Abstract
Bone marrow lesions (BML) are painful changes in subchondral bone which can be reliably identified on magnetic resonance imaging and have been identified in patients suffering from hip osteoarthritis (OA) and related conditions. Created via repetitive microdamage at the articular surface and dysregulated subchondral healing, BML have been linked to traumatic, inflammatory, degenerative, metabolic and neoplastic processes. While BML are known to be a common pathology throughout the body, BML at the hip have not been extensively studied in comparison to those at the knee. Due to the hip’s unique biomechanical architecture, function and loading, and independent risk factors leading to hip OA, hip BMLs must be independently understood. The identification of BML in the setting of a pre-osteoarthritic condition may provide a target for treatment and prevention of joint degeneration. By continuing to define and refine the relationships between BML, subchondral bone cysts and OA, prevention, diagnosis and treatment of OA could shift, leading to an improved quality of life and increased longevity of individuals’ native hips.
INTRODUCTION
One of the most prevalent musculoskeletal diseases and causes of lower extremity pain, osteoarthritis (OA) is characterized by discomfort, disability and morbidity [1]. While cartilage damage in OA has been well studied [2], the contributions of subchondral bone to this disease process have been explored only recently. Bone marrow lesions (BML), areas of altered magnetic resonance imaging signal within subchondral bone, are commonly identified in osteoarthritic patients [3]; however, BML remain poorly understood, especially about the hip.
Using detailed evaluation of femoral head radiographs, Solomon classified osteoarthritic hips based on the presence of characteristic anatomical features, destructive changes and repair phenomena. It was then suggested that OA holds some association with underlying joint pathologies, including abnormal loading forces, abnormal articular cartilage or defective subchondral support [4]. The BML, one such subchondral pathology, has been routinely identified in patients with symptomatic hip OA. While they tend to occur in patients with reduced hip cartilage volume, BML also may be found as a precursor to cartilage depletion [5] or less commonly in asymptomatic patients without OA of the hip [6]. Due to their predictable classification patterns on MRI [7], BML may become targets of future therapies to treat or delay progression of hip OA. BML in the knee have been increasingly studied [8–10]; however, due to the hip’s unique biomechanical architecture, function and loading, independent risk factors leading to hip OA [11], as well as the association between BML and multiple non-osteoarthritic diseases [12], hip BMLs must be independently understood [13]. This review will summarize the etiology, pathogenesis, radiographic findings, classification and treatment associated with BML and OA of the hip.
DEFINITIONS
Throughout life, yellow (inactive) bone marrow predictably replaces red (active) bone marrow; however, the appearance of BML on MRI is a departure from this easily identifiable and non-pathologic process of marrow replacement [3]. BML remain a key feature of OA and have strong associations with pain [3], cartilage defects [5], and indicated joint replacement [6]. In the knee, BML have been reliably shown to predate these sequelae of OA [11, 13]. But while BML do correlate with hip pain and joint space narrowing, this pathology has been far less studied than its counterpart at the knee [14].
BML were originally known as ‘bone marrow edema’, a term originally coined in 1988 to describe the characteristic MRI changes. Because edema has not been shown to contribute substantially to subchondral pathology and is not necessary to diagnose pathologic bone marrow changes either radiographically or developmentally, ‘bone marrow edema’ is a misleading term. This language has subsequently been updated to ‘bone marrow lesion’ to more accurately describe what may be a non-inflammatory, non-edematous scenario [6]. However, some literature continues to use the terms ‘bone marrow edema’ and ‘bone marrow lesion’ interchangeably [15].
In general, BML are understood to develop following osteonecrosis and pose a significant risk for development or progression of hip pain. Although reversible and treatable in early stages, BML represent microscopic collapse within necrotic subchondral bone and indicate an unfavorable prognosis [7].
One distinct yet closely related pathology is the subchondral bone cyst (SBC) [5]. Unlike early BML, SBC are irreversible and may signal degenerative progression [1], but they are identifiable both radiographically and on MRI [6]. While commonly referred to as ‘cysts’, SBC lack the epithelial lining or fluid-filled state that would truly designate them as such [6]. Therefore, SBC are sometimes more accurately described as ‘cyst-like’ lesions, ‘intraosseous’ lesions, or hollow gas-filled ‘geodes’ [16]. SBC are hallmarks of OA and have been associated with increases in osteoblasts, osteoclasts and subchondral bone turnover [5]. It has also been suggested that BML can be classified as ‘pre-cystic’ lesions with the propensity to develop into SBC over time [1]. Because of their established presence in OA, much effort has been placed upon understanding and halting SBC development.
HISTORY
In 1940, SBC were first localized along the border of osteoarthritic joint surfaces [6], but it was not suggested until 1962 that subchondral pathological changes may contribute to OA-related degenerative changes [5]. Previous to this breakthrough, OA treatment and research had been very cartilage-centric; however, more recently, cartilage has become viewed as a possible distractor due to its poor correlation with magnitude of osteoarthritic pain [2]. In addition, the theories of synovial intrusion and bone contusion have surfaced as two conflicting explanations for the presence and role of SBC in OA [6].
