Abstract
PURPOSE
The COVID-19 pandemic remains a public health emergency of global concern. Determinants of mortality in the general population are now clear, but specific data on patients with cancer remain limited, particularly in Latin America.
MATERIALS AND METHODS
A longitudinal multicenter cohort study of patients with cancer and confirmed COVID-19 from Oncoclínicas community oncology practice in Brazil was conducted. The primary end point was all-cause mortality after isolation of the SARS-CoV-2 by Real-Time Polymerase Chain Reaction (RT-PCR) in patients initially diagnosed in an outpatient environment. We performed univariate and multivariable logistic regression analysis and recursive partitioning modeling to define the baseline clinical determinants of death in the overall population.
RESULTS
From March 29 to July 4, 2020, 198 patients with COVID-19 were prospectively registered in the database, of which 167 (84%) had solid tumors and 31 (16%) had hematologic malignancies. Most patients were on active systemic therapy or radiotherapy (77%), largely for advanced or metastatic disease (64%). The overall mortality rate was 16.7% (95% CI, 11.9 to 22.7). In univariate models, factors associated with death after COVID-19 diagnosis were age ≥ 60 years, current or former smoking, coexisting comorbidities, respiratory tract cancer, and management in a noncurative setting (P < .05). In multivariable logistic regression and recursive partitioning modeling, only age, smoking history, and noncurative disease setting remained significant determinants of mortality, ranging from 1% in cancer survivors under surveillance or (neo)adjuvant therapy to 60% in elderly smokers with advanced or metastatic disease.
CONCLUSION
Mortality after COVID-19 in patients with cancer is influenced by prognostic factors that also affect outcomes of the general population. Fragile patients and smokers are entitled to active preventive measures to reduce the risk of SARS-CoV-2 infection and close monitoring in the case of exposure or COVID-19-related symptoms.
INTRODUCTION
The COVID-19 pandemic remains a public health emergency of global concern. According to the available international data, by the end of September 2020, more than 32 million cases of COVID-19 had been confirmed and over 990,000 people had died from the disease.1 At that time, Brazil had more than 4.8 million cases and close to 140,000 deaths because of COVID-19,1,2 one of the countries that have been affected more by the pandemic.3,4
CONTEXT
Key Objective
Patients with cancer have been reported to present high risk of severe complications after SARS-CoV-2 infection. We investigated the predictors of death after COVID-19 diagnosis in patients with cancer managed in a community oncology practice in Brazil.
Knowledge Generated
Some patients with cancer face increased risk of death if affected by COVID-19, which is predominantly driven by older age, heavy smoking, and the presence of active malignant disease and associated risk factors.
Relevance
Patients who are otherwise healthy and have curable malignancies present COVID-19-related mortality rates similar to the general population and should not have delayed access to cancer treatment. Measures to guarantee rigorous follow-up of patients with poor prognostic factors infected with SARS-CoV-2 are needed.
Brazil is a country with a continental dimension and a large population where socioeconomic inequalities are immense and healthcare resources are chronically deficient and unequally distributed. The federal government has assumed a position against the more intensive measures of total lockdown, which have been implemented in many developed countries during the first months of the COVID-19 pandemic.3 Indeed, control measures have been executed mostly by the state governors and municipal mayors starting in late March 2020, particularly in the regions most affected by the healthcare crisis. These included social distancing measures such as closing schools and universities, banning large events and mass gatherings, restricting travel and public transportation, quarantining and isolating cases, encouraging hand hygiene, respiratory etiquette, and the use of facemasks.3
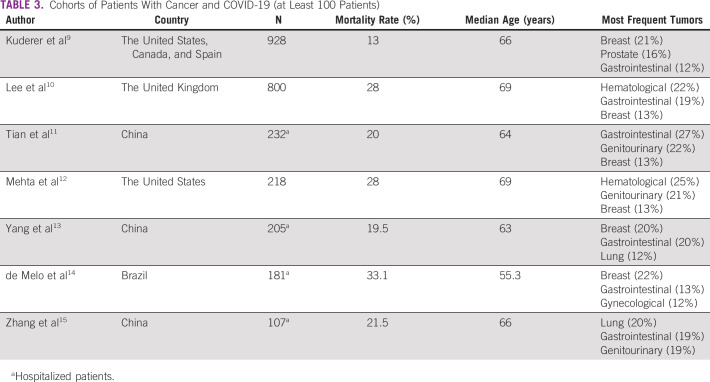
Determinants of COVID-19 mortality in the general population are now clear, but specific data on patients with cancer, who seem to have a higher risk of developing COVID-19, are still limited.5,6 Furthermore, patients with cancer have been reported to present higher risk of severe complications compared with those without cancer.7,8 The literature on COVID-19 in patients with cancer is largely biased toward single-center reports from an inpatient environment, mainly in North America, Europe, and Asia. The mortality rate in this population ranges from 13% to 33%.9-15
Oncoclínicas is the largest cancer care network in Latin America, with 68 community oncology practices distributed in four Brazilian regions, over 850 physicians, and more than 135,000 patients with cancer assisted in 2019. Shortly after the first case of SARS-CoV-2 infection in Brazil, Oncoclínicas created a crisis committee to coordinate institutional efforts to face the pandemic. This committee acquired SARS-CoV-2 RT-PCR tests and partnered with different laboratories to speed the diagnostic process and also defined internal assessment flowcharts for patients with suspicious or confirmed infection. The data generated by this initiative are being captured in a database, and all cases with diagnosis of SARS-CoV-2 infection are being closely monitored.
