13C-enriched organic compounds were formed more widely in the early solar system by ammonia-involved formose-type reaction.
Abstract
Solvent-soluble organic matter (SOM) in meteorites, which includes life’s building molecules, is suspected to originate from the cold region of the early solar system, on the basis of 13C enrichment in the molecules. Here, we demonstrate that the isotopic characteristics are reproducible in amino acid synthesis associated with a formose-type reaction in a heated aqueous solution. Both thermochemically driven formose-type reaction and photochemically driven formose-type reaction likely occurred in asteroids and ice-dust grains in the early solar system. Thus, the present results suggest that the formation of 13C-enriched SOM was not specific to the cold outer protosolar disk or the molecular cloud but occurred more widely in the early solar system.
INTRODUCTION
Carbonaceous meteorites contain various types of organic matter, including refractory macromolecules and life’s building molecules, such as amino acids, nucleobases, and sugars (1–3). This matter is crucial for understanding the astrochemical reactions of the early solar system. Furthermore, they are important for understanding chemical evolution in the origin of life, as a portion of these molecules were accreted to the prebiotic Earth.
The origin of primitive organic matter has not yet been identified, although it has been investigated extensively since its discovery in meteorites. Stable isotope ratios have been used as evidence to constrain the source and formation processes of planetary materials. Carbon in polar solvent-soluble organic matter (SOM), such as amino acids, nucleobases, aldehydes, sugar acids, and sugars, are all enriched in 13C relative to the terrestrial carbon standard (2–9). For example, the δ13C values of glycine and alanine in the Murchison meteorite exhibit highly positive values ranging from +13 to +41‰ (per mil) and from +27 to +52‰, respectively (4–6). Nucleobases and sugars also range from +38 to +45‰ and from −1 to +43‰, respectively (2, 3). Furthermore, 13C enrichment was reported for cometary glycine (10). The origin and specific processes of enrichment in 13C have not yet been identified, but they are suspected to have originated from the cold outer regions of the protosolar disk or the cold molecular cloud (5, 6, 11, 12).
Insoluble organic matter (IOM), which represents the major carbon pool in carbonaceous meteorites, is depleted in 13C relative to the terrestrial standard, as represented by negative δ13C values ranging from −34 to −17‰ for unheated Renazzo-like (CR) and Mighei-like (CM) carbonaceous chondrites (13). This depletion is also found in refractory organic macromolecules in interplanetary dust particles and cometary materials (14, 15). The origin of the distinct difference in 13C/12C between coexistent SOM and IOM is unknown, but it may assist in understanding the origin and formation processes of organic compounds in the primitive solar system.
Conflicting models for the origin of IOM and SOM have been proposed in previous studies. Some models propose that the origins of coexistent IOM and SOM are entirely different (16, 17); this is supported by the large differences in 13C/12C. Other models propose specific relations in their origins, such as the formation of SOM by IOM alteration (18, 19) and simultaneous formation in the series of a reaction (20, 21). Cody et al. (20) first proposed that formaldehyde polymerization may be responsible for the formation of IOM and cometary refractory organic solids. Similarities in the compositions of amino acids, sugars, and N-cyclic compounds in the products of formaldehyde polymerization with ammonia to those in primitive meteorites support this model as a major synthetic route of meteorite organics (3, 21–23). Formaldehyde polymerization is referred to as the formose reaction, which forms various sugars and yields caramel-like compounds in the final product (24). Although isotope fractionation in this reaction has been poorly constrained, we hypothesize that the kinetic isotope effect (KIE) in competing elemental reactions within the formose-type reaction potentially generates the large differences in 13C/12C between products.
RESULTS AND DISCUSSION
Isotope fractionation in ammonia-involved formose-type reactions
In this section, we report on 13C enrichment in amino acids and 13C depletion in synthetic IOM, simultaneously produced by a series of reactions in an ammonia-involved formose-type reaction (AFR) containing formaldehyde (δ13C = −42.6‰), glycolaldehyde (δ13C = −16.7‰), and ammonia and reacted at 80°C for up to 90 days (Fig. 1). This temperature is reasonable for parent body reactions, as suggested by several previous studies (25, 26), and similar reactions would have occurred in the early stages of aqueous processes in carbonaceous asteroids. The effect of minerals was not considered to simplify reactions, thus avoiding catalytic and adsorption effects. The isotope differences between amino acids and IOM produced in the reaction are up to 47.8‰ (Fig. 2 and table S1). This difference is consistent with or close to the enrichment observed in primitive carbonaceous meteorites: 31 to 58‰ in the Murchison meteorite, 48 to 56‰ in EET92042, 29‰ in ALH 83100, 57 to 62‰ in GRA95229, and 59 to 63‰ in QUE99177 (5, 6, 13, 27).
Fig. 1. AFR experiments and their products.