An early explanation for the development of BML considered increased flow and vascular congestion in the setting of capillary wall changes or increased intravascular pressure. It was thought that these factors and the resulting vascular leakage would lead to BML development [3]. Immunohistochemical analysis went on to dispute this theory by demonstrating increases in markers of angiogenesis in patients with BML and OA. This indicated that BML and its related pathology were not linked to capillary leakage or edema, but rather to remodeling and increasing vascularity in subchondral bone [5].
DEVELOPMENT AND HISTOLOGY
Deep to the articular cartilage in the hip joint lies subchondral bone, the site of BMLs and other pathology. This subchondral bone comprised two structures, the subchondral plate and trabeculae. The subchondral plate is a thin, porous layer that acts as the physical interface between articular cartilage and trabecular subchondral bone and also supplies the cartilage via channels containing nerves and blood vessels [17]. Subchondral trabecular bone is more porous and metabolically active than subchondral plate, and it closely correlates both biomechanically and biochemically with the overlying cartilage [6, 17].
Both components of subchondral bone respond to principal stress vectors within the joint. Channels within the subchondral plate and subchondral trabeculae adjust their concentration and orientation, respectively, to accommodate more forcefully stressed areas of the joint [17]. This dynamic adaptation and remodeling not only allows subchondral trabeculae to participate in processes such as shock absorption and joint support [6, 17] but also stiffens the bone. These same trabecular characteristics predispose to BML progression. When compared to healthy subchondral bone, osteoarthritic bone containing BML and SBC are known to possess trabecular abnormalities such as increased trabecular area [5, 18]. In addition, BML have reliably been shown to correlate with bone marrow necrosis and fibrosis in osteoarthritic human knees and hips [1, 6].
Following an improved understanding of the sequelae of SBC, two conflicting ideas have attempted to explain their development. The synovial intrusion and bone contusion theories have been explored in studies coupling histologic and radiographic observation, which suggest that both models contribute to SBC formation [19]; however, advocates of the two theories disagree as to which is the primary factor in SBC development.
The theory of synovial intrusion suggests that SBC formation is due to synovial fluid entering subchondral bone in areas of joint contact via violation of the osteochondral junction [6]. Histologic factors supporting this idea include the presence of similar fluid within joint space and SBC, abnormal articular cartilage layered above the SBC, and the uncharacteristic identification of articular cartilage within SBC. Furthermore, fluid dispersion within tissue would naturally produce the wedge shape expected in BML and SBC [19]. However, synovial intrusion alone fails to explain the formation of SBC in areas without adjacent joint contact [20].
The opposing theory of bone contusion states that in subchondral bone, the presence of atypical mechanical stresses causes microfracture and bone resorption. This leads to areas of necrosis which provide the origin of SBC [6]. The customary wedge shape can also be explained by bone contusion; the base of this wedge is known to converge about the maximum point of subchondral load, which supports microfracture as an inciting event [3]. Finite element analysis suggests that the osteoclast resorption following microfracture is the specific origin of SBC development. Interestingly, this analysis relates SBC development back to cartilage. This suggests that thin, damaged cartilage itself is the primary pathology allowing increased stress levels to induce microfracture and ensuing bone contusion, disordered bone metabolism and SBC [20].
Angiogenesis has also been indicated as a hallmark of BML development. Trabecular bone contains high numbers of mesenchymal stem cells, which are known to regulate local homeostasis and promote angiogenesis. The increased number of mesenchymal stem cells in BML and osteoarthritic bone suggest that angiogenesis, subchondral remodeling and mesenchymal stem cell recruitment and dysfunction are related; however, any causality between these factors remains poorly defined [18].
Although the various theories surrounding BML and SBC development do not provide complete clarity, their similarities do suggest overlying themes in the progression of subchondral disease. The pathologic formation of BML is due to repetitive microdamage coupled with chronic or dysregulated subchondral healing [5], which likely encompasses the processes of synovial intrusion, bone contusion and angiogenesis. Meanwhile, the pain associated with osteoarthritic disease can be reliably linked to increased intraosseous pressure and neurovascular irritation [3].
Etiologies
The first attempt at classifying the various disease states related to BML was carried out by Hofmann in 2004. While this effort primarily focused on the knee, it was expanded to include additional etiologies by Starr et al. [3]. Here, we discuss the various etiologies and corresponding diagnoses involving BML of the femoral head and acetabulum (Table I).
