Abstract
Background:
Individuals with cerebral palsy (CP) are at increased risk for obesity and obesity-related complications. Studies of total body fat in those with CP are inconsistent and studies of abdominal fat are lacking in children with CP. The objective of this study was to determine if ambulatory children with spastic CP have greater central adiposity compared to typically developing children.
Methodology:
Eighteen ambulatory children with spastic CP (n=5 girls; 8.6 ± 2.9 years) and 18 age-, sex- and race-matched typically developing children (controls; 8.9 ± 2.1 years) participated in this cross-sectional study. Children with CP were classified as I or II using the Gross Motor Function Classification System. Dual-energy x-ray absorptiometry assessed body composition, including total body, trunk and abdominal fat mass, fat-free mass, fat mass index (FMI) and fat-free mass index (FFMI).
Results:
There were no group differences in fat mass, fat-free mass, FMI, and FFMI in the total body, fat mass, fat-free mass and FFMI in the trunk, or fat mass, visceral fat mass and subcutaneous fat mass in the abdomen (p > 0.05). Compared to controls, children with CP had higher trunk FMI, abdominal FMI and visceral FMI (p < 0.05). Although marginally insignificant (p = 0.088), children with CP had higher subcutaneous FMI.
Conclusions:
Ambulatory children with spastic CP have elevated central adiposity, especially in the visceral region, despite no differences in measures of total body fat. How this relates to cardiometabolic disease progression in those with CP requires further investigation.
Keywords: Cerebral palsy, dual-energy x-ray absorptiometry, abdominal fat, visceral fat, subcutaneous fat
Introduction
Cerebral palsy (CP) results from damage or malformation of the developing brain, and is the most common physical disability of childhood (3.1 per 1,000) (1). Compared to typically developing children, children with CP have a very low level of physical activity (2, 3), which is associated with an increased accumulation of body fat (4), as well as poor cardiorespiratory fitness (5) and an underdeveloped musculoskeletal system that is infiltrated with fat (2, 3). As children with CP transition into and throughout their adult years, an additional decline in mobility has been observed (6), which further increases their risk for developing excess body fat. Furthermore, there is an alarming prevalence of obesity-related complications in the CP population (7, 8). Prior to the 3rd decade of life, young adults with CP have a 2-fold greater odds of cardiometabolic diseases compared to young adults without CP (7). By middle age (40–60 years), the multimorbidity prevalence is approximately 58%, which is 1.5–2.9 times higher than the general population of middle-aged adults (8). These findings may help to explain why cardiovascular-related mortality is higher in those with CP compared to the general population (9).
Despite a physical activity level consistent with an increased risk for developing obesity and obesity-related complications, indices commonly used to assess obesity status, such as body mass index (BMI), suggest that the obesity prevalence of children with mild to moderate CP (10) is similar to the general population of children (11). However, the accuracy of BMI as an indicator of obesity is limited in children with CP (12, 13). Furthermore, there have been inconsistent findings on the degree of total body fat (2, 3, 14).
The prevalent cardiometabolic complications coupled with inconsistent findings of total body fat have led our team and others to investigate regional fat depots in individuals with CP. Using in vivo imaging, we observed that ambulatory (3) and nonambulatory (2) children with spastic CP have elevated musculoskeletal fat infiltration of the lower extremities, but no differences in the surrounding subcutaneous fat depot or BMI compared to typically developing children. Further, adults with CP have a higher degree of abdominal fat compared to neurotypical adults (15). These findings (2, 3, 15) suggest a unique fat distribution pattern in those with CP that may go unnoticed when assessing total body fat using standard methods. Moreover, studies of the non-CP population have reported an association between abdominal fat and cardiometabolic disease risk factors independent of total body fat (16, 17). However, to date, abdominal fat distribution has not been assessed in children with CP. Identifying obesity indices in childhood may allow early detection of cardiometabolic disease risk in this population. Accordingly, the primary objective of this study was to determine if there is a higher level of abdominal fat in ambulatory children with spastic CP than typically developing children. We hypothesized that ambulatory children with spastic CP would have higher degree of central adiposity compared to age-, sex- and race-matched typically developing children.