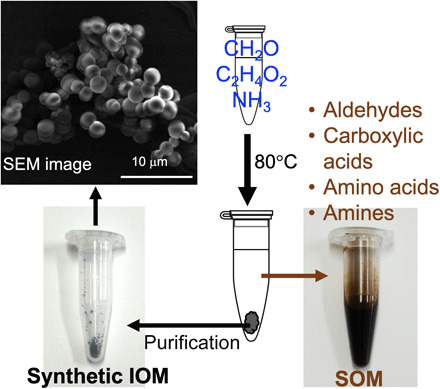
Simple heating of formaldehyde, glycolaldehyde, and ammonia with calcium hydroxide formed amino acids, amines, carboxylic acids, and synthetic IOM. Photo credit for the scanning electron microscopy (SEM) image: Yoshinari Iwasa, Tohoku University. Photo credit for the synthetic IOM and SOM: Yoshihiro Furukawa, Tohoku University.
Fig. 2. Yields and δ13C values.
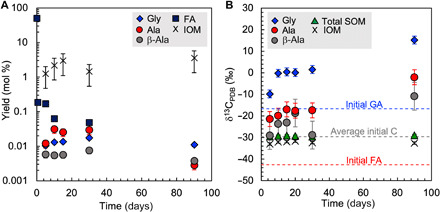
(A) Yields of amino acids and synthetic IOM. Yields are shown on the basis of molar amounts of carbon in products and total starting materials. Initial carbon-based formaldehyde (FA) content is 50%, and glycolaldehyde (GA) content is 50%. (B) Value of δ13C for amino acids, synthetic IOM, and bulk SOM. Blue, red, and gray dashed lines indicate initial δ13C values of GA, FA, and average carbon in the starting materials, respectively. The experimental reproducibility of δ13C values in glycine (Gly), alanine (Ala), β-alanine (β-Ala), total SOM, and synthetic IOM are ±1.8, ±3.5, ±6.4, ±0.4, and ± 0.5‰, respectively (1σ, n = 3).
As the formose reaction is a rapid polymerization of aldehydes, preferential consumption of 12C aldehydes by a KIE is expected, and this leaves a limited amount of 13C-enriched aldehydes in the residue. In this study, formaldehyde was rapidly consumed, decreasing to less than 0.2% after 0.5 days of the reaction (Fig. 2A). Some of the initial formaldehyde would have been used to form hexamethylenetetramine (HMT) rapidly (28). This reaction is reversible, and thus, the following formose-type reaction consumed HMT via formaldehyde (28). The AFR forms large soluble molecules from small aldehydes and ammonia in the products, which further react to form larger insoluble matter (28). Thus, in the products of the present study, most of the carbon was present as large soluble molecules. The yields of glycine, alanine, and β-alanine increased after the substantial aldehyde consumption (Fig. 2A), thereby indicating that amino acids were formed using 13C-enriched formaldehyde because of the KIE (Fig. 3A). We thus concluded that KIE during the AFR was the major factor that produced the large isotope discrimination among products of the AFR. Moreover, the 13C enrichment in the residue of a successive reaction via KIE was proposed in a previous study to explain the decrease in the δ13C value with an increasing carbon number of low–molecular weight hydrocarbons and carboxylic acids in carbonaceous meteorites (29).
Fig. 3. Schematic model of kinetic isotope fractionation in an AFR.
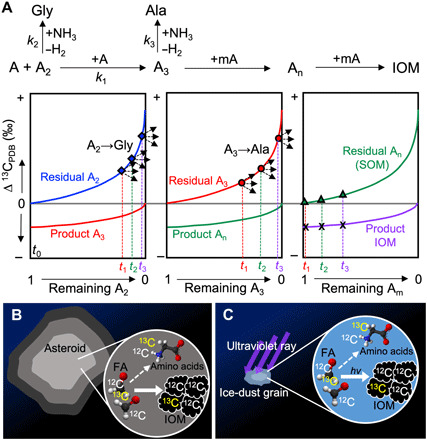
(A) Isotope fractionation in the laboratory experiment. A, A2, A3, mA, and An represent formaldehyde, glycolaldehyde, glyceraldehyde, many formaldehyde, and soluble large aldehyde molecules, respectively; k1, k2, and k3 represent the rate constants of the respective reaction. The initial δ13C values of A and A2 are shown as the same value in this figure but were different in the experiments. The rate constant of an elemental reaction in the formose reaction (k1) was significantly higher than the rate constants of amino acid formation (k2 and k3). Black dashed arrows represent potential isotope fractionation associated with amino acid formation from aldehydes. For simplification, the initial isotope ratios of A and A2 should be the same. The variables t1 to t3 represent the time of amino acid formation from the corresponding aldehyde. (B) Isotope fractionation in carbonaceous asteroids in the early solar system. (C) Isotope fractionation in ice-dust grains in the early solar system.
At temperatures less than 80°C, IOM was not formed within 5 days, whereas at higher temperatures, the yields of IOM increase with temperature and the δ13C values become closer to the bulk carbon isotope composition of the starting aldehydes (fig. S1). This characteristic is consistent with the gradual formation of IOM through the addition of kinetically fractionated, lighter to heavier carbon atoms in AFR.
