Abstract
Aims
To examine the characteristics/prognostic impact of diastolic dysfunction (DD) according to 2016 American Society of Echocardiography (ASE) and European Society of Cardiovascular Imaging (ESCVI) guidelines, and individual parameters of DD.
Methods and results
Data were derived from a large multicentre mortality-linked echocardiographic registry comprising 436 360 adults with 1 diastolic function measurement linked to 100 597 deaths during 2.2 million person-years follow-up. ASE/European Association of Cardiovascular Imaging (EACVI) algorithms could be applied in 392 009 (89.8%) cases; comprising 11.4% of cases with ‘reduced’ left ventricular ejection fraction (LVEF < 50%) and 88.6% with ‘preserved’ LVEF (≥50%). Diastolic function was indeterminate in 21.5% and 62.2% of ‘preserved’ and ‘reduced’ LVEF cases, respectively. Among preserved LVEF cases, the risk of adjusted 5-year cardiovascular-related mortality was elevated in both DD [odds ratio (OR) 1.31, 95% confidence interval (CI) 1.22–1.42; P < 0.001] and indeterminate status cases (OR 1.11, 95% CI 1.04–1.18; P < 0.001) vs. no DD. Among impaired LVEF cases, the equivalent risk of cardiovascular-related mortality was 1.51 (95% CI 1.15–1.98, P < 0.001) for increased filling pressure vs. 1.25 (95% CI 0.96–1.64, P = 0.06) for indeterminate status. Mitral E velocity, septal e’ velocity, E:e’ ratio, and LAVi all correlated with mortality. On adjusted basis, pivot-points of increased risk for cardiovascular-related mortality occurred at 90 cm/s for E wave velocity, 9 cm/s for septal e’ velocity, an E:e’ ratio of 9, and an LAVi of 32 mL/m2.
Conclusion
ASE/EACVI-classified DD is correlated with increased mortality. However, many cases remain ‘indeterminate’. Importantly, when analysed individually, mitral E velocity, septal e’ velocity, E:e’ ratio, and LAVi revealed clear pivot-points of increased risk of cardiovascular-related mortality.
Keywords: echocardiography, diastolic, function, guidelines, mortality, big, data
Introduction
Diastolic filling of the left ventricle (LV) is a highly complex process which is dependent on LV relaxation, LV compliance, and left atrial pressure. Diastolic dysfunction (DD) is associated with impaired exercise capacity1 and reduced quality of life. Moreover, in selected patient groups, such as heart failure (HF)2,3 and post-myocardial infarction,4 it has been shown to be associated with increased mortality.5 Whilst recognized as an important and common clinical entity, accurate quantification, and effective management of DD continues to challenge clinicians. The primary modality to identify and classify DD in routine clinical practice is transthoracic echocardiography.6 Accordingly, the 2016 American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI) guidelines on the clinical evaluation of diastolic function sought to simplify such assessment6 using a practical algorithm applicable to everyday clinical practice. A key feature of these guidelines is the separate assessment of preserved vs. reduced systolic function groups above and below a left ventricular ejection fraction (LVEF) threshold of 50%, and then applying a combination of seven key parameters to assess DD severity.6
Despite these best intentions, however, the assessment of DD in routine clinical practice remains challenging. For example, it has been reported that between 20% and 50% of patients have ‘indeterminate’ diastolic function if guideline algorithms are applied.7,8 Moreover, normal age-related changes are not considered. In this context identifying high-risk individuals with DD remains a priority. Unfortunately, there is a paucity of large-scale studies examining the profile and impact of DD (as derived from the ASE/EACVI algorithms and the individual diastolic parameters that inform them) across the broader patient population managed in routine clinical practice.
Applying the unique resources of the National Echocardiography Database of Australia (NEDA), therefore, we firstly aimed to describe the overall profile of DD within the large NEDA cohort according to the current ASE/EACVI guidelines. We then examined the pattern of mortality according to the current guideline-based classifications of DD among those with reduced (LVEF < 50%) or preserved (LVEF ≥ 50%) systolic function on an unadjusted and adjusted basis. Finally, consistent with previous NEDA analyses,9,10 we then examined the pattern of mortality associated with each of the main diastolic parameters measured on echocardiography in more granular detail.
Methods
Study design
As previously reported, NEDA is a large observational registry that captures routinely acquired echocardiographic data on a retrospective and prospective basis in Australia.11 Individual data linkage are then used to derive health outcomes. For this study, 23 centres throughout Australia contributed data. The study cohort are typically referred by a general practitioner or cardiologist for potential heart disease or are being followed-up with pre-existing cardiovascular disease. NEDA is registered with the Australian New Zealand Clinical Trials Registry (ACTRN12617001387314). Ethical approval has been obtained from all relevant Human Research Ethics Committees and the study adheres to the Declaration of Helsinki.
Echocardiographic profiling
All echocardiographic measurement and report data, including basic demographic profiling (biological sex and date of birth) of individuals and date of investigation collected by participating centres during the period 1 January 2000 to 21 May 2019 were transferred into a central database via an automated data extraction process. Individuals over aged 18 years were selected based on their last reported echocardiogram and based on the presence of any valid parameters of diastolic function. All data were then cleaned and transformed into standard NEDA format to generate uniform echocardiographic profiling data and to remove duplicate, inconsistent, and/or impossible measurements. All individuals contributing to NEDA receive a unique identifier linked to their echocardiograms and their anonymity protected by stringent security protocols. For the purpose of this study, data from the last recorded echocardiogram were analysed.