Methods
Participants
Eighteen ambulatory children with mild spastic CP and 19 typically developing children (controls), some of whom had participated in a previous study with details regarding study enrollment (3), that were between 4 and 12 years of age were included in this cross-sectional study. Of the 19 controls, 18 were matched to children with CP for age (± 1.5 years), sex and race. The study procedures were approved by the Institutional Review Board. Prior to testing, written consent and assent were obtained from the parents and the participants, respectively.
Anthropometrics
Height and body mass were measured while the child was in a t-shirt and shorts. Height was measured to the nearest 0.1 cm using a stadiometer (Seca 217; Seca GmbH & Co. KG., Hamburg, GER). Body mass was measured to the nearest 0.2 kg using a digital scale (Detecto, 6550, Cardinal Scale, Webb City, MO). Normative graphs published by the Centers for Disease Control and Prevention (18) were used to determine age- and sex-based percentiles of height, body mass and BMI.
Tanner staging
Sexual maturity was assessed by a physician assistant using the Tanner staging technique (19). The technique is based on a 5-point scale, with I indicating no development and V indicating full development. Pubic hair and breast development were assessed in girls. Pubic hair and testicular/penile development were assessed in boys.
Gross motor function classification system
A physician assistant determined the gross motor function of children with CP using the gross motor function classification system (GMFCS) (20). This scale ranges from I to V: GMFCS I and II reflect gross motor independence, such as walking and running, but with limited ability of speed, balance and coordination; GMFCS III reflects the use of assistive walking devices; and GMFCS IV and V reflect wheelchair-empowered mobility. Children with CP classified as GMFCS I or II were included in this study, and are considered “ambulatory” as they do not require assistive walking devices.
Dual-energy X-ray absorptiometry
Whole body dual-energy X-ray absorptiometry (DXA) scans were acquired using standard imaging and positioning protocols (Discovery W, Pediatric Whole Body Analysis; Hologic Inc., Bedord, MA), software version 12.7.3.1. To limit motion during the scan, children with CP were secured from the waist down using the BodyFIX (Medical Intelligence Inc, Schwabmunchen, Germany) and a modified procedure, as previously described (21). The modified BodyFIX procedure has no effect on body composition estimates from DXA in children (22). Abdominal fat mass was obtained based on the manufacturer’s instructions. Briefly, the android fat region was determined by an automatically defined region of interest box that was placed just above the iliac crest. The automatically determined height of the region of interest box was 20 % of the distance from the top of the iliac crest to the base of the skull while in the supine position. Abdominal fat was estimated within the android region based on the manufacturer’s automated software. Visceral fat mass was estimated within the visceral cavity and excluded the transverse abdominis. Subcutaneous fat mass was estimated by subtracting visceral fat mass and transverse abdominis fat mass from total abdominal fat mass. Studies have shown a moderate to strong correlation between abdominal fat measured using DXA and the gold standard, computed tomography, suggesting that DXA provides an accurate estimate of abdominal fat (23, 24).
To account for differences in body height, fat mass index (FMI) and fat-free mass index (FFMI) from the total body (excluding the head), trunk and abdominal regions were determined by dividing tissue mass (kg) by height (m) squared as follows:
Statistical analysis
Data were analyzed using SPSS version 24.0 (IBM Corp, Armonk, NY). All variables were checked for normality by examining skewness, kurtosis and the Shapiro-Wilk test. Group differences (CP vs. controls) were determined using an independent (unpaired) t test if the data were normally distributed or a Mann-Whitney U test if the data were non-normally distributed. One sample t tests were used to determine if height, body mass or BMI percentile were different from the 50th age- and sex-based percentiles in children with CP and controls. Values are presented as mean ± SD unless stated otherwise. Alpha level was set at 0.05. All tests were two-tailed. The magnitude of the effects were determined using Cohen’s d (d = mean difference between groups / pooled SD), with 0.2, 0.5 and 0.8 representing small, moderate and large effect sizes, respectively (25). We chose to analyze group differences using unpaired rather than paired statistical analyses because it is the more statistically conservative method in this context (26).
Results
Descriptive characteristics of the 18 age-, sex- and race-matched participants included in the study are presented in Table 1. Of the children with CP, seven were classified as GMFCS I and eleven were classified as GMFCS II. Compared to controls, children with CP had lower height percentile (p < 0.01), but there were no differences in age, Tanner stage pubic hair or testicular-penile/breast, body mass, body mass percentile, BMI or BMI percentile (all p > 0.05). Although marginally insignificant (p = 0.054), children with CP had lower height compared to controls. Furthermore, height, body mass and BMI percentile were not different from the 50th age- and sex-based percentiles in the controls (p > 0.70). While body mass and BMI percentile were not different from the 50th age- and sex-based percentiles in children with CP (p > 0.10), children with CP did have lower height percentile compared to the 50th age- and sex-based percentile (p < 0.001).