Five free amino acids were formed, including glycine, alanine, β-alanine, α-aminobutyric acid, and β-aminoisobutyric acid (fig. S2). The amounts of contaminated glycine, alanine, and β-alanine during the experiments and in the starting material were ≤0.1, ≤ 0.2, and ≤0.3% of the product (at 80°C for 5 days), respectively. Thus, the effect of contamination on the yields and δ13C values of amino acids was negligible. The δ13C values of the contaminant amino acids were most likely negative, given that they are biogenic (30). The yields of detected amino acids increased with the reaction temperature (fig. S1A). β-Alanine became the most abundant detected amino acid at the highest temperature. This was also reported by Kebukawa et al., (21) and is consistent with the amino acid compositions in aqueously altered carbonaceous chondrites (31).
Previous studies have suggested that amino acids are formed from aldehydes and ammonia through reductive amination coupled with the oxidation of aldehydes (32). The presence of sugar acids, which represent an oxidized form of sugar, in meteorites and the formation of carboxylic acids in the present study (Fig. 4) are consistent with the aldehyde oxidation in the AFR (7). The higher δ13C values of glycine (the C2 amino acid) than alanine and β-alanine (the C3 amino acids) found in the experiments support the formation of glycine from a high-δ13C glycolaldehyde (C2 aldehyde; δ13C = −16.7‰) and that of alanine and β-alanine from both high-δ13C glycolaldehyde (C2 aldehyde) and low-δ13C formaldehyde (C1 aldehyde; δ13C = −42.6‰; Fig. 2B). The difference in the δ13C values between amino acids in meteorites can provide information for relevant synthetic routes (6). The δ13C values of AFR products can be used to estimate these synthetic routes if the initial δ13C values of formaldehyde and glycolaldehyde in asteroids and comets are known.
Fig. 4. Carbon-based yields of carboxylic acids, amino acids, and synthetic IOM in the experimental product of 80°C heating for 90 days.
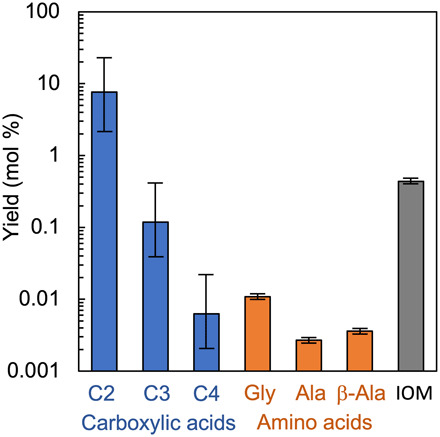
A larger signal of formic acid was detected in the gas chromatography–mass spectrometry (GC/MS) analysis, but the yield was unquantified.
Aldehyde is a major substrate used to produce amino acids with Strecker-cyanohydrin synthesis, as suggested in many previous studies on meteoritic amino acids (1, 6, 33, 34). HCN, another source material of Strecker-cyanohydrin synthesis, is found in meteorites and comets (34–36). Strecker synthesis itself does not produce 13C enrichment in the product amino acids (6); however, 13C enrichment in amino acids might have been possible if the Strecker-cyanohydrin process occurred with AFR, given that the reaction rate of the Strecker-cyanohydrin process is far lower than that of aldehyde polymerization (i.e., formose-type reaction).
IOM contains both labile aliphatic carbon and refractory aromatic carbon (37). The former is more enriched in 13C, whereas the latter is depleted in 13C (38, 39). This difference in 13C/12C has been frequently explained by the contributions of isotopically distinct carbon sources between aliphatic and aromatic carbons (38, 39). However, the difference in the δ13C value of IOM could be explained by the KIE during the aromatization of carbonaceous matter (i.e., lighter aliphatic carbons in the carbonaceous matter are preferentially consumed to form aromatic carbon; Fig. 3A).
Carbon isotope fractionation in the early solar system
The conditions of the present experiments are closer to aqueous processes in carbonaceous asteroids than to the interstellar medium (Fig. 3B). Aldehydes are present in aqueously altered carbonaceous meteorites, and those abundances decrease with an increasing aqueous alteration level (8). This suggests that aldehydes were present before aqueous processes and that AFR produced IOM and 13C-enriched small–molecular weight organics in asteroids. Carboxylic acid is one of the most abundant classes of soluble organic compounds in meteorites and its concentration is more than one order of magnitude higher than that of amino acids in the Murchison meteorite (1). Formic acid, acetic acid, propanoic acid, and butyric acid were formed in the present experiment (Fig. 4). In general, the total amount of carboxylic acids is one order of magnitude higher than that of amino acids. The concentration of total amines in the Murchison meteorite is smaller than the total concentration of amino acids, but they are of the same orders of magnitude (1). This relationship is also observed in the total yields of amines in the present experiment, which were approximately half those of the amino acids (fig. S2). Furthermore, a small amount of calcium carbonate was formed in the experiment. Carbonate is the secondary carbon pool after organic compounds in carbonaceous meteorites; for example, 0.09 weight % in the Murchison meteorite (26). The similarity in the compositions of soluble organic compounds and carbonate, as well as the isotope characteristics of amino acids and IOM between meteorites and the AFR products, indicates the substantial contribution of the AFR in the meteorite’s organic synthesis.