Table I.
| Pathology | Corresponding Imaging |
|---|---|
| Trauma |
|
| • Repetitive | |
| ○ Femoroacetabular impingement | |
| ○ Developmental dysplasia of the hip | |
| • Singular | |
| ○ Stress fracture | |
| ○ Compression fracture | |
|
| |
| Avascular necrosis |
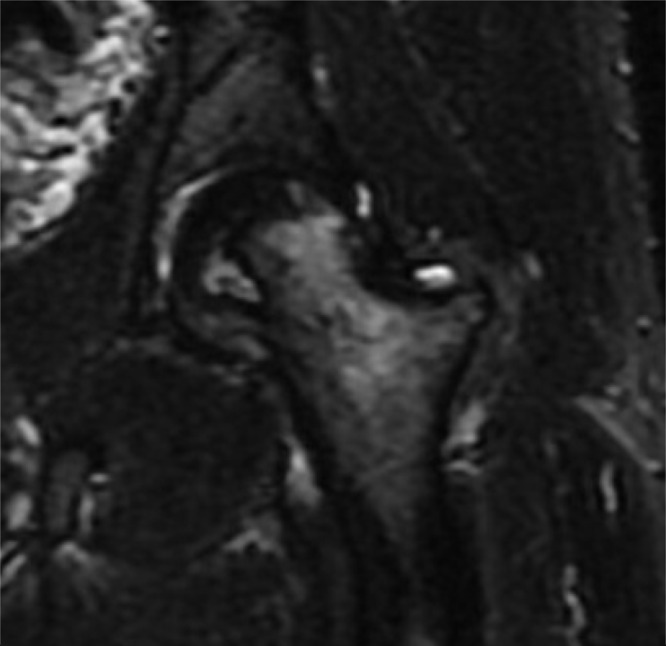
|
|
| |
| Bone marrow edema syndrome/transient osteoporosis of the hip |
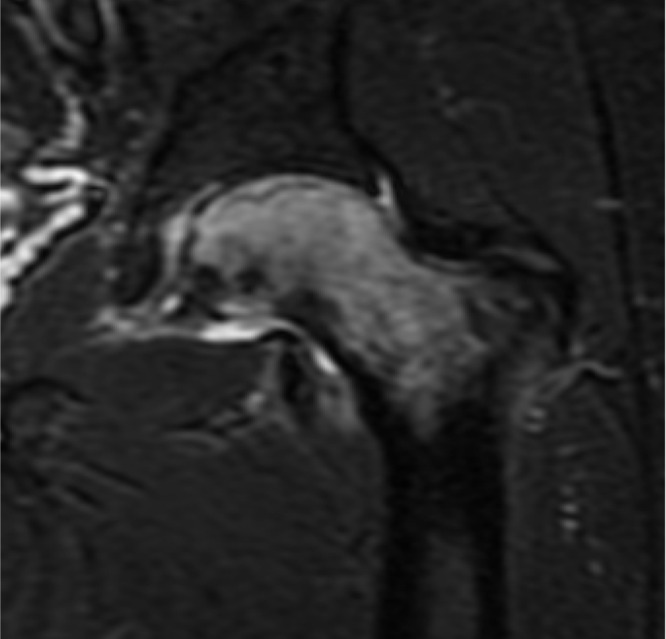
|
There have been various links established between BML and the well-established hallmarks of OA [6]. BML have been shown to hasten bone degeneration and overlying cartilage loss, and they are known to contain areas of subchondral sclerosis [2, 3, 5, 6]. In patients with advanced hip OA, increased vascularity and bone remodeling have been localized to BML-affected areas. In addition, the related characteristics of BML, cartilage defects and decreased femoral cartilage volume are more likely to be present in patients with hip OA than in those without [5, 11].
Unsurprisingly, processes that subject the hip to repetitive trauma appear likely to induce BML development. Some, such as femoroacetabular impingement (FAI) and developmental dysplasia of the hip, alter joint reactive forces, subchondral biomechanics and osseous stress [3, 6]. Stress and compression fractures similarly predispose to altered stresses and resultant BML [3].
The biomechanics of FAI relate to BML development via multiple factors. Cam and pincer deformities, the two manifestations of FAI, are defined by asphericity of the femoral head and acetabular over-coverage, respectively [21]. Acetabular over-coverage is known to predispose hips to BML development and, interestingly, is also associated with obesity [21]. This finding is significant because it indicates that obesity plays a potential risk factor in BML development. While it is well-established that obesity and the metabolic syndrome are risk factors for knee OA, these same characteristics have not been shown to reliably predict development of hip OA [21]. Furthermore, the presence of FAI has been established in up to 90% of idiopathic hip OA patients [15]. Therefore, while it has been thought that obesity does not play a reliable role in hip OA, the two may share a more complex relationship in which bone deformity and BML development play central roles.
