Abstract
In an attempt to bridge the osteoarthritis (OA) gap, this study compared biological reconstruction with traditional microfracture (MF) techniques in patients with femoroacetabular impingement and focal cartilage defects. Cohorts of two groups were investigated; age, gender and Tonnis grade matched comparison for outcomes between MF and newer biological reconstruction techniques hip arthroscopy surgery using autologous matrix-induced chondrogenesis and bone marrow aspirate combination. Outcomes investigated were pre-op and post-op mean iHOT-12 scores up to 18 months after surgery with a Kaplan–Meier survivorship analysis. Of 111 patients, 46 patients underwent MF and 65 biological reconstruction hip arthroscopy including cam/pincer osteoplasty and labral repair surgery. Age range was 20–69, mean age 45 years for both groups, Tonnis grading was as follows: Grade 0: 26% versus 30%, Grade 1: 52% versus 47% and Grade 2: 22% versus 23% in MF and biological reconstruction groups, respectively. The mean post-operative iHOT-12 score differences between MF and biological reconstruction were significant at 1-year minimum follow-up (P = 0.01, SD 2.8). Biological reconstruction allowed for an enhanced recovery protocol. The MF group had a 67.4% survivorship for conversion to hip replacement at 18 months (32.6% failure rate for any reason) and biological reconstruction had 100% survivorship at 18 months post-operatively with no failures for any reason. This study provides further support to the evidence base for biological reconstructive techniques as superior to MF in combination with joint preservation arthroscopic surgery, even in the face of focal cartilage defects and offers both surgeons and patients a potential bridging of the OA gap.
INTRODUCTION
Femoral acetabular impingement (FAI) is usually associated with chondral damage caused due to abutment of the acetabular rim and the proximal femur. Chondral lesions in the hip can also be consequences of other pathological features such as trauma, labral tears, dysplasia’s, osteonecrosis, loose bodies, dislocations, previous slipped capital femoral epiphysis, etc. [1, 2]. FAI can be either secondary to cam (femoral) or pincer (acteabular) morphology, both possibly leading to osteoarthritis (OA). In the cam type, the cartilage is pulled and sheared with a carpet-like pattern, usually at the anterosuperior acetabular region. A continuum of damage starts with the chondrolabral lesion proceeding to cartilage delamination and finally labral detachment from the subchondral bone. In the pincer variety, the chondral acetabular lesion is a typical counter-part degeneration of the posteroinferior area, or a chondral lesion on the anterior and superior area of the acetabulum, consequent to shear forces concentrating on the chondrolabral junction [3–6]. The chondropathies are a frequent cause of pain and reason for limited functional activities affecting life. These defects in the hip when are not treated tend to progress and eventually lead to arthritic damage [6]. The treatment of chondral defects in the hip is still controversial and is evolving constantly from several standpoints [2]. Several strategies have been attempted to restore large cartilage defects in the active patient, especially young adults including some with bilateral affection. Another group of particular interest are the older active population who are clinically and radiologically not severe enough to warrant a total hip replacement yet suffer significant functional disability due to their failing hip. This deficit in treatment represents an OA gap. Options for treatment include autologous chondrocyte implantation (ACI), gold standard microfracture (MF), osteochondral autografts and fresh frozen allografts [7, 8]. It has also been pointed out that none of the treatments of chondral defects are effective when the joint space is seriously compromised [6]. MF has been most commonly used in the management of chondral lesions of both the knee and ankle. It has also been used in the hip, particularly since the introduction of hip arthroscopy [2, 9–13]. Combination procedures using MF and an enhanced technique of autologous matrix-induced chondrogenesis (AMIC), have been advocated [14]. Although AMIC is an effective treatment for chondral lesions of the knee and ankle [2, 15, 16]. Only a few authors have described its use in the hip [2, 9, 16, 17]. Furthermore, no direct comparisons between AMIC and autologous concentrated bone marrow aspirate (BMAC) versus MF in the hip are currently available. The primary aim of this study was to investigate the clinical outcomes, success rates and factors affecting failure in patients with chondral lesions of the hip undergoing treatment with either BR or MF, with an attempt to identify a treatment algorithm to bridge the OA gap. The secondary aim was to evaluate whether bilateral sequential surgery, enhanced recovery protocol in the BR group allowed for faster return to normal function, as a result of the more stable construct when compared with MF.
