Abstract
Segmental bone loss continues to pose substantial clinical and technical challenges to orthopaedic surgeons. While several surgical options exist for the treatment of these complex patients, there is not a clear consensus or specific guidelines on the optimal management of these injuries as a whole. Many factors must be taken into consideration when planning surgery for these individuals. In order for these techniques to yield optimal results, each injury must be approached in a step-wise and multidisciplinary fashion to ensure that care is taken in bone and wound bed preparation, that soft tissues are healthy and free of contaminants, and that the patient's medical condition has been optimized. Through this article, we will answer relevant questions and discuss common obstacles and challenges encountered with these complex injuries. We will also review the many treatment options available or in development to address this problem.
Keywords: bone loss, lower extremity fracture, open fracture
1. Introduction
Segmental bone loss continues to pose substantial clinical and technical challenges to orthopaedic surgeons. It is most commonly observed in the traumatic setting as a result of open fractures and infection. While several surgical options exist for the treatment of these complex patients, there is not a clear consensus or specific guidelines on the optimal management of these injuries as a whole. Moreover, good long-term functional outcomes are limited by the high rates of complications and reoperations. Many factors must be taken into consideration when planning surgery for these individuals: patient factors (i.e., age, presence of chronic disease, nutritional status, psychosocial impact, smoking, etc), the state of surrounding soft tissues and blood supply, and finally the location and size of the bony defect.
Through this review, we will describe this patient demographic and how to appropriately assess and plan for operative management. We will address the concept of “critical” bone loss and the debate of what bone to keep or to discard in the initial debridement of an open fracture. Last we will review the different management options available and when they may be best utilized, along with some innovative techniques that may change how we approach this difficult orthopaedic problem.
2. When and where is bone loss a problem?
Significant bone loss is only seen in a small subset of orthopaedic fractures. In a 10-year prospective audit of admissions at the Edinburgh Orthopaedic Trauma Unit between 1988 and 1998, fractures with bone loss accounted for only 0.4% of all fractures. However, bone loss was observed in 11.4% of all open fractures[1] and was most commonly associated with Gustilo et al [2,3] IIIB and IIIC type injuries. Importantly, this was a young, active population of high-energy trauma patients with mean age of 37 years, with 71% of the cohort males.
The tibia is the most common site of traumatic bone loss. Its limited soft tissue coverage predisposes it to open fractures and bone extrusion in the high-energy setting. Open fractures of the upper extremity and axial skeleton are less common and therefore less likely to present with associated bone loss. Of all patients presenting with substantial bone loss, 68% were in the tibia, 22% in the femur, with the remainder found in other locations.[1] Based on these numbers, we will focus our discussion on bone loss in open fractures of the lower extremity.
3. Acute assessment and management
Bone defects associated with complex open fractures require a careful approach and planning. When assessing these patients in the acute setting, the initial step is to establish whether the limb is salvageable. With the advent of new surgical techniques such as bone transport,[4] acute limb shortening and lengthening,[5] massive allografts or vascularized fibular allografts,[6] induced membrane techniques (Masquelet technique)[6,7] and bone grafting with bone morphogenic protein (BMP),[8] large areas of segmental bone loss do not necessarily preclude limb-salvage. However, if the limb is severely mangled, has extensive soft tissue/muscle loss, severe neurovascular compromise, or an extensive period of ischemia, then amputation may be the best early treatment option,[9] especially if performed as an initial life-saving procedure. However, in most cases the decision between amputation and limb-salvage can be made in conjunction with the patient and their family. In appropriate cases, it is important that amputation is presented as a viable treatment option alongside limb-salvage, and not simply as a treatment failure given the lack of clear superiority of limb-salvage over amputation for most patients in the Lower Extremity Assessment Project trial.[10]
Early initiation of intravenous antibiotics is crucial.[11,12] First-generation cephalosporins should be administered promptly for Gustillo-Anderson Grade I and II open fractures. Most authors recommend additional gram-negative coverage in the form of an aminoglycoside for grade III injuries.[2,13] In the setting of fecal contamination or possible clostridial contamination, penicillin is also recommended.[14] Prophylactic antibiotics should not be continued longer than 24-hours after each surgical debridement since longer courses have not yielded a reduction in infection rates and could lead to greater rates of antibiotic resistance.[15,16]
Local antibiotic delivery into the wound at the time of surgical debridement appears to be a promising adjunct to systemic antibiotic prophylaxis alone. However, more research on this topic is necessary before stronger conclusions can be drawn.[17,18]
After initial assessment and initiation of antibiotics, the focus shifts to thorough surgical debridement and stabilization of the open injury. Debridement has often been guided by surgical principle rather than evidence-based technique. Recent literature states that open fractures should be debrided until “stable” and “all necrotic tissue and organic and inorganic contaminants have been removed.”[19] This involves working through the open fracture site, extending the wound proximally and distally and completing an excisional debridement of all nonviable skin, subcutaneous tissue, muscle, periosteum, and bone with the goal of achieving a healthy and clean surgical wound. The “tug test” can be used to assess the viability of cortical fragments within the wound[20] and consists of removing fragments that are easily removed using a pair of forceps or 2 fingers. These fragments are assumed to have insufficient viability for survival and are therefore discarded. This concept holds true primarily for cortical fragments of the diaphysis considering its poor surrounding soft tissue environment and blood supply. Metaphyseal fragments are much less commonly loose or free given the rich periosteum and musculotendinous attachments. Additionally, metaphyseal fragments may contain articular cartilage and/or critical ligamentous attachments which warrant consideration for retention. All viable fragments and reconstructible osteochondral or articular fragments should be preserved.[20–22]
In contradiction to the above, a specific scenario relevant to the concept of “critical” sized defect is the very large segmental fragment devoid of soft tissue attachment that, if removed, significantly worsens the reconstruction challenge. The decision to keep or remove this fragment requires weighing the value of the fragment and the risk-benefit ratio of both scenarios. For example, the comparison between a “low value” fragment such as a moderate sized cortical piece of diaphyseal bone, which can be managed with contemporary techniques and the “high value” fragment such as the large osteoarticular segment or whole extruded bone that may not be replaceable.[23] Methods on how to treat and reimplant large extruded segments of bone in an acute and delayed fashion in the open fracture setting have been described in the literature and have demonstrated good results despite going against popular belief; however, this evidence is largely low-level, based on case reports and case series.[24–27]
No matter the debridement technique chosen, once thorough debridement has been performed, the wound should be thoroughly irrigated in the operating room.[28] Issues such as timing of surgery, amount and type of fluid to be used, primary vs delayed closure, and timing of flap coverage are all important factors to be considered and will be covered elsewhere in this current symposium.
