Abstract
PURPOSE
Underdeveloped nations carry the burden of most cervical cancer, yet access to adequate treatment can be challenging. This report assesses the current management of cervical cancer in sub-Saharan Africa to better understand the needs of underdeveloped nations in managing cervical cancer.
METHODS
A pre- and postsurvey was sent to all centers participating in the Cervical Cancer Research Network's 4th annual symposium. The pre- and postsurvey evaluated human papillomavirus and HIV screening, resources available for workup and/or treatment, treatment logistics, outcomes, and enrollment on clinical trials. Descriptive analyses were performed on survey responses.
RESULTS
Twenty-nine centers from 12 sub-Saharan countries saw approximately 300 new cases of cervical cancer yearly. Of the countries surveyed, 55% of countries had a human papillomavirus vaccination program and 30% (range, 0%-65%) of women in each region were estimated to have participated in a cervical cancer screening program. In the workup of patients, 43% of centers had the ability to obtain a positron emission tomography and computed tomography scan and 79% had magnetic resonance imaging capabilities. When performing surgery, 88% of those centers had a surgeon with an expertise in performing oncological surgeries. Radiation therapy was available at 96% of the centers surveyed, and chemotherapy was available in 86% of centers. Clinical trials were open at 4% of centers.
CONCLUSION
There have been significant advancements being made in screening, workup, and management of patients with cervical cancer in sub-Saharan Africa; yet, improvement is still needed. Enrollment in clinical trials remains a struggle. Participants would like to enroll patients on clinical trials with Cervical Cancer Research Network's continuous support.
INTRODUCTION
With just more than half a million new diagnoses each year, cervical cancer is the fourth most frequent cancer in women. The incidence of cervical cancer is highest in southern, eastern, and western Africa.1 Although it is a preventable cancer, that is, a curable cancer when caught early, cervix cancer remains deadly in low- to middle-income countries (LMIC) where screening and early detection are not readily available. Approximately 90% of cervical cancer deaths are in LMIC.2 LMIC often lack the resources and funding to provide adequate treatment. The Gynecological Cancer Intergroup (GCIG) is a nonprofit organization with a mission to improve the care of women with cervical cancer and other gynecological malignancies through education, training, and public awareness, as well as the development of high-quality clinical trials. The Cervical Cancer Research Network (CCRN) was established by the GCIG to facilitate access to high-quality cervical cancer clinical trials in regions under-represented by the GCIG, yet, containing much of the worldwide cervical cancer burden.
CONTEXT
Key Objective
What is the current management of cervical cancer in sub-Saharan Africa and how can emerging economies be helped in managing cervical cancer?
Knowledge Generated
Twenty nine centers from 12 sub-Saharan countries were surveyed on their current practice management of cervical cancer.
Relevance
There have been significant advancements in the screening, workup, and management of patients with cervical cancer in sub-Saharan Africa; yet, enrollment in clinical trials remains a struggle. This information will help national organizations aid low- to middle-income countries in the improvement in cervical cancer management and the development and enrollment in clinical trials.
The purpose of this study was to better understand the current management of the patients with cervical cancer in sub-Saharan Africa to help the CCRN in the development and enrollment in clinical trials in LMIC.