Cam deformities may also share an indirect association with BML. This abnormality is characterized by radiographic measurement of the alpha angle in a standardized plane. The larger the alpha angle, the less spherical the femoral head, with values greater than 50° commonly indicating the presence of a cam deformity [22]. By comparing bone mineral density (BMD) with respect to alpha angle, Speirs determined that patients with both symptomatic and asymptomatic cam deformities have higher subchondral acetabular BMD than those without [15]. In a study by Ahedi measuring BMD irrespective to FAI status, it was found that acetabular BML were present in patients with decreased BMD. Although this seems to contradict Speirs’ results, Ahedi’s findings of decreased BMD were not statistically significant [14]. Furthermore, these collective results support the idea that BMD changes relative to BML development are more pronounced in patients with FAI than they are across the entire population.
One additional pathology which may fit the definition of BML is the herniation pit. These uncommon defects are thought to develop due to pressure on the superolateral femoral neck from the hip capsule, iliofemoral ligament and iliopsoas [23, 24]. This pressure then results in herniation of fluid, fibrous, and cartilaginous tissues into the subchondral space [24]. Herniation pits are usually identified incidentally via radiographic studies [23], but they do demonstrate subchondral signal changes when viewed on MRI [25]. While herniation pits have been linked to diagnosis of FAI, this relationship has been disputed and their clinical implications remain unclear [26]. Therefore, while they likely would qualify as a BML or SBC based on their characteristics on MRI, herniation pits remain difficult to classify.
Many other pathologic entities are linked to progressive joint-related disability and BML in the hip joint subchondral bone on both the femoral and acetabular sides of the joint. BML are known to speed the progression of inflammatory arthropathies such as rheumatoid arthritis [3]. Avascular necrosis (AVN), another process common to the hip joint, has been linked to BML [13]. Generally arising from compromised vascular supply to the femoral head, AVN is defined by progressive bony necrosis and leads to subchondral collapse, irreversible pain and failure of the hip joint [27]. In patients suffering from AVN, there is a high correlation between BML and hip pain, with some authors also noting the correlation between BML presence and the time of patients’ initial symptoms of AVN. In addition, BML have been linked to more rapid disease progression and increased necrotic bone volume [3].
Bone marrow edema syndrome (BMES), also known as Transient Osteoporosis of the Hip (TOH), usually manifests in middle-aged men or in women in their third trimester of pregnancy (Transient Osteoporosis of Pregnancy). This poorly categorized disease occurs most commonly in the hip and can mimic both AVN and stress fracture [27]. While one specific inciting event has not been identified, the disease is classified by unpredictable but generally self-limited 6- to 12-month episodes of hip pain in the setting of radiographically detectible edema and demineralization [27, 28]. However, because histological specimens from BMES/TOH-affected hips have not shown evidence of microfractures, these episodes are more likely due to hyperemia following necrosis than altered stress patterns [3]. Regardless of inciting event, BMES/TOH and AVN share characteristics but have unique prognoses; for this reason, great care must be taken to distinguish between the two processes [27].
Many non-arthritic processes are also linked to BML development. For example, BML have been shown to develop in the setting of infection [12]. While the volume burden of bony infections has not reliably predicted the resultant size of BML, osteomyelitis is closely associated with BML presence. Exudative and ischemic infections induce BML via vascular congestion and necrosis, respectively. In either case, BML generally border the infected bone [3].
BML are also known to develop in the setting of both benign and neoplastic tumors; however, they have shown the greatest association with benign neoplasms and in children younger than 10 years suffering from leukemia. To some degree, they are also associated with trauma, complex regional pain syndrome (CRPS), Paget’s disease, metabolic and neuropathic disorders [12]. Finally, iatrogenic causes such as surgery, radiotherapy and certain drugs provoke inflammation or other bone marrow changes which induce the onset of BML. In fact, the presence of BML prior to local surgery may impart a poorer prognosis for the postoperative period [3].
Imaging
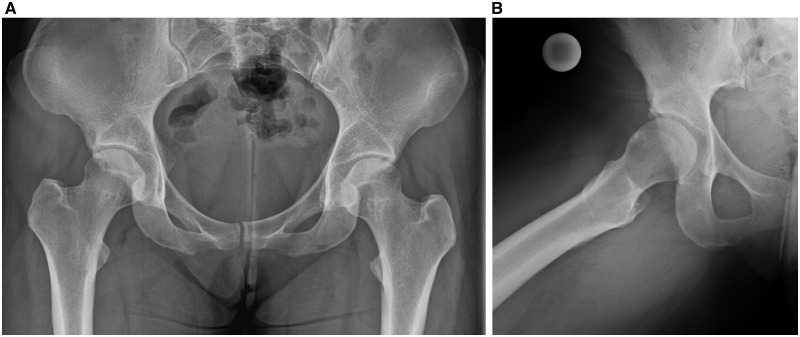
MRI remains the key imaging modality for assessing BML and other OA-related pathologies [1]. Radiographic studies are capable of detecting subchondral cysts, but they do not share the level of sensitivity offered by MRI in evaluation of BML [3, 6]. SBC correspond radiographically to well-defined areas of increased lucency with a sclerotic border (Fig. 1) [6].