When addressing the traumatic or iatrogenic bone void caused by radical bony debridement prior to closure, antibiotic impregnated polymethylmethacrylate (PMMA) spacers have provided an excellent option. They not only provide a local high concentration of antibiotic elution, but increase fracture stability, maintain adequate soft tissue tension, and prevent fibrous ingrowth. This technique is not only used in the case of staged induced membrane (Masquelet) technique, but also in cases of delayed closure where the goal is to decrease the local bio-burden prior to proceeding with primary closure or soft tissue coverage and grafting.
At the end of the initial treatment phase, these patients will have a variable amount of bone loss which will remain a management challenge. When should this defect be treated? Can it be treated with observation? Is there a “critical sized defect” that always needs a second intervention? What is best treatment for this defect? These questions all remain important issues and will be addressed below.
4. Can we define “critical” bone defect?
Bone loss can happen at the time of injury via fragment extrusion or iatrogenically during debridement of an open fracture when devitalized bone is removed. Although classification systems exist for the description of open fractures, their ability to describe the extent of bone loss is lacking.[2,3] Describing bone loss should begin with a description by anatomical bone location: diaphyseal, metaphyseal, or articular. The extent of the defect is then described by stating its length and whether the segment presents partial or complete circumferential bone loss. When Orthopaedic Trauma Association members were surveyed with regards to the definition of a “critical-sized” segmental bone defect, a precise size and volume of bone fitting this criteria was not defined.[29] Some authors have suggested that this usually occurs when the length of the defect is 2 to 2.5 times the diameter of the bone involved.[30] Others have described this entity as a defect length greater than 1 cm and greater than 50% loss of the circumference of the bone.[1,31] In general, a “critical” defect is regarded as one that will not heal spontaneously despite surgical stabilization and requires further surgical intervention, such as bone grafting.[1,32]
The SPRINT study remains one of the largest randomized control trials in surgical history, yet despite including over 1000 tibia fractures, bone loss was relatively uncommon. In this study of 1125 patients with tibia fractures, only 37 (3%) had a “critical” bone defect defined as greater than 1 cm in length and > 50% of the cortical diameter. Of these 37 patients, 47% achieved union without additional treatment, suggesting that although worrisome, this “critical” size defect may not be truly “critical.”[31] A study conducted by Haines et al may offer the most detailed assessment of fracture gap size. In their study, the average radiographic apparent bone gap (RABG) measures bone loss on all 4 cortices (Fig. 1). A RABG of < 25 mm had a union rate of 54% and a RABG >25 mm had a union rate of 0%.[33]
Figure 1.
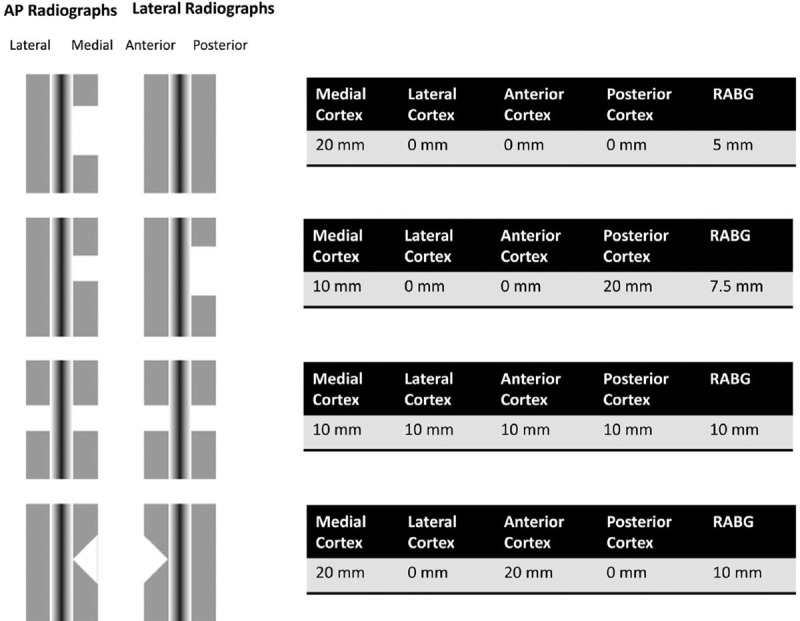
Defining the lower limit of a “Critical Bone Defect” in open diaphyseal tibial fractures. (Taken from Haines NM, Lack WD, Seymour RB, and Bosse MJ. Defining the lower limit of a “Critical bone defect” in open diaphyseal tibial fractures. J Orthop Trauma 30, e158–e163 (2016).)
It is important to understand the complex nature of bone healing. The definition of “critical sized” defect is likely oversimplified and therefore, bone gap size should not be used in isolation to make clinical decisions. Rather, multiple factors from an anatomical, biomechanical, and biological perspective all play a role in predicting nonunion. Anatomical location of the defect has a significant impact on healing potential. For example, segmental defects of the femur often present favorable soft tissue environment with spontaneous healing reported in segmental defects measuring as much as 6 to 15 cm in length.[34] In contrast to the femur, the tibia has relatively poor outcomes with lack of spontaneous healing in segments of bone loss as low as 1 cm with greater than 50% cortical circumference.[35,36] In the setting of nonunion, the biological environment is often the rate-limiting factor, with impaired cellular and molecular signaling, regardless of biomechanical stability. [1,29,31]
5. Surgical management of bone defects in open fracture
5.1. Patient optimization
Patient optimization is a crucial step that cannot be overlooked. Bone healing is predicated on both mechanical stability and a favorable biological environment; therefore, ignoring this step may be detrimental to overall outcomes. Tobacco cessation, glycemic control, nutritional optimization, and management of metabolic and endocrine abnormalities are interventions that should be optimized. In a study of 37 nonunions, patients who were referred for metabolic and endocrine testing, abnormalities such as vitamin D deficiency, thyroid, and parathyroid disorders and hypogonadism were found in > 80% of cases with otherwise unexplained nonunions.[37] With these metabolic abnormalities corrected, spontaneous healing of the nonunion without additional surgical intervention occurred in >25% of patients.[37] Other patient factors such as diabetes, radiation therapy, and alcohol abuse have also been associated with nonunion.[38] Finally, poor vascular supply to the affected extremity is invariably associated with failure of bony and soft tissue reconstruction. Consultation with a vascular surgeon is recommended for patients with compromised perfusion, as revascularization may be indicated. The treatment and optimization of these patients is necessarily multidisciplinary and the source of motivation for many centers to create limb salvage teams which are better equipped to help treat these modifiable risk factors and improve the rate of successful outcomes.
