Abstract
Context: The mainstay of treatment for acute traumatic spinal cord injury (SCI) is to artificially elevate the patient’s mean arterial pressure (MAP) to >85 mmHg to increase blood flow to the injured spinal cord for 7 days. However, the literature supporting these recommendations are only Class III evidence. In fact, the critical time window in which to elevate MAP after SCI and the optimal vasopressor to use are largely unknown, as is whether cerebrospinal fluid diversion has a role, and this leads to variability among practitioners. Also undefined is whether manipulating these parameters improves neurological outcome.
Objective: Our goal is to better delineate current clinical practice and identify gaps in knowledge surrounding the care of patients with traumatic SCI.
Methods: We undertook a systematic review of the current literature identified from PubMed on MAP elevation and spinal cord parenchymal pressure in acute SCI.
Results: The 8 articles (6 human; 2 porcine) that met our inclusion criteria were all published within the last 6 years. Four were prospective, 1 was retrospective, and 3 were review articles. Only one study was randomized. All of these studies involved small sample sizes and varying lengths of MAP elevation. Choice of vasopressor was variable as well.
Conclusions: From our literature review, we posit that norepinephrine may be the vasopressor of choice, that spinal parenchymal pressure monitors can be safely placed at the injury site, and that the combination of MAP elevation and cerebrospinal fluid drainage may improve neurologic outcome more than either intervention alone.
Keywords: Mean arterial pressure, Spinal cord perfusion pressure, Spinal cord injury, Vasopressor
Introduction
Traumatic spinal cord injury (SCI) is a devastating condition with high morbidity and public health implications. SCI affects >12,000 people in the United States each year1 and creates a tremendous burden on patients, families, and health care providers. Despite this, management of traumatic SCI has changed very little over the past 20 years. Clearly, new efficacious and evidence-based treatment strategies are needed to improve SCI patient care to promote functional recovery and enhance long-term quality of life in this patient population.
The current standard of care for treatment of patients with acute SCI is outlined in the 2013 Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injury.2 Importantly, the Guidelines for surgical management of acute SCI are based only on Level III evidence, largely extrapolated from the traumatic brain injury (TBI) literature. This “option”-level evidence suggests that artificially elevating the blood pressure to a mean arterial pressure (MAP) of >85 mmHg for 7 days after an acute SCI may improve neurologic outcome. Our current standard of care operates under the assumption that spinal cord perfusion pressure (SCPP) is equal to MAP minus cerebrospinal fluid (CSF) pressure. However, there is no direct evidence to support this claim, because there are no evidence-based studies that directly measure the effects of various MAP management strategies on outcome. This concept is extrapolated from the TBI literature even though the spinal cord is not contained in a rigid bony container like the brain.
Moreover, the actual strategy for implementation of MAP management is not standardized among physicians. It is well known that ischemia after SCI can lead to secondary damage to the already-injured spinal cord.3,4 There is evidence that aggressive treatment of hemodynamic alterations can affect neurological outcome, but there is no consensus on which vasopressor should be used and whether one is more effective than another.5 A second gap in knowledge/clinical practice is that recent studies have shown that patients with acute SCI have periods where their MAP falls below the desired 85 mmHg, but the impact of this remains unclear.6 Additionally, the role of CSF drainage (CSFD) to increase spinal cord blood flow (SCBF), although often used in repair of abdominal aortic aneurysms, has not been well studied in patients with acute traumatic SCI but has shown some promise in a recent study using a porcine model of SCI.7
Clearly, a better understanding of the physiologic changes that take place after acute SCI by MAP augmentation, CSFD, and alteration of SCPP is needed to have a better understanding of the relationship among these variables and their effect on the treatment of patients with acute traumatic SCI. We undertook a systematic review of the literature on MAP elevation and SCPP in acute SCI to better delineate current clinical practice and identify the gaps in knowledge surrounding the care of patients with a traumatic SCI.
Methods
We performed a PubMed search to find all articles containing the key words “mean arterial pressure,” “spinal cord perfusion pressure,” “acute,” and “spinal cord injury.” We reviewed the titles and abstracts of the identified articles and excluded any that did not address our questions about MAP elevation and SCPP in acute SCI. Articles that were not in English or that predated the 2013 Guidelines update were excluded. The full text of each of the remaining articles was reviewed and abstracted for our analysis.