METHODS
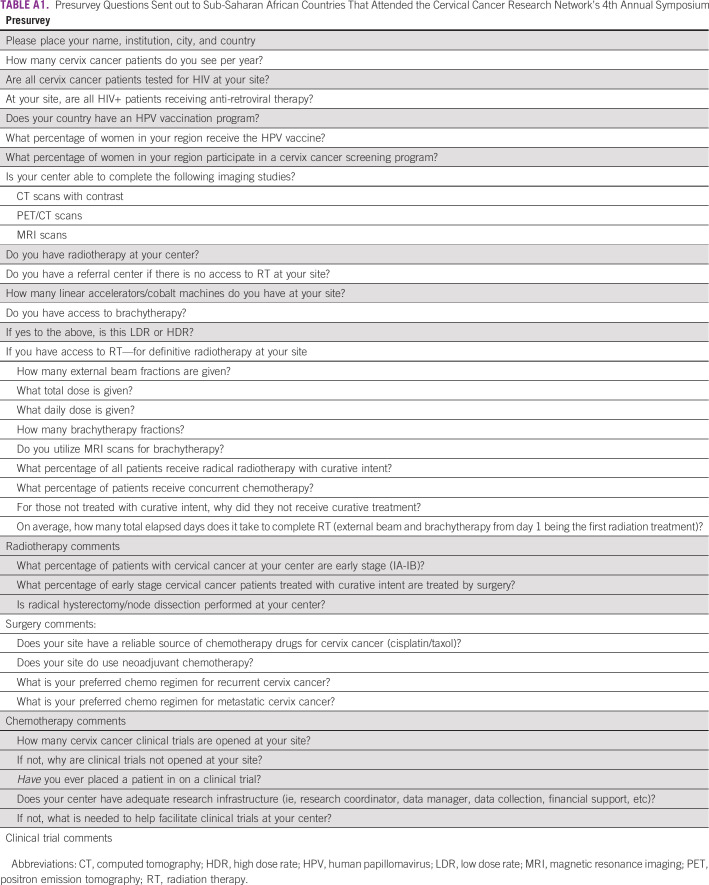
The CCRN symposium held in Johannesburg, South Africa, January 26-27, 2019, was an educational session for sub-Sahara African centers to promote adequate management of cervical cancer and encourage recruitment into CCRN clinical trials. Emphasis was on developing resource appropriate research efforts. A pre- and postsymposium survey was designed by the CCRN symposium committee to assess the current management of cervical cancer in sub-Saharan Africa. This information will be used by the CCRN for future trial development and to better understand the challenges of clinical trial enrollment in LMIC. The presymposium survey (presurvey) was sent to all centers participating in the 4th annual CCRN symposium, which included 29 sites from 12 countries across the sub-Saharan Africa: Ethiopia, Uganda, Kenya, Tanzania, Malawi, South Africa, Botswana, Zambia, Zimbabwe, Namibia, Nigeria, and Ghana. The presurvey covered the following domains: human papillomavirus (HPV) and HIV screening, treatment with surgery, chemotherapy, and radiation therapy (RT), and enrollment on clinical trials. Of the 30 surveys distributed, 29 presurveys were completed (response rate, 96.7%). A postsymposium survey was created by the CCRN symposium committee to address additional questions regarding cervical cancer management that arose from many of the participants and were not asked in the presurvey. The postsurvey covered the following domains: resources available for workup and treatment, logistics of treatment, and outcomes. Of the 30 surveys distributed, 23 postsurveys were completed (response rate of 76.7%). The presurvey was sent out to all participating centers approximately 1 month prior to the symposium, and the postsurvey was sent to all participating centers 1 week after the symposium. Each survey was sent out three times to enable ample time and notification to complete the survey. Surveys were completed voluntarily and could be done anonymously. The complete pre- and postsurvey can be viewed in Appendix Tables A1 and A2. Descriptive analyses were performed on survey responses. This study was institutional review board exempt.
RESULTS
The 29 centers from 12 sub-Saharan countries saw a large volume of cervix cancer cases annually and can be viewed in Figure 1. Each center saw approximately 300 new cases of cervical cancer each year (mean, 313; median, 300; range, 5-1,300) with late-stage disease representing 85% of the new cases seen annually.
FIG 1.
Cervix Cancer Research Network participation and Worldwide Gynecologic Cancer Intergroup.
Of the countries surveyed, approximately 30% (0%-65%) of the women in each region were estimated to have participated in a cervical screening program, 55% of countries had an HPV vaccination program, and 30% (range, 0%-94%) of women received the HPV vaccination. Two thirds of the countries surveyed tested patients with cervical cancer for HIV, and most HIV-positive patients, 86%, received antiretroviral therapy (Fig 2).
FIG 2.