MATERIALS AND METHODS
Between January 2015 and January 2018, 111 patients with FAI underwent arthroscopic treatment of an acetabular chondral lesion. FAI was diagnosed using standard radiographs and MRI as per set criteria [1]. The patients in the two groups received MF (the current gold standard) or newer BR techniques for joint preservation hip arthroscopy surgery. This was a retrospective observational cohort study. From 2015 to December 2017, only MF was carried out as treatment of chondral defects. Patients were treated a rehabilitated using the Steadman recommended post-operative protocol [18, 19]. From January 2018 onwards, BR was adopted as the treatment method for all chondral lesions and this allowed for a facilitated bilateral sequential surgery, enhanced recovery protocol. The biological reconstruction involved using ROCKSTAR Kit, Joint Operations UK, consisting of Chondro-Gide, AMIC, Giestlich, Switzerland and Bone Marrow Aspirate (MarrowCellution) combination and use of a Fibrin Glue. The senior author (R.C.) performed all the operations. The included patients fit the above and were age, gender and Tonnis grade matched. The other inclusion criteria for the study were age between 18 and 70 years; acetabular grade III and IV chondral lesions according to the Outerbridge classification [20] measuring between 2 and 8 cm2, radiographic investigation showing less than grade 2 degenerative changes according to the Tonnis classification [21] and a minimum follow-up of between 12–18 months. Exclusion criteria were patients with rheumatoid arthritis, dysplasia, axial deviation of the femoral neck, pathology affecting both the femoral head and acetabulum as a kissing lesion, coxa profunda or protrusio acetabuli.
Of the 111 patients, 46 patients underwent MF and 65 had biological reconstruction hip arthroscopy as well as cam/pincer osteoplasty and labral repair surgery. Patient with cam-type impingement had resection of the femoral head-neck with an aim of eliminating the bony prominence that impinged the labrum, in a view to improve the anatomical offset between femoral head and neck. In the pincer type impingement the acetabulum overhang was trimmed and in case of a detached labrum, this was repaired using suture anchors. Mixed impingements were surgically addressed for both.
The MFs were carried out arthroscopically. The calcified layer was removed from the subchondral bone using a motorized shaver until sharp margins were obtained. The subchondral bone was penetrated using a chondral pick. Multiple perpendicular holes ∼3–4 mm apart. The movement was from the periphery to centre. Subchondral penetration was verified by observing MF associated outflow of marrow blood, which allows clot fill of the defect. Post-operative protocol for MF was non-weight bearing with crutches for 6 weeks, followed by graduated weight bearing and muscular strengthening with joint movement as pain allowed, typically with recovery between 8 and 10 weeks.
The AMIC procedure combined MF with a resorbable collagen I/III matrix. A sized collagen matrix was prepared to fit the lesion. The matrix was slightly undersized as it swells by ∼10% when moist. The matrix consists of an intra-articular smooth surface and a porous surface, care was taken to orient this appropriate, facilitated with a skin marking. The matrix was inserted using an arthroscopic cannula and placed over the lesion under a dry arthroscopic field. The joint was put through a range of movement followed by rechecking the stability of the matrix. The matrix was then saturated with BMAC aspirate from the proximal femoral metaphysis, using the Marrow Cellution™ system and a fibrin glue was used to create a stable matrix construct. The post-operative rehabilitation involved partial weight bearing during Week 1 with crutches and full weight bearing from Week 2 onwards with independence of crutches at the end of this week. For bilateral hip affection, patients who underwent sequential surgery had the same protocol; first side surgery at Week 0, partial weight bearing with crutches for 1 week and full weight bearing for Week 2. Second side surgery took place at the beginning of Week 3 and the same protocol was followed again, allowing for independence of crutches at the end of Week 4 [18, 19].