Results
In total, 10 articles were identified from our search. One article was excluded because it was a case report that did not directly relate to MAP elevation and SCPP. One article was excluded because it related only to central cord syndrome. The remaining 8 articles that fit our inclusion criteria are outlined in Table 1. It is clear from our literature review that there are several key questions that relate to the management of a patient with an acute traumatic SCI. These questions are outlined and discussed further below.
Table 1. Articles pertaining to the relationship between MAP and SCPP in patients with acute SCI.
| Article | Study type | Subjects | Length of treatment | Outcomes | Concerns |
|---|---|---|---|---|---|
| Kong et al.8 | Prospective, non-randomized | 21 human patients | 5–7 days | All subjects had at least one recording of MAP <80 mm Hg |
|
| Martirosyan et al.7 | Prospective, non-randomized | 15 pigs | 4 h |
|
|
| Readdy et al.9 | Review | 14 human patients | 101.07 h | Complete penetrating injuries less likely than blunt injuries to improve, even with pressor use |
|
| Altaf et al.10 | Prospective, non-randomized | 11 adult human patients | 24 h | DA associated with increased ITP when compared with NE, despite stable MAP |
|
| Yue et al.11 | Review | Human | Recommended 5–7 days | N/A | N/A |
| Yue et al.12 | Review | Human | Variable | NE provided 2 mmHg increase in SCPP without differential MAP effects versus DA. In elderly SCI, more vasopressor and DA-specific complications observed. |
|
| Streijger et al.5 | Prospective, randomized | 22 pigs | 3 h | MAP augmentation with NE resulted in a modest improvement in SCBF during compression and after decompression compared with PE |
|
| Strohm et al.13 | Retrospective | 3 human patients | 48 h | Improved AIS grade after CSFD/elevated MAPs |
|
AIS, ASIA Impairment Scale; CSFD, cerebrospinal fluid drainage; DA, dopamine; ITP, intrathecal pressure; MAP, mean arterial pressure; NE, norepinephrine; PE, phenylephrine; SCBF, spinal cord blood flow; SCPP, spinal cord perfusion pressure.
The 6 human and 2 porcine articles outlined in Table 1 were all published within the last 6 years. Four of these studies were prospective, 1 was retrospective, and 3 were review articles. Only one study was randomized. All of these studies involved small sample sizes, varying lengths of treatment with MAP elevation, and choice of vasopressor. These studies suggest that norepinephrine (NE) may be the vasopressor of choice in acute traumatic SCI, and that MAP augmentation, particularly in conjunction with CSFD, may increase SCPP and SCBF and thus lead to improvement in neurologic outcome. However, because of the variability in treatment length, small patient numbers, and lack of prospective blinded study design, more work is needed to solidify new guidelines for the treatment of acute traumatic SCI. Therefore, we have tried to highlight some of the remaining existing literature throughout this paper that pertains to the questions of MAP elevation, SCPP, and CSFD after acute traumatic SCI.
Discussion
What is the optimal timing and duration of MAP elevation?
Although artificially elevating MAPs to >85 mmHg in patients with acute SCI is common practice in neurosurgical intensive care units (ICUs), there is currently no standardized method of how to do this, how long to do it for, and which vasopressor should be used. In addition, because we do not place parenchymal pressure monitors directly into injured spinal cord like we do in the brain of TBI patients for fear of causing further injury, we have no direct evidence that elevating MAPs for any period of time improves SCPP.
Kong et al.8 examined the existing clinical literature on the impact of blood pressure management after acute SCI. They noted that the goals for both the duration and elevation of MAP were widely variable (>85–90 mmHg and 24 h–7 days, respectively). There was also conflicting information on whether the MAP elevation led to improvement in neurological outcome, with many studies reporting conflicting results on this question. Therefore, it seems as though the optimal timing and duration of MAP elevation, and even the level at which to augment the MAP, remain significant unknown variables in the algorithm for treatment of patients with acute SCI.