Survey responses for HIV and HPV screening. HPV, human papillomavirus.
In the workup of a patient with cervical cancer, 43% of centers had the ability to obtain a positron emission tomography (PET) and computed tomography (CT) scan and 79% had magnetic resonance imaging (MRI) capabilities. Imaging used to evaluate for hydronephrosis included ultrasound (78%), CT (47%), and intravenous pyelogram (5%). To assess for metastatic lymph nodes, most centers used CT (73%), followed by ultrasound (52%), MRI (26%), and PET and CT (16%).
For early-stage cervical cancer, 61% of centers performed surgery. Of the centers surveyed, 82% performed a radical hysterectomy with lymph node dissection (Fig 3) and 88% of those centers had a surgeon with an expertise in performing oncological surgeries.
FIG 3.
Survey responses regarding the treatment of cervical cancer. RH + LND, radical hysterectomy and lymph node evaluation.
RT was available at 96% of the centers surveyed. There was an average wait time of 5.4 days for radiation treatment. Most patients were planned or simulated with a CT scanner (72%), and very few used a fluoroscopic simulator (22%) or no simulation (6%). The centers had between one and four linear accelerators, and at least seven centers had cobalt machines. The median dose was 48 Gy in 24 fractions (range, 45-85.7 Gy) with 76% of centers providing concurrent chemotherapy with radiation. Brachytherapy was used by 85% of centers, with 80% using high dose rate (HDR), 16% using both low dose rate (LDR) and HDR, and 4% using another form of brachytherapy. For a patient undergoing brachytherapy, four implants were used by most centers. RT was completed within 52 days on average (range, 25-90). Adjuvant RT was used for positive nodes (94%), positive margins (88%), and Sedlis criteria (tumor > 4 cm, lymphovascular space invasion (LVSI), deep invasion, 47%), and many consider it for parametrial involvement or LVSI.
Regarding chemotherapy, the majority of centers had drug availability (86%). However, a third of centers had to delay treatment or substitute a chemotherapy drug because of the lack of consistent supply. For recurrent or metastatic cervical cancer, a platinum- and taxol-based chemotherapy regimen was almost universally used; however, a couple of centers used platinum/adriamycin, platinum/irinotecan, or platinum/gemcitabine. Palliative treatments for incurable cervical cancer cases included RT (50%), chemotherapy and radiation (22%), chemotherapy alone (17%), and other methods (11%).
Most centers used standard follow-up guidelines. A third of centers ordered routine imaging in treated asymptomatic patients with cervical cancer. Approximately 40% of centers knew the percentage of patients who survived at 5 years out from treatment at their center, and 60% of the centers had a cancer registry.
Clinical trials were open at 4% of centers. Reasons for not opening clinical trials included lack of funding, too much work load, center not meeting trial requirements, and other or unknown. When asked if the center had adequate research infrastructure, such as access to a research coordinator, data manager, data collection, financial support, etc, 40% responded yes (Fig 4). Half of the physicians had placed a patient on a clinical trial in the past. Many centers seemed interested in participating in clinical trials but needed support to help train researchers, data managers, clinical trial coordinators, etc. Centers expressed needing technical support and most commonly assistance with funding of clinical research.
FIG 4.
Survey responses covering clinical trial participation.
DISCUSSION
Africa represents 20% of the world's new cervical cancer diagnoses each year, with approximately 120,000 new cases. A large fraction of women in Africa do not have access to care for cervix cancer treatment. Many centers and countries do not have expertise to perform the standard surgical procedure for early cervix cancer: a radical hysterectomy. Similarly, many countries in Africa have insufficient numbers of radiotherapy machines with several countries having none.3,4 However, some centers have a higher level of care available, see a high volume of patients, and are particularly well-suited to clinical trial enrollment. The challenges faced in sub-Saharan Africa in the treatment of cervical cancer include prevention and screening of HPV and HIV, appropriate imaging studies, access to adequate treatment (surgery, chemotherapy, and RT), proper follow-up, and survivorship support and resources to aide in clinical trial enrollment.