5.2. Induced membrane technique (Masquelet)
Although autologous bone grafting has been successful for bone defects < 5 cm in size, defects beyond this size have been associated with a high failure rate due to secondary graft resorption and consolidation failure.[7] To address this, Masquelet and Begue described an alternative induced membrane technique with 3 main benefits.[7] The first benefit is that the PMMA spacer maintains the defect space, preventing surrounding tissue contracture. Second, the membrane created is rich in growth factors including vascular endothelial growth factor, transforming growth factor-ß1, BMP-2 and core binding factor alpha-1, that improve graft consolidation by stimulating cell proliferation and differentiation into the osteoblastic lineage.[39] Finally, the membrane creates a separate compartment, protecting autograft from resorption.
The Masquelet technique occurs in 2 stages. The first stage consists of radical bony debridement followed by the placement of a PMMA cement spacer. The bone defect is then stabilized with external fixation (as originally described), internal fixation, or an intramedullary rod. To allow enough time for the membrane maturation, the second stage is delayed to allow ideal biologic and wound bed conditions. The ideal biologic timing is 4 weeks; however, the soft tissues may not be mature enough at this time and this often delays surgeon intervention this early. Typically, the second stage is completed 6 to 12 weeks following spacer placement. At this time the membrane is carefully incised and preserved while the spacer is removed. The void is then filled with autologous bone graft, with or without allograft or other bone substitutes as needed as graft extender. A graft extender should not exceed a 3:1 ratio of autograft to allograft.[7,40]
The time required prior to initiation of full weight bearing is fracture and fixation dependent. By definition, fixation of any segmental defect requires a load-bearing fixation construct, since no initial bone-to-bone contact exists at the outset to share load with the implant. Also, the time required for the bone graft to incorporate and corticate to a degree that it can bear load is often much longer than in typical fracture healing. As a result, the fixation construct must be able to withstand both high peak loads and many cycles of submaximal loads without failure. With multiplanar external fixators coupled with hydroxyapatite coated external fixation pins, locked IM nails, locking plate technology, and allograft cortical substitution, very stable and rigid fixation constructs can be created and immediate full weight bearing may be permitted. However, the optimal mechanical environment for graft healing remains unknown, though Masquelet has empirically recommended high initial fixation rigidity to promote incorporation followed by more flexible fixation to promote remodeling and cortication.[40]
Masquelet and Begue's initial series of 35 patients with defects ranging from 5 to 24 cm in length demonstrated graft incorporation and defect healing in 89% of patients.[7] This has since been replicated in other studies with success rates of 85% to 92% reported;[41] however, multiple interventions may be required to achieve union with this technique.[42–44] In summary, the Masquelet technique is reliable and allows for reconstruction of large segmental bone defects. Its advantage over distraction osteogenesis is that time to union is not dependent on the size of the bone defect, whereas its main disadvantage is the need for sufficient volumes of autologous bone graft to be successful.
5.3. Distraction osteogenesis
Management of segmental bone loss with distraction osteogenesis involves the transportation of a segment of bone in a controlled fashion, typically via the use of an external fixator or an IM device. This was initially described by Ilizarov while treating nonunions with fine wire circular frames.[45] With the advent of the spatial and monolateral frames, this technique has gained further popularity.[39] In brief, once the frame has been applied with fine wire or half pins, a metaphyseal corticotomy is performed and the transport phase begins. This may occur at a rate of up to 1 mm per day.[46] Once the transported segment reaches the docking site, it is compressed for weeks until healing occurs. The consolidation phase can often last twice as long as the transport phase.[47] The main advantages of using distraction osteogenesis for the management of segmental bone defects are reliability, ability to bear weight during reconstruction, and, most importantly, the absence of limits with regards to size of the defect that can be reconstructed. The disadvantages, however, are the length of time required to achieve consolidation (an average of 10–12 months for a defect of 10 cm in size) and the resultant physical and psychological burden on the patient with prolonged transports.
Another method of distraction osteogenesis that has minimized transport time has been that of bifocal distraction osteogenesis, which involves performing a metaphyseal corticotomy at both ends of the defect. This has been demonstrated to reduce transport time by 2.5 times as well as that of consolidation by between 1.3 and 1.9 times. However, this technique also demonstrated slower maturation of regenerated bone. Furthermore, this technique requires complex frame construct and meticulous frame management by both surgeon and patient.[47]
In a recent long-term study looking at the Ilizarov technique, although all patients eventually achieved union, the rate of union was 91.2% with a 29% reoperation rate.[48] Other studies have reported union rates between 92% and 94%,[46,49] with 1 study requiring secondary bone grafting in 36% of patients to achieve union.[46] Neurovascular complications and amputation rates have been reported at 2.2% and 2.9% respectively.[49]
Hybrid techniques in bone transport have demonstrated a decrease in time required for external fixation by stabilizing the bone with internal fixation during the consolidation phase. Kocaoglu et al[50] transported bone over an IM nail with an average defect size of 10 cm (femur) and 7 cm (tibia). The external fixator index (EFI), which is calculated by dividing the number of days in the external fixation by the total length of the defect, was 13.5 d/cm.[50] Once the transported segment reaches the docking site and the frame is removed, the segment is secured using locking screws through the nail. Oh et al,[51] using a similar technique, demonstrated 100% union with no malunion and an EFI of 13.4 days. This hybrid technique has led to increased patient comfort, decreased complications with more convenient and rapid recovery. However, these devices are significantly more costly and present an increased risk for subsequent deep infection due to the use of an internal fixation device.[39]
5.4. Acute limb shortening and lengthening
Although uncommon, if faced with segmental bone loss in the upper extremity, limb shortening is a viable option as function is not dependent on limb length symmetry.[39] This technique can also be utilized in the lower extremity, but less commonly. It is the simplest and presumably fastest treatment option for segmental bone loss. It allows for early primary closure without soft tissue tension, which can allow for delayed lengthening via distraction osteogenesis once the soft tissue envelope has healed.[52] However, a major known complication of acute shortening is kinking of the arterial system and is most commonly seen with shortening of more than 3 to 5 cm5; therefore, thorough monitoring of pulses during and after surgery is paramount to prevent this complication. Also, acute limb shortening can impair muscle contractile function if the muscle length-tension relationship is excessively altered.