At present, a variety of guidelines for management of patients with cancer with COVID-19 have been published.16-22 However, these are mostly consensus agreements, with limited information derived from prospective studies assessing specific outcomes of oncology patients diagnosed with COVID-19. Examples of some unanswered questions include possible differences in the severity of SARS-CoV-2 infection among patients with different cancer types and disease settings and the implications of active anticancer therapies on the clinical presentation and outcomes of COVID-19. In this study, we describe the experience of Oncoclínicas in the initial 3 months of the COVID-19 pandemic in Brazil, with the main objective of assessing clinical determinants of mortality in a cohort of patients with cancer with SARS-CoV-2 diagnosis in the community oncology practice.
MATERIALS AND METHODS
Study Design and Population
A longitudinal multicenter cohort study was conducted from March 29 to July 4, 2020, from all Oncoclínicas ambulatory practices. All patients of age ≥ 18 years with previous cancer history or active treatment for cancer who tested positive for SARS-CoV-2 by RT-PCR assay from a nose or throat swab, irrespective of symptoms, were prospectively registered in a centralized database. Patients were informed about the importance of contacting the Oncoclínicas medical team in the case of known exposure to the virus or symptoms compatible with COVID-19. All suspicious and SARS-CoV-2 confirmed cases were part of a proactive surveillance program with daily monitoring performed by Oncoclínicas personnel and direct communications with the medical oncologist to collect follow-up data, given the decentralized care across multiple institutions. Clinical management decisions (outpatient care, hospitalization, and intensive care unit [ICU] admission) were made by the primary oncologists together with local medical teams at each site. This project was submitted to Institutional Review Board (IRB) approval (Certificado de Apresentação de Apreciação Ética (CAAE) 32483720.7.0000.5134). The IRB approved the study and our request to waive the documentation of informed patient consent. We wanted to avoid face-to-face clinical visits of SARS-CoV-2–positive patients at Oncoclínicas sites to protect our medical team and other patients from virus transmission and followed national recommendations to discourage unnecessary travels during pandemic crisis.
Data and Outcomes
The registry started with a structured excel spreadsheet that was later combined with an electronic REDCap database and is still recruiting patients. Data on baseline characteristics, medications, cancer diagnosis and treatments, and COVID-19 course were collected. Missing information was completed by Oncoclínicas support teams at each site or abstracted from electronic medical record notes at data cutoff (July 7, 2020), without the need for data imputation.
The primary end point of this study was all-cause mortality after isolation of the SARS-CoV-2 by RT-PCR. Potential prognostic variables included were age, sex, geographical location of patient residence (region of the country), smoking status, comorbidities requiring active treatment, tumor type, disease setting (curative v noncurative), and anticancer therapy given within 4 weeks of COVID-19 diagnosis, including cytotoxic chemotherapy, targeted drugs, hormonal therapy, immunotherapy, and radiotherapy. Disease setting was defined as curative if the patient had early-stage disease and was undergoing radical, adjuvant, or neoadjuvant therapy, or if the patient was on post-treatment remission. Those with locally advanced or metastatic cancer undergoing palliative treatment or best supportive care without active anticancer therapy were classified as noncurative disease setting. Secondary outcomes were severity of the COVID-19 disease, including symptomatic versus asymptomatic infection and outpatient versus inpatient care with or without ICU support. Patients cared for in the ambulatory environment until recovery (outpatients) were considered mild COVID-19 cases.
Because of possible confounding by other infections and competing death risks, patients with suspicious COVID-19 without confirmation of SARS-CoV-2 infection were excluded from the study. Also, patients with nonmalignant neoplasms in follow-up at Oncoclínicas were excluded from this analysis. Of interest, inpatient care was not performed at Oncoclínicas sites, and detailed information about COVID-19 supportive care during hospital admissions, such as steroid use, antimicrobial medication, and mechanical ventilation, was not captured in the electronic case report form. The research team had no direct access to hospital records, and discharge notes from patients with cancer admitted for COVID-19 treatment lacked detailed information.
Statistical Analysis and Sample Size
Descriptive statistics was used to show the baseline demographic information of the participants included in our analyses. For categorical data, percentages of each variable were calculated after dichotomization based on mortality status. For age, the median and range were calculated, followed by dichotomization at age ≥ 60 years (considered elderly population in Brazil). For time-dependent variables, such as time from cancer diagnosis to SARS-CoV-2-19 infection, we calculated median and interquartile range (IQR).
Risk factors for death were assessed using logistic regression. Crude and adjusted odds ratios (ORs) were calculated. Variables with a P value < .1 at the univariate analysis were included in the multivariable model by stepwise forward selection with the entry order based on their level of significance. Because of the possibility of a small number of events (deaths), we prespecified the potential prognostic variables for the primary outcome using clinical knowledge and allowable complexity of the model (number of covariates and degrees of freedom) on the basis of an effective sample size. A recursive partitioning model with risk factors for COVID-19 mortality in univariate analysis was constructed to create a decision tree that classifies patients in different death risk populations based on dichotomous independent variables. Assuming the mortality rate of COVID-19 to be 15% in patients with cancer treated in an ambulatory clinic (about half the mortality rate seen in hospitalized patients with moderate to severe infection in Brazil14), this study would require a sample size of 196 cases to estimate this proportion with a 5% absolute precision and 95% CI. The present report represents the first planned analysis of the database. All statistical analyses were performed using R statistical software version 3.6.2, glm2 and rpart packages.