Improvement in screening and prevention can drastically decrease rates of precancerous and cervical cancer diagnosis. It is well-known that cervical cancer has been linked with the HPV, with HPV 16 and 18 accounting for more than 70% of cervical cancer and precancerous cases.5 With the development of an HPV vaccine over 10 years ago, the prevalence of HPV 16 and 18 worldwide has decreased by 83% in girls 13-19 years of age and 66% in women 20-24 years of age, leading to a decrease of cervical intraepithelial neoplasia 2+ by 52% and 31%, respectively,6 yet access to the vaccine in LMIC can be challenging.7 This study found that only around half of the centers representing sub-Saharan Africa had an HPV vaccination program in place. Increasing access to the HPV vaccination is a simple, accessible, and cost-effective approach to drastically decrease rates of cervical cancer in sub-Saharan Africa.8,9
A persistent HPV infection is more likely in HIV-infected women, and the incidence of cervical intraepithelial neoplasia is four to five times higher among HIV-infected women.10 Unfortunately, only two thirds of centers tested patients with cervical cancer for HIV. Developing better cervical cancer screening programs, access to the HPV vaccine, and HIV screening in precancerous patients and patients with cervical cancer can drastically decrease the incidence of cervical cancer and mortality in sub-Saharan Africa. Thus, it is important to continue philanthropic work in LMIC with educational programs, funding, and resource support to help improve HPV and HIV screening and vaccination programs.
Access to appropriate imaging studies in the workup of cervical cancer can be challenging for developing countries. In highly developed resource settings, a PET and CT scan is often preferred for evaluation of distant regional and metastatic disease to aide in staging. Although a recent study demonstrated that CT of abdomen and pelvis was nearly similar in identifying nodal disease as PET and CT.11 Additionally, an MRI of the pelvis can greatly help assess the local extent of disease.12 Nearly all participating sub-Saharan African countries had access to CT scans with contrast. Although access to CT scans were widely available, accessibility to pelvic MRI and PET and CT scans were low. It is important to note that the centers represented were some of the best resourced centers from their countries. However, it is likely that most sub-Saharan Africa continue to struggle with appropriate imaging for workup of patients with cervical cancer, since participants in this study were attendees of the CCRN symposium and actively working toward improving the management of cervical cancer at their center. This is particularly noteworthy since International Federation of Gynecology and Obstetrics (FIGO) staging now permits cross-sectional imaging to evaluate nodal disease.
On top of completing an appropriate workup, adequate reporting is essential for proper management of patients with cervical cancer, as treatment decisions are based on imaging and pathology findings. A timely workup and diagnosis is important for getting treatment started quickly. Multidisciplinary clinics are beneficial in helping adequately diagnose patients and improve communication among treating providers.13,14 This allows for patients to be started on treatment promptly. Unfortunately, only 50% of survey respondents discussed patients with cervical cancer at a multidisciplinary tumor board. Implementing multidisciplinary clinics in sub-Saharan African centers may significantly improve care and treatment outcomes.
Treatment of cervical cancer may include surgery, RT, chemotherapy, or a combination of modalities based on the patients with FIGO 2018 stage. Most guidelines of cervical cancer recommend either surgery or RT for tumors < 4 cm in size confined to the cervix (FIGO IB2) and chemoradiation therapy for those with more advanced disease, FIGO IB3—IVAl. A surgeon with expertise in oncological resections is essential for cervical cancer outcomes. Radical hysterectomy and lymph node evaluation is recommended in stages IA2-IIA1. Of the centers surveyed, nearly all of them performed a radical hysterectomy and lymph node evaluation by an experienced surgeon.
Adjuvant RT after hysterectomy is often offered based on the Sedlis criteria12,15 or for positive nodes, positive margins, or parametrial involvement.3,12 Almost all centers surveyed had RT available, although it is important to recognize that centers attending the CCRN symposium are not necessarily an adequate representation of sub-Saharan Africa. However, of the centers with RT available, treatment is of high quality with patients receiving adequate doses of RT within the appropriate timeframe. Given that radiotherapy is a limited resource in many settings and countries, appropriate triaging and shorter course schedules may be important or worthwhile research goals.