Table 1.
Descriptive characteristics of children with cerebral palsy (CP) and typically developing children (Con).
| CP(n = 18) | Con (n = 18) | p | d | |
|---|---|---|---|---|
| GMFCS (I/II) | 7/11 | - | ||
| Age (y) | 8.6 ± 2.9 | 8.9 ± 2.1 | 0.654 | 0.153 |
| Sex female, n (%) | 5 (28 %) | 5 (28 %) | ||
| Tanner stage (I/II/III) | ||||
| Pubic hair | 12/5/1 | 13/3/2 | 0.445 | |
| Testicular-penile/breast | 15/2/1 | 13/4/1 | 0.788 | |
| Height (m) | 1.23 ± 0.16 | 1.33 ± 0.12 | 0.054 | 0.675 |
| Height (%) | 19 ± 23 | 51 ± 29 | <0.001 | 1.242 |
| Body mass (kg) | 28.2 ± 11.4 | 29.4 ± 6.4 | 0.694 | 0.138 |
| Body mass (%) | 36 ± 34 | 50 ± 29 | 0.179 | 0.459 |
| BMI (kg/m2) | 17.9 ± 4.4 | 16.6 ± 2.2 | 0.562 | 0.387 |
| BMI (%) | 55 ± 34 | 47 ± 31 | 0.487 | 0.235 |
GMFCS, gross motor function classification system; BMI, body mass index. Differences between children with CP and Con were determined using independent t test or Mann-Whitney U test. Values are means ± SD. d = the effect size of group differences. Significant differences are bolded.
Body composition measures of the total body and trunk regions are presented in Table 2. There were no group differences in total body fat mass or fat-free mass, trunk fat mass or fat-free mass, total body FMI or FFMI, or trunk FFMI (all p > 0.05). Compared to controls, children with CP had higher trunk FMI (p = 0.019).
Table 2.
Body composition of children with cerebral palsy (CP) and typically developing children (Con).
| CP (n = 18) | Con (n = 18) | p | d | |
|---|---|---|---|---|
| Mass | ||||
| Total body fat (kg) | 8.3 ± 5.2 | 7.2 ± 3.1 | 0.474 | 0.250 |
| Total body fat-free (kg) | 16.1 ± 6.5 | 18.6 ± 4.0 | 0.189 | 0.461 |
| Trunk fat (kg) | 3.5 ± 2.4 | 2.9 ± 1.4 | 0.606 | 0.316 |
| Trunk fat-free (kg) | 8.7 ± 3.3 | 9.7 ± 1.9 | 0.267 | 0.391 |
| Mass index | ||||
| Total body FMI (kg/m2) | 5.2 ± 2.7 | 4.1 ± 1.7 | 0.133 | 0.478 |
| Total body FFMI (kg/m2) | 10.2 ± 2.0 | 10.4 ± 0.9 | 0.151 | 0.169 |
| Trunk FMI (kg/m2) | 2.2 ± 1.3 | 1.7 ± 0.8 | 0.019 | 0.512 |
| Trunk FFMI (kg/m2) | 5.5 ± 1.0 | 5.5 ± 0.4 | 0.847 | 0.067 |
FMI, fat mass index; FFMI, fat-free mass index. Differences between children with CP and Con were determined using independent t test or Mann-Whitney U test. Values are means ± SD. d = the effect size of group differences. Significant differences are bolded.