All patients were assessed pre-operatively and post- operatively using the iHOT-12 scores. Any complication or revision procedures were also recorded. A P values < 0.05 was considered statistically significant. Survival with an endpoint of further surgical intervention was assessed using Kaplan–Meier life tables comparing the two forms of treatment.
RESULTS
Of the 111 patients, 46 patients underwent MF and 65 had BR hip arthroscopy as well as cam/pincer osteoplasty and labral repair surgery, as in Table I.
Table I.
Associated pathology
| MF, n = 46 | BR, n = 65 | |
|---|---|---|
| CAM resection | 46 | 65 |
| Pincer resection | 20 | 28 |
| Labral repair | 37 | 52 |
| Labral debridement | 0 | 0 |
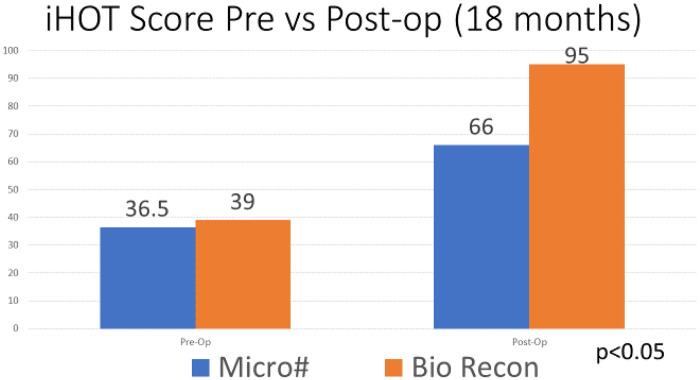
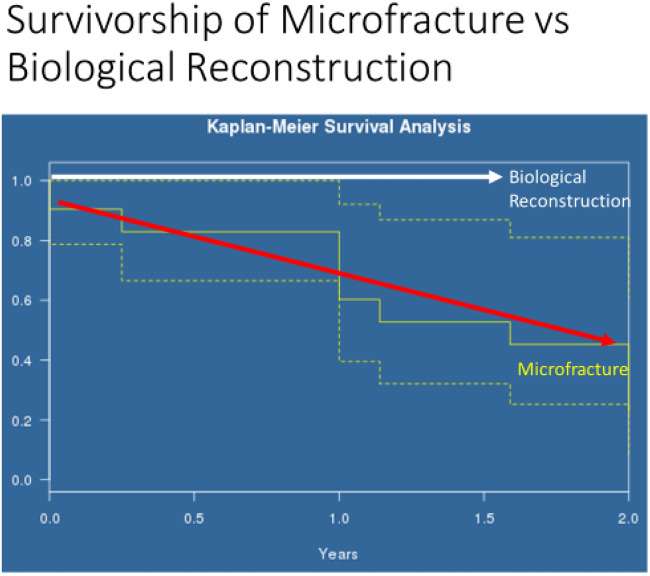
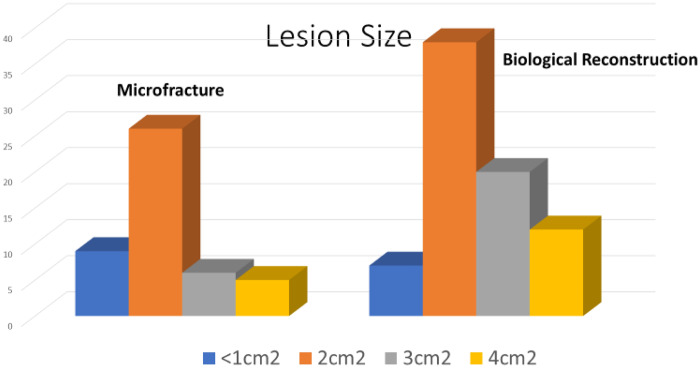
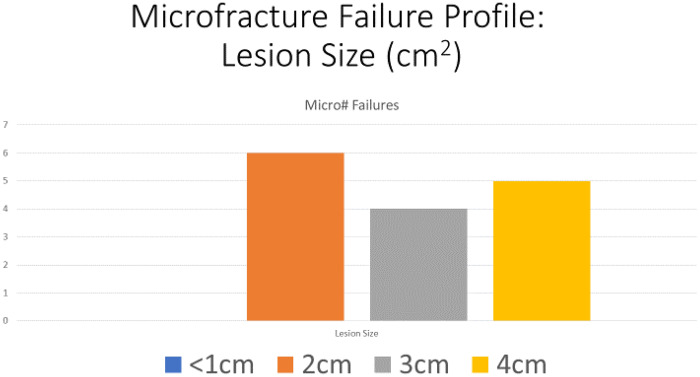
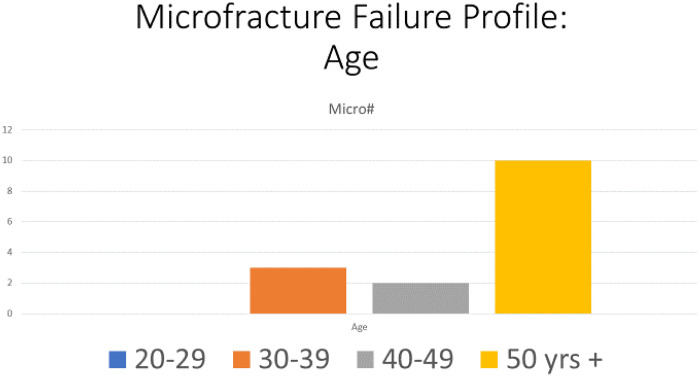
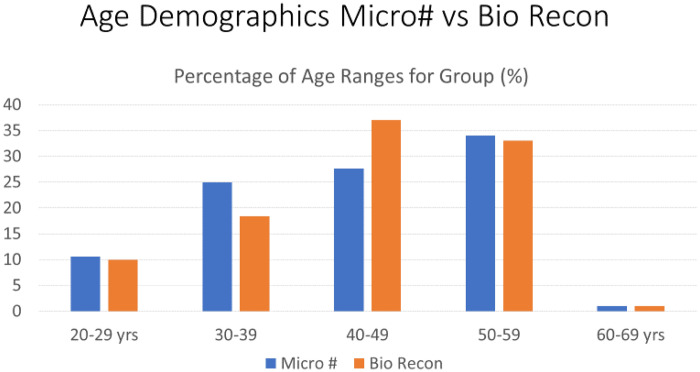
The two groups were age, gender and Tonnis matched with no significance between the two groups, Table II. Age range was 20–69, mean age 45 years for both groups, Tonnis grading was as follows: Grade 0: 26% versus 30%, Grade 1: 52% versus 47% and Grade 2: 22% versus 23% in MF and BR groups, respectively.The mean pre-operative iHOT-12 score differences between MF and BR were not significant (36.5, 95% CI (19.7–53.3) versus 39, 95% CI (35.2–45.8) respectively, P = 0.12, Mann–Whitney U test). The mean post-operative iHOT-12 score differences between MF and BR were statistically significant at 1-year minimum follow-up [66, 95% CI (46.8–85.8) versus 95, 95% CI (86–97.1) respectively, P < 0.05 Mann–Whitney U test], Fig. 1. The MF group had a 67.4% survivorship for conversion to hip replacement at 18 months (32.6% failure rate for any reason) and BR group had 100% survivorship at 18 months post-operatively with no failures for any reason—Fig. 2. The parameters used to proceed to total hip replacement were part of a shared decision process using deterioration in functional and objective clinical and radiological findings. None of the patients was self-funded. Further investigation between the MF and BR group revealed no difference in lesion size distribution as in Fig. 3. Analysis of the MF group alone for factors associated with failure showed no link to lesion size—Fig. 4. Age over 50 years showed a strong association with failure in the MF group as seen in Fig. 5, Kruskal–Wallis test, P < 0.002. To confirm that this observation link of age over 50 years and MF failure was not biased with a disproportionate elderly distribution within the MF group alone, we compared age distribution in both the MF and BR groups, which showed a similar age distribution between the two as in Fig. 6.