RESULTS
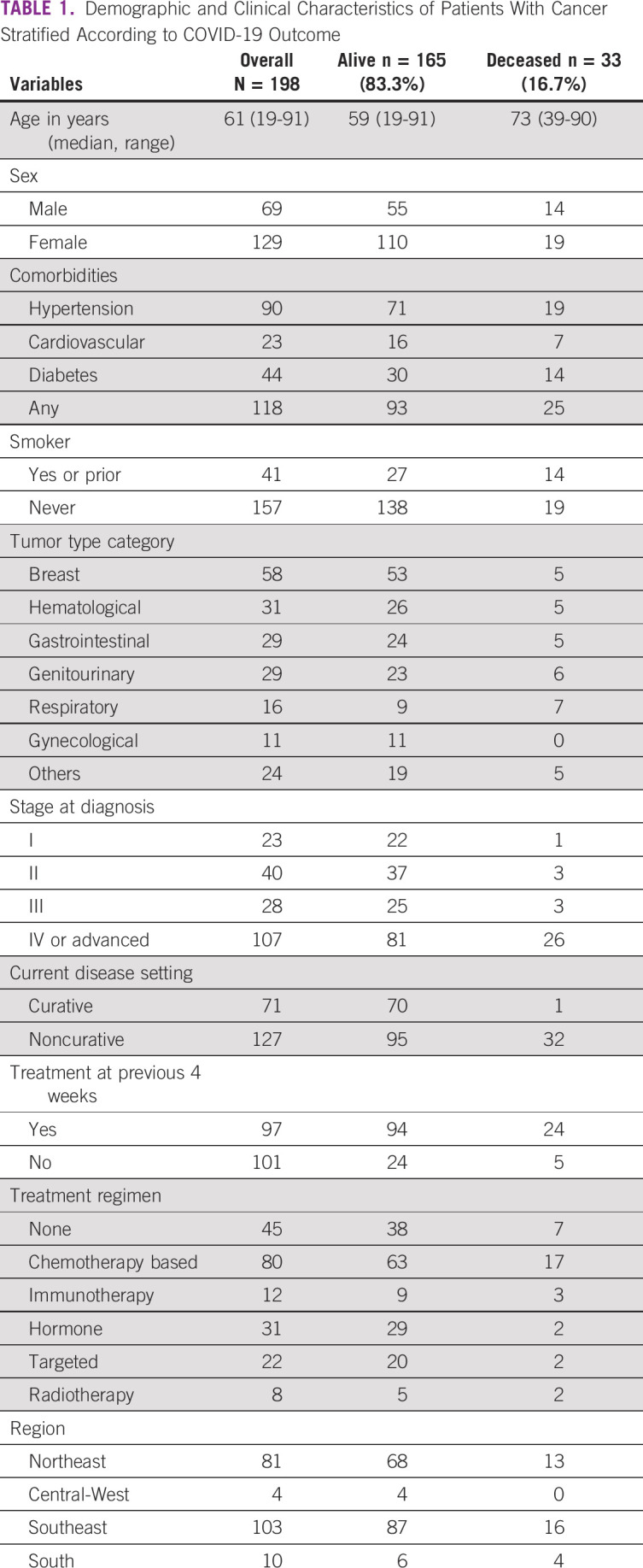
In total, 198 patients from four Brazilian regions (Southeast, Northeast, South, and Central West) were identified. Patient demographics and clinical characteristics stratified by COVID-19 outcome are shown in Table 1. The study cohort included predominantly female patients (65%) with a median age of 61 years (range, 19-91 years). Comorbidities were common, mostly hypertension (45%), diabetes (22%), and cardiovascular disease (12%), alongside history of current or former smoking (21%). Out of 41 patients with smoking history, five were active smokers and 36 stopped smoking for a median time of 14 months (IQR, 4-53). Median tobacco exposure was one pack of cigarettes per day for 23 years. Regarding cancer diagnosis and treatment, 167 patients (84%) had solid tumors and 31 (16%) had hematologic malignancies treated at community practices. The median time from cancer diagnosis to COVID-19 was 20 months (IQR, 6-47). Most frequent malignancies were breast cancer (29%) followed by prostate cancer (11%), lung cancer (8%), colorectal cancer (7%), chronic leukemia (5%), and multiple myeloma (5%). At cancer diagnosis, the majority of patients had stage IV disease or advanced hematologic malignancies (54%). Most patients were on active systemic therapy or radiotherapy (77%), largely in the noncurative setting (64%). Chemotherapy-based regimens (including combinations with immunotherapy or targeted agents) were the most frequent systemic treatments (40%), followed by hormonal therapies (16%) and targeted agents (11%). Approximately half of the study cohort (49%) received the last dose of systemic therapy or radiotherapy in the 4 weeks preceding SARS-CoV-2 isolation. In total, 23 patients (12%) were on supportive care in a palliative setting (symptom control only) and 22 patients (11%) were in post-treatment remission without active anticancer therapy.
TABLE 1.
Demographic and Clinical Characteristics of Patients With Cancer Stratified According to COVID-19 Outcome
The number of SARS-CoV-2–negative and SARS-CoV-2–positive cases during the study period is shown in Figure 1A. About half of the patients with cancer with a suspicious diagnosis of COVID-19 tested positive for SARS-CoV-2. The cumulative number of COVID-19 diagnoses by week stratified according to tumor type is depicted in Figure 1B. The most frequent was breast cancer followed by genitourinary tract cancer and hematologic malignancies. As illustrated in Figure 1C, the largest proportion of cases was diagnosed in the Southeast and Northeast regions of Brazil. In terms of COVID-19 clinical presentation, 88% were symptomatic at diagnosis and the remaining were initially tested because caregivers or close contacts were positive for SARS-CoV-2 infection. Common symptoms included fever (63%), cough (52%), dyspnea (42%), fatigue (33%), dysgeusia (23%), myalgia (18%), headache (17%), nasal discharge (14%), diarrhea (13%), sore throat (8%), and vomiting (5%). Most of the patients had mild symptoms, 55% were managed in the outpatient setting, but 45% required hospital admission because of moderate or severe infection. From these patients, 42% were admitted to ICU, as detailed in Figure 2A.