Fig. 1.
Anteroposterior (A) and lateral (B) X-rays of a young female patient showing evidence of femoroacetabular impingement, along with possible cyst in the femoral neck indicated by a sclerotic ring. Joint spaces appear maintained.
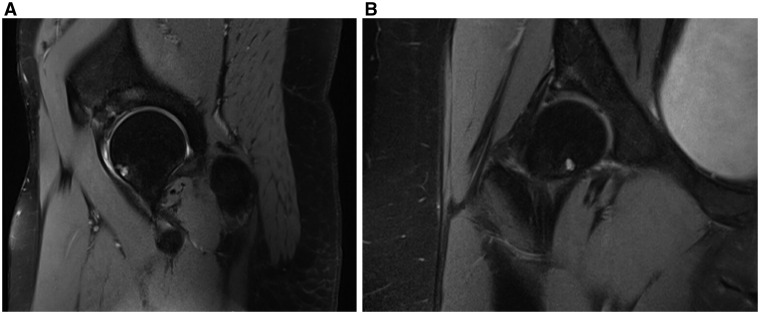
BML are defined on MRI as ill-defined areas of increased signal intensity on T2 and low signal intensity on T1 [3, 7] in subchondral bone (Fig. 2). Conversely, SBC can be identified on MRI as well-defined areas of high signal intensity in cancellous bone underlying joint cartilage [1]. These differences in definition may correspond to a spectrum of disease, corresponding to the potentially reversible nature of early BML and irreversible state of SBC [1, 7]. However, some believe that BML may progress in severity and allow SBC to develop in a similar anatomic location (Fig. 3). One hallmark of BML is the ‘water signal’, which when observed on MRI, represents increased subchondral vascularity [3].
Fig. 2.
Sagittal fast spin echo (FSE) MRI (A) of the same female patient illustrating an example of a femoral neck psuedocyst as well as evidence of bone marrow lesion in the acetabulum. Coronal FSE MRI (B), which again shows development of femoral neck pseudocyst and BML of the acetabulum.
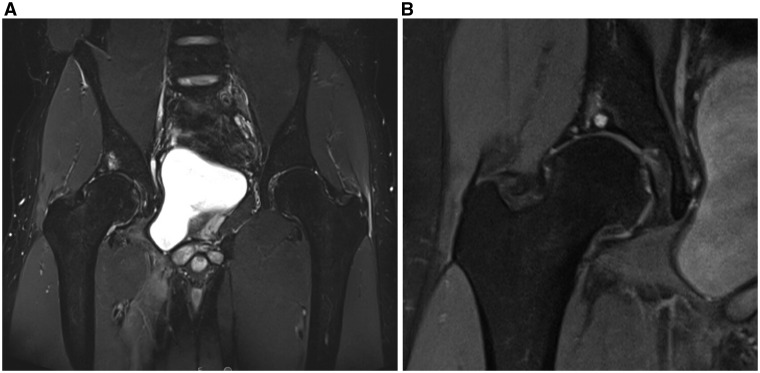
Fig. 3.
Coronal short T2 inversion recovery (STIR) image (A) of a young male patient demonstrating typical appearance of BML in acetabular subchondral weight-bearing bone. Coronal ion weighted fat saturated FSE MRI (B) of male patient, showing a common MRI appearance of a subchondral cyst with adjacent BML in the roof of the acetabulum.
Del Grande developed a classification system for BML based on their appearance on MRI. The aforementioned imaging characteristics pertain to Category I lesions, which arise in the setting of OA, trauma, CRPS, inflammatory arthropathies and AVN [12]. These trends on MRI, especially on T2, allow for development of a differential of BML-associated pathologies and etiologies; when paired with a thorough clinical history and physical exam, the imaging characteristics of BML provide the ability to properly diagnose the associated pathologic process, define disease progression, and guide treatment [1, 3]. Gadolinium MR and SPECT are both sensitive detectors of early cartilage damage; however, neither is currently utilized for BML detection [29].
As a spheroid joint, the hip’s cartilage volume and joint space are closely related. In addition, MRI slices are more easily acquired for round structures such as the hip than they are for hinge joints [2]. Therefore, MRI evaluation of joint space more accurately represents cartilage changes in the hip than in the knee. In addition, BML as observed in their early stages on MRI are known to predate the more drastic clinical sequelae of hip OA [11]. This indicates that the anatomy of the hip joint paired with the sensitivity of the MRI provides the ideal backdrop for identifying OA at the earliest point possible in the disease process. As previously mentioned, BML have shown involvement in a wide spectrum of diseases, many non-degenerative in nature; therefore, any effort to identify OA based on the presence of BML should maintain a wide differential and correlate all pathology on imaging to relevant clinical findings on history and physical examination.