5.5. Vascularized fibular allograft
The vascularized fibular graft was developed in the 1970s as the microvascular field evolved. It had been the preferred choice for defects > 10 cm; however, it has lost some of its popularity with the dawn of the improved induced membrane techniques and distraction osteogenesis. The main drawbacks of this technique are the associated donor side morbidity and the need for a microvascular trained surgeon to perform the procedure. In a study looking at its use in the treatment of type III tibia fractures, a donor site morbidity of 30% was reported.[53] Furthermore, restricted weight bearing is required to prevent fractures during fibular hypertrophy, which can take up to 2 years. Despite prolonged protected weight-bearing, approximately 20% fracture during the first year.[54] When resecting the fibula in this technique, bone can be taken as close as 4 cm to the fibular head without compromising the proximal tibiofibular joint and up to 6 cm proximal to the ankle joint without causing ankle instability.[54] Despite its significant donor site morbidity and fracture risk, union typically occurs within 6 to 9 months, which is substantially shorter than other reconstruction techniques with union rates reported as high as 97%.[53,55]
6. Modern techniques in the management of bone defects
Current treatment modalities pose a significant burden to the patients both physically and psychologically as these treatments are lengthy and cumbersome for the patient to live with on a daily basis. To address these issues, both technique and technology must evolve in order to better treat this problem and allow patients to achieve a better quality of life during treatment.
6.1. Plate-assisted bone segment transport (PABST)
Although only a case report, Barinaga et al[56] were recently able to treat a 51-year-old male presenting with a type IIIB tibia fracture with segmental bone loss with an all inside technique, involving a combination of internal fixation for bony stability and the PRECISE 2 IM Limb Lengthening System (Nuvasive), which they used for all inside distraction osteogenesis. Although this novel IM system cannot be used for all sizes of defect, it does allow for up to 80 mm of lengthening. This system may also provide more precise distraction as it is capable of bidirectional control, which allows for both distraction and compression (i.e., the accordion technique).[57–60] To their knowledge, this group is the first to describe the use of this IM system to correct a “critical” bone defect via segmental bone transport without shortening or concurrent use of an external fixation device and have coined the technique as plate-assisted bone segment transport. The advantages of this technique are that it reduces joint stiffness and muscle contracture associated with shortening and secondary lengthening,[61–63] it eliminates the need of an external fixation device which is uncomfortable for the patient and associated with several postoperative complications; and lastly, the magnetic device removes the need for subcutaneous receiver antenna;[64,65] therefore, potentially removing a source of failure. Although further work remains to be done regarding this technique, it does look promising for the future.
6.2. Three-dimensional bioprinting
Three-dimensional (3D) bioprinting is a tissue engineering fabrication method that uses spatial patterning of living cells and other biological materials assembling them in a layer-by-layer deposition approach for the construction of living tissue and organ analogs.[66] Although most of this work has been mainly in in-vitro and in-vivo animal models, if proven to be fruitful for the treatment of “critical” bone defects, it may be able to solve the problem of donor site morbidity in the case of autograft harvest as well as disease transmission, lack of osteogenicity, cost, and an already limited supply of allograft bone. To do so, 3D bioprinting combines a biocompatible scaffold that recapitulates the natural bone extracellular matrix niche, inclusion of osteogenic cells to secrete the necessary extracellular matrix, and morphogenic signals that spatiotemporally biodirect the cells to the phenotypically desirable type and sufficient vascularization to meet the growing tissue nutrient supply and metabolic needs.[66] Bioprinting also provides a much better compressive modulus, one that can exceed 500 kPa, approximating human tissue compressive moduli,[67] where conventional bone grafts possess poor compressive modulus.[68] These prints would not only be patient specific from a geometric standpoint but also the level of tissue insufficiency and the anatomy of the composite tissue as well as vascular network. As this technology evolves, this additive manufacturing technique may be used to reconstruct bone as well as articular cartilage in areas where our current available clinical technologies and tissue manufacturing strategies fail.
7. Conclusion
“Critical” bone loss associated with open fractures is a relatively uncommon but very challenging problem. The tibia and the femur are the most commonly involved sites, and bone loss in the upper extremities and articular surfaces is rare. Bone defects can vary dramatically in size and selecting the most appropriate treatment plan to address a given defect, must be carefully thought out on a case-by-case basis. Detailed discussion and shared decision making with the patient is critical since these are often life-changing injuries and surgical procedures that have lasting impacts on quality of life.
There are many techniques available to treat these complex injuries and determining the best approach for each scenario remains controversial. In order for these techniques to yield optimal results, each injury must be approached in a step-wise and multidisciplinary fashion to ensure that care is taken in bone and wound bed preparation, that soft tissues are healthy and free of contaminants, and that the patient's medical condition has been optimized. With the advances in all inside distraction osteogenesis techniques as well as 3D bioprinting with enhanced biologics, restoring satisfactory limb function may not pose as much of a burden physically, psychologically, and economically as it continues to today.
7.1. Case 1
Eighty-seven-year-old female was involved in a motor vehicle accident in July of 2017. On initial assessment, she presents an open distal femur fracture with segmental bone loss, ipsilateral intertrochanteric hip fracture (initial x-rays not available), right closed wrist fracture, left bimalleolar ankle fracture, intracranial hemorrhage, flail chest injury, and multiple spinal fractures.