FIG 1.
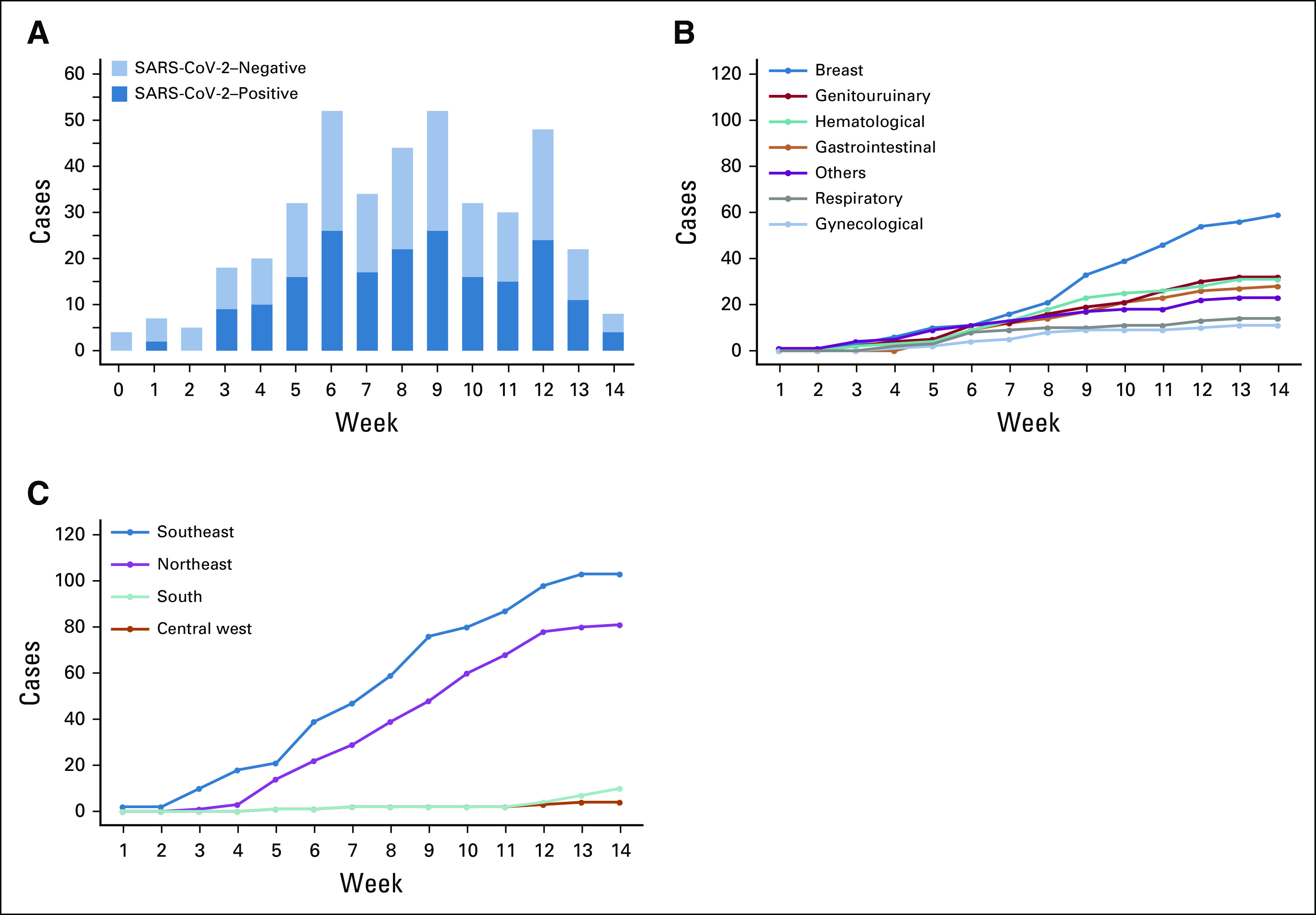
(A) Number of SARS-CoV-2–negative and SARS-CoV-2–positive diagnoses in patients with cancer during the study period. (B) Cumulative number of COVID-19 cases by week stratified according to tumor type. (C) Cumulative number of COVID-19 cases by week stratified according to geographical region of Brazil.
FIG 2.