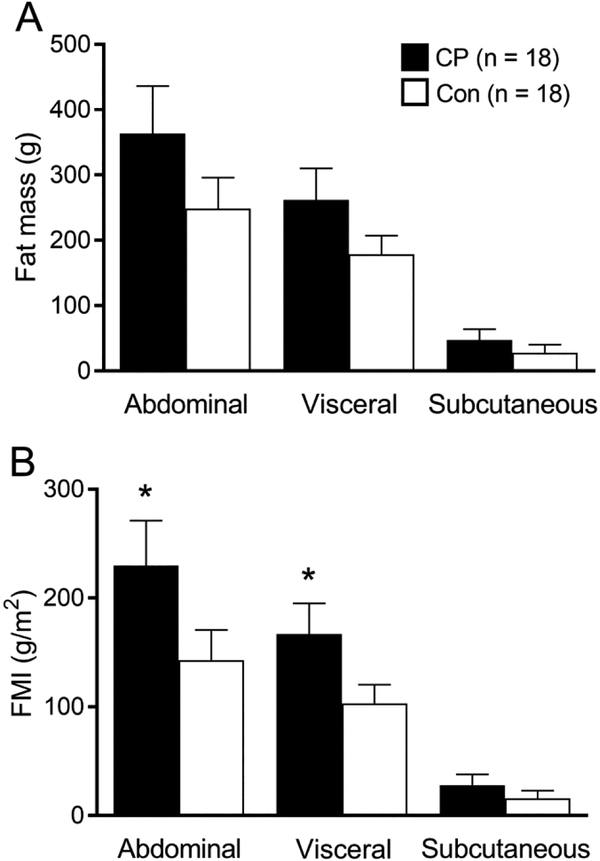
Body composition measures of the abdominal region and its visceral and subcutaneous subregions are shown in Figure 1. There were no group differences in abdominal fat mass (d = 0.449, p = 0.217), visceral fat mass (d = 0.508, p = 0.193) or subcutaneous fat mass (d = 0.312, p = 0.193) (Figure 1A). However, compared to controls, children with CP had higher abdominal FMI (d = 0.587, p = 0.002) and visceral FMI (d = 0.670, p = 0.001). Although marginally insignificant (d = 0.330, p = 0.088), children with CP had higher subcutaneous FMI compared to controls (Figure 1B).
Figure 1.
Bar graphs represent (A) fat mass and (B) fat mass index (FMI) of abdominal, visceral, and subcutaneous regions for ambulatory children with spastic cerebral palsy (CP; black bar) and typically developing children (Con; white bar). Values are means ± SE. *Different from controls, p < 0.05.
Discussion
To our knowledge, this is the first study to report greater central adiposity in children with CP compared to typically developing children. This is particularly important because adults with CP present with a high prevalence of cardiometabolic diseases and multimorbidity, which is associated with obesity status (7, 8). While an abundance of body fat in children without CP is associated with increased cardiometabolic morbidity and mortality in adulthood (27, 28), excess abdominal fat accretion may have a unique and more profound influence on cardiometabolic processes (16, 17). Therefore, showing that children with CP have greater central adiposity compared to typically developing children is clinically important. The finding is especially worrisome because it was observed in ambulatory children with CP, a relatively high functioning group. It is possible that the elevated central adiposity in children with CP is even more profound in children with CP who are nonambulatory (12).
The observation that ambulatory children with spastic CP have higher abdominal fat compared to typically developing children is consistent with previous studies in adults with CP (15, 29). Moreover, to date, there have been inconsistent findings on the degree of total body fat in children with CP (2, 3, 14). These inconsistencies may be due in part to inappropriate methodological approaches and misinterpretation of results. Children with CP are shorter and have low fat-free mass (2, 3, 14), which influences commonly used methods and formulas that estimate body fat, such as BMI and % body fat. In the current study, we found no differences in the absolute mass of total body, trunk or abdominal fat depots between groups. After we accounted for height and excluded fat-free mass by assessing FMI, we found that ambulatory children with spastic CP had a higher proportion of trunk, abdominal and visceral fat compared to typically developing children.
The elevated trunk and abdominal fat in children with CP observed in the current study may be driven by the visceral and subcutaneous fat regions. Although the elevated subcutaneous fat in children with CP was marginally insignificant (p = 0.088), these findings have important clinical implications for cardiometabolic disease risk in those with CP. Studies have found that both visceral and subcutaneous fat regions are associated with multiple cardiometabolic disease risk factors, even after adjusting for total body fat or other measures of obesity (16, 17). However, visceral fat may have a stronger association with cardiometabolic disease risk factors compared to subcutaneous fat (17). Moreover, visceral fat, but not subcutaneous fat or measures of total body fat, is associated with incident pre-diabetes and type 2 diabetes mellitus in obese patients (30).