Organic macromolecules found in Comet 81/Wild 2 particles and the Tagish Lake meteorite (the latter of which is considered to contain cold molecular cloud materials or outer protosolar disk materials) indicate that IOM formation also occurred before the accretion of asteroids (15, 40). Both formaldehyde and ammonia are contained in cometary ice (36). A photochemical formose reaction has been deemed possible (41), but isotope fractionation of 13C/12C in this type of reaction has not been investigated. Photolysis of an interstellar ice analog containing methanol, ammonia, water, and carbon monoxide forms HMT as a major product via formaldehyde (42). This suggests that a substantial amount of methanol is converted into formaldehyde in this reaction. Similar photochemical reactions also form amino acids, sugars, nucleobases, and complex insoluble organic molecules (43–46). The δ13C values of refractory organic globules found in samples of Comet 81P/Wild2 (δ13C = −35 ± 3‰) (15), in some interplanetary dust particles (mean δ13C values of clustered and individual interplanetary dust particles are −45 and − 30‰, respectively) (14), and the Tagish Lake meteorite (δ13C = −77 to +16‰, generally below −9‰) (40) all had similar values to primitive meteorites. The 13C-enriched glycine (δ13C = +29 ± 6‰) was detected from the comet-exposed foil of the Stardust mission that collected samples from Comet 81/Wild2, although it was not clear whether the detected glycine was completely pristine (10). The similar isotope gap between the present experimental products (up to 47.8‰) and the gap between the cometary glycine and cometary organic-globules (~64‰) suggest the presence of similar KIE associated with AFR in the cold protosolar disk or cold molecular cloud (Fig. 4C).
Several models have been proposed for the potential mechanism of carbon isotope fractionation in the early solar system, including the gas-phase ion-molecular reaction, isotope-selective photodissociation, and the Fischer-Tropsch–type (FTT) reaction (12, 47–49). Formations of 13C-enriched and 13C-depleted organics have been proposed for a cold molecular cloud of <10 K, resulting from the selective removal of 13C in CO through a gas-phase ion-molecular reaction (49). In contrast, the isotope-selective photodissociation of CO enriches 13C in the gas phase (47). This reaction is also expected to occur in cold regions of molecular clouds. On the basis of these proposed fractionations in the cold region of the early solar system, the linkage between meteoritic 13C-enriched SOM and the cold region of the early solar system has been widely proposed (5, 6, 12). However, these models do not explain 12C enrichment in IOM and 13C enrichment in SOM. Furthermore, these models are contradictory regarding the absence of a positive correlation between 13C enrichment and D and 15N enrichments in organic compounds in primitive solar system materials (i.e., primitive meteorites, interplanetary dust particles, and cometary materials) (14, 15, 40). The D and 15N enrichments are characteristic signatures indicating the origin of organic compounds in cold regions (50–52). The formation of SOM and IOM in AFR presented in this study is substantially different from these cold-specific models but is consistent with the absence of the positive correlation between 13C enrichment and D and 15N enrichments and with the formation of 13C-enriched SOM and 13C-depleted refractory organics found in meteorites and comets.
The FTT reaction was also proposed as a model to explain carbon isotope fractionation found in meteorites (53). In the experimental simulations of the FTT reaction, 13C-depleted long hydrocarbons and 13C-enriched CO2 were formed as a simulation of 13C-depleted IOM and 13C-enriched carbonate in meteorites. However, the produced aliphatic hydrocarbons were different from mostly aromatic IOM carbon (37). Furthermore, the isotope effect that is systematic of unreacted heavy CO and the produced light hydrocarbons from the FTT reaction is opposite to that of the light CO and heavy hydrocarbons found in the Murchison meteorite (29, 53). Meanwhile, the FTT mechanism has been proposed to explain the preponderance of 13C-depleted straight-chain amino acids (i.e., n-ω-amino acids) in thermally altered carbonaceous meteorites because of the similarity in the 13C-depleted characteristics of aliphatic hydrocarbons in FTT products (54).
The δ13C values of IOM produced in the present AFR experiment are close to the average value of the original aldehydes. This suggests that the δ13C values of IOM in meteorites, comets, and interplanetary dust particles (i.e., 12C/13C = ~90) (13–15) represent that of the bulk original polar organic compounds, including aldehydes and reactive organic compounds such as HCN and alcohols. The 12C/13C values of IOM are consistent with the homogeneous value of early solar materials, that is, 12C/13C = 90 ± 10 for the solar system and 12C/13C = 85 ± 20 for cold outer regions (55). This suggests that the 13C enrichment in polar small–molecular weight organic molecules does not have a direct relationship with the outer protosolar materials and cold molecular clouds; instead, it was probably formed more commonly by KIE in the early solar system where thermochemical or photochemical reactions occurred with volatiles, including formaldehyde and ammonia.