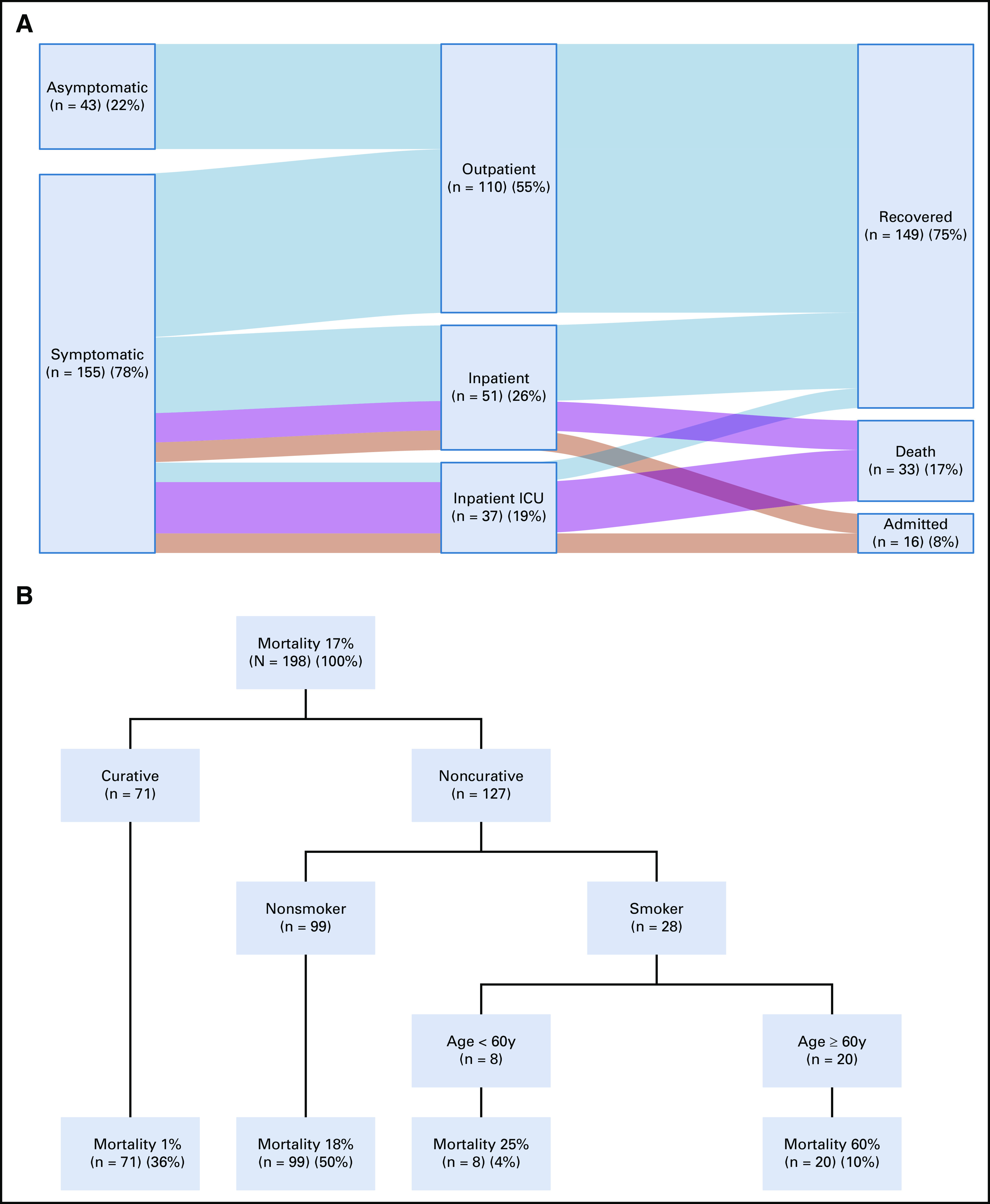
(A) Relationship of clinical presentation, patient care environment, and outcome of cancer patients with COVID-19. The vertical dark blue bars denote the patient cohort split into different groups. The light horizontal bars denote associations between the different groups, with wider bars denoting more overlap. (B) Final results of the Recursive Partitioning Multivariable Model with decision trees, which classifies patients in different death risk populations based on dichotomous independent variables. ICU, intensive care unit.
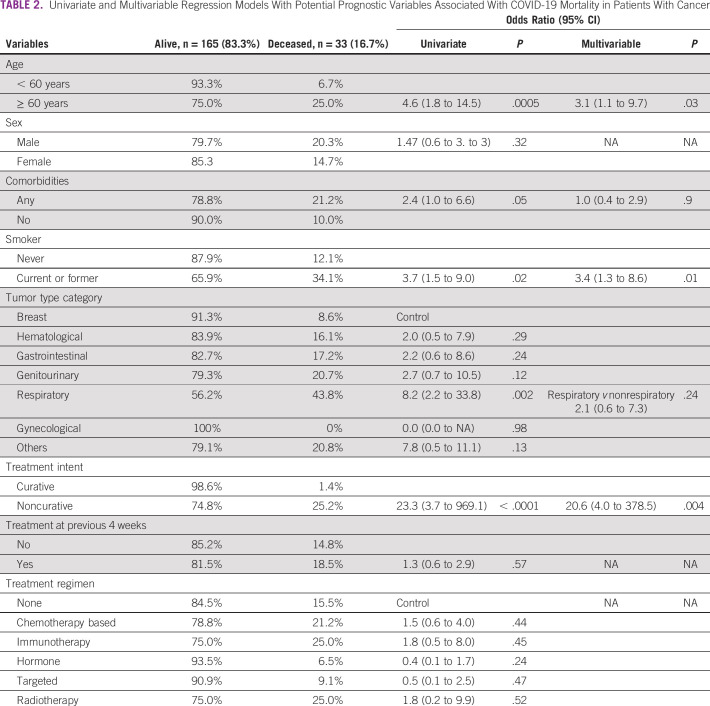
At the time of study analysis, a total of 33 patients (16.7%, 95% CI, 11.9 to 22.7) had died after COVID-19 diagnosis. Median follow-up was 61 days, and all patients were monitored until data cutoff. As shown in Table 2, univariate regression model demonstrated that compared with the remaining cohort, elderly patients had higher odds of COVID-19 mortality (25.0% v 6.7%; OR, 4.6; 95% CI, 1.8 to 14.5), as were those with prior comorbidities (21.2% v 10.0%; OR, 2.4; 95% CI, 1.0 to 6.6) and current or former smoking history (34.1% v 12.1%, OR, 3.7; 95% CI, 1.5 to 9.0). Mortality was significantly higher in patients receiving oncology care in the noncurative setting as compared with those with early-stage treated with curative intent or in remission (25.2% v 1.4%; OR, 23.3; 95% CI, 3.7 to 969.1). Compared with patients who had received systemic therapy or radiotherapy within 4 weeks of the positive test for SARS-CoV-2, those who had not received recent anticancer therapy did not suffer increased mortality when analyzed by univariate analysis (18.5% v 14.8%; OR, 1.3; 95% CI, 0.6 to 2.9). Different modalities of cancer therapy, including systemic agents (chemotherapy-based, hormone therapy, targeted therapy, and immunotherapy) or radiotherapy within 4 weeks of COVID-19, were not associated with mortality. The highest death rate was found in patients with respiratory tract malignancies (43.8%), mostly metastatic lung cancer, while case fatality was lower in patients with breast or gynecological tumors (8.6% and 0%, respectively) and intermediate in the remaining tumor types, including hematologic malignancies (16.1%).