In an article by Strohm et al.,13 3 patients who experienced an acute SCI after repair of thoracic or thoracoabdominal aortic aneurysms had lumbar drains placed for CSFD and elevation of MAPs within 24 h of recognition of the injury. All 3 patients experienced a neurologic improvement in American Spinal Injury Association (ASIA) score before discharge; however, there was no consistency in length of time for CSFD or MAP elevation, nor were the MAPs elevated to the same level. In fact, the guidelines for at-risk patients undergoing open or endovascular repair of thoracic or abdominal aortic aneurysms are vague and recommend elevating MAPs from 60 to 90–100 mmHg and using CSFD for anywhere from 36–48 h up to 5–8 days.14,15 Again, these interventions assume that SCPP = MAP – ITP (intrathecal pressure), an assumption for which we have no direct evidence in acute traumatic SCI patients.
Which vasopressor is optimal to achieve MAP goals?
Although guidelines recommending elevation of MAPs to >85 mmHg for patients with acute traumatic SCI have been in existence for over 20 years, there is still no consensus on which vasopressor to use to achieve this MAP goal, with many trauma centers favoring phenylephrine (PE) or NE16 and other centers using dopamine (DA) as their vasopressor of choice.17 Streijger et al.5 compared NE with PE in a porcine model of thoracic SCI. Their results showed a higher risk of hemorrhage at the injury site and lower SCBF levels in the PE group compared with the NE group, suggesting that perhaps NE should be used preferentially over PE in patients with acute traumatic SCI. However, this study only observed the pigs for 3 h after injury, which is a significantly shorter period of MAP elevation than is recommended in adult human patients with SCI.
Altaf et al.10 enrolled 11 adult SCI patients in a study in which NE and DA were used on all patients and ITP was evaluated via the placement of a lumbar drain. The MAP goal was >85 mmHg, and MAP and ITP were continuously monitored in an ICU setting for 3–5 days. Despite a stable MAP, DA use caused an increase in ITP and thus a decrease in SCPP. However, as is the issue with essentially all of the current literature on measurement of SCPP and ITP, the lumbar drainage catheters (and therefore measurements of ITP) were done remotely from the injury site, as these were patients with cervical or thoracic SCI. Therefore, although this study suggests that NE may be a preferred vasopressor for MAP elevation in acute traumatic SCI, there is still no direct evidence linking MAP elevation to increased SCBF directly at the site of injury. Phang and Papadopoulos18 did show that subdural intraspinal pressure was higher at the injury site and seemed to correlate with intraparenchymal pressure at the injury site, but they did not monitor changes in pressure in response to elevated MAPs or CSFD.
Yue et al.12 recently reviewed studies of vasopressor use in patients with acute traumatic SCI to assess vasopressor-associated complications. NE seemed to provide a slight increase in SCPP compared with DA, and there were more vasopressor-associated complications in elderly patients with SCI, specifically tachyarrhythmias, associated with DA. However, many of the studies on vasopressor usage in traumatic SCI patients used small sample sizes and variable time windows of MAP elevation. With this variable and inconsistent evidence, vasopressor usage after acute SCI remains institution and practitioner specific. Since administration of vasopressors is far from a benign intervention, MAP augmentation should be employed only after a discussion with the patient and their family.
Is placing spinal intraparenchymal pressure monitors directly at the injury site safe?
The Injured Spinal Cord Pressure Evaluation Study provided proof of concept that subdural intraspinal pressure at the site of injury could be safely monitored.19 This study included 18 adult patients with ASIA A–C cervical or thoracic SCI. Intraparenchymal monitoring probes were placed at the site of injury with maximal swelling based on MRI scan at the time of operative decompression and stabilization. There were no reported complications related to probe placement. Monitoring was started within 72 h of injury and continued for up to 7 days. Increasing SCPP was shown in 6 patients with the use of vasopressors to elevate MAPs to >85 mmHg. This increase in SCPP was shown to have an improvement in limb motor scores although the data were only analyzed in two of the six patients. Thus, the key finding from this study was proof of concept that spinal intraparenchymal pressure monitors could be safely placed at the site of injury, although any improvement in neurologic outcome or assumptions on MAPs relating to SCPP cannot be drawn from such a small patient sample size.
Is there a relationship between SCPP management and neurologic recovery?