MATERIALS AND METHODS
Heating experiments
Heating experiments of aldehydes solution were conducted in an electric furnace with a polytetrafluoroethylene (PTFE) bottle containing 120 mg of paraformaldehyde (≥95%; Serva), 120 mg of glycolaldehyde dimer (Merck), 30 mg of calcium hydroxide (Wako), 0.1 ml of 28% NH3aq (28% NH3 in double-distilled water, PTFE grade; Sigma-Aldrich), and 2 ml of pure water (purified with a Milli-Q Integral MT, total organic carbon: <5 parts per million, 18.2 megohm·cm). The PTFE bottle was used to avoid contamination of Na, Al, Si, and B from a glass tube because the chemical erosion rate of glass by a highly alkaline heated solution is generally high; moreover, it is known that borate ion significantly affect the formose reaction (56, 57). The PTFE bottle was washed with purified water, methanol [grade 5000 for pesticide residue and polychlorinated biphenyl (PCB) analysis; Wako], and hexane (grade 5000 for pesticide residue and PCB analysis; Wako) before use. The amounts of contaminated glycine, alanine, and β-alanine in the starting material were <0.125, <0.2, and <0.3% of the product (at 80°C for 5 days), respectively. Furthermore, the amounts of contaminated glycine, alanine, and β-alanine during the course of the experiment were <0.05, 0.2, and, 0.3% of the product (at 80°C for 5 days), respectively. The product was centrifuged to separate SOM and synthetic IOM fractions. The synthetic IOM fraction was treated with an aqueous solution of HCl, rinsed with water, methanol, and hexane, and dried in a vacuum.
At most, 0.5 to 3% of carbon in the aldehydes was converted to IOM within 90 days (Fig. 2A). The δ13C values of bulk IOM were slightly lower than the average δ13C value of formaldehyde and glycolaldehyde in the starting material (δ13C = −29.7‰) but increased slightly from −33.0 to −31.6‰ during incubation (Fig. 2B). In contrast, the δ13C values of bulk SOM (i.e., the supernatant of the reaction products, δ13C = −29.6 to −29.0‰) were slightly higher than the average δ13C value of the starting aldehydes (Fig. 2B). This isotopically balanced discrimination in 13C/12C among the starting aldehydes, SOM, and IOM indicates no leakage of carbon from the system during the experiments.
Product analyses
The synthetic IOM and an aliquot of the SOM were analyzed with an elemental analyzer isotope ratio mass spectrometer (EA-irMS; Flash 2000 connected to DELTA V; Thermo Fisher Scientific). The reproducibility of this analysis was ±0.06‰ (1σ, n = 6). Amino acids and amines were collected from an aliquot of the SOM. A part of this fraction was analyzed by ultrahigh-performance liquid chromatography–tandem mass spectrometry (Shimadzu LCMS-8040) with a reversed-phase column [Waters CORTECS; 2.1-mm inner diameter (ID), 100 mm long, 1.7-μm particles] and eluents (ammonium formate buffer and acetonitrile) as described elsewhere (58).
Another part of this fraction was purified with a cation exchange resin (AG50-X8; Bio-Rad). Then, the cation fraction was analyzed by gas chromatography–combustion–isotope ratio mass spectrometry (GC-c-irMS) with Agilent DB-5 column (30 m, 0.25-mm ID, 25-μm-thick film) for the compound-specific carbon isotopic composition of amino acids as described in (59). The reproductivity of this analysis was ±1.4‰ (1σ) (60).
Carboxylic acids were analyzed with a gas chromatograph mass spectrometer (Agilent 5977B) with an Agilent DB-FATWAX UI column (30 m, 0.25-mm ID, 25-μm-thick film). Sample injection was conducted using the solid-phase microextraction technique (polyethylene glycol phase coating). The oven temperature was initially kept at 35°C for 6 min, ramped up to 135°C at 25°C/min and then to 185°C at 1.5°C/min, and left for 10 min at 185°C.
Formaldehyde was analyzed with an ultraviolet-visible spectrophotometer (Agilent 8453) after derivatization with 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole (AHMT). A total of 2 ml of diluted supernatant of the sample was added to 2 ml of a 5 M KOH solution and 2 ml of 34.5 mM AHMT in 0.5 M HCl solution. This mixture was vortexed and left at ambient temperature (~20°C) for 20 min. Then, 2 ml of 32.6 mM KIO4 in 0.2 M KOH solution was added to the sample solution. This mixture solution was vortexed, left for ~1 min, and analyzed with the ultraviolet-visible spectrophotometer at 550 nm.