Classification of systolic and diastolic dysfunction
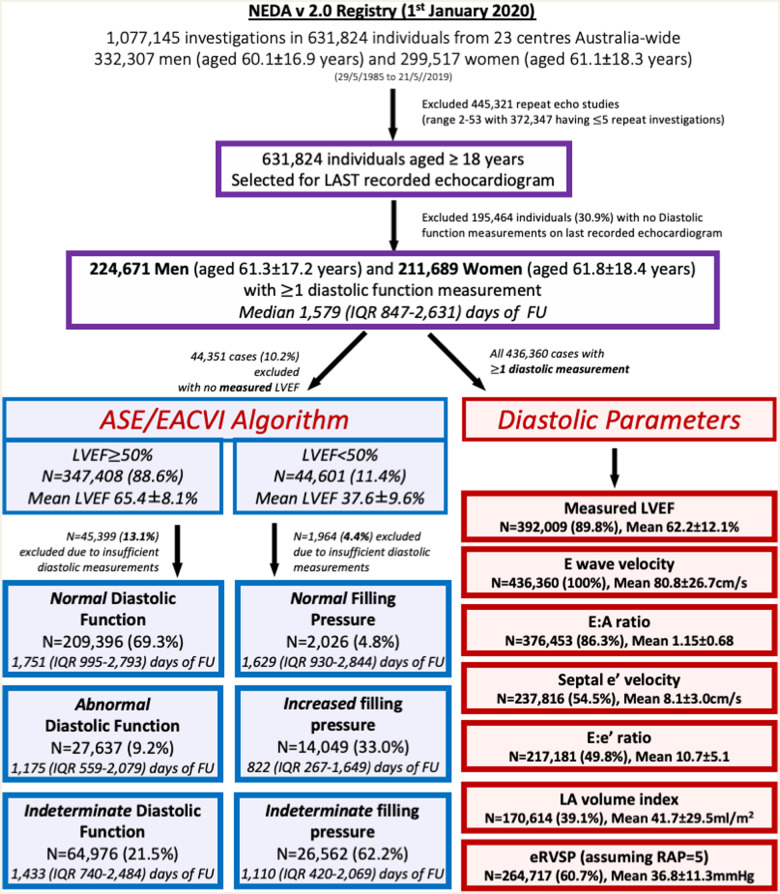
As shown in Figure 1, 436 360 individuals with at least one valid diastolic measurement were identified. Of these, 44 351 (10.2%) did not have a concurrent LVEF quantification. Subsequently, ASE/EACVI guideline-derived categories of DD were applied in 392 009 cases; of whom 347 408 (88.6%) had an LVEF ≥50%. All of these cases were then categorized according to the specific criteria outlined by the guidelines (Supplementary data online, Figure S1). Accordingly, for the ‘preserved EF’ category (LVEF ≥ 50%), the four recommended parameters were applied to derive the output categories of ‘Normal’ (diastolic function, where <50% of parameters were abnormal), ‘Indeterminate’ (50% of parameters abnormal), and ‘Abnormal’ (>50% parameters abnormal). Likewise, specific guideline criteria were applied to the ‘reduced EF’ category (LVEF <50%) to derive the output categories of ‘Normal’ (Grade I DD), ‘Indeterminate’, and ‘Increased’ filling pressure (combining Grades II and III DD). Classification into either of these categories was not based on documented rhythm (sinus rhythm, atrial fibrillation, paced rhythm, or other); however, subgroup analysis was performed for those in which the rhythm was documented (based on text comments, paced rhythm, or mitral inflow pattern).
Figure 1.
Study flowchart. This graph shows the key inclusion and exclusion criteria, including the classification of patients according to the ASE/EACVI Diastolic Function algorithm, and the individual diastolic parameters. A total of 436 360 individuals had at least one diastolic function measurement for inclusion in the cohort, with 113 725 individuals (26.1%) with all diastolic function measures available. In ASE/EACVI groups, the percentage in each diastolic function category refers to the percent of the total included in that LVEF category. FU, follow-up.
In addition to the above categories, all individuals with at least one diastolic function measurement [specifically, mitral E wave velocity, mitral A wave velocity, mitral E:A ratio, septal or lateral mitral annular e’ velocity (e’ velocity), E:e’ ratio, indexed left atrial volume (LAVi), and estimated right ventricular systolic pressure (eRVSP)] were included in the ‘diastolic parameters’ analysis. A ‘measurement’ was defined as the presence of a numerical value within the measurement section of the final echocardiographic report, as opposed to a string value within the body of the report. All data were cleaned and transformed into standard NEDA format to generate uniform echocardiographic profiling data and to remove duplicate and/or impossible measurements/investigations.
Endpoints
The primary outcomes of interest were cardiovascular-related and all-cause mortality. Mortality linkage was performed via the well-validated Australia’s National Death Index.12 Specifically, using a detailed probability matching process involving patient identifiers collected at the echocardiography study, reliable data on the survival status of individuals up to the study census date of 21 May 2019 were generated. Any listed causes of death were categorized according to ICD-10 coding. Consistent with previous analyses9,10 and based on the primary cause of death, all ICD-10AM chapter codes in the range of I00–I99 were categorized a cardiovascular-related death.
Statistical methods
NEDA data analyses and reports conform to the STROBE guidelines where possible.13 All numerators/denominators and variables used in analyses are provided; with no missing data imputed. Standard methods for describing and comparing grouped data, including means (± standard deviation), median [interquartile range (IQR)], and proportions according to baseline profiling (last echocardiogram) were applied. For these analyses, actual 5-year mortality was calculable in 242 257 out of 436 360 cases (55.5%) overall including 187 235 out of 392 009 (47.8%) with whom ASE/EACVI categories were determined. Mortality outcomes were firstly analysed according to conventional guideline-based categories of DD for those with reduced vs. preserved LVEF. After considering the statistical distribution and clinical utility of each diastolic parameter, each of the seven parameters were divided into deciles or clinically congruent unit groups (whilst ensuring a large number of cases were retained at either end of the variable distribution) for analyses.
The Kaplan–Meier method followed by Cox-proportional hazard models (proportional hazards confirmed by visual inspection of adjusted survival curves) were used to derive age- and sex-adjusted hazard ratios and 95% CI for the risk of mortality (cardiovascular-related and all-cause) during the entirety of study follow-up, according to ASE/EACVI categories and the clinical distributions of each diastolic parameter studied. For each model, the nadir of lowest overall mortality during follow-up for parameter was entered as the reference group. For the fixed time-point and outcome, actual 5-year mortality, multiple logistic regression (entry models) were used to generate adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for the ASE/EACVI categories and each diastolic parameter (Figure 1) relative to the nadir reference group. These models were then expanded to include age, sex, LVEF, the presence of sinus rhythm (vs. non-sinus rhythm), valvular heart disease, and the presence (or absence) of a mitral or aortic valve replacement had been performed. Accordingly, minimum available data determined the size of fully adjusted models and the congruence of partial vs. full models were examined before being presented. Only the four most informative individual parameters [mitral E-wave velocity, LV septal e’ velocity, septal E:e’ ratio, and indexed left atrial volume (LAVi)] were chosen for presentation. All statistical analyses were performed using SPSS (Statistical Package for the Social Sciences) version 26.0 software (SPSS Inc.). Significance was accepted at the level of P < 0.05 (two-sided).
Results
Study cohort
Table 1 summarizes the baseline profile of the study cohort of 224 671 men (aged 61.3 ± 17.2 years) and 211 689 women (aged 61.8 ± 18.4 years) according to their baseline age. Left ventricular systolic function, measured by LVEF and stroke volume index (SVi) remained similar across age groups. There were age-related changes in diastolic function measurements, with a marked increase in LAVi, and increases in indexed left ventricular mass (LVMI), mitral E and A velocities, E:e’ ratio, and eRVSP. There was a corresponding fall in the septal and lateral e’ velocity, and the E:A ratio. As expected, in addition to age-related gradients, the overall pattern of DD varied according to the distribution of systolic dysfunction (Supplementary data online, Table S1). Atrial fibrillation was specifically documented in only 32 933 individuals (8.8%), however based on the presence of a measured E-wave but not A-wave velocity atrial fibrillation (AF) was suspected in 56 649 individuals (13.0%).