Prior to arriving at our center, she is treated at an outside Level 1 Trauma Centre for her above-mentioned injuries. Primary treatment of her open femur fracture with associated 14 cm segmental bone defect involved irrigation and debridement, open reduction and internal fixation with distal femoral locking plate and placement of PMMA spacer in 1st stage Masquelet technique, as well as supplementary ipsilateral short InterTAN femur nail for treatment of her basicervical femoral neck fracture (Fig. 2).
Figure 2.
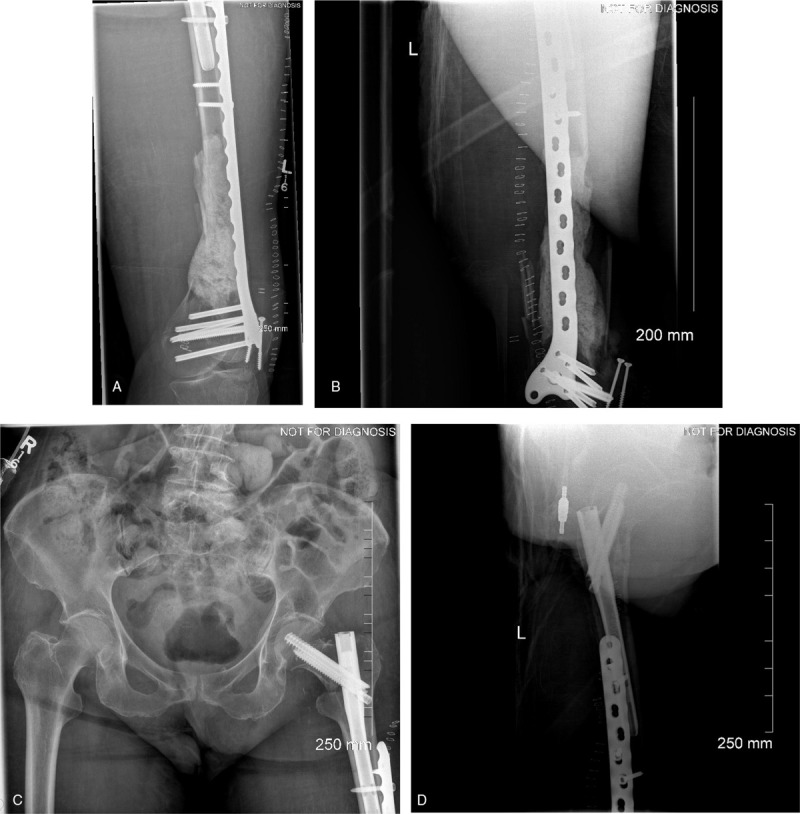
(A) AP, (B) lateral left femur (C) AP pelvis, and (D) Iateral left hip 3 weeks postop from irrigation and debridement, with open reduction internal fixation of distal femur, short InterTan and first stage Masquelet technique at an outside tertiary care center.
Due to the patients’ history of atrial fibrillation, osteoporosis, and previous left patella fracture treated with surgical fixation, the patient received an anesthesia consult, as well as an endocrinology consult for patient optimization prior to proceeding with 2nd stage Masquelet. Approximately 8 weeks following her initial injury, she was treated with removal of hardware and PMMA spacer, saucerization of distal femur, revision of left distal femoral fixation, fibular strut allograft, autogenous RIA autograft from right femur with prophylactic retrograde intramedullary nail, and with cancellous allograft with Infuse BMP graft (Fig. 3, postoperative day 1). Postoperatively she was made weight-bearing as tolerated and received standard DVT prophylaxis.
Figure 3.
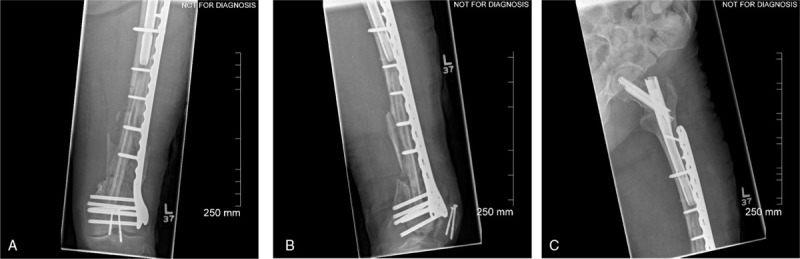
(A) AP, (B) lateral left femur, and (C) AP left hip postoperative day 1 from second stage masquelet technique with fibullar strut allograft.
Overall, the patient did very well. She was weight bearing short distances with no presence of wound issues at her 4-week follow-up. Two months postoperatively the patient presented good mobilization with limited restrictions using a 4-wheel walker. Her left knee range of motion was measured to be 0° to 100° at that time. She was fitted with a shoe lift, however, due to her associated 3.5 cm leg length discrepancy with the left leg being shorter postinjury. When she was seen for her 6-month follow-up, she had progressed with her mobilization using only a cane inside the house and even going without any walking aids on occasion. Her x-rays also demonstrated excellent consolidation of the graft (Fig. 4). Finally, when she was seen for her 1-year follow-up, the patient had progressed to mobilization without any walking aids most of the time and her fracture had demonstrated full bony union (Fig. 5). The above case and associated de-identified images were used after receiving patient consent.
Figure 4.