Acknowledgments
We thank Y. Takizawa for the support with GC-c-irMS analysis and Y. Oba, T. Kakegawa, and A. Ishida for the insightful discussion. Funding: This study was supported by Japan Society for the Promotion of Science grants to Y.F. (grant no. 18H03728) and Y.C. (grant nos. 19 K21888 and 20H00185) and by the Institute of Low-temperature Science of Hokkaido University (grant no. 20G049). Author contributions: Y.F. conceived the study. Y.I. performed the experiments, LC/MS, and GC/MS under the supervision of Y.F. Y.C. performed compound-specific isotope analysis with Y.I. and Y.F. Y.F. wrote the first draft of the paper. All authors discussed the data and agreed upon the conclusions. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/18/eabd3575/DC1
REFERENCES AND NOTES
- 1.S. Pizzarello, G. W. Cooper, G. J. Flynn, in Meteorites and the Early Solar System 2, D. S. Lauretta, H. Y. McSween Jr., Eds. (The University of Arizona Press, 2006), pp. 625–651. [Google Scholar]
- 2.Martins Z., Botta O., Fogel M. L., Sephton M. A., Glavin D. P., Watson J. S., Dworkin J. P., Schwartz A. W., Ehrenfreund P., Extraterrestrial nucleobases in the Murchison meteorite. Earth Planet. Sci. Lett. 270, 130–136 (2008). [Google Scholar]
- 3.Furukawa Y., Chikaraishi Y., Ohkouchi N., Ogawa N. O., Glavin D. P., Dworkin J. P., Abe C., Nakamura T., Extraterrestrial ribose and other sugars in primitive meteorites. Proc. Natl. Acad. Sci. U.S.A. 116, 24440–24445 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engel M. H., Macko S. A., Silfer J. A., Carbon isotope composition of individual amino acids in the Murchison meteorite. Nature 348, 47–49 (1990). [DOI] [PubMed] [Google Scholar]
- 5.Pizzarello S., Huang Y., Fuller M., The carbon isotopic distribution of Murchison amino acids. Geochim. Cosmochim. Acta 68, 4963–4969 (2004). [Google Scholar]
- 6.Elsila J. E., Charnley S. B., Burton A. S., Glavin D. P., Dworkin J. P., Compound-specific carbon, nitrogen, and hydrogen isotopic ratios for amino acids in CM and CR chondrites and their use in evaluating potential formation pathways. Meteorit. Planet. Sci. 47, 1517–1536 (2012). [Google Scholar]
- 7.Cooper G., Rios A. C., Enantiomer excesses of rare and common sugar derivatives in carbonaceous meteorites. Proc. Natl. Acad. Sci. U.S.A. 113, E3322–E3331 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aponte J. C., Whitaker D., Powner M. W., Elsila J. E., Dworkin J. P., Analyses of aliphatic aldehydes and ketones in carbonaceous chondrites. ACS Earth Space Chem. 3, 463–472 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simkus D. N., Aponte J. C., Hilts R. W., Elsila J. E., Herd C. D. K., Compound-specific carbon isotope compositions of aldehydes and ketones in the Murchison meteorite. Meteorit. Planet. Sci. 54, 142–156 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elsila J. E., Glavin D. P., Dworkin J. P., Cometary glycine detected in samples returned by Stardust. Meteorit. Planet. Sci. 44, 1323–1330 (2009). [Google Scholar]
- 11.Epstein S., Krishnamurthy R. V., Cronin J. R., Pizzarello S., Yuen G. U., Unusual stable isotope ratios in amino acid and carboxylic acid extracts from the Murchison meteorite. Nature 326, 477–479 (1987). [DOI] [PubMed] [Google Scholar]
- 12.J. R. Cronin, S. Chang, in The Chemistry of Life’s Origins, J. M. Greenberg, C. X. Mendoza-Gómez, V. Pirronello, Eds. (Springer, Dordrecht, 1993), pp. 209–258. [Google Scholar]
- 13.Alexander C. M. O’D., Fogel M., Yabuta H., Cody G. D., The origin and evolution of chondrites recorded in the elemental and isotopic compositions of their macromolecular organic matter. Geochim. Cosmochim. Acta 71, 4380–4403 (2007). [Google Scholar]
- 14.Messenger S., Identification of molecular-cloud material in interplanetary dust particles. Nature 404, 968–971 (2000). [DOI] [PubMed] [Google Scholar]
- 15.De Gregorio B. T., Stroud R. M., Nittler L. R., Alexander C. M. O’D., Kilcoyne A. L. D., Zega T. J., Isotopic anomalies in organic nanoglobules from Comet 81P/Wild 2: Comparison to Murchison nanoglobules and isotopic anomalies induced in terrestrial organics by electron irradiation. Geochim. Cosmochim. Acta 74, 4454–4470 (2010). [Google Scholar]
- 16.Remusat L., Derenne S., Robert F., Knicker H., New pyrolytic and spectroscopic data on Orgueil and Murchison insoluble organic matter: A different origin than soluble? Geochim. Cosmochim. Acta 69, 3919–3932 (2005). [Google Scholar]
- 17.Derenne S., Robert F., Model of molecular structure of the insoluble organic matter isolated from Murchison meteorite. Meteorit. Planet. Sci. 45, 1461–1475 (2010). [Google Scholar]