TREATMENTS
In 1963, Eggers suggested autogenous graft implantation as a method for reversal of acetabular SBC, suggesting that targeting subchondral lesions early in development could prevent symptoms and progression of OA altogether [30]. However, despite a growing body of literature addressing BML at the hip, the majority of treatments have focused on treatment of BML at the knee. Here, we discuss the methods currently explored and employed for BML treatment in symptomatic patients.
In patients presenting with hip pain and concern for BML, an initial course of conservative management may be appropriate. Initial evaluation should also include a standardized radiographic series. If clinical suspicion is high enough based upon the patient’s history, physical exam and initial imaging, MRI may be of value as a next step. As mentioned previously, BMES/TOH may mimic early-stage AVN both clinically and radiographically. Although many cases of BMES/TOH are self-limiting, failure to improve the patient’s symptoms and pathology does predispose to subsequent hip fracture. Resolution of symptoms with weight-bearing restrictions, analgesia, and time are not only pathognomonic of BMES/TOH but also suggests reversal of the inciting disease process. Increasing bone density by implementing pharmacologic agents such as bisphosphonates, calcitonin, or teriparatide has been shown to shorten time to resolution of BMES/TOH both symptomatically and radiographically. These agents may also reduce the risk of fracture and theoretically are useful to offset the hormonal abnormalities of pregnancy; however, many are classified as pregnancy class C and therefore demonstrate potential risk to fetuses [27]. These anti-resorptive medications do require aggressive vitamin D supplementation with their administration [31].
In a study of 34 patients with symptomatic BML at the knee, Müller compared the effect of various bisphosphonates on pain and MRI detection of disease. While a single dose of intravenous zoledronic acid was found to be the most beneficial for resolution of symptoms, it also carried the highest risk of adverse events when compared to similar agents [31]. By assessing 19 patients with symptomatic BMES/TOH via MRI of the hip at specific time points, Agarwala quantified that 84% of patients exhibited complete resolution of hip joint edema within 12 weeks of zoledronic acid administration [28]. The remainder of the patients did demonstrate improved edema within this same time period. Furthermore, none of the zoledronic acid-treated patients went on to develop AVN within a 35-month period. The average time to complete pain relief in the affected hip was 2.8 weeks. While the specific mechanism of action of bisphosphonates on BMES/TOH-affected hips is still unknown, these findings support the theory that anti-resorptive medications shorten the time course of BMES/TOH episodes when compared to conservative treatment alone [28].
Although not established as a known treatment for BMES/TOH, pulsed electromagnetic field therapy is thought to potentially increase bone mass and has been implemented as adjuvant therapy in at least one patient who made a complete recovery from BMES/TOH [32]. In theory, this therapy resolves BMES/TOH via stimulation of osteogenesis, as well as reduction of free radical production, which directly alleviates the localized edema [33].
While operative intervention has not yet been shown to improve resolution of BMES/TOH beyond the capabilities of conservative or medical management, it has proven beneficial for other sources of BML. For example, core decompression of the hip is a well-established treatment method for AVN [27]. Thus, hip pain refractory to conservative treatment warrants consideration of more invasive treatments.
By evaluating hips with and without diagnosed hip OA via MRI, Teichtahl showed that BML, cartilage defects, and decreased femoral head cartilage volume are more common in patients with OA [11]. Furthermore, this study established a link between anterior or superolateral hip BML/cartilage defects and reduced femoral head cartilage volume in patients without diagnosed OA [11]. Interestingly, this location within the acetabulum is the same quadrant in which the most pronounced increased BMD is present in those patients with radiographically apparent cam deformities [15].
Studying patients without diagnosed OA also presumably includes patients who are pre-arthritic. The correlation between BML, cartilage defects, increased BMD and decreased femoral head cartilage volume in the setting of an asymptomatic hip may indicate these pathologies as key factors in the development of OA. Therefore, BML and the associated mesenchymal stem cells have been designated as an appropriate target to explore treatment geared at the prevention of FAI and OA [18].
Due to the inability of knee arthroscopy to improve pain levels in individuals with BML in the setting of OA, Cohen and Starkey employed treatment of femoral condyle and tibial plateau BML in concert with arthroscopic debridement to simultaneously treat BML and chondral pathology at the knee. Treatment involves the injection of a bone substitute material, comprised calcium phosphate, into the BML under fluoroscopy. Ideally, this promotes improved subchondral structural integrity, acting as a scaffolding for subchondral healing and bony regeneration. Clinically, the procedure aims to decrease pain and improve joint function [22, 33], while it also appears to prolong the lifespan of the joint as it was shown to delay total knee arthroplasty by at least 2 years in 70% of patients [13, 34].
While not studied for the sole purpose of addressing BML, periacetabular osteotomy was carried out in patients with hip dysplasia, with MRI measurement of SBC preoperatively and at 10 years postoperatively. At the 10-year mark, it was found that periacetabular osteotomy had no bearing on the development or resolution of SBC in these patients [35].