The difference in associations between visceral and subcutaneous fat with cardiometabolic disease risk may have to do with the functional differences and anatomical distribution of the fat depots. Visceral fat has different adipocyte characteristics (31) and a higher inflammatory profile compared to subcutaneous fat (32). Visceral fat surrounds abdominal organs, such as the liver and pancreas, whereas subcutaneous fat is superficial to the abdominal cavity. Therefore, if elevated or metabolically altered, visceral fat may have a stronger impact on interference of important physiological processes, including insulin resistance and metabolic syndrome (31). Characterizing the abdominal fat depots in those with CP is needed to better understand the potential impact of these fat depots on cardiometabolic disease risk. Taken together, these findings and others (2, 3, 12) underscore the importance of understanding the unusual body composition profiles in children with CP. This becomes clinically important because of a recent recommendations to assess body fat and nutritional status using skinfold thickness (33, 34), which quantifies subcutaneous fat rather than visceral fat.
The present study has several strengths. First, we used DXA to assess body composition and abdominal fat distribution in children with CP. DXA provides many measures of body composition with just one or a few scans, has relatively fast scanning times and does not require labor-intensive analysis procedures like computed tomography or magnetic resonance imaging. This is important when working with children with spastic CP because they can have involuntary spasms and a difficult time holding still which can negatively affect image quality and reduce the amount of time available for scanning. Second, DXA is measuring tissue distribution within a region of the abdomen rather than at a single slice, which is widely used with in vivo imaging of abdominal fat distribution (15, 35, 36). Research has shown inconsistencies with a single slice in predicting volumetric regions of visceral and subcutaneous fat and their relationships with cardiometabolic disease risk factors (37, 38). These differences are likely due to variations of tissue distribution within the abdomen. Therefore, larger regions are more representative of tissue distribution and are more sensitive to detect differences in younger, smaller or leaner children (23), and in populations with a unique body composition, such as children with CP. Lastly, great care was taken to obtain a representative sample of typically developing control children. Specifically, typically developing children that were matched to children with CP for age, sex and race. In addition, the controls were not different from the 50th percentile for height, body mass and BMI.
The limitations of this study must also be discussed. One limitation is the small sample size and results should therefore be interpreted with caution. The region where visceral and subcutaneous fat mass were obtained may have included the inferior part of the liver. Elevated fat of the liver has been reported in populations with higher abdominal fat distributions, such as obese adolescents (39). Using the DXA method in the present study, it is not possible to segment out the liver, such as in other in vivo imaging techniques. Furthermore, it is unknown if children with CP have elevated fat infiltration of the liver. However, capturing a small portion of the liver would likely have little influence on the visceral fat measure due to its small volumetric contribution to the abdominal region of interest. Lastly, markers of cardiometabolic health were not assessed. It is unknown if the higher visceral fat in ambulatory children with CP is associated with a higher inflammatory profile or altered adipokine release, and therefore requires further attention.
Conclusion
Compared to typically developing children, ambulatory children with spastic CP have elevated abdominal fat, especially in the visceral region. The finding is consistent with the elevated risk for initiating or accelerating the development of obesity-related complications, which may not be captured by measures of total body fat in this pediatric population. Future studies are needed to track the development of obesity indices throughout growth and development, and to identify effective treatment strategies to limit the excess accumulation of fat within the abdominal region in children with CP.
Acknowledgements
This study was supported by the National Institutes of Health (HD071397 and HD090126) and by the University of Michigan Advanced Rehabilitation Research Training Program in Community Living and Participation from the National Institute on Disability, Independent Living, and Rehabilitation Research (90AR5020–0200). We thank all research participants and their families. We thank Patricia Groves and Keri DiAlessandro for their assistance with testing and Nancy Lennon for assistance with recruitment. All authors declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure:
The authors declared no conflict of interest.