- 18.Komiya M., Shimoyama A., Organic compounds from insoluble organic matter isolated from the Murchison carbonaceous chondrite by heating experiments. Bull. Chem. Soc. Jpn. 69, 53–58 (1996). [Google Scholar]
- 19.Huang Y., Alexandre M. R., Wang Y., Structure and isotopic ratios of aliphatic side chains in the insoluble organic matter of the Murchison carbonaceous chondrite. Earth Planet. Sci. Lett. 259, 517–525 (2007). [Google Scholar]
- 20.Cody G. D., Heying E., Alexander C. M. O., Nittler L. R., Kilcoyne A. L. D., Sandford S. A., Stroud R. M., Establishing a molecular relationship between chondritic and cometary organic solids. Proc. Natl. Acad. Sci. U.S.A. 108, 19171–19176 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kebukawa Y., Chan Q. H. S., Tachibana S., Kobayashi K., Zolensky M. E., One-pot synthesis of amino acid precursors with insoluble organic matter in planetesimals with aqueous activity. Sci. Adv. 3, e1602093 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koga T., Naraoka H., A new family of extraterrestrial amino acids in the Murchison meteorite. Sci. Rep. 7, 636 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naraoka H., Hashiguchi M., Distinct distribution of soluble N-heterocyclic compounds between CM and CR chondrites. Geochem. J. 53, 33–40 (2019). [Google Scholar]
- 24.Breslow R., On the mechanism of the formose reaction. Tetrahedron Lett. 1, 22–26 (1959). [Google Scholar]
- 25.Baker L., Franchi I. A., Wright I. P., Pillinger C. T., The oxygen isotopic composition of water from Tagish Lake: Its relationship to low-temperature phases and to other carbonaceous chondrites. Meteorit. Planet. Sci. 37, 977–985 (2002). [Google Scholar]
- 26.Alexander C. M. O’D., Bowden R., Fogel M. L., Howard K. T., Carbonate abundances and isotopic compositions in chondrites. Meteorit. Planet. Sci. 50, 810–833 (2015). [Google Scholar]
- 27.Martins Z., Alexander C. M. O’D., Orzechowska G. E., Fogel M. L., Ehrenfreund P., Indigenous amino acids in primitive CR meteorites. Meteorit. Planet. Sci. 42, 2125–2136 (2007). [Google Scholar]
- 28.Kebukawa Y., Nakashima S., Mita H., Muramatsu Y., Kobayashi K., Molecular evolution during hydrothermal reactions from formaldehyde and ammonia simulating aqueous alteration in meteorite parent bodies. Icarus 347, 113827 (2020). [Google Scholar]
- 29.Yuen G., Blair N., Des Marais D. J., Chang S., Carbon isotope composition of low molecular weight hydrocarbons and monocarboxylic acids from Murchison meteorite. Nature 307, 252–254 (1984). [DOI] [PubMed] [Google Scholar]
- 30.Minagawa M., Egawa S., Kabaya Y., Karasawa-Tsuru K., Carbon and nitrogen isotope analysis for amino acids from biological sample. J. Mass Spectrom. Soc. Jpn. 40, 47–56 (1992). [Google Scholar]
- 31.Glavin D. P., Callahan M. P., Dworkin J. P., Elsila J. E., The effects of parent body processes on amino acids in carbonaceous chondrites. Meteorit. Planet. Sci. 45, 1948–1972 (2010). [Google Scholar]
- 32.Yanagawa H., Kobayashi Y., Egami F., Genesis of amino acids in the primeval sea: Formation of amino acids from sugars and ammonia in a modified sea medium. J. Biochem. 87, 359–362 (1980). [DOI] [PubMed] [Google Scholar]
- 33.Peltzer E. T., Bada J. L., α-Hydroxycarboxylic acids in the Murchison meteorite. Nature 272, 443–444 (1978). [Google Scholar]
- 34.Smith K. E., House C. H., Arevalo R. D. Jr., Dworkin J. P., Callahan M. P., Organometallic compounds as carriers of extraterrestrial cyanide in primitive meteorites. Nat. Commun. 10, 2777 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pizzarello S., Hydrogen cyanide in the Murchison meteorite. Astrophys. J. Lett. 754, L27 (2012). [Google Scholar]
- 36.Le Roy L., Altwegg K., Balsiger H., Berthelier J.-J., Bieler A., Briois C., Calmonte U., Combi M. R., De Keyser J., Dhooghe F., Fiethe B., Fuselier S. A., Gasc S., Gombosi T. I., Hässig M., Jäckel A., Rubin M., Tzou C.-Y., Inventory of the volatiles on comet 67P/Churyumov-Gerasimenko from Rosetta/ROSINA. Astron. Astrophys. 583, A1 (2015). [Google Scholar]
- 37.Cody G. D., Alexander C. M. O’D., NMR studies of chemical structural variation of insoluble organic matter from different carbonaceous chondrite groups. Geochim. Cosmochim. Acta 69, 1085–1097 (2005). [Google Scholar]
- 38.Kerridge J. F., Chang S., Shipp R., Isotopic characterisation of kerogen-like material in the Murchison carbonaceous chondrite. Geochim. Cosmochim. Acta 51, 2527–2540 (1987). [DOI] [PubMed] [Google Scholar]