Chemotherapy is also an important aspect of treatment in patients with locally advanced cervical cancer. It is encouraging to see that most centers had a reliable source of chemotherapy. Nearly half of the centers used neoadjuvant chemotherapy, which is not commonly practiced in high-resource settings. One recent randomized study showed inferior survival for neoadjuvant chemotherapy in cervix cancer when compared with chemoradiation alone.16 Although not specifically addressed in the survey, centers might feel obligated to start chemotherapy in locally advanced patients if treatment with surgery and/or RT is going to be delayed.
Routine follow-up after treatment of cervical cancer often includes a gynecological exam, cervical cytology annually, and labs/imaging only if there are concerning symptoms or signs of recurrence. Most centers adhered to the appropriate follow-up guidelines; however, a third of centers ordered imaging in asymptomatic patients.
Opening and accruing to clinical trials is essential in developing countries, as this is where a majority of cervical cancers occur. The CCRN's mission is to help develop and promote implementation of resource-appropriate clinical trials. For this to be successful, it is imperative to recognize the challenges that LMIC face. The data herein identify the strengths and weaknesses present in sub-Saharan Africa today. Recommendations from this study include
Prevention of HPV with vaccination
Screening of HIV
Appropriate imaging studies
Access to adequate treatment (surgery, chemo, and RT) identified in this study at many centers but not representative of all countries
Accessible antiretroviral therapy17
Proper cancer and survivorship follow-up
Resources to aide in country specific research that can decrease cervix cancer morbidity and mortality.
The CCRN currently promotes clinical trials in LMIC by advocating for resources through the country's government and the GCIG. It also hosts the annual symposium that provides educational, networking, and advertising opportunities for clinical trials. The results from the pre- and postsurveys show the current management of cervical cancer in LMIC. The study recommendations listed above can help aid the CCRN in its future development and promotion of clinical trials.
In conclusion, as developing nations continue to carry the burden of most cervical cancer cases, access to adequate treatment in these regions is essential. Managing cervical cancer in these countries continues to be a challenge. The CCRN's mission is to help provide high-quality, low-cost research in regions struggling with adequate resources for proper treatment of cervical cancer. Enrollment in clinical trials within these nations can improve our understanding of cervical cancer and advance the quality of treatment. With the conclusion of the 4th annual symposium held in South Africa and the participation of the sub-Saharan countries, we can see significant advancements being made in screening, workup, and management of patients with cervical cancer. Clinical research is difficult worldwide in cervix cancer since cases predominate in LMIC. This is compounded in Africa where there is a marked limitation on resources. Nevertheless, participants expressed a great desire to participate in clinical studies to promote the health of women in their countries. Through educational support of physicians, data managers, and patient navigators, the CCRN hopes to improve high quality access to treatment and enrollment on clinical trials for patients with cervical cancer.
APPENDIX
TABLE A1.
Presurvey Questions Sent out to Sub-Saharan African Countries That Attended the Cervical Cancer Research Network's 4th Annual Symposium
TABLE A2.