References
- 1.Christensen D, Van Naarden Braun K, Doernberg NS, Maenner MJ, Arneson CL, Durkin MS, et al. 2014. Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning - Autism and Developmental Disabilities Monitoring Network, USA, 2008. Developmental medicine and child neurology. 56: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson DL, Miller F, Subramanian P, Modlesky CM. 2009. Adipose tissue infiltration of skeletal muscle in children with cerebral palsy. The Journal of pediatrics. 154: 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitney DG, Singh H, Miller F, Barbe MF, Slade JM, Pohlig RT, et al. 2017. Cortical bone deficit and fat infiltration of bone marrow and skeletal muscle in ambulatory children with mild spastic cerebral palsy. Bone. 94: 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson JA, Crespo NC, Sallis JF, Patterson RE, Elder JP. 2012. Dietary-related and physical activity-related predictors of obesity in children: a 2-year prospective study. Childhood obesity. 8: 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia CC, Alcocer-Gamboa A, Ruiz MP, Caballero IM, Faigenbaum AD, Esteve-Lanao J, et al. 2016. Metabolic, cardiorespiratory, and neuromuscular fitness performance in children with cerebral palsy: A comparison with healthy youth. Journal of exercise rehabilitation. 12: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day SM, Wu YW, Strauss DJ, Shavelle RM, Reynolds RJ. 2007. Change in ambulatory ability of adolescents and young adults with cerebral palsy. Developmental medicine and child neurology. 49: 647–653. [DOI] [PubMed] [Google Scholar]
- 7.Whitney DG, Hurvitz EA, Ryan JM, Devlin MJ, Caird MS, French ZP, et al. 2018. Noncommunicable disease and multimorbidity in young adults with cerebral palsy. Clinical epidemiology. 10: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cremer N, Hurvitz EA, Peterson MD. 2017. Multimorbidity in Middle-Aged Adults with Cerebral Palsy. The American journal of medicine. 130: 744 e749–744 e715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strauss D, Cable W, Shavelle R. 1999. Causes of excess mortality in cerebral palsy. Developmental medicine and child neurology. 41: 580–585. [DOI] [PubMed] [Google Scholar]
- 10.Rogozinski BM, Davids JR, Davis RB, Christopher LM, Anderson JP, Jameson GG, et al. 2007. Prevalence of obesity in ambulatory children with cerebral palsy. The Journal of bone and joint surgery American volume. 89: 2421–2426. [DOI] [PubMed] [Google Scholar]
- 11.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. 2016. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. Journal of the american medical association. 315: 2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitney DG, Miller F, Pohlig RT, Modlesky CM 2018. BMI does not capture the high fat mass index and low fat-free mass index in children with cerebral palsy and proposed statistical models that improve this accuracy. International journal of obesity. 43:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duran I, Schulze J, Martakis K, Stark C, Schoenau E. 2018. Diagnostic performance of body mass index to identify excess body fat in children with cerebral palsy. Developmental medicine and child neurology. 60: 680–686. [DOI] [PubMed] [Google Scholar]
- 14.Oftedal S, Davies PS, Boyd RN, Stevenson RD, Ware RS, Keawutan P, et al. 2017. Body composition, diet, and physical activity: a longitudinal cohort study in preschoolers with cerebral palsy. The American journal of clinical nutrition. 105: 369–378 [DOI] [PubMed] [Google Scholar]
- 15.Peterson MD, Zhang P, Haapala HJ, Wang SC, Hurvitz EA. 2015. Greater Adipose Tissue Distribution and Diminished Spinal Musculoskeletal Density in Adults With Cerebral Palsy. Archives of physical medicine and rehabilitation. 96: 1828–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly AS, Dengel DR, Hodges J, Zhang L, Moran A, Chow L, et al. 2014. The relative contributions of the abdominal visceral and subcutaneous fat depots to cardiometabolic risk in youth. Clinical obesity. 4: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Philipsen A, Jorgensen ME, Vistisen D, Sandbaek A, Almdal TP, Christiansen JS, et al. 2015. Associations between ultrasound measures of abdominal fat distribution and indices of glucose metabolism in a population at high risk of type 2 diabetes: the ADDITION-PRO study. PloS one. 10: e0123062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. 2000. CDC growth charts: United States. Advance data. 314:1–27. [PubMed] [Google Scholar]
- 19.Tanner J Growth and Adolescence, 2nd edn. Blackwell Scientific Publications: Oxford, 1962. [Google Scholar]