Table 1.
Markers of diastolic function for men and women by age quartiles
| Age quartiles | ALL | <50 years | 50–63 years | 64–75 years | >75 years | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 436 360 | n = 112 918 | n = 110 407 | n = 104 943 | n = 108 092 | ||||||
| Demographic profile | ||||||||||
| Sex, n (%) | Men | Women | Men | Women | Men | Women | Men | Women | Men | Women |
| 224 671 (51.5) | 211 689 (48.5) | 56 539 (50.1) | 56 379 (49.9) | 60 270 (54.6) | 50 137 (45.4) | 56 864 (54.2) | 48 079 (45.8) | 50 998 (47.2) | 57 094 (52.8) | |
| Age | 61.3 (17.2) | 61.8 (18.4) | 37.5 (9.4) | 36.9 (9.4) | 58.0 (4.0) | 57.9 (4.0) | 69.9 (3.1) | 70.0 (3.2) | 82.0 (4.5) | 82.8 (4.9) |
| Anthropometric data | ||||||||||
| Body weight | 86.9 (19.4) | 73.6 (19.6) | 89.4 (21.3) | 74.8 (21.5) | 90.8 (19.9) | 77.6 (20.5) | 87.0 (18.0) | 75.3 (18.6) | 78.9 (15.3) | 67.2 (15.9) |
| BMI | 28.2 (5.6) | 28.1 (7.0) | 28.0 (6.0) | 27.5 (7.5) | 29.3 (5.8) | 29.4 (7.4) | 28.6 (5.4) | 29.0 (6.9) | 26.5 (4.6) | 26.6 (5.8) |
| Vital signs | ||||||||||
| Heart rate | 69.7 (14.3) | 72.5 (13.9) | 70.1 (14.3) | 73.1 (13.6) | 69.5 (14.0) | 71.8 (13.5) | 69.4 (14.5) | 72.2 (14.0) | 69.6 (14.6) | 72.8 (14.5) |
| Blood pressure | 133.4/76.6 (20.5/11.2) | 135.3/76.8 (23.1/11.3) | 128.9/77.3 (17.6/10.8) | 122.9/74.9 (17.3/10.5) | 133.0/78.6 (20.3/10.9) | 133.8/78.2 (21.7/10.9) | 134.9/76.3 (20.6/10.8) | 140.2/77.7 (22.6/11.3) | 136.4/74.1 (22.4/11.6) | 143.5/76.8 (24.1/11.9) |
| Echocardiographic data | ||||||||||
| SVi | 41.2 (12.1) | 40.1 (11.8) | 40.9 (11.6) | 38.6 (10.3) | 40.6 (11.4) | 39.4 (10.7) | 41.4 (12.2) | 40.6 (11.7) | 41.7 (13.0) | 41.6 (13.6) |
| LVEF | 60.1 (12.8) | 64.5 (10.8) | 61.7 (9.8) | 64.4 (8.4) | 61.0 (12.1) | 65.1 (10.0) | 59.6 (13.6) | 65.0 (11.3) | 57.5 (15.0) | 63.5 (13.1) |
| LAVI | 43.4 (31.3) | 40.1 (27.6) | 32.0 (17.1) | 30.1 (14.5) | 38.8 (25.1) | 35.0 (21.4) | 46.8 (32.7) | 41.5 (27.1) | 57.5 (40.9) | 53.1 (36.1) |
| LVMI | 99.4 (30.2) | 85.7 (28.0) | 89.2 (23.5) | 74.1 (20.0) | 96.8 (27.5) | 81.9 (23.6) | 102.4 (30.6) | 88.5 (27.6) | 111.2 (34.9) | 98.7 (32.9) |
| Septal e’ | 8.0 (2.8) | 8.3 (3.1) | 10.2 (2.9) | 11.0 (3.0) | 8.0 (2.4) | 8.4 (2.5) | 7.1 (2.2) | 7.2 (2.3) | 6.4 (2.1) | 6.4 (2.2) |
| Lateral e’ | 9.5 (3.5) | 9.5 (3.7) | 12.3 (3.8) | 13.1 (3.8) | 9.4 (2.8) | 9.3 (2.6) | 8.4 (2.6) | 8.1 (2.4) | 7.9 (2.7) | 7.3 (2.4) |
| E:A ratio | 1.2 (0.7) | 1.1 (0.7) | 1.5 (0.6) | 1.5 (0.6) | 1.1 (0.6) | 1.1 (0.5) | 1.0 (0.6) | 1.0 (0.6) | 1.0 (0.9) | 1.0 (0.7) |
| E velocity | 77.9 (25.9) | 83.9 (27.3) | 78.7 (21.5) | 86.1 (22.6) | 74.7 (23.3) | 79.5 (23.9) | 76.8 (27.2) | 81.1 (28.0) | 82.3 (30.7) | 87.8 (32.3) |
| A velocity | 68.8 (26.4) | 76.6 (28.5) | 55.2 (19.7) | 60.9 (20.9) | 66.4 (23.4) | 72.9 (24.1) | 74.9 (26.0) | 82.8 (27.2) | 82.6 (29.3) | 92.4 (31.0) |
| E:e’ ratio | 10.5 (5.0) | 10.9 (5.3) | 8.1 (3.2) | 8.2 (3.2) | 9.8 (4.2) | 9.9 (4.0) | 11.2 (4.96) | 11.5 (4.9) | 13.1 (6.0) | 14.0 (6.4) |
| eRVSP | 36.9 (11.2) | 36.7 (11.4) | 31.5 (8.9) | 30.8 (9.0) | 34.7 (9.6) | 34.0 (9.7) | 37.6 (10.6) | 37.2 (10.7) | 41.3 (12.2) | 41.5 (12.1) |
Data are presented as mean (±SD) unless otherwise specified. Mean age (years) n = 436 360; body weight (kg) n = 325 045; body mass index (BMI, kg/m2, calculated using the basal 2D ASE method) n = 322 871; heart rate (bpm) n = 196 265; blood pressure n = 67 930; stroke volume index (SVi, mL/m2) n = 132 747; left ventricular ejection fraction (LVEF, %) n = 392 009; left atrial volume index (LAVI, mL/m2) n = 170 614; LV mass index (LVMI, g/m2) n = 264 271; Septal e’ velocity (cm/s) n = 237 816; Lateral e’ velocity (cm/s) n = 36 895; E:A ratio, n = 376 453; Mitral E velocity (cm/s) n = 346 360; Mitral A velocity (cm/s) n = 379 711; Septal E:e’ ratio, n = 217 181; estimated right ventricular systolic pressure (eRVSP, assuming right atrial pressure = 5 mmHg), n = 264 717.