Postsurvey Questions Sent Out to Sub-Saharan African Countries That Attended the Cervical Cancer Research Network's 4th Annual Symposium
AUTHOR CONTRIBUTIONS
Conception and design: Lindsay M. Burt, Daniel M. Kanyike, Memory Bvochora-Nsingo, Ntokozo Ndlovu, Aba A. Scott, Vinay Sharma, Catherine Nyongesa, Wondemagegnehu Tigeneh, Nazia Fakie, David K. Gaffney, Mary McCormak
Financial support: Lindsay M. Burt
Administrative support: Lindsay M. Burt, Vinay Sharma, David K. Gaffney
Provision of study materials or patients: Lindsay M. Burt, Ntokozo Ndlovu, Vinay Sharma, Catherine Nyongesa, Wondemagegnehu Tigeneh, David K. Gaffney
Collection and assembly of data: Lindsay M. Burt, Daniel M. Kanyike, Ntokozo Ndlovu, Rose I. Anorlu, Vinay Sharma, Catherine Nyongesa, Wondemagegnehu Tigeneh, David K. Gaffney
Data analysis and interpretation: Lindsay M. Burt, Fabrice Lecuru, Marie Plante, Catherine Nyongesa, Gita Suneja
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Lindsay M. Burt
Leadership: theMednet
Stock and Other Ownership Interests: theMednet
Memory Bvochora-Nsingo
Employment: Gaborone Private Hospital
Stock and Other Ownership Interests: Gaborone Private Hospital
Rose I. Anorlu
Honoraria: Sanofi, Roche
Hannah Simonds
Honoraria: Cipla
Gita Suneja
Consulting or Advisory Role: Gerson Lehrman Group
David K. Gaffney
Research Funding: Elekta
Mary McCormak
Research Funding: Roche/Genentech
Travel, Accommodations, Expenses: Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.World Health Organization : Cervix uteri fact sheet. https://gco.iarc.fr/today/data/factsheets/cancers/23-Cervix-uteri-fact-sheet.pdf
- 2.Atun R, Jaffray DA, Barton MB, et al. : Expanding global access to radiotherapy. Lancet Oncol 16:1153-1186, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Peters WA, III, Liu PY, Barrett RJ, II, et al. : Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 18:1606-1613, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Balogun O, Rodin D, Ngwa W, et al. : Challenges and prospects for providing radiation oncology services in Africa. Semin Radiat Oncol 27:184-188, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization : Human papillomavirus (HPV) and cervical cancer fact sheet. https://www.who.int/en/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
- 6.Drolet M, Bénard É, Pérez N, et al. : Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet 394:497-509, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black E, Richmond R: Prevention of cervical cancer in sub-Saharan Africa: The advantages and challenges of HPV vaccination. Vaccines (Basel) 6:61, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walwyn L, Janusz CB, Clark AD, et al. : Cost-effectiveness of HPV vaccination in Belize. Vaccine 33:A174-A181, 2015. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 9.Aguilar IB, Mendoza LO, García O, et al. : Cost-effectiveness analysis of the introduction of the human papillomavirus vaccine in Honduras. Vaccine 33:A167-A173, 2015. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 10.Moscicki AB, Ellenberg JH, Crowley-Nowick P, et al. : Risk of high-grade squamous intraepithelial lesion in HIV-infected adolescents. J Infect Dis 190:1413-1421, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Atri M, Zhang Z, Dehdashti F, et al. : Utility of PET-CT to evaluate retroperitoneal lymph node metastasis in advanced cervical cancer: Results of ACRIN6671/GOG0233 trial. Gynecol Oncol 142:413-419, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NCCN Guidelines 2019. https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
- 13.Grover S, Chiyapo SP, Puri P, et al. : Multidisciplinary gynecologic oncology clinic in Botswana: A model for multidisciplinary oncology care in low- and middle-income settings. J Glob Oncol 3:666-670, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pérez Fidalgo JA, Hernández Machancoses A, Martín González V, et al. : Treatment of cervical cancer: The importance of a multidisciplinary team approach. Clin Transl Oncol 13:431-433, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Sedlis A, Bundy BN, Rotman MZ, et al. : A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group study. Gynecol Oncol 73:177-183, 1999 [DOI] [PubMed] [Google Scholar]
- 16.da Costa SCS, Bonadio RC, Gabrielli FCG, et al. : Neoadjuvant chemotherapy with cisplatin and gemcitabine followed by chemoradiation versus chemoradiation for locally advanced cervical cancer: A randomized phase II trial. J Clin Oncol 37:3124-3131, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Grover S, Xu M, Jhingran A, et al. : Clinical trials in low and middle-income countries—Successes and challenges. Gynecol Oncol Rep 19:5-9, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]