7 and 14 days. (C) Compiled results in a bar graph for the ratio of p-MLC/GAPDH expression. (D) Compiled results in a bar graph for the ratio of MLC/GAPDH expression. (E) Compiled results in a bar graph for the ratio of p-MLC/MLC. & P < 0.05 versus 7d sham; ^ P < 0.05 versus 14d sham; * P < 0.05 versus 7d SCI; # P < 0.05 versus 14d SCI; ☆ P < 0.05 versus 7d EA; ☆ P < 0.05 versus 14d EA. Data are shown as the mean ± standard error of the mean (two-way analysis of variance and Student–Newman–Keuls post hoc test, n = 4 rats/group). sham: sham operation; SCI: SCI; EA: electroacupuncture; Y: blocking agent Y27632. MLC: myosin light chain. p-MLC: phosphorylated MLC. GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
Discussion
The key molecules in the Rho/ROCK signaling pathway involve Rho, ROCK and myosin light chain (MLC). RhoA is a major isomer of Rho family, and ROCKII is one of the most important Rho downstream effector molecule, found in the brain, spinal cord, and muscle.24,25 In our study, the mRNA and protein expression of RhoA and ROCKII in the SCI model group were significantly higher than that in the sham operation group. Separate treatment with EA or blocking agent Y27632 significantly reduced the mRNA and protein expression of RhoA and ROCKII.
The Rho/ROCK signaling pathway affects the growth of the nerve axon through regulating its downstream substrate, MLC. Activated ROCKII can increases the content of phosphorylated MLC, i.e. p-MLC directly or indirectly,26 thereby affecting the actin system and causing collapse of the axon growth cone and finally inhibiting the axon growth.27,28 In our study, we found that after SCI the expression level of p-MLC and the ratio of p-MLC/MLC exhibited the same trend as RhoA and ROCKII, while the expression level of MLC showed no obvious changes. Separate treatment with EA or blocking agent Y27632 or EA + Y27632 significantly decreased the expression level of p-MLC protein and the ratio of p-MLC/MLC but had little effect on MLC expression. The above results suggest that EA may attain the effect of treating SCI by decreasing the protein expression level of p-MLC and the ratio of p-MLC/MLC and that EA + Y27632 treatment was better than that of EA alone or Y27632 alone. Our results are consistent with the findings reported by Li and colleagues.29 However, our study indicated that it was more important for the level of p-MLC or (and) the ratio of p-MLC/MLC that affecting the myosin–actin system and collapse of the axon growth cone, not for the level of MLC.
cPLA2 plays an important role in SCI through regulating its downstream metabolite PGE.17 cPLA2 can directly or indirectly regulate cellular inflammatory response, cell signaling transduction, apoptosis and reproduction. In our study, we found that the activity of cPLA2 and the level of PGE2 were significantly increased after SCI. Y27632, a specific inhibitor of ROCKII, can reduce the activity of cPLA2 and the level of PGE2, especially after 14 days of treatment with Y27632. EA treatment exhibited the same trend as Y27632 did. Liu nai-kui et al 18,20 found that the expression and activation of cPLA2 was significantly increased in the SCI model of transgenic and gene knockout mice, which reached its peak at 7 days after model preparation and was continuously highly expressed at 14 days.
The effect of EA on SCI could achieve by down-regulating the expression of related axonal growth inhibiting factors, reducing inflammatory response, and decreasing apoptosis, and ultimately improving the tissue morphology of the injured spinal cord and promoting the recovery of lower limb movement function. 30 In the current study, we demonstrate that after treatment with EA or blocking agent Y27632 alone, or with EA + Y27632, the injured spinal cord tissue morphology was significantly improved, such as the number of neurons, the degree of neuronal swelling, and the Nissl morphology. This study showed that blocking agent Y27632 could improve behavioral scores (BBB scores) of SCI rats as well as EA treatment and BBB score of the combination EA + Y27632 treatment was higher than that EA treatment or Y27632 treatment. The results showed that EA + Y27632 treatment was superior to the single-treatment method.