ASE/EACVI categories of diastolic function
Overall, guideline-derived assessment of diastolic function was possible in 392 009 (89.8%) cases based on the availability of LVEF. This group comprised 347 408 (88.6%) and 44 601 (11.4%) cases assessed according to preserved vs. reduced systolic function criteria. However, a further 45 399 (13.1%) and 1964 (4.4%) cases in these two groups could not be further categorized due to insufficient diastolic measurements. Of the remaining 302 549 cases with preserved systolic function (87.1%), 209 936 (69.3%), and 27 637 (9.2%) were categorized as ‘normal’ or ‘abnormal’ diastolic function; leaving 64 976 (21.5%) cases categorized as ‘indeterminate’. Similarly, among the remaining 42 637 cases (95.6% of those with reduced systolic function), 2026 (4.8%) and 14 049 (33.0%) were categorized as ‘normal’ vs. ‘increased’ filling pressure; leaving 26 562 cases (62.2%) in this group with an ‘indeterminate’ classification. Table 2 summarizes the profile of those assigned to these three categories according to the LVEF status (reduced vs. preserved systolic function). In both ‘preserved’ and ‘reduced’ ejection fraction categories, those with an abnormal classification tended to be older with a higher blood pressure, a larger LAVi, increased LVMI, E:e’ ratio, and eRVSP, and lower septal and lateral e’ velocities. Overall, therefore, among the 436 360 individuals in whom at least one diastolic function measurement was obtained, 41.9% (182 712 cases) did not have classifiable diastolic function according to ASE/EACVI criteria.
Table 2.
ASE diastolic function
| Preserved EF category |
Reduced EF Category |
|||||
|---|---|---|---|---|---|---|
| Normal diastolic function (n = 209 936) | Abnormal diastolic function (n = 27 637) | Indeterminate diastolic function (n = 64 976) | Normal filling pressure (n = 2026) | Increased filling pressure (n = 14 049) | Indeterminate filling pressure (n = 26 562) | |
| Anthropometric data | ||||||
| Age at echo | 57.5 (17.8) | 74.4 (11.8) | 67.8 (15.3) | 65.1 (14.5) | 72.1 (14.7) | 67.0 (15.5) |
| Body weight | 80.1 (20.3) | 78.8 (20.4) | 78.5 (19.0) | 84.9 (22.3) | 78.9 (20.5) | 82.2 (21.4) |
| BSA | 1.9 (0.3) | 1.9 (0.3) | 1.9 (0.2) | 2.0 (0.3) | 1.9 (0.3) | 2.0 (0.3) |
| Vital signs | ||||||
| Heart rate | 69.9 (13.0) | 72.7 (14.7) | 70.4 (14.4) | 70.5 (12.8) | 74.2 (16.9) | 74.1 (17.3) |
| Blood pressure | 130.8/74.9 (20.5/11.1) | 141.3/80.1 (22.1/10.0) | 138.4/78.4 (21.5/10.7) | 126.8/73.7 (21.2/11.2) | 129.4/74.6 (23.5/13.1) | 126.5/72.4 (21.1/11.8) |
| Systolic function | ||||||
| SVi | 41.1 (11.6) | 45.1 (13.4) | 42.7 (12.7) | 35.6 (10.4) | 34.1 (11.7) | 36.1 (10.9) |
| LVEF | 64.4 (7.2) | 68.9 (10.1) | 66.8 (8.9) | 41.1 (8.1) | 35.6 (10.0) | 38.4 (9.2) |
| Diastolic function | ||||||
| LAVI | 26.4 (8.2) | 74.4 (36.6) | 54.8 (31.3) | 26.0 (5.5) | 70.7 (42.5) | 33.9 (13.7) |
| LVMI | 83.1 (22.2) | 116.3 (35.5) | 100.1 (29.2) | 106.4 (30.5) | 129.9 (37.4) | 111.0 (34.7) |
| Septal e’ velocity | 9.3 (2.7) | 5.5 (1.3) | 7.8 (3.0) | 7.2 (2.3) | 5.5 (2.1) | 6.1 (2.4) |
| Lateral e’ velocity | 11.0 (3.5) | 6.6 (1.8) | 7.6 (2.3) | 6.9 (2.4) | 7.3 (3.0) | 8.0 (3.1) |
| E:A ratio | 1.2 (0.6) | 1.0 (0.7) | 1.1 (0.7) | 0.7 (0.3) | 2.0 (1.5) | 1.0 (0.4) |
| E velocity | 79.2 (24.2) | 87.7 (30.4) | 83.7 (28.2) | 54.1 (18.1) | 96.3 (30.7) | 80.2 (31.9) |
| A velocity | 66.6 (25.2) | 95.3 (31.4) | 78.9 (28.5) | 66.6 (21.0) | 63.1 (32.7) | 78.5 (25.9) |
| Septal E:e’ ratio | 8.5 (2.5) | 16.4 (5.8) | 11.1 (4.5) | 8.8 (2.5) | 18.8 (7.8) | 11.7 (5.5) |
| eRVSP | 34.2 (10.4) | 41.6 (11.0) | 37.2 (10.3) | 34.0 (8.1) | 44.4 (12.7) | 38.8 (12.7) |
Data are presented as mean (±SD) unless otherwise specified. For the preserved EF and reduced EF categories, respectively: Age at echo (years), n = 302 009 and n = 42 637; body weight (kg), n = 248 966 and n = 31 250; body surface area (BSA, m2), n = 242 445 and n = 30 316; heart rate (bpm), n = 162 328 and n = 16 836; blood pressure (mmHg), n = 53 816 and n = 7026; stroke volume index (SVi, mL/min.m2), n = 91 624 and n = 17 608; left ventricular ejection fraction (LVEF, %), n = 302 009 and n = 42 637; left atrial volume index (LAVI, mL/m2), n = 155 097 and n = 12 090; left ventricular mass index (LVMI, g/m2, calculated using the basal 2D ASE method), n = 214 523 and n = 23 375; Septal e’ velocity (cm/s), n = 208 091 and n = 19 432; lateral e’ velocity (cm/s), n = 31 615 and n = 4419; Mitral E:A ratio, n = 271 156 and n = 31 007; Mitral E velocity (cm/s), n = 302 009 and n = 42 637; Mitral A velocity (cm/s), n = 276 436 and n = 31 479; septal E:e’ ratio, n = 189 158 and n = 17 690; estimated right ventricular systolic pressure (eRVSP, assuming RA pressure = 5 mmHg), n = 212 042 and n = 30 346. ANOVA of all groups P < 0.0001.