The lack of human evidence relating to direct measurements of SCPP and effect on neurologic outcome with increasing MAPs remains a significant shortcoming in terms of advancing treatment for patients with acute SCI. Optimal SCPP remains unknown, although Squair et al.20 showed that higher SCPP (again measured at a site remote from the injury location) does seem to correlate with an improvement in neurologic recovery. In their study, the degree of neurologic impairment in 92 patients with acute SCI was assessed at baseline and then again 6 months after injury. Patients who were exposed to a SCPP <50 mmHg were less likely to improve from their baseline level of neurologic impairment (P = 0.0056), while patients who improved in neurologic function had SCPP <50 mmHg fewer times than patient who did not improve (P = 0.012). This same effect was not noticed for MAP. Overall, 43/92 patients improved in ASIA Impairment Scale (AIS) grading (46.7%). It should be noted, however, that SCPP was measured via a lumbar drain and not via direct monitoring at the site of injury. The improvement in neurologic outcome with higher SCPP in the study held true even for patients with more severe injuries (AIS A). Their data also suggested that risk for poor neurologic improvement increases with a MAP <70 mmHg. Hawryluk et al.6 also observed better neurologic outcomes with a MAP >70–75 mmHg in the first week after injury. These studies are interesting in that they suggest that maintaining MAP goals 10–15 mmHg lower than recommended guidelines may improve neurologic recovery and potentially obviate the need for vasopressors to attain a MAP goal of >85 mmHg. Other studies have even shown that elevating MAPs does not correlate with an improvement in neurologic outcome, particularly with penetrating SCI as opposed to blunt injuries.9 Additionally, aside from the Squair study, much of the literature pertaining to SCPP is actually drawn from studies in patients who have undergone thoracic or thoracoabdominal aortic aneurysm repair rather than experienced an acute traumatic SCI.21, 22 Therefore, further studies are needed in which SCPP is measured directly at the injury site to determine an optimal pressure range in correlation with an optimal MAP goal.
What happens if the MAP goals are not met?
Despite the level III evidence to maintain MAPs > 85 mmHg after acute traumatic SCI,11 many level 1 trauma centers still fail to meet this goal. In one study done at a Canadian level 1 trauma center, patients with cervical or thoracic SCI were enrolled within 48 h of injury.8 The target MAP for this study was >80 mmHg. All 21 patients had at least one MAP recording <80 mmHg, and 18 patients had at least one MAP recording <70 mmHg. When scrutinized by injury level, patients with cervical and thoracic SCI had an average of 18.4% and 35.9% of MAP measurements <80 mmHg, respectively.
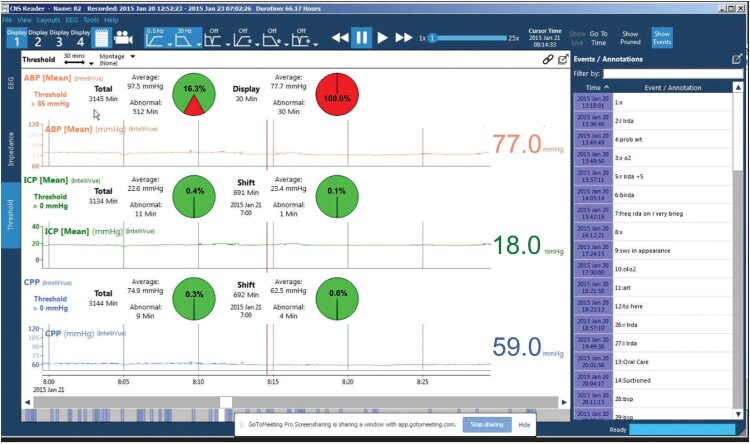
Similarly, we examined compliance with MAP goals in our acute traumatic SCI patient population. Patients were randomized within 24 h of admission to one of two groups. In the first group, MAP goals >85 mmHg were monitored via an arterial line, and vasopressors were administered as needed. In the second group, patients were similarly monitored except there was a laptop in the room with unique software (Moberg, Ambler, PA) designed to transmit an alarm if a measurement fell below 85 mmHg. The software provides immediate feedback to care providers at the bedside (Fig. 1). Our objective was to see whether the use of this software led to fewer MAP measurements out of range and thus an improvement in neurologic outcome (AIS scale) at 6 months after injury. Our overall compliance with the Guidelines of MAP >85 mmHg after acute traumatic SCI was 82.2% with 32 patients enrolled, with a trend towards significance in the patients randomized to monitoring with the specialized software. Our average time of MAP monitoring was 5.7 days, and the most common vasopressor used was PE. These studies show that when attempting to push MAP goals in accordance with recommended guidelines, even high-volume trauma centers fall short of the mark. However, it does remain unclear whether this has an effect on neurological outcome.