Limitations and future directions
The main limitation of this overview of BML of the hip is that it was not carried out as a PRISMA directed systematic review. Thus, the search for information and updates pertinent to BML was not a true systematic investigation of the literature; however, the intent is that this manuscript represents a comprehensive basic science review of this topic. Another limitation is that there is very limited quantity and quality of published clinical studies and trials addressing BML at the hip.
Although it has been established that BML at the knee are not equivalent to those at the hip, the scarce body of evidence surrounding hip BML necessitates the inclusion of knee-specific data and ideas. Over time, a concentrated effort to investigate hip BML will expand the body of evidence and allow for a systematic review of the literature to adequately address issues and advancements specific to BML at the hip.
In order to better understand hip BML, future work should focus on exploring their role as a potential target in the prevention and treatment of degenerative joint disease and OA. Employing methods to reverse BML development and assessing subsequent development of SBC and OA could not only provide methods for delaying or preventing the onset of hip OA but could also assist in establishing any concrete relationship between BML, SBC and OA at the hip. Focusing these efforts on treatment of patients with underlying FAI, DDH, or other hip-specific diagnoses could provide additional insight into the unique biomechanical landscape of the hip. First and foremost, identifying whether BML of the hip are prognostic indicators, precursors to degenerative joint disease, or simply a reactive lesion would establish a greater understanding of the subchondral femoral head and acetabulum. With this knowledge, the orthopaedic community could considerably refine and refocus current prevention and management of hip OA.
CONCLUSION
BML and SBC are closely related pathologies common to many disorders of the hip. While their specific method of development continues to be discussed and investigated, BML are readily identifiable on MRI and may share a role in the development of OA. With continued studies addressing the relationships between BML, FAI and OA, the development of hip OA can be better understood. Furthermore, by improving and refining treatments for BML, orthopaedic surgeons may be able to slow or prevent development of OA and/or AVN. Ultimately, this saves patients years of symptoms, potentially avoids the associated debility of long-term disease, and prevents the need for total hip arthroplasty. Ideally, a better understanding and treatment of BML will lead to patients enjoying improved quality of life while maintaining their native hips.
CONFLICT OF INTEREST STATEMENT
The authors received no funding for this study and report no conflicts of interest.
REFERENCES
- 1. Katsiberis G, Georgiadis P, Rigopoulou A. et al. Development of a pattern recognition system for discriminating osteoarthritic bone marrow edema like lesions on MRI. Global J Res Analy 2015; 9: 32–6. [Google Scholar]
- 2. Felson DT. Developments in the clinical understanding of osteoarthritis. Arthritis Res Ther 2009; 11: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Starr AM, Wessely MA, Albastaki U. et al. Bone marrow edema: pathophysiology, differential diagnosis, and imaging. Acta Radiol 2008; 49: 771–86. [DOI] [PubMed] [Google Scholar]
- 4. Solomon L. Patterns of osteoarthritis of the hip. J Bone Joint Surg Br 1976; 58-B: 176–83. [DOI] [PubMed] [Google Scholar]
- 5. Shabestari M, Vik J, Reseland JE. et al. Bone marrow lesions in hip osteoarthritis are characterized by increased bone turnover and enhanced angiogenesis. Osteoarthritis Cartilage 2016; 24: 1745–52. [DOI] [PubMed] [Google Scholar]
- 6. Li G, Yin J, Gao J. et al. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther 2013; 15: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ito H, Matsuno T, Minami A.. Relationship between bone marrow edema and development of symptoms in patients with osteonecrosis of the femoral head. AJR Am J Roentgenol 2006; 186: 1761–70. [DOI] [PubMed] [Google Scholar]
- 8. Antony B, Venn A, Cicuttini F. et al. Correlates of knee bone marrow lesions in younger adults. Arthritis Res Ther 2016; 18: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bettica P, Cline G, Hart DJ. et al. Evidence for increased bone resorption in patients with progressive knee osteoarthritis: longitudinal results from the Chingford study. Arthritis Rheum 2002; 46: 3178–84. [DOI] [PubMed] [Google Scholar]