- 39.Sephton M. A., Watson J. S., Meredith W., Love G. D., Gilmour I., Snape C. E., Multiple cosmic sources for meteorite macromolecules? Astrobiology 15, 779–786 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura-Messenger K., Messenger S., Keller L. P., Clemett S. J., Zolensky M. E., Organic globules in the Tagish Lake meteorite: Remnants of the protosolar disk. Science 314, 1439–1442 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Shigemasa Y., Matsuda Y., Sakazawa C., Matsuura T., Formose reactions. II. The photochemical formose reaction. Bull. Chem. Soc. Jpn. 50, 222–226 (1977). [Google Scholar]
- 42.Bernstein M. P., Sandford S. A., Allamandola L. J., Chang S., Scharberg M. A., Organic-compounds produced by photolysis of realistic interstellar and cometary ice analogs containing methanol. Astrophys. J. 454, 327–344 (1995). [Google Scholar]
- 43.Dworkin J. P., Deamer D. W., Sandford S. A., Allamandola L. J., Self-assembling amphiphilic molecules: Synthesis in simulated interstellar/precometary ices. Proc. Natl. Acad. Sci. U.S.A. 98, 815–819 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernstein M. P., Dworkin J. P., Sandford S. A., Cooper G. W., Allamandola L. J., Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues. Nature 416, 401–403 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Meinert C., Myrgorodska I., de Marcellus P., Buhse T., Nahon L., Hoffmann S. V., d’Hendecourt L. L. S., Meierhenrich U. J., Ribose and related sugars from ultraviolet irradiation of interstellar ice analogs. Science 352, 208–212 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Oba Y., Takano Y., Naraoka H., Watanabe N., Kouchi A., Nucleobase synthesis in interstellar ices. Nat. Commun. 10, 4413 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tielens A. G. G. M., Charnley S. B., Circumstellar and interstellar synthesis of organic molecules. Orig. Life Evol. Biosph. 27, 23–51 (1997). [PubMed] [Google Scholar]
- 48.Sandford S. A., Bernstein M. P., Dworkin J. P., Assessment of the interstellar processes leading to deuterium enrichment in meteoritic organics. Meteorit. Planet. Sci. 36, 1117–1133 (2001). [Google Scholar]
- 49.Charnley S. B., Ehrenfreund P., Millar T. J., Boogert A. C. A., Markwick A. J., Butner H. M., Ruiterkamp R., Rodgers S. D., Observational tests for grain chemistry: Posterior isotopic labelling. Mon. Not. R. Astron. Soc. 347, 157–162 (2004). [Google Scholar]
- 50.Millar T. J., Deuterium fractionation in interstellar clouds. Space Sci. Rev. 106, 73–86 (2003). [Google Scholar]
- 51.Watanabe N., Kouchi A., Ice surface reactions: A key to chemical evolution in space. Prog. Surf. Sci. 83, 439–489 (2008). [Google Scholar]
- 52.Rodgers S. D., Charnley S. B., Nitrogen superfractionation in dense cloud cores. Mon. Not. R. Astron. Soc. 385, L48–L52 (2008). [Google Scholar]
- 53.Lancet M. S., Anders E., Carbon isotope fractionation in the Fischer-Tropsch synthesis and in meteorites. Science 170, 980–982 (1970). [DOI] [PubMed] [Google Scholar]
- 54.Burton A. S., Elsila J. E., Callahan M. P., Martin M. G., Glavin D. P., Johnson N. M., Dworkin J. P., A propensity for n-ω-amino acids in thermally altered Antarctic meteorites. Meteorit. Planet. Sci. 47, 374–386 (2012). [Google Scholar]
- 55.Wyckoff S., Kleine M., Peterson B. A., Wehinger P. A., Ziurys L. M., Carbon isotope abundances in comets. Astrophys. J. 535, 991–999 (2000). [Google Scholar]
- 56.Kim H.-J., Ricardo A., Illangkoon H. I., Kim M. J., Carrigan M. A., Frye F., Benner S. A., Synthesis of carbohydrates in mineral-guided prebiotic cycles. J. Am. Chem. Soc. 133, 9457–9468 (2011). [DOI] [PubMed] [Google Scholar]
- 57.Furukawa Y., Horiuchi M., Kakegawa T., Selective stabilization of ribose by borate. Orig. Life Evol. Biosph. 43, 353–361 (2013). [DOI] [PubMed] [Google Scholar]
- 58.Furukawa Y., Nakazawa H., Sekine T., Kobayashi T., Kakegawa T., Nucleobase and amino acid formation through impacts of meteorites on the early ocean. Earth Planet. Sci. Lett. 429, 216–222 (2015). [Google Scholar]
- 59.Chikaraishi Y., Ogawa N. O., Kashiyama Y., Takano Y., Suga H., Tomitani A., Miyashita H., Kitazato H., Ohkouchi N., Determination of aquatic food-web structure based on compound-specific nitrogen isotopic composition of amino acids. Limnol. Oceanogr. Methods 7, 740–750 (2009). [Google Scholar]
- 60.Takizawa Y., Takano Y., Choi B., Dharampal P. S., Steffan S. A., Ogawa N. O., Ohkouchi N., Chikaraishi Y., A new insight into isotopic fractionation associated with decarboxylation in organisms: Implications for amino acid isotope approaches in biogeoscience. Prog. Earth Planet. Sci 7, 50 (2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/18/eabd3575/DC1