Figure 1.
Sample screen shot of Moberg monitor displaying physiologic parameters in real time. The percentages in red represent the amount of time the thresholds were not met for each parameter. The event/annotations down the right side indicate what was happening with the patient at the indicated time points.
Does CSFD lead to improvement in SCPP?
Another important question that needs to be answered is whether MAP elevation alone, CSFD alone, or a combination of both interventions improves SCPP. An elegant study by Martirosyan et al.7 examined each intervention separately and then together in 15 pigs with an induced mid-thoracic acute SCI. Lumbar drains were placed remotely from the injury site. PE was the vasopressor used for this study. Not surprisingly, SCBF decreased significantly (by 56%) from baseline in the control group of 3 pigs. MAP elevation alone (n = 3) resulted in an improvement in SCBF, although the result was still a 34% decrease from baseline. CSFD alone (n = 3) resulted in a 59% decrease in SCBF. However, the group of pigs (n = 3) who underwent both MAP elevation and CSFD showed a 24% increase in SCBF and also an increase in ITP. Although these results are promising, this was a small sample size and a very short monitoring period (4 h) compared with what is typically done in human SCI patients. SCBF was also measured using laser Doppler flowmetry, which is a superficial measurement and must be extrapolated to the injury site. Clearly, a human trial is warranted with longer monitoring periods and larger patient sample sizes.
Future directions
We believe that the porcine model of acute traumatic SCI is invaluable for helping to answer questions about what takes place directly at the level of the injured spinal cord. In particular, the pig spinal cord is similar in diameter to the human spinal cord (7 mm at the T10 level in swine vs. 8–9 mm in humans) and is also surrounded by a prominent layer of CSF like the human spinal cord.23 Importantly, it has also recently been shown that CSF pressures are elevated after experimental SCI in the pig and that the magnitude of CSF pressure elevations were dependent on injury severity.24,25 Porcine models have also shown that SCBF levels are decreased almost immediately after SCI, followed by recovery over the next several days. Hydrostatic pressures remain elevated for several days after injury, and lactate/pyruvate ratios increased within minutes after injury.25
Our group is currently studying acute traumatic SCI using the porcine model as well. A pressure monitor will be placed directly at the site of injury (approximately T10) and lumbar drains will also be inserted into the intrathecal space at the lumbosacral junction. The pigs will be monitored for 7 days with MAP goals >85 mmHg in accordance with the current recommendations of human acute traumatic SCI. The pigs will then be divided into 4 groups of 8 to evaluate the following outcome measures: Group 1: This will be the control group, which will not receive medical or surgical management for their SCI; Group 2: Augmented MAP; Group 3: Lowered ITP via the use of a lumbar drain; and Group 4: A combination of augmented MAP and lowered ITP.
Our first goal is to evaluate the hypothesis that spinal cord intraparenchymal pressure is elevated after a traumatic injury and that this has a negative effect on local perfusion. This study will be unique in that we will be able to measure intraparenchymal pressure directly after SCI. Preinjury values will be used as a control and measurements of intraparenchymal pressure and SCBF will be continuously recorded for 7 days after injury using laser Doppler flowmetry. The second goal is to evaluate the hypothesis that SCPP is augmented by increasing MAP and decreasing CSF pressure. We will use NE as our vasopressor of choice for this study. The pigs in groups 2 and 4 will have MAPs augmented for 7 days. The pigs in groups 3 and 4 will have ITP lowered 15 mm Hg via the lumbar drain and then titrated with response for 7 days after SCI. Finally, we will evaluate the hypothesis that optimizing MAP and CSF pressure leads to improved neurologic outcome at 3 months by assessing the pigs weekly using the Porcine Thoracic Injury Behavioral Scale (PTIBS)24 to evaluate motor function.
We posit that our study will build on previous studies to obtain direct measurements of spinal cord perfusion at the level of injury along with response to therapeutic interventions such as augmenting MAP and lowering ITP. The goal is to update current clinical practice paradigms using direct evidence from a highly relevant translational research model. Defining the relationship between blood flow, intraparenchymal pressure, and ITP on spinal cord perfusion could provide direct physiological evidence to better inform practice paradigms in the care of these patients and lead to improved functional outcomes.