Cardiovascular-related and all-cause mortality
During a total of 2.2 million person-years follow-up, there were a total of 100 597 all-cause deaths among the 436 360 cases with any measured diastolic parameters. Overall, 40 288 deaths occurred within 5 years of the last echocardiogram at which diastolic dysfunction was assessed. Cardiovascular-related mortality accounted for 49 198 (48.9%) of all deaths.
ASE/EACVI diastolic function and mortality
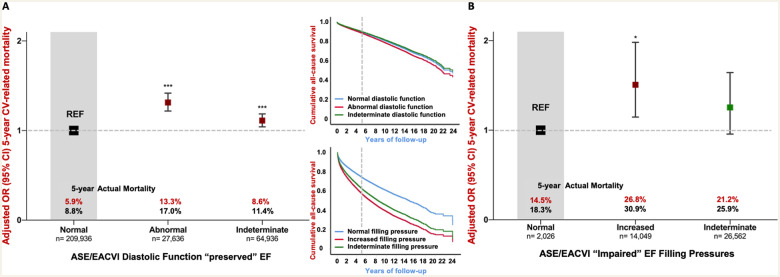
Among those 302 549 cases with preserved systolic function, a total of 15 406 (5.1%) and 58 638 (19.4%) died from cardiovascular disease and any cause during a median of 1631 days (IQR 896–2678), respectively. The equivalent figures for the remaining cases with reduced systolic function (n = 42 637) were 8582 (20.1%) and 19 333 (45.3%) cardiovascular-related and all-cause deaths during a median of 1040 days (IQR 372–1985). Figure2A and B shows the unadjusted and adjusted pattern of cardiovascular-related mortality according to ASE/EACVI criteria. Most cases with preserved LVEF had normal diastolic function and a relatively low actual 5-year cardiovascular (5.9%) and all-cause (8.8%) mortality. In fully adjusted models including age, sex, LVEF, rhythm, valvular heart disease, and the presence/absence of mitral or aortic valve replacement, the diastolic function category continued to be independently associated with 5-year cardiovascular-related mortality. The adjusted OR for cardiovascular-related mortality for those with DD and a preserved LVEF was 1.31 (95% CI 1.22–1.42, P < 0.001) vs. those with normal diastolic function. The equivalent risk for those with indeterminate diastolic function was also elevated (OR 1.11, 95% CI 1.04–1.18; P < 0.001). The corresponding long-term hazards (adjusted for age and sex) for all-cause death (upper small panel, Figure 2) were 1.12 (95% CI 1.09–1.15, P < 0.001) for DD and 0.95 (0.93–0.97, P < 0.001) for indeterminate diastolic function compared with normal diastolic function. For patients with reduced LVEF, a classification of increased filling pressure had an adjusted OR for cardiovascular-related mortality of 1.51 (95% CI 1.15–1.98, P < 0.001), whereas the difference between those categorized as indeterminate vs. normal filling pressure was of borderline significance (1.25, 95% CI 0.96–1.64; P = 0.06). The adjusted hazards for long-term mortality associated with increased and indeterminate filling pressures were 1.54 (95% CI 1.4–1.7; P < 0.001) and 1.41 (95% CI 1.29–1.53; P < 0.001), respectively.
Figure 2.
Five-year adjusted cardiovascular mortality using ASE/EACVI algorithm. This graph shows the fully adjusted pattern of actual 5-year cardiovascular-related mortality in subjects with full 5-year follow-up according to ASE/EACVI diastolic function classification for the ‘preserved EF’ (A) and ‘impaired EF’ (B) algorithms. Both groups were analysed with separate logistic regression models with full adjustment for each of the co-variates with the following odds ratios (±95% CI). Preserved EF: age (per year) OR 1.026 (1.023–1.028)***; Male sex OR 0.992 (0.938–1.049); LVEF OR per unit 0.994 (0.991–0.997)*; Non-sinus rhythm OR 1.695 (1.585–1.812)***; VHD OR 1.655 (1.552–1.766)***; prior mitral or aortic valve replacement (MVR/AVR) OR 1.526 (1.366–1.704)***. Impaired EF: age (per year) OR 1.010 (1.006–1.015)*; Male sex OR 1.063 (0.949–1.191); LVEF OR per unit 0.981 (0.976–0.986)*; Non-sinus rhythm OR 1.026 (0.922–1.142); VHD OR 1.351 (1.215–1.503)**; MVR/AVR OR 1.139 (0.953–1.362). The smaller graph inset show long-term Cox-proportional mortality hazard adjusted for age and sex. The top graph refers to preserved LVEF, and the bottom graph to impaired LVEF. LVEF, left ventricular ejection fraction (%), non-sinus rhythm is compared with patients in sinus rhythm during echocardiography; VHD, valvular heart disease, defined as an aortic valve area <1.2 cm2, mean AV or MV gradient >20 or 5 mmHg, respectively, or moderate or greater mitral or aortic regurgitation. The significance for each odds ratio is denoted by *P < 0.05, **P < 0.01, and ***P < 0.001.
Diastolic parameters and mortality
Overall, E-wave velocity was the most consistently measured diastolic parameter within the entire cohort; being recorded in all 436 360 cases studied. In contrast, A-wave velocity was far less frequently measured, usually due to the presence of an atrial arrhythmia or paced rhythm. E:A ratio was measured and recorded in 376 453 (86.3%) cases with the other key diastolic function parameters measured as follows: septal e’ velocity (237 816 cases/54.5%), E:e’ ratio (217 181/49.8%), LAVi (170 614 cases/39.1%), and eRVSP (264 717 cases/60.7%). Study follow-up of all cases studied with a minimum of a one valid diastolic measurement was a median of 1579 (IQR 847–2631) days from the index echocardiogram.