TABLE 2.
Univariate and Multivariable Regression Models With Potential Prognostic Variables Associated With COVID-19 Mortality in Patients With Cancer
The multivariable prediction model for patient mortality after COVID-19 included age, comorbidities, smoking status, tumor type (respiratory v others), and disease setting. After covariate adjustment, only disease setting (noncurative v curative, OR, 20.6; 95% CI, 4.0 to 378.5), smoking status (current or former v no smoking history; OR, 3.4; 95% CI, 1.3 to 8.6), and elderly population ( ≥ 60 years v younger, OR, 3.1; 95% CI, 1.1 to 9.7) were associated with increased risk of death (Table 2). The presence of comorbidities and tumor type did not have an impact on the mortality rate in the adjusted multivariable model.
The results of the recursive partitioning model are comparable with those of primary analysis with multivariable logistic regression, with the selection of similar variables to construct the decision tree displayed in Figure 2B. Mortality was 1% in patients under curative treatment or in remission, 18% in the noncurative setting among never smokers, 25% in the noncurative setting among smokers of age < 60 years, and 60% in the noncurative setting among smokers of age 60 years or older.
DISCUSSION
Cancer patients with COVID-19 have been reported as experiencing more ICU admissions, more mechanical ventilation support, and mortality rates when compared with the infected patients without malignancies.7,23 In general, patients with cancer are a more vulnerable group for COVID-19-related complications because of several reasons, including older age, presence of comorbidities, the immune system suppression caused by cancer itself and its treatments, and reduced pulmonary function because of primary lung tumors, pulmonary metastases, or pleural effusions.9 Moreover, patients with cancer have regular visits with physicians, nurses, and the multidisciplinary team involved in oncology care, and the occurrence of nosocomial SARS-CoV-2 infection in patients with cancer is around 10-fold higher than in individuals without malignancies.8
In the present study, age ≥ 60 years, history of heavy smoking, and active advanced or metastatic disease were the main determinants of death after diagnosis of COVID-19. The mortality rate in this cohort (16.7%) appears slightly lower than the average reported in other studies summarized in Table 3 (23.3%). However, compared with the other cohorts, the population included in our study has a younger median age and a higher proportion of patients with breast cancer, who have a lower mortality rate in the adjuvant setting. In addition, our study assessed patients initially diagnosed in an outpatient environment, in contrast to Chinese cohorts and another Brazilian cohort, which selected only hospitalized patients with higher rate of severe COVID-19 and, consequently, increased mortality rates.13,14,24 Furthermore, we believe that most SARS-CoV-2 transmissions in our population were from the community not in the healthcare setting. It is worth noting that patients with hematological cancer seem to be overrepresented as the third most frequent malignancy in the present study (16%), which is far higher than the frequency observed in regular cancer populations,25 including Oncoclínicas, where they represent < 10% of all visited patients on a yearly basis. This finding reinforces previous knowledge of the higher susceptibility of patients with hematological cancer to infections.8,12
TABLE 3.
Cohorts of Patients With Cancer and COVID-19 (at Least 100 Patients)
The analysis by a recursive partitioning multivariable model demonstrated that there is meaningful heterogeneity in the mortality risk among patients with cancer. The strongest prognostic factor in the population described here was patient management in a noncurative setting, meaning the presence of active metastatic or advanced disease, which might be explained by the frequent association with poor performance status, organ dysfunction, malnutrition, and decreased immunological function. The model also suggests that patients with early-stage disease present an average mortality risk compared with the general population with COVID-19,26 whereas heavy smokers with metastatic disease, frequently represented by patients with lung cancer, display much higher death risk when infected by the SARS-CoV-2. Our findings reinforce data from recent studies suggesting that active malignancy may be a contributive risk factor for the unfavorable prognosis of COVID-19 in patients with cancer only when combined with other risk factors, particularly heavy smoking.27,28 Indeed, most of our patients had mild COVID-19 disease and were managed in an outpatient environment until complete recovery.