Table II.
Distribution of cases in both groups with their characteristics
| MF | BR | SD | P-value | |
|---|---|---|---|---|
| Number | 46 | 65 | ||
| Gender, M/F | 19/27 | 25/40 | 0.762 | |
| Pre-op mean age (years) | 45 | 45 | 0.9 | 0.976 |
| Pre-op mean Tonnis grade (0–2) | 1.04 | 1.3 | 0.6 | 0.089 |
| Pre-op mean lesion size (1–4 cm2) | 2.15 | 2.48 | 0.85 | 0.04 |
| Pre-op mean iHOT-12 score | 36.5 | 39 | 0.67 | 0.12 |
| Post-op mean iHOT-12 score | 66 | 95 | 2.8 | <0.01 |
Fig. 1.
The average pre-operative and post-operative iHOT-12 score differences between MF and BR groups.
Fig. 2.
Kaplan–Meier survivorship analysis.
Fig. 3.
Difference in lesion size distribution in MF and BR groups.
Fig. 4.
MF failure profile depending on lesion size.
Fig. 5.
MF failure profile depending on age.
Fig. 6.
Age demographics of MF and BR groups.
DISCUSSION
Our results suggest that there is a significant difference in outcome and survivorship between MF and BR in the short term, the intermediate and long terms results for BR remain unknown. In this study the BR arm enjoyed superior iHOT function scores, with no failure for any reason. The MF group had a significantly lower iHOT functional outcome with a high failure rate, progressing to total hip replacement, suggestive of ongoing deterioration and failure of the construct stability. The current gold standard treatment for small chondral defects <2 cm2 in the hip is MF [22]. There is uncertainty in the treatment of larger defects due to various factors. In comparison to ACI, AMIC is a one-step operative procedure. Eliminating the need of chondrocyte culture, specialized centre and laboratory support, making it more cost-effective. AMIC has been used in the knee with good results, clinical study of 27 patients reported significant improvement in five different scores at 12 months and up to 24 months after the procedure [23]. Similar results are reproducible for chondral lesions of the hip. MF and AMIC techniques led to marked clinical short-term improvement in patients with chondral defects resulting from FAI in the first 2 years. However, AMIC gave significantly better results as measured by mHHS, which were maintained after 8 years, the results of MF in the hip deteriorated over time with 22% of patients undergoing conversion to THA. No patient in the AMIC group was converted to THA; the results of AMIC appeared stable over time and independent of lesion size [2, 24]. The main factor associated with failure in our results in the MF group was age over 50 years. This group is of particular interest as they represent older, active adults with limited functional activities affecting life. If not treated, they tend to progress to OA, eventually leading to a total hip replacement. MF in this group with high failure rate, created a deficit in the treatment algorithm or an OA Gap. BR showed strong results in terms of better iHOT scores and longevity of the construct stability in the short term. This phenomenon may be viewed as a potential bridge to this OA gap, from a joint preservation perspective as a good solution. This evidence of construct stability is further supported in the medium term in by de Girolamo et al. [2, 24]. Another benefit of the BR construct stability is that one can offer bilateral sequential procedures as an accelerated recovery protocol. Traditional MF demands that 6 weeks non-weight bearing is required to allow the clot covering the defect to mature and stabilize. This means that if bilateral pathology exists, the patient must rehabilitate for at least 12 weeks minimum if both sides were addressed as quickly as possible. The increased load on the contra-lateral hip also worsened the functional pain and disability whilst recovering from surgery and may have worsened the outcome due to this extra loading phase. With BR, both hips could be addressed within a 2-week window, meaning full weight bearing bilaterally at only 4 weeks. The total rehabilitation phase is