This study indicated that the BBB score after 14 days treatment of acupuncture and/or Y27632 was higher than that after 7 days treatment, and there was statistically significant. In contrast, the protein and gene expression of RhoA and ROCK II, p-MLC protein expression and p-MLC/MLC ratio, the level of PGE2 and the activity of cPLA2 enzyme after 14 days treatment were not significantly reduced, compared with 7 days of treatment. EA therapeutic effect for improving the rat hind limbs motor function was better than that for the regulation of Rho/ROCK signaling pathway. The reason probably was that EA could not only reduce the axon growth inhibition and inflammation caused by injury, but also regulate other signaling pathways or ways.31
Conclusions
The current study has revealed a potential mechanism of EA treating SCI. EA significantly improved the hind limb movement function in injured spinal cords of rats. EA was indicated to suppress mRNA and protein expression levels of RhoA and ROCKII, and consequently suppress phosphorylation of MLC and activation of cPLA2. That could inhibit the collapse of the axon growth cone and decrease the level of PGE2, and finally promote the axon growth. Thus, our study shows provides experimental basis for clinical application of EA treatment of SCI.
Abbreviations
AA, arachidonic acid; BBB, Basso-Beattie-Bresnahan: cPLA2, cytosolic phospholipase A2; EA, electroacupuncture; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MAG, myelin-associated glycoprotein; MLC, myosin light chain; NgR, Nogo protein receptor; OMgp, oligodendrocyte myelin glycoprotein; p-MLC, phosphorylated MLC; PGE, prostaglandin; Rho, Ras homolog family member; ROCK, Rho-associated kinase; RT-qPCR, Reverse transcription real-time quantitative polymerase chain reaction; SCI, spinal cord injury; SNK, Student-Newman-Keuls
Disclaimer statements
Contributors YJM conceived and designed the study and revised the paper. ESH and HHY wrote the paper, provided data and revised the paper. JS, XZ, WY, XBZ participated the experiment. All authors approved the final version of the paper.
Conflicts of interests The authors declare that they have no competing interests.
Acknowledgments
Professor Jian-ming WAN in Jiangxi University of Traditional Chinese Medicine provides the guidance of animal model building and the equipment of electric Cortical Contusion Impactor.
Funding Statement
This work was supported by the National Natural Science Foundation of China (CN) (No. 81360562, 81904289).
Ethics statement
The experiments were performed under the supervision and assessment of the Laboratory Animal Ethics Committee of Jiangxi University of Traditional Chinese Medicine, China (permit no. JZLLSC2018-0036).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.
References
- 1.Gao J, Cheng LH, Min YJ.. Clinical research in acupuncture treating the recovery period of SCI. Anmo yu Kangfu. 2015;6(3):18–21. [Google Scholar]
- 2.Gao LJ, Sun YC, Li JJ, Fan B, Pengkun L.et al.. Effects of electroacupuncture in different time on variations of fractional anisotropy mean value of diffusion tensor tractography in spinal cord injured rats. Chin J Rehabil Theory Prac. 2014;20:728–733. [Google Scholar]
- 3.Min YJ, Cheng LH, Yao HH, Liu Y, Zhiyun M, Jia P.. Effect of Santong electroacupuncture on expression of p75 neurotrophin receptor in rats with SCI. Chin J Rehabil Theory Pract 2017;23(6):621–627. [Google Scholar]