- 10. Marcacci M, Andriolo L, Kon E. et al. Aetiology and pathogenesis of bone marrow lesions and osteonecrosis of the knee. EFORT Open Rev 2016; 1: 219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Teichtahl AJ, Wang Y, Smith S. et al. Structural changes of hip osteoarthritis using magnetic resonance imaging. Arthritis Res Ther 2014; 16: 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Del Grande F, Farahani SJ, Carrino JA, Chhabra A.. Bone marrow lesions: a systematic diagnostic approach. Indian J Radiol Imaging 2014; 24: 279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klement MR, Sharkey PF.. The significance of osteoarthritis-associated bone marrow lesions in the knee. J Am Acad Orthop Surg 2019; 27: 752–9. [DOI] [PubMed] [Google Scholar]
- 14. Ahedi H, Aitken D, Blizzard L. et al. The association between hip bone marrow lesions and bone mineral density: a cross-sectional and longitudinal population-based study. Osteoarthritis Cartilage 2013; 21: 1545–9. [DOI] [PubMed] [Google Scholar]
- 15. Speirs AD, Beaule PE, Rakhra KS. et al. Increased acetabular subchondral bone density is associated with cam-type femoroacetabular impingement. Osteoarthritis Cartilage 2013; 21: 551–8. [DOI] [PubMed] [Google Scholar]
- 16. Rees RJ, Hill SO, Cassar-Pullicino V. et al. The incidence, location and distribution of degenerative subchondral acetabular cysts in primary osteoarthrosis of the hip. Hip Int 2004; 14: 24–7. [DOI] [PubMed] [Google Scholar]
- 17. Chiba K, Burghardt AJ, Osaki M. et al. Three-dimensional analysis of subchondral cysts in hip osteoarthritis: an ex vivo HR-pQCT study. Bone 2014; 66: 140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Campbell TM, Churchman SM, Gomez A. et al. Mesenchymal stem cell alterations in bone marrow lesions in patients with hip osteoarthritis. Arthritis Rheumatol 2016; 68: 1648–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Resnick D, Niwayama G, Coutts RD.. Subchondral cysts (geodes) in arthritic disorders: pathologic and radiographic appearance of the hip joint. AJR Am J Roentgenol 1977; 128: 799–806. [DOI] [PubMed] [Google Scholar]
- 20. Durr HD, Martin H, Pellengahr C. et al. The cause of subchondral bone cysts in osteoarthrosis: a finite element analysis. Acta Orthop Scand 2004; 75: 554–8. [DOI] [PubMed] [Google Scholar]
- 21. Teichtahl AJ, Wang Y, Smith S. et al. Early cartilage abnormalities at the hip are associated with obesity and body composition measures - a 3.0T MRI community-based study. Arthritis Res Ther 2015; 17: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siebelt M, Agricola R, Weinans H. et al. The role of imaging in early hip OA. Osteoarthritis Cartilage 2014; 22: 1470–80. [DOI] [PubMed] [Google Scholar]
- 23. Amjad A, Hafez AT, Nawab Ditta A, Jan W.. Synovial pit of the femoral neck: a rare disease with rare presentations. J Surg Case Rep 2020; 2020: rjaa195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Daenen B, Preidler KW, Padmanabhan S. et al. Symptomatic herniation pits of the femoral neck: anatomic and clinical study. AJR Am J Roentgenol 1997; 168: 149–53. [DOI] [PubMed] [Google Scholar]
- 25. Gao ZH, Yin JQ, Ma L. et al. Clinical imaging characteristics of herniation pits of the femoral neck. Orthop Surg 2009; 1: 189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim JA, Park JS, Jin W. et al. Herniation pits in the femoral neck: a radiographic indicator of femoroacetabular impingement? Skeletal Radiol 2011; 40: 167–72. [DOI] [PubMed] [Google Scholar]
- 27. Asadipooya K, Graves L, Greene LW.. Transient osteoporosis of the hip: review of the literature. Osteoporos Int 2017; 28: 1805–16. [DOI] [PubMed] [Google Scholar]
- 28. Agarwala S, Vijayvargiya M.. Single dose therapy of zoledronic acid for the treatment of transient osteoporosis of hip. Ann Rehabil Med 2019; 43: 314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matar WY, May O, Raymond F. et al. Bone scintigraphy in femoroacetabular impingement: a preliminary report. Clin Orthop Relat Res 2009; 467: 676–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eggers GWN, Evans EB, Blumel J. et al. Cystic change in the iliac acetabulum. J Bone Joint Surg Am 1963; 45: 669–86. [Google Scholar]
- 31. Muller F, Appelt KA, Meier C. et al. Zoledronic acid is more efficient than ibandronic acid in the treatment of symptomatic bone marrow lesions of the knee. Knee Surg Sports Traumatol Arthrosc 2020; 28: 408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scheer V. Transient osteoporosis: an unusual presentation of hip pain in a trail runner. BMJ Case Rep 2019; 12: e231005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ghasemi RA, Sadeghi S, Rahimee N. et al. Technologies in the treatment of bone marrow edema syndrome. Orthop Clin North Am 2019; 50: 131–8. [DOI] [PubMed] [Google Scholar]
- 34. Cohen SB, Sharkey PF.. Subchondroplasty for treating bone marrow lesions. J Knee Surg 2016; 29: 555–63. [DOI] [PubMed] [Google Scholar]
- 35. Mechlenburg I, Nyengaard JR, Gelineck J. et al. Cartilage thickness and cyst volume are unchanged 10 years after periacetabular osteotomy in patients without hip symptoms. Clin Orthop Relat Res 2015; 473: 2644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]