It is important to reinforce that, in our cohort, 11 out of 33 patients who died after COVID-19 diagnosis were not admitted to ICU after shared decision between family members and medical team to avoid invasive mechanical ventilation given futility of the procedure in poor prognosis patients (eight out of 11 patients received anticancer therapies within 4 weeks of SARS-CoV-2 diagnosis). The decision to pursue conservative measures is an important confounding that affects all-cause mortality for all cancer patients’ cohorts with COVID-19. In addition, given distributed care across multiple hospitals outside the Oncoclínicas network, we were not able to collect information on supportive procedures, such as early ventilatory support and steroid use, which might have a positive impact on the outcome of severe COVID-19. We also recognize the possibility of underreporting of SARS-CoV-2 testing in our cancer population. However, we believe that our study accurately reflects the first pandemic outbreaks in Southeast and Northeast regions of Brazil, where Oncoclínicas has most of the ambulatory care practices. After July 2020, data cutoff of the present analysis, the number of cases and deaths from COVID-19 has seen a gradual reduction across the country.2
The detrimental impact of the COVID-19 pandemic on cancer care is widespread, with varying magnitude among centers worldwide.29 It is essential to acknowledge that our study was conducted in the private health setting for selected health insurance covered patients with cancer in Brazil, close to 20% of the citizens. The poorest regions of the country and the population cared for under the public health system, who face significant barriers to healthcare access, were not represented in the present study. Therefore, even though there were restrictions and delays for patients treated within our institution, this impact is less pronounced in comparison with the challenges faced in the Brazilian public health system. Data on how the pandemic has and will affect patients with cancer in low- to middle-income countries are essential for the optimal guidance of future steps.
In conclusion, this study demonstrates that some patients with cancer face a higher risk of death if affected by COVID-19, which is predominantly driven by older age, smoking, and the presence of active disease and associated risk factors. Patients who are otherwise healthy and have curable malignancies present COVID-19-related mortality rates similar to the general population and should not have delayed access to cancer treatment. The oncology community is trying to thoughtfully balance fear of COVID-19 against the dire consequences of not treating cancer in an effective or timely manner.30 Our data endorse the recommendations to minimize the risk of SARS-CoV-2 infection in patients with cancer with active preventive measures, especially in subgroups of patients with recognized poor prognostic factors, and to perform close monitoring in the case of exposure to the virus or COVID-19–related symptoms.
ACKNOWLEDGMENT
We would like to thank all Oncoclínicas Group collaborators who actively monitored patients and shared information for database completion.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Márcia Menezes
Stock and Other Ownership Interests: Grupo Oncoclinicas
Pedro De Marchi
Honoraria: Sanofi
Consulting or Advisory Role: Merck Sharp & Dohme, AstraZeneca, Bristol-Myers Squibb, Roche, Bayer
Speakers' Bureau: Merck Sharp & Dohme, Bristol-Myers Squibb, Roche, AstraZeneca, Novartis, Merck, Boehringer Ingelheim
Research Funding: AstraZeneca, Amgen, Merck Sharp & Dohme, Roche
Travel, Accommodations, Expenses: Merck Sharp & Dohme, AstraZeneca, Roche, Bristol-Myers Squibb
Andréia Cristina de Melo
Honoraria: MSD Oncology, Novartis, BMS Brazil
Speakers' Bureau: BMS Brazil, MSD Oncology
Research Funding: Roche, MSD Oncology, BMS Brazil, Novartis, Clovis Oncology, AstraZeneca
Travel, Accommodations, Expenses: MSD Oncology
Alexandre A. Jácome
Speakers' Bureau: Roche, Servier
Tomás Reinert
Honoraria: Novartis, AstraZeneca, Pfizer, Lilly, Pierre Fabre, Libbs
Consulting or Advisory Role: Lilly, Novartis
Research Funding: AstraZeneca, Libbs
Rafael Duarte Paes
Speakers' Bureau: Merck Sharp & Dohm, Libbs, Amgen
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Carlos H. Barrios
Stock and Other Ownership Interests: Biomarker, MedSIR, Tummi
Honoraria: Novartis, Roche/Genentech, Pfizer, GlaxoSmithKline, Sanofi, Boehringer Ingelheim, Eisai, MSD, Lilly
Consulting or Advisory Role: Boehringer Ingelheim, Roche/Genentech, Novartis, GlaxoSmithKline, Eisai, Pfizer, AstraZeneca, Libbs, MSD Oncology, United Medical, Lilly
Research Funding: Pfizer, Novartis, Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Roche/Genentech, Lilly, Sanofi, Taiho Pharmaceutical, Mylan, Merrimack, Merck, Abbvie, Astellas Pharma, Biomarin, Bristol-Myers Squibb, Daiichi Sankyo, Abraxis BioScience, AB Science, Asana Biosciences, Medivation, Daiichi Sankyo, Exelixis, ImClone Systems, LEO Pharma, Millennium, Janssen, Atlantis Clinica, INC Research, Halozyme, Covance, Celgene, inVentiv Health
Travel, Accommodations, Expenses: Roche/Genentech, Novartis, Pfizer, BMS Brazil, AstraZeneca, MSD Oncology, Lilly
Rodrigo Dienstmann
Consulting or Advisory Role: Roche, Boehringer Ingelheim
Speakers' Bureau: Roche, Ipsen, Sanofi, MSD Oncology, SERVIER, Amgen, Merck
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Bruno L. Ferrari, Carlos Gil Ferreira, Márcia Menezes, Pedro De Marchi, Jorge Canedo, Andréia Cristina de Melo, Alexandre A. Jácome, Tomás Reinert, Bárbara Sodré, Rodrigo Dienstmann, Carlos H. Barrios
Financial support: Bruno L. Ferrari
Administrative support: Andréia Cristina de Melo, Bruno L. Ferrari
Collection and assembly of data: Bruno L. Ferrari, Carlos Gil Ferreira, Márcia Menezes, Pedro De Marchi, Rafael Duarte Paes, Bárbara Sodré, Rodrigo Dienstmann, Carlos H. Barrios