- 4.Rabchevsky AG, Patel SP, Springer JE.. Pharmacological interventions for SCI: where do we stand? How might we step forward?. Pharmacol Ther, Spinal Cord 2011;132:15–19. [DOI] [PubMed] [Google Scholar]
- 5.Wang ZQ, Min YJ. Observation of effect on thermal moxibustion treatment of incomplete SCI urinary retention. Journal of Chengdu University of TCM 2013;36(3):60–63. [Google Scholar]
- 6.Wang WC, Lu JC, Wang Q, et al. EFfect on the activities of daily life of the patients with traumatic SCI treated by the paraplegia-triple-needling method. Chinese Acupunctrue&Moxibustion 2012; 32(10):877–881. [PubMed] [Google Scholar]
- 7.Min YJ, Cheng LH, Gao J.. Comparative observations on three-unblocking acupuncture for the treatment of SCI in convalescent patients with paraplegia. Shanghai Zhenjiu Zazhi. 2013;32(10):1010–1013. [Google Scholar]
- 8.Min ZY, Cheng LH, Min YJ.. Effect of electroacupuncture on Nogo/NgR signaling pathway related factors in SCI rats. Journal of Beijing University of Traditional Chinese Medicine 2016;39(11):926–932. [Google Scholar]
- 9.Min ZY, Cheng LH, Min YJ.. REsearch progress of Nogo/NgR pathway in spinal cord nerve regeneration. Journal of Jiangxi University of TCM, 2016;28(3):103–106. [Google Scholar]
- 10.Liu BP, Cafferty WB, Budel SO, et al. Extracellular regulators of axonal growth in the adult central nervous system. Philos Trans R Soc Lond B Biol Sci 2006;361(1473):593–610. doi: 10.1098/rstb.2006.1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bito H, Furuyashiki T, Ishihara H, Shibasaki Y, Ohashi K, Mizuno K, et al. A critical role for a RhoAssociated kinase, p160 ROCK, in determining axon outgrowth in mammalian CNS neurons. Neuron 2000;26(2):431–441. doi: 10.1016/S0896-6273(00)81175-7 [DOI] [PubMed] [Google Scholar]
- 12.Wu X, Walker CL, Lu Q, Wu W, Eddelman DB., Parish JM.et al. Rhoa/Rho kinase mediates neuronal death through regulating cPLA2 activation. Mol Neurobiol 2017;54(9):6885–6895. doi: 10.1007/s12035-016-0187-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monnier PP, Sierra A, Schwah Jan M, Henke-Fahle S, Mueller BK.et al. . The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglyeans of the CNS glial scar. Mol Cell Neure sci, 2003;22(3):319–330. doi: 10.1016/S1044-7431(02)00035-0 [DOI] [PubMed] [Google Scholar]
- 14.Huang YZ, Feng DX, Li J, et al.. In vitro effect of Y27632 on dorsal root ganglion neurons axonal regeneration in neogenetic rats with SCI. Chinese Journal of Spine and SpinalCord 2010;20(9):765–770. [Google Scholar]
- 15.Kubo T, Endo M, Hata K, Taniguchi J, Kitajo K, Tomura S.et al. Myosin IIA is required for neurite outgrowth inhibition produced by repulsive guidance molecule. J Neurochem 2008; 105(1):113–126. doi: 10.1111/j.1471-4159.2007.05125.x [DOI] [PubMed] [Google Scholar]
- 16.Madura T, Yamashita T, Kubo T, Fujitani M, Hosokawa K, Tohyama M.. Activation of Rho in the injured axons following SCI. EMBO Rep 2004; 5(4):412–417. doi: 10.1038/sj.embor.7400117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elizabeth AC, Lisa AM.. Mammalian phospholipases A2: mediators of inflammation, proliferation and apoptosis. Prog Lip Res 2001;40(3):167–197. doi: 10.1016/S0163-7827(01)00002-9 [DOI] [PubMed] [Google Scholar]
- 18.Liu NK, Zhang YP, Titsworth WL, Jiang X, Han S, Lu Pei-Hua, et al. A novel role of phospholipase A2 in mediating spinal cord secondary injury. Ann Neurol 2006;59(4):606–619. doi: 10.1002/ana.20798 [DOI] [PubMed] [Google Scholar]