Conclusions
Acute traumatic SCI remains a devastating condition with significant personal and public health implications. Currently, the best evidence we have for acute management is Level III guidelines, which suggest that artificially elevating MAPs to >85 mmHg for a time period of 7 days after injury may improve neurologic outcome. However, these guidelines are based on the assumption that SCPP = MAP – ITP, a claim extrapolated from the TBI literature (CPP = MAP – ICP) for which we have no direct evidence. Indeed, significant gaps in knowledge remain in this patient population, including which vasopressor is optimal to achieve MAP goals, the duration of time to push elevated MAPs, whether or not spinal parenchymal pressure can be safely directly measured in human patients, whether CSFD alone or in conjunction with MAP elevation improves neurologic outcome, and what an optimal SCPP level should be for neurologic recovery after traumatic SCI. From our literature review, we posit that NE may be the vasopressor of choice, that spinal parenchymal pressure monitors can be safely placed at the injury site, and that the combination of MAP elevation and CSF drainage may improve neurologic outcome more than either intervention alone. However, both well-designed translational studies in large animals and appropriately powered, randomized controlled trials involving human patients are still needed to answer these important questions and to better update the guidelines for optimal care in this patient population.
Disclaimer statements
Contributors None.
Funding None.
Conflicts of interest The authors have no conflicts of interest to disclose.
Acknowledgments
The authors thank Kristin Kraus, M.Sc. for her help in the editing and preparation of this paper.
References
- 1.Spinal cord injury facts and figures at a glance. J Spinal Cord Med. 2014;37(1):117–18. doi: 10.1179/1079026813Z.000000000249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joint Section on Disorders of the Spine and Peripheral Nerves of the American Association of Neurological Surgeons and the Congress of Neurological Surgeons. Guidelines for the management of acute cervical spine and spinal cord injury. Neurosurgery. 2013;72(suppl_3):1–259. [Google Scholar]
- 3.Guha A, Tator CH, Rochon J.. Spinal cord blood flow and systemic blood pressure after experimental spinal cord injury in rats. Stroke. 1989;20(3):372–77. doi: 10.1161/01.STR.20.3.372 [DOI] [PubMed] [Google Scholar]
- 4.Dohrmann GJ, Wick KM, Bucy PC.. Spinal cord blood flow patterns in experimental traumatic paraplegia. J Neurosurg. 1973;38(1):52–8. doi: 10.3171/jns.1973.38.1.0052 [DOI] [PubMed] [Google Scholar]
- 5.Streijger F, So K, Manouchehri N, et al. A direct comparison between norepinephrine and phenylephrine for augmenting spinal cord perfusion in a porcine model of spinal cord injury. J Neurotrauma. 2018;35(12):1345–57. doi: 10.1089/neu.2017.5285 [DOI] [PubMed] [Google Scholar]
- 6.Hawryluk G, Whetstone W, Saigal R, et al. Mean arterial blood pressure correlates with neurological recovery after human spinal cord injury: analysis of high frequency physiologic data. J Neurotrauma. 2015;32(24):1958–67. doi: 10.1089/neu.2014.3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martirosyan NL, Kalani MY, Bichard WD, et al. Cerebrospinal fluid drainage and induced hypertension improve spinal cord perfusion after acute spinal cord injury in pigs. Neurosurgery. 2015;76(4):461–68; discussion 468–469. doi: 10.1227/NEU.0000000000000638 [DOI] [PubMed] [Google Scholar]
- 8.Kong CY, Hosseini AM, Belanger LM, et al. A prospective evaluation of hemodynamic management in acute spinal cord injury patients. Spinal Cord. 2013;51(6):466–71. doi: 10.1038/sc.2013.32 [DOI] [PubMed] [Google Scholar]
- 9.Readdy WJ, Saigal R, Whetstone WD, et al. Failure of mean arterial pressure goals to improve outcomes following penetrating spinal cord injury. Neurosurgery. 2016;79(5):708–14. doi: 10.1227/NEU.0000000000001249 [DOI] [PubMed] [Google Scholar]