also markedly reduced to 6 weeks. The patients who underwent this protocol all remarked on how quickly recovered and pain free they felt. The iHOT score for this group were no different to the single sided BR outcomes. The benefit of addressing bilateral pathology without overloading an already failing joint are multiple and the benefits of enhanced recovery in orthopaedics are well documented [25, 26]. The addition of BMAC and fibrin glue to the construct may also contribute to why this accelerated protocol was possible [27]. de Girolamo et al. [7, 27] reported that the addition of BMAC resulted in faster recovery rates with prompt return to activity compared with AMIC alone in the knee, as well as better Lysholm scores and lower VAS pain scores. Similar benefits of the above have been observed in our study. The socioeconomic benefits of joint preservation surgery support the use of the most stable construct for longevity. Any procedure that delays the need for a total hip replacement, a significant departure from joint preservation philosophy, should be seriously considered. BR may offset the costs and morbidity burden of a hip replacement and potential revision surgery 20 years later. If we can delay the first THR by 8–10 years or more, the likelihood of revision surgery is also reduced [28]. This study presents several positive features. Its one step nature allows early mobilization. The good clinical outcome showed that the use of AMIC+BMAC could be a promising option for the treatment of cartilage defects. The main limitations of the study include the limited number of patients and the heterogeneity. This was a retrospective study and patients were not randomized. The baseline characteristics were, however, similar in both groups. Clinical outcome was only assessed using one functional outcome score.
This study suggests that BR using AMIC+BMAC has better functional outcome than MF and supports the current literature body of evidence that is growing. Further higher quality studies are required to consolidate the evidence base.
FUNDING
This work was supported by Joint Operations, UK and Giestlich, Switzerland. The senior author (R.C.) had a consultancy agreement with Joint Operations, UK and Giestlich, Switzerland provided funding for the manuscript publication and presentation.
CONFLICT OF INTEREST STATEMENT
The senior author(R.C) has a consultancy agreement with Joint Operations UK and Giestlich, Switzerland.
REFERENCES
- 1. Fontana A, de Girolamo L.. Sustained five-year benefit of autologous matrix-induced chondrogenesis for femoral acetabular impingement-induced chondral lesions compared with microfracture treatment. Bone Joint J 2015; 97-B:628–35. [DOI] [PubMed] [Google Scholar]
- 2. de Girolamo L, Jannelli E, Fioruzzi A, Fontana A.. Acetabular chondral lesions associated with femoroacetabular impingement treated by autologous matrix-induced chondrogenesis or microfracture: a comparative study at 8-year follow-up. Arthroscopy 2018; 34:3012–23. [DOI] [PubMed] [Google Scholar]
- 3. Johnston TL, Schenker ML, Briggs KK, Philippon MJ.. Relationship between offset angle alpha and hip chondral injury in femoroacetabular impingement. Arthroscopy 2008; 24:669–75. [DOI] [PubMed] [Google Scholar]
- 4. Beck M, Kalhor M, Leunig M, Ganz R.. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br 2005; 87-B: 1012–8. [DOI] [PubMed] [Google Scholar]
- 5. Ganz R, Parvizi J, Beck M. et al. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res 2003; 466:273–20. [DOI] [PubMed] [Google Scholar]
- 6. Jannelli E, Fontana A.. Arthroscopic treatment of chondral defects in the hip: AMIC, MACI, microfragmented adipose tissue transplantation (MATT) and other options. SICOT J 2017; 3:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Girolamo L, Schönhuber H, Viganò M. et al. Autologous matrix-induced chondrogenesis (AMIC) and AMIC enhanced by autologous concentrated bone marrow aspirate (BMAC) allow for stable clinical and functional improvements at up to 9 years follow-up: results from a randomized controlled study. J Clin Med 2019; 8:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ejnisman L, Safran MR.. Biologics in hip preservation. Ann Joint 2018; 3:50. [Google Scholar]