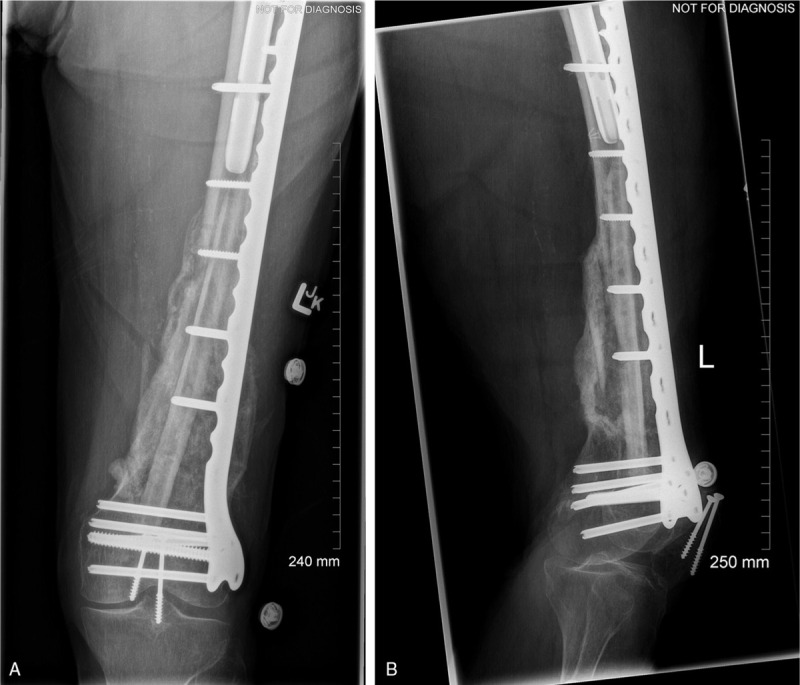
(A) AP and (B) lateral left femur 6 months postop, demonstrating maintenance of internal fixation stability, and good consolidation.
Figure 5.
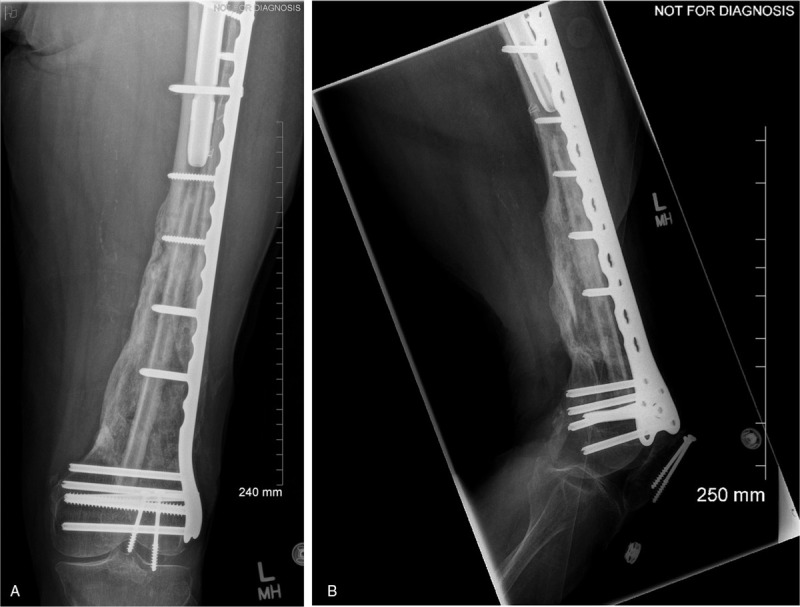
(A) AP and (B) lateral left femur 1 year postop, demonstrating maintenance of hardware fixation, and complete graft consolidation.
References
- 1.Keating JF, Simpson AH, Robinson CM. The management of fractures with bone loss. J Bone Joint Surg Br. 2005;87:142–150. [DOI] [PubMed] [Google Scholar]
- 2.Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24:742–746. [DOI] [PubMed] [Google Scholar]
- 3.Gustilo RB, Merkow RL, Templeman D. The management of open fractures. J Bone Joint Surg Am. 1990;72:299–304. [PubMed] [Google Scholar]
- 4.Cattaneo R, Catagni M, Johnson EE. The treatment of infected nonunions and segmental defects of the tibia by the methods of Ilizarov. Clin Orthop Relat Res. 1992;143–152. [PubMed] [Google Scholar]
- 5.El-Rosasy MA. Acute shortening and re-lengthening in the management of bone and soft-tissue loss in complicated fractures of the tibia. J Bone Joint Surg Br. 2007;89:80–88. [DOI] [PubMed] [Google Scholar]
- 6.Toros T, Ozaksar K. Reconstruction of traumatic tubular bone defects using vascularized fibular graft. Injury. 2019;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7.Masquelet AC, Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am. 2010;41:27–37. [DOI] [PubMed] [Google Scholar]
- 8.Johnson EE, Urist MR, Finerman GA. Resistant nonunions and partial or complete segmental defects of long bones. Treatment with implants of a composite of human bone morphogenetic protein (BMP) and autolyzed, antigen-extracted, allogeneic (AAA) bone. Clin Orthop Relat Res. 1992;229–237. [PubMed] [Google Scholar]
- 9.Southam BR, Archdeacon MT. “Iatrogenic” segmental defect: how i debride high-energy open tibial fractures. J Orthop Trauma. 2017;31 (suppl 5):S9–S15. [DOI] [PubMed] [Google Scholar]
- 10.MacKenzie EJ, Bosse MJ. Factors influencing outcome following limb-threatening lower limb trauma: lessons learned from the Lower Extremity Assessment Project (LEAP). J Am Acad Orthop Surg. 2006;14 (10 Spec No.):S205–210. [DOI] [PubMed] [Google Scholar]
- 11.Lack WD, Karunakar MA, Angerame MR, et al. Type III open tibia fractures: immediate antibiotic prophylaxis minimizes infection. J Orthop Trauma. 2015;29:1–6. [DOI] [PubMed] [Google Scholar]
- 12.Patzakis MJ, Wilkins J. Factors influencing infection rate in open fracture wounds. Clin Orthop Relat Res. 1989;36–40. [PubMed] [Google Scholar]
- 13.Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58:453–458. [PubMed] [Google Scholar]
- 14.Carver DC, Kuehn SB, Weinlein JC. Role of systemic and local antibiotics in the treatment of open fractures. Orthop Clin North Am. 2017;48:137–153. [DOI] [PubMed] [Google Scholar]
- 15.Hoff WS, Bonadies JA, Cachecho R, et al. East Practice Management Guidelines Work Group: update to practice management guidelines for prophylactic antibiotic use in open fractures. J Trauma. 2011;70:751–754. [DOI] [PubMed] [Google Scholar]
- 16.Dellinger EP, Caplan ES, Weaver LD, et al. Duration of preventive antibiotic administration for open extremity fractures. Arch Surg. 1988;123:333–339. [DOI] [PubMed] [Google Scholar]
- 17.Tennent DJ, Shiels SM, Sanchez CJ, Jr, et al. Time-dependent effectiveness of locally applied vancomycin powder in a contaminated traumatic orthopaedic wound model. J Orthop Trauma. 2016;30:531–537. [DOI] [PubMed] [Google Scholar]
- 18.Caroom C, Moore D, Mudaliar N, et al. Intrawound vancomycin powder reduces bacterial load in contaminated open fracture model. J Orthop Trauma. 2018;32:538–541. [DOI] [PubMed] [Google Scholar]
- 19.Bosse MJ, Murray CK, Carlini AR, et al. Assessment of severe extremity wound bioburden at the time of definitive wound closure or coverage: correlation with subsequent postclosure deep wound infection (Bioburden Study). J Orthop Trauma. 2017;31 (suppl 1):S3–S9. [DOI] [PubMed] [Google Scholar]
- 20.Mauffrey C, Bailey JR, Bowles RJ, et al. Acute management of open fractures: proposal of a new multidisciplinary algorithm. Orthopedics. 2012;35:877–881. [DOI] [PubMed] [Google Scholar]
- 21.Swiontkowski MF. Criteria for bone debridement in massive lower limb trauma. Clin Orthop Relat Res. 1989;41–47. [PubMed] [Google Scholar]
- 22.Zalavras CG, Marcus RE, Levin LS, et al. Management of open fractures and subsequent complications. J Bone Joint Surg Am. 2007;89:884–895. [DOI] [PubMed] [Google Scholar]