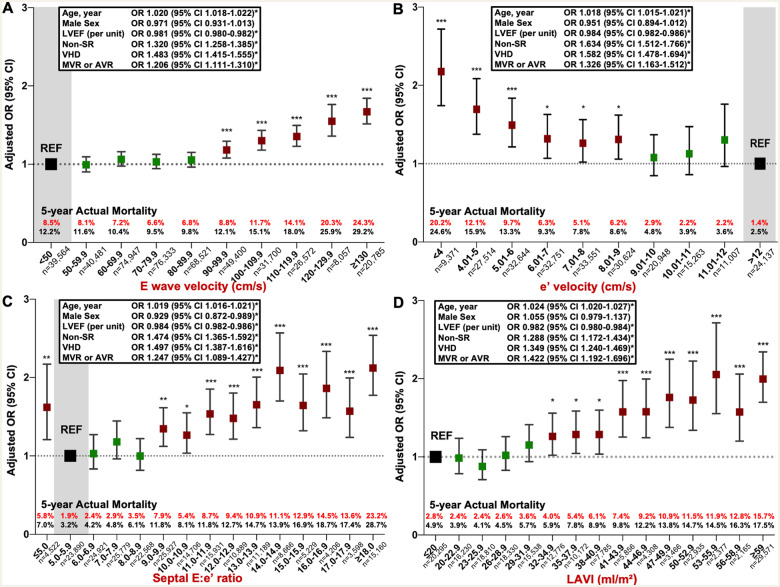
Of the seven key parameters of diastolic function specifically examined in respect to their relationship to mortality, four parameters (mitral E velocity, septal e’ velocity, septal E:e’ ratio, and LAVi) demonstrated a distinctive, differential pattern of mortality across their pre-determined/granular units of distribution. Figure 3 shows the pattern of long-term, all-cause mortality adjusted for age and sex, and according to the pre-specified unit groups within each parameter. Mitral E-wave velocity groups show similar clustering until the higher velocities, with further separation of risk above ∼120 cm/s. Similarly, septal e’ velocities cluster together around the higher velocity spectrum, with higher mortality apparent below ∼6 cm/s. The septal E:e’ ratio shows a graded mortality hazard above ∼9 cm/s, with a markedly worse mortality profile above 14. Finally, the mortality profile of LAVi worsened at around 35 mL/m2, above which a progressive rise in mortality was observed.
Figure 3.
Long-term all-cause mortality for each diastolic parameter. These graphs plot the Cox-proportional hazards for long-term all-cause mortality adjusted for age and sex, for each diastolic parameter in unit increments. Below each panel is the number of patients at risk at each time-point. (A) Mitral inflow E-wave velocity (cm/s), (B) LV septal e’ velocity (cm/s), (C) septal E:e’ ratio, and (D) indexed left atrial volume (mL/m2).
As shown in Figure 4, for E velocity (Figure 4A), there was a clear ‘pivot point’ at 90 cm/s, associated with an increase in cardiovascular-related mortality (from 6.8% in the 80.0–89.9 cm/s group to 8.8% in the 90.0–99.9 cm/s group) and in all-cause mortality (from 9.8% to 12.1%). Above this threshold there was a continuous increase in mortality. This pattern was reversed for e’ velocity (Figure 4B) where a clear threshold was demonstrated: cardiovascular-related and all-cause mortality increased from 2.9% to 6.2% and 4.8% to 8.6%, respectively below 9 cm/s. The risk of mortality remained similar between 6 and 9 cm/s but below 6 cm/s, mortality steadily increased. Figure 4C demonstrates a pivot point at 9 for E:e’ ratio and an acceleration of risk above 14. Finally, the equivalent pivot point for LAVi (Figure 4D) appears at ∼35 mL/m2, with mortality rates increasing more markedly above 44 mL/m2. In a sensitivity analysis performed on only those patients with all diastolic function parameters present (n = 113 725), the same pattern of mortality was demonstrated with similar mortality pivot-points.
Figure 4.
Five-year adjusted cardiovascular mortality for each diastolic parameter. This graph shows the fully adjusted pattern of actual 5-year cardiovascular-related mortality in subjects with full 5-year follow-up according to each of the following diastolic function parameters: (A) E-wave velocity (n = 436 360); (B) Medial mitral annular e’ velocity (n = 237 816); (C) E:e’ ratio (n = 217 181); (D) indexed left atrial volume (n = 170 614). Each of the four groups were analysed with separate logistic regression models and odds ratios (with 95% confidence intervals) for each adjustment shown in the text box. LVEF, left ventricular ejection fraction (%), non-sinus rhythm is compared with patients in sinus rhythm during echocardiography, VHD, valvular heart disease, defined as an aortic valve area <1.2 cm2, mean AV or MV gradient >20 or 5 mmHg, respectively, an MV mean gradient >5 mmHg, or moderate or greater mitral or aortic regurgitation. The significance for each odds ratio is denoted by *P < 0.05, **P < 0.01, and ***P < 0.001. In a sensitivity analysis examining only patients with all diastolic function parameters present (n = 113 725), similar mortality pivot-points were identified.
Discussion
In the largest study of diastolic function using real-world echocardiographic data from a multicultural cohort, we demonstrate that abnormal diastolic function defined by ASE/EACVI criteria is, as expected, associated with increased risk of cardiovascular-related and all-cause mortality. In addition, individual analyses of mitral E velocity, septal e’ velocity, E:e’ ratio, and LAVi revealed distinctive pivot-points of increased, adjusted risk of cardiovascular-related and all-cause mortality. Specific thresholds of increased mortality were identified at 90 cm/s for E-wave velocity, 9 cm/s for septal e’ velocity, an E:e’ ratio of 9, and an LAVi of 32 mL/m2. Examination of these individual markers is clinically relevant, since an indeterminate classification using the ESC/EACVI algorithm was common (affecting 21.5% of individuals overall and 62.2% of the reduced LVEF category), raising a clinical dilemma if the guideline recommendations are used in isolation. Our finding of a frequent indeterminate classification is similar to other studies,7,8,14 although after exclusion of patients with cardiomyopathy, valvular heart disease, and non-sinus rhythm, indeterminate diastolic function is less frequently allocated. Since DD is associated with future HF and mortality,15,16 allocation to indeterminate diastolic function has clinical relevance, especially considering our demonstration of increased mortality in the indeterminate preserved LVEF groups, and a similar demonstration by Liang et al.17 Further, although the guidelines recommend an LVEF cut-point at 50%, the distinct clinical entity associated with impaired LVEF5 is also associated with more subtle abnormalities across the LVEF spectrum, including mildly impaired systolic function (Supplementary data online, Table S1).