- 19.Min YJ, Ding LLQ, Cheng LH, Xiao Wei-ping, He Xing-wei, Zhang H, et al. Effect of electroacupuncture on the mRNA and protein expression of RhoA and RhoAssociated kinase II in SCI rats. Neural Regen Res 2017;12(2):276–282. doi: 10.4103/1673-5374.200811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu NK, Deng LX, Zhang YP, Xiao-Fei W, Jian-Guo H, Eddile oaks BS, et al. Cytosolic phospholipase A2 protein as a novel therapeutic target for SCI. Ann Neurol, Spinal Cord 2014; 75(5):644–658. doi: 10.1002/ana.24134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao J, Zhang YJ, Min YJ, et al. Study and application of acupoints in the treatment of paraplegia. Jiangxi J Tradit Chin Med 2013;44(2):54–56. [Google Scholar]
- 22.Min YJ, Deng L, Hong ES.. Orthogonal study on different acupuncture factors based on hypothalamic- pituitary-adrenal axis in rats with kindey Yang deficiency. Shanghai Zhenjiu Zazhi 2016;35:339–343. [Google Scholar]
- 23.Zhang L, Ni YQ, Li YF.. Ligustrazine facilitates hair cell regeneration in the cochlea following gentamicin ototoxicity. Neural Regen Res 2010;5(10):735–740. [Google Scholar]
- 24.Chen XL, Liu L, Wen QQ, Li P, Wang Y, Wei XR, et al. Effect of acupuncture at different acupoints on RhoA/ROCK signalinging pathway in gastric antral smooth muscle tissue of rats with diabetic gastroparesis. Shijie Huaren Xiaohua Zazhi 2016;24(23):3508–3516. [Google Scholar]
- 25.Wu XF, Chen XL, Zheng XN, Guo X, Xie ZQ, Liu L, et al. Effect of different stimulating strength of electroacupuncture on gastrointestinal motility and RhoA/ROCK signaling in gastric antral smooth muscle in diabetic gastroparesis rats. Acupuncture Research 2018;43(3):169–174. [DOI] [PubMed] [Google Scholar]
- 26.Shin HM, Je HD, Gallant C, Tao TC., Hartshorne DJ., Ito M, et al. Differential association and localization of myosin phosphatase subunits during agonist-induced signaling transduction in smooth muscle. Circ Res 2002;90(5):546–553. doi: 10.1161/01.RES.0000012822.23273.EC [DOI] [PubMed] [Google Scholar]
- 27.Khasnis M, Nakatomi A, Gumpper K, Eto M.. Reconstituted human myosin light chain phosphatase reveals distinct roles of two inhibitory phosphorylation sites of the regulatory subunit, MYPT1 . Biochemistry 2014;53(16):2701–2709. doi: 10.1021/bi5001728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khromov A, Choudhury N, Stevenson AS, Somlyo AV., Eto M.. Phosphorylation-dependent autoinhibition of myosin light chain phosphatase accounts for Ca2+ sensitization force of smooth muscle contraction. J Biol Chem 2009;284(32):21569–21579. doi: 10.1074/jbc.M109.019729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li XN, Liang XS, Wu L, Shan XC, Fu H, Mei JL, et al. Electroacupuncture improves limb locomotor function possibly by suppressing Rho-ROCKII pathway related factors in anterior horns of spinal cord in rats with acute SCI. Acupuncture Research 2018;43(7):445–460. [DOI] [PubMed] [Google Scholar]
- 30.Hu HL, Huang XL, Liu F, Renfu Q, et al. Advances in experimental studies on the mechanism of huatuo jiaji (Ex-B2) point acupuncture treatment for SCI. Shanghai J Acu-mox 2016;35(12):1480–1483. [Google Scholar]
- 31.Lü W, Yao HJ, Mo YP, et al. Nerve regeneration related signaling pathway after spinal cord injury (review). Chin J Rehabil Theory Pract 2016;22(3):293–298. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.