- 10.Altaf F, Griesdale DE, Belanger L, et al. The differential effects of norepinephrine and dopamine on cerebrospinal fluid pressure and spinal cord perfusion pressure after acute human spinal cord injury. Spinal Cord. 2017;55(1):33–8. doi: 10.1038/sc.2016.79 [DOI] [PubMed] [Google Scholar]
- 11.Yue JK, Winkler EA, Rick JW, et al. Update on critical care for acute spinal cord injury in the setting of polytrauma. Neurosurg Focus. 2017;43(5):E19. doi: 10.3171/2017.7.FOCUS17396 [DOI] [PubMed] [Google Scholar]
- 12.Yue JK, Tsolinas R, Burke JF, et al. Vasopressor support in managing acute spinal cord injury: a knowledge update. J Neurosurg Sci. in press; [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13.Strohm TA, John S, Hussain MS.. Cerebrospinal fluid drainage and blood pressure elevation to treat acute spinal cord infarct. Surg Neurol Int. 2018;9:195. doi: 10.4103/sni.sni_2_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol. 2010;55(14):e27–129. doi: 10.1016/j.jacc.2010.02.015 [DOI] [PubMed] [Google Scholar]
- 15.Ackerman LL, Traynelis VC.. Treatment of delayed-onset neurological deficit after aortic surgery with lumbar cerebrospinal fluid drainage. Neurosurgery. 2002;51(6):1414–22; discussion 1421–1412. doi: 10.1097/00006123-200212000-00011 [DOI] [PubMed] [Google Scholar]
- 16.Sookplung P, Siriussawakul A, Malakouti A, et al. Vasopressor use and effect on blood pressure after severe adult traumatic brain injury. Neurocrit Care. 2011;15(1):46–54. doi: 10.1007/s12028-010-9448-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue T, Manley GT, Patel N, Whetstone WD.. Medical and surgical management after spinal cord injury: vasopressor usage, early surgerys, and complications. J Neurotrauma. 2014;31(3):284–91. doi: 10.1089/neu.2013.3061 [DOI] [PubMed] [Google Scholar]
- 18.Phang I, Papadopoulos MC.. Intraspinal pressure monitoring in a patient with spinal cord injury reveals different intradural compartments: Injured Spinal Cord Pressure Evaluation (ISCoPE) Study. Neurocrit Care. 2015;23(3):414–18. doi: 10.1007/s12028-015-0153-6 [DOI] [PubMed] [Google Scholar]
- 19.Werndle MC, Saadoun S, Phang I, et al. Monitoring of spinal cord perfusion pressure in acute spinal cord injury: initial findings of the injured spinal cord pressure evaluation study*. Crit Care Med. 2014;42(3):646–55. doi: 10.1097/CCM.0000000000000028 [DOI] [PubMed] [Google Scholar]
- 20.Squair JW, Belanger LM, Tsang A, et al. Spinal cord perfusion pressure predicts neurologic recovery in acute spinal cord injury. Neurology. 2017;89(16):1660–67. doi: 10.1212/WNL.0000000000004519 [DOI] [PubMed] [Google Scholar]
- 21.Sandhu HK, Evans JD, Tanaka A, et al. Fluctuations in spinal cord perfusion pressure: a harbinger of delayed paraplegia after thoracoabdominal aortic repair. Semin Thorac Cardiovasc Surg. 2017;29(4):451–59. doi: 10.1053/j.semtcvs.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 22.Weigang E, Hartert M, Siegenthaler MP, et al. Perioperative management to improve neurologic outcome in thoracic or thoracoabdominal aortic stent-grafting. Ann Thorac Surg. 2006;82(5):1679–87. doi: 10.1016/j.athoracsur.2006.05.037 [DOI] [PubMed] [Google Scholar]
- 23.Bozkus H, Crawford NR, Chamberlain RH, et al. Comparative anatomy of the porcine and human thoracic spines with reference to thoracoscopic surgical techniques. Surg Endosc. 2005;19(12):1652–65. doi: 10.1007/s00464-005-0159-9 [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Jones CF, Okon EB, et al. A novel porcine model of traumatic thoracic spinal cord injury. J Neurotrauma. 2013;30(3):142–59. doi: 10.1089/neu.2012.2386 [DOI] [PubMed] [Google Scholar]
- 25.Streijger F, So K, Manouchehri N, et al. Changes in pressure, hemodynamics, and metabolism within the spinal cord during the first 7 days after injury using a porcine model. J Neurotrauma. 2017;34(24):3336–350. doi: 10.1089/neu.2017.5034 [DOI] [PubMed] [Google Scholar]