Diastolic left ventricular filling is complex, with multiple simultaneous events partially captured by echocardiographic assessment of each individual parameter. Elevated peak mitral inflow E-wave velocity may occur in elevated filling pressure18 and also in normal young people and athletes thus demonstrating biphasic association with filling pressure.19 The E:e’ ratio is a more robust marker of LV filling pressure6 and elevated E:e’ has been associated with increased mortality in a range of diseases, such as HFrEF,20 mitral and aortic regurgitation, aortic stenosis, and in hypertension.6 In our unselected cohort which included all of these diseases, E:e’ remained a marker of increased mortality with a pivot point of increased mortality at ∼9, similar to the upper limit of normal demonstrated in the NORRE study.19 Low septal e’ velocities have been strongly associated with mortality, however basal left ventricular motion is influenced by prior cardiac surgery (including aortic and mitral valve replacement), mitral annular calcification,21 and abnormal septal motion.22 Despite the heterogeneous nature of NEDA including patients with these conditions, e’ velocity showed an independent association with mortality in adjusted models, around a pivot point of 9 cm/s. The small overlap between our threshold and published reference ranges19 reinforces guideline recommendations that comprehensive diastolic function reporting should take into account all measured parameters.6
There are a number of additional factors influencing each individual diastolic function marker. Age has an important influence on each diastolic marker,23,24 and our findings reinforce this observation with demonstration of an independent association of age with cardiovascular-related and all-cause (see Table 1 and Figure 4). However, age is not currently included in the ASE/EACVI algorithm and may potentially overestimate DD in older individuals.25 Despite the differences in HF epidemiology between women and men,26 we did not confirm a significant sex-specific association between DD and mortality.
AF may directly result in increased LAVi, and conversely a large LAVi may result in atrial fibrillation.27 As noted in Figure 4, non-sinus rhythm increases the odds ratio of cardiovascular-related mortality for each parameter. Since atrial fibrillation is common, correction for underlying rhythm may potentially allow diastolic function to be applied in a broader group of patients.
The findings from our study have four important clinical implications. First, we confirm that the ASE/EACVI classification of diastolic function can meaningly separate patient groups based on mortality risk, despite a large proportion of patients in the indeterminate categories of both LVEF groups. Secondly, we have shown an important role for individual diastolic parameters, in particular, E-wave velocity, e’ velocity, E:e’ ratio, and LAVi which remain associated with cardiovascular and all-cause mortality outcomes after multi-parameter corrections, consistent with the recommendations of the HFA-PEFF diagnostic algorithm.28 Thirdly, we have shown clear mortality thresholds: Above 90 cm/s for E-wave velocity, 9 cm/s for e’ velocity, 9.0 for E:e’ ratio, and 32 mL/m2 for LAVi which, when taken together, may be helpful in individual patient assessment. However, the influence of other factors, such as age and cardiac rhythm, should be considered when applying these thresholds to individual patients. Sex does not appear to be a significant determinant of mortality in diastolic dysfunction. Fourth, it may be timely to consider new guidelines that incorporate age, rhythm, valvular heart disease or prior intervention, and the thresholds we have identified for E velocity, e’ velocity, E:e’ ratio, and LAVi, to allow for prediction of mortality across a broad patient cohort. If found to be clinically useful, a new algorithm applying these markers could be automated within echocardiography machine software.
The inherent limitations of applying and interpreting big data have been described in previous NEDA reports.9,10 For example, NEDA does not (yet) capture important clinical details on common conditions, such as coronary artery disease, ischaemic heart disease, and clinically diagnosed HF.3 At the individual patient level, these are important to interpreting the clinical implications of ASE/EACVI categories of DD. The same applies when considering the cut-points of elevated risk identified by our analyses of individual diastolic parameters. We plan to capture hospitalisation episodes in the next iteration of the registry. Similarly, we do not have salient information on the pharmacological treatment, biomarkers, and symptoms to supplement our echocardiographic profiling of each NEDA patient. For example, although the treatment for HF, such as neurohormonal modulating therapies29 may significantly influence diastolic function, we were unable to fully account for the effect of pharmacotherapies in our multivariate analyses. In the absence of specific comments on the echocardiographic reports, it is possible some patients with prior cardiac surgery (including valve intervention) were not captured. To account for this possibility, we also extracted the ‘indication for echo’. This analysis was performed on the ‘last’ echocardiogram, but a sensitivity analysis based on ‘first’ echo was performed, showing similar results. Written documentation of the underlying rhythm was not universally applied at participating sites. To improve capture, we employed a combined method that also included physician reports and the presence of mitral A waves. It is also possible that some cases of atrial fibrillation were missed, and conversely, some patients with a measured E but not a measured A wave (and thereby allocated into the ‘non-sinus rhythm’ group) may have been in sinus rhythm. Once again, this highlights the caution needed to interpret outcomes derived from a very large, heterogeneous cohort of patients at the individual level. Finally, worsening diastolic function over time has been associated with higher mortality30; however, we did not examine change in DD over time, with only the last echocardiogram for everyone included in this analysis. We plan to address these limitations in future iterations of the study.
Summary
We have demonstrated that the ASE/EACVI classification of diastolic function can successfully identify patients at increased risk of cardiovascular and all-cause mortality, in both preserved- and reduced-LVEF categories. However, many patients in both LVEF categories have ‘indeterminate’ diastolic function. Individual parameters of diastolic function, in particular, mitral E velocity, septal e’ velocity, E:e’ ratio, and LAVi were independently associated with both cardiovascular-related and all-cause mortality. Mortality thresholds were identified at 90 cm/s for E-wave velocity, 9 cm/s for septal e’ velocity, an E:e’ ratio of 9, and an LAVi of 32 mL/m2. Although these thresholds need be validated in other, more granular studies, given the size of the cohort and number of events analysed, they have strong potential to be a useful addition to future clinical guidelines on diastolic function reporting (see Central Illustration). Age-related, but not sex-specific, and rhythm-corrected changes to all measures of diastolic function also require further evaluation and potential development into new diastolic function algorithms.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Supplementary Material
Acknowledgements
The authors thank all the NEDA centres and their patients for contributing to these data.
Funding
NEDA was originally established with funding support from Actelion Pharmaceuticals, Bayer Pharmaceuticals, GlaxoSmithKline. Both NEDA (#1055214) and SS (#11358940) are supported by the National Health and Medical Research Council of Australia.
Conflict of interest: none declared.
Data availability
Original study data are not available to investigators from non-contributing centres. However, sharing of data outputs will be considered on request to D.P.