- 20.Wood E, Rosenbaum P. 2000. The gross motor function classification system for cerebral palsy: a study of reliability and stability over time. Developmental medicine and child neurology. 42: 292–296. [DOI] [PubMed] [Google Scholar]
- 21.Modlesky CM, Cavaiola ML, Smith JJ, Rowe DA, Johnson DL, Miller F. 2010. A DXA-based mathematical model predicts midthigh muscle mass from magnetic resonance imaging in typically developing children but not in those with quadriplegic cerebral palsy. The Journal of nutrition. 140: 2260–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rawal R, Miller F, Modlesky CM. 2011. Effect of a novel procedure for limiting motion on body composition and bone estimates by dual-energy X-ray absorptiometry in children. The Journal of pediatrics. 159: 691–694 e692. [DOI] [PubMed] [Google Scholar]
- 23.Bosch TA, Dengel DR, Kelly AS, Sinaiko AR, Moran A, Steinberger J. 2015. Visceral adipose tissue measured by DXA correlates with measurement by CT and is associated with cardiometabolic risk factors in children. Pediatric obesity. 10: 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaul S, Rothney MP, Peters DM, Wacker WK, Davis CE, Shapiro MD, et al. 2012. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring). 20: 1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen J Statistical power for the behavioral sciences, 2nd edn. Lawrence ErlBaum Associates: Hillsdale, NJ, 1988. [Google Scholar]
- 26.Rosner B Fundamentals of biostatistics, Seventh edn. Brooks/Cole, Cengage Learning: Boston, MA, 2010. [Google Scholar]
- 27.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. 2010. Childhood obesity, other cardiovascular risk factors, and premature death. The New England journal of medicine. 362: 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bibbins-Domingo K, Coxson P, Pletcher MJ, Lightwood J, Goldman L. 2007. Adolescent overweight and future adult coronary heart disease. The New England journal of medicine. 357: 2371–2379. [DOI] [PubMed] [Google Scholar]
- 29.Ryan JM, Crowley VE, Hensey O, McGahey A, Gormley J. 2014. Waist circumference provides an indication of numerous cardiometabolic risk factors in adults with cerebral palsy. Archives of physical medicine and rehabilitation. 95: 1540–1546. [DOI] [PubMed] [Google Scholar]
- 30.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, et al. 2012. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. Journal of the american medical association. 308: 1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kranendonk ME, van Herwaarden JA, Stupkova T, de Jager W, Vink A, Moll FL, et al. 2015. Inflammatory characteristics of distinct abdominal adipose tissue depots relate differently to metabolic risk factors for cardiovascular disease: distinct fat depots and vascular risk factors. Atherosclerosis. 239: 419–427. [DOI] [PubMed] [Google Scholar]
- 32.Tam CS, Heilbronn LK, Henegar C, Wong M, Cowell CT, Cowley MJ, et al. 2011. An early inflammatory gene profile in visceral adipose tissue in children. International journal of pediatric obesity. 6: e360–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevenson RD. 2018. Body mass index and obesity in children with cerebral palsy. Developmental medicine and child neurology. 60: 639. [DOI] [PubMed] [Google Scholar]
- 34.Finbraten AK, Martins C, Andersen GL, Skranes J, Brannsether B, Juliusson PB, et al. 2015. Assessment of body composition in children with cerebral palsy: a cross-sectional study in Norway. Developmental medicine and child neurology.57: 858–864 [DOI] [PubMed] [Google Scholar]
- 35.Huang TT, Johnson MS, Gower BA, Goran MI. 2002. Effect of changes in fat distribution on the rates of change of insulin response in children. Obesity research. 10: 978–984. [DOI] [PubMed] [Google Scholar]
- 36.Miyazaki Y, Glass L, Triplitt C, Wajcberg E, Mandarino LJ, DeFronzo RA. 2002. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. American journal of physiology endocrinology and metabolism. 283: E1135–1143. [DOI] [PubMed] [Google Scholar]
- 37.Irlbeck T, Massaro JM, Bamberg F, O’Donnell CJ, Hoffmann U, Fox CS. 2010. Association between single-slice measurements of visceral and abdominal subcutaneous adipose tissue with volumetric measurements: the Framingham Heart Study. International journal of obesity. 34: 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen W, Punyanitya M, Chen J, Gallagher D, Albu J, Pi-Sunyer X, et al. 2007. Visceral adipose tissue: relationships between single slice areas at different locations and obesity-related health risks. International journal of obesity. 31: 763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kovanlikaya A, Mittelman SD, Ward A, Geffner ME, Dorey F, Gilsanz V. 2005. Obesity and fat quantification in lean tissues using three-point Dixon MR imaging. Pediatric radiology. 35: 601–607. [DOI] [PubMed] [Google Scholar]