- 23.Toogood P, Miclau T. Critical-sized bone defects: sequence and planning. J Orthop Trauma. 2017;31 (suppl 5):S23–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meininger AK, Figuerres BF, Parameswaran AD, et al. Extruded osteoarticular distal tibia: success at 18-month follow-up with reimplantation. J Orthop Trauma. 2010;24:e102–107. [DOI] [PubMed] [Google Scholar]
- 25.Farrelly E, Ferrari L, Roland D, et al. Reimplantation of an extruded osteoarticular segment of the distal tibia in a 14-year-old girl. Case report and review of the literature. J Orthop Trauma. 2012;26:e24–28. [DOI] [PubMed] [Google Scholar]
- 26.Afshar A. Reimplantation of a large extruded segment of bone in an open fracture. J Hand Surg Am. 2017;42:128–134. [DOI] [PubMed] [Google Scholar]
- 27.Rathore S, Reddy IV, Ashwin Kumar AH. A novel technique for reimplanting extruded bone fragments in open fractures. Trauma Case Rep. 2016;4:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srour M, Inaba K, Okoye O, et al. Prospective evaluation of treatment of open fractures: effect of time to irrigation and debridement. JAMA Surg. 2015;150:332–336. [DOI] [PubMed] [Google Scholar]
- 29.Obremskey W, Molina C, Collinge C, et al. Current practice in the management of open fractures among orthopaedic trauma surgeons. Part b: management of segmental long bone defects. A Survey of Orthopaedic Trauma Association Members. J Orthop Trauma. 2014;28:e203–207. [DOI] [PubMed] [Google Scholar]
- 30.Pipitone PS, Rehman S. Management of traumatic bone loss in the lower extremity. Orthop Clin North Am. 2014;45:469–482. [DOI] [PubMed] [Google Scholar]
- 31.Sanders DW, Bhandari M, Guyatt G, et al. Critical-sized defect in the tibia: is it critical? Results from the SPRINT trial. J Orthop Trauma. 2014;28:632–635. [DOI] [PubMed] [Google Scholar]
- 32.Nauth A, Schemitsch E, Norris B, et al. Critical-size bone defects: is there a consensus for diagnosis and treatment? J Orthop Trauma. 2018;32 (suppl 1):S7–S11. [DOI] [PubMed] [Google Scholar]
- 33.Haines NM, Lack WD, Seymour RB, et al. Defining the lower limit of a “critical bone defect” in open diaphyseal tibial fractures. J Orthop Trauma. 2016;30:e158–163. [DOI] [PubMed] [Google Scholar]
- 34.Hinsche AF, Giannoudis PV, Matthews SE, et al. Spontaneous healing of large femoral cortical bone defects: does genetic predisposition play a role? Acta Orthop Belg. 2003;69:441–446. [PubMed] [Google Scholar]
- 35.Blick SS, Brumback RJ, Lakatos R, et al. Early prophylactic bone grafting of high-energy tibial fractures. Clin Orthop Relat Res. 1989;1:21–41. [PubMed] [Google Scholar]
- 36.Court-Brown CM, McQueen MM, Quaba AA, et al. Locked intramedullary nailing of open tibial fractures. J Bone Joint Surg Br. 1991;73:959–964. [DOI] [PubMed] [Google Scholar]
- 37.Brinker MR, O’Connor DP, Monla YT, et al. Metabolic and endocrine abnormalities in patients with nonunions. J Orthop Trauma. 2007;21:557–570. [DOI] [PubMed] [Google Scholar]
- 38.Niikura T, Lee SY, Sakai Y, et al. Causative factors of fracture nonunion: the proportions of mechanical, biological, patient-dependent, and patient-independent factors. J Orthop Sci. 2014;19:120–124. [DOI] [PubMed] [Google Scholar]
- 39.Mauffrey C, Barlow BT, Smith W. Management of segmental bone defects. J Am Acad Orthop Surg. 2015;23:143–153. [DOI] [PubMed] [Google Scholar]
- 40.Masquelet A, Kanakaris NK, Obert L, et al. Bone repair using the Masquelet technique. J Bone Joint Surg Am. 2019;101:1024–1036. [DOI] [PubMed] [Google Scholar]
- 41.Hartsock LA, Barfield WR, Kokko KP, et al. Randomized prospective clinical trial comparing reamer irrigator aspirator (RIA) to standard reaming (SR) in both minimally injured and multiply injured patients with closed femoral shaft fractures treated with reamed intramedullary nailing (IMN). Injury. 2010;41 (suppl 2):S94–98. [DOI] [PubMed] [Google Scholar]
- 42.Karger C, Kishi T, Schneider L, et al. Treatment of posttraumatic bone defects by the induced membrane technique. Orthop Traumatol Surg Res. 2012;98:97–102. [DOI] [PubMed] [Google Scholar]
- 43.McCall TA, Brokaw DS, Jelen BA, et al. Treatment of large segmental bone defects with reamer-irrigator-aspirator bone graft: technique and case series. Orthop Clin North Am. 2010;41:63–73. [DOI] [PubMed] [Google Scholar]
- 44.Apard T, Bigorre N, Cronier P, et al. Two-stage reconstruction of post-traumatic segmental tibia bone loss with nailing. Orthop Traumatol Surg Res. 2010;96:549–553. [DOI] [PubMed] [Google Scholar]
- 45.Ilizarov GA. The principles of the Ilizarov method. Bull Hosp Jt Dis Orthop Inst. 1988;48:1–11. [PubMed] [Google Scholar]
- 46.Chaddha M, Gulati D, Singh AP, et al. Management of massive posttraumatic bone defects in the lower limb with the Ilizarov technique. Acta Orthop Belg. 2010;76:811–820. [PubMed] [Google Scholar]