References
- 1. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA.. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018;138:861–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rossi A, Temporelli PL, Quintana M, Dini FL, Ghio S, Hillis GS. et al. ; on behalf of the MeRGE Heart Failure Collaborators. Independent relationship of left atrial size and mortality in patients with heart failure: an individual patient meta-analysis of longitudinal data (MeRGE Heart Failure). Eur J Heart Fail 2009;11:929–36. [DOI] [PubMed] [Google Scholar]
- 3. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM.. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251–9. [DOI] [PubMed] [Google Scholar]
- 4. Møller JE, Whalley GA, Dini FL, Doughty RN, Gamble GD, Klein AL. et al. Independent prognostic importance of a restrictive left ventricular filling pattern after myocardial infarction: an individual patient meta-analysis: meta-Analysis Research Group in Echocardiography acute myocardial infarction. Circulation 2008;117:2591–8. [DOI] [PubMed] [Google Scholar]
- 5. Borlaug BA, Redfield MM.. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation 2011;123:2006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T. et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–60. [DOI] [PubMed] [Google Scholar]
- 7. Gottbrecht MF, Salerno M, Aurigemma GP.. Evolution of diastolic function algorithms: implications for clinical practice. Echocardiography 2018;35:39–46. [DOI] [PubMed] [Google Scholar]
- 8. Luke P, Eggett C, Spyridopoulos I, Irvine T.. A comparative analysis of British and American Society of Echocardiography recommendations for the assessment of left ventricular diastolic function. Echo Res Practice 2018;5:139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strange G, Stewart S, Celermajer DS, Prior D, Scalia GM, Marwick TH. et al. Threshold of pulmonary hypertension associated with increased mortality. J Am Coll Cardiol 2019;73:2660–72. [DOI] [PubMed] [Google Scholar]
- 10. Strange G, Stewart S, Celermajer D, Prior D, Scalia GM, Marwick T. et al. National Echocardiography Database of Australia contributing sites. Poor long-term survival in patients with moderate aortic stenosis. J Am Coll Cardiol 2019;74:1851–63. [DOI] [PubMed] [Google Scholar]
- 11. Strange G, Celermajer DS, Marwick T, Prior D, Ilton M, Codde J. et al. The National Echocardiography Database Australia (NEDA): Rationale and methodology. Am Heart J 2018;204:186–9. [DOI] [PubMed] [Google Scholar]
- 12. Magliano D, Liew D, Pater H, Kirby A, Hunt D, Simes J. et al. Accuracy of the Australian National Death Index: comparison with adjudicated fatal outcomes among Australian participants in the Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) study. Aust N Z J Public Health 2003;27:649–53. [DOI] [PubMed] [Google Scholar]
- 13. Sharp MK, Tokalic R, Gomez G, Wager E, Altman DG, Hren D.. A cross-sectional bibliometric study showed suboptimal journal endorsement rates of STROBE and its extensions. J Clin Epidemiol 2019;107:42–50. [DOI] [PubMed] [Google Scholar]
- 14. Almeida JG, Fontes-Carvalho R, Sampaio F, Ribeiro J, Bettencourt P, Flachskampf FA. et al. Impact of the 2016 ASE/EACVI recommendations on the prevalence of diastolic dysfunction in the general population. Eur Heart J Cardiovasc Imaging 2018;19:380–6. [DOI] [PubMed] [Google Scholar]
- 15. Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ.. Burden of systolic and diastolic ventricular dysfunction in the community. JAMA 2003;289:194. [DOI] [PubMed] [Google Scholar]
- 16. Halley CM, Houghtaling PL, Khalil MK, Thomas JD, Jaber WA.. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med 2011;171:1082–7. [DOI] [PubMed] [Google Scholar]
- 17. Liang H-Y, Lo Y-C, Chiang H-Y, Chen M-F, Kuo C-C.. Validation and comparison of the 2003 and 2016 diastolic functional assessments for cardiovascular mortality in a large single-center cohort. J Am Soc Echocardiogr 2020;33:469–80. [DOI] [PubMed] [Google Scholar]
- 18. Garcia MJ, Ares MA, Asher C, Rodriguez L, Vandervoort P, Thomas JD.. An index of early left ventricular filling that combined with pulsed Doppler peak E velocity may estimate capillary wedge pressure. J Am Coll Cardiol 1997;29:448–54. [DOI] [PubMed] [Google Scholar]
- 19. Caballero L, Kou S, Dulgheru R, Gonjilashvili N, Athanassopoulos GD, Barone D. et al. Echocardiographic reference ranges for normal cardiac Doppler data: results from the NORRE Study. Eur Heart J Cardiovasc Imaging 2015;16:1031–41. [DOI] [PubMed] [Google Scholar]
- 20. Troughton RW, Prior DL, Frampton CM, Nash PJ, Pereira JJ, Martin M. et al. Usefulness of tissue doppler and color M-mode indexes of left ventricular diastolic function in predicting outcomes in systolic left ventricular heart failure (from the ADEPT study). Am J Cardiol 2005;96:257–62. [DOI] [PubMed] [Google Scholar]
- 21. Abudiab MM, Chebrolu LH, Schutt RC, Nagueh SF, Zoghbi WA.. Doppler echocardiography for the estimation of LV filling pressure in patients with mitral annular calcification. JACC Cardiovasc Imaging 2017;10:1411–20. [DOI] [PubMed] [Google Scholar]
- 22. Silbiger JJ. Pathophysiology and echocardiographic diagnosis of left ventricular diastolic dysfunction. J Am Soc Echocardiogr 2019;32:216–32.e2. [DOI] [PubMed] [Google Scholar]
- 23. Shah AM, Claggett B, Kitzman D, Biering-Sørensen T, Jensen JS, Cheng S. et al. Contemporary assessment of left ventricular diastolic function in older adults: the atherosclerosis risk in communities study. Circulation 2017;135:426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nayor M, Cooper LL, Enserro DM, Xanthakis V, Larson MG, Benjamin EJ. et al. Left ventricular diastolic dysfunction in the community: impact of diagnostic criteria on the burden, correlates, and prognosis. JAHA 2018;7:e008291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coller JM, Gong FF, McGrady M, Jelinek MV, Castro JM, Boffa U. et al. Age‐specific diastolic dysfunction improves prediction of symptomatic heart failure by Stage B heart failure. ESC Heart Fail 2019;6:747–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsutsui H, Tsuchihashi-Makaya M, Kinugawa S.. Clinical characteristics and outcomes of heart failure with preserved ejection fraction: lessons from epidemiological studies. J Cardiol 2010;55:13–22. [DOI] [PubMed] [Google Scholar]
- 27. Ball J, Carrington MJ, McMurray JJV, Stewart S.. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int J Cardiol 2013;167:1807–24. [DOI] [PubMed] [Google Scholar]
- 28. Pieske B, Tschöpe C, Boer RA, Fraser AG, Anker SD, Donal E. et al. How to diagnose heart failure with preserved ejection fraction: the HFA–PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2020;22:391–412. [DOI] [PubMed] [Google Scholar]
- 29. Dewan P, Jackson A, Lam CSP, Pfeffer MA, Zannad F, Pitt B. et al. Interactions between left ventricular ejection fraction, sex and effect of neurohumoral modulators in heart failure. Eur J Heart Fail 2020;22:898–901. [DOI] [PubMed] [Google Scholar]
- 30. Aljaroudi W, Alraies MC, Halley C, Rodriguez L, Grimm RA, Thomas JD, Jaber WA.. Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction. Circulation 2012;125:782–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original study data are not available to investigators from non-contributing centres. However, sharing of data outputs will be considered on request to D.P.