- 9. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC.. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med 2011; 39:296–303. [DOI] [PubMed] [Google Scholar]
- 10. Mardones R, Larrain C.. Cartilage restoration technique of the hip. J Hip Preserv Surg. 2015; 3:30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Byrd JWT, Jones KS.. Arthroscopic management of femoroacetabular impingement in athletes. Am J Sports Med 2011; 39:7–13S. [DOI] [PubMed] [Google Scholar]
- 12. Stubbs AJ, Howse EA, Mannava S.. Tissue engineering and the future of hip cartilage, labrum and ligamentum teres. J Hip Preserv Surg; 2015; 3:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haughom BD, Erickson BJ, Rybalko D. et al. Arthroscopic acetabular microfracture with the use of flexible drills: a technique guide. Arthrosc Tech; 2014; 3:e459–63–e463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karthikeyan S, Roberts S, Griffin D.. Microfracture for acetabular chondral defects in patients with femoroacetabular impingement: results at second-look arthroscopic surgery. Am J Sports Med 2012; 40:2725–30. [DOI] [PubMed] [Google Scholar]
- 15. Fontana A. A novel technique for treating cartilage defects in the hip: a fully arthroscopic approach to using autologous matrix-induced chondrogenesis. Arthrosc Tech; 2012; 1:e63–8–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benthien JP, Behrens P.. Autologous Matrix-Induced Chondrogenesis (AMIC): Combining Microfracturing and a Collagen I/III Matrix for Articular Cartilage Resurfacing. Cartilage 2010; 1:65–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hunziker EB, Lippuner K, Keel MJB, Shintani N.. An educational review of cartilage repair: precepts & practice—myths & misconceptions—progress & prospects. Osteoarthritis Cartilage Elsevier Ltd; 2015; 23:334–50. [DOI] [PubMed] [Google Scholar]
- 18. Steadman JR, Briggs KK, Rodrigo JJ. et al. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy 2003; 19:477–84. [DOI] [PubMed] [Google Scholar]
- 19. Kraeutler MJ, Anderson J, Chahla J. et al. Return to running after arthroscopic hip surgery: literature review and proposal of a physical therapy protocol. J Hip Preserv Surg 2020; 4:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br 1961; 43-B: 752–7. [DOI] [PubMed] [Google Scholar]
- 21. Tonnis D, Heinecke A.. Acetabular and femoral anteversion: relationship with osteoarthritis of the hip. J Bone Joint Surg Am 1999; 81:1747–70. [DOI] [PubMed] [Google Scholar]
- 22. Dallich AA, Rath E, Atzmon R. et al. Chondral lesions in the hip: a review of relevant anatomy, imaging and treatment modalities. J Hip Preserv Surg 2019; 6:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gille J, Schuseil E, Wimmer J. et al. Mid-term results of autologous matrix-induced chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc 2010; 18:1456–64. [DOI] [PubMed] [Google Scholar]
- 24. Malviya A. What the papers say. J Hip Preserv Surg 2019; 6:97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaye AD, Urman RD, Cornett EM. et al. Enhanced recovery pathways in orthopedic surgery. J Anaesthesiol Clin Pharmacol 2019; 35(Suppl 1):S35–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Soffin EM, Yadeau JT.. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth 2020; 117:iii62–72. [DOI] [PubMed] [Google Scholar]
- 27. Chahla J, Dean CS, Moatshe G. et al. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee. Orthop J Sports Med 2016; 4:232596711562548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hotham WE, Malviya A.. A systematic review of surgical methods to restore articular cartilage in the hip. Bone Joint Res 2018; 7:336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]