- 47.Borzunov DY. Long bone reconstruction using multilevel lengthening of bone defect fragments. Int Orthop. 2012;36:1695–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lowenberg DW, Buntic RF, Buncke GM, et al. Long-term results and costs of muscle flap coverage with Ilizarov bone transport in lower limb salvage. J Orthop Trauma. 2013;27:576–581. [DOI] [PubMed] [Google Scholar]
- 49.Papakostidis C, Bhandari M, Giannoudis PV. Distraction osteogenesis in the treatment of long bone defects of the lower limbs: effectiveness, complications and clinical results; a systematic review and meta-analysis. Bone Joint J. 2013;95-B:1673–1680. [DOI] [PubMed] [Google Scholar]
- 50.Kocaoglu M, Eralp L, Rashid HU, et al. Reconstruction of segmental bone defects due to chronic osteomyelitis with use of an external fixator and an intramedullary nail. J Bone Joint Surg Am. 2006;88:2137–2145. [DOI] [PubMed] [Google Scholar]
- 51.Oh CW, Apivatthakakul T, Oh JK, et al. Bone transport with an external fixator and a locking plate for segmental tibial defects. Bone Joint J. 2013;95-B:1667–1672. [DOI] [PubMed] [Google Scholar]
- 52.Sen C, Kocaoglu M, Eralp L, et al. Bifocal compression-distraction in the acute treatment of grade III open tibia fractures with bone and soft-tissue loss: a report of 24 cases. J Orthop Trauma. 2004;18:150–157. [DOI] [PubMed] [Google Scholar]
- 53.Zhen P, Hu YY, Luo ZJ, et al. One-stage treatment and reconstruction of Gustilo Type III open tibial shaft fractures with a vascularized fibular osteoseptocutaneous flap graft. J Orthop Trauma. 2010;24:745–751. [DOI] [PubMed] [Google Scholar]
- 54.Khira YM, Badawy HA. Pedicled vascularized fibular graft with Ilizarov external fixator for reconstructing a large bone defect of the tibia after tumor resection. J Orthop Traumatol. 2013;14:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cavadas PC, Landin L, Ibanez J, et al. Reconstruction of major traumatic segmental bone defects of the tibia with vascularized bone transfers. Plast Reconstr Surg. 2010;125:215–223. [DOI] [PubMed] [Google Scholar]
- 56.Barinaga G, Beason AM, Gardner MP. Novel surgical approach to segmental bone transport using a magnetic intramedullary limb lengthening system. J Am Acad Orthop Surg. 2018;26:e477–e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Catagni MA, Guerreschi F, Lovisetti L. Distraction osteogenesis for bone repair in the 21st century: lessons learned. Injury. 2011;42:580–586. [DOI] [PubMed] [Google Scholar]
- 58.De Bastiani G, Aldegheri R, Renzi-Brivio L, et al. Limb lengthening by callus distraction (callotasis). J Pediatr Orthop. 1987;7:129–134. [DOI] [PubMed] [Google Scholar]
- 59.Makhdom AM, Cartaleanu AS, Rendon JS, et al. The accordion maneuver: a noninvasive strategy for absent or delayed callus formation in cases of limb lengthening. Adv Orthop. 2015;2015:912790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ilizarov GA. Clinical application of the tension-stress effect for limb lengthening. Clin Orthop Relat Res. 1990;8–26. [PubMed] [Google Scholar]
- 61.Rigal S, Merloz P, Le Nen D, et al. Bone transport techniques in posttraumatic bone defects. Orthop Traumatol Surg Res. 2012;98:103–108. [DOI] [PubMed] [Google Scholar]
- 62.Iacobellis C, Berizzi A, Aldegheri R. Bone transport using the Ilizarov method: a review of complications in 100 consecutive cases. Strategies Trauma Limb Reconstr. 2010;5:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dahl MT, Gulli B, Berg T. Complications of limb lengthening. A learning curve. Clin Orthop Relat Res. 1994;10–18. [PubMed] [Google Scholar]
- 64.Dincyurek H, Kocaoglu M, Eralp IL, et al. Functional results of lower extremity lengthening by motorized intramedullary nails. Acta Orthop Traumatol Turc. 2012;46:42–49. [DOI] [PubMed] [Google Scholar]
- 65.Krieg AH, Lenze U, Speth BM, et al. Intramedullary leg lengthening with a motorized nail. Acta Orthop. 2011;82:344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dhawan A, Kennedy PM, Rizk EB, et al. Three-dimensional bioprinting for bone and cartilage restoration in orthopaedic surgery. J Am Acad Orthop Surg. 2019;27:e215–e226. [DOI] [PubMed] [Google Scholar]
- 67.Hoenig E, Winkler T, Mielke G, et al. High amplitude direct compressive strain enhances mechanical properties of scaffold-free tissue-engineered cartilage. Tissue Eng Part A. 2011;17:1401–1411. [DOI] [PubMed] [Google Scholar]
- 68.Khanarian NT, Jiang J, Wan LQ, et al. A hydrogel-mineral composite scaffold for osteochondral interface tissue engineering. Tissue Eng Part A. 2012;18:533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]