Data analysis and interpretation: Bruno L. Ferrari, Carlos Gil Ferreira, Pedro De Marchi, Jorge Canedo, Andréia Cristina de Melo, Rafael Duarte Paes, Bárbara Sodré, Rodrigo Dienstmann, Carlos H. Barrios
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
REFERENCES
- 1.WHO : Coronavirus Disease (COVID-19) Pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports [Google Scholar]
- 2.Brasil MdS : Coronavírus Brasil. https://covid.saude.gov.br/ [Google Scholar]
- 3.Aquino EML, Silveira IH, Pescarini JM, et al. : Social distancing measures to control the COVID-19 pandemic: Potential impacts and challenges in Brazil. Cien Saude Colet 25:2423-2446, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Marson FAL: COVID-19—6 million cases worldwide and an overview of the diagnosis in Brazil: A tragedy to be announced. Diagn Microbiol Infect Dis 98:115113, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J, Ouyang W, Chua MLK, et al. : SARS-CoV-2 transmission in patients with cancer at a Tertiary Care Hospital in Wuhan, China. JAMA Oncol 6:1108-1110, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai A, Sachdeva S, Parekh T, et al. : COVID-19 and cancer: Lessons from a pooled meta-analysis. JCO Glob Oncol 6:557-559, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang W, Guan W, Chen R, et al. : Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol 21:335-337, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai M, Liu D, Liu M, et al. : Patients with cancer appear more vulnerable to SARS-CoV-2: A multicenter study during the COVID-19 outbreak. Cancer Discov 10:783-791, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuderer NM, Choueiri TK, Shah DP, et al. : Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 395:1907-1918, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee LYW, Cazier JB, Starkey T, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet 395:1919-1926, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian Y, Qiu X, Wang C, et al. : Cancer associates with risk and severe events of COVID-19: A systematic review and meta-analysis. Int J Cancer (in press) [DOI] [PMC free article] [PubMed]
- 12.Mehta V, Goel S, Kabarriti R, et al. : Case fatality rate of cancer patients with COVID-19 in a New York Hospital System. Cancer Discov 10:935-941, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang K, Sheng Y, Huang C, et al. : Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: A multicentre, retrospective, cohort study. Lancet Oncol 21:904-913, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Melo AC, Thuler LCS, da Silva JL, et al. : Cancer inpatient with COVID-19: A report from the Brazilian National Cancer Institute. medRxiv. doi: 10.1101/2020.06.27.20141499 [DOI] [PMC free article] [PubMed]
- 15.Zhang H, Wang L, Chen Y, et al. : Outcomes of novel coronavirus disease 2019 (COVID-19) infection in 107 patients with cancer from Wuhan, China. Cancer 126:4023-4031, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dingemans AC, Soo RA, Jazieh AR, et al. : Treatment guidance for patients with lung cancer during the Coronavirus 2019 pandemic. J Thorac Oncol 15:1119-1136, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaves ALF, Castro AF, Marta GN, et al. : Emergency changes in international guidelines on treatment for head and neck cancer patients during the COVID-19 pandemic. Oral Oncol 107:104734, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burki TK. Cancer guidelines during the COVID-19 pandemic. Lancet Oncol 21:629-630, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curigliano G, Cardoso MJ, Poortmans P, et al. : Recommendations for triage, prioritization and treatment of breast cancer patients during the COVID-19 pandemic. Breast 52:8-16, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinkove R, McQuilten ZK, Adler J, et al. : Managing haematology and oncology patients during the COVID-19 pandemic: Interim consensus guidance. Med J Aust 212:481-489, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guckenberger M, Belka C, Bezjak A, et al. : Practice recommendations for lung cancer radiotherapy during the COVID-19 pandemic: An ESTRO-ASTRO consensus statement. Radiother Oncol 146:223-229, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramirez PT, Chiva L, Eriksson AGZ, et al. : COVID-19 global pandemic: Options for management of gynecologic cancers. Int J Gynecol Cancer 30:561-563, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Zhu F, Xie L, et al. : Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann Oncol 31:894-901, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian J, Yuan X, Xiao J, et al. : Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: A multicentre, retrospective, cohort study. Lancet Oncol 21:893-903, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2020. CA Cancer J Clin 70:7-30, 2020 [DOI] [PubMed] [Google Scholar]
- 26.Prevention CfDCa : Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19)—United States, February 12-March 16, 2020. http://www.ecie.com.ar/images/paginas/COVID-19/4MMWRSevere_Outcomes_Among_Patients_with_Coronavirus_Disease_2019_COVID-19-United_States_February_12-March_16_2020.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brar G, Pinheiro LC, Shusterman M, et al. : COVID-19 severity and outcomes in patients with cancer: A matched cohort study. J Clin Oncol 2020:JCO2001580. doi:10.1200JCO.20.01580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garassino MC, Whisenant JG, Huang LC, et al. : COVID-19 in patients with thoracic malignancies (TERAVOLT): First results of an international, registry-based, cohort study. Lancet Oncol 21:914-922, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jazieh AR, Akbulut H, Curigliano G, et al. : Impact of the COVID-19 pandemic on cancer care: A global collaborative study. JCO Glob Oncol 6:1428-1438, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cannistra SA, Haffty BG, Ballman K: Challenges faced by medical journals during the COVID-19 pandemic. J Clin Oncol 38:2206-2207, 2020 [DOI] [PubMed] [Google Scholar]